Abstract
Structural colours, nature's most pure and intense colours, originate when light is scattered via nanoscale modulations of the refractive index. Original colours in fossils illuminate the ecological interactions among extinct organisms and functional evolution of colours. Here, we report multiple examples of vivid metallic colours in diverse insects from mid-Cretaceous amber. Scanning and transmission electron microscopy revealed a smooth outer surface and five alternating electron-dense and electron-lucent layers in the epicuticle of a fossil wasp, suggesting that multilayer reflectors, the most common biophotonic nanostructure in animals and even plants, are responsible for the exceptional preservation of colour in amber fossils. Based on theoretical modelling of the reflectance spectra, a reflective peak of wavelength of 514 nm was calculated, corresponding to the bluish-green colour observed under white light. The green to blue structural colours in fossil wasps, beetles and a fly most likely functioned as camouflage, although other functions such as thermoregulation cannot be ruled out. This discovery not only provides critical evidence of evolution of structural colours in arthropods, but also sheds light on the preservation potential of nanostructures of ancient animals through geological time.
Keywords: Insecta, cuckoo wasps, colour, Cretaceous, amber, taphonomy
1. Introduction
Nature's colours principally derive from three sources, i.e. bioluminescence, pigments and structural colours. Structural colours, produced by nanoscale biophotonic structures, are the most pure and intense colours in nature [1,2]. Original colours in fossils illuminate the visual communication strategies and functional evolution of colours [3,4]. Direct visual evidence of original coloration in fossils is exceedingly rare [4] because the original colours are easily altered during fossilization and most often lost altogether [5]. Our knowledge about original coloration in fossil animals is largely based on reconstructions of melanin- or carotenoid-based reptile integument and plumage of fossil birds and feathered dinosaurs [6–10], and biophotonic nanostructures of fossil insects [11–14]. To date, all known visual metallic structural colours are confined to the Cenozoic, not earlier than 48 million years ago (Ma) [3,12,15]. Metallic coloured fossil insects in resin occur in Quaternary Colombian copal, Miocene Mexican amber and Eocene Baltic amber [12,16,17], but the cause and fidelity of fossil colours remain elusive. D'Alba et al. [14] have examined the nanostructure and colour of extant and amber-entombed gold-coloured representatives of micropterigid moths (Lepidoptera) and springtails (Collembola), demonstrating that the golden coloration is produced by diffraction gratings. Here, we show diverse metallic colours in multiple insect lineages (representing three orders) in approximately 99 Myr-old amber, which are caused by multilayer reflectors (MLs). Our electron microscopy analysis of the coloured insects, though long thought to be improbable [16], reveals the cause of colour preservation in Cretaceous insects, with implications for the taphonomy of nanostructures in amber.
2. Results
(a). Metallic coloured insects in Cretaceous Burmese amber
The studied material (35 specimens) are inclusions from mid-Cretaceous Burmese amber, approximately 99 Myr old [18], including a diversity of insects belonging to three orders (Coleoptera, Diptera and Hymenoptera) and at least seven families (identified by C.C.). Most of these insects are brightly coloured throughout the whole body or have only some body parts coloured. They display vivid green, blue, bluish-green, yellowish-green and occasionally purple, metallic colours (figure 1; electronic supplementary material, figures S1–S4) under strong reflected light, especially when observed with a black background.
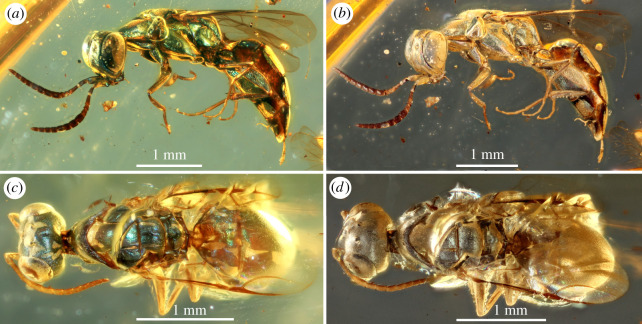
Figure 1.
Diverse structural-coloured insects in mid-Cretaceous amber from northern Myanmar. (a) A metallic green cleptine cuckoo wasp and a non-metallic brown stem-group ant, NIGP166126. (b) Wasp with metallic bluish-green head and green mesosoma, NIGP166127. (c) Cuckoo wasp with bluish-green head and mesosoma, NIGP166128. (d) Metallic green head and mesosoma of cleptine wasp, NIGP166129. (e) Cleptine wasp with bluish-green head, mesosoma and femora, NIGP166130. (f) Chalcid wasp with metallic blue head and mesosoma, NIGP166131. (g) Aculeata wasp with green head and mesosoma, NIGP166132. (h) Primitive soldier fly with bluish-green thorax, NIGP166146. (i) Elongate bark-gnawing beetle with metallic blue head, pronotum and elytra, FXBA10102. (Online version in colour.)
The most distinctively coloured insects (29 specimens) are represented by cuckoo wasps (family Chrysididae) and chalcid wasps (superfamily Chalcidoidea), which are usually of small body sizes, making them ideal candidates for preservation in amber. Like their modern relatives (electronic supplementary material, figure S5a–h), the diverse fossil chrysidids (figure 1a,c–e; electronic supplementary material, figures S1, S2 and S3a–c) have metallic bluish-green, yellowish-green, purplish-blue or green colours on the head, mesosoma, antennal scapes and pedicels, femora, tibiae, and the whole or partial metasoma. By contrast, other body parts such as the wings, flagella, tarsi and occasionally the apical metasoma are not metallic but brown, showing the colour of chitinous cuticle. As in most extant coloured chalcidoids, metallic dark-blue colour of the fossil (figure 1f) is confined to the head and mesosoma; other parts such as antennae, legs and metasoma are generally dark brown. The coloured fossil beetles (figure 1i; electronic supplementary material, figure S4), represented by five specimens, belong to three extant families: Trogossitidae (bark-gnawing beetles), Helotidae, and Staphylinidae (rove beetles). The trogossitids, with an elongate body and truncate elytra, are close to the extant genus Nemozoma (tribe Trogossitini). The body is wholly blue, but the elytra are either purple or blue in different species. Similar metallic colours, though uncommon, are found in the extant genus Temnoscheila (electronic supplementary material, figure S5h). The whole body of the helotid is metallic green, similar to the tropical Southeast Asian Metahelotella marthae (electronic supplementary material, figure S5i). The fossil rove beetle, belonging to the subfamily Phloeocharinae (near Vicelva), has brilliant metallic greenish-blue colour on the elytra. The fossil fly (figure 1h) is a soldier fly (Stratiomyidae), representing a primitive form as evidenced by its wing venation. The dorsal side of the thorax is metallic dark-green, very similar to that of some extant members of Beridinae and Sarginae [19].
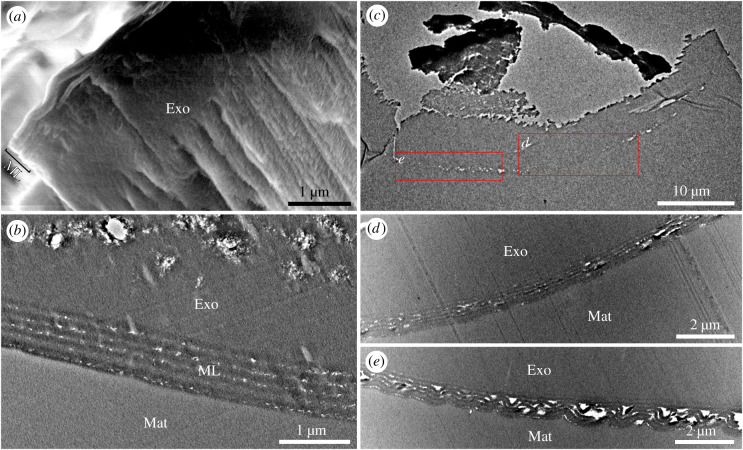
Bright metallic colours, occurring in diverse animals such as birds, butterflies and fishes, result from MLs [1,2]. Among insects, iridescent coloration of cuckoo wasps is generated by an epicuticular ML [20], and this biophotonic nanostructure is also the most common iridescence mechanism in beetles [21,22]. To investigate the cause of metallic colours of amber-entombed insects, we used light, scanning and transmission electron microscopy (SEM and TEM) and calculated the reflectance spectra. Two fossil chrysidids (NIGP-S01 and NIGP-S02) were analysed in detail using electron microscopy. Light microscopy revealed that the head, lateral pronotum, mesopleuron and femur of the sampled wasp (NIGP-S02) shone metallic bluish-green, whereas the pronotum and mesoscutum were non-metallic black under strong light. No obvious colour difference was observed when the body was viewed from different angles. SEM of the cuticle of the mesoscutum of the other analysed chrysidid (NIGP-S01) revealed the regular layering of the cuticle (figure 2a): the cuticle is divided into a finely layered epicuticle and the unlayered mummified exocuticle, as in those found in extant Chrysididae [20]. TEM of the cuticle of the mesopleuron (figure 2b; electronic supplementary material, figure S6a,c,e) revealed the presence of multiple layers in the epicuticle and the somewhat altered exocuticle, the latter probably caused by dehydration [23]. The surface of the epicuticle is smooth, and no corrugations were detected (figure 2b; electronic supplementary material, figure S6e). Owing to the mummified nature of the epicuticle, it is difficult to measure the true thickness of the exocuticle. The epicuticle, namely the outermost part of the cuticle, has a total thickness of about 770 nm, including a 50 nm surface layer and five alternating electron-lucent (low refractive indices about 29 nm each) and electron-dense (high refractive indices about 115 nm each) layers. The laminar array within the epicuticle has a periodicity of about 144 nm, allowing it to generate a structural colour, and this photonic nanostructure corresponds to a typical ‘non-ideal’ multilayer [24]. Because there is no evidence of a diffraction grating or of three-dimensional photonic crystals on the epicuticle [12], the metallic colour is caused by the identified ML in the epicuticle, demonstrating, to our knowledge, the first example of ML in an amber fossil.
Figure 2.
Scanning and transmission electron microscopy (SEM and TEM) of sampled amber-entombed wasps. (a) SEM of cuticle of silvery cleptine wasp, NIGP-S01, showing multi-layered epicuticle and unlayered exocuticle. (b) TEM of metallic bluish-green mesopleuron of cleptine wasp, showing multilayer reflector in epicuticle, NIGP-S02. (c) TEM of dull cuticle of mesoscutum of cleptine wasp, NIGP-S02. (d) Enlargement of (c) showing slightly altered multilayer reflector. (e) Enlargement of (c) showing strongly corrugated multilayer reflector. Exo, exocuticle; Mat, matrix; ML, multilayer reflector. (Online version in colour.)
To understand why structural coloration is not always preserved in amber inclusions, sections of a dull cuticle sample extracted from the mesoscutum of specimen NIGP-S02 were studied. TEM of the non-iridescent cuticle (figure 2c–e; electronic supplementary material, figure S6b,d,f) revealed the presence of altered ML within the epicuticle: it comprised five alternating electron-lucent and electron-dense layers, similar to those in the epicuticle of the coloured mesopleuron, but the ML varied in thickness (ca 746–970 nm) and was strongly corrugated, causing irregular electron-lucent spaces and a wavy cuticle surface, suggestive of possible dehydration during fossilization [23,25]. Apparently, the nanostructure of the altered ML has been damaged, explaining the absence of metallic colours and the dull black colour of the mesoscutum. Our TEM results demonstrate that the quality of preservation may vary drastically in different body parts of a single specimen.
(b). Theoretical modelling of the reflectance spectra of a fossil multilayer reflector
To test whether the observed colours of the fossil wasp NIGPS-02 were caused by ML and whether they may have been altered during the fossil diagenesis in amber, the reflective peak of wavelength of the preserved ML structure in specimen NIGPS-02 was calculated based on theoretical modelling of the reflectance spectra. Theoretically, in a ‘non-ideal’ multilayer system, the first-order reflectance peak occurs at λmax = 2(nada + nbdb), where λ is the wavelength, na and nb are the refractive indices of the light and dense layers, and da and db are the actual thicknesses, i.e. at twice the sum of the optical thicknesses of the light and dense layer-pairs [24]. In the fossil ML of the coloured area, there is no evidence that the epicuticle experienced diagenetic alterations, as the amber resin is usually excluded by the impermeable cuticle of insects [23]. Therefore, the fossil ML provides a reliable model for determining the preservation fidelity of cuticular nanostructures. Based on the analogous data for the ML within the epicuticle of extant and fossil insects [12,15,20], the high- and low-index layers of the ML are assigned the refractive index values of 1.73 and 1.4, respectively. We calculated the reflectance spectra for wavelengths ranging from 350 to 800 nm using the OpenFilters software [26]. The model revealed a spectrum with one dominant reflectance wavelength at 514 nm under normal incidence (θ = 0°) (figure 3). These values are qualitatively similar to the observed bluish-green colour of the sampled part of the fossil wasp.
Figure 3.
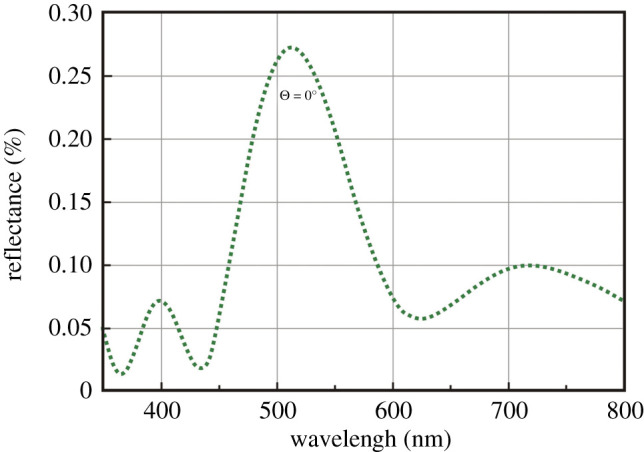
Computed reflectance spectrum for the epicuticular multilayer reflector in the bluish-green mesopleuron of a cleptine wasp. (Online version in colour.)
3. Discussion
The colour displayed by fossils can often be misleading because fine nanostructures responsible for coloration can be altered during fossilization. However, the original colour of fossils can be reconstructed using theoretical modelling [11]. The calculated reflectance peaks match the observed metallic bluish-green coloration of the mesopleuron of our studied wasp, confirming that extremely fine nanostructures can be preserved in Mesozoic amber. While this in itself does not necessarily indicate that the colour preserved in the fossils is original, there are several factors that suggest that this may indeed be the case. Unlike the non-iridescent wasp mesoscutum (NIGP-S02) which displayed damaged cuticular nanostructure, the nanostructure from the strikingly iridescent sample NIGP-S01 is clearly non-corrugated. The layers are not superimposed, flexed or fractured. There are no diagenetic desiccation cracks, which would be expected to form in rapidly mummified insects. Moreover, there is no evidence of the expansion of the nanostructure, as spacers between layers are continuous and uniform. Taken together, our observations strongly suggest that the colour preserved in some amber inclusions may be the same as displayed by the insects when alive, some 99 Ma. This is moreover corroborated by the fact that metallic blue–green coloration is frequently found in extant cuckoo wasps [20].
This rare quality of preservation seems surprising; as Burmese amber originates from mudstone that was subjected to high pressure during formation [27]. Amber inclusions from the same deposit show an incredible variation in preservation quality, some being represented by mere carbonized films or pyrite replacements. On the other end of the spectrum, amber can preserve some organisms with exceptional fidelity as dried-out mummies, even conserving their internal organs [28]. The fossils studied by us appear to fall towards this end of the spectrum of preservation, as indicated by their symmetrical, undistorted body parts and intact cuticle. Thus, our study provides new information about the taphonomy of amber inclusions.
The colours of visible light produced by the biophotonic nanostructure are determined by the periodicity of the ML structure and the refractive indices of alternating layers [12,22]. Based on our electron microscopy data on different parts of the wasp and the high-fidelity preservation of amber inclusions [5], the ultrastructures in, at least, parts of the fossil epicuticles persisted during fossilization. Maturation experiments using extant structurally coloured beetles suggested that high temperature contributes significantly to the colour change during burial of compressions [29]. There is no evidence showing modifications of the epicuticle ultrastructure of the coloured part, and subsequently of the refractive index of the epicuticle. In extant insects, the refractive index of the cuticle depends on its biomolecular composition [30]. Therefore, it is likely that the refractive index of the biomacromolecules in fossil epicuticles remain unchanged through long geological time, as there is evidence that chitin can be preserved in 25 Myr-old fossil beetles [31]. Although most fossil arthropod cuticles are chemically altered during diagenesis [30], a recent discovery demonstrated that traces of chitin may have survived since as early as the Palaeozoic [32–34]. Geochemical analyses of a Pleistocene structurally coloured beetle also indicated the presence of original macromolecules, including chitin [15]. In Tertiary ambers, exquisite ultrastructures such as muscles and even cell organelles can be preserved [23,25,35], but it was suggested that more decay-resistant chitin in the Miocene Dominican amber cannot have survived [36]; the latter study was based on a small random sample of amber fossils, so its conclusions might not be true for other exceptional amber deposits, organisms or particular body parts. Therefore, further exploration of the biomolecular composition of the ML in the epicuticle of amber inclusions represents a promising avenue for future research.
The metallic colours of the insects in Burmese amber appear permanent, but under some circumstances are subject to alteration. When we prepared the studied amber pieces (trimmed, ground and polished with water-fed sandpapers and polishing powder), most of them retained the original colours, whereas some lost them, and partially or wholly turned to metallic silver after several days (figure 4; electronic supplementary material, figure S7). Once the colours changed, the changes are irreversible, obviously differing from reversible colour change in some extant beetles [22]. Based on a comparative observation between coloured and silver amber pieces, we discovered a possible factor contributing to the unexpected alteration of the structural colour: the silver amber inclusions were either covered with air bubbles touching the body or their non-metallic body parts (e.g. legs) were slightly worn out, during preparation, making them partly exposed to water and air. Amber-entombed insects are usually mummified, and their body cavities and appendages are partially or entirely hollow, which allows water and/or air to penetrate into the body and eventually the epicuticle, causing the alteration of the ML structures and/or the refractive index. In theory, the alteration of the refractive index, but not the ML, would cause a shift of the reflectance peaks of wavelengths [12] as found in some extant beetles [22], but it would not produce a silver colour. Alternatively, the silver colour may be caused by a very thin layer of air created between the insect and the amber during preparation. In extant insects, silver structural colour is known in ruteline beetles, with their cuticle characterized by a broadband reflector (chirped ML) [22,37]. In such ML, layers gradually increase in thickness towards the surface and reflect most wavelengths of light simultaneously [22]. Unlike the black colour caused by corrugated ML layering, the ML nanostructure of silver insects should have survived, but its ultrastructures and precise photonic properties need further confirmation. Our discovery of silvery colour in multiple specimens indicates that they were probably initially metallic coloured, contradicting the hypothesis that their silvery appearance is caused by the cuticle partially pulling away from the external mould formed by the amber [16].
Figure 4.
Comparisons between original and altered metallic colours in cleptine wasps. (a) Metallic green wasp before preparation, NIGP166133. (b) The same as (a) silvery wasp after preparation. (c) Metallic blue wasp before preparation, NIGP166134. (d) The same as (c) silvery wasp after preparation. (Online version in colour.)
Structural colours are widespread in modern birds and insects [2,21] and they possess different functions in various groups [22,38]. The ecological functions of structural colours in modern wasps have long been elusive [20]. Most extant chrysidid wasps lack a stinger and are not known to be noxious [39], ruling out aposematism as a cause of their coloration. If the observed structural colours played a role in intraspecific communication or signalling male quality, as has been suggested in some butterflies [40], then males would be expected to be brightly coloured while the females would have dull coloration. However, both male and female chrysidid wasps are brightly coloured [39]. This is especially puzzling given that females of many recent cuckoo wasps are, as their name suggests, kleptoparasites and lay their eggs into unguarded nests of other hymenopterans [39]. As cuckoo wasps often perch near their host's nests [41], it would be expected that female chrysidid wasps should be under strong selection pressure to avoid detection. Recent studies have brought to forefront a seemingly counterintuitive explanation of structural coloration in insects—as a form of camouflage. Studies with human, bird and insect models have shown that iridescent objects may be difficult to identify against a similarly coloured background, such as green leaves, and make the coloured insect more difficult to target [42,43]. Notably, it has been shown that bright iridescence can make it difficult for some hymenopterans to recognize shapes [43], explaining how the striking coloration of cuckoo wasps may conceal them from their hosts. Nevertheless, at present, it cannot be ruled out that the brilliant metallic blue green and yellowish green colours present in fossil cuckoo and chalcid wasps, as well as in their extant counterparts also has non-cryptic functions such as thermoregulation [20]. In any way, the fossils hint at the possibility that the intricate relationship between cuckoo wasps and their hymenopteran host may have evolved at least by the mid-Cretaceous.
The fossil rove beetle and helotid are characterized by their metallic green colour, which is also the most common multilayer colour in extant beetles. In beetles, iridescent cuticle appears to occur more often in diurnal species than in night-active species, suggesting that the coloration has an adaptive function [44]. It is probable that, just like in cuckoo wasps, this form of coloration may make the beetles more difficult to detect in the forest environment. Given that bark-gnawing beetles are mostly associated with wood [45], the purple and blue hue of the elytra of the fossil species may have likewise provided camouflage in bark and timber. The green colour on the thorax of the fossil fly probably had the same cryptic function as in cuckoo wasps and beetles. The occurrence of structural colours in several unrelated lineages of wasps and beetles demonstrates multiple repeated evolutionary origins of ML in these groups by about 99 Ma.
Our discovery of vivid structural colours and the earliest ML ultrastructure illuminate the early evolution of structural colours and camouflage strategies in Mesozoic arthropods. It confirms the existence of exquisite biophotonic nanostructures in deep time.
4. Material and methods
(a). Materials and deposits
A total of 35 fossil specimens are included in this paper. All of them are preserved in mid-Cretaceous amber from northern Myanmar, approximately 99 Myr old [18]. All fossil specimens, except FXBA10102, are housed at the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences; FXBA10102 is deposited in the Lingpoge Amber Museum in Shanghai. All studied specimens, except for NIGP173193–NIGP173201 with structural colour patchy or indistinct, are figured in the paper and the electronic supplementary material.
(b). Specimen preparation and imaging
For standard observation, fossils were polished using different grades of sandpaper and diatomite powder, to get as close to the inclusions as possible without damaging them. Where it aided observation, some pieces were polished into very thin slices, making the insect inclusions clearly observable and the surrounding amber matrix almost transparent in bright light. Photographs against various backgrounds were taken using a Canon EOS 5D Mark III digital camera, equipped with a Canon MP-E 65 mm macro lens (F2.8, 1–5X), and with an attached Canon MT-24EX twin flash. Focus stacking software (Zerene Stacker, v. 1.04) was used to increase the depth of field. The resultant images were edited in Photoshop only to correct brightness and contrast. Two amber-entombed wasps (metallic coloured and silvery) were selected for ultrastructural analysis of the cuticles. Ultrathin (70 nm thick) sections were cut with a Diatome 3 mm 45° diamond knife, mounted on formvar-coated copper grids, and air-dried. Unstained sections were imaged using a JEM-2100 Plus transmission electron microscope (at Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences) at 80 keV. The sectioned samples were mounted on stubs with double-sided carbon tape. The sectioned surface was imaged with a LEO 1530 VP analytical scanning electron microscope controlled by JEOL InTouchScope v. 1.05A software, using the variable-pressure secondary electron detector at low vacuum and at 18 keV. The theoretical reflectance spectrum of the MLs in the epicuticle of the sampled wasp was directly calculated based on the dimensions of layers and the values of their complex refractive indices. The theoretical spectrum was produced using the OpenFilters software (v. 1.0). While previous studies of structural colour in compression fossils have made use of complementary spectrometric measurements to quantify the spectra of observed colours [3,11,12], this could not be performed on our specimens, as it was not possible to separate the delicate outer layer of the cuticle from the amber matrix.
Supplementary Material
Acknowledgements
We are grateful to P. Rosa for identifying extant chrysidids and to M. E. McNamara for helpful discussions.
Data accessibility
The figures supporting this article have been uploaded as part of the electronic supplementary material, SI File 01.
Authors' contributions
C.C. and Y.P. conceived the project. C.C. drafted the manuscript, to which Y.P., D.H. and E.T. contributed. C.C. photographed fossil specimens and performed theoretical modelling of the multilayer reflector. Y.P. performed scanning- and transmission electron microscopic imaging of the amber inclusions. All authors participated in morphological studies and discussion, commented on the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The work has been supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant nos XDB26000000, XDB18000000), the National Natural Science Foundation of China (grant nos 41688103 and 91514302), and the Youth Innovation Promotion Association of the CAS (grant no. 2018347).
References
- 1.Vukusic P, Sambles JR. 2003. Photonic structures in biology. Nature 424, 852–855. ( 10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Bhushan B, Tong J. 2013. Structural colouration in nature. RSC Adv. 3, 14 862–14 889. ( 10.1039/C3RA41096J) [DOI] [Google Scholar]
- 3.Parker AR, McKenzie DR. 2003. The cause of 50 million-year-old colour. Biol. Lett. 270, S151–S153. ( 10.1098/rsbl.2003.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinther J. 2015. A guide to the field of palaeo colour. Bioessays 37, 643–656. ( 10.1002/bies.201500018) [DOI] [PubMed] [Google Scholar]
- 5.Martínez-Délclòs X, Briggs DEG, Penlaver E. 2004. Taphonomy of insects in carbonates and amber. Palaeogeogr. Palaeoclimatol. Palaeoecol. 203, 19–64. ( 10.1016/S0031-0182(03)00643-6) [DOI] [Google Scholar]
- 6.Zhang F, Kearns SL, Orr PJ, Benton MJ, Zhou Z, Johnson D, Xu X, Wang X. 2010. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463, 1075–1078. ( 10.1038/nature08740) [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Gao KQ, Vinther J, Shawkey MD, Clarke JA, D'alba L, Meng Q, Briggs DE, Prum RO. 2010. Plumage colour patterns of an extinct dinosaur. Science 327, 1369–1372. ( 10.1126/science.1186290) [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Clarke JA, Gao KQ, Zhou CF, Meng Q, Li D, D'Alba L, Shawkey MD. 2014. Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature 507, 350–353. ( 10.1038/nature12973) [DOI] [PubMed] [Google Scholar]
- 9.Lindgren J, et al. 2014. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 506, 484–488. ( 10.1038/nature12899) [DOI] [PubMed] [Google Scholar]
- 10.McNamara ME, Orr PJ, Kearns SL, Alcalá L, Anadón P, Peñalver E. 2016. Reconstructing carotenoid-based and structural coloration in fossil skin. Curr. Biol. 26, 1075–1082. ( 10.1016/j.cub.2016.02.038) [DOI] [PubMed] [Google Scholar]
- 11.McNamara ME, Briggs DE, Orr PJ, Wedmann S, Noh H, Cao H. 2011. Fossilized biophotonic nanostructures reveal the original colours of 47-million-year-old moths. PLoS Biol. 9, e1001200 ( 10.1371/journal.pbio.1001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara ME, Briggs DEG, Orr PJ, Noh H, Cao H. 2012. The original colours of fossil beetles. Proc. R. Soc. B 279, 1114–1121. ( 10.1098/rspb.2011.1677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, et al. 2018. Fossil scales illuminate the early evolution of lepidopterans and structural colors. Sci. Adv. 4, e1700988 ( 10.1126/sciadv.1700988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alba L, Wang B, Vanthournout B, Shawkey MD. 2019. The golden age of arthropods: ancient mechanisms of colour production in body scales. J. R. Soc. Interface 16, 20190366 ( 10.1098/rsif.2019.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka G, Taniguchi H, Maeda H, Nomura SI. 2010. Original structural colour preserved in an ancient leaf beetle. Geology 38, 127–130. ( 10.1130/G25353.1) [DOI] [Google Scholar]
- 16.McKellar RC, Engel MS. 2014. New bethylid and chrysidid wasps (Hymenoptera: Chrysidoidea) from Canadian Late Cretaceous amber. Paläont. Z. 88, 433–451. ( 10.1007/s12542-013-0208-y) [DOI] [Google Scholar]
- 17.Wille A. 1959. A new fossil stingless bee (Meliponini) from the amber of Chiapas, Mexico. J. Paleontol. 33, 849–852. [Google Scholar]
- 18.Shi G, Grimaldi DA, Harlow GE, Wang J, Wang J, Yang M, Lei W, Li Q, Li X. 2012. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretaceous Res. 37, 155–163. ( 10.1016/j.cretres.2012.03.014) [DOI] [Google Scholar]
- 19.Woodley NE. 2001. A world catalog of the Stratiomyidae (Insecta: Diptera). Myia 11, 1–473. [Google Scholar]
- 20.Kroiss J, Strohm E, Vandenbem C, Vigneron JP. 2009. An epicuticular multilayer reflector generates the iridescent coloration in chrysidid wasps (Hymenoptera, Chrysididae). Naturwissenschaften 96, 983–986. ( 10.1007/s00114-009-0553-6) [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita S, Yoshioka S. 2005. Structural colours in nature: the role of regularity and irregularity in the structure. Chem. Phys. Chem. 6, 1442–1459. ( 10.1002/cphc.200500007) [DOI] [PubMed] [Google Scholar]
- 22.Seago AE, Brady P, Vigneron J-P, Schultz TD. 2009. Gold bugs and beyond: a review of iridescence and structural colour mechanisms in beetles (Coleoptera). J. R. Soc. Interface 6, S165–S184. ( 10.1098/rsif.2008.0354.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henwood A. 1992. Exceptional preservation of dipteran flight muscle and the taphonomy of insects in amber. Palaios 7, 203–212. ( 10.2307/3514931) [DOI] [Google Scholar]
- 24.Land MF. 1972. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 24, 75–106. ( 10.1016/0079-6107(72)90004-1) [DOI] [PubMed] [Google Scholar]
- 25.Poinar GO Jr, Hess R. 1982. Ultrastructure of 40-million-year-old insect tissue. Science 215, 1241–1242. ( 10.1126/science.215.4537.1241) [DOI] [PubMed] [Google Scholar]
- 26.Larouche S, Martinu L. 2008. OpenFilters: open-source software for the design, optimization, and synthesis of optical filters. Appl. Optics 47, C219–C230. ( 10.1364/AO.47.00C219) [DOI] [PubMed] [Google Scholar]
- 27.Cruickshank RD, Ko K. 2003. Geology of an amber locality in the Hukawng Valley, Northern Myanmar. J. Asian Earth Sci. 21, 441–455. ( 10.1016/S1367-9120(02)00044-5) [DOI] [Google Scholar]
- 28.Huang DY., et al. 2016. New fossil insect order Permopsocida elucidates major radiation and evolution of suction feeding in hemimetabolous insects (Hexapoda: Acercaria). Sci. Rep. 6, 1–9. ( 10.1038/srep23004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamara ME, et al. 2013. The fossil record of insect colour illuminated by maturation experiments. Geology 41, 487–490. ( 10.1130/G33836.1) [DOI] [Google Scholar]
- 30.Schultz TD, Rankin MA. 1985. The ultrastructure of the epicuticular interference reflectors of tiger beetles (Cicindela). J. Exp. Biol. 117, 87–110. [Google Scholar]
- 31.Stankiewicz BA, Briggs DEG, Evershed RP, Flannery MB, Wuttke M. 1997. Preservation of chitin in 25-million-year-old fossils. Science 276, 1541–1543. ( 10.1126/science.276.5318.1541) [DOI] [Google Scholar]
- 32.Briggs DEG. 1999. Molecular taphonomy of animal and plant cuticles: selective preservation and diagenesis. Phil. Trans. R. Soc. Lond. B 354, 7–17. ( 10.1098/rstb.1999.0356) [DOI] [Google Scholar]
- 33.Cody GD, Gupta NS, Briggs DE, Kilcoyne AL, Summons RE, Kenig F, Plotnick RE, Scott AC. 2011. Molecular signature of chitin-protein complex in Paleozoic arthropods. Geology 39, 255–258. ( 10.1130/G31648.1) [DOI] [Google Scholar]
- 34.Ehrlich H., et al. 2013. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 3, 3497 ( 10.1038/srep03497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka G, Parker AR, Siveter DJ, Maeda H, Furutani M. 2009. An exceptionally well-preserved Eocene dolichopodid fly eye: function and evolutionary significance. Proc. R. Soc. B 276, 1015–1019. ( 10.1098/rspb.2008.1467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stankiewicz BA, Poinar HN, Briggs DE, Evershed RP, Poinar GO. 1998. Chemical preservation of plants and insects in natural resins. Proc. R. Soc. B 265, 641–647. ( 10.1098/rspb.1998.0342) [DOI] [Google Scholar]
- 37.Parker AR, McKenzie DR, Large MCJ. 1998. Multilayer reflectors in animals using green and gold beetles as contrasting examples. J. Exp. Biol. 201, 1307–1313. [DOI] [PubMed] [Google Scholar]
- 38.Doucet SM, Meadows MG. 2009. Iridescence: a functional perspective. J. R. Soc. Interface 6, S115–S132. ( 10.1098/rsif.2008.0395.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimsey LS, Bohart RM. 1990. The chrysidid wasps of the world, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 40.Kinoshita S, Yoshioka S, Kawagoe K. 2002. Mechanisms of structural colour in the Morpho butterfly: cooperation of regularity and irregularity in an iridescent scale. Proc. R. Soc. B 269, 1417–1421. ( 10.1098/rspb.2002.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strohm E, Kroiss J, Herzner G, Laurien-Kehnen C, Boland W, Schreier P, Schmitt T. 2008. A cuckoo in wolves' clothing? Chemical mimicry in a specialized cuckoo wasp of the European beewolf (Hymenoptera, Chrysididae and Crabronidae). Front. Zool. 5, 2 ( 10.1186/1742-9994-5-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kjernsmo K, Whitney HM, Scott-Samuel NE, Hall JR, Knowles H, Talas L, Cuthill IC. 2020. Iridescence as camouflage. Curr. Biol. 30, 551–555.e3. ( 10.1016/j.cub.2019.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kjernsmo K, Hall JR, Doyle C, Khuzayim N, Cuthill IC, Scott-Samuel NE, Whitney HM. 2018. Iridescence impairs object recognition in bumblebees. Sci. Rep. 8, 1–5. ( 10.1038/s41598-018-26571-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lövei GL, Sunderland KD. 1996. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 41, 231–256. ( 10.1146/annurev.en.41.010196.001311) [DOI] [PubMed] [Google Scholar]
- 45.Kolibáč J, Leschen RAB. 2016. Trogossitidae Fabricius, 1801. In Handbook of zoology, vol 4/38: coleoptera, beetles, vol. 1 morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim) (eds Beutel RG, Leschen RAB), pp. 241–247, 2nd edn Berlin, Germany: W DeGruyter. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The figures supporting this article have been uploaded as part of the electronic supplementary material, SI File 01.