Background:
Although the efficacy of ursodeoxycholic acid (UDCA) and obeticholic acid (OCA) for primary biliary cholangitis (PBC) has been suggested by small trials, a meta-analysis to summarize the evidence has not yet been carried out. The aim of this study was to evaluate the clinical outcomes of the combination therapy of UDCA and OCA compared with UDCA monotherapy in patients with PBC.
Methods and materials:
We searched the PubMed, EMBASE, the web of science, and the Cochrane Library databases for English-language studies published before September 2018. Studies were included if they were randomized controlled trials (RCTs) and reported relative risk (RR) estimates with 95% confidence intervals (CIs) or related data for the clinical outcomes of different therapies in patients with PBC.
Results:
Of the 1169 titles identified, two studies meeting the inclusion criteria were included in the meta-analysis. Approximately 222 patients with PBC were included in this analysis. The results of this study indicated that combination therapy was significantly superior to monotherapy in reducing serum alanine transaminase (mean difference: –15.63 IU/L; 95% CI, –21.59 to –9.68), aspartate transaminase (mean difference: –6.63 IU/L; 95% CI, –11.03 to –2.24), gamma-glutamyl transpeptidase (mean difference: –131.30 IU/L; 95% CI, –177.52 to –85.08), and C-reactive protein (mean difference = –1.17 mg/L; 95% CI, –2.19 to –0.14), but NS in improving primary endpoints of alkaline phosphatase level with 15.0% reduction from baseline, and equal or higher than the upper limit of normal serum total bilirubin (RR = 2.75; 95% CI, 0.43–17.68), conjugated bilirubin (mean difference = –0.06 mg/dL; 95% CI, –0.28 to 0.15), IgM (mean difference = –41.18 mg/dL; 95% CI, –244.45 to 162.09), and adverse events (P > 0.05).
Conclusion:
This meta-analysis demonstrated that combination therapy with UDCA and OCA provided satisfactory clinical outcomes, which may be a promising alternative for patients with PBC who had an inadequate response to UDCA therapy. Therefore, high-quality RCTs on the safety and efficacy of the combination therapy of UDCA and OCA compared with UDCA monotherapy in patients with PBC should be performed in the future.
Keywords: randomized controlled trials, combination therapy, monotherapy, primary biliary cholangitis, obeticholic acid, ursodeoxycholic acid
Introduction
Primary biliary cholangitis (PBC) is a chronic, autoimmune cholestatic liver disease that predominantly occurs in middle-aged women [1]. One crucial and unique feature of PBC is the progressive destruction of small intrahepatic bile ducts, resulting in the development of fibrosis and potential cirrhosis [2]. The prevalence ranges from 1.91 to 40.20 per 100 000 population according to different countries and has increased over time [1]. The etiology of PBC is unknown but presumed to be correlated with a combination of environmental triggers and genetically susceptible hosts [3].
Current evidence-based guidelines recommended that all patients with PBC should receive lifetime treatment. Because serum alkaline phosphatase and bilirubin are strongly associated with clinical outcomes (death or liver transplantation) in patients with PBC [4]. The only approved drug for PBC is ursodeoxycholic acid (UDCA), which slackens liver biochemical parameters and prolongs the time to liver transplantation [5,6]. However, nearly 40% of UDCA-treated patients do not have the adequate response in liver biochemistry parameters reduction and higher mortality compared to the favorable respondent, indicating a pressing unmet medical need for additional therapies [7,8].
Obeticholic acid (OCA), a selective farnesoid X receptor (FXR) agonist, has received accelerated approval in the USA in May 2016 for the treatment of patients with PBC who do not have an adequate response to UDCA, or as monotherapy in adults unable to tolerate UDCA [9,10]. Recent studies of refractory PBC to UDCA monotherapy demonstrated that patients receiving OCA for salvage treatment may improve certain biochemical indexes, immunological indicators, and clinical symptoms, but the results from different institutions appeared to be inconsistent [8–11]. The differences mentioned between the studies may be explained partially by the sample size, methodology, eligibility criteria, outcome measures, etc. The aim of this meta-analysis was to evaluate the clinical outcomes of the combination of UDCA and OCA compared with UDCA monotherapy in patients with PBC.
Methods and materials
Search strategy and selection criteria
A literature search of PubMed, EMBASE, the web of science, and the Cochrane Library, with no language restrictions, was conducted for studies published before September 2018 using the combination of the terms: PBC; randomized controlled trial (RCT); OCA. Titles and abstracts were screened for relative and duplicate references and the full texts of remained studies were retrieved for further assessment. We considered researches as eligible studies only if they met the following criteria: (1) population included in the studies were patients with PBC who incompletely responded to UDCA therapy; (2) patients in the intervention group were treated with combination therapy of OCA and UDCA; (3) patients in the control group were treated with placebo and UDCA or just UDCA; (4) RCT design; and (5) Effect size such as mean difference, relative risk (RR) with 95% confidence intervals (CIs) (or with data to calculate them) available. Reasons for exclusion were discussed by two reviewers (Li and Liao) and discrepancies were solved by other authors. The searching strategy is shown in Fig. 1.
Fig. 1.
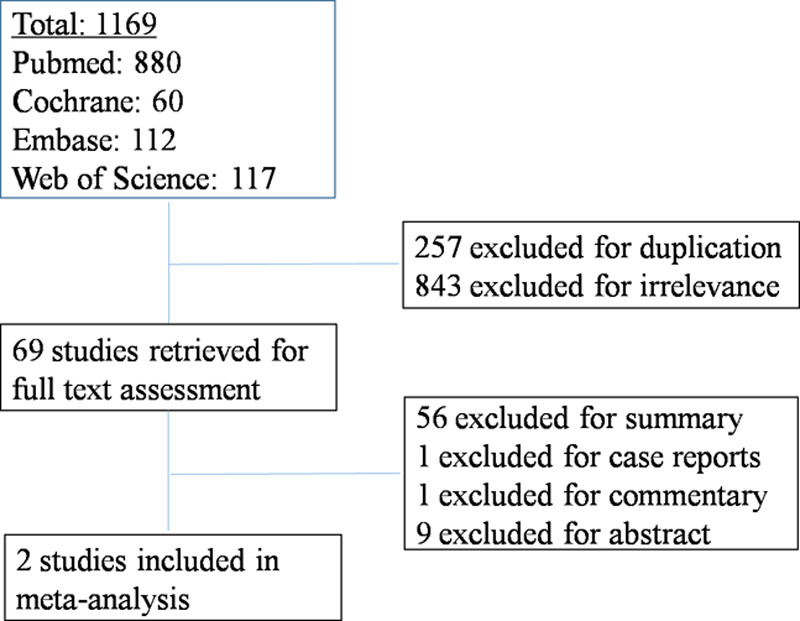
Literature search results.
Quality assessment
Quality assessment was performed, using the ‘Risk of bias table’ recommended by the Cochrane Collaboration. The table consisted of six components: subject randomization, allocation concealment of treatment, blinding, incomplete outcome data, selective reporting, and other sources of bias. Trials with high or unclear risk for bias for one of the first three components were considered as trials with high risks of bias [12]. This process was conducted independently by the reviewers (Xue and Cheng) who extracted data included authors, sample size, publication type, characteristics of the study population (including sex, age, treatment duration, and dose), and outcomes reported in all included studies. Any disagreement was resolved by consensus.
Statistical analyses
We selected the mean difference for continuous outcomes and the RR for dichotomous outcomes as the effect sizes, all were displayed with 95% CI. Data reported as final measurement values of trials were combined in the meta-analysis to calculate mean difference. Any outcome published as median (interquartile range) was converted as mean ± SD by the method recommended by Luo and Wan [13,14]. Using the χ2 and I2 tests, we detected the heterogeneity of all outcomes among included trials, and P-value <0.10 or an I2 value >50% was considered as statistically significant heterogeneity [12]. A fixed-effect model was applied to calculate the effect size when there was no heterogeneity of the results. Otherwise, the random-effect model was used.
All statistical analyses were performed with RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
A total of 1169 studies were recognized in our primary search, 1100 of which were excluded based on the screening of titles and abstracts (Fig. 1). The remained 69 publications were retrieved for full-text assessment. After excluding summary, case report, commentary, and abstract, we finally identified two RCTs that met the inclusion criteria.
The two studies were published in the year of 2015 and 2016, respectively, and the total sample size was n = 381 patients with PBC. A total of 222 patients were included in the analysis according to the inclusion criteria. The baseline demographic and clinical, laboratory characteristics of the included studies are shown in Table 1. The population in these two studies was patients with PBC who incompletely responded to the regular therapy of UDCA (13–15 mg/kg/day) initially. Then, these patients were randomly assigned to receive OCA therapy at different doses (5–25 mg/day). The mean age was 54.9 years and the ratio of male/female was 28/373. The duration of treatment of these two trials was 85 days and 12 months, respectively. The two trials included in the meta-analysis were considered with low quality as there are high or unclear risks for bias for at least one of the first three components in the risk bias table (Fig. 2).
Table 1.
Baseline characteristics of the included studies

Fig. 2.
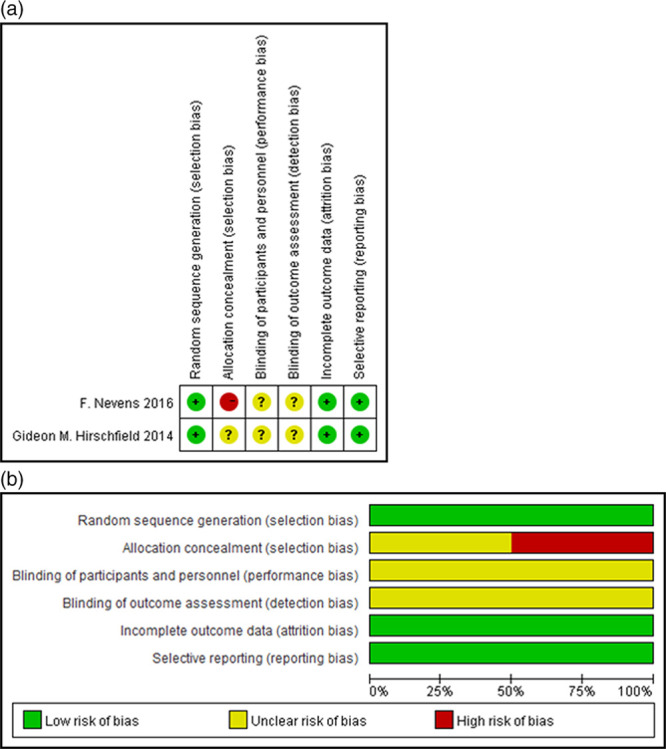
Quality assessment. (a) Risk of bias in the included studies. +: low risks;?: unclear risks; –: high risks. (b) Risk of bias graph: review of the authors’ judgments regarding each risk of bias item presented as percentages across all included studies. Low risk of bias is labeled as green, high risk of bias is labeled as red and unclear risk of bias is labeled as yellow.
Primary endpoints
Two randomised controlled trials comprising 222 patients had reported data for the primary endpoints (<1.67 times the upper limit of normal serum alkaline phosphatase with 15% reduction from baseline, and equal or higher than the upper limit of normal serum total bilirubin at the end of trials). Fifty-eight of 111 patients in the combination therapy groups and 24 of 111 patients in the monotherapy groups had the primary endpoints, There were no significant differences between the groups (RR: 2.75; 95% CI, 0.43–17.68, P = 0.29; Fig. 3). There was significant heterogeneity between studies (I2 = 93%, P < 0.001).
Fig. 3.
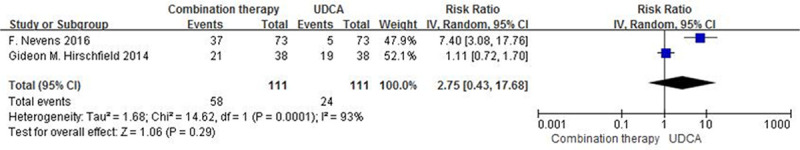
Primary endpoints (alkaline phosphatase ≤1.67*ULN and tBili ≤ULN) in patients with PBC treated with combination therapy vs. monotherapy. UDCA, ursodeoxycholic acid; M-H, Mante–Haenszel; CI, confidence interval; df, degrees of freedom; ULN, upper limit of the normal range; tBili, total bilirubin; PBC, primary biliary cholangitis.
Secondary endpoints
Liver biochemistry
The two included studies both reported the level of alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase (GGT), and conjugated bilirubin. We combined the data of the final measurement of trials and found that levels of alanine aminotransferase (mean difference: –15.63 IU/L; 95% CI, –21.59 to –9.68; P < 0.001), aspartate aminotransferase (mean difference: –6.63 IU/L; 95% CI, –11.03 to –2.24; P = 0.003), and GGT (mean difference: –131.30 IU/L; 95% CI, –177.52 to –85.08; P < 0.001) in patients with combination therapy were significantly lower than those in UDCA monotherapy (Fig. 4). Due to the high heterogeneity (I2 = 92%, P < 0.001), we used the random-effect model to evaluate the mean difference for conjugated bilirubin. There was no significant difference between combination therapy groups and monotherapy groups (mean difference = –0.06 mg/dL; 95% CI, –0.28 to 0.15; P = 0.56).
Fig. 4.
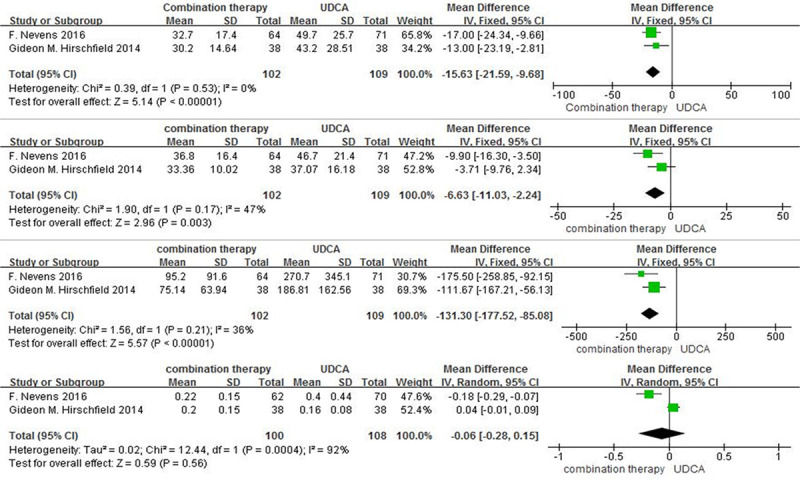
Liver chemistry (a) alanine aminotransferase (IU/L); (b) aspartate aminotransferase (IU/L); (c) GGT (IU/L); (d) conjugated bilirubin (mg/dL) levels in patients with PBC treated with combination therapy versus monotherapy. UDCA, ursodeoxycholic acid; IV, inverse-variance; CI, confidence interval; df, degrees of freedom; B: GGT, gamma-glutamyl transpeptidase; PBC, primary biliary cholangitis.
Immunological parameters
As shown in Fig. 5, the levels of C-reactive protein (CRP) and immunoglobulin M (IgM) reported in these two trials. As for the level CRP, there was no significant heterogeneity between the included studies (I2 = 9%, P = 0.30). We pooled the data using a fix-effect model and found that the level of serum CRP exposed to combination therapy was significantly lower than that in controls (mean difference = –1.17 mg/L; 95% CI, –2.19 to –0.14; P = 0.03). In the random-effect model, the pooled mean difference of IgM was –41.18 mg/dL (P = 0.69) in the combined therapy when compared to controls.
Fig. 5.
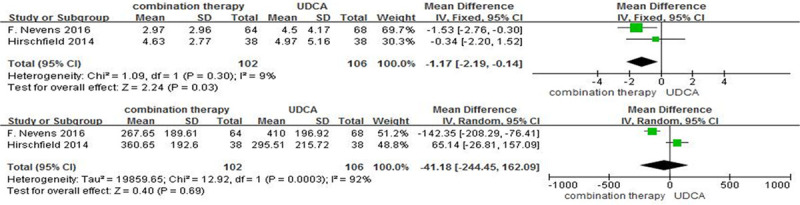
Inflammatory markers (a) CRP (mg/L); (b) IgM (mg/dL) in patients with PBC treated with combination therapy vs. monotherapy. UDCA, ursodeoxycholic acid; IV, inverse-variance; CI, confidence interval; df, degrees of freedom; CRP, C-reactive protein; IgM, immunoglobulin M; PBC, primary biliary cholangitis.
Adverse events
Adverse events for therapy, including pruritus, nasopharyngitis, headache, fatigue, nausea, diarrhea, upper respiratory tract infection, urinary tract infection, dyspepsia, and arthralgia, were also recorded in the two trials. Due to no significant heterogeneity (all I2 values were <50% and all P values were >0.1), fix-effect models were performed to evaluate associations between different therapies and adverse events, except for pruritus, and dyspepsia. The results showed that no significant association with increased risks of adverse events was found between patients with different therapies (Fig. 6).
Fig. 6.
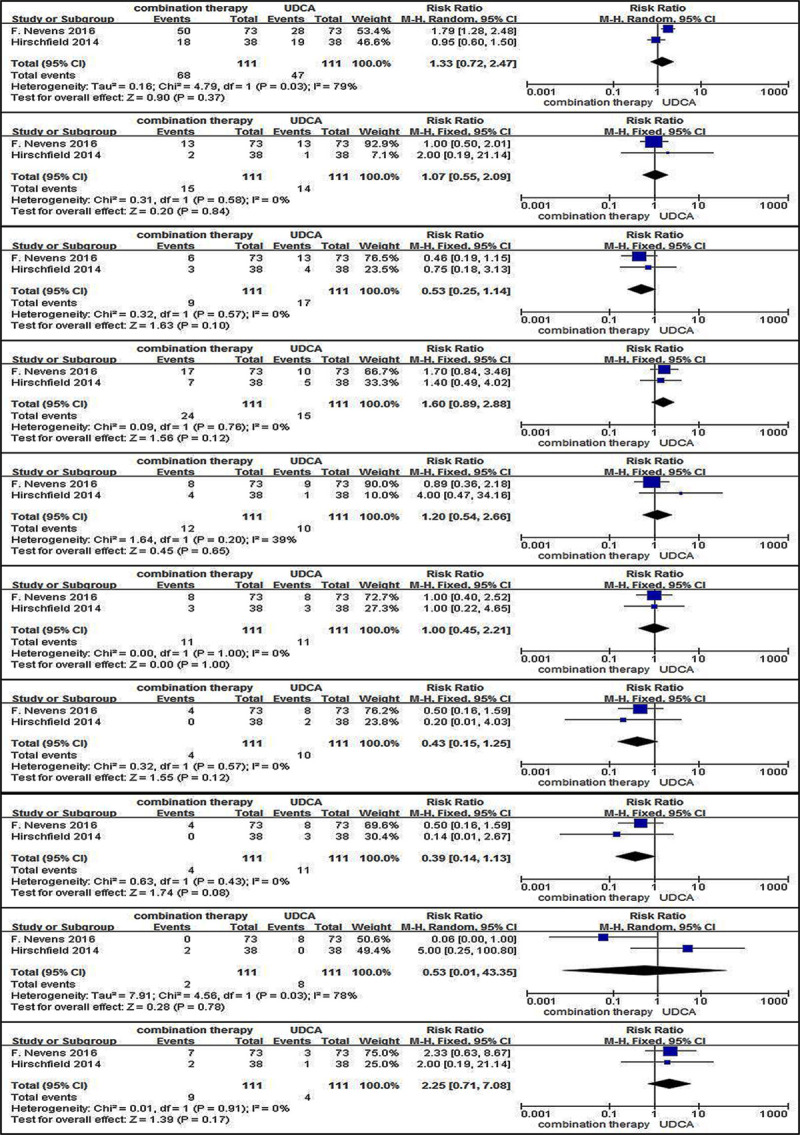
Adverse events (a) pruritus; (b) nasopharyngitis; (c) headache; (d) fatigue; (e) nausea; (f) diarrhea; (g) upper respiratory tract infection; (h) urinary tract infection; (i) dyspepsia; (j) arthralgia in patients with PBC treated with combination therapy vs. monotherapy. UDCA, ursodeoxycholic acid; M-H, Mante–Haenszel; CI, confidence interval; df, degrees of freedom; PBC, primary biliary cholangitis.
Discussion
In cholestasis, the primary mode of action of UDCA [9], an epimer of chenodeoxycholic acid (CDCA) but no FXR agonist properties, is thought to be choleretic by facilitating bile flow rather than suppressing its synthesis and buffering the bile. In contrast, OCA is a modified bile acid and is a potent agonist of the FXR [8], has marked suppressing bile salt synthesis and modulating the immune response in cholestatic liver diseases. Clinical trials testing OCA in PBC indicates good toleration and substantial improvements in liver biochemical indexes associated with cholestasis. Therefore, based on the different action mode between UDCA and OCA, rational combination therapy for refractory PBC would serve as a promising strategy. To our knowledge, this meta-analysis first evaluated the clinical outcomes of the combination therapy of UDCA and OCA compared with UDCA monotherapy in patients with PBC. The results of this study indicated that combination therapy did not differ significantly from monotherapy in improving primary endpoints, conjugated bilirubin, IgM, or adverse events, but was significantly superior to monotherapy in reducing liver biochemical indexes including alanine aminotransferase, aspartate aminotransferase, GGT, and immunological parameter CRP.
PBC is a chronic cholestatic disease that can lead to end-stage liver disease and impairs the quality of life, and risk stratification and disease management are crucial for long-term prognosis. Alkaline phosphatase [15] and bilirubin [4], along with aminotransferases, have been used in previous response criteria and primary endpoints for clinical trials [7]. Several studies have demonstrated that the rate of the primary endpoints was higher in the combination therapy group (61.3%) than in the monotherapy group (42.3%) and importantly treatment with OCA was associated with decreases in the levels of serum IgM [2,11,16]. Interestingly, serum CRP levels in patients with PBC who receive UDCA or OCA treatment have yielded diverse results. Some trial data have revealed that CRP was not different between OCA and the placebo [17]; however, in other studies, OCA has been associated with significant improvements in CRP [8,11]. The present study, via a meta-analysis of published studies, confirmed that the level of serum CRP in combination therapy was significantly lower than that in UDCA monotherapy.
Pruritus was the most common adverse event in patients with PBC, but neither its incidence nor its severity correlates with disease stage [18,19]. However, pruritus was OCA dose-related [20], with a higher incidence reported in the combination therapy group (61.3%) than in the monotherapy group (42.3%). The mechanism of OCA-related pruritus remains unclear, two putative mechanisms have been proposed: the activation of TGR5 [21–23] and activation of the autotoxin pathway [24,25]. Moreover, this meta-analysis did not show any significant association with increased risks of adverse events between different therapies. However, the conclusion should be addressed with caution, because subgroup meta-analysis could not be performed in this study due to the limitation of patient size and eligibility criteria biases.
Some weaknesses in the present work should be mentioned. First, only two eligible RCT studies were included in the meta-analysis and of low quality through the ‘Risk of bias table’. Second, given the limited numbers of studies, sensitivity analysis was not performed to explore heterogeneous sources. Furthermore, for the publication bias, the funnel plot could not be performed because we did not have the recommended minimum number of five trials in the meta-analysis [26].
In summary, the present meta-analysis demonstrated that combination therapy with UDCA and OCA provided satisfactory clinical outcomes, which may be a promising alternative for patients with PBC who have an inadequate response to UDCA therapy. Therefore, high-quality RCTs on the safety and efficacy of the combination therapy of UDCA and OCA compared with UDCA or OCA monotherapy in patients with PBC should be performed in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation for Excellent Young Scholars of China (81922012), the National Natural Science Foundation of China (81770583, 81703043), the Natural Science Foundation of Chongqing (cstc2016jcyjA0149), Third Military Medical University (2017MPRC-13) Science Foundation of Outstanding Youth, and Research Foundation of Education Bureau of Hunan Province (17B216).
Conflicts of interest
There are no conflicts of interest.
All of the authors conceived the study, performed the literature search, quality assessment, and performed the statistical analysis. All of the authors were involved in manuscript writing and preparation. All of the authors have read and approved of the final manuscript.
Footnotes
Jiaquan Qu and Jin Chai contributed equally to the writing of this article.
References
- 1.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012; 56:1181–1188 [DOI] [PubMed] [Google Scholar]
- 2.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015; 386:1565–1575 [DOI] [PubMed] [Google Scholar]
- 3.Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004; 127:485–492 [DOI] [PubMed] [Google Scholar]
- 4.Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, et al. ; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014; 147:1338–49.e5; quiz e15 [DOI] [PubMed] [Google Scholar]
- 5.Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997; 113:884–890 [DOI] [PubMed] [Google Scholar]
- 6.Milkiewicz M. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009; 51:821–822 [DOI] [PubMed] [Google Scholar]
- 7.Corpechot C, Chazouillères O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011; 55:1361–1367 [DOI] [PubMed] [Google Scholar]
- 8.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015; 148:751–61.e8 [DOI] [PubMed] [Google Scholar]
- 9.Floreani A, Franceschet I, Perini L, Cazzagon N, Gershwin ME, Bowlus CL. New therapies for primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015; 48:263–272 [DOI] [PubMed] [Google Scholar]
- 10.Talwalkar JA, Angulo P, Keach JC, Petz JL, Jorgensen RA, Lindor KD. Mycophenolate mofetil for the treatment of primary biliary cirrhosis in patients with an incomplete response to ursodeoxycholic acid. J Clin Gastroenterol. 2005; 39:168–171 [PubMed] [Google Scholar]
- 11.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. ; POISE Study Group. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016; 375:631–643 [DOI] [PubMed] [Google Scholar]
- 12.Savović J, Weeks L, Sterne JA, Turner L, Altman DG, Moher D, Higgins JP. Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014; 3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018; 27:1785–1805 [DOI] [PubMed] [Google Scholar]
- 15.Mantaka A, Koulentaki M, Samonakis D, Sifaki-Pistolla D, Voumvouraki A, Tzardi M, Kouroumalis E. Association of smoking with liver fibrosis and mortality in primary biliary cholangitis. Eur J Gastroenterol Hepatol. 2018; 30:1461–1469 [DOI] [PubMed] [Google Scholar]
- 16.Hirschfield GM, Dyson JK, Alexander GJM, Chapman MH, Collier J, Hübscher S, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018; 67:1568–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, et al. ; Obeticholic Acid PBC Monotherapy Study Group. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018; 67:1890–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolier AR, Peri S, Oude Elferink RP, Beuers U. The challenge of cholestatic pruritus. Acta Gastroenterol Belg. 2012; 75:399–404 [PubMed] [Google Scholar]
- 19.Bergasa NV. The pruritus of cholestasis: facts. Hepatology. 2015; 61:2114. [DOI] [PubMed] [Google Scholar]
- 20.Talwalkar JA, Souto E, Jorgensen RA, Lindor KD. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2003; 1:297–302 [PubMed] [Google Scholar]
- 21.Dawson PA, Karpen SJ. Bile acids reach out to the spinal cord: new insights to the pathogenesis of itch and analgesia in cholestatic liver disease. Hepatology. 2014; 59:1638–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010; 78:617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farzad A, Edwin K, Poole DP, Tinamarie L, Victoria L, Fiore C, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013; 123:1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012; 56:1391–1400 [DOI] [PubMed] [Google Scholar]
- 25.Kremer AE, Martens JJ, Kulik W, Ruëff F, Kuiper EM, van Buuren HR, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010; 139:1008–18 [DOI] [PubMed] [Google Scholar]
- 26.Viechtbauer W. Publication bias in meta-analysis: prevention, assessment and adjustments. Psychometrika. 2007; 72:269–271 [Google Scholar]


