Aim
To compare the effectiveness and safety of transjugular intrahepatic portosystemic shunt (TIPS) with endoscopic therapy plus non-selective β-blockers (NSBBs) for secondary prevention of gasroesophageal variceal bleeding (GEVB) in cirrhotic patients with high-risk factors of treatment failure.
Methods and material:
A total of 122 cirrhotic patients with history of gasroesophageal variceal bleeding and high factors including hepatic vein pressure gradient (HVPG) ≥ 20 mmHg, portal vein thrombosis (PVT), gastrorenal shunt (GRS), or extraluminal para-gastric veins (ep-GVs) detected by endoscopic ultrasound, were analyzed retrospectively. Seventy-seven patients underwent TIPS with PTFE-covered stent (group A) and 102 patients received endoscopic therapy combined with nonselective β-blockers (NSBBs) (group B). According to above high-risk factors, both groups were stratified into four paired subgroups (A1–A4 and B1–B4). Two-year rebleeding rate, overt hepatic encephalopathy, overall survival, and procedure-related adverse events were compared between the two groups and paired subgroups.
Results:
The 2-year cumulative probability of free of variceal rebleeding was higher in group A than group B (93 vs. 62%, P < 0.001). Similarly, the 2-year cumulative probability of free of variceal rebleeding was also higher in the subgroups A1–A4 than the subgroups B1–B4 (91 vs. 67%, P = 0.022, 90 vs. 67%, P = 0.021, 94 vs. 59%, P = 0.029, and 90 vs. 58%, P = 0.016, respectively). There was no significant difference between the two groups and corresponding subgroups in overt hepatic encephalopathy and survival.
Conclusion:
Compared to secondary prophylaxis with endoscopic therapy plus NSBBs, polytetrafluoroethylene-covered TIPS could significantly reduce the variceal rebleeding rate in cirrhotic patients with HVPG ≥ 20 mmHg, PVT, GRS, or ep-GVs, without increasing the incidence of hepatic encephalopathy.
Keywords: endoscopic therapy, hepatic encephalopathy, portal hypertension, transjugular intrahepatic portosystemic shunt, variceal bleeding
Introduction
Gasroesophageal variceal bleeding (GEVB) is a major and severe complication of portal hypertension [1]. Patients who survived the first episode of GEVB had a high rebleeding rate (60% in the first year), with a mortality of up to 33% [2]. Therefore, implementation of effective measures to prevent variceal rebleeding should be actively performed in patients with a history of GEVB [3]. According to the clinical practice guidelines [2,3], the first-line treatment to prevent recurrent GEVB is endoscopic therapy plus nonselective β-blockers (NSBBs). Transjugular intrahepatic portosystemic shunt (TIPS) is recommended for patients who failed endoscopic therapy plus NSBBs. However, even with appropriate secondary prophylactic therapy, the rate of gastroesophageal variceal rebleeding remains at a concerning 42–43% [4]. Nowadays, risk stratification, individual management, and multidisciplinary cooperation are gradually becoming the mainstream approaches for treating portal hypertensive bleeding. A previous study had shown that acute GEVB patient with high risk factors of treatment failure can benefit from the early use of TIPS [5]. However, it remains unclear whether patients with high-risk factors of treatment failure for endoscopic therapy plus NSBBs would benefit from using TIPS as the first-line treatment to prevent recurrent GEVB?
Measurement of hepatic venous pressure gradient (HVPG) is a useful tool to predict prognosis and survival of cirrhotic patients [6]. One-year incidence rate of rebleeding and mortality of acute GEVB in patients with HVPG ≥20 mmHg is higher compared with those with a lower HVPG [7]. Prevalence of portal vein thrombosis (PVT) in cirrhotic patients is about 10–23% [8]. The patient with PVT undergoing endoscopic therapy may need longer time to achieve complete variceal eradication. In addition, NSBBs treatment may induce thrombus formation by reducing splanchnic blood flow and cause further portal vein stasis. The incidence of gastric varices accompanied by gastrorenal shunt (GRS) is up to 65–85% [9,10]. Endoscopic injection of n-butyl-2-cyanoacrylate or isobutyl-2-cyanoacrylate is used as the standard treatment for gastric varices bleeding [11]. However, due to the coexistence of high flow GRS, severe embolization complications of aberrant organs have raised concerns for the safety of endoscopic glue injection [12,13]. Extraluminal para-gastric veins (ep-GVs) detected by endoscopic ultrasound (EUS) were reported to be associated with a higher variceal rebleeding rate and poor response to endoscopic therapy [14].
Several authors reported their experiences of performing TIPS to prevent variceal rebleeding in cirrhotic patients with HVPG ≥20 mmHg or PVT [15–17]. However, the stent used in their studies was not the currently recommended polytetrafluoroethylene-covered stents. To our knowledge, there is still not enough evidence comparing polytetrafluoroethylene-covered TIPS to endoscopic combined with pharmaceutical therapy for preventing variceal rebleeding in patients with GRS or ep-GVs. Thus, we performed this study to evaluate the effectiveness and safety of TIPS using polytetrafluoroethylene-covered stent for preventing recurrence of GEVB in patients with HVPG ≥20 mmHg, PVT, GRS, or ep-GVs.
Materials and methods
Study design
This single-center retrospective study protocol was approved by local institutional review board. Medical records of consecutive patients with history of endoscopy-proven GEVB who were admitted to our hospital between November 2015 and December 2017 were analyzed.
Patients selection and grouping
Patients were considered eligible for this study if they met the following criteria: (1) liver cirrhosis (diagnosed by clinical presentations, laboratory tests, images, or liver biopsies); (2) age between 18 and 75 years; (3) GEVB occurred 5–42 days ago; (4) adequate liver and renal function: Child-Pugh score ≤ 9, aspartate aminotransferase and alanine aminotransferase <5 × upper limit of normal, alkaline phosphatase <4 × upper limit of normal, total bilirubin <51 µmol/L, serum creatinine ≤115 µmol/L; (5) presented with HVPG ≥ 20 mmHg/PVT/GRS or ep-GVs; and (6) written informed consent was submitted. Exclusion criteria included as follows: (1) the patients simultaneously having two or more the above-mentioned high-risk factors; (2) already received primary prophylaxis with standard treatment, TIPS placement or shunt surgery; (3) PVT < 50% of the main portal vein (PVT with less than half of the vessel lumen occluded may have little impact on portal flow; (4) severe cardiopulmonary diseases; (5) hepatocellular carcinoma or other extrahepatic malignancy; and (6) contraindications for propranolol, anticoagulation, or TIPS. Before therapy was initiated, the benefits and potential adverse events related to TIPS or endoscopic therapy plus NSBBs were elaborated thoroughly for the patients and their family members. The final decision to receive what kind of therapy was completely up to them.
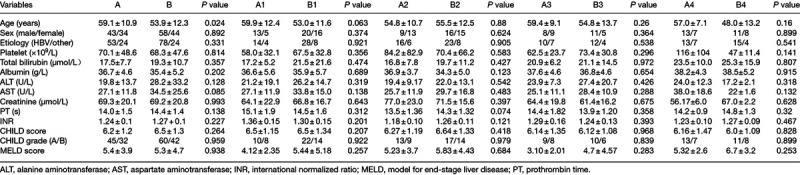
According to above-mentioned criteria, 179 patients were included. Seventy-seven patients received TIPS (group A) and 102 cases underwent endoscopic therapy plus NSBBs (group B) (Fig. 1). Baseline characteristics are presented in Table 1.
Fig. 1.
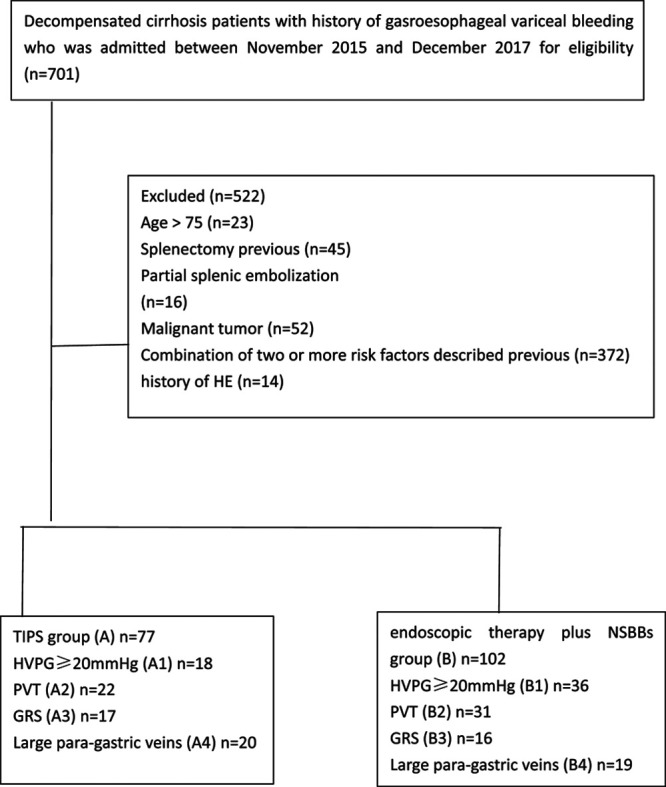
Flow chart demonstrating the study design and enrollment course of the patient. GRS, gastrorenal shunt; HVPG, hepatic venous pressure gradient; NSBBs, nonselective β-blockers; PVT, portal vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt.
Table 1.
Baseline characteristics between the groups and paired subgroups
Treatments
The TIPS creation and endoscopic therapy plus NSBBs technique had been described previously [18–21].
TIPS was performed with 8 mm polytetrafluoroethylene-covered stents (Viatorr Endoprosthesis, GORE and Associates, Flag- staff, Arizona, USA) by Z.P.Y., J.J.L., W.Z., and J.Q.M. (with 27, 18, 5, and 4 years of experience with the TIPS, respectively). Coils and cyanoacrylate were combined or separated use to embolize detected collateral. The final portography and pressure measurement was performed in the main portal and the right atrium. Anticoagulation was used immediately after the TIPS procedure only for patients with PVT. Low molecular weight heparin 4100 U was injected subcutaneously twice daily for 3–5 days and then followed by orally taken warfarin. Warfarin was started with an initial dosage of 2.5 mg daily and titrated carefully to achieve a target international normalized ratio of 2–3.
Endoscopic band ligation (EBL) and cyanoacrylate injection were performed by S.Y.C. and J.W. (with 23 and 19 years of experience with the procedure, respectively) for treatment of esophageal and gastric varices. These therapies were repeated every 2 weeks until variceal eradication was achieved. Propranolol was given continuously, with an initial dosage of 20 mg twice daily and then with increasing doses until 55 beats per minute or a 25% decrease in heart rate was achieved. Only for patients with PVT, the anticoagulation protocol was just as that used in TIPS group and implemented after variceal eradication was achieved.
Follow-up
Follow-up visits, including clinical, biochemical, Doppler ultrasound, and computed tomography evaluations, were scheduled at 1, 3, and 6 months and then every 6 months thereafter or whenever there was clinical recurrence of portal hypertension. In TIPS group, if shunt dysfunction was suspected, angioplasty and another covered stent placement was performed. For patients received endoscopic combined with drug therapy, further endoscopic variceal ligation (EVL) and glue injection was implemented if new varices were detected.
Efficacy and safety assessment
Two-year rebleeding and overt hepatic encephalopathy (OHE) rate, overall survival, and procedure-related adverse events were compared between the two groups.
The variceal rebleeding was defined as recommended in the Baveno V consesus [21], and OHE was diagnosed and graded acoording to current guidelines [22].
Statistical analysis
Continuous variables were presented as mean values ± SD and compared by the independent or paired sample t test. Categorical variables were presented as frequencies and compared using the chi-square test. The overall survival, free of variceal bleeding/hepatic encephalopathy survival, was analyzed with the Kaplan–Meier curves and log-rank test. P value <0.05 indicated a significant difference. Variables that achieved statistical significance (P < 0.1) in univariate analysis were subsequently assessed by multivariate analysis with use of a Cox proportional hazards model. A stepwise regression procedure was used to determine which factors were major independent predictors for survival. Statistical software (SPSS version 19.0; SPSS, Chicago, Illinois, USA) was used for analysis.
Results
Patient characteristics
Between November 2015 and December 2017, a total of 966 patients who fulfilled the inclusion criterion were enrolled, and 787 patients were excluded. Finally, 179 patients were included in this study (77 in group A and 102 in group B), and both groups were stratified into four paired subgroups based on different risk factors (Fig. 1).
There were no significant difference of clinical baseline characteristics between the groups and paired subgroups except age. The patients in group A were older than those in group B (59.1 ± 10.9 vs. 53.9 ± 12.3, P = 0.024) (Table 1). Nine patients in group B transferred to other treatment (rescuing TIPS in eight patients and liver transplantation in one patient) due to the development of rebleeding. The mean HVPG measurement before procedure was 16.58 ± 3.96 mmHg in group A and 17.41 ± 4.30 mmHg in subgroup B, with no significant difference (P = 0.190). The median follow-up time was 20 months, (20 and 21 months in group A and group B, respectively).
In group A, TIPS was successfully performed in all patients. The mean portal-systemic pressure gradient dropped from 21.7 ± 6.35 before the procedure to 10.0 ± 3.06 mmHg (P < 0.001) after the procedure. Fifty-seven patients with significant varices underwent variceal embolization during the TIPS procedure.
In group B, 86 patients (84.3%) achieved variceal eradication after a mean of 2.6 ± 1.41 sessions (range 1–6) and 6.4 ± 7.05 months (range 3–14) by EBL and cyanoacrylate injection, including 12 patients after variceal rebleeding. In the remaining 28 patients, variceal eradication was not obtained for the reason of death (n = 6), turning into rescuing TIPS (n = 8), and liver transplantation (n = 2). All patients in group B received propranolol, with a mean dose of 62.7 ± 32.1 mg/day.
Variceal rebleeding
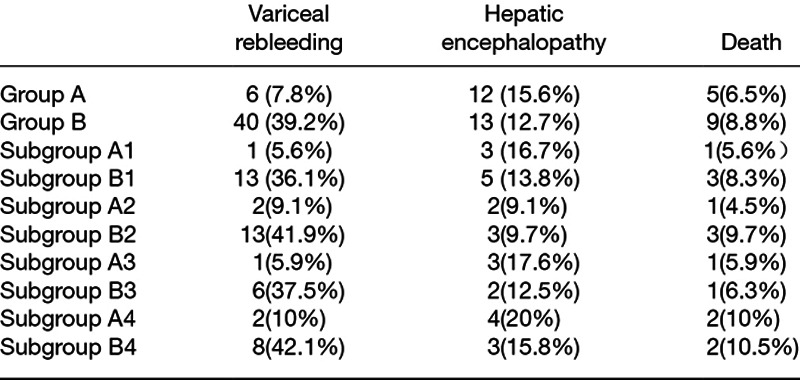
During the follow-up period, a total of 64 variceal rebleeding episodes occurred in 46 patients (25.7%): eight episodes from six patients (7.8%) in group A and 56 episodes from 40 patients (39.2%) in group B. In group A, six patients (7.79%) with variceal rebleeding were found by endoscopy and confirmed by portography as TIPS shunt dysfunction (stent stenosis), and all of them received TIPS revisions successfully. In group B, endoscopic hemostasis was successfully achieved in 25 patients, and eight patients underwent rescuing TIPS, and the remaining seven died for massive bleeding. The two-year cumulative variceal rebleeding free rate was significantly higher in the group A than in group B (93 vs. 62%, P < 0.001, log-rank test) (Fig. 2).
Fig. 2.
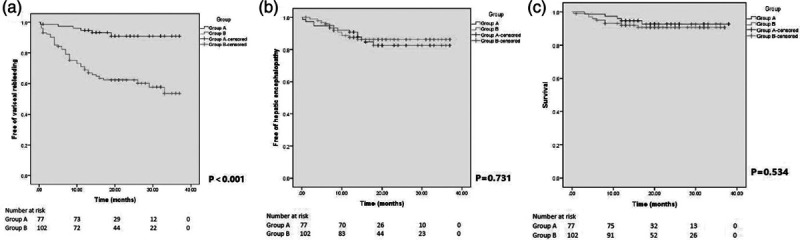
Kaplan–Meier curve shows the probalility of free of vairceal rebleeding (a), free of hepatic encephalopathy (b), and survival (c) between the group A and group B.
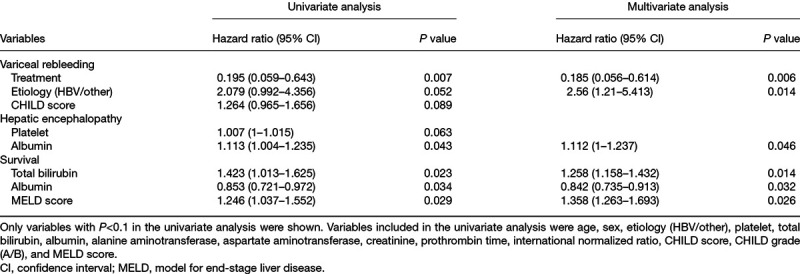
In univariate analysis, the treatment allocation, etiology (hepatitis B virus [HBV]) and Child-Pugh Grade were associated with variceal rebleeding. But only the treatment allocation (P = 0.006) and etiology (HBV) (P = 0.014) became the variables independently predicting variceal rebleeding in the multivariate analysis (Table 2).
Table 2.
Outcomes of groups and paired subgroups
Hepatic encephalopathy
During the follow-up, 25 patients (14.0%) developed hepatic encephalopathy, with 12 patients (15.6%) in group A and 13 patients (12.7%) in group B. Severe hepatic encephalopathy occurred in two patients from group A. Hepatic encephalopathy was effectively controlled by the medicine in all patients except one patient in group A who died for the progressive electrolyte disorder. There was no significant difference of the two-year cumulative hepatic encephalopathy free rate between the groups (84 vs. 88%, P = 0.731, log-rank test) (Fig. 2).
In the univariate analysis, platelet and albumin were related with hepatic encephalopathy. When platelet and albumin were included in multivariate analysis, only albumin (P = 0.046) was the independent predictors of hepatic encephalopathy (Table 2).
Survival
Fourteen patients (7.8%) died during the follow-up period, five (6.5%) in group A and nine (8.8%) in group B. Four patients from group A and two patients from group B died due to hepatic failure. Other causes of death incorporated massive bleeding (seven patients from group B) and severe hepatic encephalopathy (one patient from group A). There was no significant difference of the cumulative two-year survival rate between the two groups (92.7 vs. 90.7%, P = 0.534, log-rank test) (Fig. 2).
After univariate and multivariate analysis, total bilirubin (P = 0.014), albumin (P = 0.032), and model for end-stage liver disease (MELD) score (0.026) were associated with survival (Table 2).
Adverse event
During the follow-up period, 45 (58%) patients in group A and 57 (56%) patients in group B experienced at least one adverse event (Table 3). There was no significant difference in the number of the patients (P = 0.732).
Table 3.
Univariate and multivariate analysis
Hepatic venous pressure gradient ≥20 mmHg
Fifty-four patients with HVPG ≥20 mmHg were included in subgroups (18 in subgroup A1 and 36 in subgroup B1) (Fig. 1). The mean HVPG measurement before procedure was 22.06 ± 0.73 mmHg in subgroup A1 and 22.47 ± 2.09 mmHg in subgroup B1, with no significant difference (P = 0.124). The median follow-up time was 20 months (20 and 20.5 months in subgroup A1 and subgroup B1, respectively).
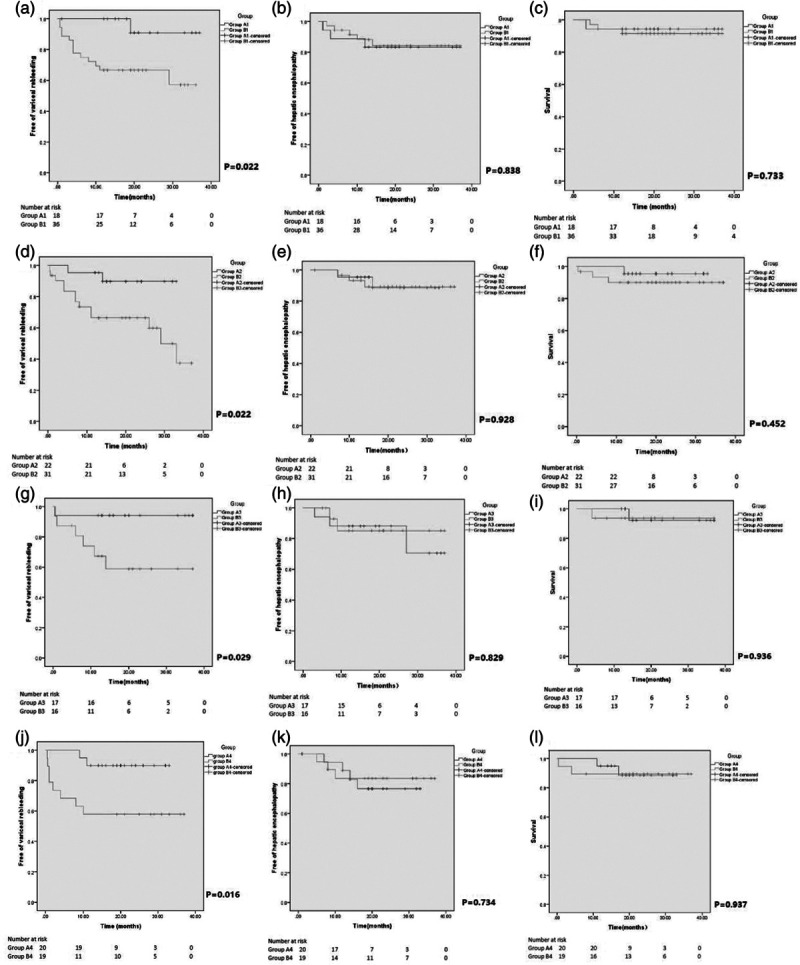
The patients with reaching endpoint of study in subgroups A1 and B1 are shown in Table 4. The two-year cumulative variceal rebleeding free rate was significantly higher in the subgroup A1 than in subgroup B1 (91 vs. 67%, P = 0.022, log-rank test). There was no significant difference of the two-year hepatic encephalopathy free rate or two-year survival rate between two subgroups (83 vs. 84%, P = 0.838, log-rank test, and 94 vs. 92%, P = 0.733, log-rank test, in subgroup A1 and subgroup B1, respectively) (Fig. 3).
Table 4.
Adverse events between group A and group B
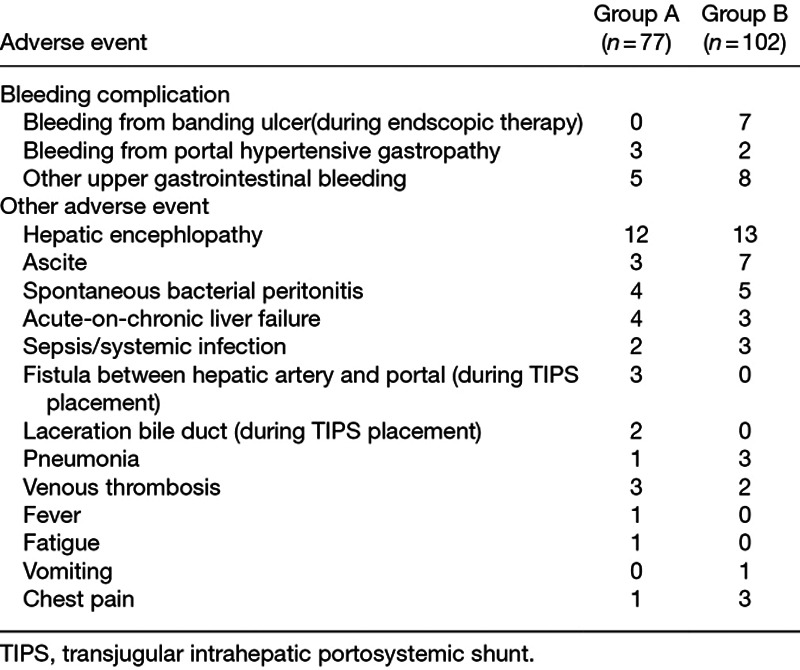
Fig. 3.
Kaplan–Meier curve shows the probalility of free of vairceal rebleeding (A1–A4) free of hepatic encephalopathy (B1–B4) and survival (C1–C4) between the subgroups A1–A4 and subgroups B1–B4.
Portal vein thrombosis
Fifty-three patients with PVT were enrolled in subgroups (22 in subgroup A2 and 31 in subgroup B2) (Fig. 1). The mean HVPG measurement before procedure was 15.45 ± 2.73 mmHg in subgroup A2 and 15.06 ± 1.94 mmHg in subgroup B2, with no significant difference (P = 0.547). The median follow-up time was 20 months (18 and 21 months in subgroup A2 and subgroup B2, respectively).
The patients with reaching endpoint of study in subgroups A2 and B2 are shown in Table 4. Likewise, variceal rebleeding was significantly lower in subgroup A2, with the two-year cumulative variceal rebleeding free rate (90 vs. 67%, P = 0.021, log-rank test). There was no significant difference in terms of the occurrence of hepatic encephalopathy or survival between two subgroups, with two-year hepatic encephalopathy free rate and two-year survival rate (93 vs. 89%, P = 0.928, log-rank test; 96 vs. 90%, P = 0.452, log-rank test in subgroup A2 and subgroup B2, respectively) (Fig. 3).
Gastrorenal shunt
Thirty-three patients with GRS were incorporated in subgroups (17 in subgroup A3 and 16 in subgroup B3) (Fig. 1). The mean HVPG measurement before procedure was 15.29 ± 2.61 mmHg in subgroup A3 and 14.18 ± 2.31 mmHg in subgroup B3, with no significant difference (P = 0.209).The median follow-up time was 18 months, (16 and 19 months in subgroup A3 and subgroup B3, respectively).
The patients with reaching endpoint of the study in subgroups A3 and B3 are shown in Table 4. The two-year cumulative variceal rebleeding free rate was 94% in subgroups A3 and 59% in subgroups B3 (P = 0.029, log-rank test), indicating lower incident of variceal rebleeding in subgroup A3. The comparisons of two-year hepatic encephalopathy free rate and two-year survival rate were 88 vs. 85% (P = 0.829, log-rank test) and 92 vs. 94% (P = 0.936, log-rank test), respectively, with no significant difference (Fig. 3).
Extraluminal para-gastric veins
Thirty-nine patients with ep-GVs were comprised in subgroups (20 in subgroup A4 and 19 in subgroup B4) (Fig. 1). The mean HVPG measurement before procedure was 14.25 ± 2.24 mmHg in subgroup A4 and 14.73 ± 1.93 mmHg in subgroup B4, with no significant difference (P = 0.474). The mean follow-up time was 23 months (20.5 and 25 months in subgroup A4 and subgroup B4, respectively).
The patients with reaching endpoint of the study in subgroups A4 and B4 are shown in Table 4. The two-year cumulative variceal rebleeding free rate was significantly higher in the subgroup A4 than subgroup B4 (90 vs. 58%, P = 0.016, log-rank test). No significant differences were found in the aspect of the two-year hepatic encephalopathy free rate or two-year survival rate between two groups (77 vs. 84%, P = 0.734, log-rank test and 89 vs. 89%, P = 0.937, log-rank test, respectively) (Fig. 3).
Discussion
Prevention for variceal rebleeding in patients with cirrhosis is mandatory after the first episode, with extensively acknowledged. Nevertheless, the optimal treatment strategy for this purpose remains controversial [3]. The combination of endoscopic therapy and NSBBs is recommended as the first-line treatment for the prophylaxis of variceal rebleeding, and TIPS is considered as the alternative choice for the patients who failed endoscopic therapy plus NSSBs. Currently, polytetrafluoroethylene-covered stents have been used widely, with the benefit of prolonged patency [23]. Recent studies and meta-analysis [16,24,25] comparing TIPS with covered stents with endoscopic therapy plus NSBBs demonstrated a significant benefit of preventing variceal rebleeding, without increasing the overall survival or risk of hepatic encephalopathy. A previous inspiring study [5] proved that early TIPS is superior to endoscopic and pharmacological therapy in terms of preventing variceal rebleeding and survival for treatment of acute variceal bleeding in patients with high risk factors, which was either Child-Pugh class C disease or Child-Pugh class B disease with active bleeding. This triggered the discussion whether TIPS can also have advantage in both preventing variceal rebleeding and survival in some patients with several high-risk factors of treatment failure for endoscopic therapy plus NSBBs.
Our retrospective study showed that covered TIPS was more effective for preventing variceal rebleeding in cirrhotic patients with high-risk factors compared with endoscopic therapy combined with NSBBs, without increasing the incidence of hepatic encephalopathy. However, it did not perform superiority in terms of survival. Similar results were observed in all subgroups. Basing on above findings, our results suggested that TIPS perhaps can act a good alternative as the first-line treatment for secondary prevention for GEVB in some selected patients with a high HVPG (≥20 mmHg), PVT, GRS, or splenic-renal shunt, and ep-GVs.
HVPG, as a golden measurement for assessing portal pressure, plays an important role in patients with cirrhosis, including diagnosis of portal hypertension, classification of portal hypertension assessment of disease severity, and prognosis and guidance of therapy [26]. Comparing the treatment of endoscopic therapy plus NSBBs, TIPS could directly and immediately reduce the portal pressure which is the main cause of variceal rupture. The previous trial by Monescillo et al. [28]demonstrated that early TIPS placement reduces treatment failure and mortality in the patients of acute variceal bleeding with HVPG ≥20 mmHg. Another recent study [17] by Zhang showed that uncovered TIPS effectively reducing variceal rebleeding than EVL plus propranolol in cirrhosis patients with HVPG >20 mmHg, and also no difference in survival, but high hepatic encephalopathy events in the TIPS group.
The current recommended treatment for the prophylaxis of variceal rebleeding was based on result of some randomized controlled trials (RCTs) which excluded the patients with PVT. Therefore, the optimal therapy in patients with PVT has not been established so far. PVT deteriorated portal hypertension and decreased the portal perfusion, with increasing the risk of the rupture of the varices and aggravating liver function. Physicians become more hesitate to choose the anticoagulation therapy for PVT in patients with decompensated cirrhosis, especially in those with variceal bleeding history [27]. TIPS could effectively recanalize the thrombosed portal venous and decrease the pressure of portal vein. Previous studies [15,16] had shown that TIPS placement in patients with decompensated cirrhosis and PVT were more effective in reducing variceal rebleeding than EVL combined with propranolol, without increasing the risk of hepatic encephalopathy. However, the currently recommended covered stents were not used in their studies.
GRS is not the frequent complication in cirrhotic patients, with the prevalence ranging from 15 to 20% [29,30]. However, the incidence of gastric varices accompanied by GRS is up to 65–85% [9, 10]. Several guidelines [2,3] recommend the combination of NSBBs and endoscopic therapy (EVL or N-butyl cyanoacrylate injection) as the first-line therapy to prevent rebleeding for gastric varices. The patients with gastric varices accompanied by GRS had the risk of systemic embolization especially pulmonary embolism, stroke, and multiorgan collapses, when receiving endoscopic therapy [31]. Moreover, GRS has not been removed after the procedure. Several previous studies [32] had showed that GRS is associated with a higher risk of hepatic encephalopathy and worse liver function. TIPS is a well tolerated and effective treatment in patients with GRS for preventing variceal rebleeding, with the additional benefit of occluding the GRS during the operation.
A previous study [32] had shown the diameter of para-esophageal varices was a powerful predictive indicator for esophageal variceal recurrence in the course of EBL. Another report [14] demonstrated the presence of para-gastric veins increased the risk of variceal rebleeding when receiving endoscopic therapy. In our study, TIPS is superior to endoscopic therapy combined with NSBBs for preventing variceal rebleeding. The results in our study possibly due to the advantage of TIPS to directly reducing the portal pressure and embolization of para-gastric veins during the procedure. The existence of para-gastric veins indicated a relatively higher portal pressure and could not been completely eradicated by the endoscopic therapy, with higher risk of variceal rebleeding.
Our results confirmed several previous studies [15,16,25,26,33] with covered stent revealing that TIPS had more advantage for preventing variceal rebleeding in patients, with no significant difference in survival. However, our outcomes of hepatic encephalopathy were similar with some reports but different with another report [25] which showed higher incident of hepatic encephalopathy in the TIPS group. The benefit of preventing variceal rebleeding of TIPS did not translate into improved survival, indicating other elements rather than the treatment allocation may predict survival in both groups. In our Cox regression analysis, total bilirubin, albumin, and MELD score were associated with survival. What is more, eight patients received rescuing TIPS, which may cover up the potential advantage of TIPS for survival.
There were several limitations in our study. First, it is a single-center retrospective study with small sample especially in subgroups. Second, the follow-up time is perhaps relatively short. Third, we do not stratify the type of the PVT and the GRS. Further prospective studies and RCTs are needed to certify these results.
In conclusion, polytetrafluoroethylene-covered TIPS, compared with secondary prophylaxis with endoscopic therapy plus NSBBs, could significantly reduce the variceal rebleeding rate in cirrhotic patients with HVPG ≥20 mmHg, PVT, GRS, or ep-GVs without increasing the incidence of hepatic encephalopathy.
Acknowledgements
We thank Dr Junmiao Wen from the Department of Radiation Oncology, Fudan University Shanghai Cancer Center for editing this manuscript.
This work was supported by the clinical research special fund from Zhongshan Hospital, Fudan University (2018ZSLC23).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Yongjie Zhou, Wen Zhang, Junying Gu and Zihan Zhang contributed equally to the writing of this article.
References
- 1.de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015; 63:743–752 [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2017; 65:310–335 [DOI] [PubMed] [Google Scholar]
- 3.EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018; 69:406–460 [DOI] [PubMed] [Google Scholar]
- 4.Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003; 361:952–954 [DOI] [PubMed] [Google Scholar]
- 5.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. ; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010; 362:2370–2379 [DOI] [PubMed] [Google Scholar]
- 6.Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014; 20:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moitinho E, Escorsell A, Bandi JC, Salmerón JM, García-Pagán JC, Rodés J, Bosch J. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999; 117:626–631 [DOI] [PubMed] [Google Scholar]
- 8.Qi X, Han G, Fan D. Management of portal vein thrombosis in liver cirrhosis. Nat Rev Gastroenterol Hepatol. 2014; 11:435–446 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988; 95:434–440 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto A, Hamamoto N, Nomura T, Hongou Y, Arisaka Y, Morikawa H, et al. Balloon-occluded retrograde transvenous obliteration of high risk gastric fundal varices. Am J Gastroenterol. 1999; 94:643–649 [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell A. Management of gastric varices. Clin Gastroenterol Hepatol. 2014; 12:919–928.e1; quiz e51 [DOI] [PubMed] [Google Scholar]
- 12.Bhat YM, Banerjee S, Barth BA, Chauhan SS, Gottlieb KT, Konda V, et al. ; ASGE Technology Committee. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointest Endosc. 2013; 78:209–215 [DOI] [PubMed] [Google Scholar]
- 13.Kahloon A, Chalasani N, DeWitt J, Liangpunsakul S, Vinayek R, Vuppalanchi R, et al. Endoscopic therapy with 2-octyl-cyanoacrylate for the treatment of gastric varices. Dig Dis Sci. 2014; 59:2178–2183 [DOI] [PubMed] [Google Scholar]
- 14.Tseng Y, Ma L, Luo T, Zeng X, Li F, Li N, et al. Patient response to endoscopic therapy for gastroesophageal varices based on endoscopic ultrasound findings. Gut Liver. 2018; 12:562–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo X, Wang Z, Tsauo J, Zhou B, Zhang H, Li X. Advanced cirrhosis combined with portal vein thrombosis: a randomized trial of TIPS versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal variceal bleeding. Radiology. 2015; 276:286–293 [DOI] [PubMed] [Google Scholar]
- 16.Lv Y, Qi X, He C, Wang Z, Yin Z, Niu J, et al. ; PVT-TIPS Study Group.. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut. 2018; 67:2156–2168 [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Wang G, Zhao L, Wu Z, Zhang W, Zhang C. Second prophylaxis of variceal bleeding in cirrhotic patients with a high HVPG. Scand J Gastroenterol. 2016; 51:1502–1506 [DOI] [PubMed] [Google Scholar]
- 18.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK., Jr The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012; 199:746–755 [DOI] [PubMed] [Google Scholar]
- 19.Rössle M. TIPS: 25 years later. J Hepatol. 2013; 59:1081–1093 [DOI] [PubMed] [Google Scholar]
- 20.Chen SL, Hu P, Lin ZP, Zhao JB. The effect of puncture sites of portal vein in TIPS with ePTFE-covered stents on postoperative long-term clinical efficacy. Gastroenterol Res Pract. 2019; 2019:2935498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Franchis R; Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010; 53:762–768 [DOI] [PubMed] [Google Scholar]
- 22.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014; 60:715–735 [DOI] [PubMed] [Google Scholar]
- 23.Perarnau JM, Le Gouge A, Nicolas C, d’Alteroche L, Borentain P, Saliba F, et al. ; STIC-TIPS Group. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014; 60:962–968 [DOI] [PubMed] [Google Scholar]
- 24.Holster IL, Tjwa ET, Moelker A, Wils A, Hansen BE, Vermeijden JR, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016; 63:581–589 [DOI] [PubMed] [Google Scholar]
- 25.Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rössle M, Panther E, et al. ; German Study Group for Prophylaxis of Variceal Rebleeding. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology. 2015; 149:660–668.e1 [DOI] [PubMed] [Google Scholar]
- 26.Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009; 6:573–582 [DOI] [PubMed] [Google Scholar]
- 27.Campbell S, Lachlan NJ. Anticoagulation for cirrhotic portal vein thrombosis: bold, brave, and possibly beneficial. Clin Gastroenterol Hepatol. 2012; 10:784–785 [DOI] [PubMed] [Google Scholar]
- 28.Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004; 40:793–801 [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, Shen L, Chu J, Ma X, Jin B, Meng F, et al. Characterization of uncommon portosystemic collateral circulations in patients with hepatic cirrhosis. Oncol Lett. 2015; 9:347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarantino G, Citro V, Conca P, Riccio A, Tarantino M, Capone D, et al. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009; 9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi X, Qi X, Zhang Y, Shao X, Wu C, Wang Y, et al. Prevalence and clinical characteristics of spontaneous splenorenal shunt in liver cirrhosis: a retrospective observational study based on contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) scans. Med Sci Monit. 2017; 23:2527–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irisawa A, Saito A, Obara K, Shibukawa G, Takagi T, Shishido H, et al. Endoscopic recurrence of esophageal varices is associated with the specific EUS abnormalities: severe periesophageal collateral veins and large perforating veins. Gastrointest Endosc. 2001; 53:77–84 [DOI] [PubMed] [Google Scholar]
- 33.Qi X, Tian Y, Zhang W, Zhao H, Han G, Guo X. Covered TIPS for secondary prophylaxis of variceal bleeding in liver cirrhosis: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016; 95:e5680. [DOI] [PMC free article] [PubMed] [Google Scholar]


