Abstract:
The 2017 consensus report of the Asia Dry Eye Society (ADES) on the definition and diagnosis of dry eyes described dry eye disease as “Dry eye is a multifactorial disease characterized by unstable tear film causing a variety of symptoms and/or visual impairment, potentially accompanied by ocular surface damage.” The report emphasized the instability of tear film and the importance of visual dysfunction in association with dry eyes, highlighting the importance of the evaluation of tear film stability. This report also discussed the concept of tear film–oriented therapy, which stemmed from the definition, and which is centered on provision of insufficient components in each tear film layer and ocular surface epithelium. The current ADES report proposes a simple classification of dry eyes based on the concept of tear film–oriented diagnosis and suggests that there are three types of dry eye: aqueous-deficient, decreased wettability, and increased evaporation. It is suggested that these three types respectively coincide with the problems of each layer: aqueous, membrane-associated mucins, and lipid/secretory mucin. Although each component cannot be quantitatively evaluated with the current technology, a practical diagnosis based on the patterns of fluorescein breakup is recommended. The Asia Dry Eye Society classification report suggests that for a practical use of the definition, diagnostic criteria and classification system should be integrated and be simple to use. The classification system proposed by ADES is a straightforward tool and simple to use, only through use of fluorescein, which is available even to non-dry eye specialists, and which is believed to contribute to an effective diagnosis and treatment of dry eyes.
Key Words: Asia Dry Eye Society, Classification, Dry eye, Tear film–oriented therapy, Tear film stability
The Asia Dry Eye Society (ADES) was founded in 2012 in Tokyo by Korean, Chinese, and Japanese dry eye specialists (http://asia-dry-eye.biz). The aim of the society was to facilitate collaborative research, encourage mutual communication, and generate agreements on the essential fields such as the definition of dry eye, diagnostic criteria, and classification. The first agreement on the definition was achieved in October 2014 in Tokyo after which the new definition and diagnostic criteria proposal were published in the January 2017 issue of The Ocular Surface.1 This consensus was made through extensive discussion over the years with ADES member countries. The definition emphasized the importance of an unstable tear film as the most important mechanism for the development of dry eye. Because the normal corneal sensation is associated with a stable and healthy tear film, an unstable tear film induces discomfort and pain via a possible increase of tear osmolality. An unstable tear film is also known to affect vision, because an irregular tear film over the visual axis has been reported in studies using the tear film stability assessment system, ocular aberrometers, and functional visual acuity systems.2–4
With the emergence of new aqueous tears and/or mucin secretagogue eye drops containing diquafosol sodium (Diquas) or rebamipide (Mucosta) and based on the accumulating evidence in relation to their favorable effects on the tear film and ocular surface epithelium,5–31 we took further steps to emphasize the importance of a stable tear film of dry eye patients in Asia. These two eye drops work to increase tear breakup time by increasing aqueous and or mucin components to the ocular surface.5,32 Many earlier reports have pointed out the importance of a stable tear film in ocular surface health.33–35
In this consensus paper, we would like to introduce our new classification system for dry eye based on the ADES definition.1 For the daily practice of dry eyes, a simple and practical definition and evidence-based diagnostic criteria are mandatory. Because there are two types of dry eye that includes aqueous deficient and evaporative dry eye, the latter for which meibomian gland dysfunction (MGD) is responsible as the major cause, the clinicians need a straightforward classification, which may be useful for determining the most useful treatment. Thus, discussion on classification was initiated in ADES to fulfill this need.
Along with the definition consensus meetings, the classification discussion also began concurrently with preliminary discussion in November 2014. After completion of the definition consensus, we continued the discussion of classification. However, because TFOS DEWS II started in April 2015, we decided to postpone the classification discussion until TFOS DEWS II was published to avoid the possible conflict of interest. The final meeting was held in Osaka on October 20, 2017.
BACKGROUND OF ASIAN COUNTRIES FOR A REGIONAL CONSENSUS
First, in Asia, optometrists are not involved in clinics of dry eye disease, but only ophthalmologists. The base membership of ADES believed that there was a need for simple and effective examination methods that could appeal to ophthalmologists and be used as a guide in Asian countries. Second, in Asian countries, in contrast to North America and continental Europe, it has become possible to prescribe secretagogue eye drops that can treat dry eye disease. Another important point is that Japan and most Asian countries have access to diagnostic tools and imaging technology that help to view and assess the lipid and the aqueous layers. These led to the evolution of the concept of tear film–oriented therapy (TFOT), that is, evaluation and treatment tailored to the problems in each layer. Third, epidemiological studies have shown that dry eye prevalence in Asia is higher than in Europe and the United States. Moreover, their epidemiological studies showed that short BUT type of dry eyes are prevalent in Japan and in other Asian countries, compared with other types of dry eye disease.36,37 With such a background, ADES was launched in 2014, and Asian dry eye clinicians met together, and the current definition of dry eye and diagnostic criteria were reported.1 After that, discussions continued further in ADES, leading to the present classification.
HISTORY OF THE CLASSIFICATION OF DRY EYE
The first comprehensive classification of dry eye was published in 1995 on the basis of consensus from the NEI/Industry working group on Clinical Trials in Dry Eye.38 In the 1995 report, dry eye was divided into 2 primary categories; tear-deficient and evaporative. These two subgroups were further subclassified according to a range of intrinsic and extrinsic etiological factors. It is of note that in this report, dry eye was defined as “a disorder of the tear film due to tear deficiency or excessive tear evaporation” suggesting that dry eye caused either by tear deficiency or excessive evaporation were exclusively considered to be the main categories of dry eye. Since then, this classification scheme has had a great impact on dry eye research and clinical practice. The scheme was basically retained in the DEWS report published in 2007 (Fig. 1).39 Aqueous deficient dry eye (coined as “tear-deficient” in the NEI report) was further classified into Sjogren and non-Sjogren categories. Evaporative dry eye was sub classified into intrinsic and extrinsic categories, and they were further classified according to etiological factors.
FIG. 1.
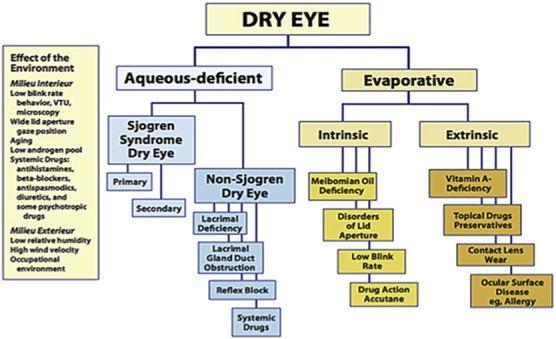
Major etiological cause of dry eye in TFOS DEWS report in 2007. Reprinted from the ocular surface, Vol 5(2), the definition and classification of dry eye disease: Report of the definition and classification subcommittee of the International Dry Eye Workshop (2007), pages No. 75–92, copyright 2007, with permission from Elsevier.
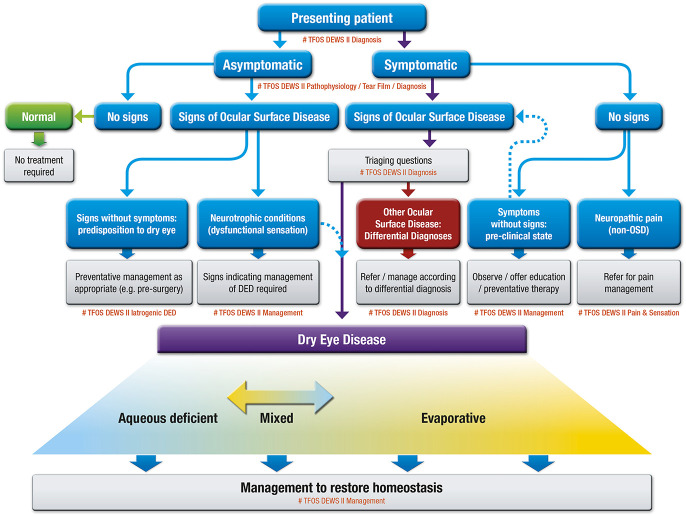
The classification system also took its place in the recent TFOS DEWS II report with some modification.40 The newly proposed classification scheme considers the cases where patients exhibit dry eye symptoms without evidence of obvious signs, or present with marked signs, but lack of dry eye symptoms. The former includes cases with neuropathic pain where the somatosensory system is affected, and the latter is related to the reduced corneal sensitivity (neurotrophic condition) (Fig. 2). Eyes with both signs and symptoms were classified into either aqueous deficient or evaporative dry eyes. Although this part of classification is basically the same as the previous classifications, there is a slight modification to clarify that there are a significant number of eyes that have both aqueous deficient and evaporative components, and that these two subcategories were not exclusive.
FIG. 2.
Classification of dry eye disease in TFOS DEWS II. Reprinted from the ocular surface, Vol 15(3), Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report, pages 276–283, copyright 2017, with permission from Elsevier.
There are other groups which have proposed various classification systems. In Asia, Chinese scholars proposed their dry eye classification in 2004.41 It proposed a method based on the structure of tear film and tear dynamics. Dry eye was divided into five types, including lipid deficient dry eye (evaporative dry eye), aqueous deficient dry eye, mucin deficient dry eye, abnormal tear dynamics dry eye, and mixed dry eye. The Chinese dry eye consensus subsequently adopted this classification.42 The classification based on the structure and tear film dynamics is very helpful for the treatment of different types of dry eye. In 2005, Murube et al.43 published an article entitled “The triple classification of dry eye for practical use”. His classification consisted of the three following aspects: etiopathogenesis, affected glands/tissues, and grade of severity. In relation to etiopathogenesis, dry eye was classified into the following 10 subcategories; age-related, hormonal, pharmacologic, immunopathic, hyponutritional, dysgenetic, adenitic, traumatic, neurologic, and tantalic. In a classification according to the affected glands/tissues, there were the following five subcategories; aqueous deficient, lipid deficient, mucin-deficient, epitheliopathic, and nonocular exocrine-deficient. Murube's classification systems emphasized more of the physiological or pathological abnormalities in dry eye compared with those proposed by those in NEI/DEWS/TFOS DEWS II.
There is also a classification system, the Delphi approach, proposed by dry eye specialists in the United States and Europe.44 In their report, dry eye is viewed in relation to the presence or absence of lid margin disease, and tear distribution abnormalities (Fig. 3). The Delphi classification was based on the treatment algorithm for dry eye patients, and different treatment methods were described in each subcategory.
FIG. 3.
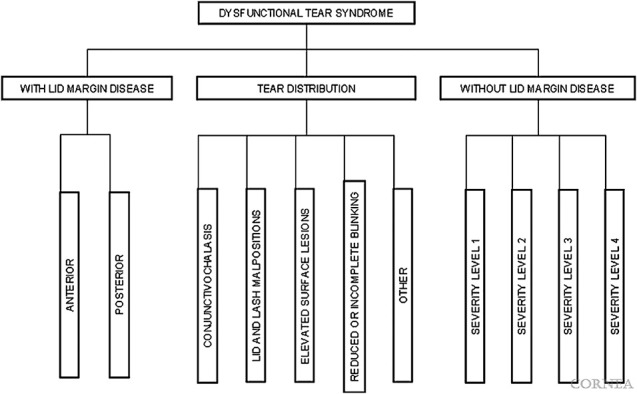
Dry eye disease classification in Delphi approach (2006). Reprinted from cornea, Vol 25, Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: A Delphi approach to treatment recommendations, pages 900–7, copyright 2006, with permission from Wolters Kluwer.
NEW DRY EYE CLASSIFICATION BASED ON THE TEAR FILM–ORIENTED DIAGNOSIS CONCEPT
Dry eye was reported as Sjogren syndrome related dry eye in 1933. This is a typical form of decreased aqueous secretion and was considered the classic type of dry eye. However, according to the recent epidemiological study targeting office workers, most dry eye showed tear film instability and ocular surface abnormalities without reduction in tear secretion, and this short BUT type is now more dominant than the so-called classic type.45
An unstable tear film can be caused by several mechanisms. Any ocular surface components comprising those of tear film and surface epithelium can affect the tear film stability, including lipids, aqueous/secretory mucins, and membrane-associated mucins. An abnormality in the lipid components is thought to accelerate tear evaporation, resulting in an unstable tear film, despite big discussions as to the suppression of the tear film lipid layer on the evaporation of aqueous tears from aqueous layer.46–52 Aqueous tear deficiency is the classical type of dry eye, including Sjogren syndrome, which of course is associated with an unstable tear film due to aqueous tear deficiency. Decrease of secretory mucins may be involved in the unstable tear film.53–56 Deficiency of membrane-associated mucin decreases the wettability of the cornea and conjunctiva, and may contribute to the stability of tear film and their deficiency may shorten the tear film breakup time. Asia Dry Eye Society discussions led to a dry eye classification based on the components of two-layered tear film and of the surface epithelium (Fig. 4). However, a mixed type of dry eye may exist. It should be noted that TFOS DEWS II classification has shown that it is difficult to strictly distinguish between the aqueous deficient type dry eye and the increased evaporative type, and a hybrid form has been proposed. Asia Dry Eye Society classification scheme is valuable for the concept of TFOT (Fig. 5). Because this classification is principally based on the abnormalities of the components of each tear film layer and the ocular surface epithelium, the diagnosis itself automatically leads us to TFOT. When the lipid layer is abnormal, such as due to MGD, then treatment for MGD becomes the obvious approach for dry eye. When the aqueous tear secretion is deficient such as in Sjogren or non-Sjogren aqueous tear-deficient type of dry eyes, aqueous components should be provided by artificial tears, hyaluronic acid, or tear secretagogues such as diquafosol sodium, or using punctal plugs in combination with eye drops. When the secretory mucin and membrane-associated mucin is abnormal, the mucin components should be provided to the ocular surface such as by mucin secretagogues such as diquafosol sodium or rebamipide. Our classification scheme automatically finds the way to an appropriate treatment modality (Fig. 6). Although we categorized dry eye disease into three types according to the tear film abnormality or epithelial surface abnormality, not all patients are diagnosed with only one of these categories and a mixed type diagnosis can exist. In the TFOS DEWS II report, patients may have aqueous tear deficiency and MGD together, or they may have MGD and a decrease of membrane-type mucin abnormalities together. Therefore, it may be necessary to go through several steps in treatment as well.
FIG. 4.
New dry eye disease classification by the Asia Dry Eye Society.
FIG. 5.
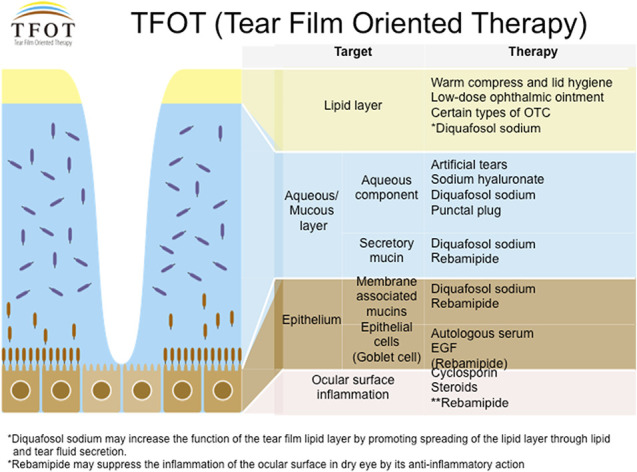
The concept of tear film-oriented therapy. *Diquafosol sodium may increase the function of the tear film lipid layer by promoting spreading of the lipid layer through lipid and tear fluid secretion. **Rebamipide may suppress the inflammation of the ocular surface in dry eye by its anti-inflammatory action. Figure provided by Dry Eye Society Japan.
FIG. 6.
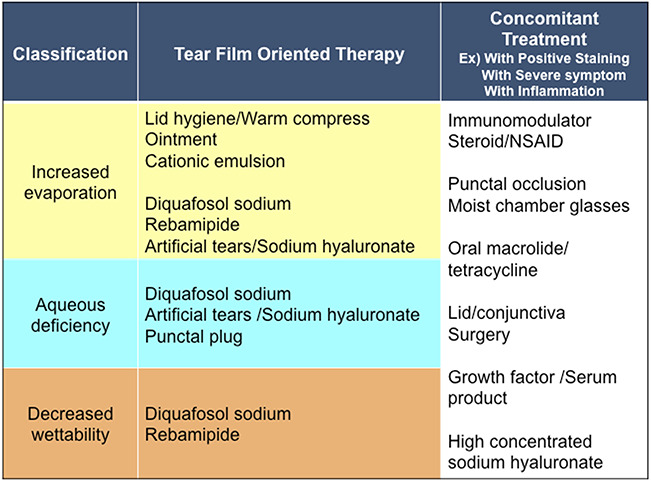
Tear film-oriented therapy based on dry eye disease classification by ADES. ADES, Asia Dry Eye Society.
Tear film–oriented diagnosis (TFOD) concept is important not only from the aspect of discomfort, but also from the impairment of vision which is related to dry eye, because tear film instability disturbs the light entering the eyes, by increasing the scattering and optical aberrations. It is well known that dry eye decreases the quality of vision in daily life from driving to reading. An unstable tear film can explain such deterioration in visual experiences. Many studies on functional visual acuity in dry eye conditions and increased aberrations as assessed by aberrometers support this hypothesis.57–61
Asia Dry Eye Society also discussed the dynamic aspects of dry eye disease in relation to the etiology or classification. A decreased blink rate in visual display terminal (VDT) workers may increase tear evaporation,1,62,63 thus this type can be categorized as evaporative dry eye. Lagophthalmos is a condition in which the eye remains open all the time, thus too much evaporation occurs and consequently damages the ocular surface epithelium and thus it can be categorized as evaporative dry eye. So, whatever the reason for the particular classification, we can still classify dry eye based on the abnormalities in two-layer components of tear film and in ocular surface epithelium. A more detailed discussion is provided in each section of the three classifications (Fig. 4).
Subjective symptoms and objective findings-oriented severity scales have been considered in the diagnosis and treatment of dry eyes. In the 2007 DEWS report, there is a table showing the severity of objective findings and severity of subjective symptoms.39 In the TFOS DEWS II report, the severity matrix table was eliminated. Because the signs and symptoms do not always match in dry eye disease,64–66 this table was not regarded as practical by ADES, although there are definitely mild, moderate, and severe dry eyes based on the symptoms. According to the ADES agreement in the previous consensus paper, the dry eye disease severity was not thought to be related to the ocular surface condition. As we will cover in the final section of this paper, the severity is more related to the neuropathic pain components. Thus, it was decided not to include severity as a classification component. The subjective severity is rather the “target” for the treatment whether the selected treatment is effective or not.
In ADES classification, dry eye is classified into three subtypes: aqueous deficient type, decreased wettability type, and increased evaporation type. It should be noted that TFOS DEWS II classification has shown that it is difficult to strictly distinguish between the aqueous deficient type and the increased evaporative type, and a hybrid form has been proposed.
TEAR FILM BREAKUP PATTERNS AND THE CLASSIFICATION OF DRY EYE
A major breakthrough reported by Yokoi et al.67 for the classification of dry eye was based on the fluorescein breakup patterns. Tear film breakup occurs because of various reasons including increased evaporation, decreased aqueous tear volume, and decreased wettability of corneal surface, which resulted in different patterns of fluorescein breakup (Fig. 7). The detailed pattern of each classification is described later, but the fundamental point is very simple. If dry eye occurs because of aqueous deficiency, line or area breakup patterns are dominant. When the dry eye occurs because of a lipid deficiency, the breakup pattern is the random type. Decreased wettability type results in showing spot or dimple breakup patterns as representative breakup patterns.
FIG. 7.
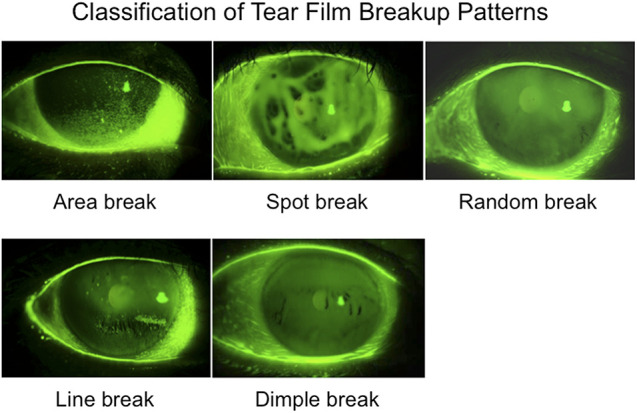
Classification of fluorescein breakup patterns.
To obtain a reproducible breakup pattern, it is necessary to put a small amount of fluorescein into the eye. Specifically, after wetting the fluorescein test paper, it is shaken to remove excess dye, and the test paper is placed on the edge of the lower eyelid. Also, by having the patient open their eyes quickly, it is possible to detect any hidden abnormality in the tear film.
The dry eye diagnosis in ADES concept is also tear film-oriented, because diagnosis of aqueous deficiency, decreased corneal wettability, or increased evaporation is done from the breakup pattern of the tear film. The TFOS DEWS II report suggests that the Schirmer test is an invasive test. In contrast, the current consensus acknowledges that aqueous deficiency can be detected by Schirmer test without anesthesia, meniscometry, and strip meniscometry. In dry eye clinical routine, Schirmer test without anesthesia should be done at least once to evaluate the possibility of aqueous deficiency. Moreover, for the diagnosis of lipid deficiency, lipid layer interferometry is to be used to evaluate decreased lipid thickness in patients with dry eye, but it is not widely used. In a practical way, presence of MGD and poor Meibomian gland expressibility determined by manual pressure to the eyelid can be used to diagnose lipid deficiency. The current challenge for the TFOD is the decreased wettability dry eye, namely the deficiency of membrane-associated mucin.
Although several studies have provided evidence that the short BUT type of dry eye, which includes evaporative and decreased wettability dry eye is related to decreased mucin components in the tear film layer and the surface epithelium,45,68,69 there is no easy method to evaluate the mucin layer. This area should be investigated further in the near future, but for the time being, tear film breakup patterns can provide a clue not only to the diagnosis of evaporative and decreased wettability dry eye (Fig. 7). Moreover, repeatability of tear film breakup pattern should be studied in future studies.
Aqueous Deficient Dry Eye
This classic type of dry eye was first described in association with Sjogren syndrome in 1933 and later with non-Sjogren aqueous deficiency type. Because aqueous components decrease, the tear film layer becomes incompletely established with very unstable tear film. When tears are almost nonexistent as in Sjogren syndrome, the tear film layer is not established after a blink. In this situation, “area break” can be observed (Figs. 4 and 7). The aqueous deficient dry eye with limited or no reflex tear secretion is also associated with severe dry eyes such as seen in patients with chronic graft-versus-host disease (cGVHD), ocular cicatricial pemphigoid, or Stevens-Johnson syndrome. According to the compensation theory of the tear film, this type of dry eye may have a very thick lipid layer compensating for the aqueous tear components, occasionally accompanied by increased mucin production resulting in the too much discharge.70 Aqueous tear deficiency is known to be associated with thicker lipid layer and accumulated mucus. This accompaniment is supported by the compensation theory,70 where decreased production of aqueous is compensated by the increased production of lipids from meibomian glands and increased mucins from goblet cells. However, this may be explained by the delayed aqueous tear clearance.71,72
Typical aqueous tear-deficient dry eye shows a “line break” as the breakup patterns (Fig. 7). The “line break” can be observed during the upward movement of fluorescein-stained aqueous tear after the eye is opened at the inferior part of the cornea. Aqueous tear-deficient dry eye is often accompanied by the superficial punctate keratopathy and this should be the result of the repeated breakup. The aqueous deficient dry eye is diagnosed by Schirmer test without anesthesia. According to the ADES consensus, Schirmer test without anesthesia of less than or equal to 5 mm in 5 min is considered to be aqueous tear-deficient. When it is more than 5 mm, but less than or equal to 10 mm, it is considered to be moderate aqueous tear deficiency, because a normal value is more than 10 mm. Video interferometry is also a useful methodology in this diagnosis. Video interferometer mainly observes the lipid layer, but it can predict the aqueous condition because the lipid layer spreads by each blink when the proper aqueous components exist. If the aqueous components are absent, we can see that the upwardly spreading lipid layer is undetectable by interferometry.73
Tear meniscus observation also provides a clue for tear deficiency via meniscometry (or strip meniscometry).73–75 When the strip meniscometry is less than 4 mm in 5 sec, a diagnosis of aqueous tear deficiency can be made.76
The tear-deficient type of dry eye includes Sjogren syndrome, ocular cicatricial pemphigoid, Stevens-Johnson syndrome, cGVHD, and non-Sjogren type dry eye, such as seen in long-term VDT users.77–89 The mechanism of non-Sjogren type aqueous deficiency is unknown, but there is a hypothesis that long-term use of VDT induces a lacrimal gland hypo-function resulting in accumulation of excess secretary vesicles in the acinar cells which cannot be secreted with normal stimulation (Fig. 8).77,79
FIG. 8.
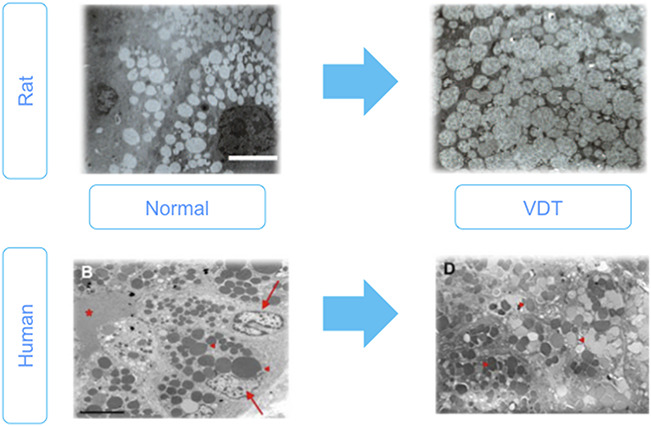
Lacrimal gland hypofunction in visual display terminal (VDT) users and animal model. Upper Figures: Rat VDT user model causes alterations in lacrimal gland morphology. Electron microscopic analysis of acinar cells of normal and VDT rat model. Images showing expanded acinar cells accompanied by accumulated enlarged secretory vesicle in the cytoplasm, decreased endoplasmic reticulum. Scale bar = 10 micrometers. Lower Figures: Electron microscopic findings of the lacrimal gland acinus in human normal and VDT user. Homogeneous secretory vesicles in normal controls. Scale bar = 5 micrometers. High magnification view of secretory vesicles in the VDT user. Red arrows, arrowheads and asterisk show nuclei, secretory vesicles and ductal lumen, respectively. Figures from77,79 Nakamura et al. and Kamoi et al., respectively. These are open access articles distributed under the terms of the Creative Commons Attribution License.
In treatment, aqueous deficiency type of dry eye requires replacement of necessary aqueous components. Eye drops such as the artificial tear substitutes also supply the lacking components of the tear film. Hyaluronic acid and carboxymethylcellulose are eye drops that supplement the aqueous layer, and these negatively-charged and high molecular weight polymers can retain water in the aqueous layer. Artificial tears or hyaluronic acid eye drops are usually preferred as the initial line of therapy, but their residence time for the ocular surface are temporary, say 3 or 5 min.73 Tear secretagogues such as diquafosol sodium are currently the first choice of treatment (Fig. 6). Studies have shown that the staining score decreased compared with baseline from 2 weeks of diquafosol treatment,90,91 and efficacy increased with longer use of diquafosol sodium.91 When a quicker therapeutic efficacy is necessary, punctal plug becomes the choice of treatment. There are proven evidences which showed the efficacy of punctal plug treatment for aqueous deficient type dry eye disease.73,92,93 Punctal occlusion improves not only the aqueous components, but the lipid and mucin components as well, because it sustains all the three tear film layer components because of the delayed aqueous tear clearance as stated above. A combination of punctal plug with diquafosol sodium may provide immediate and long-term relief, and is also a viable treatment approach.
Anti-inflammatory treatment is also important, and it is believed that instability of the tear film increases the friction between the eyelids and the eye, which will result in ocular inflammation due to epithelial damage. T cells play an important role in dry eye onset. Cyclosporin A reduces T-cell activation via IL-2. Several studies have reported improvement in symptoms, improvement in tear stability, and improvement in tear secretion. Also, recently, 5% lifitegrast has been FDA approved. This drug mimics the ICAM-1 cell adhesion molecule and blocks the interaction between ICAM-1 and lymphocyte function-related antigen (LFA-1), which influences T-cell migration and activation. Phase 3 clinical trials reported improvement in dry eye symptoms and vital staining scores.94
Patients with Sjogren syndrome, cGVHD, ocular cicatricial pemphigoid, or Stevens-Johnson syndrome may require special attention because of the severity and immune-orientated disease. For those patients, control of autoimmune reaction by steroids or immunosuppressants such as cyclosporine may become necessary.88,95–99 For the severe aqueous tear-deficient dry eye, autologous serum eye drops are sometimes necessary to provide the essential tear components to the ocular surface epithelium.96,100,101 In addition, consultation with an internal medicine specialist for the systemic evaluation is recommendable.
Increased Evaporation Dry Eye
This type of dry eye may be caused by the lack of lipid or abnormal quality of lipid resulting in an unstable tear film. The most frequent representative condition is thought to be MGD.102–105 This type provides the “random break” as a breakup pattern (Figs. 4 and 7), because pure lipid deficiency type has normal aqueous and mucins. According to the compensation hypothesis, pure MGD patients have an increased aqueous tear volume to compensate the lipid layer deficiency to try to stabilize the tear film.70 In addition to the pattern of “random break,” lipid layer interferometry is the supplementary equipment to observe the lipid deficiency type of dry eye. The lipid layer is measured as more than 40 µm in this system, but it is less than 40 µm when lipid deficiency occurs. Video interferometer is also a useful tool for the observation of lipid deficiency in addition to the “random break” pattern observed during BUT testing.
For the treatment, the primary target is thought to be the meibomian gland, although meibomian lipids may have limited action to suppress aqueous evaporation from the tear film.46 Lid hygiene is the first choice of treatment using lid scrubs or eye shampoo.106–112 Small amounts of lipid or lipid-containing eye drops are reported to be effective.108 Recently, washing with an ointment is reported to be effective in keeping the meibomian orifices open.113
Warming of the lids by heated towels is also a simple and long-standing treatment. Eye warming devices such as infrared LED devices or disposable eye warming masks are gaining in popularity in Asia.114–116 Since the problem of the lipid deficiency resulting in excessive evaporation, suppression of evaporation by increased ambient humidity is another strategy. Not only increasing the room humidity, but the local environment around the eye is an important target. Goggles, moisture glasses and moisture devices are the choices of treatment for lipid deficiency type of dry eye.
Decreased Wettability Dry Eye
This type of dry eye may be increasing with the increase in VDT users.45 The characteristic phenotype is the so-called short BUT type dry eye in which BUT is short but the tear production is normal and staining is none or slight. Toda et al.33 originally reported short BUT dry eye in 1995 which mainly included this type of dry eye and increased evaporation dry eye.
Because the membrane-associated mucin is deficient or abnormal, the wettability of the surface of the cornea becomes impaired. According to the DEWS or TFOS DEWS II consensus reports of dry eye, this type is categorized as “evaporative” with lipid deficiency type, but the fundamental mechanism of the disease is completely different. The representative patterns of tear breakup in this category are “spot break” or “dimple break.” These types of breakup pattern appear respectively during the phase of deposition of aqueous tears on the cornea immediately after eye opening or during the phase of upward movement of aqueous tears before the establishment of precorneal tear film. Especially for the “spot break” (Fig. 7), BUT is very short corresponding to 0 sec. In this breakup pattern, breakup occurs during eye opening, which means breakup already exists when eye opening was completed. In the recent reports, rapid expansion of the breakup is proposed as a modification of some breakup patterns that are possibly associated with a decreased corneal wettability mechanism and are often seen in the short BUT type dry eye.117,118 The patient's complaint of decreased wettability dry eye is relatively obvious and was usually difficult to treat except by punctal plug. Recently, it has been reported that the amount of ocular surface glycocalyx significantly correlated with tear film breakup time (Fig. 9).119 From this result, eyes with “spot break” as the breakup patterns should have a localized membrane-type mucin deficiency at the cornea. After the introduction of rebamipide and diquafosol sodium, the mucin layer deficiency can now be treated adequately120 and thus the unstable tear film due to the mucin deficiency has now become treatable.
FIG. 9.
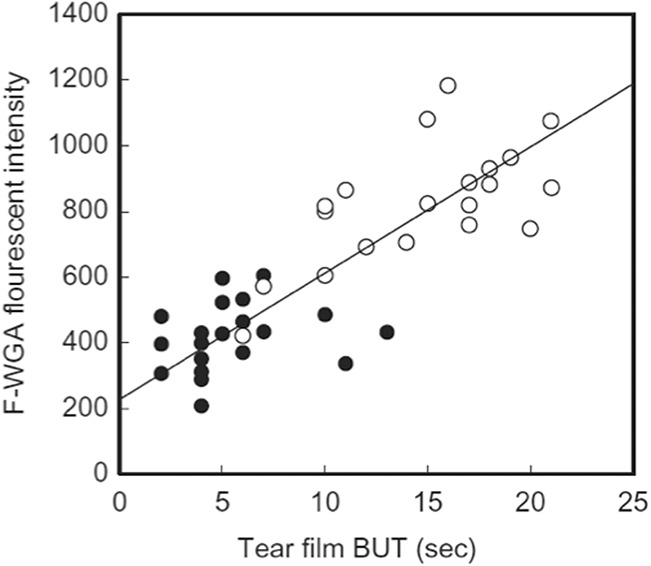
Correlation between tear film breakup time and amount of glycocalyx at corneal surface. Glycocalyx at corneal surface was evaluated using a fluorescein-labeled wheat germ agglutinin (F-WGA) as a marker. Reprinted from current eye research, Vol 41, Fukui M, Yamada M, Akune Y, et al. Fluorophotometric analysis of the ocular surface glycocalyx in Soft Contact Lens Wearers, pages 9–14, copyright 2015, with permission from Elsevier.
Tear Dynamics and Dry Eye Development
In addition to the three-layer classification that includes the two-layered tear film and the surface epithelial layer, the ADES report emphasized the role of tear dynamics such as decreased blink, lid deformity, low environmental humidity, lagophthalmos, conjunctivochalasis, and the other ocular surface diseases with decreased tear clearance. Those factors may affect the tear film stability and thus can constitute to an independent classification. However, the treatment for decreased blinking or excessive evaporation eventually falls into one or two layers of tear film including lipid layer and aqueous layer. If excessive tear evaporation occurs because of less blink or lagophthalmos, the basic treatment is the same, in addition to targeting each specific treatment such as lid closure or ointment application during sleep for lagophthalmos. Visual display terminal users with decreased blink may need education on the importance of blinks.
Special Subtypes of Dry Eye
As we have described earlier, special subtypes of dry eye exist such as Sjogren syndrome, GVHD, ocular cicatricial pemphigoid, and Stevens-Johnson syndrome for which special attention should be paid. In addition, there are certain types of conditions that affect the stability of the tear film. These are conjunctivochalasis, contact lens wear, superior limbic keratoconjunctivitis, and nocturnal lagophthalmos.
NEUROPATHIC PAIN COMPONENT AND SUBJECTIVE SEVERITY
The Asia Dry Eye Society discussed about a possible classification for the severity of the dry eye disease with subcategories such as mild, moderate, and severe dry eyes. For therapeutic purposes, the severity does not give us enough information, because the symptoms do not directly correlate with the severity of the sign(s). An original report of the short-BUT-type of dry eye in 1995 clearly showed that the symptoms of a patient can be almost as severe as that in a patient with Sjogren syndrome, except that there was no observable ocular surface damage.33 Now it is speculated that neuropathic components exist even in the ordinary type of dry eye, where the neuropathic components determine the severity of symptoms. Thus, we concluded that subjective severity could be used as a marker for the therapeutic efficacy, but not for the purpose of classification. In other words, we classified dry eyes according to the two tear film layers and the surface epithelium, increased evaporation, aqueous-deficient and decreased wettability types for various treatment options, but the therapeutic efficacy should be evaluated by the symptoms such as dry eye–related pain, discomfort, or visual disturbances.
CONCLUSION
The ADES proposes a simple classification of dry eyes based on the concept of TFOD according to the definition report proposed previously.1 There are basically three types of dry eye: increased evaporation, aqueous-deficient, and decreased wettability. These three types coincide with the problems of each layer: lipid, aqueous/secretory mucin, and membrane-associated mucin. Although each component cannot be quantitatively evaluated with exact precision with the current technology, we can make a practical diagnosis using the patterns of tear film breakup simply using fluorescein. The “random break” corresponds to the increased evaporation dry eye (evaporative dry eye), and “line break” and “area break” correspond to the aqueous deficient dry eye respectively of mild-to-moderate and severe in their severity. Decreased wettability dry eye is related to “spot break” and “dimple break” as representative patterns. These three simple classifications lead us to select the choices of treatment to target the important layer of tear film and the surface epithelium, each of which maintains tear film stability.
For a practical use of the diagnostic criteria system, definition, diagnostic criteria, and classification should be integrated and be simple to use. The classification system proposed by ADES is a straightforward tool and practical, because it only uses fluorescein, even for non-dry eye specialists, which can contribute to an effective diagnosis and treatment of dry eyes.
Because the fluorescein breakup pattern is one of the tear film-oriented diagnostic methods derived from physical theory and clinical findings, the relationship with the ocular surface mucins and the tear film lipid layer needs be clarified in future studies. We are convinced that the suggestions of this paper may be used as a guide, and serve as a basis for further discussions for the future dry eye workshop meetings.
ACKNOWLEDGMENTS
Members of Asia Dry Eye Society: China: Z. Liu, X. Sun, W. Chen, Yingping Deng, Jing Hong, Ying Jie, M. Li, Wei Li, Ying Li, L. Liang, Fan Lu, Hong Qi, Hua Wang, Xiaoming Yan, Wenzhao Yang, Yufeng Ye, Jin Yuan, Hong Zhang, Hui Zhan, Mingchang Zhang, Shaozhen Zhao. Korea: H.-M. Kim, H.-W. Tchah, Chul Young Choi, Si Hwan Choi, Eui-Sang Chung, So-Hyang Chung, Tae Young Chung, Young keun Han, J. Y. Hyon, Hong Kyun Kim, Hyung Joon Kim, Jae Yong Kim, Jin Hyoung Kim, Mee Kum Kim, Myoung Joon Kim, Tae-Im Kim, Do-Hyung Lee, Hyung-Keun Lee, Jong Soo Lee, Sang-Bumm Lee, Woo Chan Park, K. Y. Seo, Jong Suk Song, K.-C. Yoon, In Cheon You. Japan: K. Tsubota, N. Yokoi, Shiro Amano, Reiko Arita, M. Dogru, Masatoshi Hirayama, Yuichi Hori, Osama Ibrahim, Minako Kaido, Tetsuya Kawakita, Motoko Kawashima, Shizuka Koh, T. Kojima, Aoi Komuro, Naoyuki Morishige, Kohji Nishida, Yoko Ogawa, J. Shimazaki, Chika Shigeyasu, Miki Uchino, Yuichi Uchino, H. Watanabe, M. Yamada, Masahiko Yamaguchi. Singapore: L. Tong. Philippines: Jessica Marie Abaño, Victor Leido Caparas, Irwin Yang Cua, Ivo John Dualan, Reynaldo Santos, R. Lim Bon Siong. Thailand: Pinnita Tanthuvanit, V. Puangsricharern, Ngamjit Kasetsuwan, Olan Suwan-apichon, Wimolwan Tangpagasit. Malaysia: Shamala Ratnasabapathy, Chandramalar T. Santhirathelagan, T. K. Yong. Taiwan: Shu-Wen Chang, F.-R. Hu, Chi-Chin Sun. Hong Kong: Kendrick Co Shih, Vishal Jhanji. Vietnam: Pham Ngoc Dong.
Footnotes
Outside the submitted work, these authors report COI as follows: K. Tsubota reports research funding and consultancies from Santen Pharmaceutical Co, Ltd and Otsuka Pharmaceutical Co, Ltd, and holds the patent right for the method and apparatus used for the functional visual acuity measurement system (US patent no: 7470026 by Kowa Company); and is a consultant for Shire. N. Yokoi reports personal fees from Santen Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, and consultancies from Rhoto Co, Ltd, Alcon Japan Co, Ltd, and patents for ophthalmologic apparatus with Kowa Co, Ltd. H. Watanabe reports personal fees from Santen Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co, Ltd, Senju Pharmaceutical, Alcon and Pfizer Inc. M. Dogru reports personal fees from Echo Electricity, Santen Pharmaceutical, and Otsuka Pharmaceutical Co, Ltd. T. Kojima reports personal fees from Staar Surgical, Santen Pharmaceutical, Otsuka Pharmaceutical, Johnson & Johnson, and Alcon. M. Yamada reports research funding from Santen Pharmaceutical Co, Ltd, and personal fees from Santen Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Johnson & Johnson Vision Care Co, and Alcon Co. S. Kinoshita reports research funding and consultancies from Santen Pharmaceutical Co, Ltd Otsuka Pharmaceutical Co, Ltd, Senju Pharmaceutical Co, Ltd, and KOWA Co, Ltd, research funding from Oncolys Biopharma Inc, HOYA Corporation, and Lion Corporation, personal fees from Alcon Japan and AMO Inc. J. Y. Hyon reports researching funding and consultancy with Santen Pharmaceutical Co, Ltd. K. C. Yoon reports research funding and consultancies from Santen Pharmaceutical Co, Ltd and Pfizer. K. Y. Seo reports personal fees from Santen Pharmaceutical Co, Ltd and Lumenis Korea Ltd. L. Tong reports funding for research, advisory boards and conference related travels from Alcon-Novartis, Allergan, Bausch and Lomb, and Santen Pharmaceutical Co, Ltd. V. Puangsricharern reports honorarium as a speaker for Santen, Alcon and Allergan. R. Lim-Bon-Siong is a member of the Santen Advisory Board and receives educational and research grants from Santen Pharmaceutical Co, Ltd. T. K. Yong received travel grants from Santen and Allergan. Z. Liu reports research funding or consultancies or travel grants from: Alcon, Allergen, Novartis Johnson & Johnson Vision, Santen, Senju, Yuejia, Xingqi, Zhuhaiyisheng, Reilin, Dakai. J. Shimazaki reports research funding and consultancies from Santen Pharmaceutical Co, Ltd and Otsuka Pharmaceutical Co, Ltd. The remaining authors have no conflicts of interest to disclose.
The Asia Dry Eye Society is partially supported by Santen Pharmaceutical Co, Ltd.
REFERENCES
- 1.Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia dry eye society. Ocul Surf 2017;15:65–76. [DOI] [PubMed] [Google Scholar]
- 2.Goto T, Zheng X, Okamoto S, et al. Tear film stability analysis system: Introducing a new application for videokeratography. Cornea 2004;23:S65–S70. [DOI] [PubMed] [Google Scholar]
- 3.Kaido M, Ishida R, Dogru M, et al. The relation of functional visual acuity measurement methodology to tear functions and ocular surface status. Jpn J Ophthalmol 2011;55:451–459. [DOI] [PubMed] [Google Scholar]
- 4.Kojima T, Ishida R, Dogru M, et al. A new noninvasive tear stability analysis system for the assessment of dry eyes. Invest Ophthalmol Vis Sci 2004;45:1369–1374. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita S, Awamura S, Oshiden K, et al. Rebamipide (OPC-12759) in the treatment of dry eye: A randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology 2012;119:2471–2478. [DOI] [PubMed] [Google Scholar]
- 6.Takeji Y, Urashima H, Aoki A, et al. Rebamipide increases the mucin-like glycoprotein production in corneal epithelial cells. J Ocul Pharmacol Ther 2012;28:259–263. [DOI] [PubMed] [Google Scholar]
- 7.Kaido M, Uchino M, Kojima T, et al. Effects of diquafosol tetrasodium administration on visual function in short break-up time dry eye. J Ocul Pharmacol Ther 2013;29:595–603. [DOI] [PubMed] [Google Scholar]
- 8.Kimura K, Morita Y, Orita T, et al. Protection of human corneal epithelial cells from TNF-alpha-induced disruption of barrier function by rebamipide. Invest Ophthalmol Vis Sci 2013;54:2572–2760. [DOI] [PubMed] [Google Scholar]
- 9.Koh S, Inoue Y, Sugmimoto T, et al. Effect of rebamipide ophthalmic suspension on optical quality in the short break-up time type of dry eye. Cornea 2013;32:1219–1223. [DOI] [PubMed] [Google Scholar]
- 10.Ohguchi T, Kojima T, Ibrahim OM, et al. The effects of 2% rebamipide ophthalmic solution on the tear functions and ocular surface of the superoxide dismutase-1 (sod1) knockout mice. Invest Ophthalmol Vis Sci 2013;54:7793–7802. [DOI] [PubMed] [Google Scholar]
- 11.Shimazaki-Den S, Iseda H, Dogru M, et al. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea 2013;32:1120–1125. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Fukuda K, Ishida W, et al. Rebamipide increases barrier function and attenuates TNFalpha-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br J Ophthalmol 2013;97:912–916. [DOI] [PubMed] [Google Scholar]
- 13.Arimoto A, Kitagawa K, Mita N, et al. Effect of rebamipide ophthalmic suspension on signs and symptoms of keratoconjunctivitis sicca in Sjögren syndrome patients with or without punctal occlusions. Cornea 2014;33:806–811. [DOI] [PubMed] [Google Scholar]
- 14.Kaido M, Ishida R, Dogru M, et al. Short-term effects of instillation of a rebamipide suspension on visual function. J Ocul Pharmacol Ther 2014;30:313–318. [DOI] [PubMed] [Google Scholar]
- 15.Kashima T, Itakura H, Akiyama H, et al. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: A critical appraisal. Clin Ophthalmol 2014;8:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinoshita S, Awamura S, Nakamichi N, et al. A multicenter, open-label, 52-week study of 2% rebamipide (OPC-12759) ophthalmic suspension in patients with dry eye. Am J Ophthalmol 2014;157:576–583.e1. [DOI] [PubMed] [Google Scholar]
- 17.Koh S, Maeda N, Ikeda C, et al. Effect of diquafosol ophthalmic solution on the optical quality of the eyes in patients with aqueous-deficient dry eye. Acta Ophthalmol 2014;92:e671–e675. [DOI] [PubMed] [Google Scholar]
- 18.Kojima T, Dogru M, Ibrahim OM, et al. The effects of 3% diquafosol sodium application on the tear functions and ocular surface of the Cu, Zn-superoxide dismutase-1 (Sod1)-knockout mice. Mol Vis 2014;20:929–938. [PMC free article] [PubMed] [Google Scholar]
- 19.Igarashi A, Kamiya K, Kobashi H, et al. Effect of rebamipide ophthalmic suspension on intraocular light scattering for dry eye after corneal refractive surgery. Cornea 2015;34:895–900. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T, Fujita M, Yamada Y, et al. Improvements in signs and symptoms of dry eye after instillation of 2% rebamipide. J Nippon Med Sch 2015;82:229–236. [DOI] [PubMed] [Google Scholar]
- 21.Shigeyasu C, Hirano S, Akune Y, et al. Diquafosol tetrasodium increases the concentration of mucin-like substances in tears of healthy human subjects. Curr Eye Res 2015;40:878–883. [DOI] [PubMed] [Google Scholar]
- 22.Ueda K, Matsumiya W, Otsuka K, et al. Effectiveness and relevant factors of 2% rebamipide ophthalmic suspension treatment in dry eye. BMC Ophthalmol 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, Chen WQ, Li R, et al. Efficacy and safety of topical diquafosol ophthalmic solution for treatment of dry eye: A systematic review of randomized clinical trials. Cornea 2015;34:644–650. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Nishijima T, Shimazaki J, et al. Real-world assessment of diquafosol in dry eye patients with risk factors such as contact lens, meibomian gland dysfunction, and conjunctivochalasis: Subgroup analysis from a prospective observational study. Clin Ophthalmol 2015;9:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamane M, Ogawa Y, Fukui M, et al. Long-term rebamipide and diquafosol in two cases of immune-mediated dry eye. Optom Vis Sci 2015;92:S25–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon HS, Hyon JY. The efficacy of diquafosol ophthalmic solution in non-sjögren and sjögren syndrome dry eye patients unresponsive to artificial tear. J Ocul Pharmacol Ther 2016;32:463–468. [DOI] [PubMed] [Google Scholar]
- 27.Yokoi N, Kato H, Kinoshita S. The increase of aqueous tear volume by diquafosol sodium in dry-eye patients with sjögren's syndrome: A pilot study. Eye (Lond) 2016;30:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue Y, Ochi S. Effects of 3% diquafosol sodium ophthalmic solution on higher-order aberrations in patients diagnosed with dry eye after cataract surgery. Clin Ophthalmol 2017;11:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kase S, Shinohara T, Kase M, et al. Effect of topical rebamipide on goblet cells in the lid wiper of human conjunctiva. Exp Ther Med 2017;13:3516–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobashi H, Kamiya K, Shimizu K. Randomized comparison between rebamipide ophthalmic suspension and diquafosol ophthalmic solution for dry eye after penetrating keratoplasty. J Ocul Pharmacol Ther 2017;33:13–18. [DOI] [PubMed] [Google Scholar]
- 31.Lee H, Kim SM, Choi S, et al. Effect of diquafosol three per cent ophthalmic solution on tear film and corneal aberrations after cataract surgery. Clin Exp Optom 2017;100:590–594. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Xia S, Chen Y. Comparison of the efficacy between topical diquafosol and artificial tears in the treatment of dry eye following cataract surgery: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toda I, Shimazaki J, Tsubota K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology 1995;102:302–309. [DOI] [PubMed] [Google Scholar]
- 34.Khurana AK, Moudgil SS, Parmar IP, et al. Tear film flow and stability in acute and chronic conjunctivitis. Acta Ophthalmol (Copenh) 1987;65:303–305. [DOI] [PubMed] [Google Scholar]
- 35.Zengin N, Tol H, Gündüz K, et al. Meibomian gland dysfunction and tear film abnormalities in rosacea. Cornea 1995;14:144–146. [PubMed] [Google Scholar]
- 36.Kawashima M, Yamada M, Suwaki K, et al. A clinic-based survey of clinical characteristics and practice pattern of dry eye in Japan. Adv Ther 2017;34:732–743. [DOI] [PubMed] [Google Scholar]
- 37.Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka study. Am J Ophthalmol 2013;156:759–766. [DOI] [PubMed] [Google Scholar]
- 38.Lemp MA. Report of the National eye institute/industry workshop on clinical trials in dry eyes. CLAO J 1995;21:221–232. [PubMed] [Google Scholar]
- 39.The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international dry eye workshop (2007). Ocul Surf 2007;5:75–92. [DOI] [PubMed] [Google Scholar]
- 40.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15:276–283. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z. The preliminary recommendations on the name and classification of dry eye [in Chinese]. Chin J Eye Otolaryngol 2004;3:4–5. [Google Scholar]
- 42.Society TCC. The consensus on clinical diagnosis and treatment of dry eye [in Chinese]. Chin J Ophthalmol 2013;49:73–75. [Google Scholar]
- 43.Murube J, Nemeth J, Hoh H, et al. The triple classification of dry eye for practical clinical use. Eur J Ophthalmol 2005;15:660–667. [DOI] [PubMed] [Google Scholar]
- 44.Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: A Delphi approach to treatment recommendations. Cornea 2006;25:900–907. [DOI] [PubMed] [Google Scholar]
- 45.Uchino Y, Uchino M, Yokoi N, et al. Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol 2014;132:985–992. [DOI] [PubMed] [Google Scholar]
- 46.Herok GH, Mudgil P, Millar TJ. The effect of Meibomian lipids and tear proteins on evaporation rate under controlled in vitro conditions. Curr Eye Res 2009;34:589–597. [DOI] [PubMed] [Google Scholar]
- 47.Rantamaki AH, Javanainen M, Vattulainen I, et al. Do lipids retard the evaporation of the tear fluid? Invest Ophthalmol Vis Sci 2012;53:6442–6447. [DOI] [PubMed] [Google Scholar]
- 48.Borchman D, Foulks GN, Yappert MC, et al. Factors affecting evaporation rates of tear film components measured in vitro. Eye Contact Lens 2009;35:32–37. [DOI] [PubMed] [Google Scholar]
- 49.Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film—A review. Exp Eye Res 2015;137:125–138. [DOI] [PubMed] [Google Scholar]
- 50.Brown SI, Dervichian DG. The oils of the meibomian glands. Physical and surface characteristics. Arch Ophthalmol 1969;82:537–540. [DOI] [PubMed] [Google Scholar]
- 51.Cerretani CF, Ho NH, Radke CJ. Water-evaporation reduction by duplex films: Application to the human tear film. Adv Colloid Interface Sci 2013;197–198:33–57. [DOI] [PubMed] [Google Scholar]
- 52.Kulovesi P, Rantamäki AH, Holopainen JM. Surface properties of artificial tear film lipid layers: Effects of wax esters. Invest Ophthalmol Vis Sci 2014;55:4448–4454. [DOI] [PubMed] [Google Scholar]
- 53.Mantelli F, Tiberi E, Micera A, et al. MUC5AC overexpression in tear film of neonates. Graefes Arch Clin Exp Ophthalmol 2007;245:1377–1381. [DOI] [PubMed] [Google Scholar]
- 54.Gipson IK, Argüeso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol 2003;231:1–49. [DOI] [PubMed] [Google Scholar]
- 55.Inatomi T, Spurr-Michaud S, Tisdale AS, et al. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci 1995;36:1818–1827. [PubMed] [Google Scholar]
- 56.Argüeso P, Spurr-Michaud S, Russo CL, et al. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci 2003;44:2487–2495. [DOI] [PubMed] [Google Scholar]
- 57.Kaido M, Uchino M, Yokoi N, et al. Dry-eye screening by using a functional visual acuity measurement system: The Osaka study. Invest Ophthalmol Vis Sci 2014;55:3275–3281. [DOI] [PubMed] [Google Scholar]
- 58.Koh S, Maeda N, Hirohara Y, et al. Serial measurements of higher-order aberrations after blinking in patients with dry eye. Invest Ophthalmol Vis Sci 2008;49:133–138. [DOI] [PubMed] [Google Scholar]
- 59.Koh S, Maeda N, Hori Y, et al. Effects of suppression of blinking on quality of vision in borderline cases of evaporative dry eye. Cornea 2008;27:275–278. [DOI] [PubMed] [Google Scholar]
- 60.Koh S, Tung C, Aquavella J, et al. Simultaneous measurement of tear film dynamics using wavefront sensor and optical coherence tomography. Invest Ophthalmol Vis Sci 2010;51:3441–3448. [DOI] [PubMed] [Google Scholar]
- 61.Dogru M, Ward SK, Wakamatsu T, et al. The effects of 2 week senofilcon-A silicone hydrogel contact lens daily wear on tear functions and ocular surface health status. Cont Lens Anterior Eye 2011;34:77–82. [DOI] [PubMed] [Google Scholar]
- 62.Hirayama M, Murat D, Liu Y, et al. Efficacy of a novel moist cool air device in office workers with dry eye disease. Acta Ophthalmol 2013;91:756–762. [DOI] [PubMed] [Google Scholar]
- 63.Tsubota K, Toda I, Nakamori K. Poor illumination, VDTs, and desiccated eyes. Lancet 1996;347:768–769. [DOI] [PubMed] [Google Scholar]
- 64.Bartlett JD, Keith MS, Sudharshan L, et al. Associations between signs and symptoms of dry eye disease: A systematic review. Clin Ophthalmol 2015;9:1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan BD, Crews LA, Messmer EM, et al. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: Clinical implications. Acta Ophthalmol 2014;92:161–166. [DOI] [PubMed] [Google Scholar]
- 66.Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004;23:762–770. [DOI] [PubMed] [Google Scholar]
- 67.Yokoi N, Georgiev GA, Kato H, et al. Classification of fluorescein breakup patterns: A Novel method of differential diagnosis for Dry Eye. Am J Ophthalmol 2017;180:72–85. [DOI] [PubMed] [Google Scholar]
- 68.Shimazaki-Den S, Dogru M, Higa K, et al. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea 2013;32:1211–1218. [DOI] [PubMed] [Google Scholar]
- 69.Hori Y, Kageyama T, Sakamoto A, et al. Comparison of short-term effects of diquafosol and rebamipide on mucin 5AC level on the rabbit ocular surface. J Ocul Pharmacol Ther 2017;33:493–497. [DOI] [PubMed] [Google Scholar]
- 70.Arita R, Morishige N, Koh S, et al. Increased tear fluid production as a compensatory response to meibomian gland loss: A multicenter cross-sectional study. Ophthalmology 2015;122:925–933. [DOI] [PubMed] [Google Scholar]
- 71.Adams AD. The morphology of human conjunctival mucus. Arch Ophthalmol 1979;97:730–734. [DOI] [PubMed] [Google Scholar]
- 72.Yokoi N, Mossa F, Tiffany JM, et al. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol 1999;117:723–729. [DOI] [PubMed] [Google Scholar]
- 73.Yokoi N, Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res 2004;78:399–407. [DOI] [PubMed] [Google Scholar]
- 74.Yokoi N, Bron A, Tiffany J, et al. Reflective meniscometry: A non-invasive method to measure tear meniscus curvature. Br J Ophthalmol 1999;83:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yokoi N, Bron AJ, Tiffany JM, et al. Reflective meniscometry: A new field of dry eye assessment. Cornea 2000;19:S37–S43. [DOI] [PubMed] [Google Scholar]
- 76.Shinzawa M, Dogru M, Miyasaka K, et al. Application of CASIA SS-1000 optical coherence tomography tear meniscus imaging in testing the efficacy of new strip meniscometry in dry eye diagnosis. Eye Contact Lens 2018;44(Suppl 1):S44–S49. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura S, Kinoshita S, Yokoi N, et al. Lacrimal hypofunction as a new mechanism of dry eye in visual display terminal users. PLoS One 2010;5:e11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008;115:1982–1988. [DOI] [PubMed] [Google Scholar]
- 79.Kamoi M, Ogawa Y, Nakamura S, et al. Accumulation of secretory vesicles in the lacrimal gland epithelia is related to non-Sjögren's type dry eye in visual display terminal users. PLoS One 2012;7:e43688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uchino M, Nishiwaki Y, Michikawa T, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology 2011;118:2361–2367. [DOI] [PubMed] [Google Scholar]
- 81.Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012;31:472–478. [DOI] [PubMed] [Google Scholar]
- 82.Dart J. Cicatricial pemphigoid and dry eye. Semin Ophthalmol 2005;20:95–100. [DOI] [PubMed] [Google Scholar]
- 83.Pflugfelder SC, Huang AJ, Feuer W, et al. Conjunctival cytologic features of primary Sjögren's syndrome. Ophthalmology 1990;97:985–991. [DOI] [PubMed] [Google Scholar]
- 84.Jain R, Sharma N, Basu S, et al. Stevens-Johnson syndrome: The role of an ophthalmologist. Surv Ophthalmol 2016;61:369–399. [DOI] [PubMed] [Google Scholar]
- 85.Lemp MA. The mucin-deficient dry eye. Int Ophthalmol Clin 1973;13:185–189. [DOI] [PubMed] [Google Scholar]
- 86.Roujeau JC, Phlippoteau C, Koso M, et al. Sjögren-like syndrome after drug-induced toxic epidermal necrolysis. Lancet 1985;1:609–611. [DOI] [PubMed] [Google Scholar]
- 87.Ogawa Y, Okamoto S, Wakui M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol 1999;83:1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Ogawa Y, Dogru M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant 2008;41:293–302. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Ogawa Y, Dogru M, et al. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone Marrow Transplant 2010;45:1077–1083. [DOI] [PubMed] [Google Scholar]
- 90.Matsumoto Y, Ohashi Y, Watanabe H, et al. Diquafosol ophthalmic solution phase 2 study G. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: A Japanese phase 2 clinical trial. Ophthalmology 2012;119:1954–1960. [DOI] [PubMed] [Google Scholar]
- 91.Yamaguchi M, Tsubota K, Watanabe H, et al. The safety and efficacy of long-term treatment with 3% diquafosol ophthalmic solution for dry eye. Atarashii Ganka 2012;29:527–535. [Google Scholar]
- 92.Kojima T, Matsumoto Y, Ibrahim OM, et al. Evaluation of a thermosensitive atelocollagen punctal plug treatment for dry eye disease. Am J Ophthalmol 2014;157:311–317.e311. [DOI] [PubMed] [Google Scholar]
- 93.Ervin AM, Law A, Pucker AD. Punctal occlusion for dry eye syndrome: Summary of a cochrane systematic review. Br J Ophthalmol 2019;103:301–306. [DOI] [PubMed] [Google Scholar]
- 94.Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: Results of the OPUS-1 phase 3 study. Ophthalmology 2014;121:475–483. [DOI] [PubMed] [Google Scholar]
- 95.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol 2002;120:330–337. [DOI] [PubMed] [Google Scholar]
- 96.Foulks GN, Forstot SL, Donshik PC, et al. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf 2015;13:118–132. [DOI] [PubMed] [Google Scholar]
- 97.Moscovici BK, Holzchuh R, Chiacchio BB, et al. Clinical treatment of dry eye using 0.03% tacrolimus eye drops. Cornea 2012;31:945–949. [DOI] [PubMed] [Google Scholar]
- 98.Moscovici BK, Holzchuh R, Sakassegawa-Naves FE, et al. Treatment of Sjögren's syndrome dry eye using 0.03% tacrolimus eye drop: Prospective double-blind randomized study. Cont Lens Anterior Eye 2015;38:373–378. [DOI] [PubMed] [Google Scholar]
- 99.Ogawa Y, Okamoto S, Kuwana M, et al. Successful treatment of dry eye in two patients with chronic graft-versus-host disease with systemic administration of FK506 and corticosteroids. Cornea 2001;20:430–434. [DOI] [PubMed] [Google Scholar]
- 100.Rocha EM, Pelegrino FS, de Paiva CS, et al. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant 2000;25:1101–1103. [DOI] [PubMed] [Google Scholar]
- 101.Tahmaz V, Gehlsen U, Sauerbier L, et al. Treatment of severe chronic ocular graft-versus-host disease using 100% autologous serum eye drops from a sealed manufacturing system: A retrospective cohort study. Br J Ophthalmol 2017;101:322–326. [DOI] [PubMed] [Google Scholar]
- 102.Cuevas M, González-García MJ, Castellanos E, et al. Correlations among symptoms, signs, and clinical tests in evaporative-type dry eye disease caused by Meibomian gland dysfunction (MGD). Curr Eye Res 2012;37:855–863. [DOI] [PubMed] [Google Scholar]
- 103.Foulks GN, Borchman D. Meibomian gland dysfunction: The past, present, and future. Eye Contact Lens 2010;36:249–253. [DOI] [PubMed] [Google Scholar]
- 104.Uchino M, Dogru M, Yagi Y, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci 2006;83:797–802. [DOI] [PubMed] [Google Scholar]
- 105.Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52:1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi A, Ide T, Fukumoto T, et al. Effects of a new eyelid shampoo on lid hygiene and eyelash length in patients with meibomian gland dysfunction: A comparative open study. J Ophthalmol 2016;2016:4292570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guillon M, Maissa C, Wong S. Eyelid margin modification associated with eyelid hygiene in anterior blepharitis and meibomian gland dysfunction. Eye Contact Lens 2012;38:319–325. [DOI] [PubMed] [Google Scholar]
- 108.Goto E, Dogru M, Fukagawa K, et al. Successful tear lipid layer treatment for refractory dry eye in office workers by low-dose lipid application on the full-length eyelid margin. Am J Ophthalmol 2006;142:264–270. [DOI] [PubMed] [Google Scholar]
- 109.Garrigue JS, Amrane M, Faure MO, et al. Relevance of lipid-based products in the management of dry eye disease. J Ocul Pharmacol Ther 2017;33:647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baudouin C, Galarreta DJ, Mrukwa-Kominek E, et al. Clinical evaluation of an oil-based lubricant eyedrop in dry eye patients with lipid deficiency. Eur J Ophthalmol 2017;27:122–128. [DOI] [PubMed] [Google Scholar]
- 111.Lim A, Wenk MR, Tong L. Lipid-based therapy for ocular surface inflammation and disease. Trends Mol Med 2015;21:736–748. [DOI] [PubMed] [Google Scholar]
- 112.Chung SH, Lim SA, Tchach H. Efficacy and safety of carbomer-based lipid-containing artificial tear formulations in patients with dry eye syndrome. Cornea 2016;35:181–186. [DOI] [PubMed] [Google Scholar]
- 113.Kaido M, Ibrahim OM, Kawashima M, et al. Eyelid cleansing with ointment for obstructive meibomian gland dysfunction. Jpn J Ophthalmol 2017;61:124–130. [DOI] [PubMed] [Google Scholar]
- 114.Ishida R, Matsumoto Y, Onguchi T, et al. Tear film with “Orgahexa EyeMasks” in patients with meibomian gland dysfunction. Optom Vis Sci 2008;85:684–691. [DOI] [PubMed] [Google Scholar]
- 115.Mori A, Shimazaki J, Shimmura S, et al. Disposable eyelid-warming device for the treatment of meibomian gland dysfunction. Jpn J Ophthalmol 2003;47:578–586. [DOI] [PubMed] [Google Scholar]
- 116.Matsumoto Y, Dogru M, Goto E, et al. Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea 2006;25:644–650. [DOI] [PubMed] [Google Scholar]
- 117.Yokoi N, Georgiev GA. Tear film-oriented diagnosis and tear film-oriented therapy for dry eye based on tear film dynamics. Invest Ophthalmol Vis Sci 2018;59:DES13–DES22. [DOI] [PubMed] [Google Scholar]
- 118.Yokoi N, Georgiev GA. Tear-film-oriented diagnosis for dry eye. Jpn J Ophthalmol 2019;63:127–136. [DOI] [PubMed] [Google Scholar]
- 119.Fukui M, Yamada M, Akune Y, et al. Fluorophotometric analysis of the ocular surface glycocalyx in Soft contact lens Wearers. Curr Eye Res 2016;41:9–14. [DOI] [PubMed] [Google Scholar]
- 120.Yokoi N, Sonomura Y, Kato H, et al. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjögren's syndrome. Eye (Lond) 2015;29:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]