Abstract
Introduction
No therapy has proven to be effective yet to reduce mortality and/or invasive mechanical ventilation (IMV) requirement in COVID-19. Tocilizumab (TCZ) in patients with severe COVID-19 could be an effective treatment.
Methods
We conducted a retrospective case-control study in the Nord Franche-Comté Hospital, France. We compared the outcome of patients treated with TCZ and patients without TCZ considering a combined primary endpoint: mortality and/or IMV requirement.
Results
Thirty patients were treated with TCZ and 176 patients were treated without TCZ. TCZ was used in patients in critical condition (oxygen therapy flow at TCZ onset was 10.5 L/min and 14/30 patients had ≥ 50% lung involvement on CT scan) as a rescue treatment (8/30 patients who died were not admitted in USC in regard to their comorbidities). However, mortality and/or IMV requirement were lower in patients with TCZ than in patients without TCZ (27% vs 52%, p = 0.009).
Conclusion
Despite the small sample size in the TCZ group, this result suggests that TCZ reduces mortality and/or IMV requirement in patients with severe SARS-CoV-2 pneumonia. This notion needs to be confirmed and spread in the medical community.
Keywords: COVID-19, SARS-CoV-2, Tocilizumab, Mortality, Invasive mechanical ventilation
Introduction
The spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has now been threatening human health for months. Intensive care unit (ICU) capacities are challenged to face this outbreak (Phua et al., 2020). Data is particularly needed on treatments able to reduce mortality and the number of critically ill patients (Weiss and Murdoch, 2020). Death mainly results from acute respiratory distress syndrome (ARDS) (Xu et al., 2020). Markers of inflammation such as C-reactive-protein (CRP), ferritin, and interleukin-6 are significantly associated with mortality (Henry et al., 2020, Ruan et al., 2020). Coronavirus disease 2019 (COVID-19)-related multiple-organ failure and ARDS are mainly caused by cytokine storm (Ye et al., 2020). Post-viral hyper-inflammation, which begins in the second week of the disease, seems to explain disease severity (Siddiqi and Mehra, 2020). Tocilizumab (TCZ) is a recombinant humanized anti-interleukin-6 receptor (IL-6R) monoclonal antibody used in the treatment of rheumatoid arthritis and systemic lupus erythematosus. Several arguments show that TCZ administered to patients with severe COVID-19 could be an effective treatment to reduce mortality. By neutralizing a key inflammatory factor in the cytokine release syndrome (CRS), this molecule may block the cytokine storm during the systemic hyperinflammation stage and reduce disease severity (Fu et al., 2020, Zhang et al., 2020a). Studies comparing the outcomes of patients treated with and without TCZ are scarce, and include small numbers of patients (Capra et al., 2020). We have recently published a retrospective study including 45 patients treated in our hospital, which shows that TCZ seems to reduce the number of COVID-19 severe cases and/or mortality (Klopfenstein et al., 2020). In this work, we aim to extend this study to our entire patient population with confirmed COVID-19 to compare the outcome, especially in terms of need for invasive mechanical ventilation (IMV) and/or mortality, between patients treated with TCZ and without TCZ.
Method
We have conducted a retrospective case-control study in NFC (Nord Franche-Comté) Hospital. On March 1st, a first case of COVID-19 was confirmed in our hospital. “Standard treatment” was administered to patients requiring oxygen therapy: hydroxychloroquine or lopinavir-ritonavir therapy or corticosteroids and antibiotics. On April 1st, in relation with the increasing medical literature data, the NFC hospital scientific medical committee including infectious diseases specialists, ICU specialists, rheumatologists, biologists, and pharmacists, approved the off-label use of TCZ in patients with general status deterioration despite well-conducted standard care. Daily “tocilizumab multidisciplinary team meetings” were organized to discuss patients’ eligibility to receive TCZ. Based on the medical literature, we checked several criteria before starting TCZ treatment: no contraindication to TCZ, confirmed COVID-19 with real-time reverse transcription (RT)-PCR SARS-CoV-2 RNA, period since symptoms onset ≥5 days, oxygen therapy ≥4 L/min, ≥25% of lung damages on chest computed tomography (CT) scan, and ≥2 parameters of inflammation or biological markers of mortality (with a high level) such as ferritin, CRP, d-dimer, lymphopenia, and/or lactate dehydrogenase.
The present work compares two groups of patients
The “tocilizumab group” (TCZ group) included all patients (except patients already in intensive care unit with IMV) who received standard treatment and TCZ (8 mg/kg per dose, 1 or 2 doses). Between April 1st and May 11th, 2020, we enrolled all adult patients who received TCZ for confirmed COVID-19 by RT-PCR SARS-CoV-2 RNA. All patients receiving TCZ were informed that this prescription was used outside of its marketing authorization indications; they were also informed that they could deny the administration of TCZ. In practice, several patients received TCZ when they were in critical condition as a rescue treatment; in order to judge the effectiveness of TCZ administration we excluded patients who had received the first dose of TCZ less than 24 h before intubation and/or death.
The standard treatment group (ST group) included patients receiving standard treatment but without TCZ. On average, patients received TCZ seven days after admission in our hospital (Klopfenstein et al., 2020), so we stopped the inclusion in the ST group one week before TCZ availability. We excluded patients who had not received the standard treatment. This group included all hospitalized adult patients with confirmed COVID-19 RT-PCR SARS-CoV-2 RNA between March 1st and March 24th, 2020. Because patients from the TCZ group were all critically ill patients, and for comparative purposes between the two groups, we excluded from the control group the patients with moderate disease (i.e. those hospitalized for less than 48 h and/or patients without any COVID-19 symptoms) and patients who were less than 50 years old (as none of the patients in the TCZ group was younger than 50 years old).
In all patients, diagnosis of COVID-19 was confirmed by RT-PCR on respiratory samples. Briefly, viral RNA was extracted using the NucleoSpin®RNA Virus kit (Macherey-Nagel) according to the manufacturer’s instructions, and amplified by RT-PCR protocols developed by the Charité (E gene) (Corman et al., 2020) and the Institut Pasteur (RdRp gene) (Bernard Stoecklin et al., 2020) on LightCycler 480® (Roche).
We collected the following data from the medical files of patients in both groups: demographic characteristics, comorbidities and outcome. To increase statistical power, we chose a combined primary endpoint (mortality and/or IMV requirement) to compare the two groups. Continuous variables were expressed as mean and standard deviation (SD) and compared with ANOVA test. Categorical variables were expressed as number (%) and compared by χ2 test or Fisher’s exact test between the two groups. A P-value < 0.05 was considered significant. We used the SPSS v24.0 software (IBM, Armonk, NY, USA).
Results
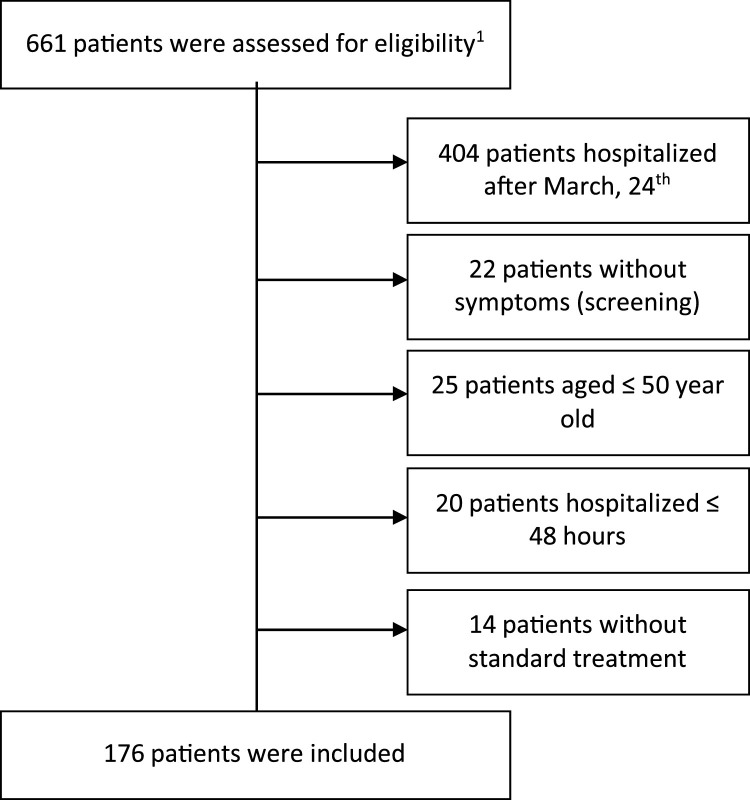
We have included 30 patients in the TCZ group. Thirty-three patients with confirmed COVID-19 were treated with TCZ before intubation between March 1st and May 11th, 2020. Three patients intubated less than 24 h after first TCZ administration were excluded. We included 176 patients out of 661 patients who were assessed for eligibility in the ST group (Figure 1 ).
Figure 1.
Inclusion criteria in Standard Treatment group.
1All hospitalized adult patients with confirmed COVID-19 RT-PCR SARS-CoV-2 RNA without Tocilizumab administration.
Concerning the TCZ group, oxygen therapy flow at TCZ onset was 10.5 L/min [1.5–15], and the time of first symptoms and of admission to TCZ onset was respectively 11.7 days [5–21] and 6.5 days [1–21]. Patients had high serum levels of C reactive protein (CRP) (mean 142 mg/l) and ferritin (mean 1496 ng/mL) at TCZ onset. Forty-seven percent of patients (14/30) had ≥ 50% lung involvement on CT scan. Concerning TCZ administration, 27 patients had 2 doses of TCZ (second dose given 24–72 hours after the first dose) and only 3 patients received a single dose.
No statistical differences were observed between the two groups (TCZ and ST) with regard to age, sex, and comorbidities (Table 1 ).
Table 1.
Comparison of demographic and clinical findings and outcomes of both groups.
|
Characteristics |
TCZ group (n = 30) | ST group (n = 176) | p-value | ||
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (y) (mean, extremes, SD) | 75.6 [52−93]± 11.3 | 74.3 [51−97]± 11 | 0.559 | ||
| [51−60] | 4 (13.3%) | 23(13.1%) | 0.580 | ||
| [61−70] | 7 (23.3%) | 47 (26.7%) | 0.698 | ||
| [71−80] | 6 (20%) | 49 (27.8%) | 0.370 | ||
| >80 | 13 (43.3%) | 57 (32.4%) | 0.242 | ||
| Male (Number, %) | 21 (70%) | 104 (59.1%) | 0.258 | ||
| Comorbidities (Number, %) | No comorbidities | 3 (10%) | 36 (20.5%) | 0.177 | |
| Number of comorbidities | 1.9 [0−5]±1.3 | 1.7 [0−6]±1.5 | 0.525 | ||
| Obesitya | 4 (13.3%) | 21 (11.9%) | 0.511 | ||
| Hypertension | 18 (60%) | 90 (51.1%) | 0.369 | ||
| Cardio-vascular diseasesb | 12 (40%) | 84(47.7%) | 0.433 | ||
| Diabetes mellitus | 5 (16.7%) | 50 (28.4%) | 0.179 | ||
| COPDc | 3 (10%) | 19 (10.8%) | 0.598 | ||
| Immunosuppressiond | 3 (10%) | 5 (2.8%) | 0.094 | ||
| Malignancy | 4 (13.3%) | 12 (6.8%) | 0.188 | ||
| Malnutritiona | 5 (17%) | 25 (14.2%) | 0.779 | ||
| Characteristics at admission | |||||
| Clinical characteristics at admission | |||||
| Mean arterial pressure < 65 mmHg | 0 | 5 (2.8%) | 1 | ||
| Respiratory rate > 30 (Number, %) | 4 (13.3%) | 10 (5.7%) | 0.128 | ||
| PaO2/FiO2 (mmHg -mean, SD-) | 267 ± 73 | 277 ± 133 | 0.725 | ||
| Biological characteristics at admission | |||||
| Hemoglobin (g/dl) | 12.95 [10.3−16.9] | 12.98 [6.9−19.3] | 0.934 | ||
| Lymphocytes (Giga/L) | 0.74 [0.21−1.73] | 0.87 [0.15−5.81] | 0.21 | ||
| Platelets (Giga/L) | 208 [56−366] | 213 [49−658] | 0.807 | ||
| C-reactive protein (mg/L) | 125 [11−282] | 118 [1−539] | 0.649 | ||
| Ferritin (ng/mL) | 1496 [156−4024] | 952 [23−5549] | 0.009 | ||
| d-dimer (ng/mL) | 8 344 [286-66,670] | 2 532 [214-18,810] | 0.033 | ||
| Fibrinogen (g/l) | 5.6 [2.3−8.3] | 5.6 [2−8.8] | 0.854 | ||
| IL-6 (pg/mL) | 549 [3−4156] | 179 [66−399] | 0.531 | ||
| Lactate dehydrogenase (U/L) | 482 [236−770] | 409 [160−854] | 0.024 | ||
| AST (U/L) | 63.6 [23−196] | 57.9 [9−323] | 0.508 | ||
| ALT (U/L) | 40.7 [13−117] | 44.3 [9−454] | 0.684 | ||
| Creatinine (μmol/l) | 101 [32−354] | 97 [32−447] | 0.727 | ||
| Characteristics during hospitalization | |||||
| Highest level of oxygen therapy (L/min) | 11.8 | 8.1 | <0.001 | ||
| Hydroxychloroquine | 25 (83%) | 86 (49%) | 0.001 | ||
| Lopinavir/ritonavir | 0 | 37 (21%) | 0.003 | ||
| Corticosteroids | 16 (53%) | 39 (22%) | <0.001 | ||
| Antibiotics | 158 (90%) | 30 (100%) | 0.081 | ||
| Outcome | |||||
| Death and/or IMV | 8 (26.7%) | 92 (52.3%) | 0.009 | ||
| Death | 8 (26.7%) | 66 (37.5 %) | 0.253 | ||
| Invasive mechanical ventilation (IMV) | 0 | 39 (22.2%) | 0.004 | ||
| Patients still hospitalizede | 3 (10%) | 6 (3.4%) | 0.127 | ||
| Transferred to Physical Medicine and Rehabilitation Service | 3 (10%) | 23 (13.1%) | 0.454 | ||
| Discharge | 16 (53.3%) | 82 (46.6%) | 0.556 | ||
| Duration of hospitalization (days) | 17 [4−42]± 10.1 | 15.2 [3−55]± 12 | 0.464 | ||
Abbreviations: TCZTocilizumab; STStandard Treatment.
Obesity defined by Body Mass Index (BMI) >30 kg/m (Weiss and Murdoch, 2020) and malnutrition by BMI <18kg/m (Weiss and Murdoch, 2020).
Cardio-vascular diseases defined by cardiac failure, cardiac arrhythmia, coronary heart disease, stroke, peripheral arterial obstructive disease and thromboembolic disease.
COPD: chronic obstructive pulmonary disease.
Immunosuppression defined by transplantation, cirrhosis, agranulocytosis, bone marrow aplasia and hypogammaglobulinemia.
For both groups we collected outcome data until May 18th 2020.
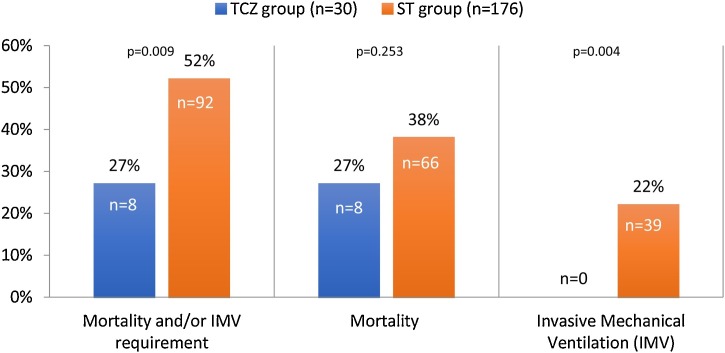
Our combined primary endpoint (mortality and/or IMV requirement) was higher in the ST group than in the TCZ group (52% vs 27%, p = 0.009) (Figure 2 ). Patients in the ST group clearly required IMV more often than patients in the TCZ group (22% vs 0, p = 0.004); however, no statistical difference was observed between the two groups in terms of mortality. The 8 patients who died in the TCZ group were not admitted in USC in regard to their comorbidities.
Figure 2.
Outcome in the tocilizumab (TCZ) group and in the standard treatment (ST) group.
It is interesting to note that none of the three patients intubated less than 24 h after TCZ first administration died (two were discharged and one was still hospitalized). Furthermore, if we included these 3 patients, mortality and/or IMV requirement was still higher in the ST group than in the TCZ group (92/176 [52%] vs 11/33 [33%], p = 0.046).
Discussion
Despite the small sample size of the TCZ group, the latter strongly suggests that TCZ may reduce the number of mortalities and/or IMVs in patients with severe SARS-CoV-2 pneumonia.
Our population is older, with more comorbidities, and a higher level of mortality than other studies with COVID-19 patients (Ruan et al., 2020, Zhou et al., 2020). These results are probably explained by the exclusion of patients without hospitalization criteria and less than 50 years old (in order to have a comparable population with the TCZ group).
Patients in the TCZ group seemed to be more severe than patients in the ST group. In the TCZ group, patients had a higher respiratory rate and a lower PaO2/FiO2 ratio at admission than the ST group but without statistical differences. Biological findings at admission were worse in the TCZ group than the ST group especially for ferritin, d-dimer and Lactate dehydrogenase which are known on a high level as predictive of poor outcome. Furthermore, during hospitalization patients with TCZ required a higher level of oxygen therapy (L/min) than patients with ST (11.8 vs 8.1, p < 0.001).
The two groups differ regarding standard treatment. Lopinavir/ritonavir was only administered in the “Standard Treatment” group. On the contrary, hydroxychloroquine and corticosteroids were more often administered in the TCZ group. This is explained by the local management during the crisis; in our hospital lopinavir/ritonavir was recommended in the beginning of the outbreak and after a few weeks was replaced by hydroxychloroquine. Then, in the end of the first period of inclusion, corticosteroids were an alternative treatment.
TCZ was administered on average 12 days after COVID-19 symptoms onset. It was prescribed on average 6.5 days after admission, after standard treatment failure in most cases, in patients presenting comorbidities in 83% of cases and who were critically ill (mean oxygen flow of 10.5 L/min). However, compared with the ST group, the occurrence of mortality and/or IMV requirement was clearly lower (27% vs 52%, p = 0.009).
None of our 30 TCZ-treated patients needed IMV. Finding enough ICU beds is highly challenging during the present COVID-19 pandemic (Phua et al., 2020); TCZ could be the key in the treatment of COVID-19 cases to reduce ICU admissions. It could also have a huge public health impact as well as an impact on reducing the human and economic cost of the outbreak.
Case reports and series have reported that repeated doses of TCZ may improve the condition of critical patients (Fu et al., 2020, Anon, 2020, Michot et al., 2020, Zhang et al., 2020b, Mihai et al., 2020, Somers et al., 2020, Rossotti et al., 2020). Capra et al. reported a case-control study which shows that TCZ decreases mortality rate in patients with COVID-19 related pneumonia (Capra et al., 2020). In their study, two out of 62 patients of the TCZ group and 11 out of 23 in the control group died; patients receiving TCZ showed a significantly greater survival rate as compared to control patients with a hazard ratio for death at 0.035 (95% confidence interval, 0.004 to 0.347; p = 0.004), adjusting for baseline clinical characteristics. In a recent observational, controlled study of 154 patients with severe COVID-19 illness requiring mechanical ventilation, tocilizumab was associated with a 45% reduction in the hazard of death (Somers et al., 2020). The same kind of results were found by Rossotti et al. in a comparative analysis between seventy-four patients treated with TCZ and 148 patient controls (Rossotti et al., 2020). The low number of patients included in our work in the TCZ group may explain that the difference in mortality is not significant because of a lack of statistical power. However, the main reason is probably that we administered TCZ in many cases as a rescue treatment in critical patients who were too old and comorbid to be transferred to the ICU. Therefore, the 8 patients who received TCZ and died were not admitted to the ICU due to their comorbidities. Furthermore, we have already published that our patients who received TCZ were older, presented more comorbidities and were more critically ill than a control group selected with the same methodology but in a shorter period of time (Klopfenstein et al., 2020).
The post-viral hyperinflammation onset on the second week of the disease seems to explain COVID-19 disease severity. By neutralizing a key inflammatory factor in the cytokine release syndrome, TCZ may block the cytokine storm during the systemic hyperinflammation stage and reduce disease severity. We do not have data about the right timing for TCZ administration; in our study TCZ was administered on average 12 days after onset of symptoms. However, TCZ should probably be administrated earlier in the second week of the disease.
Our study is retrospective with a low number of patients in the TCZ group. Larger prospective randomized trials are required to confirm these findings.
Conclusion
Our results suggest that tocilizumab reduces mortality and/or invasive mechanical ventilation requirements in patients with severe SARS-CoV-2 pneumonia. This idea needs to be confirmed and spread in the medical community.
Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Conflict of interest
The authors declare that they have no conflict of interests.
Funding source
The authors declare that they have no funding sources.
Ethical approval
Due to the retrospective nature of the study, the Ethics & Scientific Committee of Nord Franche-Comté Hospital determined that patients consent was required only for the off-label use Tocilizumab.
Informed consent
Due to the retrospective nature of the study, the Ethics Committee determined that patient consent was not required. We make sure to keep patient data confidential and in compliance with the Declaration of Helsinki.
Acknowledgements
The authors thank especially Emmanuel Siess, Azzedine Rahmani, Charlotte Bourgoin, Elodie Bouvier, Julien Lorenne and Frederic Deuze for their strong implication in the present work.
They also thank the management team of the Hospital Nord Franche-Comté for having made available Tocilizumab outside its approved indication and each member of the HNF Hospital Tocilizumab multidisciplinary team.
Special acknowledgements to all the physicians, caregivers (nurses and orderlies) and patients.
References
- Phua J., Weng L., Ling L., Egi M., Lim C.-M., Divatia J.V., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;6 doi: 10.1016/S2213-2600(20)30161-2. avr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet Lond Engl. 2020;28(395(10229)):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med [Internet] 2020;18 doi: 10.1016/S2213-2600(20)30076-X. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7164771/ févr [cité 26 avr 2020]; Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;10 doi: 10.1515/cclm-2020-0369. avr. [DOI] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;6 doi: 10.1007/s00134-020-05991-x. avr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. avr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J Heart Lung Transplant [Internet] 2020 doi: 10.1016/j.healun.2020.03.012. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7118652/ 20 mars [cité 29 avr 2020]; Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med [Internet] 2020 doi: 10.1186/s12967-020-02339-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7154566/ 14 avr [cité 26 avr 2020];18. Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortaliJ.-W.y. Int J Antimicrob Agents. 2020;29 doi: 10.1016/j.ijantimicag.2020.105954. mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P., et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med [Internet] 2020 doi: 10.1016/j.ejim.2020.05.009. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7219361/ 13 mai cité 25 mai 2020]; Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Zayet S., Lohse A., Balblanc J.-C., Badie J., Royer P.-Y., et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect [Internet] 2020 doi: 10.1016/j.medmal.2020.05.001. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7202806/ 6 mai [cité 25 mai 2020]; Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard Stoecklin S., Rolland P., Silue Y., Mailles A., Campese C., Simondon A., et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(6) doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 28 mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon . 2020. Tocilizumab treatment in COVID-19: A single center experience. - PubMed - NCBI [Internet]https://www.ncbi.nlm.nih.gov/pubmed/?term=Luo+P%2C+Liu+Y%2C+Qiu+L%2C+Liu+X%2C+Liu+D%2C+Li+J.+Tocilizumab+treatment+in+COVID-19%3A+A+single+center+experience [cité 29 avr 2020]. Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michot J.-M., Albiges L., Chaput N., Saada V., Pommeret F., Griscelli F., et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol Off J Eur Soc Med Oncol. 2020;2 doi: 10.1016/j.annonc.2020.03.300. avr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307–1310. doi: 10.1182/bloodadvances.2020001907. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihai C., Dobrota R., Schröder M., Garaiman A., Jordan S., Becker Mo, et al. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. 2020;79(5):668–669. doi: 10.1136/annrheumdis-2020-217442. [DOI] [PubMed] [Google Scholar]
- Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;11 doi: 10.1093/cid/ciaa954. juill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossotti R., Travi G., Ughi N., Corradin M., Baiguera C., Fumagalli R., et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis. J Infect. 2020;8 doi: 10.1016/j.jinf.2020.07.008. juill. [DOI] [PMC free article] [PubMed] [Google Scholar]