Abstract
The vaccine BCG has been reported to offer protection against SARS-CoV-2 infection. It has been hypothesized this is based on nonspecific enhancement of innate immunity. This study addressed whether there is strong homology between a SARS-CoV-2 capsid protein and a Mycobacterium bovis protein that would allow for stronger, more specific immune protection. The study also showed the utility of immunohistochemistry in the diagnostic pathology laboratory for elucidating this information. Immunohistochemistry documented that an antibody directed against the SARS-CoV-2 envelope, but not the spike or membrane proteins, strongly cross hybridized to 11/11 Mycobacterial species tested, including M. bovis. BlastP analysis showed high homology of the SARS-CoV-2 envelope protein with 12 consecutive amino acids of the protein LytR C, which is a consensus protein unique to Mycobacteria. Six additional cases of human tuberculosis with few organisms showed that the viral envelope specific antibody (5/6) was more accurate than the AFB stain (2/6) for diagnostic purposes. These data indicate BCG vaccination induces a specific immunity against SARS CoV-2 that targets the viral envelope protein that is essential for infectivity. Thus, a concurrent booster or first use of the BCG vaccine may reduce the severity of the current COVID-19 pandemic. The data also suggests the value of using the SARS-CoV-2 envelope antibody in the diagnosis of Mycobacterial infections in formalin fixed, paraffin embedded tissues by the diagnostic pathologist.
Keywords: BCG, COVID-19, Envelope protein, AFB, SARS-CoV-2
Highlights
-
•
The envelope protein of SARS-CoV2 is strongly homologous with a consensus Mycobacterial protein.
-
•
Immunohistochemistry with an antibody against the viral envelope, thus, can detect Mycobacterial infections.
-
•
This test is superior to the AFB stain for Mycobacterial detection by the anatomic pathologist.
-
•
The BCG vaccine offers a strong, viral specific immunity against COVID-19 due to this strong homology.
1. Introduction
SARS-CoV-2 is the causative agent of coronavirus disease 2019 (COVID-19) the worst worldwide pandemic since the H1N1 Spanish flu of 1918 [1]. The pathology of severe COVID-19 is one of a pauci-inflammatory complement mediated thrombotic microangiopathy affecting the lung's alveolar septal capillaries and other organs including the skin, heart, and liver [2].
Many countries mandate Bacillus Calmette-Guerin (BCG) vaccination, a live attenuated strain of Mycobacterium bovis, to newborns due to its effectiveness against tuberculosis and leprosy [1,3,4]. It is has been reported that countries with long-standing mandatory BCG vaccination have less cases of COVID-19 per capita and a lower death rate compared to countries such as Italy, Spain, and the United States where BCG vaccines are rarely given [3]. Hence, BCG vaccination is being touted as a treatment for reducing the complications of SARS-CoV-2 [1,[3], [4], [5]]. It has been assumed that the protective effect of BCG vaccination is due to an epigenetic enhanced “trained innate immunity” whereby macrophages and natural killer cells are primed from the exposure to Mycobacterium bovis to eliminate any micro-pathogens [[6], [7], [8], [9]].
Another possibility is that there may be heterologous immunity due to a protein in SARS CoV-2 that is homologous to a protein in Mycobacterium bovis. Such heterologous immunity has been documented between other pathogens, such as adenovirus and hepatitis C virus [10]. The presence of such immunity between a SARS-CoV-2 capsid protein and a protein of Mycobacterium bovis would allow for a much stronger and specific immunity against the virus subsequent to BCG vaccination. Immunohistochemistry is a powerful method well suited to detect such potential heterologous immunity since it allows for specific cellular localization of the relevant antibody-antigen complex in the context of the known distribution of the latter, in this case defined by the AFB stain [11]. Signal intensity between the primary antibody and heterologous antigen is related to the degree of homology and co-localization documents that the primary antibody and antigen are within an area of 150 nm [11,12].
The diagnosis of Mycobacterium species by the anatomic pathologist can be problematic because of the relatively few numbers of organisms in a given sample. There are two main tools available to the anatomic pathologic to diagnose Mycobacterial infection: the AFB stain and direct fluorescent microscopy using an antibody directed against Mycobacterial species. A study, using bacterial culture and PCR-based confirmation of Mycobacterial infection in 55 human samples, found that the AFB test gave false negative results in 64% of cases and fluorescent microscopy missed the diagnosis in 20% of cases [13]. Although Mycobacterial infection in patients with AIDS often have many organisms, it is still clear that better tests are needed by the diagnostic pathologist to diagnose Mycobacterial infections in either formalin fixed, paraffin embedded tissues or cytology specimens that can easily be fixed and processed for immunohistochemistry.
This study documented the strong cross reactivity between a SARS-CoV-2 protein and a consensus protein of Mycobacteria. The data offers a more sensitive test to diagnose Mycobacterial infections for the diagnostic pathologist and, clearly, indicates the BCG vaccine can offer immediate, specific immunity that could potentially much reduce the increasing death rate in the current COVID-19 pandemic.
2. Methods
2.1. Tissue samples
Formalin fixed, paraffin embedded tissues from cases confirmed to contain Mycobacterial infections were obtained from various sources. Eleven such cases were identified and were positive for: Mycobacterium bovis (n = 2), Mycobacterium leprae (n = 2), Mycobacterium avium-intracellulare (n = 3), and Mycobacterium tuberculosis (n = 4). Also studied were six cases of PCR-documented Mycobacterium infection chosen as they were reported to contain very few microorganisms. All samples were obtained prior to 2018, and, thus, could not have contained SARS-CoV-2.
2.2. Immunohistochemistry
Our immunohistochemistry method for the detection of SARS-CoV-2 proteins has been published [2]. The automated Leica Bond Max platform was used with DAB as the chromogen. The optimal conditions included antigen retrieval for 30 min with the EDTA solution from Leica, dilutions of 1:4000 (spike Ab), 1:500 (membrane Ab), 1:250 (envelope Ab, each from ProSci, Poway, CA) and the use of the horseradish peroxidase conjugate from Enzo Life Sciences in place of the equivalent product from Leica as this reduced background [11,12]. In selected cases the chromogen Fast Red (with the alkaline phosphatase reporter enzyme) was used in place of DAB by using the Leica Fast Red kit. Positive and negative controls were lung tissues from people who had died of COVID-19 and normal lung tissue obtained prior to 2018.
2.3. Other testing
The AFB stain was done per a standard protocol [12]. All AFB testing was done using serial sections to the ones tested for homology with the different SARS-CoV-2 antibodies. The BlastP analyses were done using the EMBL-EBI search and sequence analysis tools [14].
3. Results
3.1. Immunohistochemistry with SARS-CoV-2 capsid antibodies and Mycobacterial positive samples
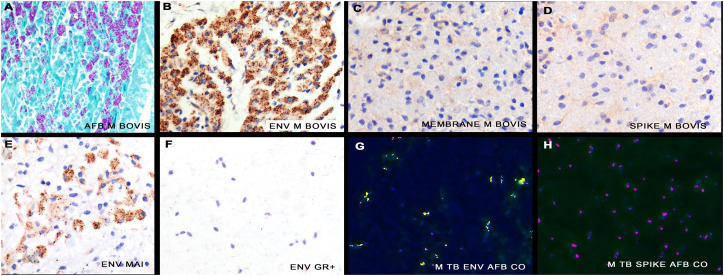
First, the question as to whether any of the SARS CoV-2 capsid antibodies targeting the spike, envelope, and membrane proteins respectively would be able using immunohistochemistry to detect Mycobacteria in the formalin fixed, paraffin embedded tissues from the eleven cases that were each strongly AFB positive was addressed. The testing was done blinded with regards to the specific viral capsid protein on eleven pre-COVID-19 archival tissue samples of Mycobacterial infection (four of Mycobacterium tuberculosis, three Mycobacterium avium-intracellulare, and two each of Mycobacterium bovis plus Mycobacterium leprae). Acid-fast bacillus (AFB) stains indeed highlighted Mycobacterial organisms in each tissue. As seen in Fig. 1 , antibodies targeting the SARS-CoV-2 spike or membrane proteins yielded no signals in the Mycobacterial samples. However, 11/11 (100%) of the Mycobacterium cases showed strong immunoreactivity with SARS CoV-2 specific envelope antibody with a similar distribution and staining intensity as the AFB stain, indicative of strong homology. No signal was evident between the SARS-CoV-2 envelope antibody and bacteria including Escherichia coli, Bacillus cereus, Streptococcus faecalis, and Lactobacillus species (Fig. 1). Using a published protocol [2,12], co-localization experiments documented that the SARS-CoV-2 envelope protein and AFB signals co-expressed (Fig. 1).
Fig. 1.
Cross hybridization between the covid-19 envelope protein and a consensus protein of Mycobacteria. An AFB stain shows many intracellular Mycobacterium bovis bacteria (panel A). A strong signal was seen with the antibody against SARS-CoV-2 envelope protein indicative of strong homology (panel B) whereas no signal was seen with antibodies specific for the membrane (panel C) or spike proteins (panel D). An equivalent signal was evident after immunohistochemistry with the SARS-CoV-2 envelope Ab and Mycobacterium avium-intracellulare (panel E) whereas no signal was seen with various gram positive and negative bacteria (panel F). The SARS-CoV-2 specific antibody based signal (green) strongly co-localized with the AFB stain (red) (panel G, yellow defines co-expression) in a case of Mycobacterium tuberculosis whereas AFB and the SARS-CoV-2 spike antibody showed no co-expression (panel H). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
BlastP analyses documented that SARS-CoV-2 envelope protein from the region used to generate the antibody shares high homology with a stretch of 12 amino acids present in Mycobacterium bovis, called tuberculin-like proteins, also known as the LytRC-terminal domain-containing protein (Fig. 2 ). Mycobacterial LytRC proteins are highly conserved in different Mycobacterium species (Fig. 2). This LytRC domain is rarely found in gram positive or negative bacteria [15] and does not show homology with other non-Mycobacterial species (data not shown). The AFB stain, specific for the mycolic acid-rich Mycobacterial cell wall, gave no signal with SARS-CoV-2 cases (data not shown).
Fig. 2.
BLASTP analysis indicating region of homology between SARS-CoV-2 envelope protein and Mycobacterium sp.
A stretch of 12 amino acids (17–29 of YP_009724392 sequence) of SARS-CoV-2 envelope protein (dark red line) has high homology to LytR C-terminal domain-containing proteins of Mycobacterium taxa and variants as indicated. Also note the strong homology among the different species of Mycobacteria. An identical amino acid residue between the SARS-CoV-2 envelope and Mycobacteria is shown with an *, a : marks a residue with strongly similar properties, and a . denotes weakly similar properties. Sequence identities as follows: envelope protein (Sars-CoV-2) - YP_009724392; 1-WP_003873789; 2-WP_156147206; 3-YP_009357768 variant bovis AF2122/97; 4-CAL70455 M.tub. variant bovis BCG strain Pasteur 1173P2; 5-AHM06124, M.tub. variant bovis BCG strain ATCC 35743; 6-WP_003898448 M.tub. H37Rv tuberculin-like peptide; 7-WP_031685486; 8-AYP10944. Alignment was done using CLUSTAL O (1.2.4) multiple sequence alignment at EMBL-EBI [12]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Direct comparison of the AFB test and immunohistochemistry using the SARS-CoV-2 envelope specific antibody for detection of Mycobacteria in formalin fixed, paraffin embedded tissues
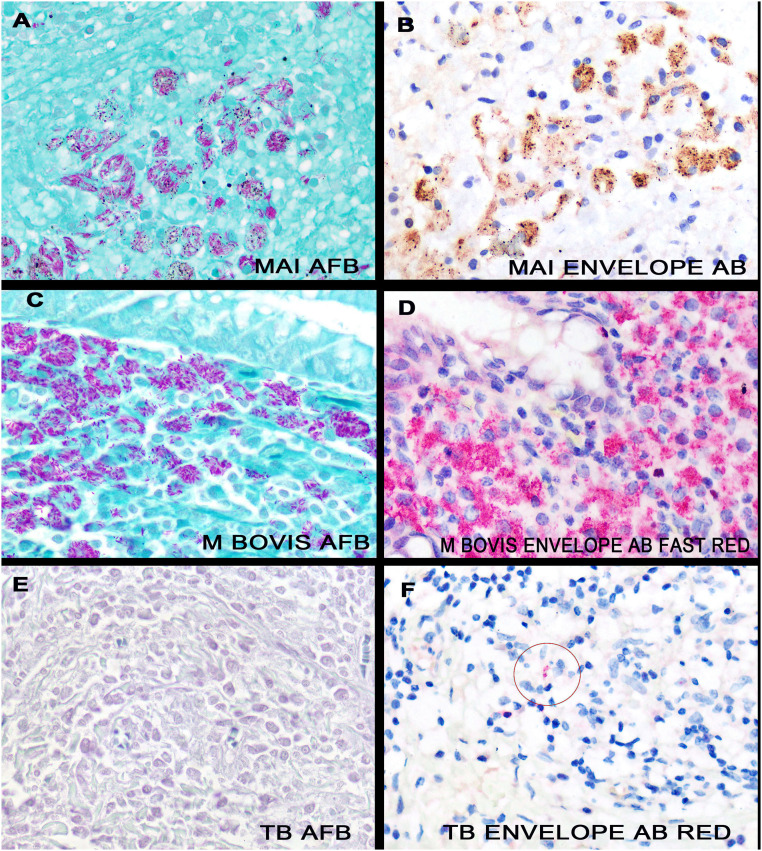
The initial eleven Mycobacteria positive cases had many Mycobacterium in a given case. Fig. 3 shows serial section analyses of one of the Mycobacterium avium-intracellulare cases tested after AFB staining (panel A) and immunohistochemistry for the viral envelope protein (panel B). Note the typical slender pink/red rods with the AFB stain. In comparison, using DAB as the chromogen, the SARS-CoV-2 envelope protein produces an intense punctate brown signal (panel B), suggesting that the homologous protein is compartmentalized. Panels C and D are also serial sections photographed at the same area in which the chromogen Fast Red is used for the immunohistochemistry; hence each signal is red. Note that the signal intensity is stronger with the viral envelope-specific antibody compared to the AFB test.
Fig. 3.
Direct comparison of the AFB test and immunohistochemistry directed against the envelope protein of SARS-CoV-2 for the detection of Mycobacterial infections. Panels A/B are serial sections of a case of Mycobacterium avium-intracellulare tested by AFB (panel A) and by immunohistochemistry against the SARS-CoV-2 envelope protein (panel B, DAB). Note the similar intracellular distribution. Similarly, panels C/D are serial sections using these two different assays, but where the chromogen for the immunohistochemistry is Fast Red. Note the stronger signal intensity for the immunohistochemistry test (panel D). Panels E/F are serial sections of a case of documented tuberculosis where bacteria were not evident with the AFB test (panel E) but were noted with the immunohistochemistry test against the SARS-CoV-2 envelope protein (panel F, circle), using the Fast Red chromogen. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To do a more stringent test of the sensitivity of each test, six formalin fixed, paraffin embedded tissues from different cases of confirmed Mycobacterium tuberculosis infection, but where the AFB test was “negative” or “equivocal” were re-tested with each assay. The AFB test was positive in 2/6 cases. In comparison, the immunohistochemistry test using the viral envelope specific antibody was positive in 5/6 cases; the one negative case was mostly necrotic. Representative images are shown in Fig. 3.
4. Discussion
The main findings of this study to the diagnostic pathologist are threefold: 1) immunohistochemistry is a highly effective tool to demonstrate strong homology between epitopes on two disparate proteins, in this case the SARS-CoV-2 envelope protein and the protein LytR C, which is a consensus protein unique to Mycobacteria. The antibodies used routinely in the diagnostic laboratory commonly recognize an epitope from 5 to 6 amino acids in length [12]. In this case, there was indeed a 5 amino acid sequence that was identical in each of the Mycobacterium species tested protein LytR C and the region of the SARS-CoV-2 envelope protein used to generate the antibody. 2) It is well known that the AFB test has relatively poor sensitivity for the diagnostic pathologist, especially in cytology specimens or in formalin fixed, paraffin embedded tissues. It was shown that the immunohistochemistry using the antibody directed against the viral envelope was more sensitive for the diagnosis of Mycobacterial infections than the AFB test. Also, the AFB stain does not show the tissue morphology well and tends to show background in plasma cells; immunohistochemistry shows tissue morphology well and background with this test was minimal. 3) The pathology laboratory setting up a diagnostic test for SARS-CoV-2 can use immunohistochemistry and an antibody directed against the envelope protein with formalin fixed, paraffin embedded tissues from Mycobacterial infections as the positive control. This will greatly shorten the time to optimize the assay since COVID-19 autopsy material is often difficult to obtain and the viral protein distribution is very heterogeneous [2].
Despite these three important points for the diagnostic pathologist, clearly the most significant finding in this study is that the BCG vaccine, derived from Mycobacterium bovis, should indeed be effective against the current COVID-19 pandemic. SARS-CoV-2 has four major structural proteins: the spike, nucleocapsid, membrane and envelope proteins. The envelope protein is integral to the pathogenicity of the virus [[1], [2], [3]]. The data in this paper strongly suggests that BCG vaccination induces a specific immunity directed against the SARS-CoV-2 envelope protein that, in turn, is integral to the pathogenicity of the virus. Repeat BCG vaccination to those who have already received it, typically in childhood, can within several days reactivate the immune system memory directed against the Mycobacterial antigens that would include the heterologous immunity against the SARS-CoV-2 envelope protein [16]. Large numbers of new COVID-19 cases are still being reported daily. Thus, prospective trials examining whether there is an inverse correlation between a concurrent booster BCG vaccine in countries where this vaccine is mandatory at birth, as well as concurrent BCG vaccination to those who never have had the vaccine, and the incidence/severity of SARS-CoV-2 disease may well underscore the specific value of vaccination against Mycobacterium bovis to reducing the severity of the current pandemic.
In sum, BCG vaccination is being touted as a treatment for reducing the complications of SARS-CoV-2 [1,[3], [4], [5]]. The main clinical finding of this study is that BCG vaccination offers a specific heterologous immunity against infection by SARS-CoV-2 by inducing an adaptive immunity response against a protein essential to the virus's infectivity. This allows one to predict that BCG vaccination boosters should induce a strong anti-viral protection specifically against the disease COVID-19.
CRediT authorship contribution statement
GJN (hypothesis, testing, manuscript), EM (hypothesis and testing), LH (testing), DS (sample contribution, manuscript), ET (BlastP analyses, manuscript), CMM (testing, manuscript).
Declaration of competing interest
The authors have no competing interests to report.
Acknowledgements
The authors thank Dr. Margaret Nuovo for the microphotographs as well as Toni Schaffer, Jordan Fehr, Jason Bice, and Adel Mikhail for helpful comments.
References
- 1.O’Neill L.A.J., Netea M.G. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magro C.M., Mulvey J., Berlin D., Nuovo G.J., Salvatore S., Harp J. Complement activation is associated with the thrombotic microvascular injury of severe COVID-19 disease. Transl Res. 2020 doi: 10.1016/j.trsl.2020.04.007. (April 15). OI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller A. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042937. [DOI] [Google Scholar]
- 4.Redelman-Sidi G. Could BCG be used to protect against COVID-19? Nat Rev Urol. 2020 doi: 10.1038/s41585-020-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorlag S. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Kleinnijenhuis J. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netea M.G. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arts R.J.W. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Nemes E. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal B. Heterologous immunity between adenoviruses and hepatitis C virus (HCV): recombinant adenovirus vaccine vectors containing antigens from unrelated pathogens induce cross-reactive immunity against HCV antigens. Cells. 2019;8(5) doi: 10.3390/cells8050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuovo G.J. False positive results in diagnostic immunohistochemistry are related to the horseradish peroxidase conjugates in commercially available assays. Ann Diagn Pathol. 2016;25:54–59. doi: 10.1016/j.anndiagpath.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Nuovo G.J. 2nd ed. Elsevier; San Diego, CA: 2020. In situ molecular pathology and co-expression analyses: an integrated approach. [Google Scholar]
- 13.Riello F.N., Brígido R.T., Araújo S., Moreira T.A., Goulart L.R., Goulart I.M. Diagnosis of mycobacterial infections based on acid-fast bacilli test and bacterial growth time and implications on treatment and disease outcome. BMC Infect Dis. Apr 1 2016;16:142. doi: 10.1186/s12879-016-1474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madeira F. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Gebali S. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashyap R.S., Husain A.A., Morey S.H. Assessment of immune response to repeat stimulation with BCG vaccine using in vitro PBMC model. J Immune Based Ther Vaccines. 2010;8:3–10. doi: 10.1186/1476-8518-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]