The cerebellum was recently found in animal models to have a role in modulating complex reward processing1–5 that was thought to be predominantly controlled by the dopaminergic system in ventral tegmental area, ventral striatum, and prefrontal cortex. Interestingly, these brain areas of classical reward processing and reinforcement learning have dense connections with the cerebellum; thus, the cerebellum may modulate the activity in these brain regions. Within the cerebellum, each neuronal component can be responsible for modulating the reward processing: the granule neuronal firing patterns encode reward delivery and anticipation,5 whereas the climbing fiber firing patterns convey the information of reward prediction error.3 Moreover, Purkinje cell firing patterns are directly associated with reward processing via reinforcement learning.6
Whether the cerebellum contributes to reward processing in humans remains unknown. Based on the cerebellar reward-modulating hypothesis, we predict that patients with cerebellar dysfunction will have abnormal reward processing, leading to impulsivity and compulsivity, the classical symptoms related to abnormal reward processing. We thus conducted a single-center, cross-sectional study to include 50 individuals with cerebellar ataxia and 50 age-matched controls. We used the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale to measure the impulsivity and compulsivity.7
We found that cerebellar ataxia cases have 1.87-fold higher Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale scores than controls (19.36 ± 12.80 vs. 10.36 ± 8.21, P = 0.007; Fig. 1A, Supplemental Table 1), supporting the fact that individuals with cerebellar ataxia are more impulsive and compulsive. These impulsive and compulsive behaviors are domain specific: ataxia cases have 2.83-fold higher in gambling (P = 0.001), 2.51-fold in hobbyism punding (P < 0.001), and 9.15-fold in excessive medication use (P < 0.001) when compared with controls, but there is no difference in sex, buying, or eating (Fig. 1B, Supplemental Table 1). We found that impulsivity and compulsivity do not correlate with ataxia severity (Fig 1C, Supplemental Table 2), but correlate with the degree of depression (Fig. 1D, Supplemental Table 2), a common nonmotor symptom in ataxia patients.
Figure 1.
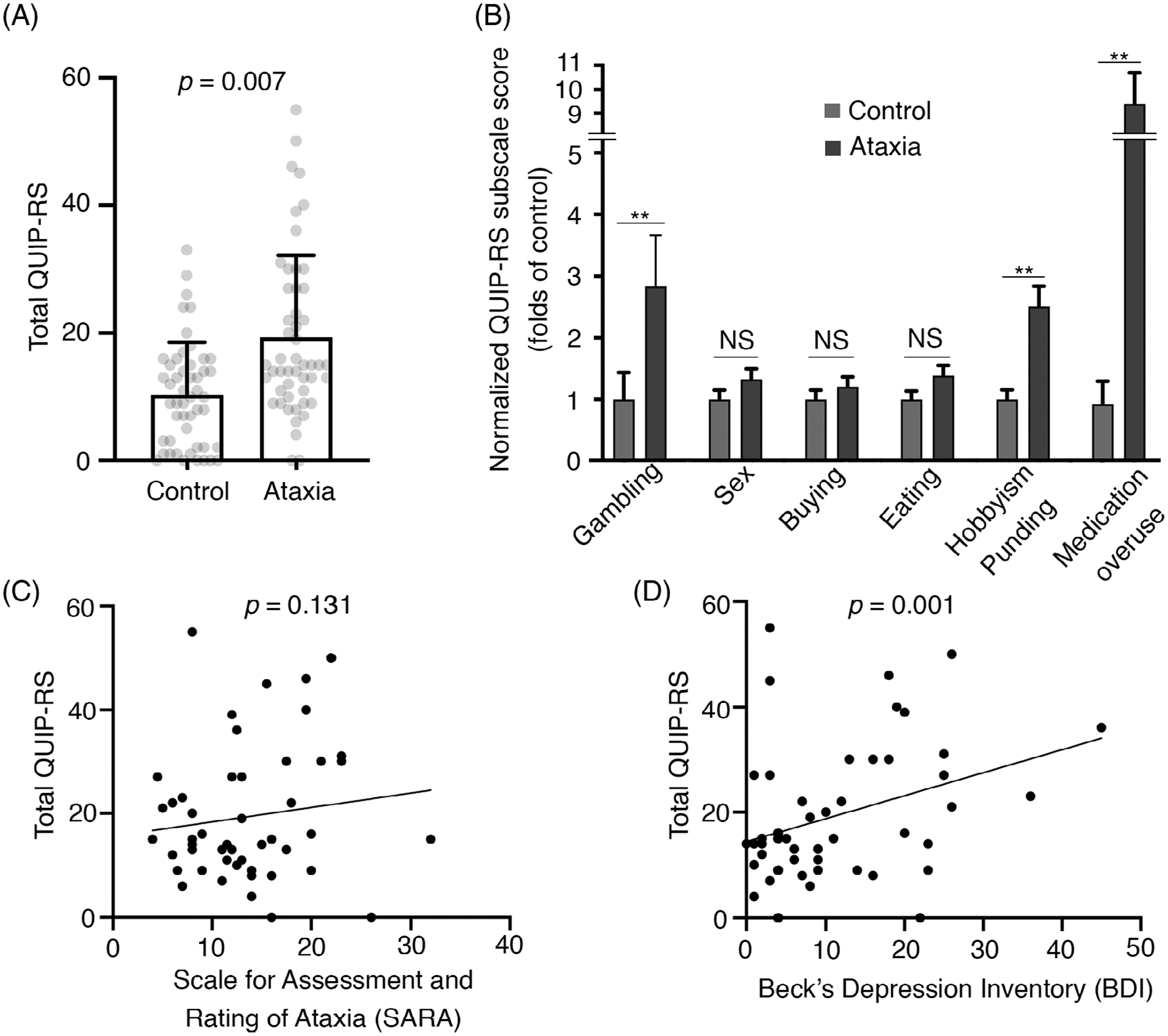
(A) Total scores of Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale (QUIP-RS) in controls and individuals with cerebellar ataxia (mean ± standard deviation). (B) Normalized QUIP-RS subscores between ataxia cases and controls (mean ± standard error)., Bonferroni correction was conducted for 6 groups: *P < 0.008; **P < 0.0016; NS, not significant. Note that the medication overuse subscale was measured by asking participants whether they have compulsive use of any medications rather than specifically of Parkinson’s disease medications as the original QUIP-RS. (C) The correlation between the total score of QUIP-RS and ataxia severity, measured by the Scale for the Assessment and Rating of Ataxia. (D) The correlation between the total score of QUIP-RS and the degree of depression, measured by Beck’s Depression Inventory.
To exclude the potential dopaminergic effect, we conducted a subgroup analysis by excluding 1 spinocerebellar ataxia patient taking levodopa and 9 multiple system atrophy patients, and the results remained similar (Supplemental Table 3), as were the results from spinocerebellar ataxia patients or patients with idiopathic late-onset cerebellar ataxia (Supplemental Table 4). These results demonstrate that impulsive and compulsive behaviors are common in ataxia patients regardless of age, gender, or the underlying cause for ataxia (Supplemental Tables 5, 6).
In the present study, we found that individuals with cerebellar ataxias are more impulsive and compulsive, and these behaviors are domain specific, suggesting that the cerebellum might modulate some, but not all, of the impulsive and compulsive behaviors. These behaviors correlate with depression, a common nonmotor symptom of cerebellar ataxia, suggesting that impulsivity and compulsivity could be a broader part of the cerebellar cognitive affective syndrome. Our data support the role of the cerebellum in the reward-processing circuits in humans. Future studies including additional neuropsychological tests and using the reward paradigm will be important to further understand the cognitive function of the cerebellum.
Supplementary Material
Funding agencies:
Dr. Kuo has received funding from the National Institutes of Health: National Institute of Neurological Disorders and Stroke (NINDS) R01 NS104423 (principal investigator), NINDS R03 NS114871 (principal investigator), Brain Research Foundation, National Ataxia Foundation, Parkinson’s Foundation, and International Essential Tremor Foundation. Dr. Pan has received funding from Ministry of Science and Technology in Taiwan, grants MOST 104-2314-B-002-076-MY3 (principal investigator), Ministry of Science and Technology, Taiwan (MOST) 107-2321-B-002-020 (principal investigator), MOST 108-2321-B-002-011 (principal investigator), and MOST-108-2321-B-002-059-MY2 (principal investigator), National Taiwan University Hospital, grants 105-N3227 and 108-039, and Yin-Lin branch of the hospital, grant NTUHYL104.N007.
Footnotes
Relevant conflicts of interests/financial disclosures: Nothing to report.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Carta I, Chen CH, Schott AL, et al. Cerebellar modulation of the reward circuitry and social behavior. Science 2019;363(6424):eaav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heffley W, Song EY, Xu Z, et al. Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci 2018;21(10):1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostadinov D, Beau M, Blanco-Pozo M, et al. Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci 2019;22(6):950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sendhilnathan N, Ipata AE, Goldberg ME. Neural correlates of reinforcement learning in mid-lateral cerebellum. Neuron 2020;106(1): 188–198.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner MJ, Kim TH, Savall J, et al. Cerebellar granule cells encode the expectation of reward. Nature 2017;544(7648):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohmae S, Medina JF. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci 2015;18(12):1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weintraub D, Mamikonyan E, Papay K, et al. Questionnaire for impulsive-compulsive disorders in Parkinson’s Disease-Rating Scale. Mov Disord 2012;27(2):242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


