Abstract
Purpose
To provide a detailed description of practical approaches to dose escalation in pancreatic cancer.
Methods and Materials
The current paper represents an international collaborative effort of radiation oncologists from the MR-linac consortium with expertise in pancreatic dose escalation.
Results
A 15-fraction hypofractionated intensity modulated radiation therapy (67.5 Gy in 15 fractions) and 5-fraction stereotactic body radiation therapy case (50 Gy in 5 fractions) are presented with information regarding patient selection, target volumes, organs at risk, dose constraints, and specific considerations regarding quality assurance. Additionally, we address barriers to dose escalation and briefly discuss future directions in dose escalation for pancreatic cancer, including particle therapy and magnetic resonance guided radiation therapy.
Conclusions
This article on dose escalation for pancreatic cancer may help to guide academic and community based physicians and to serve as a reference for future therapeutic trials.
Background
Locally advanced pancreatic cancer (LAPC) primarily causes death by 2 main mechanisms: early metastatic spread and uncontrolled local growth.1 Although the systemic control of the disease remains a major challenge, advances in systemic therapy have improved overall survival.2 Historically, inferior chemotherapy when coupled with standard doses of radiation (RT) failed to improve survival; however, it is accepted that standard doses of RT for pancreatic cancer are insufficient for tumor control.3,4 Thus, new studies with increasingly effective systemic agents (eg, gemcitabine and nab-paclitaxel and folinic acid, 5-fluorouracil, irinotecan, oxaliplatin [FOLFIRINOX]) coupled with escalated-dose radiation are needed. Indeed, as systemic therapy leads to better distant disease control, local tumor control would be expected to become of greater importance, similar to the evolution of breast cancer therapy.5 Further, although patients with unresectable disease may not be cured, they may experience a better quality of life and prolongation of survival through better local tumor control, as local progression can cause significant morbidity and eventual death. Improvements in radiation technique (eg, intensity modulated radiation therapy [IMRT], 3-dimensional image guidance, immobilization, motion management) have resulted in decreased toxicity and improved tolerability of standard dose radiation with concurrent chemotherapy, and strong interest exists in using these techniques for dose escalation to ultimately achieve better local control for pancreatic ductal adenocarcinoma.6
Recently published, the American Society for Radiation Oncology clinical practice guidelines for pancreatic cancer cite the need for prospective dose-escalation studies in the face of multiple series reporting improved local control with higher biologically effective doses. Further, for cases of LAPC, not appropriate for downstaging to future surgery, a conditional recommendation with 85% consensus includes dose-escalated chemoradiation or multifraction SBRT after systemic chemotherapy as definitive treatment options.7
Evidence for dose-escalation
Data regarding the benefits of dose escalation in pancreatic ductal adenocarcinoma as well as the use of stereotactic body radiation therapy (SBRT) are emerging. For example, freedom from local progression has been demonstrated in rates in excess of 80% to 90% at 12 months in patients with LAPC treated with SBRT (25 Gy × 1).8–10 As to be expected with dose escalation, the major concern, based on observations from early experiences, has been duodenal toxicity. More recently, fractionated SBRT appears to be favored at most institutions to safely meet constraints.9,11 In a multi-institutional, prospective phase 2 trial, Herman et al treated 49 patients with SBRT (33 Gy in 5 fractions [fx]) and reported a freedom-from-local-progression rate of 78% at 1 year, as well as a low rate of acute and late gastrointestinal toxicity.12 Shaib et al recently published results of a phase-1 dose-escalation study of SBRT for borderline resectable patients who did not reach dose-limiting toxicity at the highest dose level (45 Gy/3 fx).13 In terms of hypofractionated intensity modulated radiation therapy (IMRT), a Korean study evaluating almost 500 patients with pancreatic cancer found that patients receiving ≥61 Gy had improved freedom from local failure, progression-free survival, and overall survival.14 Further, the experience at MD Anderson Cancer Center with escalated-dose radiation (EDR, defined as a biological equivalent dose [BED] > 70 Gy), using IMRT planning in 15 to 28 fx (67.5–70 Gy), yielded better tolerability as far as gastrointestinal and overall toxicities compared with standard-dose radiation (50.4 Gy/28 fx) with concurrent chemotherapy in addition to improved overall survival and local- and regional-recurrence-free survival.15,16 Colbert et al published a dosimetric feasibility study suggesting that 60 Gy/5 fx may be feasible while still meeting dose constraints to organs at risk (OARs) and maintaining adequate coverage of the gross tumor volume (GTV) in well-selected patients. Such an approach requires reduced planning target volumes (PTVs) made possible by utilization of image guidance (IGRT) and motion management techniques, and these doses are currently being evaluated in a prospective clinical trial (NCT: NCT003340974).17 Finally, EDR has been delivered safely with concurrent chemotherapy (capecitabine and gemcitabine) in multiple studies.14,16
Simultaneous-integrated boost and tumor vessel interface
A common approach to dose escalation for LAPC is using a simultaneous integrated boost (SIB), in which the totality of the tumor is encompassed by a set dose while a volume that spares bowel structures receives a higher dose during the same fraction (ie, dose painting). From a dosimetric and biological point of view, SIB has the practical benefit of saving time over a sequential boost. Because most SIB fractionation regimens use 15 to 28 treatments, higher doses to the tumor and surrounding nodes can be achieved compared with SBRT in general.18 Some have argued that an SIB approach to the hypoxic center of a tumor may have benefits for treating resistant clones while also allowing for lower radiation doses that are both safe for normal tissues and effective for microscopic disease (eg, in areas abutting the gastrointestinal tract).4 The tumor–evessel interface (TVI) has been found to be a region at high risk for recurrence and may lie outside of the primary tumor volume. Zhu et al retrospectively reviewed 217 locoregional recurrences after SBRT and found that approximately one-third of patients recurred near the celiac trunk and superior mesenteric artery (SMA).19 Additionally, Kharofa et al studied patterns of marginal local failures in a phase 2 prospective trial of neoadjuvant chemotherapy and 5-fraction SBRT for locally advanced pancreatic cancer. In this trial, “PTV33” was defined as a 3-mm expansion from the GTV and included tumor and vessel in contact with tumor. They found that local failures were mostly observed outside of the “PTV33,” but within volumes that would have been treated with conventional radiation (areas of clinical microscopic risk). This is consistent with what is known about the neurotropic spread of LAPC along mesenteric vessels and the high rate of perivascular recurrences in patients after resection. The authors concluded that omission of mesenteric vasculature may predispose patients to a higher risk of recurrence, thus favoring techniques providing coverage of at-risk vascular target volumes.20 Finally, in addition to lowering the risk of local recurrence, it is thought that targeting the TVI may also improve the likelihood of a negative surgical margin in patients who eventually undergo surgery. The utilization of SIB and targeting of the TVI in SBRT are referenced in the recent American Society for Radiation Oncology guidelines.7
Case 1: 15-Fraction Hypofractionated IMRT With SIB
Patient history and selection
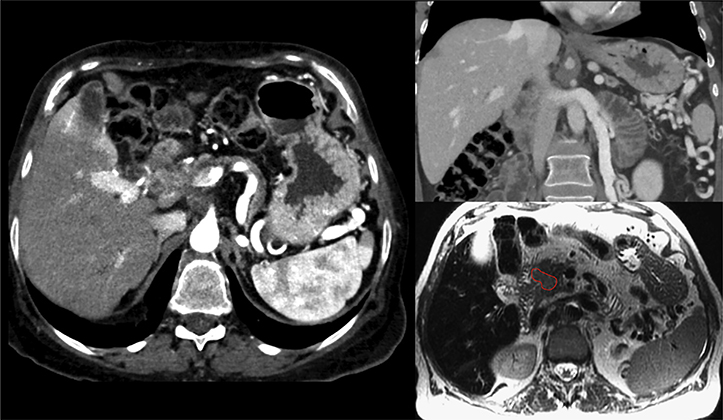
A 70-year-old woman presented with decreased appetite, abdominal pain, and weight loss. Computed tomography (CT) of the abdomen revealed a 2.9-cm mass extending from the head of the pancreas to superiorly and inferiorly surround the celiac axis (CA) and abut the SMA by 180°. The superior mesenteric vein (SMV), portal vein, and splenic vein were free from tumor, although mild abutment existed at the confluence. No lymphadenopathy was appreciated. After neoadjuvant chemotherapy with gemcitabine and abraxane for 8 cycles, the patient maintained a stable pancreatic head mass with continued encasement of the celiac trunk and abutment of the SMA. In addition, MRI revealed probable abutment of the common hepatic and splenic artery and the SMV and splenoportal confluence (Fig 1). At this point, given stable, unresectable disease without distant metastasis, the patient was referred for chemoradiation with concurrent capecitabine and was identified as a candidate for EDR and planned for treatment to 6750 cGy in 15 fx.
Figure 1.
Case 1: Patient selection for hypofractionated dose escalation in locally advanced pancreatic cancer. A 2.9-cm mass extends from the head of the pancreas surrounding the celiac axis and abutting the superior mesenteric artery by 180°. No lymphadenopathy is appreciated. After neoadjuvant chemotherapy, the patient maintained a stable pancreatic head mass with continued encasement of the celiac axis and abutment of the superior mesenteric artery. In addition, magnetic resonance imaging revealed probable abutment of the common hepatic and splenic artery, superior mesenteric vein, and splenoportal confluence.
CT simulation and breath-hold
The patient was simulated under the following conditions: 3 hours NPO, supine position with arms up, immobilized with a custom cradle in a wing board to ensure setup reproducibility. IV contrast was infused at a fixed rate (5 cm3/s) and coordinated with the acquisition of the images, with timing similar to a triphasic, pancreas-protocol CT scan.21 Respiratory correlated breath-hold CT was used in which a marker in combination with video feedback guided the patient into a series of reproducible breath-holds. A series of CTs were acquired to test patient suitability for treatment with breath-hold and the potential benefits of this technique in terms of reductions to organs at risk. For this patient, IGRT was planned using CT-on-Rails with setup to GTV daily.
Target volume creation (internal GTV, CTV, and OAR)
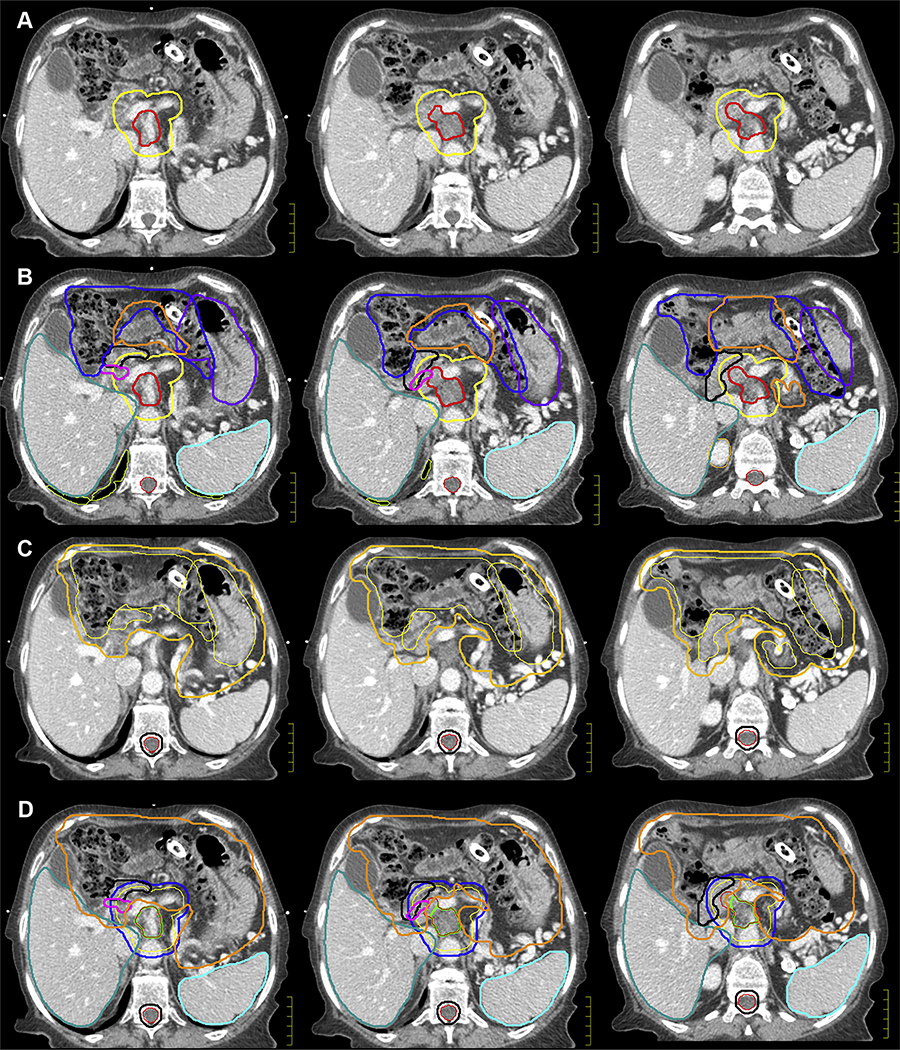
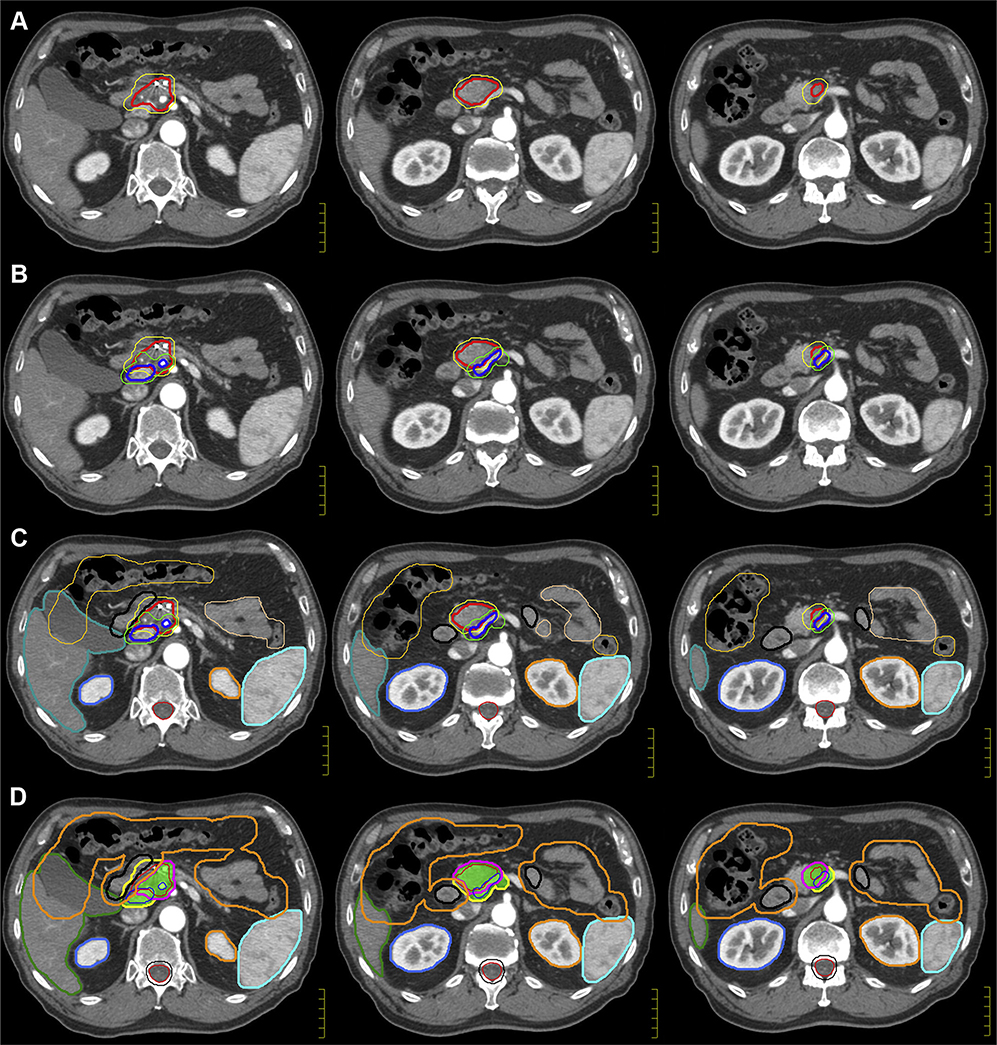
To fully appreciate the location of the GTV, the tumor is evaluated on all triphasic diagnostic scans (CT and magnetic resonance imaging [MRI]) and on CT simulation images to create an internal GTV (iGTV). The iGTV is defined by the complete extent of tumor, accounting for variability in tumor position among the breath-hold scans. In this case of EDR, the clinical target volume (CTV) comprises a 10-mm uniform expansion from the iGTV and may need to be further expanded to include areas at risk of microscopic extension and nodal elective irradiation around the CA, SMA, and SMV (generally starting at the origin of the arteries and including the vessels at the same axial slice levels as the GTV). The CTV can extend into bowel structures, ensuring coverage of microscopic risk of disease in the adjacent duodenum and nodal basins. The goal is to treat this comprehensive volume to a lower dose level (3750 cGy), thereby respecting the duodenum tolerance (Dmax 45 Gy). Meanwhile, no CTV is defined for the high-dose target (6750 cGy); rather, a planning structure is constructed directly from the iGTV (with minimal or no expansion) and modified based on adjacent, dose-limiting duodenum and other luminal OARs. The iGTV and CTV are displayed in Figure 2A on axial slices at the level between the CA and SMA. The duodenum, small and large bowel, stomach, liver, common bile duct, and spleen are contoured as OARs (Fig 2B). Of note, the bowel structures are contoured on all respiratory gated scans (typically 3–4 scans) and are referred to as iBowel.
Figure 2.
Case 1: Target volume creation (internal GTV, clinical target volume, and organ at risk) and planning volume creation (planning target volume and PRV). (A, B) The internal GTV (red) defines the complete extent of the tumor, accounting for motion throughout the respiratory cycle. In escalated-dose fractionated radiation, the clinical target volume (yellow) comprises a 10-mm uniform expansion from the GTV and areas at risk of microscopic extension and may be further expanded to provide comprehensive, elective nodal irradiation around the celiac axis and superior mesenteric artery. The duodenum (black), small bowel (orange), large bowel (blue), stomach (purple), liver (teal), common bile duct (magenta), and spleen (cyan) are contoured as organs-at-risk. Of note, the bowel structures are contoured on all respiratory gated scans and are referred to as iBowel (small and large), iDuodenum, and iStomach. (C, D) A collective PRV structure is created as a 5-mm expansion from all bowel structures (gastrointestinal PRV) (orange). For hypofractionated, escalated-dose radiation therapy, 2 planning treatment volumes will be constructed. The PTV_Low (blue) is intended to comprehensively cover, with margin, the complete extent of the tumor, areas of possible microscopic extension, and elective nodal basins; therefore, the dose will be lower to this volume (3750 cGy in 15 fractions) with a uniform, unmodified, 5-mm expansion. The zPTV_High (green) will encompass the planning volume for dose-escalation (6750 cGy in 15 fractions); thus, this planning target volume will have no expansion and will consist of subtracting the gastrointestinal PRV from the internal GTV. Note: The patient’s pancreatic stent migrated into the bowel and was in the large bowel by the time of simulation. She passed it without complications. This emphasizes that stents may not be reliable for daily setup for radiation. Abbreviations: GTV = gross tumor volume; PRV = planning organ-at-risk volume.
Distinguishing the anatomy of the gastrointestinal tract allows for selection of optimized beam angles for IMRT to reduce toxicity. For instance, the small and large bowel have different radiation tolerances, and it may be useful or necessary to contour the duodenum to prevent hotspots. Indeed, one of the more worrisome toxicities from high doses near the bowel is a bleeding ulcer. Although gastric and proximal duodenal ulcers could potentially be repaired by an endoscopic approach, distal duodenum and jejunal lesions would likely require an operative procedure, potentially increasing the risk of morbidity. Therefore, although a structure that defines the potential bowel space (ie, bowel bag) may be convenient to contour and can be used to assist with planning, it should not be the sole structure used. Contouring the space and cavity as opposed to the actual bowel trades off valuable anatomic information that can be used to optimize dosimetry.
Planning volume creation (PTV and PRV)
A collective planning organ-at-risk volume (PRV) is created as a 5-mm expansion from all bowel structures (gastrointestinal [GI] PRV), as seen in Figure 2C. For hypofractionated EDR, 2 planning treatment volumes are constructed (Fig 2D). PTV_Low is intended to comprehensively cover the tumor with margin and areas of possible microscopic extension and elective nodal basins; therefore, the dose will be lower to this volume (3750 cGy/15 fx) with a uniform, unmodified, 5-mm expansion. Meanwhile, a high-dose planning structure, zPTV_High, will encompass the planning volume for dose escalation (6750 cGy/15 fx). This planning volume is modified to respect the adjacent OAR limits and created by subtracting the GI PRV from the iGTV. Because this is technically a modified planning structure, it should not be used for dose evaluation and is designated with a “z” prefix as per the American Association of Physicists in Medicine (AAPM) Task Group 263 report.22 Rather, the coverage of the iGTV should be used to evaluate high-dose coverage. Importantly, contouring the bowel will not only be critical to gauging the safety of the plan on the dose–volume histogram but also to determining the coverage of the GTV with high-dose radiation because the bowel determines the PRV, which in turn determines the zPTV_High.
RT plan evaluation
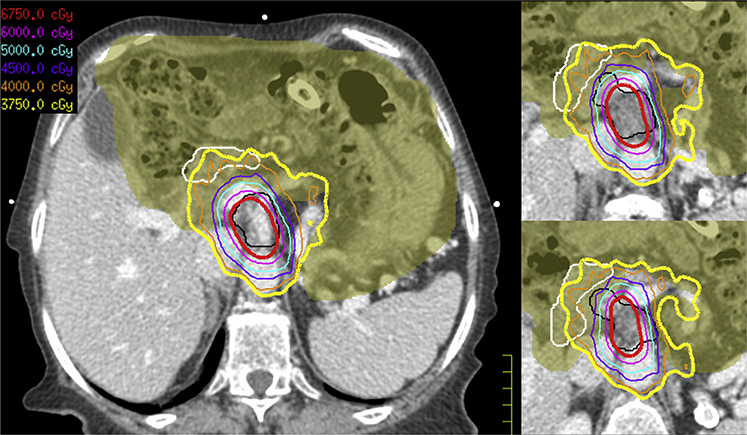
Several considerations are necessary during the RT planning process. Tumor coverage, for example, should be maximized for PTV_Low, and for this patient, note the 3750 cGy line encompasses part of the duodenum and bowel structures (Fig 3). When evaluating high dose, scrutiny of dose to OARs should take precedence over maximizing coverage, although the physician and dosimetrist should aim to cover the area of iGTV that does not overlap with GI PRV to a minimum of 45 Gy. Full coverage (≥95%) of the iGTV to escalated prescription dose (6750 cGy) is often not feasible, and goals must be individualized based on differing anatomy and tumor shape and location among patients. Given that zPTV_High provides no expansion from the tumor and consists of the GI PRV subtracted from the iGTV, planning may intentionally avoid part of the original tumor. In this patient, the 6750 cGy covers the central tumor but avoids the anterior lateral extent extending toward the duodenum while the near total extent of tumor is intentionally covered by an intermediate 4500 cGy (Fig 3). Table 1 summarizes the previously mentioned targets, expansions, and dose levels and suggested coverage goals and recommended constraints. For certain patients, fractionation beyond 15 fractions (to improve the therapeutic ratio) may be required to achieve ablative BED while respecting dose constraints to the OARs.15
Figure 3.
Case 1: Hypofractionated escalated-dose radiation therapy plan with isodose lines. Tumor coverage should be maximized for the PTV_Low, and for this patient, note the 3750 cGy isodose lines (yellow) encompass part of duodenum (white) and bowel structures (olive color wash). Given that the zPTV_High (red) provides no expansion from the tumor and consists of the gastrointestinal planning organ-at-risk volume subtracted from the internal gross tumor volume (black), planning may intentionally avoid part of the original tumor. In this patient, the 6750 cGy covers the central tumor but avoids the anterior lateral extent near the duodenum, whereas the near total extent of tumor is intentionally covered by an intermediate 4500 cGy (purple).
Table 1.
Dosimetric objectives and constraints
| 5-Fraction SBRT dose escalation | ||
| PTV_Low | zPTV_High* | |
| Target | ([iGTV + TVI] + 3 mm) | ([iGTV + TVI] + 3 mm)–GI PRV |
| Total dose, cGy | 3300 | 5000 |
| Objective | >98% coverage | 1. ≥98% coverage of iGTV to 4000 cGy |
| 2. ≥95% coverage of TVI to 4000 cGy | ||
| 3. ≥90%–95% coverage of iGTV to 5000 cGy† | ||
| 15-Fraction IMRT dose escalation | ||
| PTV_Low | zPTV_High* | |
| Target | (iGTV + CA/SMA‡) + 10 mm | iGTV–GI PRV |
| Expansion | CTV + 5 mm | None |
| Total dose | 3750 cGy | 6750 cGy |
| Objective | >98% coverage | 1. ≥98% coverage of iGTV to 4500 cGy |
| 2. ≥90%–95% coverage of iGTV to 6750 cGy† | ||
| Organ at Risk | 15-fraction (IMRT) constraint | 5-fraction (SBRT) constraint |
| Spinal cord | Dmax <30 Gy | V20 <1 cm3 |
| Liver | 700 cm3 <24 Gy | V12 <50% |
| Mean <24 Gy | ||
| Kidneys (left and right) | V20 <33% | V12 <75% |
| iStomach | Dmax < 45 Gy | V40 <0.5 cm3 |
| V35 <1 cm3 | ||
| V30 <2 cm3 | ||
| iDuodenum | Dmax <45 Gy | V40 <0.5 cm3 |
| V35 <1 cm3 | ||
| V30 <3 cm3 | ||
| iBowel (small) | Dmax <45 Gy | V40 <0.5 cm3 |
| V35 <1 cm3 | ||
| V30 <2 cm3 | ||
| iBowel (large) | Dmax <50 Gy | V35 <1 cm3 |
| Bile duct | Dmax <70 Gy | Max 55 Gy |
| Spleen | Mean <6 Gy | Mean <2 Gy |
| Heart | V40 < 10%, V50 <1 cm3, Max 55 Gy | Max 30 Gy |
Abbreviations: CA = celiac axis; CTV = clinical target volume; Dmax = maximum dose; GI PRV = gastrointestinal planning organ-at-risk volume; iGTV = internal gross tumor volume; IMRT = intensity modulated radiation therapy; PTV = planning target volume; SBRT = stereotactic body radiation therapy; SMA = superior mesenteric artery; TVI = tumor–vessel interface.
Modified planning target volume is indicated by prefix “z” and is not to be used for plan evaluation.
Represents an ideal target objective and may not be attainable in all cases.
Areas at high clinical risk of microscopic disease.
Patient follow-up
The patient tolerated treatment without significant (grade ≥3) acute or chronic toxicity. At 3 years from diagnosis and 2 years from EDR, this patient’s pancreatic head mass remains stable without local or distant progression. The patient’s most recent MRI reveals a residual, treated pancreatic head tumor with unchanged soft tissue thickening along the CA and SMA. The patient’s performance status is excellent (Eastern Cooperative Oncology Group 1) with only mild fatigue and residual neuropathy secondary to chemotherapy.
Case 2: 5-Fraction SBRT
Patient history and selection
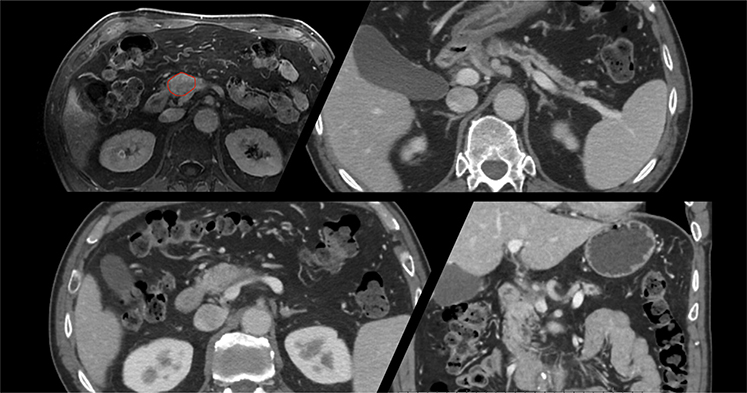
A 60-year-old man was found to have a 3-cm pancreatic head mass encasing the common hepatic and splenic arteries and invasion of the portal vein and superior mesenteric vein. No regional adenopathy was appreciated. The patient was treated with 8 cycles of FOLFIRINOX with an excellent clinical response and a decrease in size of the mass and vein involvement; however, there was continued involvement of the common hepatic artery and the origin of the splenic artery. A baseline electrocardiogram, triphasic CT, and MRI (to determine resectability and for radiation planning) were ordered (Fig 4). Patient was discussed at multidisciplinary tumor board as locally advanced and ineligible for subtotal pancreatectomy. Patient was evaluated for a dose-escalation SBRT protocol after meeting inclusion criteria (Table 2). Treatment dose and fractionation selected was 5000 cGy/5 fx with most of the total prescription dose confined to the tumor with a small margin. As surgical considerations were a future possibility for this patient, collaboration with the surgical oncologist ensured that areas of future anastomosis were spared of high-dose radiation in case the patient eventually became resectable.
Figure 4.
Case 2: Patient selection for stereotactic body radiation therapy for locally advanced pancreatic cancer. A 3-cm pancreatic head mass encases the common hepatic and splenic artery and invades the portal and superior mesenteric veins. No regional adenopathy is appreciated. After neoadjuvant chemotherapy with excellent clinical response (decrease in size of the mass and vein involvement), there was continued involvement of the common hepatic artery and origin of the splenic artery. The patient was selected for a dose-escalation stereotactic body radiation therapy protocol after meeting inclusion criteria (Table 2). Treatment dose and fractionation selected was 5000 cGy in 5 fractions with prescription dose to be confined to the tumor with small margin as surgical considerations were a future possibility for this patient.
Table 2.
SBRT selection criteria for dose escalation
| Suggested SBRT (5 Fractions) dose-escalation inclusion criteria |
| Cytologic- and biopsy-confirmed adenocarcinoma |
| Locally advanced, unresectable, or borderline (abutment of SMA, celiac axis, SMV, or PV involvement*) |
| ECOG ≤2 |
| Pancreatic tumor size ≤5 cm |
| No regional adenopathy† |
| No evidence of distant metastasis |
| Metal stent in place if duodenal stent required |
| Reproducible motion management (encompass tumor motion ≤5 mm) |
| Adequate hematologic function (ANC ≥ 1500/mm3, Hgb ≥ 8 g/dL, PLT ≥ 100,000/mm3) |
| Adequate renal function (creatinine ≤1.5 X ULN) |
| Adequate liver function (total bilirubin ≤ 1.5 X ULN; AST, ALT, and ALP ≤ 2.5 X ULN) |
| Exclusion criteria |
| Prior overlapping abdominal radiation treatment |
| Prior surgical resection of pancreatic tumor |
| Active or uncontrolled gastric or duodenal ulcer |
| Direct invasion of duodenum by tumor |
| Residual or persistent grade 3 chemotherapy toxicity |
| Therapeutic anticoagulation |
Abbreviations: ALP = alkaline phosphatase; ALT = alanine aminotransferase; ANC = absolute neutrophil count; AST = aspartate aminotransferase; ECOG = Eastern Coppoeative Oncology Group; PLT = platelet; PV = portal vein; SMA = superior mesenteric artery; SMV = superior mesenteric vein; SBRT = stereotactic body radiation therapy; ULN = upper limit of normal.
Involvement requiring vascular reconstruction.
SBRT may be possible for select patients with nodal disease.
CT simulation
Gold fiducials were endoscopically placed 48 hours before simulation. The patient was simulated under the following conditions: 3 hours NPO, in the supine position with arms up, immobilized with a custom cradle and wing board to ensure reproducibility of setup for radiation treatments. IV contrast was infused at a fixed rate (5 cm3/s) and coordinated with the acquisition of the images per triphasic pancreatic protocol. Respiratory correlated breath-hold CT was used, in which a marker in combination with video feedback guided the patient into a series of reproducible breath-holds. A series of CTs were acquired to test the patient suitability for treatment with breath-hold and the potential benefits of this technique in terms of reductions to organs at risk. Planned IGRT was breath-hold cone beam CT with setup to fiducials daily.
Target volume creation (GTV and TVI)
Similar to the hypofractionated approach, the location of the GTV was evaluated on all biphasic IV contrast diagnostic scans (CT and MRI) and on CT simulation with IV contrast. Accurate identification of the primary tumor is critical for SBRT given the expansion to PTV with minimal clinical margin. In cases where resection is planned, collaboration with the surgical oncologist is critical. For example, if a jump graft for vascular reconstruction is planned there may a section of vessel that is specifically spared dose to improve the likelihood of revascularization. Consultation with an abdominal radiologist may be necessary and is encouraged in situations in which the primary tumor is difficult to discern. Depending on technique (ie, breath-hold or other motion management), contours from the appropriate phases should be considered to create an iGTV (Fig 5A). For SBRT, the TVI should be contoured. The TVI encompasses any portion of the vessel with direct contact to tumor. These involved vessels should be contoured to the full radial extent when abutting tumor (eg, in the extent of 180° abutment, the full 360° extent of a vessel would be included). The superior and inferior extent of the TVI should be delineated on all axial slices on which iGTV is contoured. As previously mentioned, patterns of failure indicate locoregional failures often occur around the CA and SMA, and consideration of neurotropic spread and nodal coverage around these vessels, akin to the IMRT approach, should be done on a case-by-case basis. In this patient, given contact of the pancreatic head mass with the common hepatic and splenic artery, these contoured vessels represent the TVI (Fig 5B). Nodal regions are generally not included in SBRT; thus, for patients with regional lymphadenopathy, in proximity to tumor, a more fractionated approach should be considered. SBRT should also be strongly cautioned when the tumor abuts the bowel radiographically, and endoscopy can help determine whether the tumor invades the bowel. In cases of bowel invasion, SBRT is an absolute contraindication secondary to the risk of potentially fatal gastrointestinal bleeding.
Figure 5.
Case 2: Target volume creation (internal gross tumor volume and TVI) and planning volume creation (organ at risk, planning target volume, and planning organ-at-risk volume). (A, B) Similarly to the hypofractionated approach, contours from the appropriate phases should be considered to create an iGTV (red). For stereotactic body radiation therapy, the TVI should be contoured (blue). The TVI encompasses any portion of the vessel with direct contact to tumor. These involved vessels should be contoured to the full radial extent when abutting tumor (eg, in the extent of 180° abutment, the full 360° extent of a vessel will be included). The superior and inferior extent of the TVI should be delineated on all axial slices on which iGTV is contoured and will also be expanded by 3 mm (green). In this patient, given contact of the pancreatic head mass with the common hepatic and splenic artery, these contoured vessels represent the TVI. (C, D) A 2-volume approach is used for planning that considers iGTV (red), the TVI (blue), and GI PRV (orange). GI PRV represents a 5-mm expansion from all bowel structures, including the small (peach) and large (apricot) iBowel. PTV_Low (yellow) represents the low-dose level at 3300 cGy and encompasses a 3-mm expansion of both, the iGTV, and TVI to ensure minimum dose encompasses these structures without regard to normal structures. As with the 15-fraction regimen, a modified, high-dose planning volume, zPTV_High (green color wash), is created from (iGTV + TVI) + 3 mm with the GI PRV subtracted. Essentially, the PTV_Low modified by the luminal organs at risk represents the targeted area for maximum dose (5000 cGy) to the tumor and TVI. Abbreviations: GI PRV = gastrointestinal planning organ-at-risk volume; iGTV = internal gross tumor volume; TVI = tumor–vessel interface.
Planning volume creation (OAR, PTV, and PRV)
A 2-volume approach was again used for planning, which considers iGTV, TVI, and GI PRV. As with hypofractionated treatment, the GI PRV represents a 5-mm expansion from all bowel structures (Fig 5C). PTV_Low represents the low-dose level (3300 cGy) and encompasses a 3-mm expansion of both the iGTV and TVI to ensure the minimum dose encompasses these structures without regard to normal structures. As with the 15 fx regimen, a modified, high-dose planning volume, zPTV_High, is created from (iGTV + TVI) + 3 mm with the GI PRV subtracted; in essence, this is the PTV_Low modified by the luminal OARs and will represent the targeted area for maximum dose (5000 cGy) to the tumor and TVI (Fig 5D). In the past, 3 dose levels were used with a third PTV representing an intermediate dose (~4000 cGy), and although this is a reasonable approach, recent dosimetric planning studies have demonstrated that plans of similar conformality and dose drop-off can be achieved with only 2 dose levels, which we recommend for simplicity. Plan evaluation, however, will be critical to ensure dose is distributed appropriately.
RT plan evaluation
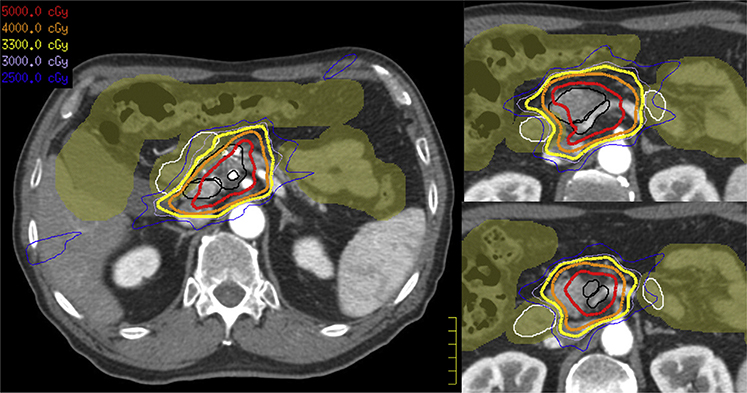
The patient’s SBRT plan is presented in Figure 6. Of note, the total prescription dose (5000 cGy) does not cover the tumor and TVI entirely, given the modified zPTV_High. During evaluation, we appreciate that the low dose (3300 cGy) approaches complete coverage of PTV_Low (ie, tumor and TVI), and an intermediate dose (4000 cGy) provides near complete coverage of tumor. Although we do not use an intermediate dose target volume during the planning process, we still recommend evaluating the intermediate isodose level during plan evaluation. As this patient had a reduction in tumor size after neoadjuvant chemotherapy, it would also be prudent to assess the dose to the prechemotherapy volume and ensure coverage with meaningful low dose (3000–3300 cGy). Overall, the plan appears relatively conformal, with dose pushed appropriately away from the duodenum and bowel. Table 1 summarizes the previously mentioned targets, expansions, and dose levels in addition to suggested coverage goals and recommended constraints.
Figure 6.
Case 2: Radiation therapy plan with IDLs. The patient’s stereotactic body radiation therapy plan is presented. Of note, the total prescription dose (5000 cGy, red IDLs) does not completely cover the tumor and tumor–vessel interface (both in black) given the modifications to zPTV_High. During evaluation, we appreciate that the low dose (3300 cGy, yellow IDLs) approaches complete coverage of the tumor and tumor–vessel interface while the intermediate dose (4000 cGy, orange IDLs) provides near complete coverage of the tumor. The plan appears relatively conformal, with dose pushed appropriately away from the duodenum (white) and bowel structures (olive color wash). Abbreviation: IDL = isodose line.
Patient follow-up
The patient’s disease remained stable at 6-week imaging post-SBRT, and the patient underwent a pancreaticoduodenectomy (Whipple procedure) and distal splenic artery reconstruction. Pathology revealed a moderately differentiated pancreatic ductal adenocarcinoma with 40% viability. Margins were negative, and 2 out of 37 lymph nodes were involved (ypT3N1). Pancreatic fibrosis and atrophy were noted. The patient was dispositioned for adjuvant chemotherapy.
Challenges and Barriers to Dose Escalation
Defining the target
Questions remain regarding the appropriate target volumes for SBRT. Properly delineating the GTV can be difficult in certain cases, and multidisciplinary review with an experienced abdominal radiologist may be warranted. Given that our technique includes meticulously delineating the bowel structures and OARs to create PRVs or a “buffer zone” when planning high-dose radiation, the radiation oncologist should avoid the temptation to “undercontour” the GTV, which lends itself to poor plan evaluation and coverage. As previously mentioned, recurrences along the mesenteric vessels are a pattern of local failure seen in patients undergoing dose-escalated treatment, and currently, most dose-escalated SBRT techniques do not purposefully target the regions of microscopic risk around the CA and SMA. At least 1 prospective phase 2 trial has implemented generous expansions to cover the SMA and CA in addition to the peripancreatic lymph nodes at risk to ~25–30 Gy in addition to the TVI (NCT:03563248), but currently the question remains unanswered for dose-escalated SBRT. At the current time, for a 5-fx SBRT regimen, we recommend coverage of the TVI by a minimum of 3300 cGy, ideally 4000 cGy, and an assessment of the dose falloff and nodal coverage, specifically the 2500 cGy and 3000 cGy isodose lines. For patients with nodal disease, we recommend a hypofractionated regimen (eg, 15 fx) if dose escalation is employed to comprehensively cover the nodal basins to meaningful BED. Although SBRT could be considered on a case-by-case basis when nodes are involved, achieving target coverage and maintaining dose constraints can be challenging. Finally, for the minority of patients with significant tumor downsizing after chemotherapy, prechemotherapy imaging may add significant value to treatment planning.
Challenging anatomy
Despite improved localization and imaging techniques, many cases of LAPC are not candidates for dose escalation with SBRT or hypofractionation, secondary to tumor size, location, or patient anatomy. Given the location of the pancreatic head, many lesions in this section of the pancreas will abut or invade the duodenum, making dose escalation challenging. Further, when patients undergo treatment, the radiation oncologist must be willing to postpone treatment if conditions are unfavorable for dose escalation. For example, when gas or the position of the bowel is problematic, treatment later in the day or on a subsequent day with antigas medication (ie, simethicone) is preferred. In some cases, resimulation or an adaptive plan may be necessary to deliver all fractions safely.
Radiomodulating agents or hydrogel spacers, if found to be safe and effective, may eventually address the issue of duodenal proximity, thereby increasing the number of cases eligible for, and the safety of, dose-escalated therapy.23 Technical considerations for implementation of dose escalation are provided (Appendix E1, available online at https://doi.org/10.1016/j.prro.2020.01.012).
Future Directions
Clinical trials are needed to define the value of dose-escalated RT and SBRT for LAPC. Currently, a phase 3 trial is evaluating the addition of SBRT to a modified regimen of FOLFIRINOX to determine safety and efficacy in terms of progression-free survival (NCT:01926197).24 Dose-escalated SBRT is also being combined with radio-modulating agents. For example, a phase 1 trial evaluated the use of nelfinavir with concurrent SBRT (up to 40 Gy/5 fx).25 A multi-institutional phase 1/2 trial is evaluating adaptive dose escalation with SBRT for LAPC with the radiomodulator GC4419 or placebo (NCT003340974). Additionally, technological advances in particle therapy, image guidance, and adaptive planning may improve therapeutic effectiveness.
Data on particle therapy are emerging, but results are mixed. One study of dose escalation with protons reported a high incidence of radiation-induced ulcers in the stomach and duodenum,26,27 although a different study reported favorable proton dose-escalation outcomes with minimal toxicity.28 A multi-institutional study from Japan using carbon therapy has found low rates of toxicity at ~1 year median follow-up, and a prospective trial is underway.29
MRgRT provides improved soft tissue delineation and the ability to visualize the target in real-time, potentially improving target coverage while helping to minimize toxicity.30–32 A prospective trial is actively recruiting (NCT: NCT03621644) and will serve to build on the promising outcomes reported in a multi-institutional, retrospective analysis of patients treated with adaptive, dose-escalated MRgRT for inoperable LAPC.33
Conclusions
In conclusion, a new era for LAPC and radiation therapy may be emerging. Although prior studies with older chemotherapy regimens failed to show improved survival with standard doses of radiation for locally advanced disease,3 new studies with more effective combinations of chemotherapy such as gemcitabine and nab-paclitaxel and FOLFIRINOX with EDR are needed. The expectation is that with improved systemic therapies for LAPC, local tumor control will reemerge as an important consideration for patients with this disease. Ultimately, appropriate patient selection, technical considerations with treatment delivery, advanced image guidance, and respiratory management techniques are required to safely deliver higher radiation doses.
Supplementary Material
Acknowledgments
Sources of support: We gratefully acknowledge support from the Andrew Sabin Family Fellowship, the Sheikh Ahmed Center for Pancreatic Cancer Research, institutional funds from the University of Texas MD Anderson Cancer Center, the Khalifa Foundation, equipment support by GE Healthcare and the Center of Advanced Biomedical Imaging, Philips Healthcare, and Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute to MD Anderson. Dr Eugene Koay was supported by the National Institutes of Health (1R01CA218004-01A1, 1R01CA221971-01A1, U54CA210181-01, U54CA143837 and U01CA196403).
Disclosures: Dr Koay reports grants from the National Institutes of Health, grants from Elekta, grants from Philips Healthcare, grants from GE, and grants from MD Anderson Cancer Center, during the conduct of the study; and personal fees from Taylor and Francis, LLC, outside the submitted work. Dr Hall reports other from Elekta, during the conduct of the study; and other from Siemens Healthineers, other from Accuray, and other from Manteia Medical Technologies, outside the submitted work. Dr Taniguchi reports other from Accuray, outside the submitted work. Dr Aitken reports personal fees and nonfinancial support from Elekta, outside the submitted work. Dr Myrehaug reports personal fees from Ipsen and grants and personal fees from Novartis, outside the submitted work. Dr Herman reports other from Augmenix, other from BTG, other from Oncosil, other from Medtronic, other from Sirtex, and other from Boston Scientific, outside the submitted work. Dr Erickson reports other from Elekta, during the conduct of the study; and other from Siemens Healthineers, other from Accuray, and other from Manteia Medical Technologies, outside the submitted work.
Footnotes
Supplementary Data
Supplementary material for this article can be found at https://doi.org/10.1016/j.prro.2020.01.012.
References
- 1.Yachida S, Iacobuzio-Donahue CA. The Pathology and Genetics of Metastatic Pancreatic Cancer. Vol 133 Available at: https://www.archivesofpathology.org/doi/pdf/10.1043/1543-2165-133.3.413. Accessed March 7, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammel P, Huguet F, van Laethem J-L, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib. JAMA. 2016;315:1844. [DOI] [PubMed] [Google Scholar]
- 4.Crane CH. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res. 2016;57:i53–i57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badiyan SN, Olsen JR, Lee AY, et al. Induction chemotherapy followed by concurrent full-dose gemcitabine and intensity-modulated radiation therapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2016;39:1–7. [DOI] [PubMed] [Google Scholar]
- 7.Palta M, Godfrey D, Goodman KA, et al. Radiation therapy for pancreatic cancer: Executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. 2019;9:322–332. [DOI] [PubMed] [Google Scholar]
- 8.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115: 665–672. [DOI] [PubMed] [Google Scholar]
- 9.Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol. 2011;81:181–188. [DOI] [PubMed] [Google Scholar]
- 10.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol. 2004;58:1017–1021. [DOI] [PubMed] [Google Scholar]
- 11.Murphy JD, Christman-Skieller C, Kim J, Dieterich S, Chang DT, Koong AC. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol. 2010;78:1420–1426. [DOI] [PubMed] [Google Scholar]
- 12.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaib WL, Hawk N, Cassidy RJ, et al. A phase 1 study of stereotactic body radiation therapy dose escalation for borderline resectable pancreatic cancer after modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol. 2016;96:296–303. [DOI] [PubMed] [Google Scholar]
- 14.Chung SY, Chang JS, Lee BM, Kim KH, Lee KJ, Seong J. Dose escalation in locally advanced pancreatic cancer patients receiving chemoradiotherapy. Radiother Oncol. 2017;123:438–445. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan S, Chadha AS, Suh Y, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol. 2016;94:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colbert LE, Moningi S, Chadha A, et al. Dose escalation with an IMRT technique in 15 to 28 fractions is better tolerated than standard doses of 3DCRT for LAPC. Adv Radiat Oncol. 2017;2:403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colbert LE, Rebueno N, Moningi S, et al. Dose escalation for locally advanced pancreatic cancer: How high can we go? Adv Radiat Oncol. 2018;3:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Reznik R, Fraass BA, et al. Dosimetric evaluation of simultaneous integrated boost during stereotactic body radiation therapy for pancreatic cancer. Med Dosim. 2015;40:47–52. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Ju X, Cao Y, et al. Patterns of local failure after stereotactic body radiation therapy and sequential chemotherapy as initial treatment for pancreatic cancer: Implications of target volume design. Int J Radiat Oncol Biol Phys. 2019;104:101–110. [DOI] [PubMed] [Google Scholar]
- 20.Kharofa J, Mierzwa M, Olowokure O, et al. Pattern of marginal local failure in a phase ii trial of neoadjuvant chemotherapy and stereotactic body radiation therapy for resectable and borderline resectable pancreas cancer. Am J Clin Oncol. 2019;42: 247–252. [DOI] [PubMed] [Google Scholar]
- 21.Qayyum A, Tamm EP, Kamel IR, et al. ACR Appropriateness Criteria ® 2 Staging of Pancreatic Ductal Adenocarcinoma STAGING OF PANCREATIC DUCTAL ADENOCARCINOMA Expert Panel on Gastrointestinal Imaging. Available at: https://acsearch.acr.org/docs/3099847/Narrative/. Accessed June 24, 2019. [Google Scholar]
- 22.Task Group A. Standardizing Nomenclatures in Radiation Oncology: The Report of AAPM Task Group; 263 Available at: https://www.aapm.org/pubs/reports/RPT_263.pdf. Accessed June 27, 2019. [Google Scholar]
- 23.Rao AD, Feng Z, Shin EJ, et al. A novel absorbable radiopaque hydrogel spacer to separate the head of the pancreas and duodenum in radiation therapy for pancreatic cancer. Int J Radiat Oncol. 2017; 99:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phase III FOLFIRINOX (mFFX) +/− SBRT in locally advanced pancreatic cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT01926197. Accessed June 24, 2019.
- 25.Lin C, Verma V, Ly QP, et al. Phase I trial of concurrent stereotactic body radiotherapy and nelfinavir for locally advanced borderline or unresectable pancreatic adenocarcinoma. Radiother Oncol. 2019; 132:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terashima K, Demizu Y, Hashimoto N, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol. 2012; 103:25–31. [DOI] [PubMed] [Google Scholar]
- 27.Takatori K, Terashima K, Yoshida R, et al. Upper gastrointestinal complications associated with gemcitabine-concurrent proton radiotherapy for inoperable pancreatic cancer. J Gastroenterol. 2014;49:1074–1080. [DOI] [PubMed] [Google Scholar]
- 28.Hiroshima Y, Fukumitsu N, Saito T, et al. Concurrent chemoradiotherapy using proton beams for unresectable locally advanced pancreatic cancer. Radiother Oncol. 2019;136:37–43. [DOI] [PubMed] [Google Scholar]
- 29.Kawashiro S, Yamada S, Okamoto M, et al. Multi-institutional study of carbon-ion radiotherapy for locally advanced pancreatic cancer: Japan carbon-ion Radiation Oncology Study Group (JCROS) study 1403 pancreas. Int J Radiat Oncol Biol Phys. 2018; 101:1212–1221. [DOI] [PubMed] [Google Scholar]
- 30.Henke LE, Contreras JA, Green OL, et al. Magnetic resonance image-guided radiotherapy (MRIgRT): A 4.5-year clinical experience. Clin Oncol. 2018;30:720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard JM, Wen Z, Sadagopan R, Wang J, Ibbott GS. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017; 90:20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Sörnsen de Koste JR, Palacios MA, Bruynzeel AME, Slotman BJ, Senan S, Lagerwaard FJ. MR-guided gated stereotactic radiation therapy delivery for lung, adrenal, and pancreatic tumors: A geometric analysis. Int J Radiat Oncol. 2018;102:858–866. [DOI] [PubMed] [Google Scholar]
- 33.Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8: 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.