Abstract
Background:
Opioid analgesics are often prescribed to manage pain following bariatric surgery, which may develop into chronic prescription opioid use (CPOU) in opioid-naïve patients. Bariatric surgery may affect opioid use in those with or without pre-surgical CPOU.
Objective:
To compare CPOU persistence and incidence in a large multi-site cohort of Veterans undergoing bariatric surgery (open Roux-en-Y gastric bypass (ORYGB), laparoscopic RYGB (LRYGB), or laparoscopic sleeve gastrectomy (LSG)) and matched non-surgical controls.
Setting and Methods:
In a retrospective cohort study, we matched 1,117 surgical patients with baseline CPOU to 9,531 non-surgical controls and 2,822 surgical patients without CPOU at baseline to 26,392 non-surgical controls using sequential stratification. CPOU persistence in Veterans with baseline CPOU was estimated using generalized estimating equations by procedure type. CPOU incidence in Veterans without baseline CPOU was estimated in Cox regression models by procedure type because post-operative pain, complications and absorption may differ by procedure.
Results:
In Veterans with baseline CPOU, post-surgical CPOU declined over time for each surgical procedure; these trends did not differ between surgical patients and non-surgical controls. In Veterans without baseline CPOU, compared to non-surgical controls, bariatric patients had higher CPOU incidence within 5 years following ORYGB (hazard ratio (HR)=1.19; 95% confidence interval (CI), 1.06-1.34) or LRYGB (HR=1.22, 95% CI: 1.06-1.41). Veterans undergoing LSG had higher CPOU incidence 1-to-5 years following surgery (HR=1.28; 95% CI, 1.05-1.56) than non-surgical controls.
Conclusions:
Bariatric surgery was associated with greater risk of CPOU incidence in patients without baseline CPOU but was not associated with greater CPOU persistence.
Keywords: Opioid, Medication, Obesity, Surgery, Bariatric, Gastric bypass, Sleeve gastrectomy, Veterans, Matching
1.0. Introduction
Opioid use and misuse increased in the US in the early 2000s as clinical guidelines called for better management of chronic pain associated with cancer and other pain-related conditions (e.g., osteoarthritis) and to treat acute pain after major surgery. Pain was classified as the “fifth vital sign,” implying that clinicians were required to treat it when present.1 All this coincided with acceptance of opioids to treat non-cancer pain. Retail sales of opioids nearly doubled between 1997-20062 and the average dose per person of prescribed opioids increased from ~100 morphine milligram equivalents (MMEs) to ~700 between 1997-2007.3 As a result, 37% of US adults had one or more prescription opioids in 20154, as did 37% of older adults in 2012.5
Veterans have been shown to be more likely to report pain than non-Veterans, and more likely to report severe pain, defined as pain that occurs “most days” or “every day” that bothers the person “a lot”.6 Management of acute pain with opioids can transition to prolonged opioid use if dosages aren’t tapered once acute pain subsides. Chronic prescription opioid use (CPOU) increases the risk for developing an opioid use disorder.6
Surgical patients are often prescribed opioids to treat acute pain following major surgery.7-9 Prior studies have shown that a majority of patients undergoing common surgical procedures are prescribed opioids and that 6-7% of opioid-naïve patients develop CPOU.10-12 Patients with a history of CPOU are at particular risk for post-surgical CPOU. Prior evidence about opioid use in patients undergoing non-bariatric procedures may not generalize to bariatric surgery in general or to Veterans receiving bariatric surgery, because male patients with obesity have been shown to be less likely to fill opioid prescriptions than normal weight patients.13
The prior studies examining CPOU following bariatric surgery found one-year rates of CPOU initiation of 4%14, 6%15 and 6.3%.16 The only study examining long-term CPOU initiation found that 14.2% of bariatric patients were using opioids chronically 7 years after surgery,15 suggesting that some surgical patients develop chronic pain syndromes that may not resolve after bariatric surgery. These prior studies lacked non-surgical controls, so it is unclear how post-surgical CPOU differs between surgical patients and non-surgical controls. Further, these prior studies included few patients undergoing laparoscopic sleeve gastrectomy (LSG), which is now the most commonly performed procedure. To determine if bariatric surgery reduces the likelihood that patients require CPOU, it is necessary to compare them to a non-surgical control group. To address these evidence gaps, we examined time until CPOU incidence between surgical patients and matched controls without CPOU at baseline from a large, multi-site cohort with severe obesity. Among Veterans with CPOU at baseline, we also examined whether CPOU trends differed between surgical patients and non-surgical controls up to 5 years after surgery.
2. Materials and Methods
2.1. Study Design and Study Population
We conducted a retrospective cohort study of Veterans Affairs (VA) bariatric surgery patients and a matched non-surgical cohort of Veterans with severe obesity, which was approved by Institutional Review Boards of the Durham VAMC and Kaiser Permanente Washington Health Research Institute. We identified 10,653 Veterans with procedure codes for four bariatric surgical procedures (open Roux-en-Y gastric bypass (ORYGB), laparoscopic RYGB (LRYGB), LSG, and adjustable gastric band (AGB)) in any VA bariatric center nationwide between 10/1/2000—9/30/2016. After exclusions (eFigure 1), the final surgical cohort included 3,939 patients who underwent ORYGB, LRYGB or LSG.
Potential non-surgical controls for bariatric surgery patients were identified from VA electronic health record data using sequential stratification matching to accommodate the time-varying nature of surgical eligibility characteristics (e.g., body mass index (BMI)).17-19 The data were organized into a series of “n of 1” randomized trials, where the trial start date (index date) was the surgical patient’s date of surgery.20 For each surgical patient, we identified a pool of potential matches who had not had bariatric surgery and were similar to the surgical patient in characteristics that influence long-term outcomes and the likelihood of receiving bariatric surgery. The categorical variables on which potential non-surgical matches were required to match exactly included sex, white versus non-white race, current diabetes (yes/no), CPOU in the prior year, depression treatment in the prior year, unhealthy alcohol use (Alcohol Use Disorders Identification Test (AUDIT-C score) > 4 for men or > 3 for women) in the prior two years, and VA network. Surgical patients and potential matches must have been within 5 years of age and had BMIs within a radius that increased with the BMI of the surgical patient. For matching BMIs, surgical patients with higher BMIs were less likely to have a match with a close BMI, so we used bands that differed based on the surgical patient’s baseline BMI, with tighter bands at lower BMIs. BMIs of potential matches were required to be within 5 kg/m2 when surgical cases had a BMI between 35.0 and 45.0 kg/m2, within 10 kg/m2 when surgical cases have a BMI between 45.0 and 60.0 kg/m2, or within 15 kg/m2 when surgical cases have a BMI of 60.0 kg/m2 or greater. Patients who underwent surgery without a representative exact match were excluded (n=134, eFigure 1). After identifying potential controls, up to 10 matches with the closest BMI measurements were selected for each surgical patient.
Potential non-surgical controls often had many BMI measurements over the study period, so they could match to multiple surgical patients. The matching process was not contingent upon future information, so 469 control patients (representing 552 matches) subsequently received bariatric surgery and contributed person-time to the control group in models until they received bariatric surgery. Their follow-up time as a non-surgical control was censored at their date of surgery. The final control cohort included 31,971 individual patients representing 35,923 matches.
The final analytic cohort was stratified by evidence of CPOU at baseline and by surgical procedure type (ORYGB, LRYGB, LSG) because post-operative pain and complications requiring opioids may be different between open and laparoscopic RYGB. Also, prior pharmacokinetic work has found more rapid post-operative absorption of opioids following both subtypes of RYGB than following LSG.21 Using the definition from Raebel et al.,11 evidence of CPOU at baseline was defined by having 10 or more fills of Schedule II and non-Schedule II outpatient opioids or ≥120-day supply of Schedule II or non-Schedule II outpatient opioids filled in VA in the 12 months prior to and including index date. We matched 2,822 surgical patients without CPOU at baseline to 26,392 non-surgical controls without baseline CPOU. We also matched 1,117 surgical patients with baseline CPOU to 9,531 non-surgical controls with CPOU at baseline for evaluation of persistence of CPOU after bariatric surgery. Over 98% of these cohorts received VA care one year after the index date and 94% received VA care 5 years after index.
2.2. Opioid Use Outcomes
2.2.1. Persistent CPOU:
Among Veterans with CPOU at baseline, the primary outcome focused on CPOU persistence in the first 5 years after surgery, defined as having ≥10 VA outpatient fills or ≥120-day supply in each 12 month period. CPOU persistence was also analyzed up to 15 years following surgery. Annual binary indicators of CPOU persistence following surgery (1-365 days, 366-730 days, etc.) were defined for each year using the same criteria as for baseline CPOU.
2.2.2. Incident CPOU:
Among Veterans without CPOU at baseline, we examined whether the time until incident CPOU differed between surgical patients and non-surgical controls. Incidence was defined using the criteria for baseline CPOU and used the earlier of the date of 10th fill or the date of the fill providing 120th day of supply for determining days between bariatric patient’s hospital discharge and day of post-surgical incident CPOU. The criteria for incident CPOU had to be met within a rolling 12-month window. CPOU incidence was censored at the earliest of the following dates: death, 5 years post-surgery, the end of follow-up (7/31/2018) or when a bariatric procedure occurred (if previously a non-surgical control).
2.3. Statistical Analysis
Covariate balance between surgical patients and non-surgical controls was evaluated using standardized mean differences (SMD), with a value <0.2 indicating reasonable balance.22 For each of the 3 bariatric procedures, CPOU persistence up to five years after surgery in surgical and matched non-surgical patients with baseline CPOU was estimated by logistic regression fit using generalized estimating equations (GEEs). Based on minimizing Akaike Information Criterion (AIC), cubic time trends best fit the data and were included in interactions with an indicator of receipt of surgery. We also described unadjusted CPOU patterns after surgery to illustrate variation in the timing of CPOU discontinuation and conducted an analysis of CPOU persistence up to 15 years after surgery in a secondary analysis.
The association between bariatric surgery and CPOU incidence among Veterans withoutbaseline CPOU was examined descriptively using Kaplan-Meier estimators for each of the three procedure types (ORYGB, LRYGB, LSG). CPOU incidence by procedure type was compared between surgical and non-surgical patients without baseline CPOU using a Cox model analysis with a robust sandwich variance estimator to account for the possibility that the same non-surgical control matched to multiple surgical patients.23 Inspection of log(−log[survival]) curves and Schoenfeld residuals revealed that the proportional hazards assumption was satisfied for ORYGB and LRYGB procedures at all times, and for LSG when time was differentiated into the first year following surgery and the remainder of the follow-up period.24 Therefore, the estimated hazard ratio was constant across all follow-up years for ORYGB and LRYGB procedures, while differing for LSG by time period. All analyses were conducted in SAS 9.4 using a 0.05 a priori level of statistical significance. Some descriptive statistics were produced using R version 3.5.2.
3.0. Results
3.1. CPOU Persistence: Matched Cohorts with CPOU at Baseline
3.1.1. Characteristics of Patients with Baseline CPOU:
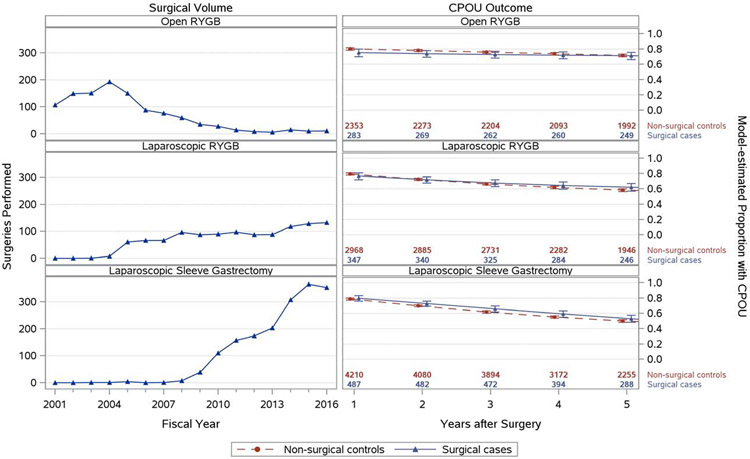
Marked temporal trends in volume of the 3 bariatric procedures were observed (Figure 1). ORYGB was the dominant VA procedure in 2001-2007, but LRYGB then became dominant until LSG became the most common procedure starting in 2010 (Figure 1).
Figure 1.
Trends in Bariatric Surgical Volume for All Patients and Model-estimated CPOU Persistence: Proportion of Patients with Post-operative CPOU up to 5 Years in Matched Bariatric Surgical Patients and Non-Surgical Patients with Chronic Prescription Opioid Use at Baseline, by Bariatric Procedure
Note: Figures on the left-hand side showing trends in surgical volume include patients with and without CPOU at baseline.
In patients undergoing ORYGB (n=283), mean follow-up was 10.8 years (median=11.9) and 9.7 years (median=10.4) for their matched non-surgical controls (n=2,353). Patients undergoing ORYGB and matched controls were similar in most observed characteristics (Table 1). Mean BMI was higher in ORYGB patients (48.4 kg/m2 vs. 46.4 kg/m2, standardized mean difference (SMD)=0.32) than matched controls.
Table 1.
CPOU Persistence Cohorts: Baseline Characteristics of Matched Cohorts with Chronic Prescription Opioid Use at Baseline
| Open RYGB | Laparoscopic RYGB | Laparoscopic Sleeve Gastrectomy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-surgical (n=2,353) |
Surgical (n=283) |
SMD | Non- surgical(n=2,968) |
Surgical (n=347) |
SMD | Non-surgical (n=4,210) |
Surgical (n=487) |
SMD | |
| VARIABLES USED IN MATCH | |||||||||
| Female, N (%) | 155 (6.6) † | 34 (12.0) | 0.19 | 430 (14.5) † | 71 (20.5) | 0.16 | 549 (13.0) † | 84 (17.2) | 0.12 |
| Male, N (%) | 2,198 (93.4) | 249 (88.0) | 2,538 (85.5) | 276 (79.5) | 3,661 (87.0) | 403 (82.8) | |||
| Age, Mean (SD) | 54.8 (6.6) † | 53.7 (7.3) | 0.16 | 55.1 (7.8) † | 53.8 (8.2) | 0.17 | 56.2 (8.5) † | 55.2 (8.8) | 0.11 |
| BMI, Mean (SD) | 46.4 (6.0) †† | 48.4 (6.6) | 0.32 | 43.6 (5.6) † | 44.7 (6.4) | 0.19 | 43.0 (5.3) †† | 44.2 (6.3) | 0.21 |
| Race, White, N (%) | 2,073 (88.1) | 245 (86.6) | 0.05 | 2,551 (86.0) | 288 (83.0) | 0.08 | 3,485 (82.8) | 388 (79.7) | 0.08 |
| Diagnosed Diabetes, N (%) | 1,693 (72.0) | 195 (68.9) | 0.07 | 1,946 (65.6) | 217 (62.5) | 0.06 | 2,661 (63.2) | 300 (61.6) | 0.03 |
| Unhealthy alcohol use at baseline, N (%) | 18 (0.8) | 2 (0.7) | 0.01 | 91 (3.1) | 14 (4.0) | 0.05 | 219 (5.2) | 37 (7.6) | 0.10 |
| Depression treatment at baseline, N (%) | 1,081 (45.9) | 135 (47.7) | 0.04 | 1,431 (48.2) | 172 (49.6) | 0.03 | 2,243 (53.3) | 265 (54.4) | 0.02 |
| Diagnosed alcohol use disorder at baseline, N (%) | 80 (3.4) | 14 (4.9) | 0.08 | 169 (5.7) | 28 (8.1) | 0.09 | 275 (6.5) | 40 (8.2) | 0.06 |
| Diagnosed opioid use disorder at baseline, N (%) | 10 (0.4) | 2 (0.7) | 0.04 | 10 (0.3) | 3 (0.9) | 0.07 | 5 (0.1) | 2 (0.4) | 0.06 |
| VARIABLES NOT USED IN MATCH | |||||||||
| NOSOS risk score in 2006-2015, Mean (SD) | 2.2 (1.3) | 2.1 (1.1) | 0.06 | 2.0 (1.2) | 2.0 (1.0) | 0.07 | 2.0 (1.3) | 2.0 (1.1) | 0.03 |
| DCG risk score in 2000-2005, Mean (SD) | 1.0 (0.9) | 0.9 (0.8) | 0.09 | 0.8 (0.7) | 0.6 (0.3) | 0.52 | 1.1 (0.8) | -- | NA |
| Average MME per day, Mean (SD) | 39.8 (60.6) | 38.9 (52.9) | 0.02 | 38.1 (55.8) | 39.0 (58.7) | 0.01 | 38.0 (55.4) | 33.8 (47.9) | 0.08 |
| Married, N (%) | 1,100 (46.7) | 157 (55.5) | 1,327 (44.7) | 182 (52.4) | 1,894 (45.0) | 255 (52.4) | |||
| Previously married, N (%) | 623 (26.5) †† | 95 (33.6) | 0.41 | 966 (32.5) †† | 116 (33.4) | 0.23 | 1,342 (31.9) †† | 170 (34.9) | 0.28 |
| Unmarried or unknown, N (%) | 630 (26.8) | 31 (11.0) | 675 (22.7) | 49 (14.1) | 974 (23.1) | 62 (12.7) | |||
| VA Outpatient Mental Health Visits, Mean (SD) | 7.0 (18.2) | 8.5 (17.3) | 0.08 | 9.2 (20.3) | 11.9 (21.2) | 0.13 | 10.8 (22.5) | 12.2 (20.5) | 0.07 |
| VA Outpatient Visits, Mean (SD) | 24.7 (18.6) | 26.3 (16.2) | 0.09 | 21.5 (17.9) †† | 25.8 (15.8) | 0.26 | 21.5 (17.2) †† | 26.0 (16.0) | 0.27 |
| Non-VA Outpatient Visits, Mean (SD) | 0.8 (6.2) | 1.8 (15.9) | 0.09 | 0.6 (3.2) | 0.8 (2.0) | 0.08 | 0.6 (2.7) | 0.6 (2.5) | 0.00 |
| VA reliance, Mean (SD) | 1.0 (0.1) | 1.0 (0.1) | 0.06 | 1.0 (0.1) | 1.0 (0.1) | 0.08 | 1.0 (0.1) | 1.0 (0.0) | 0.06 |
| Diagnosed Hypertension, N (%) | 1,861 (79.1) | 226 (79.9) | 0.02 | 2,295 (77.3) | 275 (79.3) | 0.05 | 3,229 (76.7) | 384 (78.9) | 0.05 |
| Diagnosed Asthma, N (%) | 206 (8.8) | 29 (10.2) | 0.05 | 277 (9.3) | 43 (12.4) | 0.10 | 410 (9.7) | 42 (8.6) | 0.04 |
| Diagnosed Fatty Liver, N (%) | 14 (0.6) | 3 (1.1) | 0.05 | 32 (1.1) | 7 (2.0) | 0.08 | 79 (1.9) | 14 (2.9) | 0.07 |
| Diagnosed PTSD, N (%) | 458 (19.5) | 63 (22.3) | 0.07 | 637 (21.5) | 88 (25.4) | 0.09 | 1,067 (25.3) | 132 (27.1) | 0.04 |
| Diagnosed Cannabis disorder, N (%) | 21 (0.9) | 4 (1.4) | 0.05 | 53 (1.8) | 3 (0.9) | 0.08 | 82 (1.9) | 4 (0.8) | 0.10 |
| Diagnosed Other drug disorder, N (%) | 40 (1.7) † | 11 (3.9) | 0.13 | 132 (4.4) | 10 (2.9) | 0.08 | 186 (4.4) | 17 (3.5) | 0.05 |
| Diagnosed Anxiety, N (%) | 306 (13.0) | 31 (11.0) | 0.06 | 539 (18.2) | 66 (19.0) | 0.02 | 909 (21.6) | 100 (20.5) | 0.03 |
| Diagnosed Bipolar, N (%) | 92 (3.9) | 12 (4.2) | 0.02 | 188 (6.3) | 25 (7.2) | 0.03 | 255 (6.1) | 31 (6.4) | 0.01 |
| Diagnosed Psychosis, N (%) | 28 (1.2) | 1 (0.4) | 0.10 | 34 (1.1) | 2 (0.6) | 0.06 | 77 (1.8) † | 2 (0.4) | 0.14 |
| Diagnosed Schizophrenia, N (%) | 60 (2.5) † | 3 (1.1) | 0.11 | 97 (3.3) † | 3 (0.9) | 0.17 | 108 (2.6) † | 4 (0.8) | 0.14 |
| Diagnosed Eating disorder, N (%) | cno00 | 4 (1.4) | 0.12 | cnoo\ | 6 (1.7) | 0.14 | 17 (0.4) † | 12 (2.5) | 0.17 |
| Diagnosed Tobacco Use disorder, N (%) | 404 (17.2) † | 33 (11.7) | 0.16 | 618 (20.8) †† | 36 (10.4) | 0.29 | 887 (21.1) † | 66 (13.6) | 0.20 |
| Diagnosed CAD, N (%) | 671 (28.5) † | 62 (21.9) | 0.15 | 575 (19.4) | 59 (17.0) | 0.06 | 841 (20.0) | 103 (21.1) | 0.03 |
| Diagnosed dyslipidemia, N (%) | 1,405 (59.7) | 156 (55.1) | 0.09 | 1,973 (66.5) | 232 (66.9) | 0.01 | 2,841 (67.5) | 328 (67.4) | 0.00 |
| Diagnosed GERD, N (%) | 554 (23.5) | 75 (26.5) | 0.07 | 756 (25.5) † | 117 (33.7) | 0.18 | 1,118 (26.6) | 143 (29.4) | 0.06 |
| Non-recent depression diagnosis, N (%) | 763 (32.4) | 100 (35.3) | 0.06 | 1,632 (55.0) | 190 (54.8) | 0.00 | 2,484 (59.0) | 291 (59.8) | 0.02 |
Abbreviations: SD, standard deviation; BMI, body mass index; PTSD, post-traumatic stress disorder; RYGB, Roux-en-Y gastric bypass; DCG, diagnosis cost group; MME, morphine milligram equivalent; SMD, standardized mean difference; GERD, gastroesophageal reflux disorder; CPOU, chronic prescription opioid use.
Note: Risk measures were not available the entire study period, so we relied on DCG from 2000-2005 and NOSOS from 2006-2015. Descriptive statistics for DCG score in LSG could not be calculated due to lack of prevalence.
standardized mean difference > 0.10;
standardized mean difference > 0.20; a value <0.2 indicates reasonable balance.
All diagnoses were identified from inpatient and outpatient visit records using ICD-9 and ICD-10 codes.
Standardized mean differences compare each covariate’s mean or proportion between the surgical cases and non-surgical controls in units of the pooled standard deviation.22
In patients undergoing LRYGB (n=347), mean follow-up was 6.5 years (median=6.1) and 5.9 years (median=5.4) for their matched non-surgical controls (n=2,968). LRYGB and match control patients were similar in all observed characteristics on which they were matched and most other covariates on which they were not matched (Table 1). LRYGB patients had a mean age of 53.8 years, a mean BMI of 44.7 kg/m2 and 79.5% were male, while their matches had mean age of 55.1 years, mean BMI of 43.6 kg/m2 and 85.5% were male.
In patients undergoing LSG (n=487), mean follow-up was 4.9 years (median=4.5) and 4.6 years (median=4.2) for their matched non-surgical controls (n=4,210). The cohorts were well matched, except for higher mean BMIs in surgical patients (44.2 kg/m2 vs. 43.0 kg/m2, SMD=0.21).
3.1.2. Unadjusted and Estimated Trends in CPOU Persistence:
In cohorts with baseline CPOU, trends in model-estimated post-surgical CPOU decreased with similar trends between surgical patients and non-surgical controls. Unadjusted CPOU persistence decreased from 75-80% of patients in all 3 surgical cohorts at 1 year, to 71% in ORYGB patients, 62% in LRYGB patients, and 53% in LSG patients at 5 years (eTable 2). Regression analyses confirmed that post-surgical CPOU trends were similar between surgical patients and matched controls (Figure 1)and both groups declined over time.
Within-person trends in CPOU persistence exhibited considerable heterogeneity (eFigure 2 but persistence in all observed years was the most common pattern for surgical patients and non-surgical controls. Transitioning between use and non-use was rare. In a sensitivity analysis extending follow-up to 15 years, patients undergoing LRYGB or ORYGB had higher rates of CPOU than matched controls 10-15 years after surgery (eFigure 3).
3.2. CPOU Incidence: Matched Cohorts without CPOU at Baseline
3.2.1. Characteristics of Patients without CPOU at Baseline:
In patients undergoing ORYGB (n=812), mean follow-up was 11.8 years (median=13.1) and 11.1 years (median=12.3) for their matched non-surgical controls (n=7,424). Surgical patients and matched controls were similar in nearly all observed characteristics (Table 2), except BMI was higher in surgical patients (48.7 kg/m2 vs. 46.9 kg/m2, SMD=0.24).
Table 2.
CPOU Incidence Cohorts: Baseline Characteristics of Matched Cohorts without Chronic Prescription Opioid Use at Baseline
| Open RYGB | Laparoscopic RYGB | Laparoscopic Sleeve Gastrectomy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-surgical (n=7,424) |
Surgical (n=812) |
SMD | Non-surgical (n=7,216) |
Surgical (n=775) |
SMD | Non-surgical (n=11,752) |
Surgical (n=1,235) |
SMD | |
| VARIABLES USED IN MATCH | |||||||||
| Female, N (%) | 1,486 (20.0) | 187 (23.0) | 0.07 | 1,907 (26.4) | 225 (29.0) | 0.06 | 3,027 (25.8) | 341 (27.6) | 0.04 |
| Male, N (%) | 5,938 (80.0) | 625 (77.0) | 5,309 (73.6) | 550 (71.0) | 8,725 (74.2) | 894 (72.4) | |||
| Age, Mean (SD) | 52.4 (8.3) | 51.8 (8.5) | 0.07 | 53.3 (9.9) | 52.6 (10.1) | 0.07 | 52.7 (10.4) | 52.1 (10.4) | 0.05 |
| BMI, Mean (SD) | 46.9 (6.6) †† | 48.7 (7.8) | 0.24 | 43.6 (5.6) † | 44.5 (6.6) | 0.15 | 42.8 (5.3) | 43.4 (5.7) | 0.10 |
| Race, White, N (%) | 5,924 (79.8) | 636 (78.3) | 0.04 | 5,702 (79.0) | 602 (77.7) | 0.03 | 8,247 (70.2) | 856 (69.3) | 0.02 |
| Diagnosed Diabetes, N (%) | 4,026 (54.2) | 437 (53.8) | 0.01 | 4,244 (58.8) | 454 (58.6) | 0.00 | 6,336 (53.9) | 667 (54.0) | 0.00 |
| Unhealthy alcohol use at baseline, N (%) | 50 (0.7) | 7 (0.9) | 0.02 | 356 (4.9) | 43 (5.5) | 0.03 | 797 (6.8) | 98 (7.9) | 0.04 |
| Depression treatment at baseline, N (%) | 2,726 (36.7) | 318 (39.2) | 0.05 | 2,854 (39.6) | 315 (40.6) | 0.02 | 4,676 (39.8) | 504 (40.8) | 0.02 |
| Diagnosed alcohol use disorder at baseline, N (%) | 261 (3.5) | 39 (4.8) | 0.06 | 314 (4.4) | 42 (5.4) | 0.05 | 534 (4.5) | 72 (5.8) | 0.06 |
| Diagnosed opioid use disorder at baseline, N (%) | 3 (0.0) | 1 (0.1) | 0.03 | 6 (0.1) | 4 (0.5) | 0.08 | 9 (0.1) | 6 (0.5) | 0.08 |
| VARIABLES NOT USED IN MATCH | |||||||||
| NOSOS risk score in 20062015, Mean (SD) | 1.5 (1.0)† | 1.6 (0.9) | 0.15 | 1.4 (1.0) † | 1.5 (0.9) | 0.12 | 1.4 (1.0) | 1.5 (0.8) | 0.08 |
| DCG risk score in 2000-2005, Mean (SD) | 0.6 (0.6) | 0.6 (0.5) | 0.02 | 0.7 (0.6)† | 0.6 (0.5) | 0.16 | 0.9 (1.5) †† | 0.4 (0.2) | 0.44 |
| Married, N (%) | 3,127 (42.1) | 425 (52.3) | 3,144 (43.6) | 402 (51.9) | 5,014 (42.7) | 607 (49.1) | |||
| Previously married, N (%) | 2,044 (27.5) †† | 246 (30.3) | 0.31 | 2,116 (29.3) †† | 260 (33.5) | 0.31 | 3,576 (30.4) †† | 415 (33.6) | 0.24 |
| Unmarried or unknown, N (%) | 2,253 (30.3) | 141 (17.4) | 1,955 (27.1) | 113 (14.6) | 3,161 (26.9) | 213 (17.2) | |||
| VA Outpatient Mental Health Visits, Mean (SD) | 6.1 (19.2) | 6.6 (13.6) | 0.03 | 8.1 (20.4) | 9.4 (19.1) | 0.06 | 9.2 (22.4) | 9.2 (16.4) | 0.00 |
| VA Outpatient Visits, Mean (SD) | 14.1 (15.4) †† | 17.9 (14.4) | 0.25 | 14.1 (15.0) †† | 18.9 (13.6) | 0.34 | 14.0 (14.8) †† | 18.6 (13.4) | 0.32 |
| Non-VA Outpatient Visits, Mean (SD) | 0.3 (4.6) | 0.3 (1.6) | 0.01 | 0.4 (6.8) | 0.4 (1.2) | 0.01 | 0.3 (2.4) | 0.4 (1.0) | 0.02 |
| VA reliance, Mean (SD) | 1.0 (0.1) | 1.0 (0.1) | 0.01 | 1.0 (0.1) | 1.0 (0.1) | 0.03 | 1.0 (0.1) | 1.0 (0.1) | 0.00 |
| Diagnosed Hypertension, N (%) | 4,821 (64.9) †† | 603 (74.3) | 0.20 | 4,688 (65.0) † | 556 (71.7) | 0.15 | 7,373 (62.7) † | 863 (69.9) | 0.15 |
| Diagnosed Asthma, N (%) | 502 (6.8) | 61 (7.5) | 0.03 | 578 (8.0) | 80 (10.3) | 0.08 | 1,015 (8.6) | 131 (10.6) | 0.07 |
| Diagnosed Fatty Liver, N (%) | 20 (0.3) † | 11 (1.4) | 0.12 | 71 (1.0) | 13 (1.7) | 0.06 | 162 (1.4) | 30 (2.4) | 0.08 |
| Diagnosed PTSD, N (%) | 1,040 (14.0) | 116 (14.3) | 0.01 | 1,339 (18.6) | 152 (19.6) | 0.03 | 2,392 (20.4) | 261 (21.1) | 0.02 |
| Diagnosed Cannabis disorder, N (%) | 40 (0.5) | 7 (0.9) | 0.04 | 71 (1.0) | 5 (0.6) | 0.04 | 146 (1.2) | 6 (0.5) | 0.08 |
| Diagnosed Other drug disorder, N (%) | 142 (1.9) | 22 (2.7) | 0.05 | 165 (2.3) | 9 (1.2) | 0.09 | 315 (2.7) | 29 (2.3) | 0.02 |
| Diagnosed Anxiety, N (%) | 605 (8.1) | 67 (8.3) | 0.00 | 954 (13.2) | 121 (15.6) | 0.07 | 1,790 (15.2) | 188 (15.2) | 0.00 |
| Diagnosed Bipolar, N (%) | 291 (3.9) | 44 (5.4) | 0.07 | 354 (4.9) | 43 (5.5) | 0.03 | 609 (5.2) | 60 (4.9) | 0.01 |
| Diagnosed Psychosis, N (%) | 88 (1.2) | 7 (0.9) | 0.03 | 77 (1.1) | 6 (0.8) | 0.03 | 166 (1.4) † | 4 (0.3) | 0.12 |
| Diagnosed Schizophrenia, N (%) | 312 (4.2) † | 18 (2.2) | 0.11 | 276 (3.8) | 7 (0.9) | 0.19 | 395 (3.4) † | 17 (1.4) | 0.13 |
| Diagnosed Eating disorder, N (%) | 39 (0.5) | 12 (1.5) | 0.10 | 23 (0.3) | 13 (1.7) | 0.14 | 45 (0.4) † | 17 (1.4) | 0.11 |
| Diagnosed Tobacco Use disorder, N (%) | 807 (10.9) † | 59 (7.3) | 0.13 | 865 (12.0) †† | 42 (5.4) | 0.23 | 1,446 (12.3) † | 80 (6.5) | 0.20 |
| Diagnosed CAD, N (%) | 1,158 (15.6) | 124 (15.3) | 0.01 | 1,004 (13.9) | 94 (12.1) | 0.05 | 1,427 (12.1) | 140 (11.3) | 0.03 |
| Diagnosed dyslipidemia, N (%) | 3,256 (43.9) | 382 (47.0) | 0.06 | 4,173 (57.8) † | 497 (64.1) | 0.13 | 6,499 (55.3) | 741 (60.0) | 0.10 |
| Diagnosed GERD, N (%) | 1,098 (14.8) † | 173 (21.3) | 0.17 | 1,195 (16.6) †† | 234 (30.2) | 0.33 | 2,192 (18.7) † | 335 (27.1) | 0.20 |
| Non-recent depression diagnosis, N (%) | 1,514 (20.4) † | 211 (26.0) | 0.13 | 2,764 (38.3) | 328 (42.3) | 0.08 | 5,023 (42.7) | 577 (46.7) | 0.08 |
Abbreviations: SD, standard deviation; BMI, body mass index; PTSD, post-traumatic stress disorder; RYGB, Roux-en-Y gastric bypass; DCG, diagnosis cost group; MME, morphine milligram equivalent; SMD, standardized mean difference; GERD, gastroesophageal reflux disorder; CPOU, chronic prescription opioid use.
Note: Risk measures were not available the entire study period, so we relied on DCG from 2000-2005 and NOSOS from 2006-2015
standardized mean difference > 0.10;
standardized mean difference > 0.20; a value <0.2 indicates reasonable balance.
All diagnoses were identified from inpatient and outpatient visit records using ICD-9 codes.
Standardized mean differences compare each covariate’s mean or proportion between the surgical cases and non-surgical controls in units of the pooled standard deviation.22
In patients undergoing LRYGB (n=775), mean follow-up was 6.9 years (median=6.6) and 6.4 years (median=5.9) for their matched non-surgical controls (n=7,216). Surgical patients undergoing LRYGB and matched controls were similar in all observed characteristics (Table 2). LRYGB patients had a mean age of 52.6 years, a mean BMI of 44.5 kg/m2 and 71.0% were male, while their controls had mean age of 53.3 years, mean BMI of 43.6 kg/m2 and 73.6% were male.
In patients undergoing LSG (n=1,235), mean follow-up was 4.5 years (median=4.0) and years (median=3.9) for their matched controls (n=11,752). Surgical patients and matched controls with baseline CPOU were well-matched on age (mean: 52.1 years for LSG, 52.7 years for controls), BMI (mean: 43.4 kg/m2 for LSG, 42.8 kg/m2 for controls) and sex (72.4% for LSG, 74.2% for controls). 20.4% in controls) and diagnosed anxiety (15.2% in both cases and controls) were less common.
3.2.2. Unadjusted and Adjusted Trends in CPOU Incidence:
The Kaplan-Meier estimated 1-year CPOU initiation rates were 13.3% for ORYGB patients and 10.5% for matched controls, 5-year rates were 37.6% for ORYGB patients and 31.8% for matched controls. One-year (9.0% for LRYGB, 8.4% for controls) and 5-year (28.0% for LRYGB, 22.2% for controls) CPOU initiation rates were higher for surgical patients than matched controls (Figure 2). One-year (6.3% for LSG, 6.6% for controls) and 5-year (16.9% for LSG, 15.7% for controls) CPOU initiation rates were similar for surgical patients and matched controls.
Figure 2.
Unadjusted CPOU Incidence: Kaplan-Meier Estimated Cumulative Incidence of Post-operative CPOU up to 5 Years in Matched Bariatric Surgical Patients and Non-Surgical Patients without Chronic Prescription Opioid Use at Baseline, by Bariatric Procedure
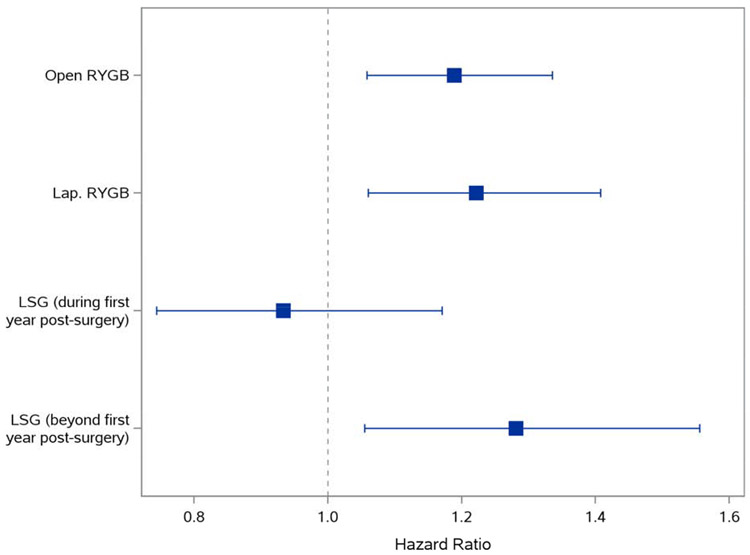
In Cox regression (Figure 3), ORYGB was associated with higher CPOU incidence compared to matched controls (hazard ratio (HR)=1.19, 95% confidence interval (CI): 1.06-1.34), as was LRYGB (HR=1.22; CI: 1.06-1.41). LSG was not associated with CPOU incidence in the first year of follow-up compared to matched controls (HR=0.93; 95% CI: 0.74-1.17) but was associated with higher incidence 1-to-5 years following surgery (HR=1.28; 95% CI: 1.05-1.56).
Figure 3.
Adjusted CPOU Incidence: Association and 95% Confidence Intervals of Bariatric Surgery and CPOU Incidence up to 5 Years after Surgery in Patients without Chronic Prescription Opioid Use at Baseline
Note: The proportional hazards assumption was not met for patients receiving LSG, hazard ratios were estimated separately for the first year of following and the remaining years 1-5. Proportional hazards assumption was met for patients undergoing RYGB procedures, so hazard ratios were estimated for the entire 5-year period for these cohorts. Hazard ratios and 95% confidence intervals are from Cox models in matched surgical patients and non-surgical controls without chronic prescription opioid use at baseline. HR=hazard ratio; RYGB=Roux-en-Y gastric bypass; LSG=laparoscopic sleeve gastrectomy.
4.0. Discussion
In this analysis of Veterans receiving bariatric surgery in 2001-2016, among the majority of patients who were not chronically on opioids at baseline, we found that there was increased incidence of CPOU at 5 years among those undergoing bariatric surgery compared with matched patients who did not. The hazard ratios of CPOU incidence were similar between the 3 procedure types (ORYGB, LRYGB or LSG), counter to our original hypothesis.21 Similarity in hazard ratios may be due to close management of opioids by VA providers.
Among patients with baseline CPOU, there was no association between bariatric surgery (ORYGB, LRYGB or LSG), compared to matched controls without bariatric surgery, and persistence of CPOU during the first 5 years of follow-up. This finding is reassuring, suggesting that bariatric surgery does not increase the risk of CPOU persistence. It was important to examine post-surgical CPOU trends separately by surgical procedure because post-operative pain, complications and absorption of some opioid formulations have been shown to differ. In fact, secondary analyses suggested that patients undergoing open or laparoscopic RYGB might have increased risk of new CPOU over the long term (10-15 years after surgery), which will require corroboration in future studies.
These results are notable because prior studies of post-surgical opioid use only examined surgical patients and included few laparoscopic sleeve gastrectomies that are now the most common type of procedure performed.11,14,15 The estimated one-year rates of CPOU incidence of 6% for LSG patients and 9% for LRYGB patients are slightly higher than one-year incidence rates found in prior studies with varying CPOU definitions14-16, which ranged from 4% to 6%. However, the 13% rate for ORYGB patients at one year is much higher prior estimates. The 7-year CPOU incidence of 14% from a large uncontrolled prospective study14 is similar to the 16.9% rate observed in the LSG patients in this cohort, but is much lower than the 28% rate in LRYGB patients and 38% rate in ORYGB patients.
These CPOU incidence rates observed in this Veteran cohort may differ from prior studies for several reasons. First, this age and sex composition of this cohort differs markedly (e.g., predominantly men in their 50s) from cohorts in prior studies (e.g., predominantly women in their 30s or 40s). Second, prior research suggests that Veterans are more likely to report pain than non-Veterans.6 Third, it is possible that opioid prescribing was less conservative in VA than non-VA settings before the VA implemented the Opioid Safety Initiative in 2013. These are the first long-term findings about CPOU in LSG patients and require further exploration in non-Veteran cohorts.
This study has several limitations that should be acknowledged. First, unobserved confounding in this retrospective cohort study was reduced via sequential stratification matching but residual confounding may persist after matching.25 Given sample size and statistical constraints related to the number of variables that could be accommodated in the matching process, we could not match on every available characteristic and BMI imbalances in two cohorts were statistically but not clinically significant. For example, VA collects race and ethnicity information in greater detail than white/non-white examined here, but we were unable to match on and examine opioid use in more granular detail. Due to the observational design, the estimated hazard ratios represent associations and not necessarily the causal effect of bariatric surgery on CPOU outcomes. Second, opioid use data was identified from VA claims only, so opioids obtained through Medicaid, Medicare or commercial coverage, or opioids obtained from other sources including illicitly, were not ascertained. Third, due to changes in bariatric procedures used over time, as well as changes in prescription opioid use for chronic pain, surgical procedure and the persistence and incidence of CPOU associated with each procedure, were confounded by calendar year. In addition, LSG only came into widespread use in VA after 2010, so secondary analyses of outcomes after 10-15 years were not possible for LSG, which in 2016 constituted the majority of bariatric surgeries performed in VA. Finally, it is possible that opioid use was initiated in either cohort due to receipt of non-bariatric procedures in the follow-up period.
These results underscore the risk of administering opioids to patients after surgery, which should be considered in preoperative discussions as part of the risk-benefit assessment of these surgical procedures in individual patients. Bariatric surgery has substantial and sustained effects on weight, obesity-related comorbidities, quality of life and long-term survival.26-31 Patients may be aware of these clinical benefits, but may not be aware of the risks, including risk of managing short-term pain in the perioperative period with opioids that could lead to CPOU for some patients. Post-operative CPOU might increase their risk for developing opioid dependence and subsequent complications including overdose-related hospitalizations and death.32-34 The analysis highlights the importance of mitigation efforts aimed at decreasing CPOU initiation after bariatric surgery. In recent years, the American Society for Metabolic and Bariatric Surgery have promoted Enhanced Recovery after Surgery (ERAS) protocols to reduce narcotic use, including preoperative discussion with patients about pain management and use of narcotics only for breakthrough pain.35,36
5.0. Conclusion
Bariatric surgery was associated with greater risk of incident CPOU in patients without baseline CPOU but was not associated with CPOU persistence in patients with baseline CPOU. Results underscore the ongoing substantial risk for CPOU associated with major surgical procedures, including bariatric surgery, and the need to mitigate this risk.
Supplementary Material
Highlight.
Short-term opioid analgesics are often prescribed to manage pain following bariatric surgery, which may develop into chronic prescription opioid use (CPOU) in opioid-naïve patients.
In a retrospective cohort study of 1,117 surgical patients and 9,531 non-surgical controls with baseline CPOU, post-surgical CPOU declined over time and these trends did not differ between surgical patients and non-surgical controls.
In 2,822 surgical patients and 26,392 non-surgical controls without CPOU at baseline, bariatric patients were more likely to initiate CPOU within 5 years following bariatric surgery.
Acknowledgments
Funding/Support: This research was funded by the NIH NIDA R01 DA040056. Dr. Maciejewski was also supported by a Research Career Scientist award from the Department of Veterans Affairs (RCS 10-391) and by the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham VA Health Care System. Effort on this study and manuscript were made possible by an American College of Surgeons George H.A. Clowes Career Development Award and a VA Career Development Award to Dr. Funk (CDA 015-060).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Dr. Maciejewski reports research grants for National Institutes of Health (NIH), Veterans Affairs Health Services Research and Development Service (VA HSR&D) and National Committee for Quality Assurance (NCQA) and ownership of Amgen stock due to his spouse’s employment. Dr. Arterburn reports research grants from NIH and PCORI outside of the submitted work. Dr. Bradley reports research grants from NIH, Agency for Healthcare Research and Quality (AHRQ) and NCQA outside of the submitted work. All other authors have no conflicts of interest to disclose.
Role of the Sponsor: The National Institute on Drug Abuse had no role in the design, conduct, collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs, the United States Government, Duke University, the Kaiser Permanente Washington Health Research Institute, or the University of Washington.
REFERENCES
- 1.Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physician. 2008;11(2 Suppl):S5–S62. [PubMed] [Google Scholar]
- 2.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011. ;377(9784):2226–2235. [DOI] [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Weisler RH, Patkar AA. A national epidemic of unintentional prescription opioid overdose deaths: how physicians can help control it. J Clin Psychiatry. 2011. ;72(5):589–592. [DOI] [PubMed] [Google Scholar]
- 4.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 5.Axeen S Trends in Opioid Use and Prescribing in Medicare, 2006-2012. Health Serv Res. 2018;53(5):3309–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jena AB, Goldman D, Karaca-Mandic P. Hospital Prescribing of Opioids to Medicare Beneficiaries. JAMA Intern Med. 2016;176(7):990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369–1376. [DOI] [PubMed] [Google Scholar]
- 12.Mudumbai SC, Oliva EM, Lewis ET, et al. Time-to-Cessation of Postoperative Opioids: A Population-Level Analysis of the Veterans Affairs Health Care System. Pain medicine. 2016;17(9):1732–1743. [DOI] [PubMed] [Google Scholar]
- 13.Barry D, Petry NM. Associations between body mass index and substance use disorders differ by gender: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addictive behaviors. 2009;34(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raebel MA, Newcomer SR, Bayliss EA, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf 2014;23(12):1247–1257. [DOI] [PubMed] [Google Scholar]
- 15.King WC, Chen JY, Belle SH, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith ME, Lee JS, Bonham A, et al. Effect of new persistent opioid use on physiologic and psychologic outcomes following bariatric surgery. Surg Endosc. 2019;33(8):2649–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy EH, Taylor JM, Schaubel DE, Williams S. The effect of salvage therapy on survival in a longitudinal study with treatment by indication. Stat Med. 010;29(25):2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YP, Propert KJ, Rosenbaum PR. Balanced Risk Set Matching. Journal of the American Statistical Association. 2001;96(455):870–882. [Google Scholar]
- 19.Lu B Propensity Score Matching with Time-Dependent Covariates. Biometrics. 2005;61(3):721–728. [DOI] [PubMed] [Google Scholar]
- 20.Mirza RD, Guyatt GH. A Randomized Clinical Trial of n-of-1 Trials-Tribulations of a Trial. JAMA Intern Med. 2018;178(10):1378–1379. [DOI] [PubMed] [Google Scholar]
- 21.Lloret-Linares C, Hirt D, Bardin C, et al. Effect of a Roux-en-Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin Pharmacokinet. 2014;53(10):919–930. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin DY, Wei LJ. The Robust Inference for the Proportional Hazards Model. Journal of the American Statistical Association. 1989;84(408):1074–1078. [Google Scholar]
- 24.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 25.Flum DR. Administrative data analyses in bariatric surgery--limits of the technique. Surg Obes Relat Dis. 2006;2(2):78–81. [DOI] [PubMed] [Google Scholar]
- 26.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The Effectiveness and Risks of Bariatric Surgery: An Updated Systematic Review and Meta-analysis, 2003-2012. JAMA Surg. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjostrom L Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. Journal of internal medicine. 2013;273(3):219–234. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int JObes (Lond). 2007;31(8):1248–1261. [DOI] [PubMed] [Google Scholar]
- 29.Kolotkin RL, Davidson LE, Crosby RD, Hunt SC, Adams TD. Six-year changes in health-related quality of life in gastric bypass patients versus obese comparison groups. Surg Obes Relat Dis. 2012;8(5):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. NEngl J Med. 2007;357(8):741–752. [DOI] [PubMed] [Google Scholar]
- 31.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. [DOI] [PubMed] [Google Scholar]
- 32.Carey CM, Jena AB, Barnett ML. Patterns of Potential Opioid Misuse and Subsequent Adverse Outcomes in Medicare, 2008 to 2012. Ann Intern Med. 2018;168(12):837–845. [DOI] [PubMed] [Google Scholar]
- 33.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. The Clinical journal of pain. 2008;24(6):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort Study of the Impact of High-Dose Opioid Analgesics on Overdose Mortality. Pain medicine. 2016;17(1):85–98. [DOI] [PubMed] [Google Scholar]
- 35.Brethauer SA, Grieco A, Fraker T, et al. Employing Enhanced Recovery Goals in Bariatric Surgery (ENERGY): a national quality improvement project using the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. Surg Obes Relat Dis. 2019;15(11):1977–1989. [DOI] [PubMed] [Google Scholar]
- 36.Thorell A, MacCormick AD, Awad S, et al. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40(9):2065–2083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.