Abstract
Background
Decontaminating and reusing filtering facepiece respirators (FFRs) for healthcare workers is a potential solution to address inadequate FFR supply during a global pandemic.
Aim
The objective of this review was to synthesize existing data on the effectiveness and safety of using chemical disinfectants to decontaminate N95 FFRs.
Methods
A systematic review was conducted on disinfectants to decontaminate N95 FFRs using Embase, Medline, Global Health, Google Scholar, WHO feed, and MedRxiv. Two reviewers independently determined study eligibility and extracted predefined data fields. Original research reporting on N95 FFR function, decontamination, safety, or FFR fit following decontamination with a disinfectant was included.
Findings and Conclusion
A single cycle of vaporized hydrogen peroxide (H2O2) successfully removes viral pathogens without affecting airflow resistance or fit, and maintains an initial filter penetration of <5%, with little change in FFR appearance. Residual hydrogen peroxide levels following decontamination were within safe limits. More than one decontamination cycle of vaporized H2O2 may be possible but further information is required on how multiple cycles would affect FFR fit in a real-world setting before the upper limit can be established. Although immersion in liquid H2O2 does not appear to adversely affect FFR function, there is no available data on its ability to remove infectious pathogens from FFRs or its impact on FFR fit. Sodium hypochlorite, ethanol, isopropyl alcohol, and ethylene oxide are not recommended due to safety concerns or negative effects on FFR function.
Keywords: Personal protective equipment, Decontamination, Respirator, N95, Disinfectant, COVID-19, Filtering facepiece respirator
Introduction
Shortages of personal protective equipment (PPE), including N95 filtering facepiece respirators (FFRs), are common during pandemics. The Centers for Disease Control and Prevention (CDC) recommend N95 FFRs, which filter 95% of airborne particles, as the preferred PPE when entering the room of a patient with suspected or confirmed coronavirus disease 2019 (COVID-19), and that an N95 FFR should be worn during all aerosol-generating procedures [1,2]. Unfortunately, during the ongoing COVID-19 pandemic, some hospitals and healthcare workers are faced with an inadequate supply of N95 FFRs while also dealing with an increase in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive patients [3,4]. Project N95, a COVID-19 initiative that transfers PPE from manufacturers and other disciplines to healthcare institutions in need, received requests for more than 253 million units of equipment from 6962 health centres globally since March 20th, 2020 [5]. Consequently, addressing the N95 FFR shortage has become a matter of increasing urgency as cases of COVID-19 continue to rise.
A potential solution to FFR shortages would be to decontaminate and reuse FFRs. However, prior to utilizing this strategy, it is essential to demonstrate that decontamination does not compromise structural integrity, fit, filter efficiency (aerosol penetration), and airflow resistance of the FFR [6]. Several decontamination methods have been previously investigated, including energetic (e.g. microwave irradiation, ultraviolet germicidal irradiation (UVGI) [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]]), gaseous (e.g. ethylene oxide, vaporized hydrogen peroxide [7,9,11,19,20]), and liquid (e.g. hydrogen peroxide, sodium hypochlorite [7,9,[11], [12], [13],18,19,21,22]) protocols. The CDC recently released crisis standards of care decontamination recommendations, with a brief summary of evidence for several of these approaches [23]. However, detailed information on the safety and efficacy of a variety of decontamination methods is essential to allow hospital decision-makers to evaluate the evidence and determine the feasibility of rapidly implementing different protocols at their institutions.
To help inform FFR-reuse policies and procedures, our team has conducted three systematic reviews to synthesize existing published data regarding the effectiveness of UVGI, heat and microwave irradiation, and chemical disinfectants for decontamination of National Institute for Occupational Safety and Health (NIOSH)-approved N95 FFRs [24,25]. This review focuses on chemical disinfectants, with the following objectives: (1) to assess the impact of each disinfectant method on FFR performance, with a specific focus on aerosol penetration and airflow resistance; (2) to determine the effectiveness of each disinfectant method at reducing viral or bacterial load; (3) to describe observations related to changes in physical traits following decontamination with a disinfectant; (4) to determine the impact of each disinfectant on FFR fit; and (5) to describe findings or observations related to potential health risks or irritation from residual disinfectant remaining on FFRs following decontamination.
Methods
The study protocol and objectives were established a priori and registered on PROSPERO on April 5th, 2020 (CRD42020178440), and reported here according to the PRISMA guidelines for systematic reviews (Appendix A) [26]. The protocol was also uploaded as a preprint to OSF on April 5th, 2020 (https://osf.io/8usx6/).
Eligibility criteria
Studies were eligible for inclusion in this systematic review if they satisfied all of the following criteria: (1) original publication or systematic review; (2) study reported on decontamination procedures for NIOSH-approved N95 (including surgical N95 (SN95)) FFRs or their components; (3) at least one of the decontamination procedures evaluated used one of the following chemical disinfectants: sodium hypochlorite; liquid hydrogen peroxide; vaporized hydrogen peroxide, hydrogen peroxide gas plasma, or ionized hydrogen peroxide; ethanol or isopropyl alcohol; (4) the study reported on at least one of the following outcomes of interest: (i) impact of the disinfectant on FFR performance, with a specific focus on aerosol penetration and airflow resistance (drop in pressure); (ii) effectiveness of the disinfectant at removing viral or bacterial load; (iii) observations related to changes in physical traits following decontamination with a disinfectant; (iv) impact of each disinfectant on FFR fit; or (v) findings or observations related to user safety or irritation. Only studies published in English or French were included. Studies published prior to 1972, the year that the N95 FFR was invented, were excluded [27]. Peer-reviewed literature and non-peer-reviewed preprints were included. Editorials, narrative reviews, book chapters, patents, and non-peer-reviewed commissioned reports were excluded.
Search and selection
The following databases were searched by two health sciences librarians (L.S. and M.S.) during the electronic component of the systematic review: Medline and Medline in Process via OVID (1946 to March 31, 2020), Embase Classic + Embase via OVID (1947 to March 31st, 2020), and Global Health via CAB Direct (1913 to March 31st, 2020). A search strategy was developed in Medline, reviewed by a second librarian, and then translated into the other databases, as appropriate (Appendix B). All databases were searched from January 1st, 1972 to March 31st, 2020. Additional restrictions were English or French language and a publication date starting from 1972, the year N95 FFRs were invented [27].
Two journals were also hand-searched, as they were particularly relevant to the review but are not indexed in any of the databases searched: Journal of the International Society for Respiratory Protection, and Journal of Engineered Fibers and Fabrics. A search of Google Scholar (March 31st, 2020) through Publish or Perish was screened until 50 consecutive apparently irrelevant records were found. Results to that point were saved as an RIS file and edited to remove patents, reports, and books. The World Health Organization (WHO) database on COVID-19 was downloaded and searched within Reference Manager. Disaster Lit: Database for Disaster Medicine and Public Health, MedRxiv and OSF Preprints were searched March 31st, 2020, for the term ‘N95’ and records pertaining to decontamination were selected and downloaded.
Citation screening and data extraction
Citations were uploaded to InsightScope (www.insightscope.ca) for title and abstract screening and full text review. At both title/abstract and full text screening levels, citations were assessed in duplicate and independently. Before citation screening was initiated, each reviewer was asked to read the published protocol for this systematic review to familiarize themselves with the review objectives and citation screening process. Next, to ensure that the reviewers understood the citation eligibility criteria, the study lead (K.O.) created a test set of 30 citations. The test set included five true positives (i.e. citations that met the eligibility criteria to be included in this systematic review) and 25 true negatives (i.e. citations that did not meet eligibility criteria to be included in this systematic review) [28]. Each reviewer (J.G., R.N.) was then required to complete the test set by assessing the same 30 citations. Reviewers had to achieve a sensitivity in excess of 80% on the test set before they were given access to title/abstract screening. At both title/abstract and full text review, records were removed only if both reviewers agreed to exclude. Cases with screening conflicts were resolved by review by the study lead (K.O.). At the completion of full text review, the study lead (K.O.) reviewed the eligible citations to identify potential duplicates and confirm eligibility. The reference lists of included studies were reviewed to identify any potentially relevant studies not included in the screening set.
A data extraction tool was developed in REDCap by the study lead (K.O.) and piloted on five eligible studies [29,30]. Eligible studies were divided equally among the reviewers for duplicate, independent data extraction into REDCap and Microsoft Excel, followed by conflict resolution by the study lead. When necessary, data was extracted from figures using SourceForge Plot Digitizer (http://plotdigitizer.sourceforge.net/).
Outcome data is reported for NIOSH-approved N95 (particulate, including surgical) FFRs or their components only. Other respirator types (e.g. R- or P-filter type) were not included in the analysis. FFR ‘component’ was defined as a piece of an N95 FFR that had been cut out with all layers still intact. Intervention arms described by authors as vaporized hydrogen peroxide, hydrogen peroxide gas plasma, and ionized hydrogen peroxide were analysed together and, for convenience, are collectively referred to as vaporized hydrogen peroxide for the remainder of this review.
Risk of bias
Risk of bias was assessed for each study by outcome using a predetermined evaluation matrix which included evaluation based on study design, methodological consistency, population heterogeneity, sampling bias, outcome evaluation, and selective reporting (Appendix C).
Study analysis and statistics
All statistical analyses were performed using the R statistical programming language [31]. Data was meta-analysed using a random effects model with the R package ‘metafor’ [32]. Random effects meta-analyses were employed to present either the pooled absolute value pre/post chemical disinfectant intervention or relative change (from control or no treatment arm).
For both the aerosol penetration and airflow resistance outcomes, the data was presented as an absolute value. For the germicidal outcome, the data was presented as a relative change in viral load on a logarithmic scale. The majority of studies evaluated the germicidal effect of chemical disinfectants using viruses; to improve comparability, studies on bacteria or bacteriophage decontamination were removed from the germicidal analysis and are instead presented descriptively. Random effects meta-analysis was used to calculate the effect size for each type of chemical disinfectant. Heterogeneity was assessed by calculating an I 2 statistic from a fixed effect model. The standard deviation for each control and treatment arm within a chemical disinfectant class was calculated from the pooled absolute values post intervention. The sample size represents the total number of replicates for all N95 FFR models included.
Results
Identification of eligible studies
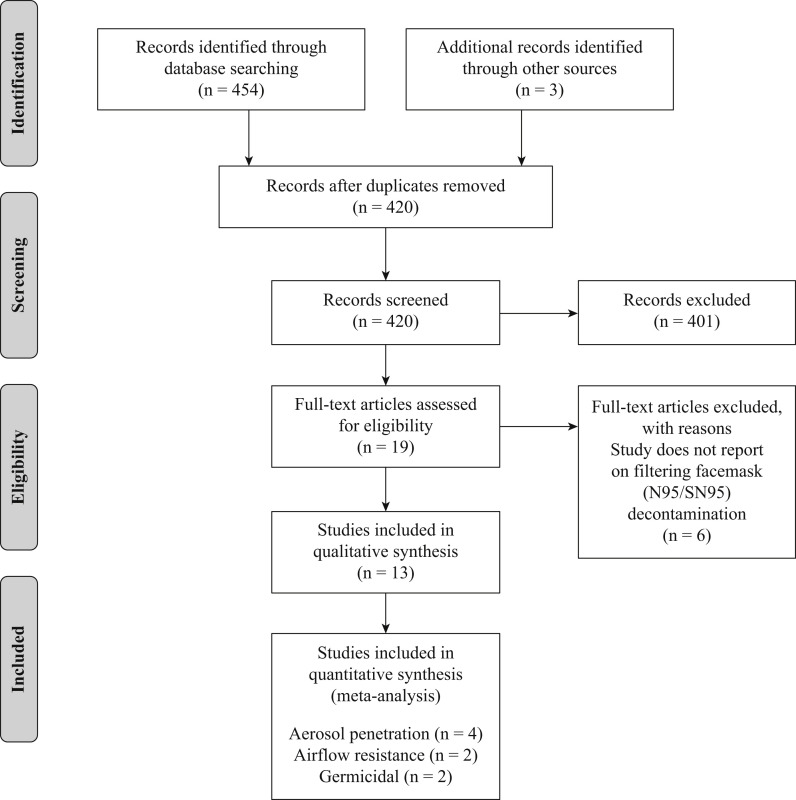
A total of 454 records were identified through the initial database search. After removing duplicate citations in Endnote, there were 417 citations remaining. Both reviewers correctly identified all true positives and true negatives in the test set. Title and abstract screening excluded 401, with the review team achieving a kappa of 0.9. At the full text level, the reviewers excluded six of the records, with a kappa of 0.86. An additional three preprint articles were identified following the initial search, screened in duplicate and included in the analysis, resulting in a total of 13 eligible articles [7,9,[11], [12], [13],[18], [19], [20], [21], [22],[33], [34], [35]]. An overview of the search process, results and reason for exclusions are shown in the PRISMA diagram (Figure 1 ).
Figure 1.
PRISMA diagram. Overview of the citation screening process, results, and reason for exclusions.
Study demographics
Nine studies originated from the USA, three from East Asia, and the remaining one originated from Canada. The studies included a total of 58 intervention arms, including sodium hypochlorite (N = 21), liquid hydrogen peroxide (liquid H2O2, N = 4), vaporized hydrogen peroxide (vaporized H2O2, N = 12), ethanol (N = 9), isopropyl alcohol (N = 3), ethylene oxide (EtO, N = 6), or other (N = 3). Thirty-five N95 models were evaluated across the 13 studies. The most common models studied were the 3M 1870 (N = 7), 3M 1860 (N = 6), and the 3M 8210 (N = 5). The number of articles evaluating the main study outcomes were aerosol penetration (N = 5), airflow resistance (N = 3), germicidal activity (N = 8), fit (N = 2), changes in physical traits (N = 6), and safety/irritation (N = 3). A summary of the intervention arms, outcomes, and number of N95 FFR models evaluated is presented in Table I . The majority of studies reported on outcomes following a single cycle of decontamination. Bergman et al. evaluated aerosol penetration and airflow resistance following three decontamination cycles, and Kenney et al. reported on changes in physical traits following five cycles [7,20]. Fischer et al. and Kumar et al. reported on FFR fit following three decontamination cycles and 1, 3, 5, 10 and 20 cycles, respectively [35,34]. Three studies were published as preprints [20,34,35], the remaining were peer-reviewed publications.
Table I.
Characteristics of studies included in this systematic review of decontaminating N95 filtering facepiece respirators using disinfectants
| Study | Intervention arms |
Outcomes evaluated |
No. of N95 models | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaOCl or ClO− | Liquid H2O2 | Vaporized H2O2 | EtOH | IP | EtO | Othera | Aerosol penetration | Airflow resistance | Germicidal activity | Fit | Physical traits | Potential health risks | ||
| Bergman et al. (2010) [7] | 1 | 1 | 2 | 1 | Yes | Yes | No | No | Yes | No | 6 | |||
| Cheng et al. (2020) [33] | 1 | No | No | Yes | No | No | Yes | 2 | ||||||
| Fischer et al. (2020) [34] | 1 | 1 | No | No | Yes | Yes | No | No | 2 | |||||
| Fisher et al. (2009) [12] | 4 | No | No | Yes | No | No | No | 1 | ||||||
| Heimbuch et al. (2014) [13] | 1 | 1 | Yes | No | Yes | No | No | No | 3 | |||||
| Kenney et al. (2020) [20] | 2 | No | No | Yes | No | Yes | No | 1 | ||||||
| Kumar et al. (2020) [35] | 2 | 1 | No | No | Yes | Yes | No | No | 4 | |||||
| Lin et al. (2017) [21] | 1 | 1 | 1 | Yes | Yes | No | No | Yes | No | 1 | ||||
| Lin et al. (2018) [22] | 3 | 7 | No | No | Yes | No | No | No | 1 | |||||
| Salter et al. (2010) [19] | 1 | 1 | 1 | 1 | 2 | No | No | No | No | Yes | Yes | 6 | ||
| Viscusi et al. (2007) [11] | 2 | 2 | 2 | 2 | 2 | Yes | No | No | No | Yes | No | 1 | ||
| Viscusi et al. (2009) [9] | 1 | 1 | 1 | Yes | Yes | No | No | Yes | Yes | 6 | ||||
| Vo et al. (2009) [18] | 7 | No | No | Yes | No | No | No | 1 | ||||||
NaOCL, sodium hypochlorite; ClO–, hypochlorite; H2O2, hydrogen peroxide; EtOH, ethanol; IP, isopropyl alcohol; EtO, ethylene oxide.
Other includes benzalkonium chloride; mixed oxidants (10% oxone, 6% sodium chloride, 5% sodium bicarbonate); and dimethyl dioxirane (10% oxone, 10% acetone, 5% sodium bicarbonate).
Aerosol penetration
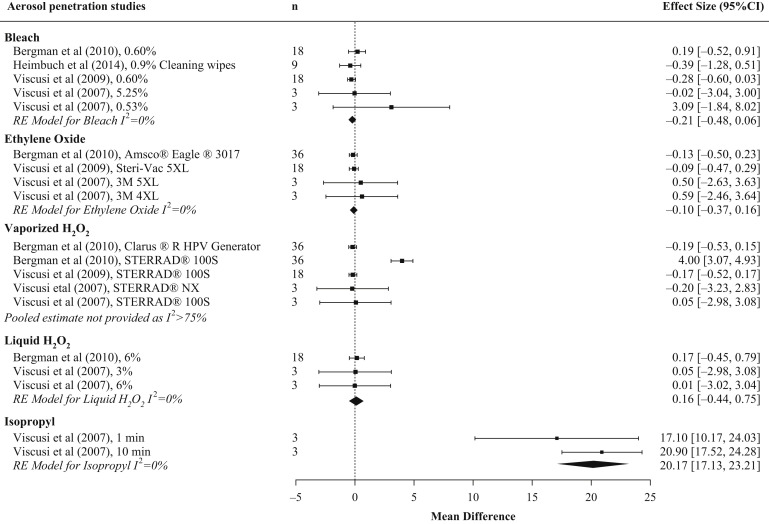
Five studies were identified that evaluated aerosol penetration following decontamination with a disinfectant, including intervention arms that evaluated sodium hypochlorite (N = 6), liquid H2O2 (N = 3), vaporized H2O2 (N = 5), ethanol (N = 1), isopropyl alcohol (N = 3), and EtO (N = 3) (Table II ) [7,9,11,13,21]. The majority of studies measured initial aerosol penetration. This was done using a continuous airflow of 85 L/min and an aerosol of sodium chloride with a count median diameter of 0.075 ± 0.020 μm, geometric size deviation of <1.86 and mass median aerodynamic diameter ∼300 nm in accordance with 42 CFR 84.174 for NIOSH certification testing [36]. The exception was Lin et al. who used a challenge aerosol with a count median diameter of 101 ± 10 and a geometric size deviation of 2.01 ± 0.08 [21]. Analysis was limited to studies that used a testing aerosol that adhered to NIOSH certification testing standards. Some of the included studies, such as Viscusi et al. and Bergman et al., evaluated different concentrations of sodium hypochlorite or different methodologies for vaporized H2O2 and therefore had more than one arm included [7,11]. We choose not to provide a pooled estimate of aerosol penetration between chemical sterilization, as this metric would not be relevant for decision-makers. Instead, the mean difference from the random effects model and the I 2 from a fixed effects model were calculated for each decontamination type (Figure 2 ). Studies on sodium hypochlorite showed no change in aerosol penetration post sterilization, with low study heterogeneity. Studies on ethylene oxide and liquid H2O2 yielded consistent findings of no change in aerosol penetration post sterilization, with low between-study heterogeneity. Studies on vaporized H2O2 showed no change in aerosol penetration post sterilization, with large between-study heterogeneity driven by three decontamination cycles using the Sterrad® 100S H2O2 Gas Plasma Sterilizer in Bergman et al. [7]. Studies on isopropyl provided consistent findings that filtration efficiency post sterilization was impaired, with aerosol penetration exceeding 5% (pooled aerosol penetration estimate 20.17% (95% CI: 17.13, 23.21). A summary of the aerosol penetration results for each chemical disinfectant type is shown in Table III .
Table II.
Disinfectants and N95 filtering facepiece respirators used to evaluate the effects of decontamination using chemical disinfectants on FFR aerosol penetration and airflow resistance
| Study | Intervention arm | Description | Function outcome(s) evaluated | N95 FFR modelsa |
|---|---|---|---|---|
| Bergman et al. (2010) [7] | Sodium hypochlorite | 0.6%, 30 min submersion | Aerosol penetration Airflow resistance |
3M 8210 3M 8000 Moldex 2201 KC PFR95-174 3M 1870 3M 1860s |
| Liquid H2O2 | 6%, 30 min submersion | |||
| Vaporized H2O2 | Arm 1: Four portable modules: the Clarus® R HPV generator (utilizing 30% H2O2), the Clarus R20 aeration unit, an instrumentation module and a control computer. The Clarus R was placed in a room (64 m3). The HPV concentration, temperature and relative humidity within the room were measured by the instrumentation module and monitored by a control computer situated outside the room. Room concentration: 8 g/m3, 15 min dwell, 125 min total cycle time. Following HPV exposure, the Clarus R20 aeration unit was run overnight inside the room to catalytically convert the HPV into oxygen and water vapour. Arm 2: Sterrad® 100S H2O2 Gas Plasma Sterilizer, 59% H2O2, cycle time ∼55 min (short cycle); 45–50°C. |
|||
| EtO | Amsco® Eagle® 3017 100% EtO Sterilizer/Aerator on HI-TEMP setting (55°C, 736.4 mg/L). EtO exposure for 1 h followed by 12 h aeration. | |||
| Heimbuch et al. (2014) [13] | Hypochlorite | 0.9% hypochlorite wipes, wiped 3 times, left to dry 15 min | Aerosol penetration | 3M 1860 3M 1870 KC PFR |
| Other | Benzalkonium chloride wipes, wiped 3 times, left to dry 15 min | |||
| Lin et al. (2017) [21] | Sodium hypochlorite | 0.5%, 10 min submersion | Aerosol penetration Airflow resistanceb |
3M 8210 |
| Isopropyl alcohol | 100%, 10 min submersion | |||
| Ethanol | 70%, 10 min submersion | |||
| Viscusi et al. (2007) [11] | Sodium hypochlorite | Arm 1: 5.25%, 30 min submersion Arm 2: 0.525%, 30 min submersion |
Aerosol penetration | 3M 8000 |
| Liquid H2O2 | Arm 1: 3%, 30 min submersion Arm 2: 6%, 30 min submersion |
|||
| Vaporized H2O2 | Arm 1: Sterrad NX Standard cycle, 28 min cycle Arm 2: Sterrad 100S Standard cycle, 55 min cycle |
|||
| EtO | Arm 1: EtO 3M Steri-Vac 4XL, warm cycle of 55°C and 883 mg/L ethylene oxide gas Arm 2:EtO 3M Steri-Vac 5XL, warm cycle of 55°C and 725 mg/L ethylene oxide gas EtO exposure for 1 h followed by 4 h aeration |
|||
| Isopropyl alcohol | Arm 1: 70%, 1 min submersion Arm 2: 70%, 10 min submersion |
|||
| Viscusi et al. (2009) [9] | Sodium hypochlorite | 0.6%, 30 min submersion. Dried overnight | Aerosol penetration Airflow resistance |
3M 8210 3M 8000 Moldex 2201 KC PFR95-174 3M 1870 3M 1860s |
| Vaporized H2O2 | Sterrad 100S H2O2 Gas Plasma Sterilizer, 55 min cycle | |||
| EtO | Steri-Vac 5XL sterilizer. Single warm cycle (55°C, 725 mg/L 100% EtO Gas). EtO exposure for 1 h followed by 4 h of aeration. |
Figure 2.
Pooled results of studies assessing aerosol penetration by chemical disinfectant type. Forest plot of initial aerosol penetration in N95 filtering facepiece respirators (FFR) across chemical disinfectant types. Where aerosol penetration overlaps 0, evidence suggests that this safety metric is not impaired by decontamination. FFR type examined varied by study. n represents the total number of replicates for all N95 FFR models included in the experimental arm. Results are only shown for studies that used a testing aerosol adhering to NIOSH certification testing procedures. CI, confidence interval.
Table III.
Summary of results following N95 FFR decontamination for each outcome of interest and chemical disinfectant type
| Disinfectant | Aerosol penetration | Airflow resistance | Viral/bacterial load | Fit | Physical traits | Potential health risks |
|---|---|---|---|---|---|---|
| Sodium hypochlorite | No change in aerosol penetration, aerosol penetration <5% maintained (N = 4) | No change in airflow resistance, NIOSH standards maintained (N = 3) | Log10 reduction in viral levels of 0.66 ± 0.47 to 4.37 ± 0.4 depending on concentration used (N = 2) | Not assessed | Tarnished metallic nosepieces (N = 4) | Potential for low-level chlorine exposure when FFR is rehydrated during respiration (N = 1) |
| Aerosol penetration exceeded 5% for particles >63 nm (N = 1)a | 0% relative survival of bacteria (N = 1) | Oxidized staples (N = 2) | ||||
| Mean reduction in bacteria levels >99% (N = 1) | Yellowing of nose pads (N = 1) | |||||
| Bleeding of lettering (N = 1) | ||||||
| Stiffening of filter media and elastic straps (N = 1) | ||||||
| Dissolved nose pad (50%) (N = 1) | ||||||
| Bleach odour following treatment (N = 3) | ||||||
| Liquid H2O2 | No change in aerosol penetration, aerosol penetration <5% maintained (N = 2) | No change in airflow resistance, NIOSH standards maintained (N = 1) | Not assessed | Not assessed | Oxidized staples (N = 1) | Did not deposit significant quantities of toxic residues on the FFRs (N = 1) |
| Slight fading of the label lettering (N = 1) | ||||||
| No changes in odour (N = 1) | ||||||
| Vaporized H2O2 | No change in aerosol penetration, aerosol penetration <5% maintained following one (N = 2) and three decontamination cycles (N = 1) | No change in airflow resistance, NIOSH standards maintained (N = 2) | No viable virus, or virus or bacteriophage below detectable assay limit (N = 4) | Fit factor >100 achieved following 1, 3, 5, 10 cycles with the VHP ARD system, but only following one cycle using the Sterrad 100NX (N = 1)b | Slight tarnishing of metallic nosebands (N = 2) | Did not deposit significant quantities of toxic residues on the FFRs (N = 2) |
| Aerosol penetration exceeded 5% for four out of six FFR following three decontamination cycles (N = 1) | Fit factor >100 achieved following 3 × 2 h wear + decontamination cycles (N = 1) | No changes in physical appearance (N = 3) | ||||
| No changes in odour (N = 3) | ||||||
| Ethanol | Aerosol penetration post sterilization exceeded 5% for particles >63 nm (N = 1)a | NIOSH standards maintained post sterilization (N = 1) | Relative bacterial survival rates of 68–89% depending on ethanol concentration (N = 1) | Fit factor >100 achieved for six replicate FFRs following one decontamination cycle, but not all six replicates achieved a fit factor >100 following two and three decontamination cycles (N = 1) | No changes in physical appearance (N =1) | Not assessed |
| Relative bacterial survival rates of 20–33% depending on ethanol concentration 24 h post sterilization (N = 1) | ||||||
| Viral levels below detectable assay limit (N = 1) | ||||||
| Isopropyl alcohol | Aerosol penetration exceeded 5% for particle penetration (N = 1) | Not assessed | Not assessed | Not assessed | Fading of ink strap (N = 1) | Not assessed |
| Aerosol penetration post sterilization exceeded 5% for particles >76 nm (N = 1)a | No changes in physical appearance (N = 1) | |||||
| EtO | No change in aerosol penetration post sterilization, aerosol penetration <5% maintained (N = 3) | No change in airflow resistance post sterilization, NIOSH standards maintained (N = 2) | No viable virus (N = 1) | Fit factor >100 achieved for four FFR models following one and three decontamination cycles (N = 1)b | No changes in physical appearance (N = 4) | Presence of ethylene glycol monoacetate on FFR straps, safety currently unclear (N = 1) |
| No changes in odour (N = 3) |
FFR, filtering facepiece respirator; H2O2, hydrogen peroxide; EtO, ethylene oxide.
N refers to number of studies.
Lin et al. used an challenge aerosol with a count median diameter of 101 ± 10 and a geometric size deviation of 2.01 ± 0.08, and evaluated penetration of particle sizes from 14.6 to 594 nm.
Based on normal breathing and deep breathing exercises only.
Airflow resistance
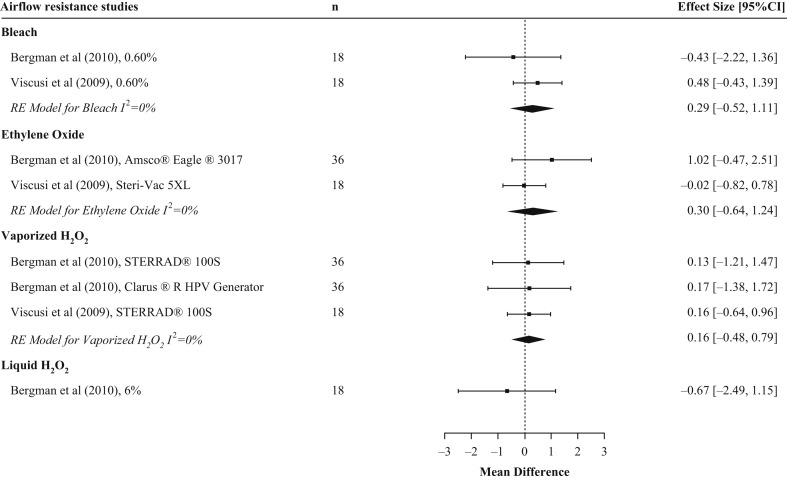
Three studies reported on airflow resistance, including intervention arms that evaluated sodium hypochlorite (N = 3), liquid H2O2 (N = 1), vaporized H2O2 (N = 3), ethanol (N = 1), and EtO (N = 2) (Table II) [7,9,21]. The mean difference for sodium hypochlorite was 0.29 mmH2O (95% CI: –0.52, 1.11) with an I 2 of ∼0%. It was not possible to include the results from Lin et al. in the analysis for sodium hypochlorite as there was no standard deviation (SD) for the treatment arm [21]. The mean difference for EtO was 0.30 mmH2O (95% CI: –0.64, 1.24) with an I 2 of 0%. The mean difference for vaporized H2O2 was 0.16 mmH2O (95% CI: –0.48, 0.79), with an I 2 of 0%. The estimate for liquid H2O2 (N = 1) was –0.67 mmH2O (95% CI: –2.49, 1.15) (Figure 3 ). The study by Lin et al. evaluated ethanol but did not supply SD for the treatment and therefore could not be evaluated [21]. In Lin et al.’s study, FFRs treated with ethanol maintained NIOSH airflow resistance standards (9.69 mmH2O). For airflow resistance, all meta-analyses showed consistent findings of no change post decontamination, and all FFRs maintained NIOSH certification standards irrespective of the chemical disinfectant type (sodium hypochlorite, EtO, liquid H2O2, and vaporized H2O2). A summary of the airflow resistance results for each disinfectant type is shown in Table III.
Figure 3.
Pooled results of studies assessing airflow resistance by chemical disinfectant type. Forest plot of airflow resistance in N95 filtering facepiece respirators (FFR) across chemical disinfectant types. FFR type examined varied by study. n represents the total number of replicates for all N95 FFR models included in the experimental arm.
Germicidal
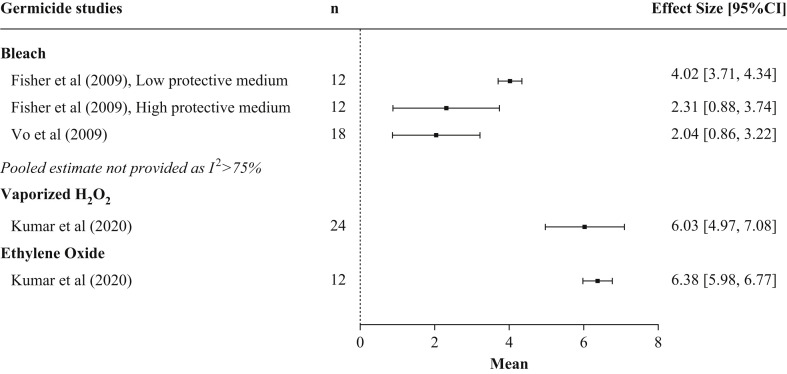
Eight studies evaluated the germicidal impact of one or more disinfectants, including two studies on bacteria [13,22], five studies on viruses [12,18,[33], [34], [35]], and one study on bacteriophages [20]. Intervention arms included in the eight studies were sodium hypochlorite (N = 15), vaporized H2O2 (N = 6), ethanol (N = 8), and EtO (N = 1) (Table IV ). No studies evaluated germicidal removal following decontamination with liquid H2O2 or isopropyl alcohol. Whereas all of the studies showed a reduction in viral load post decontamination, there were large differences in the magnitude of the effect, ranging from log10 2.04 to 6.38 (Figure 4 ). N95 FFRs that were inoculated with SARS-CoV-2 virus, then sprayed with 70% ethanol until saturation, showed viral levels below the limit of detection of the assay (<100.5 TCID50/mL) at 5, 10, 30, and 60 min post sterilization [34]. However, ethanol was not as effective at eradicating bacteria. Immersion in an ethanol solution for 10 min at concentrations of 50%, 70%, 80%, and 95% resulted in relative survival rates of Bacillus subtilis of 89 ± 6%, 72.01 ± 10.69%, 68 ± 3%, and 73 ± 7% respectively. By 24 h post sterilization, survival rates had declined to 33 ± 8%, 22.28 ± 7.88%, 20 ± 2%, and 26 ± 7% [21].
Table IV.
Disinfectants and N95 filtering facepiece respirators used to evaluate the effects of decontamination using disinfectants on reductions in infectious pathogens
| Study | Intervention arm | Intervention arm description | Infectious pathogen | Conditions | N95 FFR modelsa |
|---|---|---|---|---|---|
| Cheng et al. (2020) [33] | Vaporized H2O2 | SteraMist Surface Unit | Influenza A virus subtype H1N1 | 1,000,000 TCID50/mL viral concentration 100,000 TCID50/mL viral concentration 10,000 TCID50/mL viral concentration |
3M 1860 3M 1870 |
| Fischer et al. (2020) [34] | Ethanol | 70%, sprayed until saturated | SARS-CoV-2 | – | AO Safety N9504C |
| Vaporized H2O2 | Panasonic 146 MCO-19AIC-PT | ||||
| Fisher et al. (2009) [12] | Sodium hypochlorite | Arm 1: 0.0006%, submerged 10 min, left to dry 2 min Arm 2: 0.006%, submerged 10 min, left to dry 2 min Arm 3: 0.06%, submerged 10 min, left to dry 2 min Arm 4: 0.60%, submerged 10 min, left to dry 2 min |
MS2 | Low Protective Factor Aerosol Medium (1% ATCC 271) High Protective Factor aerosol medium (100% ATCC 271) |
Cardinal Healthb |
| Heimbuch et al. (2014) [13] | Hypochlorite | 0.9% hypochlorite wipes, wiped three times, left to dry 15 min | Staphylococcus aureus | – | 3M 1860 3M 1870 KC PFR |
| Other | Benzalkonium chloride wipes, wiped three times, left to dry 15 min | ||||
| Kenney et al. (2020) [20] | Vaporized H2O2 | Arm 1: Bioquell BQ-50 Arm 2: Bioquell BQ-50 + 5 min steam sterilization (135°C) |
Phage phi-6 Phage T1 Phage T7 |
– | 3M 1870 |
| Kumar et al. (2020) [35] | Vaporized H2O2 | Arm 1: Steris VHP ARD System Arm 2: Sterrad 100NX device |
VSV SARS-CoV-2 |
– | 3M 1860 3M Aura 1870 3M Vflex 1804 AO Safety 1054 |
| EtO | 5XLP Steri-Vac Sterilizer/Aerator, 1 h exposure, 12 hr aeration | ||||
| Lin et al. (2018) [22] | Sodium hypochlorite | Arm 1: 5.4%, applied and left to dry for 10 min Arm 2: 2.7%, applied and left to dry for 10 min Arm 3: 0.54%, applied and left to dry for 10 min |
Bacillus subtilis | Measured immediately after treatment 24 h incubation at 95% RH, 37°C |
3M 8210 |
| Ethanol | Arm 1: 50%, applied, left to dry 10 min Arm 2: 70%, 0.082 packing densityc, applied, left to dry 10 min Arm 3: 70%, 0.23 packing density, applied, left to dry 10 min Arm 4: 70%, 0.44 packing density, applied, left to dry 10 min Arm 5: 70%, 0.87 packing density, applied, left to dry 10 min Arm 6: 80%, applied, left to dry for 10 min Arm 7: 95%, applied, left to dry for 10 min |
||||
| Vo et al. (2009) [18] | Sodium hypochlorite | Arm 1: 0.005%, submerged 10 min, left to dry 2 min Arm 2: 0.01%, submerged 10 min, left to dry 2 min Arm 3: 0.05% %, submerged 10 min, left to dry 2 min Arm 4: 0.1%, submerged 10 min, left to dry 2 min Arm 5: 0.25%, submerged 10 min, left to dry 2 min Arm 6: 0.5%, submerged 10 min, left to dry 2 min Arm 7: 0.75%, submerged 10 min, left to dry 2 min |
MS2 | – | Wilson N1105 |
FFR, filtering facepiece respirators; H2O2, hydrogen peroxide; VSV, vesicular stomatitis virus; RH, relative humidity.
FFR models for the following studies obtained through private correspondence: Fisher et al. [12].
No longer commercially available.
Packing density (Vdisinfectant/VFFR).
Figure 4.
Pooled results of studies assessing the germicidal effect of chemical disinfectants on viruses by disinfectant type. Forest plot of germicidal effectiveness across chemical disinfectant type. Larger values of mean log10 change in viral load suggests greater decontamination effect. Filtering facepiece respirator (FFR) type examined varied by study. n represents the total number of replicates for all N95 FFR models included in the experimental arm. Only studies that reported germicidal outcome data as a log10 change are included in the figure.
EtO decontamination removed all viable vesicular stomatitis virus (VSV) from four N95 FFR models. In the same study, a single cycle of vaporized H2O2 also removed all viable VSV and SARS-CoV-2 [35]. One cycle of vaporized H2O2 also resulted in bacteriophage levels (<10 plaque-forming units [20]) or viral levels (<100.5 TCID50/mL [34]) below the detectable limit of the assay on one N95 FFR model each, and no growth of H1N1 on two N95 FFR models at seven days following decontamination with vaporized H2O2 [33].
A summary of the germicidal results for each disinfectant type is presented in Table III.
Physical traits
Six studies were identified that evaluated changes in FFR physical traits following decontamination (Table V ). Sodium hypochlorite and liquid H2O2 consistently resulted in changes in FFR appearance, including tarnished metallic nosepieces [7,9,11,19], oxidized staples [7,19], yellowing of nose pads [7,9], bleeding or fading of lettering [7,11], stiffening of filter media and elastic straps [11], or dissolving nosepieces [7]. The majority of studies that evaluated physical appearance following exposure to vaporized H2O2, isopropyl alcohol, ethanol, and EtO reported that FFR appearance was unchanged.
Table V.
Disinfectants and N95 filtering facepiece respirators used to evaluate the effects of decontamination using disinfectants on physical traits
| Study | N95 FFR modelsa | Intervention arm | Changes in physical appearance following visual inspection of decontaminated FFRs | Changes in odour after sniffing decontaminated FFRs |
|---|---|---|---|---|
| Bergman et al. (2010) [7] | 3M 8210 3M 8000 Moldex 2201 KC PFR95-174 3M 1870 3M 1860s |
Sodium hypochlorite | All FFR models: Metallic nosebands slightly tarnished 3M 8210: Discoloured (yellowed) nose pads 3M 8000: Staples oxidized Moldex 2201: Staples oxidized KC PFR95-174: Area adjacent to nose clip discoloured 3M 1870: Staples oxidized, discoloured (yellowed) nose pads, 50% of nose pad dissolved, material adjacent to nose pad became yellowed 3M 1860s: Staples oxidized, discoloured (yellowed) nose pads, bleeding of printed ink lettering |
Bleach odour |
| Liquid H2O2 | 3M 8000: Staples oxidized Moldex 2201: Staples oxidized 3M 1870: Staples oxidized 3M 1860s: Staples oxidized |
No changes reported | ||
| Vaporized H2O2 | No observable physical changes | No changes reported | ||
| EtO | No observable physical changes | No changes reported | ||
| Kenney et al. (2020) [20] | 3M 1870 | Vaporized H2O2 | Appeared similar to new with no deformity | Not assessed |
| Vaporized H2O2 + steam | Degradation observed | Not assessed | ||
| Lin et al. (2017) [21] | 3M 8210 | Sodium hypochlorite | No changes reported | Not assessed |
| Isopropyl alcohol | No changes reported | Not assessed | ||
| Ethanol | No changes reported | Not assessed | ||
| Salter et al. (2010) [19]b | 3M 8000 3M 8210 Moldex 1500 3M 1860 3M 1870 Kimberly Clark PFR |
Sodium hypochlorite | Corrosion and/or discolouration of metal parts of the FFRs (staples, nosepieces, etc.) | Bleach odour |
| Liquid H2O2 | No changes reported | No changes reported | ||
| Vaporized H2O2 | No changes reported | No changes reported | ||
| EtO | No changes reported | No changes reported | ||
| Viscusi et al. (2007) [11] | 3M 8000 | Sodium hypochlorite | Arm 1 (0.525%): Metallic nosebands slightly tarnished Arm 2 (5.25%): Metallic nosebands slightly tarnished, stiffening of filter media and elastic straps |
Not assessed |
| Liquid H2O2 | Arm 1 (3%): No visible changes Arm 2 (6%): Label ink slightly faded |
Not assessed | ||
| HPV | Arm 1 (Sterrad NX): Metallic nosebands slightly tarnished Arm 2 (Sterrad 100S): Metallic nosebands slightly tarnished |
Not assessed | ||
| EtO | No visible changes reported for either EtO intervention arm | Not assessed | ||
| Isopropyl alcohol | Fading of strap ink | Not assessed | ||
| Viscusi et al. (2009) [9] | 3M 8210 3M 8000 Moldex 2201 KC PFR95-174 3M 1870 3M 1860s |
Sodium hypochlorite | All FFR models: Metallic nosebands slightly tarnished 3M 1870: Inner nose comfort cushion discolored. |
Bleach odour |
| Vaporized H2O2 | All FFR models: Metallic nosebands slightly tarnished | No changes reported | ||
| EtO | All FFR models: No visible changes observed for all samples | No changes reported |
H2O2, hydrogen peroxide; EtO, ethylene oxide; FFR, filtering facepiece respirator.
FFR models for the following studies obtained through private correspondence: Bergman et al. (2010) [7], Salter et al. (2010) [19], Viscusi et al. (2007) [11], Viscusi et al. (2009) [9].
Salter et al. also evaluated two other disinfectants developed by the US Department of Defense that are not widely distributed. These results are not reported in the table.
Changes in FFR odour were evaluated in three studies following disinfectant treatment with sodium hypochlorite, EtO, liquid H2O2, and vaporized H2O2. Sodium hypochlorite left a characteristic bleach odour on the FFRs [7,9,19]. None of the other chemical disinfectants assessed resulted in changes in FFR odour.
A summary of the results of evaluations on changes in physical traits for each disinfectant type is presented in Table III.
FFR fit
Two studies evaluated FFR fit following decontamination, and included the following intervention arms: EtO (N = 1), vaporized H2O2 (N = 3), and ethanol (N = 1). Kumar et al. evaluated FFR fit using the PortaCount Fit Tester and two exercises (normal and deep breathing) following multiple decontamination bouts on new (unworn) FFRs [35]. Four FFR models were exposed to multiple bouts of decontamination (EtO: one and three cycles; vaporized H2O2: one, five, and 10 cycles using the VHP ARD System; one, five, 10, and 20 cycles using the Sterrad 100NX sterilizer). A single fit test was then performed with each of the four FFR models for each of the decontamination methods and number of cycles described above. All four FFR models passed the fit test (achieved a fit factor >100 as per Occupational Safety and Health Administration (OSHA) standards) following one, three, five, and 10 decontamination cycles of vaporized H2O2 using the VHP ARD System, but not with the standard cycle of the Sterrad 100NX sterilizer (fit factor >100 after one cycle, but <100 following five, 10, and 20 cycles). A passing fit factor score was achieved following both one and three decontamination cycles of EtO.
Fischer et al. evaluated FFR fit following three cycles of 2 h wear and decontamination using a 3M Aura 9211+/37193. After each wear–decontamination cycle, six fit tests were performed using six replicate FFRs. A fit factor >100 was achieved following one, two, and three vaporized H2O2 wear–decontamination cycles. The fit factor ranged from 112 to 200 after one wear–decontamination cycle, from 167 to 200 after two cycles, and from 100 to 200 after three cycles. The six replicate FFRs achieved a fit factor >100 following one ethanol wear–decontamination cycle (fit factor range: 153–200), but two out of the six replicate FFRs did not achieve a passing fit factor score following two ethanol wear–decontamination cycles (fit factor range 54.7–200), and four replicate FFRs had a fit factor <100 following three cycles (range 29.5–200) [34].
A summary of the fit test results for each disinfectant type is presented in Table III.
Potential health risks
Potential health risks associated with disinfectant decontamination were evaluated in three studies. Viscusi et al. reported that, while letting sodium hypochlorite-treated FFRs air-dry overnight significantly reduced off-gassing (2–12 ppm when wet vs 0–∼0.05 ppm), low-level chlorine off-gassing (∼0.1 ppm) was observed when the FFR was rehydrated [9]. Salter et al. measured the oxidant remaining on FFRs following decontamination with three different chemical disinfectants and found that the mass of oxidant remaining on the treated FFRs varied by FFR model and disinfectant [19]. For each disinfectant, the FFR with the highest oxidant masses evaluated using iodometric back titrations at 18 h following decontamination were as follows: vaporized H2O2 (3M 1860: 1.23 mg; 95% CI: 0.68, 1.77); sodium hypochlorite (3M 1820: 1.66 mg; 95% CI: –2.03, 5.34); and liquid H2O2 (Moldex 1500: 0.70 mg; 95% CI: 0.38, 1.02). FFRs treated with ethylene oxide were evaluated using gas chromatography–mass spectrometry. Although no residual EtO was detected on any of the FFR, ethylene glycol monoacetate was detected on FFR straps on 15 occasions. Cheng et al. evaluated residual H2O2 following decontamination using vaporized H2O2. The level of H2O2 on the FFRs' inner surface at 2 h was 0.6 ppm and undetectable at 3 h [33]. A summary of the results of evaluations for potential safety risks is presented in Table III.
Risk of bias
A detailed risk-of-bias assessment is presented in Appendix D. Overall risk of bias for aerosol penetration was low in all studies. Risk was moderate in one study for airflow resistance due to population heterogeneity (i.e. FFRs not obtained from the same lot) and potential selective reporting. Moderate risk of bias for all germicidal outcomes was primarily due to the use of visual assays and population heterogeneity, whereas moderate risks for fit evaluations were due to population heterogeneity and missing methodological details. Physical trait assessments had moderate to high risks of bias for various reasons, but unblinded outcome evaluation was common across all studies. For safety/irritation evaluations, no controls were used and two studies lacked enough details to assess all risk categories, resulting in moderate to high risks of bias for this outcome.
Discussion
This is the first systematic review to synthesize the existing evidence on using chemical disinfectants to decontaminate N95 FFRs. It was found that a single cycle of vaporized H2O2 successfully removes viral pathogens without affecting airflow resistance or fit, and maintains an initial filter penetration of <5%, with little change in FFR physical traits. Further research is required before the acceptability of decontamination using liquid H2O2 can be determined. Sodium hypochlorite, ethanol, isopropyl alcohol, and EtO are not recommended due to safety concerns and/or adverse effects on FFR function.
This systematic review identified five studies that reported on changes in aerosol penetration following decontamination with a chemical disinfectant. NIOSH has established a 95% filter efficiency standard (i.e. aerosol penetration of <5%) for N95 FFR [37]. Results showed that filter efficiency >95% was generally maintained following decontamination with sodium hypochlorite, liquid H2O2, vaporized H2O2, and EtO under laboratory test conditions. However, though the majority of studies evaluating sodium hypochlorite and vaporized H2O2 reported a post-decontamination aerosol penetration of <5%, there were conflicting findings in one study for each method. Four of the five studies that evaluated sodium hypochlorite reported that filter penetration of <5% was maintained following submersion (0.3%–5.25%, 30 min submersion) [7,11,12] or wiping (three times) with hypochlorite wipes [13]; however, the study by Lin et al., which used a different aerosol penetration testing protocol, reported penetration values that exceeded 5% for particle sizes >60 nm (0.5%, 10 min submersion) [21]. Vaporized H2O2 maintained filter performance in the majority of the studies where it was evaluated, with the exception of Bergman et al. [7]. In this study, three 55 min cycles with the Sterrad 100S H2O2 Gas Plasma Sterilizer resulted in filter penetration levels >5% in four of six FFR models (3M 8000, Moldex 2201, Kimberly Clark KC PFR95-174, 3M 1860), but this was not observed following three cycles with the Clarus R HPV Generator. Degradation in filter performance seemed to be related to level of exposure to the vaporized H2O2 during processing (i.e. FFRs packed near the top or bottom of the pouch during treatment). Interestingly, a single cycle with the same Sterrad 100S H2O2 Gas Plasma Sterilizer and almost identical FFR models did not adversely affect filter efficiency in a previous study [9]. These observations suggest the effects of vaporized H2O2 on filter performance and the number of decontamination cycles that can be employed are dependent on the system used. A recent US Food and Drug Administration (FDA)-commissioned report on the Bioquell Clarus C HPV generator provides further evidence on the impact of repeated vaporized H2O2 cycles on filter performance [38]. Using NIOSH testing standards, the report showed that filter efficiency of the 3M 1860 FFR was 99.6% ± 0.2 following 50 cycles of vaporized H2O2, safely exceeding the NIOSH requirement of 95%. Findings from the included studies showed that submersion in ethanol and isopropyl alcohol resulted in significantly higher filter penetration values that exceeded the levels permissible by NIOSH. This is not surprising, as solvents such as isopropyl alcohol have been shown to eliminate the electrostatic charges on the FFR filter [39,40], and the filter efficiency of uncharged media is typically 10-fold lower than charged media [41].
In addition to standards for aerosol penetration, NIOSH has also established standards for airflow resistance of N95 FFRs (peak average inhalation of 35mm H2O pressure (343.2 Pa) and an exhalation resistance to airflow 25 mmH2O (245.1 Pa)) [37]. These standards ensure that breathing is not impaired while wearing an N95 FFR. Evaluations from three studies showed that measured values for airflow resistance were within the NIOSH standards following a single cycle (sodium hypochlorite, ethanol, EtO, vaporized H2O2 [9,21]) or three decontamination cycles (sodium hypochlorite, liquid H2O2, vaporized H2O2, EtO [7]) across three different FFR models.
A successful decontamination protocol must also remove infectious pathogens from the FFR surface. Published literature on the ability of disinfectants to eradicate germicidal activity from N95 FFRs was only available for four of the disinfectants of interest (sodium hypochlorite, ethanol, EtO, and vaporized H2O2). The ability of other methods (liquid H2O2, isopropyl alcohol) to reduce pathogen load on N95 FFRs is not known at this time. Ethanol (at a sufficiently high concentration), sodium hypochlorite, and EtO can effectively remove viral pathogens from FFR surfaces; however, given the negative impact of ethanol on filter performance and inadequate ability to reduce bacteria from FFR, and the potential health risks associated with sodium hypochlorite and EtO, vaporized H2O2 is the most promising of the four disinfectants evaluated. A single cycle of five different vaporized H2O2 protocols effectively removed infectious pathogens (see Appendix E for details of the vaporized H2O2 protocols used in all studies included in this review) [20,[33], [34], [35]]. The FDA-commissioned Bioquell report further confirms the germicidal efficacy of vaporized H2O2, reporting a 6-log reduction in G. stearothermophilus spore viability [38]. The ability of vaporized H2O2 to eradicate virus and bacteria from N95 FFRs could be further enhanced by allowing the FFR to sit for an extended time-period between uses. It is well established that bacteria and virus levels on surfaces decrease over time [42,43]. For example, the infectiveness of SARS-CoV-2 declines by a factor of ≥10 every 24 h across a variety of surfaces [44]. Therefore, building a ‘holding period’ into the decontamination protocol, where FFRs sit for five to seven days following exposure to vaporized H2O2, would further guarantee the absence of viable viral particles.
FFR fit is an important outcome of interest when considering whether a decontamination protocol is acceptable to use. Improper fit results in an inadequate seal of the FFR against the wearer's face, reducing the FFR's ability to prevent particle penetration [45]. Two studies evaluated FFR fit following decontamination. FFR fit was not affected by one cycle of decontamination with ethanol [34] or by multiple cycles of EtO or vaporized H2O2 [34,35]. The multiple decontamination cycle results from Kumar et al. should be interpreted with some caution, however, as FFR fit was evaluated using only normal breathing and deep breathing exercises [35]. The additional exercises from the Occupational Safety and Health Administration (OHSA) protocol that involve movement of the mouth, head and body (to mimic movements performed by healthcare workers) were not included in the evaluation. Therefore, it is not clear whether a fit factor >100 would have still been achieved had the full OHSA protocol been used. Additionally, the FFRs that were evaluated were new and had not been worn by healthcare workers prior to decontamination, or between decontamination cycles. There is evidence that FFR fit deteriorates through repeated donning and doffing [46]; therefore the number of decontamination and reuse cycles that can be applied to a FFR will be limited by physical stress imposed by both decontamination and donning and doffing. The FDA-commissioned Bioquell report [38] also evaluated fit following multiple decontamination cycles using vaporized H2O2 and achieved similar results to Kumar et al. (fit was maintained following 10 and 20 decontamination cycles). However, these findings are limited by the fact that fit testing was performed using a mannequin and, by 30 vaporized H2O2 cycles, FFR straps degraded such that fit testing was no longer possible. The methods used by Fischer et al. are more representative of the real world, as they involved three cycles of decontamination and 2 h wearing [34]. The FFR model they evaluated (3M Aura 9211+/37193) maintained a fit factor >100 following three cycles of vaporized H2O2; however, they used a small sample size (six replicates of one FFR model). Further testing should be conducted using FFRs worn by healthcare workers prior to and between decontamination cycles in order to confirm whether FFRs maintain fit in the real-world setting following multiple decontamination cycles with vaporized H2O2.
Sodium hypochlorite consistently resulted in significant changes to FFR appearance. Some of the changes reported were substantial enough that they could result in changes in FFR fit or comfort, such as stiffening of filter media and elastic straps and dissolving of half of the FFR nosepiece. Sodium hypochlorite also resulted in a bleach odour on the FFRs that would be unpleasant to the wearer. Some chlorine off-gassing was observed on FFRs that had been submerged in sodium hypochlorite and rehydrated, which the authors felt could be significant since rehydration of the FFR could be compared with moisture in the exhaled breath of an individual wearing the FFR [9]. Therefore, as low-level exposure to chlorine may occur when wearing an FFR that has been decontaminated by submersion in sodium hypochlorite, sodium hypochlorite is not recommended for FFR decontamination and reuse. There are also potential safety concerns with the use of EtO. EtO in itself is hazardous and a known human carcinogen, and decontamination with EtO requires a lengthy aeration process to remove residual EtO [47]. Following a 12 h aeration cycle, Salter et al. found no EtO on six FFR models, but did find traces of a hazardous contaminant, ethylene glycol monacetate [19]. Whether residual EtO remains on N95 FFRs when using a shorter aeration cycle, such as the 4 h cycle used by Viscusi et al., is unknown [9,11]. Given the potential safety concerns and the impracticality of a lengthy aeration cycle, the results of this review do not endorse EtO sterilization for N95 decontamination, which is consistent with the CDC recommendations [48]. Residual levels of liquid and vaporized H2O2 were reported in one and two studies respectively, though they remained within established safety limits, and in the case of vaporized H2O2, decreased over time. A ‘holding period’ following decontamination, as described above, would provide extra assurance that hydrogen peroxide levels were under the permissible exposure limit set by OHSA (1 ppm) [49].
The moderate overall risks of bias noted for germicidal outcomes are largely due to the use of unblinded assays to quantify bacterial and viral loads; although these are generally accepted as appropriate means of pathogen quantification, counting of plaques and colonies may be subjective. Assessments of physical appearance and odour were unblinded in all cases and results were often reported as additional comments about observations of damage, rather than through a systematic procedure for all FFR models, which makes it unclear to what degree sampling and assessment biases may have influenced these outcomes. The potential for bias in evaluations of post-decontamination safety was increased by the lack of control group in any study, which reduces confidence in accuracy of the machine measurements.
Although this systematic review provides valuable information regarding the use of chemical disinfectants for the decontamination of N95 FFRs, a number of limitations must be acknowledged. Each study used a different combination of FFR types. In order to address this, we aggregated across FFR types within each study, treating the pooled replicates across FFR types as our statistically independent sampling unit. This is appropriate for our research question aimed at performance of FFRs in general (where we assume little difference between FFR types). If there are large differences between FFR types, our approach might artificially inflate our sample size; if this were the case it would unlikely change our findings, due to the consistency of these studies' conclusions and low heterogeneity. For ease of completion, our review was limited to studies available in English or French, therefore evidence available in other languages is not included in our analysis. With the exception of the fit data from Fischer et al., all of the reported outcomes were evaluated on new, unworn FFRs [33]. It is not clear whether extended FFR use prior to decontamination would alter our findings. Future work evaluating decontamination of N95 FFRs should perform testing in real-world conditions, using N95 FFRs that have been worn by healthcare workers in the clinical setting prior to decontamination. Finally, we did not have access to unpublished data, such as quality assurance work performed at hospitals or work performed by industry and submitted to regulatory agencies. For example, the FDA recently reissued Emergency Use Authorizations (EUA) and no longer authorize decontamination or reuse of respirators that have exhalation valves. In the current published literature, the reasons for this EUA are not evident [50].
Conclusion
We identified 13 studies that evaluated decontamination of NIOSH-approved N95 FFRs using a chemical disinfectant. Of these, the most promising chemical disinfectant evaluated was vaporized H2O2. A single cycle of vaporized H2O2 successfully removes viral pathogens without affecting airflow resistance or fit, and maintains an initial filter penetration of <5%, with little change in FFR appearance. Residual hydrogen peroxide levels following decontamination were within safe limits. More than one decontamination cycle of vaporized H2O2 may be possible but further information is required on how multiple cycles would affect FFR fit in a real-world setting before the upper limit can be established. Although immersion in liquid H2O2 does not appear to adversely affect FFR function, there is no available data on its ability to remove infectious pathogens from FFRs or its impact on FFR fit. Sodium hypochlorite, ethanol, isopropyl alcohol, and EtO are not recommended due to safety concerns or negative effects on FFR function.
Future directions
This systematic review provides valuable data on the efficacy and safety of decontaminating N95 FFRs using chemical disinfectants. At the time of this review, published data only supports one disinfectant approach. Literature in this area is rapidly evolving as researchers work to recognize solutions to widespread shortages in N95 FFRs. These circumstances are amenable to a living review that allows for the rapid identification and incorporation of important new data. Recognizing this need, our group has initiated a living review with an open-access database [51]; the most recent scoping review update on June 25th, 2020, identified 11 new publications evaluating decontamination of NIOSH-approved N95 FFRs using chemical disinfectants, none of which would significantly alter the findings of this systematic review [[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]].
It is important that future studies on decontamination employ approved N95 FFRs and techniques for testing (e.g. NIOSH testing standards for aerosol penetration and airflow resistance). This is particularly relevant when evaluating germicidal effects, where significant heterogeneity in pathogen selection, application procedures, and assays were observed. It would be prudent for researchers working in this area to both consider the available literature on disinfectant efficacy and consult with their regulatory agencies about the most up-to-date testing requirements.
Conflict of interest statement
None declared.
Funding sources
This research was supported by funding provided by the Ontario Ministry of Health and Long-Term Care, received from the successful application to the CHAMO-COVID-19 grant opportunity.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.08.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bałazy A., Toivola M., Adhikari A., Sivasubramani S., Reponen T., Grinshpun S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:49–56. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 2.US Centers for Disease Control and Prevention . 2020. Interim infection prevention and control recommendations for patients with suspected or confirmed Coronavirus Disease 2019 (COVID-19) in healthcare settings.https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html Available at: [last accessed April 2020] [Google Scholar]
- 3.Patel A., D’Alessandro M.M., Ireland K.J., Burel W.G., Wencil E.B., Rasmussen S.A. Personal protective equipment supply chain: lessons learned from recent public health emergency responses. Health Secur. 2017;15:244–252. doi: 10.1089/hs.2016.0129. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan A., Jernign D.B., Liedtke L., Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: views from a national survey of infectious diseases consultants. Clin Infect Dis. 2004;39:272–274. doi: 10.1086/421777. [DOI] [PubMed] [Google Scholar]
- 5.Project N95. The national COVID-19 medical equipment clearinghouse.
- 6.Bauchner H., Fontanarosa P.B., Livingston E.H. Conserving supply of personal protective equipment – a call for ideas. JAMA. 2020 Mar 20 doi: 10.1001/jama.2020.4770. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Sambol A.R. DigitalCommons@University of Nebraska; Lincoln: 2010. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. [Google Scholar]
- 8.Bergman M.S., Viscusi D.J., Palmiero A.J., Powell J.B., Shaffer R.E. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J Int Soc Resp Protect. 2011;28:48–59. [Google Scholar]
- 9.Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viscusi D.J., Bergman M.S., Novak D.A., Faulkner K.A., Palmiero A., Powell J., et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 11.Viscusi D.J., King W.P., Shaffer R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Resp Protect. 2007;24:93–107. [Google Scholar]
- 12.Fisher E., Rengasamy S., Viscusi D., Vo E., Shaffer R. Development of a test system to apply virus-containing particles to filtering facepiece respirators for the evaluation of decontamination procedures. Appl Environ Microbiol. 2009;75:1500–1507. doi: 10.1128/AEM.01653-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimbuch B.K., Kinney K., Lumley A.E., Harnish D.A., Bergman M., Wander J.D. Cleaning of filtering facepiece respirators contaminated with mucin and Staphylococcus aureus. Am J Infect Control. 2014;42:265–270. doi: 10.1016/j.ajic.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lore M.B., Heimbuch B.K., Brown T.L., Wander J.D., Hinrichs S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 15.Lindsley W.G., Martin S.B., Jr., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R., et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills D., Harnish D.A., Lawrence C. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo M.H., Grippin A., Anwar D., Smith T. Effects of relative humidity and spraying medium on UV decontamination of filters loaded with viral aerosols. Appl Environ Microbiol. 2012;78:5781–5787. doi: 10.1128/AEM.00465-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vo E., Rengasamy S., Shaffer R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl Environ Microbiol. 2009;75:7303–7309. doi: 10.1128/AEM.00799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salter W.B., Kinney K., Wallace W.H., Lumley A.E., Heimbuch B.K., Wander J.D. Analysis of residual chemicals on filtering facepiece respirators after decontamination. J Occup Environ Hyg. 2010;7:437–445. doi: 10.1080/15459624.2010.484794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney P., Chan B.K., Kortright K., Cintron M., Havill N., Russi M., et al. Hydrogen peroxide vapor sterilization of N95 respirators for reuse. medRxiv. 2020 Mar 27 doi: 10.1101/2020.03.24.20041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin T.H., Chen C.C., Huang S.H., Kuo C.W., Lai C.Y., Lin W.Y. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: effects of five decontamination methods. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin T.H., Tang F.C., Hung P.C., Hua Z.C., Lai C.Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;31:31. doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Centers for Disease Control and Prevention Decontamination and reuse of filtering facepiece respirators. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at: [last accessed April 2020]
- 24.O’Hearn K., Gertsman S., Sampson M., Webster R.J., Tsampalieros A., Ng R., et al. Decontaminating N95 and SN95 masks with ultraviolet germicidal irradiation (UVGI) does not impair mask efficacy and safety: a systematic review. J Hosp Infect. 2020;106:163–175. doi: 10.1016/j.jhin.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gertsman S., Agarwal A., O’Hearn K., Webster R., Tsampalieros A., Barrowman N., et al. Microwave- and heat-based decontamination of n95 filtering facepiece respirators (FFR): a systematic review. OSF Preprints. 2020 doi: 10.31219/osf.io/4whsx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Wilson M. The untold origin story of the N95 mask. 2020. https://www.fastcompany.com/90479846/the-untold-origin-story-of-the-n95-mask Available at: [last accessed April 2020]
- 28.Nama N., Barrowman N., O’Hearn K., Sampson M., Zemek R., McNally J.D. Quality control for crowdsourcing citation screening: the importance of assessment number and qualification set size. J Clin Epidemiol. 2020;122:160–162. doi: 10.1016/j.jclinepi.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team . R Foundation for Statistical Computing; Vienna: 2012. R: a language and environment for statistical computing.http://www.R-project.org/ Available at: [Google Scholar]
- 32.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statist Software. 2010;36 [Google Scholar]
- 33.Cheng V., Wong S.-C., Kwan G., Hui W.-T., Yuen K.-Y. Disinfection of N95 respirators by ionized hydrogen peroxide in pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer R., Morris D.H., van Doremalen N., Sarchette S., Matson J., Bushmaker T., et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv. 2020 April 24 doi: 10.1101/2020.04.11.20062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A., Kasloff S.B., Leung A., Cutts T., Strong J.E., Hills K., et al. N95 Mask decontamination using standard hospital sterilization technologies. medRxiv. 2020;2020 doi: 10.1101/2020.04.05.20049346. 2004.2005.20049346. [DOI] [Google Scholar]
- 36.Electronic Code of Federal Regulations (42 CFR 84.174) Filter efficiency level determination test – non-powered series N, R, and P filtration. https://www.ecfr.gov/cgi-bin/text-idx?SID=5b2666d4940f00b38d815af01a2e7044&mc=true&node=pt42.1.84&rgn=div5#se42.1.84_1174 Available at: [last accessed August 2020]
- 37.National Institute for Occupational Safety and Health . 1996. NIOSH guide to the selection and use of particulate respirators.https://www.cdc.gov/niosh/docs/96-101/default.html Available at: [last accessed March 2020] [Google Scholar]
- 38.Battelle . 2016. Final report for Bioquell HPV decontamination for the reuse of N95 respirators. Columbus, OH. [Google Scholar]
- 39.Chen C.C., Huang S.H. The effects of particle charge on the performance of a filtering facepiece. Am Indust Hyg Assoc J. 1998;59:227–233. doi: 10.1080/15428119891010488. [DOI] [PubMed] [Google Scholar]
- 40.Kim J., Hinestroza J.P., Jasper W., Barker R.L. Effect of solvent exposure on the filtration performance of electrostatically charged polypropylene filter media. Textile Res J. 2009;74:343–350. doi: 10.1177/0040517508090887. [DOI] [Google Scholar]
- 41.Tsai P.P. Information and FAQs on the performance, protection, and sterilization of face mask materials. 2020. https://utrf.tennessee.edu/information-faqs-performanceprotection-sterilization-of-face-mask-materials/#abstract_link Available at: [last accessed April 2020]
- 42.Bean B., Moore B.M., Sterner B., Peterson L.R., Gerding D.N., Balfour H.H., Jr. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146:47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 43.Yeargin T., Buckley D., Fraser A., Jiang X. The survival and inactivation of enteric viruses on soft surfaces: a systematic review of the literature. Am J Infect Control. 2016;44:1365–1373. doi: 10.1016/j.ajic.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 44.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reponen T., Lee S.A., Grinshpun S.A., Johnson E., McKay R. Effect of fit testing on the protection offered by n95 filtering facepiece respirators against fine particles in a laboratory setting. Ann Occup Hyg. 2011;55:264–271. doi: 10.1093/annhyg/meq085. [DOI] [PubMed] [Google Scholar]
- 46.Bergman M.S., Viscusi D.J., Zhuang Z., Palmiero A.J., Powell J.B., Shaffer R.E. Impact of multiple consecutive donnings on filtering facepiece respirator fit. Am J Infect Control. 2012;40:375–380. doi: 10.1016/j.ajic.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 47.US Centers for Disease Control and Prevention Ethylene Oxide “Gas” Sterilization. 2016. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/ethylene-oxide.html Available at: [last accessed April 2020]
- 48.US Centers for Disease Control and Prevention Strategies for optimizing the supply of N95 respirators: crisis/alternate strategies. https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/crisis-alternate-strategies.html Available at: [last accessed March 2020]
- 49.Occupational Safety and Health Administration Permissible exposure limits. https://www.osha.gov/dsg/annotated-pels/tablez-1.html [last accessed April 2020]
- 50.US Food and Drug Administration Coronavirus (COVID-19) Update: FDA reissues emergency use authorizations revising which types of respirators can be decontaminated for reuse. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-reissues-emergency-use-authorizations-revising-which-types Available at: [last accessed July 2020]
- 51.O’Hearn K., Agarwal A., Choong K., Gertsman S., Nama N., Sampson M., et al. Live scoping review of N95 and surgical facemask decontamination and reuse: a scoping review protocol. OSF Preprints. 2020 doi: 10.31219/osf.io/rpc27. [DOI] [Google Scholar]
- 52.Derr T.H., James M.A., Kuny C.V., Kandel P.P., Beckman M.D., Hockett K.L., et al. Aerosolized hydrogen peroxide decontamination of N95 respirators, with fit-testing and virologic confirmation of suitability for re-use during the COVID-19 pandemic. MedRxiv. 2020 April 22 doi: 10.1128/msphere.00303-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nazeeri A.I., Hilburn I.A., Wu D.A., Mohammed K.A., Badal D.Y., Chan M.H.W., et al. Ethanol-drying regeneration of N95 respirators. MedRxiv. 2020 July 3 [Google Scholar]
- 54.Cramer A., Plana D., Yang H.L., Carmack M., Tian E., Sinha M.S., et al. Analysis of SteraMist ionized hydrogen peroxide technology in the sterilization of N95 respirators and other PPE: a quality improvement study. MedRxiv. 2020 Apr 23 doi: 10.1038/s41598-021-81365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dave N., Pascavis K.S., Patterson J.M., Wallace D.W., Chowdhury A., Abbaszadegan M., et al. Characterization of a novel, low-cost, scalable vaporized hydrogen peroxide system for sterilization of N95 respirators and other COVID-19 related personal protective equipment. MedRxiv. 2020 June 26 [Google Scholar]
- 56.Ludwig-Begall L.F., Wielick C., Dams L., Nauwynck H., Demeuldre P.F., Napp A., et al. Decontamination of face masks and filtering facepiece respirators via ultraviolet germicidal irradiation, hydrogen peroxide vaporisation, and use of dry heat inactivates an infectious SARS-CoV-2 surrogate virus. MedRxiv. 2020 June 5 [Google Scholar]
- 57.Saini V., Sikri K., Batra S.D., Kalra P., Gautam K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathogens. 2020;12:29. doi: 10.1186/s13099-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cadnum J.L., Li D.F., Redmond S.N., John A.R., Pearlmutter B., Donskey C.J. Effectiveness of ultraviolet-C light and a high-level disinfection cabinet for decontamination of N95 respirators. Path Immun. 2020;5:52–67. doi: 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai C., Floyd E.L. Effects of sterilization with hydrogen peroxide and chlorine dioxide on the filtration efficiency of N95, KN95, and surgical face masks. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.John A.R., Raju S., Cadnum J.L., Lee K., McClellan P., Akkus O., et al. Scalable in-hospital decontamination of N95 filtering facepiece respirator with a peracetic acid room disinfection system. MedRxiv. 2020 May 29 doi: 10.1017/ice.2020.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wigginton K.R., Arts P.J., Clack H., Fitzsimmons W.J., Gamba M., Harrison K.R., et al. Validation of N95 filtering facepiece respirator decontamination methods available at a large university hospital. MedRxiv. 2020 April 30 doi: 10.1093/ofid/ofaa610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith J.S., Hanseler H., Welle J., Rattray R., Campbell M., Brotherton T., et al. Effect of various decontamination procedures on disposable N95 mask integrity and SARS-CoV-2 infectivity. MedRxiv. 2020 May 7 doi: 10.1017/cts.2020.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.