Abstract
Objective:
To examine trends and correlates of frequency of self-reported alcohol and nicotine use among pregnant women.
Methods:
Cross-sectional study of 363,240 pregnancies from 2009–2017 screened for self-reported substance use at their first prenatal visit in Kaiser Permanente Northern California. Poisson regression with a log link function was used to estimate the annual prevalences of self-reported daily, weekly, and ≤monthly alcohol and nicotine use, adjusting for socio-demographics. Generalized estimating equation models were used to estimate the adjusted odds ratios (aOR) of any self-reported prenatal alcohol or nicotine use among those who self-reported use in the year prior to pregnancy, by frequency of pre-pregnancy substance use and socio-demographics.
Results:
The sample was 64% non-White [mean (SD) age=30.1 (5.6)]. From 2009 to 2017, alcohol use before pregnancy increased from 63.4% to 65.9% (trend p-value=.008), and prenatal alcohol use decreased from 11.6% to 8.8% (trend p-value<.0001). Nicotine use before pregnancy decreased from 12.7% to 7.7% (trend p-value<.0001), and prenatal use decreased from 4.3% to 2.0% (trend p-value<.0001). Trends by use frequency were similar to overall trends. The odds of continued use of alcohol and nicotine during pregnancy were higher among those who used daily or weekly (versus monthly or less) in the year before pregnancy and varied with socio-demographics.
Discussion:
Prenatal alcohol and nicotine use decreased from 2009–2017. More frequent pre-pregnancy use predicted higher odds of prenatal use. Results suggest that interventions and education about the harms of prenatal substance use for frequent users prior to conception may reduce substance use during pregnancy.
Keywords: Alcohol, Nicotine, Pregnancy, Prenatal, Trends, Women, Screening
1. Introduction
Substance use during pregnancy is a critical public health concern with significant consequences to both mother and infant (Forray, 2016). Women who use substances during pregnancy are at increased risk for poor perinatal outcomes, including preterm labor, low birth weight, congenital abnormalities, and stillbirths, and there can be additional long-lasting physical, mental, behavioral and neurodevelopmental consequences for their children (Austin et al., 2020; Creanga et al., 2012; Forray, 2016; Metz and Borgelt, 2018; Patrick et al., 2015; Ruisch et al., 2018). Recognizing prenatal substance use as a primary cause of preventable birth defects, US guidelines consider substance use screening and referral to be essential for prenatal care (American College of Obstetricians and Gynecologists, 2020).
Alcohol and nicotine are among the most commonly used substances by women before and during pregnancy. Prenatal alcohol use is associated with structural impairments, increased risk for adverse birth outcomes (e.g., intrauterine growth restriction and stillbirth), fetal alcohol spectrum disorder and fetal alcohol syndrome, and neurodevelopmental problems in childhood (Dejong et al., 2019; McQuire et al., 2020; Meyer-Leu et al., 2011; Moise, 2019; O’Leary et al., 2009). Prenatal nicotine use is associated with pregnancy complications (e.g., placenta previa and abruption), poor infant outcomes (e.g., preterm delivery, low offspring birthweight), sudden infant death syndrome, birth defects, and long-term health issues in childhood (American College of Obstetricians and Gynecologists, 2020; Crume, 2019; Gaysina et al., 2013; Moore et al., 2020; Oga et al., 2018).
National data indicate that alcohol use is increasing over time, and nicotine use is decreasing over time, among US women of reproductive age (Hasin et al., 2019; Nighbor et al., 2018). However, corresponding with growing awareness of the potential harms of alcohol and nicotine use during pregnancy, initial data suggest that prenatal alcohol and nicotine use are decreasing over time (Centers for Disease and Prevention, 2009; Hasin et al., 2019; Tan et al., 2015; Tong et al., 2013). For example, data from the National Survey of Drug Use and Health indicate that among US adult pregnant women, any past-month use of alcohol during pregnancy decreased non-significantly from 9.6% in 2002 to 8.4% in 2016, and any past-month cigarette smoking decreased significantly from 17.5% in 2002 to 10.3% in 2016 (Agrawal et al., 2019).
Past-month alcohol and nicotine use is most common during the first trimester of pregnancy (Agrawal et al., 2019; Ethen et al., 2009), during which time women may not realize that they are pregnant. Although healthcare systems are well poised to screen women of reproductive age for substance use, it is challenging to predict which women are at risk for using nicotine and alcohol when they become pregnant, and health care systems have limited resources and require better data to prioritize whom to target with education about prenatal substance use prior to conception. Initial data from nationally representative studies indicate that lower socioeconomic status, lower education, White race, and serious psychological distress are associated with higher risk of nicotine use during pregnancy (Goodwin et al., 2017; Kurti et al., 2017), while younger age, other substance use, depression, higher socioeconomic status, higher education, and being unmarried are associated with greater risk of alcohol use during pregnancy (Shmulewitz and Hasin, 2019). Less is known about risk factors that are associated with continued use versus quitting alcohol or nicotine among those who use these substances prior to pregnancy.
Given the health risks associated with alcohol and nicotine use during pregnancy, and the changing prevalence and patterns of use of these substances among women of reproductive age in the US (Hasin et al., 2019; Nighbor et al., 2018), research is needed to better understand trends in prenatal use of alcohol and nicotine and to identify factors associated with quitting versus continuing to use these substances during pregnancy. The primary objective of this study was to examine trends in daily, weekly, and monthly or less self-reported use of alcohol and nicotine in the year before pregnancy and during pregnancy from 2009 to 2017, among a diverse population of pregnant women within a large healthcare system with screening for substance use as part of standard prenatal care. We also examined whether frequency of alcohol and nicotine use in the year before pregnancy were associated with continued use of these substances during pregnancy.
2. Methods
2.1. Data source and study population
Kaiser Permanente Northern California (KPNC) is an integrated multispecialty healthcare delivery system that provides care to more than 4 million diverse members who are representative of the Northern California region (Gordon, 2013, 2015; Selby et al., 2005). During standard prenatal care, pregnant women are screened for frequency of substance use in the year before pregnancy and during pregnancy via a self-administered questionnaire at the first prenatal visit (at ~8 weeks gestation). Our study sample included all pregnant women aged 11 years and older who completed the self-administered questions about use of alcohol and nicotine in the year before and during pregnancy from January 1, 2009 to December 31, 2017. KPNC’s Institutional Review Board approved this study with waiver of informed consent.
2.2. Measures
Pregnant women’s self-reported frequency of alcohol and nicotine use in the year before pregnancy and since the start of pregnancy were assessed using the self-administered questionnaire completed at the first prenatal visit. The frequency variable included four response levels [daily (at least once per day), weekly (at least once per week but less than daily), monthly or less (at least once during the timeframe but less than weekly), none].
We created an additional variable to reflect whether women with self-reported alcohol use in the year before pregnancy quit alcohol use prior to pregnancy (i.e., reported no use during pregnancy), decreased their frequency of alcohol use during pregnancy (i.e., went from daily to weekly or monthly or less, or from weekly to monthly or less), maintained their frequency of alcohol use during pregnancy (i.e., maintained daily, weekly, or monthly or less use), or increased their frequency of alcohol use during pregnancy (i.e., went from monthly or less to weekly or daily, or from weekly to daily). A similar variable was created to reflect changes in nicotine use frequency during pregnancy among women who self-reported nicotine use in the year before pregnancy.
Socio-demographic variables that were available in the electronic health record (EHR) that have been associated with prenatal alcohol or nicotine use in the literature were also examined, including age, self-reported race/ethnicity, and median neighborhood household income quartiles based on census data.
2.3. Statistical analysis
2.3.1. Frequency of self-reported alcohol and nicotine use
All analyses were conducted first for alcohol use and then for nicotine use. We first described the frequency of self-reported alcohol use in both the year before pregnancy and during pregnancy and examined socio-demographic differences in alcohol use frequency using Chi-square tests.
2.3.2. Linear trends in self-reported alcohol and nicotine use over time
We then used Poisson regression with a log link function to estimate the adjusted prevalence of self-reported daily, weekly, and monthly or less alcohol use in the year before pregnancy and during pregnancy annually. Each outcome was modeled separately. Socio-demographics were adjusted for in these analyses using the average covariate distributions across the study period. In order to estimate the annual prevalence of alcohol use in the year before and during pregnancy for the population, women with more than one pregnancy during the study period could contribute to the analysis more than once. Linear trends in alcohol use frequency before and during pregnancy were modeled using a linear term for calendar year to estimate the annual relative rate of change with 95% confidence intervals, and the significance of the trend was calculated using a Wald test.
2.3.3. Continued alcohol and nicotine use in pregnancy
Next, among women who self-reported alcohol use in the year before pregnancy, we used generalized estimating equation (GEE) models with a logit link to estimate the adjusted odds ratio (aOR) and 95% confidence interval of any self-reported alcohol use during pregnancy across years by frequency of alcohol use in the year before pregnancy and by socio-demographic factors, accounting for the correlation among women who were pregnant more than once during the study. All models were adjusted for calendar year. We then extended this analysis to examine the categorical outcome of change in frequency of alcohol use from the year before pregnancy to during pregnancy, defined as 3 categories: (1) quit during pregnancy, (2) reduced frequency of use, and (3) maintained or increased frequency of use. We used GEE models for multinomial outcomes to estimate the aOR and 95% confidence interval for each category of use and by socio-demographic factors, accounting for the correlation among women who were pregnant more than once during the study. Using Poisson regression with a log link function, we also estimated the adjusted prevalence of any alcohol use during pregnancy, and changes in frequency of alcohol use during pregnancy, among those who self-reported use in the year before pregnancy.
This series of analyses was repeated for self-reported nicotine use in the year before and during pregnancy. Analyses were conducted using SAS 9.4 and a two-sided P-value <0.05 was considered as statistically significant.
3. Results
Among 414,028 pregnancies in the KPNC system from 2009 to 2017, the final study sample included 363,240 pregnancies from 274,456 unique women; 73,965 (26.9%) women had more than one pregnancy during the study period. The excluded pregnancies consisted of 44,474 pregnancies (10.7%) without a prenatal substance use screening questionnaire and 6,314 pregnancies (1.7%) with a partially completed questionnaire that was missing a response to one or more question on alcohol or nicotine use. Pregnancies excluded for missing data did not differ significantly from those included on the age of the women [mean (SD) = 30.1 (6.2) versus 30.1 (5.6), p=.21], but they had a slightly lower median neighborhood household income [mean (SD) = $72,340 ($30,882) versus $74,386 ($30,789), p<.0001]. Pregnancies in Black women were more likely to be excluded (18%) than pregnancies in women with Asian (11%), Hispanic (13%), White (11%), or other (13%) race/ethnicity (p<.0001) due to missing data.
The sample was 36.0% White, 27.9% Hispanic, 16.7% Asian, 6.0% Black, and 13.5% other race/ethnicity; 1.2% were aged 11–17, 15.4% were aged 18–24, 61.5% were aged 25 to 34 years, and 22.0% were aged 34 or older. The median neighborhood household income was $70,476 (inter quartile range $51,593-$92,647) (Table 1 and eAppendix 1). Most pregnancies were screened for self-reported substance use during the first trimester of pregnancy (84%) at a median (IQR) gestation age of 8.7 (3.6) weeks.
Table 1.
Frequency of Alcohol and Nicotine Use in the Year Before Pregnancy and During Pregnancy by Socio-Demographics.
| Total | Alcohol Use in Year Before Pregnancy | Nicotine Use in Year Before Pregnancy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N = 363,240 | None N (%) = 125,102 (34.4) | ≤ Monthly N (%) = 159,527 (43.9) | Weekly N (%) = 72,731 (20.0) | Daily N (%) = 5,880 (1.6) | None N (%) = 326,569 (89.9) | ≤ Monthly N (%) = 12,236 (3.4) | Weekly N (%) = 5,285 (1.5) | Daily N (%) = 19,150 (5.3) |
| % | % | % | % | % | % | % | % | % | |
| Age | |||||||||
| 11–17 | 1.2 | 67.1 | 27.5 | 4.8 | 0.6 | 82.7 | 6.3 | 3.2 | 7.8 |
| 18–24 | 15.4 | 39.4 | 48.2 | 11.7 | 0.7 | 82.2 | 5.3 | 2.6 | 9.9 |
| 25–34 | 61.5 | 32.3 | 44.9 | 21.2 | 1.6 | 90.8 | 3.2 | 1.3 | 4.7 |
| >34 | 22.0 | 35.1 | 39.1 | 23.3 | 2.4 | 93.1 | 2.4 | 1.0 | 3.5 |
| Race/ethnicity | |||||||||
| Asian | 16.7 | 49.6 | 40.3 | 9.4 | 0.7 | 94.1 | 2.5 | 0.8 | 2.6 |
| Black | 6.0 | 37.0 | 48.0 | 13.8 | 1.3 | 86.4 | 3.4 | 2.3 | 7.9 |
| Hispanic | 27.9 | 37.8 | 48.1 | 13.3 | 0.8 | 91.2 | 3.7 | 1.4 | 3.7 |
| Other | 13.5 | 44.7 | 40.0 | 14.2 | 1.1 | 90.5 | 3.0 | 1.4 | 5.2 |
| White | 36.0 | 20.5 | 43.2 | 33.3 | 3.0 | 87.3 | 3.6 | 1.7 | 7.3 |
| Neighborhood income | |||||||||
| <$51,593 | 25.0 | 38.6 | 43.9 | 16.0 | 1.5 | 87.4 | 3.8 | 1.9 | 7.0 |
| $51,593–<$70,476 | 25.0 | 34.3 | 44.9 | 19.2 | 1.6 | 89.1 | 3.5 | 1.5 | 5.9 |
| $70,476–<$92,647 | 25.0 | 33.3 | 44.2 | 21.0 | 1.5 | 90.7 | 3.3 | 1.4 | 4.6 |
| ≥$92,647 | 25.0 | 31.6 | 42.7 | 23.9 | 1.9 | 92.4 | 2.9 | 1.1 | 3.6 |
| Total | Alcohol Use During Pregnancy | Nicotine Use During Pregnancy | |||||||
| Characteristics | N = 363,240 | None N (%) = 328,644 (90.5) | ≤ Monthly N (%) = 27,818 (7.7) | Weekly N (%) = 5,996 (1.7) | Daily N (%) = 782 (0.2) | None N (%) = 352,596 (97.1) | ≤ Monthly N (%) = 3,382 (0.9) | Weekly N (%) = 2,067 (0.6) | Daily N (%) = 5,195 (1.4) |
| % | % | % | % | % | % | % | % | % | |
| Age | |||||||||
| 11–17 | 1.2 | 93.7 | 5.8 | 0.5 | 0.1 | 93.9 | 2.6 | 1.5 | 2.0 |
| 18–24 | 15.4 | 90.6 | 8.1 | 1.2 | 0.1 | 94.3 | 1.9 | 1.3 | 2.6 |
| 25–34 | 61.5 | 90.6 | 7.6 | 1.6 | 0.2 | 97.5 | 0.8 | 0.5 | 1.2 |
| >34 | 22.0 | 89.9 | 7.8 | 2.0 | 0.3 | 97.9 | 0.6 | 0.4 | 1.2 |
| Race/ethnicity | |||||||||
| Asian | 16.7 | 92.3 | 6.7 | 0.9 | 0.1 | 98.7 | 0.6 | 0.2 | 0.5 |
| Black | 6.0 | 90.8 | 7.6 | 1.5 | 0.2 | 94.8 | 1.7 | 1.2 | 2.3 |
| Hispanic | 27.9 | 91.0 | 7.6 | 1.3 | 0.1 | 98.0 | 0.8 | 0.4 | 0.8 |
| Other | 13.5 | 92.0 | 6.6 | 1.3 | 0.2 | 97.0 | 0.9 | 0.6 | 1.5 |
| White | 36.0 | 88.6 | 8.6 | 2.5 | 0.4 | 96.0 | 1.1 | 0.8 | 2.2 |
| Median household income | |||||||||
| < $51,593 | 25.0 | 91.1 | 7.3 | 1.5 | 0.2 | 96.0 | 1.2 | 0.8 | 2.1 |
| $51,593–<$70,476 | 25.0 | 90.4 | 7.9 | 1.6 | 0.2 | 97.0 | 1.0 | 0.6 | 1.6 |
| $70,476–<$92,647 | 25.0 | 90.4 | 7.7 | 1.8 | 0.2 | 97.4 | 0.9 | 0.5 | 1.2 |
| ≥$92,647 | 25.0 | 90.0 | 7.9 | 1.8 | 0.3 | 98.0 | 0.7 | 0.4 | 0.9 |
Notes. Results reflect column percentages for the Total column and row percentages for frequency of alcohol and nicotine use. Data on median household income was missing for 850 (0.2%) pregnancies. P-values for differences in frequency of alcohol and nicotine use by demographics based on Chi-square tests are all significant at p-value<.0001.
3.1. Frequency of self-reported alcohol and nicotine use
During the study period, 65.6% of women self-reported alcohol use in the year before pregnancy (43.9% monthly or less, 20.0% weekly, 1.6% daily), and 9.5% self-reported alcohol use during pregnancy (7.7% monthly or less, 1.7% weekly, and 0.2% daily) (Table 1). More frequent alcohol use both in the year before pregnancy and during pregnancy was associated with older age, White race/ethnicity, and higher income. Overall, 56.2% of women self-reported alcohol use only during the year before pregnancy, 0.2% self-reported alcohol use only during pregnancy, and 9.3% self-reported alcohol use both in the year before pregnancy and during pregnancy.
Across years, 10.1% of women self-reported nicotine use in the year before pregnancy (3.4% monthly or less, 1.5% weekly, 5.3% daily) and 2.9% self-reported use during pregnancy (0.9% monthly or less, 0.6% weekly, and 1.4% daily) (Table 1). Higher nicotine use frequency both in the year before pregnancy and during pregnancy was associated with younger age, Black or White race/ethnicity, and lower income. Overall, 7.2% of women self-reported nicotine use only during the year before pregnancy, 0.1% self-reported nicotine use only during pregnancy, and 2.9% self-reported nicotine use both in the year before pregnancy and during pregnancy.
In terms of co-use, 8.8% of women reported use of both nicotine and alcohol in the year before pregnancy and 1.2% of women reported use of both nicotine and alcohol during pregnancy.
3.2. Linear trends in self-reported alcohol use frequency
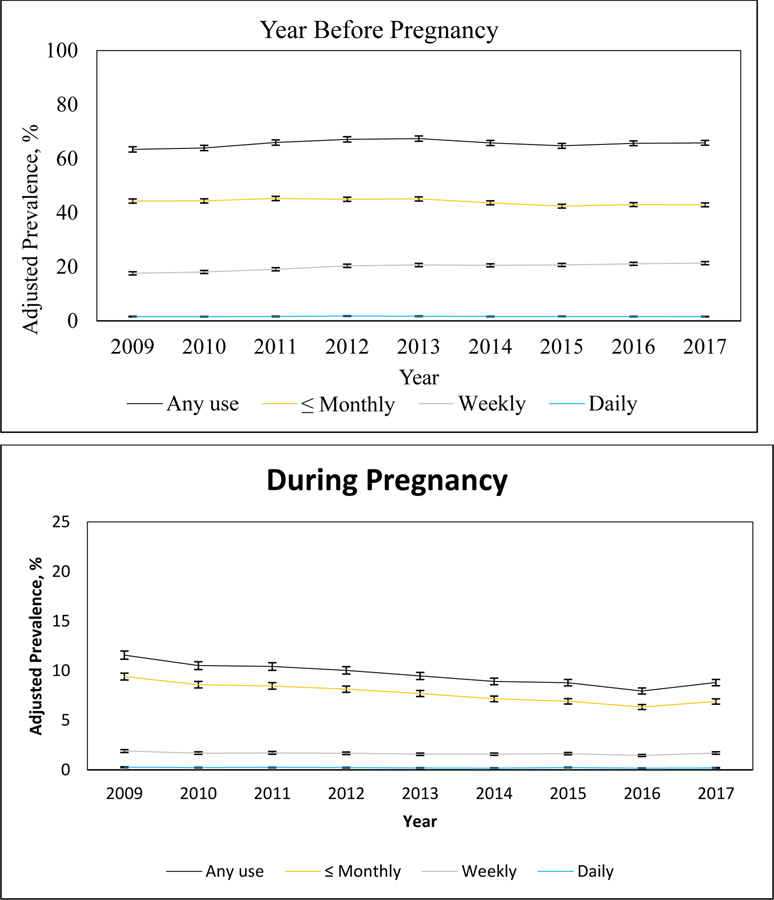
From 2009 to 2017, the adjusted prevalence of any self-reported alcohol use in the year before pregnancy increased slightly from 63.41% (95% CI: 62.44%−64.38%) to 65.85% (95% CI: 64.97%−66.73%) (Figure 1, eAppendix 2). This increase appeared to be driven by a modest increase in self-reported weekly use of alcohol in the year before pregnancy, which increased significantly at an annual relative rate of 1.023 (95% CI: 1.019–1.027), from 17.56% (95% CI: 16.98%−18.14%) in 2009 to 21.35% (95% CI: 20.78%−21.92%) in 2017. In contrast, monthly or less alcohol use in the year before pregnancy decreased slightly at an annual relative rate of 0.994 (95% CI: 0.991–0.996), from 44.31% (95% CI: 43.52%−45.10%) in 2009 to 42.91% (95% CI: 42.21%−43.60%) in 2017, and daily alcohol use in the year before pregnancy did not change significantly over the study period [annual relative rate of change 0.999 (95% CI: 0.990–1.008); 1.57% (95%CI: 1.46%−1.69%) in 2009 and 1.56% (95% CI: 1.45%−1.66%) in 2017].
Figure 1.
Adjusted Prevalence of Self-Reported Alcohol Use in the Year Before Pregnancy and During Pregnancy, by Frequency of Use, 2009–2017 (N = 363,240).
Notes: Estimated annual relative rates of change (95% CI) for alcohol use in the year before pregnancy: Any use [1.003 (1.001, 1.005); p-value = 0.0080], ≤ Monthly use [0.994 (0.991, 0.996); p-value <.0001], Weekly use [1.023 (1.019, 1.027); p-value <.0001], Daily use [0.999 (0.990, 1.008); p-value = 0.7801]. Estimated annual relative rates of change (95% CI) for alcohol use during pregnancy: Any use [0.961 (0.956, 0.966); p-value <.0001], ≤ Monthly use [0.956 (0.951, 0.961); p-value <.0001], Weekly use [0.983 (0.974, 0.993); p-value = 0.0006], Daily use [0.962 (0.941, 0.982); p-value = 0.0003].
During pregnancy, the adjusted prevalence of any self-reported alcohol use decreased significantly from 11.57% (95% CI: 11.16%−11.98%) in 2009 to 8.80% (95% CI: 8.48%−9.12%) in 2017 by 3.9% per year (annual rate=0.961, 95% CI: 0.959–0.966) (Figure 1 and eAppendix 2). From 2009 to 2017, monthly or less alcohol use during pregnancy decreased significantly at an annual relative rate of 0.956 (95% CI: 0.951–0.961), from 9.41% (95% CI: 9.06%−9.75%) to 6.90% (95% CI: 6.63%−7.16%), weekly alcohol use during pregnancy decreased significantly at an annual relative rate of 0.983 (95% CI: 0.974–0.993), from 1.90% (95% CI: 1.76%−2.04%) to 1.70% (95% CI: 1.58%−1.82%), and daily alcohol use during pregnancy decreased significantly at an annual relative rate of 0.962 (95% CI: 0.941–0.982), from 0.27% (95% CI: 0.22%−0.31%) to 0.20% (95% CI: 0.17%−0.23%).
3.3. Linear trends in self-reported nicotine use frequency
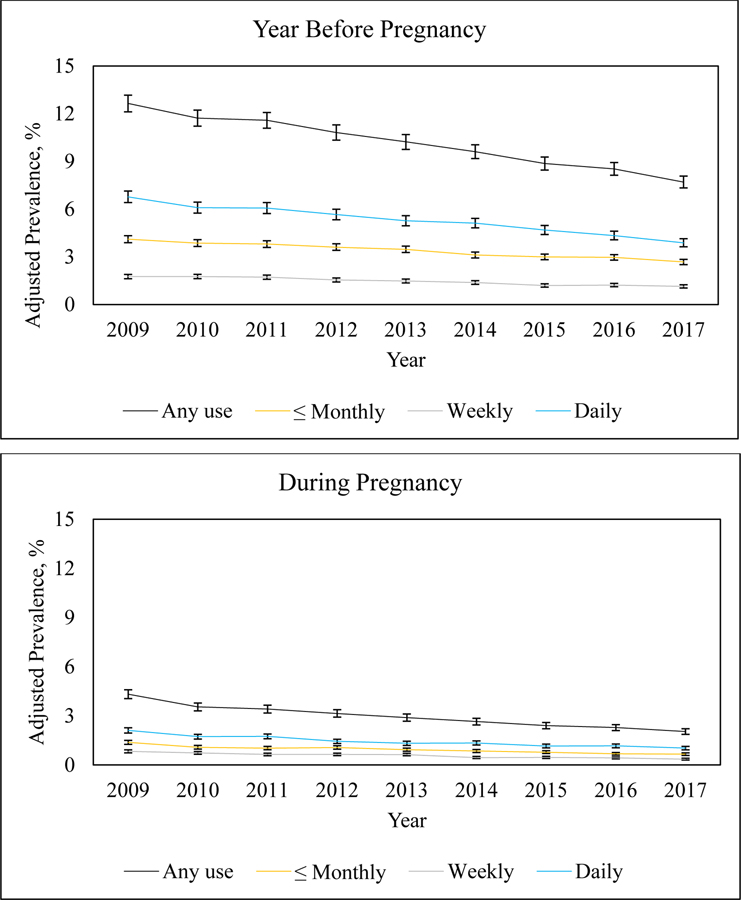
From 2009 to 2017, the adjusted prevalence of any self-reported nicotine use in the year before pregnancy decreased from 12.65% (95% CI: 12.12%−13.17%) to 7.70% (95% CI: 7.33%−8.08%) (Figure 2, eAppendix 3). Monthly or less nicotine use in the year before pregnancy decreased significantly at an annual relative rate of 0.949 (95% CI: 0.943–0.956), from 4.11% (95% CI: 3.89%−4.33%) to 2.68% (95% CI: 2.51%−2.84%), weekly nicotine use in the year before pregnancy decreased significantly at an annual relative rate of 0.941 (95% CI: 0.932–0.951), from 1.76% (95% CI: 1.62%−1.89%) to 1.14% (95% CI: 1.04%−1.24%), and daily nicotine use in the year before pregnancy decreased significantly at an annual relative rate of 0.938 (95% CI: 0.931–0.946), from 6.77% (95% CI: 6.41%−7.14%) to 3.88% (95% CI: 3.63%−4.14%).
Figure 2.
Adjusted Prevalence of Self-Reported Nicotine Use in the Year Before Pregnancy and During Pregnancy, by Frequency of Use, 2009–2017 (N = 363,240).
Notes: Estimated annual relative rates of change (95% CI) for nicotine use in the year before pregnancy: Any use [0.942 (0.937, 0.948); p-value <.0001], ≤ Monthly use [0.949 (0.943, 0.956); p-value <.0001], Weekly use [0.941 (0.932, 0.951); p-value <.0001], Daily use [0.938 (0.931, 0.946); p-value <.0001]. Estimated annual relative rates of change (95% CI) for nicotine use during pregnancy: Any use [0.916 (0.908, 0.925); p-value <.0001], ≤ Monthly use [0.918 (0.906, 0.930); p-value <.0001], Weekly use [0.906 (0.891, 0.922); p-value <.0001], Daily use [0.919 (0.908, 0.930); p-value <.0001].
The adjusted prevalence of any self-reported nicotine use during pregnancy decreased significantly from 4.32% (95% CI: 4.05%−4.59%) to 2.04% (95% CI: 1.87%−2.21%) (Figure 2, eAppendix 3). Monthly or less nicotine use during pregnancy decreased significantly at an annual relative rate of 0.918 (95% CI: 0.906–0.930), from 1.38% (95% CI: 1.26%−1.50%) to 0.66% (95% CI: 0.58%−0.74%), weekly nicotine use during pregnancy decreased significantly at an annual relative rate of 0.906 (95% CI: 0.891–0.922), from 0.83% (95% CI: 0.74%−0.92%) to 0.35% (95% CI: 0.30%−0.41%), and daily nicotine use during pregnancy decreased significantly at an annual relative rate of 0.919 (95% CI: 0.908–0.930), from 2.11% (95% CI: 1.95%−2.27%) to 1.03% (95% CI: 0.92%−1.13%).
3.4. Continued alcohol use in pregnancy
Among the 238,138 women who self-reported any alcohol use in the year before pregnancy, 85.8% self-reported no use during pregnancy and 14.2% self-reported use during pregnancy. The odds of continued alcohol use during pregnancy were higher among women who reported daily (aOR=3.82, 95% CI: 3.60–4.06) or weekly (aOR=2.30, 95% CI: 2.24–2.36) alcohol use compared to those who reported monthly or less alcohol use in the year before pregnancy (Table 2). In addition, demographic factors associated with higher odds of continued alcohol use during pregnancy included being age 11–24 (versus >34), non-White race, having a median neighborhood household income in the first or second (versus fourth) quartile, and having a pregnancy in an earlier study year (Table 2). The adjusted prevalences in continued alcohol use are provided in eAppendix 4.
Table 2.
Estimating the Odds of Any Self-Reported Alcohol or Nicotine Use During Pregnancy Among Women with Self-Reported Alcohol Use (N = 238,138) or Nicotine Use (N = 36,671) in the Year Before Pregnancy.
| Alcohol | Nicotine | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Total (N=238,138) | Any Prenatal Alcohol Use | Total (N=36,671) | Any Prenatal Nicotine Use | ||||
| % | % Yes | % No | OR (95% CI) | % | % Yes | % No | OR (95% CI) | |
| Overall | 100.0 | 14.2 | 85.8 | 100.0 | 28.3 | 71.7 | ||
| Age | ||||||||
| 11–17 | 0.6 | 18.3 | 81.8 | 1.34 (1.16, 1.54) | 2.1 | 34.2 | 65.8 | 1.25 (1.05, 1.49) |
| 18–24 | 14.2 | 15.1 | 84.9 | 1.13 (1.09, 1.18) | 27.1 | 31.4 | 68.6 | 0.96 (0.89, 1.04) |
| 25–34 | 63.5 | 13.7 | 86.3 | 0.93 (0.90, 0.96) | 55.9 | 26.2 | 73.8 | 0.80 (0.74, 0.86) |
| >34 | 21.7 | 15.3 | 84.8 | Reference | 15.0 | 29.7 | 70.3 | Reference |
| Race/ethnicity | ||||||||
| Asian | 12.8 | 14.9 | 85.1 | 1.38 (1.33, 1.43) | 9.7 | 22.0 | 78.0 | 0.78 (0.71, 0.86) |
| Black | 5.7 | 14.2 | 85.8 | 1.16 (1.10, 1.22) | 8.0 | 37.1 | 62.9 | 1.24 (1.13, 1.36) |
| Hispanic | 26.5 | 14.1 | 85.9 | 1.20 (1.17, 1.24) | 24.4 | 22.1 | 77.9 | 0.76 (0.71, 0.81) |
| Other | 11.4 | 14.2 | 85.8 | 1.19 (1.14, 1.24) | 12.7 | 30.5 | 69.6 | 1.02 (0.94, 1.10) |
| White | 43.6 | 14.2 | 85.8 | Reference | 45.2 | 30.9 | 69.1 | Reference |
| Neighborhood Income | ||||||||
| Quartile 1 | 23.4 | 14.1 | 85.9 | 1.04 (1.01, 1.08) | 31.2 | 31.2 | 68.8 | 1.17 (1.08, 1.26) |
| Quartile 2 | 25.0 | 14.4 | 85.7 | 1.05 (1.02, 1.09) | 26.8 | 28.2 | 71.8 | 1.05 (0.97, 1.13) |
| Quartile 3 | 25.4 | 14.1 | 85.9 | 1.02 (0.99, 1.05) | 23.0 | 26.9 | 73.1 | 1.05 (0.97, 1.14) |
| Quartile 4 | 26.0 | 14.3 | 85.7 | Reference | 18.7 | 25.4 | 74.7 | Reference |
| Pre-pregnancy use | ||||||||
| Daily | 2.5 | 29.7 | 70.3 | 3.82 (3.60, 4.06) | 52.2 | 41.7 | 58.3 | 6.03 (5.64, 6.45) |
| Weekly | 30.5 | 20.4 | 79.6 | 2.30 (2.24, 2.36) | 14.4 | 22.5 | 77.5 | 2.55 (2.34, 2.79) |
| Monthly or less | 67.0 | 10.9 | 89.1 | Reference | 33.4 | 9.9 | 90.1 | Reference |
| Calendar year | 100.0 | 14.2 | 85.8 | 0.94 (0.94, 0.94) | 100.0 | 28.3 | 71.7 | 0.95 (0.94, 0.96) |
Notes: Results reflect column percentages for the Total column and row percentages for any prenatal alcohol or nicotine use. Among those women with self-reported alcohol use in the year before pregnancy, neighborhood household income information was missing for 514 (0.2%) pregnancies. The neighborhood household income quartiles among 238,138 pregnancies were as follows: <$52,609 represented 1st quartile, $52,609–<$71,676 represented 2nd quartile, $71,676–<$94,318 represented 3rd quartile, ≥$94,318 represented highest quartile. Among those women with self-reported nicotine use in the year before pregnancy, neighborhood household income information was missing for 95 (0.3%) pregnancies. The neighborhood household income quartiles among 36,671 pregnancies were as follows: <$46,975 represented 1st quartile, $46,975–<$64,485 represented 2nd quartile, $64,485–<$85,870 represented 3rd quartile, ≥$85,870 represented highest quartile.
However, demographic patterns among women with continued alcohol use during pregnancy slightly varied by whether they maintained or increased their frequency of alcohol use (9.7%) or decreased their frequency of alcohol use (4.5%) (Table 3). Similar to the overall associations in Table 2, women aged 11–24 (versus >34), non-White women, and those with a lower median neighborhood household income had slightly higher odds of increasing or maintaining their frequency of alcohol use versus quitting, although the association with income was only statistically significant for the second versus fourth quartile of income (Table 3). In contrast, older women (>34 years), White women, and those with a greater median household neighborhood income had significantly higher odds of decreasing their frequency of alcohol use versus quitting alcohol use during pregnancy, although the association with income was only significant for the third versus fourth quartile of income (Table 3). eAppendix 5 shows the adjusted prevalence of changes in alcohol use frequency.
Table 3.
Estimating the Odds of Changes in Alcohol or Nicotine Use Frequency During Pregnancy Among Women with Self-Reported Alcohol Use (N = 238,138) or Nicotine Use (N = 36,671) in the Year Before Pregnancy.
| Prenatal Alcohol Use | ||||||
|---|---|---|---|---|---|---|
| Increase/maintain (N=23,123, 9.7%) | Decrease (N=10,771, 4.5%) | Quit (N=204,244, 85.8%) | ||||
| Characteristics | % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) |
| Age | ||||||
| 11–17 | 14.7 | 1.33 (1.15, 1.55) | 3.5 | 0.73 (0.55, 0.97) | 81.8 | Reference |
| 18–24 | 11.5 | 1.09 (1.04, 1.15) | 3.6 | 0.71 (0.66, 0.77) | 84.9 | Reference |
| 25–34 | 9.2 | 0.90 (0.87, 0.93) | 4.5 | 0.83 (0.79, 0.87) | 86.3 | Reference |
| >34 | 9.9 | Reference | 5.3 | Reference | 84.8 | Reference |
| Race/ethnicity | ||||||
| Asian | 11.7 | 1.46 (1.40, 1.52) | 3.2 | 0.54 (0.50, 0.58) | 85.1 | Reference |
| Black | 10.4 | 1.21 (1.14, 1.29) | 3.8 | 0.66 (0.60, 0.72) | 85.8 | Reference |
| Hispanic | 10.8 | 1.28 (1.23, 1.33) | 3.3 | 0.58 (0.55, 0.61) | 85.9 | Reference |
| Other | 10.2 | 1.24 (1.18, 1.30) | 4.0 | 0.70 (0.65, 0.74) | 85.8 | Reference |
| White | 8.3 | Reference | 5.9 | Reference | 85.8 | Reference |
| Neighborhood Income | ||||||
| <$52,609 | 10.0 | 1.03 (0.99, 1.07) | 4.2 | 0.94 (0.89, 1.00) | 85.9 | Reference |
| $52,609–<$71,676 | 9.9 | 1.04 (1.00, 1.09) | 4.5 | 0.96 (0.91, 1.02) | 85.7 | Reference |
| $71,676–<$94,318 | 9.6 | 1.02 (0.98, 1.06) | 4.5 | 0.93 (0.88, 0.99) | 85.9 | Reference |
| ≥$94,318 | 9.4 | Reference | 4.9 | Reference | 85.7 | Reference |
| Calendar year | 9.7 | 0.95 (0.95, 0.96) | 4.5 | 0.94 (0.94, 0.95) | 85.8 | Reference |
| Prenatal Nicotine Use | ||||||
| Increase/maintain (N=6,883, 18.8%) | Decrease (N=3,497, 9.5%) | Quit (N=26,291, 71.7%) | ||||
| Characteristics | % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) |
| Age | ||||||
| 11–17 | 19.5 | 0.93 (0.76, 1.14) | 14.8 | 1.92 (1.52, 2.43) | 65.8 | Reference |
| 18–24 | 19.1 | 0.87 (0.80, 0.95) | 12.3 | 1.57 (1.39, 1.77) | 68.6 | Reference |
| 25–34 | 17.7 | 0.75 (0.69, 0.81) | 8.5 | 1.02 (0.91, 1.14) | 73.8 | Reference |
| >34 | 22.0 | Reference | 7.7 | Reference | 70.3 | Reference |
| Race/ethnicity | ||||||
| Asian | 15.4 | 0.67 (0.61, 0.75) | 6.6 | 0.63 (0.55, 0.73) | 78.0 | Reference |
| Black | 22.0 | 1.06 (0.96, 1.18) | 15.1 | 1.53 (1.35, 1.73) | 62.9 | Reference |
| Hispanic | 14.3 | 0.58 (0.54, 0.63) | 7.8 | 0.63 (0.58, 0.70) | 77.9 | Reference |
| Other | 20.4 | 0.96 (0.88, 1.05) | 10.1 | 1.00 (0.89, 1.12) | 69.6 | Reference |
| White | 20.9 | Reference | 10.0 | Reference | 69.1 | Reference |
| Neighborhood Income | ||||||
| <$46,975 | 20.8 | 1.38 (1.27, 1.50) | 10.4 | 1.17 (1.05, 1.30) | 68.8 | Reference |
| $46,975–<$64,485 | 18.5 | 1.17 (1.08, 1.28) | 9.7 | 1.11 (1.00, 1.24) | 71.8 | Reference |
| $64,485–<$85,870 | 18.0 | 1.12 (1.03, 1.23) | 8.9 | 1.03 (0.91, 1.15) | 73.1 | Reference |
| ≥$85,870 | 16.7 | Reference | 8.7 | Reference | 74.7 | Reference |
| Calendar year | 18.8 | 0.96 (0.95, 0.97) | 9.5 | 0.94 (0.93, 0.96) | 71.7 | Reference |
Notes: Results reflect row percentages. Among those women with self-reported alcohol use in the year before pregnancy, neighborhood household income information was missing for 514 (0.2%) pregnancies. Among women with self-reported nicotine use in the year before pregnancy, neighborhood household income information was missing for 95 (0.3%) pregnancies.
3.5. Continued nicotine use during pregnancy
Among the 36,671 women who self-reported any nicotine in the year before pregnancy, 71.7% self-reported no use during pregnancy and 28.3% self-reported nicotine use during pregnancy. Compared to women who self-reported monthly or less nicotine use in the year before pregnancy, those who self-reported daily (aOR = 6.03, 95% CI: 5.64–6.45) or weekly nicotine use (aOR = 2.55, 95% CI: 2.34–2.79) in the year before pregnancy had higher odds of any versus no nicotine use in pregnancy (Table 2). In addition, those aged 11–17 (versus >34), Black women (versus White women), and those with the lowest (versus highest) median neighborhood household income quartile had higher odds of any versus no continued nicotine use during pregnancy, while those aged 25–34 (versus >34), Asian and Hispanic women (versus White women), and those with pregnancies in later study years had lower odds of any versus no continued nicotine use during pregnancy (Table 2). The adjusted prevalences in continued nicotine use are provided in eAppendix 4.
Most of these patterns persisted when continued use was divided into two groups: those who increased or maintained their pre-pregnancy frequency of nicotine use (18.8%) and those who decreased their pre-pregnancy frequency of nicotine use (9.5%) (Table 3). Women ages 18 to 34 (versus >34) and Asian and Hispanic (versus White) women were significantly less likely to increase/maintain their pre-pregnancy frequency of nicotine use (versus quit), and those with lower median neighborhood household incomes were more likely to increase/maintain their pre-pregnancy frequency of nicotine use. In addition, those aged 11–24 (versus >34), Black women, and those with a median neighborhood household income in the first quartile (versus fourth quartile) were more likely to decrease their pre-pregnancy frequency of nicotine use (versus quit), while Asian and Hispanic (versus White women) were significantly less likely to decrease their pre-pregnancy frequency of nicotine use. The adjusted prevalences of change in nicotine use frequency are provided in eAppendix 5.
4. Discussion
Using data from a large study of pregnant women screened for self-reported alcohol and nicotine use in the year before and during pregnancy as part of standard prenatal care from 2009 to 2017, we found that the adjusted prevalence of any alcohol use in the year before pregnancy increased slightly over time, driven by modest increases in weekly alcohol use. However, there were significant decreases in the adjusted prevalence of alcohol use during pregnancy and in the adjusted prevalence of nicotine use both in the year before pregnancy and during pregnancy, with significant decreases in daily, weekly, and monthly or less use. These findings are somewhat consistent with national data from 2002 to 2016 (Agrawal et al., 2019), indicating significant declines in any cigarette smoking during pregnancy, and non-significant decreases in any alcohol use during pregnancy. Notably, the prevalence of nicotine use in our sample was lower than national estimates, consistent with lower smoking rates in California in general (Truth Initiative, 2019).
Health care systems could benefit from additional information about which reproductive-aged women might be at greatest risk for using alcohol or nicotine during pregnancy. Results from the current study indicate that subgroups of women at risk for more frequent pre-conception and prenatal alcohol use include older women, White women, and those with higher neighborhood incomes. These demographic differences might be important for developing interventions to prevent or limit drinking during pregnancy. As these subgroups are not traditionally seen as “at-risk”, results emphasize the importance of universal substance use screening and highlight that women’s health providers should be aware of any unconscious bias or assumptions they make about which women are at risk for alcohol use during pregnancy.
Most women who self-reported alcohol use in the year before pregnancy reported no alcohol use during pregnancy; however, 4.5% reported decreasing their alcohol use frequency during pregnancy, and 9.7% reported increasing or maintaining their frequency of use during pregnancy. Among those who drank in the year before pregnancy, continued alcohol use during pregnancy was most likely among women who drank daily or weekly versus monthly or less, consistent with prior research indicating that pre-pregnancy substance use is a strong predictor of prenatal use (Chang et al., 2006). Further, among those who drank alcohol in the year prior to pregnancy, younger women, non-White women, and those with a lower neighborhood income were more likely to increase or maintain their frequency of alcohol use. These findings indicate that the factors associated with more frequent alcohol use (before and during pregnancy) may be different than those associated with maintaining or increasing pre-pregnancy frequency of use during pregnancy, and subgroups with a lower prevalence of use (younger women, non-White women, those with a lower neighborhood income) may have greater difficulty cutting down or quitting use during pregnancy.
Consistent with prior studies, more frequent nicotine use both in the year before pregnancy and during pregnancy was associated with younger age, Black and White race/ethnicity, and lower income (Goodwin et al., 2017; Kurti et al., 2017). Among women who self-reported any nicotine use in the year before pregnancy, most self-reported no use during pregnancy; however, 9.5% reported decreasing their pre-pregnancy frequency of use during pregnancy, and 18.8% reported increasing or maintaining their pre-pregnancy frequency of use during pregnancy. It is notable that the percentage of women with continued use during pregnancy was twice as high for nicotine versus alcohol, reflecting the highly addictive and reinforcing nature of nicotine addiction. Factors associated with continued nicotine use during pregnancy among those who used in the past year were similar to those associated with greater frequency of prenatal use, including daily or weekly versus monthly or less pre-pregnancy nicotine use, adolescent age group, Black race/ethnicity, and having a lower neighborhood income.
Although most women stop using substances when they learn they are pregnant, many continue to use in the weeks after conception before they realize they are pregnant, and some continue to use throughout pregnancy (Salas-Wright et al., 2015). Research has shown that earlier discontinuation of substance use may decrease risks to the woman and the developing fetus, particularly during the first trimester (American College of Obstetricians and Gynecologists, 2020; Polakowski et al., 2009; Reichert et al., 2009). Notably, 98% of women who self-reported alcohol or nicotine use during pregnancy also reported use in the year prior to pregnancy, and consistent with prior studies (Chang et al., 2006), pre-pregnancy substance use was a strong predictor of prenatal use. Given that few women initiate alcohol or nicotine use during pregnancy, our findings highlight an important window of opportunity to provide education about prenatal substance use to women of reproductive age prior to conception, as it is not possible to offer pregnant patients education about prenatal substance use and advice to quit during the early weeks of pregnancy if they do not know they are pregnant or have not yet initiated prenatal care. In particular, targeted education about the risks of prenatal alcohol and nicotine use, advice to cut down or quit use while trying to conceive, and referrals for substance use interventions and resources for women of reproductive age who report daily or weekly use of alcohol or nicotine could be beneficial.
Substance use screening for women of reproductive age prior to conception may be useful for identifying frequent users who are most at risk for continued use during pregnancy. Screening and documentation of patients use of tobacco and delivery of brief cessation counseling is now routine in many US healthcare systems (Tan et al., 2018), and some healthcare systems now also screen all patients for unhealthy alcohol use as part of standard primary care (Sterling et al., 2020). Our results indicate that screening, along with brief interventions and referrals to treatment, may be particularly important for identifying and providing early intervention for women of reproductive age prior to conception who may be at greater risk for prenatal alcohol and nicotine use. It is important that these conversations are supportive and non-punitive, focused on providing education and support to help women make informed decisions about substance use to increase the likelihood of future substance-free pregnancies. Women’s health clinicians should also discuss risks associated with prenatal substance use at prenatal intake appointments to ensure that all patients receive the recommendation for complete abstinence throughout the pregnancy. Further, education about tracking one’s menstrual cycle for earlier recognition of pregnancy could potentially help women stop alcohol or nicotine use earlier, particularly in cases where women are not actively trying to conceive. Future studies that examine pregnancy intentions may be useful to understand whether trends in prenatal alcohol and nicotine use vary among women whose pregnancies are intended versus unintended.
In contrast to declines in the prevalence and frequency of alcohol and nicotine use among pregnant women seen in the current study, recent studies have found increases in the frequency (Young-Wolff et al., 2019) and prevalence of cannabis use during pregnancy (Agrawal et al., 2019; Brown et al., 2017; Young-Wolff et al., 2017) . Cannabis use during pregnancy commonly co-occurs with alcohol and nicotine use (Goler et al., 2018), and additional studies are needed to better understand patterns of co-use of alcohol, nicotine and cannabis among pregnant women over time.
4.1. Strengths and limitations
This study has a number of strengths, including a large sample of diverse pregnant women universally screened for alcohol and nicotine use as part of standard prenatal care, data on self-reported frequency of use (rather than simply yes or no) both in the year before pregnancy and during pregnancy, and repeated cross-sectional data spanning nine years.
There are also several study limitations. Our sample was limited to pregnant women who completed the self-reported substance use screening questionnaire as part of standard prenatal care. Findings may not be generalizable to pregnant women who did not complete the self-reported substance use screening questionnaire or to those who do not receive prenatal care, who may be more likely to use substances during pregnancy. Data on self-reported alcohol and nicotine use came from the initial prenatal visit (at ~8 weeks gestation), and do not reflect continued use throughout pregnancy. We were unable to differentiate alcohol and nicotine use in pregnancy that occurred before versus after women realized they were pregnant, and many women in our sample who used alcohol or nicotine while pregnancy may have stopped as soon as they became aware of their pregnancy. Finally, our study may underestimate both the prevalence and frequency of alcohol and nicotine use before and during pregnancy as women may choose not to disclose their use to their healthcare provider.
4.2. Conclusions
The prevalence and frequency of self-reported alcohol and nicotine use during pregnancy has decreased significantly over time among women in California, potentially leading to reductions in poor perinatal outcomes. While most pregnant women who self-report alcohol or nicotine use in the year before pregnancy do not report any use of these substances during pregnancy, self-reported daily or weekly pre-pregnancy use is a strong and consistent predictor of alcohol and nicotine use during early pregnancy. Preconception resources and smoking and alcohol education efforts and brief interventions for women who use nicotine or alcohol daily or weekly prior to pregnancy may hold promise for supporting at-risk women in stopping use prior to pregnancy and improving pregnancy and neonatal outcomes.
Supplementary Material
Highlights.
From 2009 to 2017, self-reported pre-pregnancy alcohol use increased.
Pre-pregnancy nicotine use and prenatal alcohol and nicotine use decreased.
Among pre-pregnancy users, frequent pre-pregnancy use increased odds of prenatal use.
Among pre-pregnancy users, socio-demographics were associated with prenatal use.
Prenatal substance use education prior to conception may reduce use in pregnancy
Acknowledgments
We are grateful to Agatha Hinman for her preparation of the manuscript.
Role of Funding Source
This study was supported by a NIH NIDA K01 Award (DA043604). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Declaration of Competing Interest
The authors report no conflict of interests.
References
- Agrawal A, Rogers CE, Lessov-Schlaggar CN, Carter EB, Lenze SN, Grucza RA, 2019. Alcohol, cigarette, and cannabis use between 2002 and 2016 in pregnant women from a nationally representative sample. JAMA Pediatr 173, 95–96. 10.1001/jamapediatrics.2018.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2020. Tobacco and nicotine cessation during pregnancy: ACOG Committee Opinion Summary, Number 807. Obstet. Gynecol 135, 1244–1246. 10.1097/AOG.0000000000003825. [DOI] [PubMed] [Google Scholar]
- Austin AE, Berkoff MC, Shanahan ME, 2020. Incidence of injury, maltreatment, and developmental disorders among substance exposed infants. Child Maltreat, 1077559520930818 10.1177/1077559520930818. [DOI] [PubMed]
- Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS, 2017. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. JAMA 317, 207–209. 10.1001/jama.2016.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C., Prevention, 2009. Alcohol use among pregnant and nonpregnant women of childbearing age - United States, 1991–2005. MMWR Morb Mortal Wkly Rep 58, 529–532. [PubMed] [Google Scholar]
- Chang G, McNamara TK, Orav EJ, Wilkins-Haug L, 2006. Alcohol use by pregnant women: partners, knowledge, and other predictors. J Stud Alcohol 67, 245–251. 10.15288/jsa.2006.67.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanga AA, Sabel JC, Ko JY, Wasserman CR, Shapiro-Mendoza CK, Taylor P, Barfield W, Cawthon L, Paulozzi LJ, 2012. Maternal drug use and its effect on neonates: a population-based study in Washington State. Obstet. Gynecol 119, 924–933. 10.1097/AOG.0b013e31824ea276. [DOI] [PubMed] [Google Scholar]
- Crume T, 2019. Tobacco use during pregnancy. Clin. Obstet. Gynecol 62, 128–141. 10.1097/GRF.0000000000000413. [DOI] [PubMed] [Google Scholar]
- Dejong K, Olyaei A, Lo JO, 2019. Alcohol use in pregnancy. Clin. Obstet. Gynecol 62, 142–155. 10.1097/GRF.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA, National Birth Defects Prevention, S., 2009. Alcohol consumption by women before and during pregnancy. Matern Child Health J 13, 274–285. 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A, 2016. Substance use during pregnancy. F1000Res 5 10.12688/f1000research.7645.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Elam KK, Natsuaki MN, Neiderhiser JM, Harold GT, 2013. Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry 70, 956–963. 10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goler N, Conway A, Young-Wolff KC, 2018. Data are needed on the potential adverse effects of marijuana use in pregnancy. Ann. Intern. Med 169, 492–493. 10.7326/M18-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Cheslack-Postava K, Nelson DB, Smith PH, Hasin DS, Janevic T, Bakoyiannis N, Wall MM, 2017. Serious psychological distress and smoking during pregnancy in the United States: 2008–2014. Nicotine Tob Res 19, 605–614. 10.1093/ntr/ntw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NP, 2013. Characteristics of adult members in Kaiser Permanente’s Northern California Region, as estimated from the 2011 Kaiser Permanente Adult Member Health Survey, Kaiser Permanente California, Oakland CA, Oakland CA, https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/mhs11reg.pdf. (accessed on March 10, 2020). [Google Scholar]
- Gordon NP, 2015. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2011–2012 California Health Interview Survey, Kaiser Permanente Division of Research, Oakland CA, https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf. (accessed on March 24, 2020). [Google Scholar]
- Hasin DS, Shmulewitz D, Keyes K, 2019. Alcohol use and binge drinking among U.S. men, pregnant and non-pregnant women ages 18–44: 2002–2017. Drug Alcohol Depend 205, 107590 10.1016/j.drugalcdep.2019.107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti AN, Redner R, Lopez AA, Keith DR, Villanti AC, Stanton CA, Gaalema DE, Bunn JY, Doogan NJ, Cepeda-Benito A, Roberts ME, Phillips J, Higgins ST, 2017. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med 104, 50–56. 10.1016/j.ypmed.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuire C, Daniel R, Hurt L, Kemp A, Paranjothy S, 2020. The causal web of foetal alcohol spectrum disorders: a review and causal diagram. Eur. Child Adolesc. Psychiatry 29, 575–594. 10.1007/s00787-018-1264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz TD, Borgelt LM, 2018. Marijuana use in pregnancy and while breastfeeding. Obstet. Gynecol 10.1097/AOG.0000000000002878. [DOI] [PMC free article] [PubMed]
- Meyer-Leu Y, Lemola S, Daeppen JB, Deriaz O, Gerber S, 2011. Association of moderate alcohol use and binge drinking during pregnancy with neonatal health. Alcohol. Clin. Exp. Res 35, 1669–1677. 10.1111/j.1530-0277.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- Moise IK, 2019. Alcohol use, pregnancy and associated risk factors: a pilot cross-sectional study of pregnant women attending prenatal care in an urban city. BMC Pregnancy Childbirth 19, 472 10.1186/s12884-019-2652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BF, Shapiro AL, Wilkening G, Magzamen S, Starling AP, Allshouse WB, Adgate JL, Dabelea D, 2020. Prenatal exposure to tobacco and offspring neurocognitive development in the Healthy Start Study. J. Pediatr 218, 28–34 e22. 10.1016/j.jpeds.2019.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nighbor TD, Doogan NJ, Roberts ME, Cepeda-Benito A, Kurti AN, Priest JS, Johnson HK, Lopez AA, Stanton CA, Gaalema DE, Redner R, Parker MA, Keith DR, Quisenberry AJ, Higgins ST, 2018. Smoking prevalence and trends among a U.S. national sample of women of reproductive age in rural versus urban settings. PLoS One 13, e0207818 10.1371/journal.pone.0207818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary CM, Nassar N, Kurinczuk JJ, Bower C, 2009. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG 116, 390–400. 10.1111/j.1471-0528.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- Oga EA, Mark K, Coleman-Cowger VH, 2018. Cigarette smoking status and substance use in pregnancy. Matern Child Health J 22, 1477–1483. 10.1007/s10995-018-2543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Dudley J, Martin PR, Harrell FE, Warren MD, Hartmann KE, Ely EW, Grijalva CG, Cooper WO, 2015. Prescription opioid epidemic and infant outcomes. Pediatrics 135, 842–850. 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakowski LL, Akinbami LJ, Mendola P, 2009. Prenatal smoking cessation and the risk of delivering preterm and small-for-gestational-age newborns. Obstet. Gynecol 114, 318–325. 10.1097/AOG.0b013e3181ae9e9c. [DOI] [PubMed] [Google Scholar]
- Reichert V, Xue X, Bartscherer D, Jacobsen D, Fardellone C, Folan P, Kohn N, Talwar A, Metz CN, 2009. A pilot study to examine the effects of smoking cessation on serum markers of inflammation in women at risk for cardiovascular disease. Chest 136, 212–219. 10.1378/chest.08-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisch IH, Dietrich A, Glennon JC, Buitelaar JK, Hoekstra PJ, 2018. Maternal substance use during pregnancy and offspring conduct problems: A meta-analysis. Neurosci. Biobehav. Rev 84, 325–336. 10.1016/j.neubiorev.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Salas-Wright CP, Vaughn MG, Ugalde J, Todic J, 2015. Substance use and teen pregnancy in the United States: evidence from the NSDUH 2002–2012. Addict. Behav 45, 218–225. 10.1016/j.addbeh.2015.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD, McFarland BH, 2005. Kaiser Permanente Medical Care Program In: Strom BL (Ed.), Pharmacoepidemiology Wiley, New York: pp. 241–259. [Google Scholar]
- Shmulewitz D, Hasin DS, 2019. Risk factors for alcohol use among pregnant women, ages 15–44, in the United States, 2002 to 2017. Prev Med 124, 75–83. 10.1016/j.ypmed.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling SA, Palzes VA, Lu Y, Kline-Simon AH, Parthasarathy S, Ross T, Elson J, Weisner C, Maxim C, Chi FW, 2020. Associations between medical conditions and alcohol consumption levels in an adult primary care population. JAMA Netw Open 3, e204687 10.1001/jamanetworkopen.2020.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ASL, Young-Wolff KC, Carter-Harris L, Salloum RG, Banerjee SC, 2018. Disparities in the receipt of tobacco treatment counseling within the US context of the Affordable Care Act and Meaningful Use implementation. Nicotine Tob. Res 20, 1474–1480. 10.1093/ntr/ntx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D, 2015. Alcohol use and binge drinking among women of childbearing age - United States, 2011–2013. MMWR Morb Mortal Wkly Rep 64, 1042–1046. 10.15585/mmwr.mm6437a3. [DOI] [PubMed] [Google Scholar]
- Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, England LJ, Centers for Disease, C., Prevention, 2013. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill. Summ 62, 1–19. [PubMed] [Google Scholar]
- Truth Initiative, 2019. Tobacco use in California 2019, https://truthinitiative.org/research-resources/smoking-region/tobacco-use-california-2019. (accessed on March 24, 2020).
- Young-Wolff KC, Sarovar V, Tucker LY, Conway A, Alexeeff S, Weisner C, Armstrong MA, Goler N, 2019. Self-reported daily, weekly, and monthly cannabis use among women before and during pregnancy. JAMA Netw Open 2, e196471 10.1001/jamanetworkopen.2019.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Tucker LY, Alexeeff S, Armstrong MA, Conway A, Weisner C, Goler N, 2017. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009–2016. JAMA 318, 2490–2491. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.