Abstract
An improved understanding of life-history responses to current environmental variability is required to predict species-specific responses to anthopogenic climate change. Previous research has suggested that cooperation in social groups may buffer individuals against some of the negative effects of unpredictable climates. We use a 15-year dataset on a cooperative breeding arid zone bird, the southern pied babbler Turdoides bicolor, to test (i) whether environmental conditions and group size correlate with survival of young during three development stages (egg, nestling, fledgling) and (ii) whether group size mitigates the impacts of adverse environmental conditions on survival of young. Exposure to high mean daily maximum temperatures (mean Tmax) during early development was associated with reduced survival probabilities of young in all three development stages. No young survived when mean Tmax > 38°C, across all group sizes. Low survival of young at high temperatures has broad implications for recruitment and population persistence in avian communities given the rapid pace of advancing climate change. Impacts of high temperatures on survival of young were not moderated by group size, suggesting that the availability of more helpers in a group is unlikely to buffer against compromised offspring survival as average and maximum temperatures increase with rapid anthropogenic climate change.
Keywords: climate change, cooperative breeding, dryland ecology, environmental variability, survival of young, southern pied babbler
1. Introduction
Anthropogenic climate change has altered weather patterns in every ecosystem on Earth [1,2], with far-reaching consequences for population dynamics across taxa [3]. An improved understanding of life-history responses to current environmental variability is required to predict species-specific responses to climate change [4]. While cooperative breeders occur in diverse habitats [5,6], comparative research has demonstrated that both cooperatively breeding birds [7] and mammals [8] occur with disproportionate frequency in regions characterized by high spatial and temporal variability in environmental conditions. This implies that group living enhances the ability to persist in challenging environments [9]. To date, however, there are few empirical studies that explicitly test the extent to which group living mitigates the effects of climate variability on reproduction [10–14]. Some of these studies [10,11,14] explore impacts of temperature alongside variation in group size, but only Covas et al. [13] consider offspring survival across more than one development stage. This latter point is important, because specific drivers of survival can differ substantially between development stages [15–17].
Recent changes in temperature and rainfall patterns have led to adjustments to the timing and success of breeding in some bird species [18,19]. For birds in arid environments, higher rainfall is often associated with improved offspring survival [20,21], droughts with reduced offspring survival [22,23], and periods of very hot weather with lower nest survival rates [15] and nestling growth rates [24–26]. Offspring survival and population persistence of birds in arid environments will be further impacted as regions become hotter and drier under climate change [27,28].
Cooperative breeding, where more than two individuals rear a single brood [29], occurs in approximately 9% of bird species [30]. Benefits of cooperation include earlier fledging age and more broods raised per season [31], reduced costs of breeding for females [11,32], enhanced egg investment [33], increased fledgling recruitment [17,34], and the ability to raise overlapping broods [12,35]. Global comparative studies suggest that cooperative breeding evolved in unpredictable environments [36], facilitated the colonization of such environments [37] or prevented extinction under increasingly harsh conditions [38]. One prominent explanation for the occurrence of cooperative breeding in birds in variable environments is that it represents a ‘bet-hedging’ strategy [36], reducing interannual variation in offspring survival in response to unpredictable rainfall and food availability [9]. This implies that cooperation might buffer breeding attempts from failure during adverse environmental conditions [13,39], via load-lightening [40].
Load-lightening refers to individual reductions in workload in response to the presence of additional group members. Load-lightening has been observed in a number of cooperatively breeding species [11,16,41] and may operate via task-partitioning [35,42] or improving access to resources [43]. In larger groups, more individuals can assist with breeding attempts, leading to either load-lightening among individual group members [11,35] or cumulatively greater investment in young [34,44]. Both are potential benefits of group living that may be particularly advantageous when unfavourable rainfall or temperature conditions are experienced. Specifically, larger groups may be better able to maintain adequate levels of parental care to eggs, nestlings and/or fledglings at high temperatures or during periods of low rainfall, despite individual declines in investment in parental care behaviours.
We use a comprehensive 15-year dataset on southern pied babblers Turdoides bicolor (hereafter ‘pied babblers’), a cooperatively breeding passerine endemic to the Kalahari in southern Africa, to explore the impacts of temperature, rainfall and group size (the number of adults in the group, indicating the potential for the breeding pair to receive help) on the survival of young, including fledgling survival. Specifically, we test for effects of these parameters on survival of young from (i) initiation of incubation to hatching, (ii) hatching to fledging and (iii) fledging to nutritional independence at 90 days of age. We expected high temperatures to reduce survival and high rainfall and larger group sizes to enhance survival during each development stage. If the presence of helpers buffers the effect of environmental variation on reproduction [9], then we would expect an interaction between environmental factors and group size, such that weaker impacts of adverse climatic conditions on reproduction are observed in larger groups.
2. Material and methods
(a). Study site and system
Fieldwork was conducted at the Kuruman River Reserve (33 km2, KRR; 26°58′S, 21°49′E) in the southern Kalahari. Mean summer daily maximum temperatures at the study site, from 1995 to 2015, averaged 34.7 ± 9.7°C and mean annual precipitation averaged 186.2 ± 87.5 mm [45]. The Kalahari region is characterized by hot summers and periodic droughts [46], with extremely variable rainfall between years [47], and increases in both the frequency and severity of high temperature extremes over the last 20 years [48]. Pied babblers weigh 60–90 g and live in territorial groups ranging in size from 3 to 15 adults [49]. Groups consist of a single breeding pair with subordinate helpers [50], and all adult group members (individuals greater than 1 year old) engage in cooperative behaviours, including territory defence and parental care [51,52]. Pied babblers breed during the austral summer, from September to March [51]. Previous research has shown that high temperatures and drought negatively affect many aspects of this species's ecology, including foraging efficiency, body mass maintenance, interannual survival and provisioning of young [53–55].
Birds in the study population are marked as nestlings with a metal band (engraved with a unique number) and a unique combination of up to three colour rings for individual identification and are habituated to observation at distances of 1–5 m [52]. Habituated groups are visited weekly during the breeding season to check group composition and record life-history events, including breeding activity.
(b). Data collection
Data were collected for each austral summer breeding season from September 2005 to February 2019 (14 breeding seasons in total).
(i). Nest life-history data
Nest monitoring (locating nests, determining incubation, hatch and fledge or failure dates, recording group size and brood size) followed Ridley & van den Heuvel [31]. Nests were located by observing nest-building, and incubation start, hatch and fledge dates were determined by checking nests every 2–3 days. Breeding attempts were considered to have failed when nests were no longer attended, or when dependent fledglings were not seen on two consecutive visits. Failure dates were calculated as the midpoint between the date of the last pre-fail nest/group check and the date when the nest was no longer attended or the fledgling was missing. In most cases, it was not possible to determine the proximate cause of nest failure or death, although common causes of nest failure in this species include predation, abandonment and nestling starvation [49,56].
Group size (mean = 4.2 ± 1.5, range: 2–10 adults) was recorded for each nest incubated. Brood size (mean = 2.7 ± 0.8, range: 1–5 nestlings) was recorded 11 days after hatching, when nestlings were ringed. We defined early development as the period between initiation of incubation and nutritional independence at 90 days of age [52]. Average time from initiation of incubation to hatching is 14 ± 1.2 days and from hatching to fledging is 15.4 ± 1.7 days. Pied babblers are nutritionally independent (receiving less than 1 feed per hour) by 90 days of age [52].
(ii). Sexing and nestling mass
Pied babblers are sexually monomorphic [51] and molecular sexing was used to determine the sex of individuals (sensu Fridolfsson & Ellegren [57]). Blood samples were collected by brachial venipuncture and stored in Longmire's lysis buffer. Nestlings were ringed, blood sampled and weighed to 0.1 g (Mass11) on a top-pan scale 11 days post-hatching.
(iii). Temperature and rainfall
Daily maximum temperature (°C) and rainfall (mm) data were collected from an on-site weather station (Vantage Pro2, Davis Instruments, Hayward, USA). Missing weather data from 2009, 2010 and 2011 were sourced from a nearby South African Weather Services weather station (Van Zylsrus, 28 km away), which produces significantly repeatable temperature measurements and moderately repeatable rainfall measurements, adequately detecting wet versus dry periods, in comparison with the on-site weather station [58].
Daily minimum (Tmin) and maximum (Tmax) temperatures, and daily temperature variation (Tmax − Tmin), were averaged for each development stage: incubation (mean TminInc, mean TmaxInc, mean TvarInc), nestling (mean TminBrood, mean TmaxBrood, mean TvarBrood) and fledgling (mean Tmin90, mean Tmax90, mean Tvar90). Rainfall was summed for the 60 days prior to initiation of incubation (Rain60), and for the period between fledging and independence (Rain90).
(c). Statistical analyses
Statistical analyses were conducted in R version 3.6.0 [59]. All continuous explanatory variables were scaled by centreing and standardizing by the mean [60,61]. All explanatory variables were tested for correlation with one another [62]. Mean Tmax90 and mean Tmin90 were correlated (VIF = 2.8, correlation coefficient = 0.77) and all other explanatory variables were not correlated with each other (all VIF < 1.3, correlation coefficients less than 0.30). Correlated variables were not included in the same additive models. Unless otherwise indicated, summary statistics are presented as mean ± one standard deviation. Analyses exclude groups greater than 8 due to small sample sizes for groups of 9 (n = 5) and 10 (n = 1) over 15 years of records. A quadratic term for temperature (T2) was included as a predictor variable only when no main linear effect of temperature was found and visual inspection of the data suggested a nonlinear relationship. Sensitivity power analyses, using the package pwr [63], indicated sufficient statistical power to detect effects of two-way interactions given our sample sizes [64,65], see electronic supplementary material table S1. We tested for temporal trends in environmental (temperature and rainfall) and reproductive (nest success, fledgling survival) parameters using univariate linear models with breeding season as the only predictor. Covariates exhibiting temporal trends were detrended using the detrend function in the package pracma [66].
(i). Survival probabilities during each development stage
Pied babbler survival probabilities are not constant across time during early development [51] (see electronic supplementary material, figure S1), and covariates are unlikely to have the same relationship with survival during all three early development stages. We used generalized linear mixed effects models (GLMMs) with a binomial distribution and a logit link function in the package lme4 [67] to determine which variables best predicted survival probabilities during each development stage. These analyses were undertaken at the level of the breeding attempt (i.e. clutch or brood) because individual offspring were only ringed for individual identification from the 11th day after hatching, by which time approximately 60% of monitored breeding attempts had failed. Model selection using Akaike's information criterion corrected for small sample size (AICc) with maximum-likelihood estimation was used to test a series of models to determine which best explained patterns of variation in the data [60]. Where several models were within five AICc of the top model, top model sets were averaged using the package MuMIn [68]. Model terms with confidence intervals not intersecting zero were considered to explain significant patterns in our data [69]. Binomial model fits were tested against the dispersion parameter in the package RVAideMemoire [70].
We considered the influence of the following parameters on (i) the probability of at least one egg per clutch surviving to hatch, (ii) the probability of at least one nestling per brood surviving to fledge and(iii) the probability of at least one fledgling per brood surviving to nutritional independence: for (i) group size, Rain60, mean TminInc, mean TmaxInc and mean TvarInc, (ii) group size, Rain60, mean TminBrood, mean TmaxBrood, mean TmaxBrood2 and mean TvarBrood, and (iii) group size, Rain90, mean Tmin90, mean Tmax90, mean Tmax902 and mean Tvar90. We tested two-way interactions between rainfall, Tmax, and group size variables in each analysis, and, in order to account for non-independence of data, included group identity as a random term in all three analyses.
In order to contribute data that can be incorporated into mechanistic modelling of the effects of climate change on avian populations [4], we further sought to identify the threshold Tmax (breakpoints) above which survival was compromised during each development stage. We used the package segmented [71] to apply a Davies test for a non-zero difference-in-slope and, when the regression parameter in the linear predictor was non-constant, to identify a point estimate and 95% confidence interval for the breakpoint. We then fitted simple linear regression models for the data above and below the identified breakpoints with temperature (mean TmaxInc, mean TmaxBrood and mean Tmax90, respectively) as the only predictor, and survival per breeding attempt as the response. In the segmented regressions, we used a continuous form of the survival response, specifically the number of days between (i) initiation of incubation and either the hatching of at least one egg or failure of the breeding attempt before hatching (age at hatch/fail), (ii) hatching and fledging at least one nestling from a brood or failure (age at fledge/fail) and (iii) fledging and at least one fledgling surviving to nutritional independence or failure (age at survival/fail).
(ii). Influence of nestling mass on fledgling survival
In addition to survival data at the scale of the breeding attempt, we have detailed individual-level survival data for 372 fledglings weighed and banded as 11-day-old nestlings. Larger nestling mass is commonly associated with higher survival probabilities in birds [16,72]. Prior research on pied babblers has shown that nestling mass is influenced by environmental factors such as temperature and rainfall [54]. We therefore used a confirmatory path analysis [73,74] to test for indirect effects of environmental and group size factors on survival to nutritional independence in known individual fledglings mediated via their mass as a nestling (Mass11). We computed the path analysis using the R package piecewiseSEM [75], which can accommodate multiple error structures. This capacity is important because the response terms of our component models have different distributions (see below). Path analysis allowed us to specify and simultaneously quantify all hypothesized relationships of interest, including the indirect effects of weather and group size on survival via nestling mass. Path coefficients are partial regression coefficients and can be interpreted similarly to simple and multiple regression outputs. Statistical significance was taken as p < 0.05. We hypothesized that:
Survival would be negatively affected by (i) high temperatures during the nestling and fledgling stages, (ii) low rainfall between fledging and independence, (iii) smaller group size and (iv) low nestling body mass (model with binomial error structure).
Nestling body mass would be negatively affected by (i) high mean temperatures during the nestling period, (ii) low rainfall prior to the nestling period and (iii) smaller group size (model with Gaussian error structure).
3. Results
(a). Temporal patterns in temperature, rainfall and reproduction
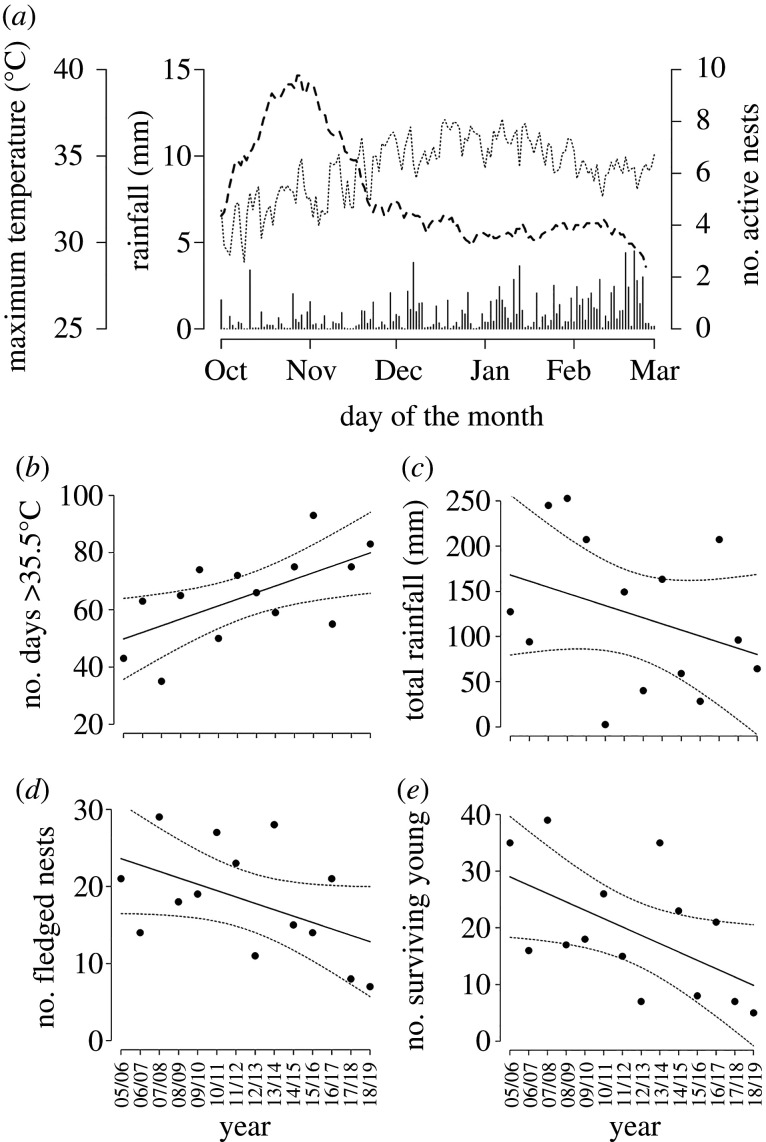
Most rain falls between December and February (72%), when temperatures are high (figure 1). Most pied babbler breeding activity occurs between October and December (68%), when conditions are generally drier and cooler than later in the season (figure 1a). The total number of days (Oct–Mar) exceeding 35.5°C, identified as a critical temperature threshold in pied babblers [53,54], has increased significantly at the study site since 2005 (F1,12 = 7.448, p = 0.018; figure 1b). Total summer rainfall (Oct–Mar) over the same time period was highly variable but showed a declining, non-statistically significant trend (F1,12 = 1.616, p = 0.228; figure 1c). Both the number of nests fledged (a non-significant trend; F1,12 = 3.747, p = 0.077; figure 1d) and the number of surviving young produced (F1,12 = 5,285, p = 0.040; figure 1e) have declined at the study site since 2005, despite the number of groups monitored remaining relatively constant between years (coefficient of variation = 0.17).
Figure 1.
(a) Breeding activity between October and March (average number of active nests per day: dashed line), relative to temperature (average daily maximum temperature in °C per day: dotted line) and rainfall (average rainfall in mm per day: vertical bars). (b) the number of days >35.5°C at the study site (c) total summer rainfall, (d) number of southern pied babbler Turdoides bicolor nests fledged in the study population and (e) number of surviving young produced in the study population per breeding season per year (austral summer: 1 October to 1 March) since 2005. Black lines in (b–e) represent predictions from the linear regression models, and dashed lines the 95% confidence intervals.
(b). Survival probabilities during each development stage
Overall, 31.4 ± 10.9% of breeding attempts produced at least one fledgling that survived to nutritional independence. Mean (±s.e.) survival probabilities of young differed between life stages (incubation, nestling, dependent fledgling), with lower survival probabilities during early development in the nest than post-fledging (electronic supplementary material, figure S1c).
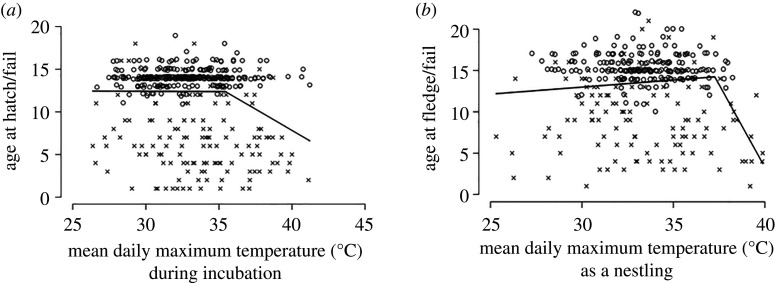
Of 489 breeding attempts by 50 groups over 14 breeding seasons, 339 hatched (69.3%). The probability of at least one egg per clutch hatching decreased as mean TmaxInc increased (electronic supplementary material, table S2a). We found no evidence that mean TminInc, mean TvarInc, Rain60 or group size, or interactions between environmental conditions, influenced the probability of hatching (see electronic supplementary material, table S3 for full model output). For the period between initiation of incubation and hatching, a breakpoint was detected at 35.4°C (95% CI: 33.9, 36.9): there was no effect of mean TmaxInc on age at hatch/fail below 35.4°C (F1,399 = 0.008, p = 0.926), whereas above 35.4°C, age at hatch/fail significantly declined with increasing temperature (F1,85 = 9.490, p = 0.003, figure 2a).
Figure 2.
Survival from (a) initiation of incubation to hatch and (b) hatch to fledge as a function of mean daily maximum air temperature during the corresponding time period. Lines represent segmented linear regressions for the relationship between survival age and air temperature above and below the detected temperature thresholds. Open circles indicate that the clutch (a) or brood (b) transitioned to the next development stage; crosses indicate failure of the clutch (a) or brood (b).
Of 339 hatched nests by 46 groups over 14 breeding seasons, 210 fledged at least one chick (61.9%). The probability of at least one nestling per brood surviving to fledge increased with increasing mean TmaxBrood until approximately 33.1°C, above which survival probability decreased (electronic supplementary material, table S2b). We found no evidence that mean TminBrood, mean TvarBrood, Rain60 or group size, or interactions between group size and environmental conditions, influenced the probability of fledging (see electronic supplementary material, table S4 for full model output). For the period between hatching and fledging, a breakpoint was detected at 37.3°C (95% CI: 36.5, 38.0). Age at fledge/fail tended to increase with increasing mean TmaxBrood until 37.3°C (F1,317 = 3.239, p = 0.073), above which age at fledge/fail declined significantly with increasing temperature (F1,20 = 13.370, p = 0.002, figure 2b). At mean TmaxBrood > 38°C (n = 12), no nests fledged young.
Of 198 fledged broods with complete weather data by 36 groups over 14 breeding seasons, 160 produced at least one fledgling that survived to nutritional independence (80.8%). The probability of surviving to nutritional independence increased as Rain90 increased (electronic supplementary material, table S2c). We found no evidence that mean Tmin90, mean Tmax90, mean Tvar90, group size or interactions between group size and environmental conditions influenced the probability of fledgling survival to independence (see electronic supplementary material, table S5 for full model output). We also found no evidence for a breakpoint in the data related to variation in mean Tmax90 for the period between fledging and independence (Davies test p = 0.288). While temperature was not a significant predictor of survival to nutritional independence overall, no breeding attempts produced surviving young at mean Tmax90 > 38°C (n = 8).
(c). Influence of nestling mass on fledgling survival
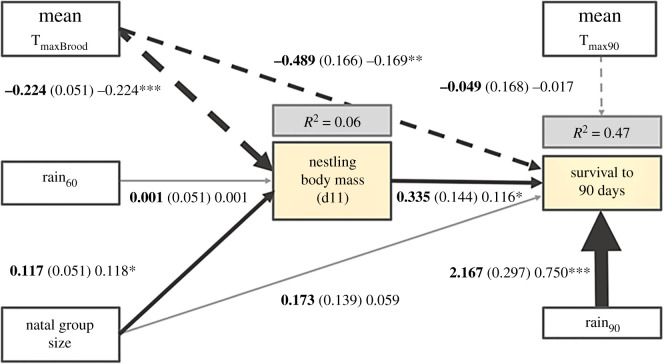
The confirmatory path analysis model explained 47% of the variation in survival from fledging to independence (figure 3; X2 = 0.689, p = 0.708). Higher Rain90 was directly associated with an increased probability of surviving to independence, and larger group sizes were indirectly associated with increased survival via the positive effect of larger group size on nestling Mass11. High mean TmaxBrood was associated with reduced survival both directly and indirectly (high mean TmaxBrood was associated with reduced nestling mass, which in turn predicted reduced survival). There was no evidence for a direct effect of either mean Tmax90 or natal group size on survival to independence, or an effect of Rain60 on Mass11.
Figure 3.
Confirmatory path analysis exploring the effects of environmental factors (temperature and rainfall) and group size on nestling body mass and survival to nutritional independence (90 days). Boxes represent measured variables. Arrows represent hypothesized unidirectional relationships among variables. Solid arrows denote positive relationships, dashed arrows negative relationships. Unstandardized path coefficients are shown in bold, followed by standard errors in parentheses, standardized estimates and an indicator of statistical significance of the effect (asterisks). Non-significant paths are grey. The thickness of significant paths has been scaled relative to the absolute magnitude of the standardized estimates, such that stronger effects have thicker arrows. R2 for component models are given (grey shaded boxes) in the grey boxes above response variables (orange shaded boxes). (Online version in colour.)
Specifically, larger nestlings were more likely to survive to independence (Est = 0.116, p = 0.019), as were fledglings that experienced higher Rain90 (Est = 0.750, p < 0.001). However, fledglings were less likely to survive (Est = −0.169, p = 0.003) when they had experienced higher mean TmaxBrood. Nestlings were heavier when raised by larger groups (Est = 0.118, p = 0.021) and lighter when they experienced higher mean TmaxBrood (Est = −0.224, p < 0.001). There was an indirect negative effect of mean TmaxBrood on survival via nestling mass [Est = −0.026 (calculated by multiplying standardized estimates for each component of the indirect path: −0.244 × 0.116; figure 3)]. The combined direct and indirect effect of mean TmaxBrood (via nestling mass) on survival was negative [−0.195, calculated by summing standardized estimates for direct and indirect paths: −0.169 + (−0.026); figure 3]. The direct effect of mean TmaxBrood was more prominent (approx. 87% of the combined effect) than the indirect effect via nestling mass (approx. 13% of the combined effect). Natal group size had an indirect positive effect on survival via nestling mass [Est = 0.014 (=0.118 × 0.116)], with an overall effect of natal group size = 0.132 (0.059 + 0.014).
4. Discussion
We investigated the impacts of environmental conditions and the potential for larger group sizes to buffer against these impacts in a cooperatively breeding bird, focusing on egg, nestling and fledgling survival. Exposure to high mean daily maximum temperatures during early development was associated with significant reductions in survival probabilities, in keeping with other recent studies [23,24,26]. Both environmental (largely direct) and social (indirect) factors were important for predicting survival during different development stages. Contrary to our expectations, we found no evidence that effects of Tmax and rainfall on reproduction were moderated by group size, despite considerable statistical power to detect such interactions. Taken together with evidence that temperatures are increasing and rainfall decreasing at the study site, and that the number of surviving young produced in the study population showed similar declines, the impacts of high temperatures on mortality during early development are concerning.
(a) Impacts of high temperatures during early development
In pied babblers, high mean daily maximum temperatures during early development were associated with a significantly increased risk of mortality. Adverse weather is known to impair egg [76] and nestling development [77]. For example, survival to fledging can be compromised both by sub-optimally cool [78] and sub-optimally hot conditions [24,26]. For the early development stages before fledging, we identified temperature thresholds in the mid- to high-30 s (35.4°C during incubation, and 37.3°C for nestlings) above which survival of eggs and young became significantly less likely. These temperatures are within approximately 2°C of an apparent upper limit (38°C) above which we recorded no successful breeding in this species over 15 years of research. While we did not detect a direct effect of high temperatures on post-fledging mortality (i.e. between fledging and independence) at the brood scale, path analysis revealed that the probability of individual fledglings surviving to independence was influenced by high temperatures experienced as a nestling (Mean TmaxBrood), both directly and indirectly via the effect of high temperatures on nestling Mass11. This suggests that dependent pied babbler fledglings, similar to the young of other species [79,80], are influenced by carryover effects of high temperatures they experienced while still in the nest. With temperatures increasing rapidly in the Kalahari [28,46], the 38°C limit for successful breeding in this species suggests that pied babblers may increasingly experience conditions that do not allow successful breeding. This could undermine population growth and ultimately lead to local extinctions for this species, although the effect may be mitigated by behavioural adjustments such as breeding earlier in the season or engaging in compensatory breeding during good years [58].
(b). Different drivers of survival for each early development stage
The primary climatic (temperature and rainfall) and social (group size) drivers of survival probability were different across the three development stages. Mean daily maximum temperature was the strongest predictor of survival probability during both the incubation and nestling development stages. At high temperatures over prolonged periods, incubating birds may not be able to sustain nest attendance to regulate egg temperature [81], leaving eggs vulnerable to overheating and becoming unviable [15,76]. Likewise, several studies have reported that high temperatures constrain nestling growth [24,77,82], result in smaller nestlings overall [25,83,84], alter corticosterone levels [85,86] and reduce nestling survival probabilities [87,88].
Rainfall was the strongest predictor of survival probability during the dependent fledgling stage. Higher rainfall periods are associated with greater food availability [20,89], which likely enhanced both provisioning rates to fledglings [90] and their ability to find food for themselves [91,92]. In cooperative breeders, survival of young during this stage often improves with increasing group size [34,51]: larger groups may provision more regularly [17] (but see [54]), better detect and repel predators [49], or access higher quality territories or nest sites [16]. We did not find a direct effect of group size on survival to independence at the brood scale. However, path analysis indicated that group size influenced individual fledgling survival probabilities indirectly, via a positive effect on nestling mass. Larger nestlings are more mobile and better developed at fledging, enabling them to forage more effectively, avoid predators and survive longer [93]. The presence of both direct and indirect (via negative effects on nestling Mass11) effects of mean Tmax during the nestling period on survival to independence suggests that carryover effects of high temperatures during early development continue to impact individual survival probabilities post-fledging [94,95].
(c). Buffering effect of group size
We found a lack of a buffering effect of group size on the effects of high mean Tmax on offspring survival. While, as discussed above, we found an indirect positive effect of larger group size on survival from fledging to independence, group size did not interact with the large and persistent negative effects of high mean Tmax on survival observed across all development stages to buffer the detrimental effects of high temperatures on survival from one early development stage to the next. This suggests that physiological tolerance limits [96] and resource constraints [97] at high temperatures may exceed any potential buffering effect of group size on offspring survival in cooperative breeders in arid and semi-arid environments [10].
5. Conclusion
In this study, negative effects of adverse climate conditions on breeding success in a cooperative breeder were not moderated by group size, suggesting that reproduction in pied babblers is constrained by available resources and physiology at high temperatures and low rainfall, regardless of group size. Climate change is one of the defining challenges of our time, posing a serious threat to biodiversity [3] and society [1]. Hot extremes will continue to become more frequent, and the length, frequency and intensity of heatwaves will continue to increase over most land masses [28,98]. At higher average and extreme temperatures, arid zone birds may increasingly experience temperatures that preclude successful breeding. We have observed both increasing temperatures and declining rainfall, along with declining offspring survival rates, at the study site over the last 15 years. Over time, the negative effects of these high temperatures on offspring survival could limit population recruitment and lead to local extinctions. Despite the intuitive appeal of the hypothesis that cooperative breeding may buffer against some of these effects, we found no evidence this will be the case in pied babblers. Our findings suggest that the presence of more helpers in a group is unlikely to provide a buffer against reproductive failure as average and maximum temperatures increase with advancing anthropogenic climate change and raise concerns for the long-term persistence of arid zone species in the face of rapidly changing environmental conditions.
Supplementary Material
Acknowledgements
We thank the Kuruman River Reserve (KRR) and surrounding farms, Van Zylsrus, South Africa, for making the work possible. Thanks to Sello Matjee, Paige Ezzey and Lesedi Moagi for fieldwork during 2016–2019, and all past and present staff and students of the Pied Babbler Research Project for data collected since 2003. The opinions, findings and conclusions are those of the authors alone, and the National Research Foundation accepts no liability in this regard. We thank the associate editor and two anonymous reviewers for their thorough and thoughtful comments which helped us to improve the manuscript.
Ethics
All data were collected under animal ethics permit numbers R2012/2006/V15/AR and 2016/V6/SC from the University of Cape Town and 1216/2016 from the Department of Environment and Nature Conservation, and blood samples were collected and birds banded by licenced professionals [blood sampling was authorised by the South African Veterinary Council (AL17/15885) and bird banding licences were issued by SAFRING (11663)].
Data accessibility
The datasets underlying all analyses presented in this study have been archived at the University of Cape Town's open access institutional data repository, ZivaHub (a figshare platform), where they are publicly available at doi:10.25375/uct.12441899.v1.
Authors' contributions
A.R.R., S.J.C., A.R.B. and C.N.S. conceived the study and secured funding. A.R.R. started habituation of the study animals and collection of life-history data in 2003 and has maintained it ever since; this was central to making the study possible. A.R.B. undertook all fieldwork from 2016 onwards. A.R.B. and A.R.R. analysed the data. A.R.B. drafted the manuscript. All authors contributed substantially to revisions and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The K.R.R. was financed by the Universities of Cambridge and Zurich, the MAVA Foundation and the European Research Council (grant no. 294494 to Tim Clutton-Brock) and received logistical support from the Mammal Research Institute, University of Pretoria. Work was funded by the DST-NRF Centre of Excellence at the FitzPatrick Institute of African Ornithology, the University of Cape Town, the Oppenheimer Memorial Trust (grant no. 20747/01 to A.R.B.), the British Ornithologists’ Union, the Australian Research Council (grant no. FT110100188 to ARR), a BBSRC David Phillips Fellowship (BB/J014109/1 to C.N.S.) and the National Research Foundation of South Africa (grant no. 99050 to S.J.C.).
References
- 1.Scheffers BR, et al. 2016. The broad footprint of climate change from genes to biomes to people. Science 354, 719–731. ( 10.1126/science.aaf7671) [DOI] [PubMed] [Google Scholar]
- 2.Stillman JH. 2019. Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34, 86–100. ( 10.1152/physiol.00040.2018) [DOI] [PubMed] [Google Scholar]
- 3.Spooner FEB, Pearson RG, Freeman R. 2018. Rapid warming is associated with population decline among terrestrial birds and mammals globally. Glob. Chang. Biol. 24, 4521–4531. ( 10.1111/gcb.14361) [DOI] [PubMed] [Google Scholar]
- 4.Conradie S, Woodborne SM, Cunningham SJ, McKechnie AE. 2019. Chronic, sublethal effects of high temperatures will cause severe declines in southern African arid-zone birds during the 21st century. Proc. Natl Acad. Sci. 116, 14 065–14 070. ( 10.1073/pnas.1821312116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen S-F, Emlen ST, Koenig WD, Rubenstein DR. 2017. The ecology of cooperative breeding behaviour. Ecol. Lett. 20, 708–720. ( 10.1111/ele.12774) [DOI] [PubMed] [Google Scholar]
- 6.Lin Y-H, Chan S-F, Rubenstein DR, Liu M, Shen S-F. 2019. Resolving the paradox of environmental quality and sociality: the ecological causes and consequences of cooperative breeding in two lineages of birds. Am. Nat. 194, 207–216. ( 10.1086/704090) [DOI] [PubMed] [Google Scholar]
- 7.Jetz W, Rubenstein DR. 2011. Environmental uncertainty and the biogeography of cooperative breeding in birds. Curr. Biol. 21, 72–78. ( 10.1016/j.cub.2010.11.075) [DOI] [PubMed] [Google Scholar]
- 8.Lukas D, Clutton-Brock T. 2017. Climate and the distribution of cooperative breeding in mammals. R. Soc. Open Sci. 4, 160897 ( 10.1098/rsos.160897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenstein DR, Lovette IJ. 2007. Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr. Biol. 17, 1414–1419. ( 10.1016/j.cub.2007.07.032) [DOI] [PubMed] [Google Scholar]
- 10.van de Ven TMFN, Fuller A, Clutton-Brock T. 2020. Effects of climate change on pup growth and survival in a cooperative mammal, the meerkat. Funct. Ecol. 34, 194–202. ( 10.1111/1365-2435.13468) [DOI] [Google Scholar]
- 11.Langmore NE, Bailey LD, Heinsohn RG, Russell AF, Kilner RM. 2016. Egg size investment in superb fairy-wrens: helper effects are modulated by climate. Proc. R. Soc. B 283, 10–12. ( 10.1098/rspb.2016.1875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guindre-Parker S, Rubenstein DR. 2018. The oxidative costs of parental care in cooperative and pair-breeding African starlings. Oecologia 188, 53–63. ( 10.1007/s00442-018-4178-3) [DOI] [PubMed] [Google Scholar]
- 13.Covas R, Du Plessis MA, Doutrelant C. 2008. Helpers in colonial cooperatively breeding sociable weavers Philetairus socius contribute to buffer the effects of adverse breeding conditions. Behav. Ecol. Sociobiol. 63, 103–112. ( 10.1007/s00265-008-0640-2) [DOI] [Google Scholar]
- 14.Bourne AR, Cunningham SJ, Spottiswoode CN, Ridley AR. 2020. Hot droughts compromise interannual survival across all group sizes in a cooperatively breeding bird. bioRxiv ( 10.1101/2020.06.11.147538) [DOI]
- 15.DuRant SE, Willson JD, Carroll RB. 2019. Parental effects and climate change: will avian incubation behavior shield embryos from increasing environmental temperatures? Integr. Comp. Biol. 59, 1068–1080. ( 10.1093/icb/icz083) [DOI] [PubMed] [Google Scholar]
- 16.Mumme RL, Bowman R, Pruett MS, Fitzpatrick JW. 2015. Natal territory size, group size, and body mass affect lifetime fitness in the cooperatively breeding Florida scrubjay. Auk 132, 634–646. ( 10.1642/AUK-14-258.1) [DOI] [Google Scholar]
- 17.Meade J, Nam KB, Beckerman AP, Hatchwell BJ. 2010. Consequences of ‘load-lightening’ for future indirect fitness gains by helpers in a cooperatively breeding bird. J. Anim. Ecol. 79, 529–537. ( 10.1111/j.1365-2656.2009.01656.x) [DOI] [PubMed] [Google Scholar]
- 18.Wingfield JC, Pérez JH, Krause JS, Word KR, González-Gómez PL, Lisovski S, Chmura HE. 2017. How birds cope physiologically and behaviourally with extreme climatic events. Phil. Trans. R. Soc. B 372, 20160140 ( 10.1098/rstb.2016.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson IR, Bryant DM. 2000. Avian phenology: climate change and constraints on breeding. Nature 406, 366–367. ( 10.1038/35019151) [DOI] [PubMed] [Google Scholar]
- 20.Hidalgo Aranzamendi N, Hall ML, Kingma SA, van de Pol M, Peters A. 2019. Rapid plastic breeding response to rain matches peak prey abundance in a tropical savannah bird. J. Anim. Ecol. 88, 1799–1811. ( 10.1111/1365-2656.13068) [DOI] [PubMed] [Google Scholar]
- 21.Skagen SK, Yackel Adams AA. 2012. Weather effects on avian breeding performance and implications of climate change. Ecol. Appl. 22, 1131–1145. ( 10.1890/11-0291.1) [DOI] [PubMed] [Google Scholar]
- 22.Conrey RY, Skagen SK, Yackel Adams AA, Panjabi AO. 2016. Extremes of heat, drought and precipitation depress reproductive performance in shortgrass prairie passerines. Ibis (Lond. 1859) 158, 614–629. ( 10.1111/ibi.12373) [DOI] [Google Scholar]
- 23.Cruz-McDonnell KK, Wolf BO. 2016. Rapid warming and drought negatively impact population size and reproductive dynamics of an avian predator in the arid southwest. Glob. Chang. Biol. 22, 237–253. ( 10.1111/gcb.13092) [DOI] [PubMed] [Google Scholar]
- 24.Cunningham SJ, Martin RO, Hojem CL, Hockey PAR. 2013. Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: a study of common fiscals. PLoS ONE 8, e74613 ( 10.1371/journal.pone.0074613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salaberria C, Celis P, López-Rull I, Gil D. 2014. Effects of temperature and nest heat exposure on nestling growth, dehydration and survival in a Mediterranean hole-nesting passerine. Ibis (Lond. 1859) 156, 265–275. ( 10.1111/ibi.12121) [DOI] [Google Scholar]
- 26.van de Ven TMFN, McKechnie AE, Er S, Cunningham SJ. 2020. High temperatures are associated with substantial reductions in breeding success and offspring quality in an arid-zone bird. Oecologia 193, 225–235. ( 10.1007/s00442-020-04644-6) [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Yu H, Dai A, Wei Y, Kang L. 2017. Drylands face potential threat under 2°C global warming target. Nat. Clim. Chang. 7, 417–422. ( 10.1038/nclimate3275) [DOI] [Google Scholar]
- 28.Mbokodo I, Bopape M-J, Chikoore H, Engelbrecht F, Nethengwe N. 2020. Heatwaves in the future warmer climate of South Africa. Atmosphere (Basel) 11, 712 ( 10.3390/atmos11070712) [DOI] [Google Scholar]
- 29.Cockburn A. 2002. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177. ( 10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 30.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383. ( 10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridley AR, van den Heuvel I. 2012. Is there a difference in reproductive performance between cooperative and non-cooperative species? A southern African comparison. Behaviour 8, 821–848. ( 10.1163/1568539X-00003005) [DOI] [Google Scholar]
- 32.Cockburn A, Sims RA, Osmond HL, Green DJ, Double MC, Mulder RA. 2008. Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol. 77, 430–438. ( 10.1111/j.1365-2656.2007.01351.x) [DOI] [PubMed] [Google Scholar]
- 33.Valencia J, Mateos C, de la Cruz C, Carranza J. 2016. Maternal allocation in eggs when counting on helpers in a cooperatively breeding bird. J. Avian Biol. 48, 536–543. ( 10.1111/jav.01020) [DOI] [Google Scholar]
- 34.Canestrari D, Marcos JM, Baglione V. 2008. Reproductive success increases with group size in cooperative carrion crows, Corvus corone corone. Anim. Behav. 75, 403–416. ( 10.1016/j.anbehav.2007.05.005) [DOI] [Google Scholar]
- 35.Ridley AR, Raihani NJ. 2008. Task partitioning increases reproductive output in a cooperative bird. Behav. Ecol. 19, 1136–1142. ( 10.1093/beheco/arn097) [DOI] [Google Scholar]
- 36.Rubenstein DR. 2011. Spatiotemporal environmental variation, risk aversion, and the evolution of cooperative breeding as a bet-hedging strategy. Proc. Natl Acad. Sci. USA 108, 10 816–10 822. ( 10.1073/pnas.1100303108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornwallis CK, Botero CA, Rubenstein DR, Downing PA, West SA, Griffin AS. 2017. Cooperation facilitates the colonization of harsh environments. Nat. Ecol. Evol. 1, 0057 ( 10.1038/s41559-016-0057) [DOI] [PubMed] [Google Scholar]
- 38.Griesser M, Drobniak SM, Nakagawa S, Botero CA. 2017. Family living sets the stage for cooperative breeding and ecological resilience in birds. PLoS Biol. 15, e2000483 ( 10.1371/journal.pbio.2000483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenig WD, Walters EL. 2018. Causes of seasonal decline in reproduction of the cooperatively-breeding acorn woodpecker. J. Avian Biol. 49, e01784 ( 10.1111/jav.01784) [DOI] [Google Scholar]
- 40.Crick HP. 1992. Load-lightening in cooperatively breeding birds and the cost of reproduction. Ibis (Lond. 1859) 1, 56–61. [Google Scholar]
- 41.Hatchwell BJ. 1999. Investment strategies of breeders in avian cooperative breeding systems. Am. Nat. 154, 205–219. ( 10.1086/303227) [DOI] [PubMed] [Google Scholar]
- 42.Clutton-Brock TH, Russell AF, Sharpe LL. 2004. Behavioural tactics of breeders in cooperative meerkats. Anim. Behav. 68, 1029–1040. ( 10.1016/j.anbehav.2003.10.024) [DOI] [Google Scholar]
- 43.Ebensperger LA, Correa LA, León C, Ramírez-Estrada J, Abades S, Villegas Á, Hayes LD. 2016. The modulating role of group stability on fitness effects of group size is different in females and males of a communally rearing rodent. J. Anim. Ecol. 85, 1502–1515. ( 10.1111/1365-2656.12566) [DOI] [PubMed] [Google Scholar]
- 44.Pike KN, Ashton BJ, Morgan KV, Ridley AR. 2019. Social and individual factors influence variation in offspring care in the cooperatively breeding Western Australian magpie. Front. Ecol. Evol. 7, 201900092. [Google Scholar]
- 45.van de Ven TMFN, McKechnie AE, Cunningham SJ. 2019. The costs of keeping cool: behavioural trade-offs between foraging and thermoregulation are associated with significant mass losses in an arid-zone bird. Oecologia 191, 205–215. ( 10.1007/s00442-019-04486-x) [DOI] [PubMed] [Google Scholar]
- 46.van Wilgen NJ, Goodall V, Holness S, Chown SL, McGeoch MA. 2016. Rising temperatures and changing rainfall patterns in South Africa's national parks. Int. J. Climatol. 36, 706–721. ( 10.1002/joc.4377) [DOI] [Google Scholar]
- 47.MacKellar N, New M, Jack C. 2014. Observed and modelled trends in rainfall and temperature for South Africa: 1960–2010. S. Afr. J. Sci. 110, 1–13. ( 10.1590/sajs.2014/20130353) [DOI] [Google Scholar]
- 48.Kruger AC, Sekele SS. 2013. Trends in extreme temperature indices in South Africa: 1962–2009. Int. J. Climatol. 33, 661–676. ( 10.1002/joc.3455) [DOI] [Google Scholar]
- 49.Raihani NJ, Ridley AR. 2007. Variable fledging age according to group size: trade-offs in a cooperatively breeding bird. Biol. Lett. 3, 624–627. ( 10.1098/rsbl.2007.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson-Flower MJ, Hockey PAR, O'Ryan C, Raihani NJ, Du Plessis MA, Ridley AR. 2011. Monogamous dominant pairs monopolize reproduction in the cooperatively breeding pied babbler. Behav. Ecol. 22, 559–565. ( 10.1093/beheco/arr018) [DOI] [Google Scholar]
- 51.Ridley AR. 2016. Southern pied babblers: The dynamics of conflict and cooperation in a group-living society. In Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior (eds Dickinson JL, Koenig W), pp. 115–132. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 52.Ridley AR, Raihani NJ. 2007. Variable postfledging care in a cooperative bird: causes and consequences. Behav. Ecol. 18, 994–1000. ( 10.1093/beheco/arm074) [DOI] [Google Scholar]
- 53.du Plessis KL, Martin RO, Hockey PAR, Cunningham SJ, Ridley AR. 2012. The costs of keeping cool in a warming world: Implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob. Chang. Biol. 18, 3063–3070. ( 10.1111/j.1365-2486.2012.02778.x) [DOI] [PubMed] [Google Scholar]
- 54.Wiley EM, Ridley AR. 2016. The effects of temperature on offspring provisioning in a cooperative breeder. Anim. Behav. 117, 187–195. ( 10.1016/j.anbehav.2016.05.009) [DOI] [Google Scholar]
- 55.Bourne AR, Cunningham SJ, Nupen LJ, McKechnie AE, Ridley AR. 2018. Male and female Southern Pied Babbler Turdoides bicolor nestlings respond similarly to heat stress. See https://www.bou.org.uk/wp-content/uploads/2019/08/bou-funded-project-report-bourne.pdf (accessed on 1 June 2020).
- 56.Ridley AR, Thompson AM. 2011. Heterospecific egg destruction by Wattled Starlings and the impact on Pied Babbler reproductive success. Ostrich 82, 201–205. ( 10.2989/00306525.2011.618247) [DOI] [Google Scholar]
- 57.Fridolfsson A-K, Ellegren H. 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121. ( 10.2307/3677252) [DOI] [Google Scholar]
- 58.Bourne AR, Cunningham SJ, Spottiswoode CN, Ridley AR. 2020. Compensatory breeding in years following drought in a desert-dwelling cooperative breeder. Front. Ecol. Evol. 8, 190 ( 10.3389/fevo.2020.00190) [DOI] [Google Scholar]
- 59.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.r-project.org. [Google Scholar]
- 60.Harrison XA, Donaldson L, Correa-cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R. 2018. A brief introduction to mixed effects modelling and multi-model inference in ecology. Peer J 6, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. ( 10.1111/j.2041-210x.2010.00012.x) [DOI] [Google Scholar]
- 62.Fox J, Monette G. 1992. Generalised collinearity diagnostics. J. Am. Stat. Assoc. 87, 178–183. ( 10.1080/01621459.1992.10475190) [DOI] [Google Scholar]
- 63.Champely S, Ekstrom C, Dalgaard P, Gill J, Weibelzahl S, Anandkumar A, Ford C, Volcic R, De Rosario H. 2018. pwr: Basic functions for power analysis. See https://github.com/heliosdrm/pwr.
- 64.Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd Edition New York, NY: Lawrence Erlbaum Associates. [Google Scholar]
- 65.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG. 2016. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur. J. Epidemiol. 31, 337–350. ( 10.1007/s10654-016-0149-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borchers HW. 2019. pracma: practical numerical math functions. See https://cran.r-project.org/web/packages/pracma/index.html.
- 67.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 68.Barton K. 2015. MuMIn: Multi-model inference. See https://cran.r-project.org/package=MuMin (accessed on 1 June 2020).
- 69.Grueber CE, Nakagawa S, Laws RS, Jamieson IG. 2011. Multimodal inference in ecology and evolution: challenges and solution. J. Evol. Biol. 24, 699–711. ( 10.1111/j.1420-9101.2010.02210.x) [DOI] [PubMed] [Google Scholar]
- 70.Herve M. 2019. RVAideMemoire: testing and plotting procedures for biostatistics cran.R-project.org. See https://cran.r-project.org/package=RVAideMemoire (accessed on 4 March 2020).
- 71.Muggeo VM. 2008. Segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25. [Google Scholar]
- 72.Kruuk LEB, Osmond HL, Cockburn A. 2015. Contrasting effects of climate on juvenile body size in a Southern Hemisphere passerine bird. Glob. Chang. Biol. 21, 2929–2941. ( 10.1111/gcb.12926) [DOI] [PubMed] [Google Scholar]
- 73.Shipley B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90, 363–368. ( 10.1890/08-1034.1) [DOI] [PubMed] [Google Scholar]
- 74.Larson JE, Sheley RL, Hardegree SP, Doescher PS, James JJ. 2015. Seed and seedling traits affecting critical life stage transitions and recruitment outcomes in dryland grasses. J. Appl. Ecol. 52, 199–209. ( 10.1111/1365-2664.12350) [DOI] [Google Scholar]
- 75.Lefcheck JS. 2016. piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 76.Ospina EA, Merrill L, Benson TJ. 2018. Incubation temperature impacts nestling growth and survival in an open-cup nesting passerine. Ecol. Evol. 8, 3270–3279. ( 10.1002/ece3.3911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mainwaring MC, Hartley IR. 2016. Local weather conditions have complex effects on the growth of blue tit nestlings. J. Therm. Biol. 60, 12–19. ( 10.1016/j.jtherbio.2016.05.005) [DOI] [PubMed] [Google Scholar]
- 78.Vafidis JO, Vaughan IP, Jones TH, Facey RJ, Parry R, Thomas RJ. 2016. The effects of supplementary food on the breeding performance of Eurasian reed warblers Acrocephalus scirpaceus: implications for climate change impacts. PLoS ONE 11, e0159933 ( 10.1371/journal.pone.0159933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 80.Moore MP, Martin RA. 2019. On the evolution of carry-over effects. J. Anim. Ecol. 88, 1832–1844. ( 10.1111/1365-2656.13081) [DOI] [PubMed] [Google Scholar]
- 81.Carroll RL, Davis CA, Fuhlendorf SD, Elmore RD, DuRant SE, Carroll JM. 2018. Avian parental behavior and nest success influenced by temperature fluctuations. J. Therm. Biol. 74, 140–148. ( 10.1016/j.jtherbio.2018.03.020) [DOI] [PubMed] [Google Scholar]
- 82.Andreasson F, Nord A, Nilsson JÅ. 2018. Experimentally increased nest temperature affects body temperature, growth and apparent survival in blue tit nestlings. J. Avian Biol. 49, 1–14. ( 10.1111/jav.01620) [DOI] [Google Scholar]
- 83.Wada H, Kriengwatana B, Allen N, Schmidt KL, Soma KK, MacDougall-Shackleton SA. 2015. Transient and permanent effects of suboptimal incubation temperatures on growth, metabolic rate, immune function and adrenocortical responses in zebra finches. J. Exp. Biol. 218, 2847–2855. ( 10.1242/jeb.114108) [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez S, Barba E. 2016. Nestling growth is impaired by heat stress: an experimental study in a Mediterranean great tit population. Zool. Stud. 40, 2016.55-40 ( 10.6620/ZS.2016.55-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crino OL, Driscoll SC, Brandl HB, Buchanan KL, Griffith SC. 2020. Under the weather: corticosterone levels in wild nestlings are associated with ambient temperature and wind. Gen. Comp. Endocrinol. 285, 113247 ( 10.1016/j.ygcen.2019.113247) [DOI] [PubMed] [Google Scholar]
- 86.Newberry GN, Swanson DL. 2018. Elevated temperatures are associated with stress in rooftop-nesting common nighthawk (Chordeiles minor) chicks. Conserv. Physiol. 6, coy010 ( 10.1093/conphys/coy010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greño JL, Belda EJ, Barba E. 2008. Influence of temperatures during the nestling period on post-fledging survival of great tit Parus major in a Mediterranean habitat. J. Avian Biol. 39, 41–49. ( 10.1111/j.0908-8857.2008.04120.x) [DOI] [Google Scholar]
- 88.Zuckerberg B, Ribic CA, McCauley LA. 2018. Effects of temperature and precipitation on grassland bird nesting success as mediated by patch size. Conserv. Biol. 32, 872–882. ( 10.1111/cobi.13089) [DOI] [PubMed] [Google Scholar]
- 89.Cumming GS, Bernard RTF. 1997. Rainfall, food abundance and timing of parturition in African bats. Oecologia 111, 309–317. ( 10.1007/s004420050240) [DOI] [PubMed] [Google Scholar]
- 90.Russell AF, Clutton-Brock TH, Brotherton PNM, Sharpe LL, McIlrath G, Dalerum FD, Cameron EZ, Barnard JA. 2002. Factors affecting pup growth and survival in cooperatively breeding meerkats Suricatta suricata. J. Anim. Ecol. 71, 700–709. ( 10.1046/j.1365-2656.2002.00636.x) [DOI] [Google Scholar]
- 91.Wheelwright NT, Templeton JJ. 2003. Development of foraging skills and the transition to independence in juvenile savannah sparrows. Condor 105, 279–287. ( 10.1093/condor/105.2.279) [DOI] [Google Scholar]
- 92.Naef-Daenzer B, Grüebler MU. 2016. Post-fledging survival of altricial birds: ecological determinants and adaptation. J. F. Ornithol. 87, 227–250. ( 10.1111/jofo.12157) [DOI] [Google Scholar]
- 93.Martin TE, Tobalske B, Riordan MM, Case SB, Dial KP. 2018. Age and performance at fledging are a cause and consequence of juvenile mortality between life stages. Sci. Adv. 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blomberg EJ, Sedinger JS, Gibson D, Coates PS, Casazza ML. 2014. Carryover effects and climatic conditions influence the postfledging survival of greater sage-grouse. Ecol. Evol. 4, 4488–4499. ( 10.1002/ece3.1139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones TM, Ward MP, Benson TJ, Brawn JD. 2017. Variation in nestling body condition and wing development predict cause-specific mortality in fledgling dickcissels. J. Avian Biol. 48, 439–447. ( 10.1111/jav.01143) [DOI] [Google Scholar]
- 96.Smit B, Whitfield MC, Talbot WA, Gerson AR, McKechnie AE, Wolf BO. 2018. Avian thermoregulation in the heat: phylogenetic variation among avian orders in evaporative cooling capacity and heat tolerance. J. Exp. Biol. 221, jeb174870 (doi:jeb174870) [DOI] [PubMed] [Google Scholar]
- 97.Nowakowski AJ, Frishkoff LO, Agha M, Todd BD, Scheffers BR. 2018. Changing thermal landscapes: merging climate science and landscape ecology through thermal biology. Curr. Landsc. Ecol. Reports 3, 57–72. ( 10.1007/s40823-018-0034-8) [DOI] [Google Scholar]
- 98.IPCC. 2013. Climate change 2013: The Intergovernmental Panel on Climate Change fifth assessment report. Cambridge, UK: Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets underlying all analyses presented in this study have been archived at the University of Cape Town's open access institutional data repository, ZivaHub (a figshare platform), where they are publicly available at doi:10.25375/uct.12441899.v1.