Abstract
In species with internal fertilization, the female genital tract appears challenging to sperm, possibly resulting from selection on for example ovarian fluid to control sperm behaviour and, ultimately, fertilization. Few studies, however, have examined the effects of swimming media viscosities on sperm performance. We quantified effects of media viscosities on sperm velocity in promiscuous willow warblers Phylloscopus trochilus. We used both a reaction norm and a character-state approach to model phenotypic plasticity of sperm behaviour across three experimental media of different viscosities. Compared with a standard medium (Dulbecco's Modified Eagle Medium, DMEM), media enriched with 1% or 2% w/v methyl cellulose decreased sperm velocity by up to about 50%. Spermatozoa from experimental ejaculates of different males responded similarly to different viscosities, and a lack of covariance between elevations and slopes of individual velocity-by-viscosity reaction norms indicated that spermatozoa from high- and low-velocity ejaculates were slowed down by a similar degree when confronted with high-viscosity environments. Positive cross-environment (1% versus 2% cellulose) covariances of sperm velocity under the character-state approach suggested that sperm performance represents a transitive trait, with rank order of individual ejaculates maintained when expressed against different environmental backgrounds. Importantly, however, a lack of significant covariances in sperm velocity involving a cellulose concentration of 0% indicated that pure DMEM represented a qualitatively different environment, questioning the validity of this widely used standard medium for assaying sperm performance. Enriching sperm environments along ecologically relevant gradients prior to assessing sperm performance will strengthen explanatory power of in vitro studies of sperm behaviour.
Keywords: cryptic female choice, ovarian fluid viscosity, phenotypic plasticity, Phylloscopus trochilus, sperm competition, sperm motility
1. Background
In species with internal fertilization, spermatozoa typically have to migrate through the female genital tract to reach and eventually fertilize eggs. On this long and challenging journey, spermatozoa face a highly complex and selective environment [1,2]. While the original adaptive function of such an environment was possibly rooted in pathogen defence, selection may have favoured any extension of sophisticated discrimination and control mechanisms of non-self cells to include control over sperm behaviour, and thereby, ultimately, fertilization. In particular in promiscuous species, sexually antagonistic coevolution of loci coding for male versus female traits in control of fertilization [3] predicts the evolution of cryptic female choice enabling females to select spermatozoa among and within ejaculates (reviewed in [4,5]).
Obstacles impeding spermatozoa from reaching the egg may include sperm ejection by females [6–8], immunological (e.g. phagocytosis) as well as physico-chemical barriers (e.g. acidic pH and ovarian fluid viscosity and composition, structure of the cervix [1,9]). As a result, only a tiny proportion of the usually vast number of spermatozoa inseminated will ever get close to the site of fertilization. In galliform birds, for example, it has been shown that only about 1–2% of the inseminated spermatozoa enter the sperm-storage tubules located at the uterovaginal junction of the female genital tract [10,11]. Traversing the vagina thus seems to represent a major barrier for avian sperm to overcome and recent evidence suggest that the vagina is indeed an important site for sperm selection [12]. A non-random sub-population of fast-swimming spermatozoa reaches the ovum in the zebra finch Taenopygia guttata, suggesting that sperm swimming velocity is a highly important trait that enables fast-swimming spermatozoa to have a greater chance of migrating through the vagina and entering the sperm-storage tubules [13]. Selection for high sperm velocity is expected to be stronger in more promiscuous species (cf. [14,15]), since sperm in this case will have to compete more intensely with spermatozoa from other males. Sperm selection is likely to be mediated by a multitude of different filter mechanisms [16], yet detailed knowledge about these mechanisms is currently very limited (reviewed in [17]).
One potential mechanism likely to affect sperm transit through the female genital tract that so far has received little attention is ovarian fluid viscosity. The sperm swimming environment within the female genital tract is highly viscous, although there may be considerable temporal and spatial variation [18,19]. Ovarian fluid viscosity may have a strong impact on sperm swimming performance (e.g. [18–21]) and could thus greatly influence the ability of spermatozoa to migrate through the vagina, to enter the sperm-storage tubules, to leave the tubules at the optimal time and ultimately to fertilize eggs. Furthermore, given the potentially strong effect of ovarian fluid viscosity on sperm velocity and the potential for ovarian fluid viscosity to vary in time and space, there may be selection on spermatozoa to be able to perform in different viscosity environments.
While there is a growing body of evidence indicating taxonomically widespread phenotypic plasticity in sperm morphology (e.g. [22–24]), relatively few studies have addressed plasticity in sperm behaviour. In fish, for example, males were found to produce slower sperm when experimentally promoted to higher ranks in social dominance hierarchies [25] or males responded to perceived sperm competition intensity by producing longer lived [26] or faster sperm [27]. In birds, sperm motility traits varied in a phenotypically plastic manner with male social status [28], female attractiveness [29] or season [30]. Even fewer studies have quantified reaction norms in sperm performance in response to experimentally modified swimming environments, for example, across gradients of temperature [31] or pH [32,33] or when contrasting sperm activated in ovarian fluid versus water [34]. In mice, spermatozoa chemo-attracted by progesterone showed erratic trajectories and non-progressive movement in low-viscosity media, but linear trajectories and more progressive movement in high-viscosity media, suggesting the latter should be used for in vitro assessment of mammal sperm behaviour to better simulate conditions experienced by sperm in vivo [35]. In birds, advanced sperm mobility assays in poultry science made use of media of different traversability to more efficiently discriminate between males with different siring potential (e.g. [36]).
Vernon & Woolley [20] used media of two different viscosities (standard medium versus medium enriched with 2% w/v methyl cellulose) for an analysis of sperm swimming behaviour in selected wild bird species, including a passerine bird, the starling Sturnus vulgaris. Their analyses were purely qualitative, however, and focused on an in-depth description of the functional morphology of sperm propulsion in non-passerine versus passerine birds [20]. Interestingly, nevertheless, the authors stated that ‘the actual shape of the sperm head is adapted for the screw-like motion seen in high-viscosity media rather than for propulsion through low-viscosity salines' [20]. This emphasizes the need of incorporating ecologically relevant properties of the sperm swimming environment like viscosity into the quantitative analysis of sperm behaviour. We are, however, unaware of any studies that have examined effects of media viscosity on sperm performance quantitatively in natural bird populations.
Female extra-pair mating is common and widespread in passerine birds (reviewed in [37–39]). As a consequence, passerines have become the most widely used vertebrate model system to study the ecology and evolution of male reproductive traits under post-copulatory sexual selection. Passerine birds are particularly well suited to study sperm traits due to the ease of non-invasive sperm sampling via cloacal massage [40]. We here used wild willow warblers Phylloscopus trochilus to study phenotypic plasticity in sperm velocity in response to three experimental sperm swimming environments characterized by different viscosities. The willow warbler is a widespread and common socially monogamous passerine bird with a high frequency of extra-pair paternity [41–43], suggesting significant post-copulatory sexual selection on sperm competitiveness.
We took two different statistical approaches allowing complementary inference: a reaction norm (RN) and a character-state (CS) approach [44]. Our RN approach fits one parameter for individual variation in average trait values (in the form of a random intercept variance) and one parameter for individual variation in response to different viscosities (in the form of a random slope variance). Both parameters (i.e. elevation and slope of the reaction norm) are interpretable in evolutionary terms, if we think of phenotypic plasticity as a trait that itself can be the target of natural or sexual selection [44]. Our CS approach, by contrast, treats phenotypes expressed in different environments as different characters/traits and fits separate inter-individual variance parameters for each environment (in the form of random intercept variances). Individual variation in phenotypic plasticity is then modelled indirectly as cross-environmental correlations of sperm performance from individual ejaculates. A perfect correlation among environments suggests no variation in plasticity, while the perfect independence of performance in different environments is characterized by zero correlation among environments and reflects maximum variation in plasticity. The evolutionary motivation for the CS approach is the conception that the trait might have been selected in different environments and phenotypic plasticity arises as a consequence of differential selection in different environments [44]. Despite these different conceptual perspectives on variation in plasticity, the two approaches are mathematically interchangeable in the case of two discrete environments [44].
2. Material and methods
(a). Study population and field methods
Fieldwork was carried out in the Pasvik Valley (69°28′ N, 29°50′ W) in northern Norway at the onset of the breeding season on 15–16 June 2012. Male willow warblers (n = 28) were captured with playback and mist-nets. To avoid inadvertent re-sampling of individuals, each male was ringed with a uniquely numbered aluminium ring provided by the Norwegian Bird Ringing Centre at Stavanger Museum. One sperm sample per male was obtained by gently massaging the cloacal protuberance as described in detail elsewhere [40] and immediately diluted in 20 µl pre-warmed (38°C) Dulbecco's Modified Eagle Medium (advanced DMEM, Invitrogen). From this stock solution, 4.5 µl was transferred to either (i) 20 µl pre-warmed (38°C) DMEM with 1% w/v methyl cellulose (sodium carboxymethyl cellulose, Sigma-Aldrich; hereafter: cellulose), or (ii) 20 µl pre-warmed (38°C) DMEM with 2% w/v cellulose or (iii) 20 µl pre-warmed (38°C) DMEM without cellulose. An aliquot of 4.5 µl of each of the three sperm solutions was then deposited on a pre-heated (38°C) microscope count slide (2 chambers, 20 µm, Leja, Nieuw-Vennep, The Netherlands) mounted on a MiniTherm stage warmer (Hamilton Thorne, Beverly, MA, USA) set to 38°C.
Sperm velocity declines with increasing time interval since sampling. Thus to avoid any systematic bias in sperm velocities due to the order in which treatment levels (i.e. 0%, 1% or 2% cellulose) of any experimental ejaculate were recorded, their sequence was alternated and randomized with respect to male/ejaculate identity. Sperm velocity was recorded within two minutes of sampling the experimental ejaculate from a male using a CCD black and white video camera (XCST50CE PAL, Sony) mounted on a negative phase-contrast microscope (CH30, Olympus) with a 10 x objective. For each slide chamber/video recording, multiple independent video takes (between one and ten), each lasting for up to a maximum of 5 s, were recorded in quick succession to increase the number of different spermatozoa measured. Representative video recordings (one for each of three cellulose concentrations) are provided in the electronic supplementary material (electronic supplementary material, video files S1–S3). Birds were released immediately after video recording was finished.
(b). Computer-assisted sperm analysis
Videos were analysed using the sperm tracker software HTM-CEROS v.12 (Hamilton Thorne, Beverly, MA, USA). The image analyser was set at a frame rate of 50 Hz and 25 frames (i.e. spermatozoa were tracked for 0.5 s). Each video recording was visually examined and cell-detection parameters were adjusted using two interactive quality control plots as well as directly from visual examination of each recording. Video recordings were analysed with MiniDV with a resolution of 720 × 576 (PAL). The minimum size setting for sperm detection was set to nine pixels. The computer-assisted sperm analysis (CASA) system recorded by default curvilinear velocity (VCL), average path velocity (VAP) and straight line velocity (VSL). Spermatozoa with VSL less than 15 µm s–1 were counted as static and excluded from the motility analyses, along with spermatozoa tracked for < 15 frames. We also excluded from analysis any tracked objects that were spherical (elongation value > 60) as willow warblers have highly elongated sperm heads, see electronic supplementary material, figure S1. VCL, VAP and VSL were highly correlated (Pearson's r for VCL/VAP: 0.94; VCL/VSL: 0.84; VAP/VSL: 0.96); we therefore decided to focus on just one of these. With no attractant (e.g. egg or chemical gradient) present in our in vitro assays, swimming trajectories of spermatozoa were not expected to be straight and we therefore used VCL as the least derived variable [45]. Sperm video recordings were analysed blindly with respect to experimental treatment and ejaculate identity by a single observer (G.R.).
(c). Statistical analysis
The multi-level hierarchical structure of the data and our specific interest in estimating variance components required a mixed effects modelling approach, the rationale of which we explain in detail below. We took two complementary statistical modelling approaches to analyse phenotypic plasticity of sperm behaviour across experimental environments. First, we adopted a RN perspective that models sperm velocity as a function of environmental variation in a linear mixed effects model random regression framework [46]. Second, we took a character-state approach, which treats sperm velocities at different cellulose concentrations as different characters/traits and allows the estimation of sperm velocity variances within and covariances among the three different media [47].
Besides cellulose concentration as our fixed treatment effect, we were mostly interested in among-ejaculate random variation in sperm velocity in order to test the idea that spermatozoa from ejaculates of different males may specialize in their performance in different swimming environments and thereby trade-off high velocity in a low-viscosity environment with their ability to show high velocity in a high-viscosity environment, or vice versa. In the following, we therefore focus on analysis of random effects. Note that we had sampled only a single experimental ejaculate per male, such that our ejaculate identity variance term includes both among-individual variation but also among-ejaculate variation caused by uncontrolled environmental effects (e.g. seasonal plasticity in sperm phenotype). We used log-transformed VCL as our dependent variable in all analyses.
(d). Reaction norm approach
Under the RN approach, we tested for effects of experimental media viscosities on sperm velocity by means of linear mixed effects models and used random regression analyses to test for variation in phenotypic plasticity among ejaculates. Linear mixed effects models were fitted in R v. 3.5.1 [48] using the function lmer from the package lme4 [49].
As explanatory fixed effects, we included cellulose concentration (ranging from 0% over 1% to 2% w/v) as a continuous variable which we mean-centred to obtain biologically meaningful estimates for the intercept and corresponding intercept variances of the random effects described below. As a cellulose concentration of 1% represents the average experimental environment, the intercept of the models thus describes the mean velocity-by-cellulose concentration reaction norm elevation (defined as the predicted phenotype in the average environment experienced) and the corresponding random intercept variances describe the among-ejaculate variation in reaction norm elevations. Furthermore, we included order of measurement as an ordered factor (including a linear and quadratic term) to account for slightly different time intervals between obtaining an experimental ejaculate and the start of the three corresponding video recordings (one recording for each of the three cellulose concentrations). We defined the second-order position to be used for estimation of the intercept. Furthermore, we included a cellulose-by-order interaction term.
As random effects, we included video take identity nested within video recording identity nested within experimental ejaculate identity as random intercept effects to account for the non-independence in the hierarchical data structure and to estimate the respective variance components (random intercept model). To test for differences in slopes of sperm velocity of spermatozoa from different ejaculates across the range of experimental environments, we added a cellulose-by-ejaculate random slope term (random intercept and slope model). To test for potential trade-offs between average sperm velocity and the response in sperm velocity to the cellulose gradient, we evaluated the covariance between the ejaculate random intercept term (reflecting random variation in the reaction norm elevation) and the cellulose-by-ejaculate random slope term in our random intercept and slope model.
Significance of fixed effects was determined by likelihood ratio tests after removing the focal term from a maximum-likelihood (ML) fit of our random intercept model. Significance of random effects (random intercepts, random slopes and covariance among random intercepts and slopes) was determined by likelihood ratio tests comparing models before and after removing the focal terms from restricted maximum-likelihood (REML) fits of the respective more complex models. All statistical tests were two-tailed and we rejected the null hypothesis at p < 0.05.
(e). Character-state approach
The CS approach treats sperm velocity at different cellulose concentrations as different characters/traits and allows the estimation of velocity variances and covariances within and among the three different cellulose concentrations. We therefore fitted a multi-response mixed effects model that controls for average sperm velocity at each cellulose concentration and for the order of measurement in the fixed effects part of the model separately for the three environments (thus effectively including a cellulose-by-order interaction effect).
The random effects part includes the variances within environments and covariances among environments. For each of the random effects, these are estimated as 3 × 3 variance–covariance matrices for the three cellulose concentrations. Covariances can be easily converted to correlations by dividing the covariance by the geometric mean of the two respective variances. The random effect for which all variances and covariances could be estimated was ejaculate identity. Furthermore, we fitted video take identity as another random effect. Note, however, that for video take identity the cross-environment covariances were undefined, because each video take was nested within a single video recording and thus viscosity treatment level. For our experimental design, video recording identity was confounded with video take identity in a model that treats the environments separately and therefore fitting video recording identity was not necessary under the CS approach. Residual covariances were undefined by design. We fitted the multi-response models using the software ASReml 3.0 [50]. The significance of fixed and random effects was tested by likelihood ratio tests comparing nested models.
(f). Comparison of approaches
While the three different cellulose concentrations were treated as snapshots of a continuous environmental gradient in the RN approach, they represent three discrete environments in the CS approach (with three cross-environmental covariances). Thus, the two approaches are mathematically not interchangeable for our experimental design and the CS approach estimates three more parameters than the RN approach. In contrast with the CS approach, our RN model makes the assumption that the relationship between cellulose concentrations and sperm velocity is linear, which appears to be approximately true for our data after log-transformation. The two approaches also differ in their null hypotheses for significance testing. In the RN approach, the null hypothesis for the random slope term is no among-ejaculate variation in plasticity while in the CS approach the null hypothesis for the covariance term is no correlation among environments (i.e. the opposite). While it is technically possible to test against a null hypothesis of a perfect correlation between environments (e.g. by likelihood ratio tests), such tests suffer from the fact that both sampling variation and measurement error tend to reduce any actually existing correlation, rendering a significance test against H0: r = 1 largely meaningless; we therefore refrain from applying such a test under the CS approach. Finally, we note that the multi-response model estimates separate residual variances for each environment, unlike the random slope model of the RN approach that fits a single residual variance and thus assumes residuals to be identically distributed across all observations.
3. Results
(a). Fixed effects
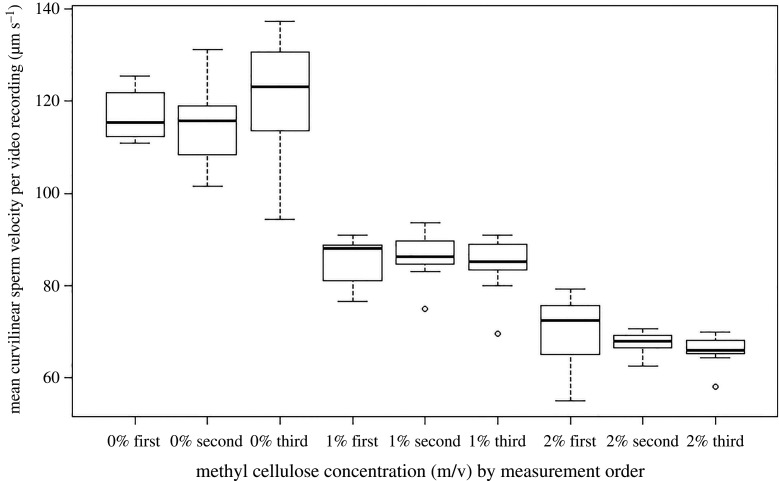
The final sample size consisted of 10 908 individual spermatozoa originating from 582 video takes of 84 video recordings of 28 experimental ejaculates of 28 males. On average (± s.d.), 19 ± 18 spermatozoa were tracked per video take, 130 ± 125 per video recording and 390 ± 239 per experimental ejaculate. Fixed effects estimates turned out to be very similar whether estimated under the RN or the CS approach and we therefore only report details from the RN approach in the main text (see electronic supplementary material, tables S1 and S2 for comprehensive model outputs from both approaches). There was no significant effect of order of measurement on sperm velocity (random intercept model: χ2 = 3.33, d.f. = 2, p = 0.19; figure 1; see electronic supplementary material, table S1a for detail). Sperm velocity, however, decreased by nearly 50% from the lowest to the highest cellulose concentration (random intercept model: χ2 = 183.1, d.f. = 1, p < 0.001, figures 1 and 2, electronic supplementary material, table S1a; note that this decline represents the mean population-wide sperm velocity-by-cellulose concentration reaction norm slope). The strength of the observed decrease in velocity was independent of the order in which video recordings associated with specific cellulose concentrations were taken within experimental ejaculates (random intercept model: cellulose-by-order interaction: χ2 = 0.87, d.f. = 2, p = 0.65, figure 1). Subsequent analyses of random effects were therefore based on a fixed effects structure represented by the additive effects of cellulose concentration and order of measurement.
Figure 1.
Mean curvilinear sperm velocity per video recording as a function of cellulose concentration and order of measurement for n = 84 video recordings of 28 experimental willow warbler ejaculates/males. Boxplots show median, interquartile range (box) and data within 1.5 times the interquartile range (whiskers). Note that boxplots are based on aggregated raw data (per recording), while statistical analyses were based on log-transformed values of sperm velocity for individual spermatozoa.
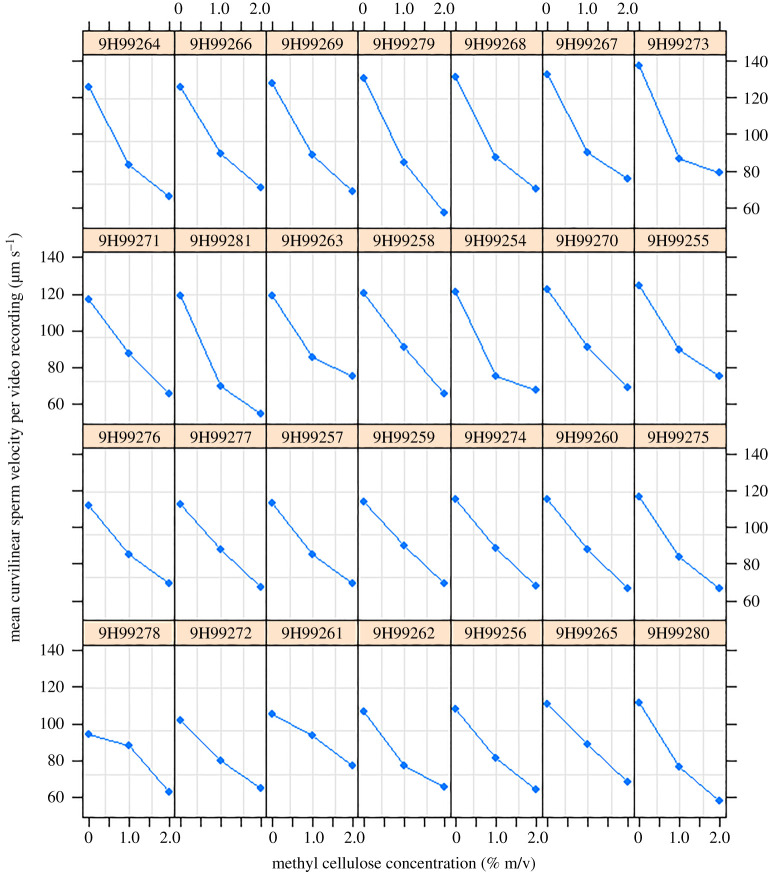
Figure 2.
Mean curvilinear sperm velocity per video recording as a function of cellulose concentration for n = 28 experimental willow warbler ejaculates/males (identified by ring numbers). Note that on display are aggregated raw data (per recording) which in contrast with statistical analysis are not controlled for the (non-significant) effects of order of measurement. (Online version in colour.)
(b). Random effects under a reaction norm approach
We found significant among-video take variance (χ2 = 47.9, d.f. = 1, p < 0.001), among-video recording variance (χ2 = 33.8, d.f. = 1, p < 0.001) and among-ejaculate variance (χ2 = 9.5, d.f. = 2, p = 0.002) for the sperm velocity-by-cellulose concentration reaction norm elevation (electronic supplementary material, table S1a). However, conditional on the fixed effects cellulose concentration and order of measurement, these variance components explained only 2.3%, 2.8% and 2.8% of the total phenotypic variance, respectively. Including a cellulose-by-ejaculate random slope term did not significantly increase model fit (χ2 = 0.13, d.f. = 2, p = 0.94; electronic supplementary material, table S1b). This indicates that spermatozoa from different ejaculates respond in a similar way to changes in cellulose concentrations (figure 2). The correlation between the random intercept term for ejaculate identity and the cellulose-by-ejaculate random slope term in our random intercept and slope model was negative (r = −0.27) but not significantly different from zero (χ2 = 0.08, d.f. = 1, p = 0.77; electronic supplementary material, table S1b). This suggests that spermatozoa from ejaculates with different elevations of the sperm velocity-by-cellulose reaction norm did not differentially decrease in sperm velocity across the experimental cellulose gradient. Thus, spermatozoa from ejaculates with high mean sperm velocity in the mean environment experienced (cellulose concentration = 1%) were affected by changing cellulose concentrations in a similar way to spermatozoa from ejaculates with low mean sperm velocity.
(c). Random effects under a character-state approach
Controlling for the fixed effects of cellulose concentration and order of measurement (see electronic supplementary material, table S2 for details), there was significant among-ejaculate variation in sperm velocities, accounting for 5–11% of the variance in the three different cellulose concentrations (lowest among-ejaculate contribution at 1%, highest at 0%, table 1). Furthermore, we found low, but significant, variation in sperm velocities among different video takes within ejaculates, accounting for 1–4% of the phenotypic variance (after controlling for fixed effects, table 1). All between-environment correlations were estimated positive, but only the correlation in sperm velocities between cellulose concentrations of 1% and 2% was strong (r = 0.88 ± 0.11) and highly significant (χ2 = 12.14, d.f. = 1, p = 0.0005, table 2). By contrast, both correlations involving a cellulose concentration of 0% were substantially weaker (r = 0.17 ± 0.26 and 0.47 ± 0.22, respectively) and not significantly different from zero (0% versus 1%, χ2 = 0.36, d.f. = 1, p = 0.55, 0% versus 2%, χ2 = 2.94, d.f. = 1, p = 0.09). This indicates that a cellulose concentration of 0% (i.e. pure DMEM without dissolved cellulose) represented a qualitatively different environment compared to the other two treatments. By allowing for separate residual variances in the three cellulose concentrations, our CS approach also confirmed a decrease in the residual variance from lower to higher cellulose concentrations (χ2 = 6.00, d.f. = 2, p < 0.05) when comparing the full model to a model where residual variances were constrained to be identical for the three environments (figure 1; see also table 1).
Table 1.
Variance components and ratios of variance components (±s.e.) of curvilinear sperm velocity of 10 908 spermatozoa from 582 video takes from 84 video recordings of 28 experimental willow warbler Phylloscopus trochilus experimental ejaculates/males in three experimental media of different viscosities (cellulose concentrations). Estimates are from a multi-response mixed model under a character-state approach (see methods for details).
| cellulose (w/v) | phenotypic variance VP | among ejaculate variance VEJ |
among video take variance VVT |
residual (within video take) variance VR |
|||
|---|---|---|---|---|---|---|---|
| VP | VEJ | VEJ/VP | VVT | VVT/VP | VR | VR/VP | |
| 0% | 731.51 ± 31.23 | 78.75 ± 26.65 | 0.11 ± 0.03 | 22.33 ± 7.07 | 0.03 ± 0.01 | 630.43 ± 16.29 | 0.86 ± 0.03 |
| 1% | 455.97 ± 13.12 | 24.63 ± 8.97 | 0.05 ± 0.02 | 6.27 ± 2.62 | 0.01 ± 0.01 | 425.07 ± 9.64 | 0.93 ± 0.02 |
| 2% | 277.36 ± 9.76 | 21.40 ± 7.76 | 0.08 ± 0.03 | 11.91 ± 2.91 | 0.04 ± 0.01 | 244.05 ± 5.77 | 0.88 ± 0.03 |
Table 2.
Within-ejaculate, cross-environment phenotypic covariances (±s.e.; lower left) and correlations (±s.e.; upper right) of curvilinear sperm velocity of 10 908 spermatozoa from 582 video takes from 84 video recordings of 28 experimental willow warbler Phylloscopus trochilus experimental ejaculates/males in three experimental media of different viscosities (cellulose concentrations). Estimates are from a multi-response mixed model under a character-state approach (see methods for details).
| cellulose (w/v) | 0% | 1% | 2% |
|---|---|---|---|
| 0% | — | 0.168 ± 0.255 | 0.467 ± 0.215 |
| 1% | 7.40 ± 11.56 | — | 0.876 ± 0.108 |
| 2% | 19.18 ± 11.12 | 20.11 ± 7.29 | — |
4. Discussion
Although ovarian fluid viscosity may exert a strong influence on sperm swimming behaviour (see introduction), few studies have examined this relationship empirically, in particular in birds. In the poultry industry, sperm mobility tests used media of different traversability with the goal to better discriminate between males with different siring potential (e.g. [36]). Vernon & Woolley [20] used media of two different viscosities (standard medium versus 2% cellulose) for their analysis of sperm swimming behaviour in selected bird species, including a passerine, the starling Sturnus vulgaris. But their analyses were purely qualitative and focused on a detailed description of the functional morphology of sperm propulsion in non-passerine versus passerine birds [20]. Our study, therefore, represents the first quantitative analysis of sperm behaviour as a function of media viscosities in any passerine bird. By combining a reaction norm with a character-state approach, our study revealed four major findings: First, sperm velocity sharply decreased with increasing levels of media viscosities; second, ejaculates of different males responded in a very similar way when exposed to different viscosities; third, a lack of covariance between elevations and slopes of reaction norms indicated that spermatozoa from high-velocity ejaculates were not slowed down more strongly than spermatozoa from low-velocity ejaculates and fourth, spermatozoa from different ejaculates demonstrated positive cross-environment correlations in velocity in 1% versus 2% cellulose but none with 0% cellulose.
(a). Main effects of media viscosities on sperm velocity
Sperm velocity decreased substantially with increasing media viscosities, featuring a 50% decline in sperm swimming velocity from our lowest to highest experimental media viscosities. This has important methodological and biological implications.
In internally fertilizing species, spermatozoa are unlikely to be confronted with swimming environments that resemble, in terms of viscosity, the standard cell culture media commonly used for assaying sperm performance (velocity, motility). Several authors have speculated that the female genital tract, portrayed as challenging to sperm in general [1], must possibly represent a high-viscosity environment [18,20]. In fact, the nearly universal cork-screw design of the passerine sperm head (electronic supplementary material, figure S1; see also [51,52]) and the highly distinctive style of twist-drill swimming of passerine sperm [20] are strongly suggestive for high-viscosity fluids being a defining feature of the selective environment to which passerine spermatozoa are exposed [15,53]. Thus, enriching sperm environments along potentially relevant gradients prior to assessing their performance will help strengthening the reliability and explanatory power of in vitro studies of sperm performance in birds and other taxa (for mammals see e.g. [35]). Indeed, recently this step has sometimes been incorporated into experimental designs. For example, Cramer et al. [54] used experimental media containing female genital tract fluid to test for differential sperm performance across (sub-) species boundaries of closely related Ficedula flycatchers.
The striking changes in sperm velocity in response to the viscosity of the medium might also be of substantial evolutionary significance, in particular in the context of cryptic female choice [5,55]. The willow warbler is a highly promiscuous species [41–43] and patterns of variation in sperm length suggest that sperm competition is generally high across the wide distribution ranges of two subspecies [56]. Thus, a predicted high ovarian fluid viscosity in the female genital tract may contribute to better informed cryptic female choices of preferred spermatozoa within-ejaculates but in particular among competing ejaculates (the latter in a similar way poultry science has used media of different traversability with the goal to better differentiate between males with different siring potential, see [36]). High fluid viscosity may here serve as one of several filter mechanisms weeding out spermatozoa with inferior swimming performance and/or enable females to generally slow down sperm swimming velocity in order to allow other probing mechanisms, for example the immune system, to function best. Furthermore, our results show that with only small changes in ovarian fluid viscosity females may be able to impose large effects on sperm behaviour (cf. figure 2), potentially allowing them to fine-tune selectivity in response to e.g. specific copulation partners or general social and environmental conditions. Unfortunately, to our knowledge, no information is available with respect to fluid viscosity in the genital tract of female birds. Examining this represents an important line for future research including the study of potential temporal dynamics for example in relation to the female reproductive cycle. Theoretical considerations and the visual inspection of individual reaction norms in our results suggest that the effects of media viscosities on sperm velocity may not be linear, but rather a decelerating function (figure 2). Only three experimental cellulose concentrations are, however, insufficient to allow further inference regarding the potential curvature of this effect. Future work should expose spermatozoa to a better resolved and also wider range of experimental cellulose concentrations. Furthermore, as cellulose concentration may not scale linearly with viscosity, it would also be informative to measure the actual viscosity of experimental (and of course natural, see above) sperm swimming environments.
(b). Sources of random variation and covariation in sperm velocity
In both statistical frameworks, we found low, but significant, among-video take variance within video recordings in sperm velocity. This pattern suggests small-scale variation e.g. in cellulose concentration within slide chambers or artefacts due to edge effects, for example, when the slide chamber is not fully or not equally filled such that some of the probed grids start drying from the margins earlier than others. While such variance components are often not accounted for in statistical models applied to CASA data, our results suggest they should. For any split-ejaculate designs, the same reasoning applies to the low but significant among-video recording variance within experimental ejaculates detected under the RN approach.
Interestingly, we found significant among-ejaculate variance in sperm velocity both in our random slope model and in each of the three environments under the CS approach. In promiscuous species in particular, sperm velocity is a potentially highly relevant trait in determining competitive fertilization success [15] and testing for consistent differences among individual males in natural populations is therefore important. In our dataset, however, male and ejaculate identity are confounded as just one experimental ejaculate per male had been sampled. Thus, it remains unclear at present whether among-ejaculate variance in our models actually maps to individual male phenotypes (or even genotypes) rather than individual ejaculates. While some evidence for consistent among-individual variation in sperm velocity suggests the former (e.g. [40,57]), only routinely sampling replicate ejaculates within males will allow evaluating the relative contribution of among-ejaculate and among-male variation in this key sperm trait.
In our RN approach, we found no evidence that spermatozoa from different ejaculates responded differentially to changes in media viscosity. This corresponds well with the visual representation of our aggregated raw data (figure 2) and may suggest biomechanical constraints on cross-environment sperm performance and/or selection acting on the slope of the velocity-by-viscosity reaction norm, resulting in little genetic and/or phenotypic variation in this trait. It remains unclear, however, whether any random slope variation would refer to individual male phenotypes (or even genotypes) rather than individual ejaculates (as discussed above). An extended sampling regime with higher replication on under-sampled grouping levels would be necessary to firmly exclude the possibility of among-male random slope variation. Such a sampling regime may be more feasible in captive rather than natural bird populations. Under the RN approach, we found that spermatozoa from ejaculates with different elevations of their velocity-by-cellulose reaction norm (i.e. that have relatively fast or slow spermatozoa in the mean experimental environment experienced) did not differentially decrease in sperm velocity across the experimental cellulose concentration gradient. Thus, there is also no evidence for any kind of trade-off in sperm performance (which could lead to specialization), for example, such that generally fast spermatozoa tire out sooner in environments where they meet with more resistance.
The CS approach estimated all correlations in sperm velocity among cellulose concentrations to be positive, but both correlations involving the lowest cellulose concentration (i.e. 0%) were low and non-significant. This indicates that sperm performance in a medium without cellulose was strongly independent from sperm performance in the presence of cellulose, which questions the validity of inference from sperm performance assays in such standard media. In a within-species context, for example, a reported lack of significant associations between (i) sperm morphology and sperm velocity as assessed in standard media (e.g. [58]) or (ii) sperm velocity as assessed in standard media and competitive fertilization success (e.g. [59]) may then represent false-negative results. The correlation among viscosities of 1% and 2%, however, was strong and highly significant, emphasizing the importance of among-ejaculate variation in overall swimming velocity rather than specialization to these different media viscosities. This result suggests that sperm velocity of individual ejaculates/males represents a transitive trait, the rank order of which is maintained when expressed across different environmental backgrounds. In line with this and the results from the RN approach, since none of the correlations was estimated to be negative, there is no indication for specialization, which would be the case if different ejaculates contained sperm that perform well in some environments, but poorly in others.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the members of the Stats Club of Bielefeld University's Evolutionary Biology and Animal Behaviour departments for discussion and Steven Ramm and two anonymous reviewers for helpful comments on a previous version of this manuscript.
Ethics
Permits to capture, handle and ring the birds were issued by the Norwegian Directorate for Nature Management to O.K. (A-license, 1082). Sperm was collected in adherence to the Norwegian regulations for the use of animals in research.
Data accessibility
Data including R and ASReml code are available as electronic supplementary material.
Authors' contributions
O.K. and G.R. conceived the study and conducted the fieldwork. G.R. performed the computer-assisted sperm analysis. T.S. analysed the data (reaction norm approach) and led the writing of the manuscript. H.S. analysed the data (character-state approach). All authors contributed critically to the drafts and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Financial support was received from The Fram Centre and the Norwegian Institute for Nature Research. H.S. was supported by an Emmy Noether fellowship from the German Research Foundation (DFG; SCHI 1188/1-1). Data analysis and interpretation by T.S. and H.S. benefitted from discussions within the Collaborative Research Center TRR 212 (NC³) funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - Projektnummer 316099922 - TRR 212.
References
- 1.Birkhead TR, Møller AP, Sutherland WJ. 1993. Why do females make it so difficult for males to fertilize their eggs? J. Theor. Biol. 161, 51–60. ( 10.1006/jtbi.1993.1039) [DOI] [Google Scholar]
- 2.Miller DJ. 2018. Review: the epic journey of sperm through the female reproductive tract. Animal 12, S110-S120. ( 10.1017/s1751731118000526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice WR. 2000. Dangerous liaisons. Proc. Natl Acad. Sci. USA 97, 12 953–12 955. ( 10.1073/pnas.97.24.12953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberhard W. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Firman RC, Gasparini C, Manier MK, Pizzari T. 2017. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 32, 368–382. ( 10.1016/j.tree.2017.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies NB. 1983. Polyandry, cloaca-pecking and sperm competition in dunnocks. Nature 302, 334–336. ( 10.1038/302334a0) [DOI] [Google Scholar]
- 7.Pizzari T, Birkhead TR. 2000. Female feral fowl eject sperm of subdominant males. Nature 405, 787–789. [DOI] [PubMed] [Google Scholar]
- 8.Snook RR, Hosken DJ. 2004. Sperm death and dumping in Drosophila. Nature 428, 939–941. ( 10.1038/nature02455) [DOI] [PubMed] [Google Scholar]
- 9.Suarez SS, Pacey AA. 2006. Sperm transport in the female reproductive tract. Hum. Reprod. Update 12, 23–37. ( 10.1093/humupd/dmi047) [DOI] [PubMed] [Google Scholar]
- 10.Brillard JP, Bakst MR. 1990. Quantification of spermatozoa in the sperm-storage tubules of turkey hens and the relation to sperm numbers in the perivitelline layer of eggs. Biol. Reprod. 43, 271–275. ( 10.1095/biolreprod43.2.271) [DOI] [PubMed] [Google Scholar]
- 11.Brillard JP. 1993. Sperm storage and transport following natural mating and artificial insemination. Poult. Sci. 72, 923–928. ( 10.3382/ps.0720923) [DOI] [PubMed] [Google Scholar]
- 12.Hemmings N, Birkhead T. 2017. Differential sperm storage by female zebra finches Taeniopygia guttata. Proc. R. Soc. B 284, 20171032 ( 10.1098/rspb.2017.1032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmings N, Bennison C, Birkhead TR. 2016. Intra-ejaculate sperm selection in female zebra finches. Biol. Lett. 12, 20160220 ( 10.1098/rsbl.2016.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S. 2009. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132. ( 10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleven O, Fossøy F, Laskemoen T, Robertson RJ, Rudolfsen G, Lifjeld JT. 2009. Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution 63, 2466–2473. ( 10.1111/j.1558-5646.2009.00725.x) [DOI] [PubMed] [Google Scholar]
- 16.Holt WV, Fazeli A. 2016. Sperm selection in the female mammalian reproductive tract. Focus on the oviduct: hypotheses, mechanisms, and new opportunities. Theriogenology 85, 105–112. ( 10.1016/j.theriogenology.2015.07.019) [DOI] [PubMed] [Google Scholar]
- 17.Lüpold S, Pitnick S. 2018. Sperm form and function: what do we know about the role of sexual selection? Reproduction 155, R229–R243. ( 10.1530/rep-17-0536) [DOI] [PubMed] [Google Scholar]
- 18.Hunter RHF, Coy P, Gadea J, Rath D. 2011. Considerations of viscosity in the preliminaries to mammalian fertilisation. J. Assist. Reprod. Genet. 28, 191–197. ( 10.1007/s10815-010-9531-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkman-Brown JC, Smith DJ. 2011. Sperm motility: is viscosity fundamental to progress? Mol. Hum. Reprod. 17, 539–544. ( 10.1093/molehr/gar043) [DOI] [PubMed] [Google Scholar]
- 20.Vernon GG, Woolley DM. 1999. Three-dimensional motion of avian spermatozoa. Cell Motil. Cytoskeleton 42, 149–161. [DOI] [PubMed] [Google Scholar]
- 21.Moore H, Dvorakova K, Jenkins N, Breed W. 2002. Exceptional sperm cooperation in the wood mouse. Nature 418, 174–177. ( 10.1038/nature00832) [DOI] [PubMed] [Google Scholar]
- 22.Marshall DJ. 2015. Environmentally induced (co)variance in sperm and offspring phenotypes as a source of epigenetic effects. J. Exp. Biol. 218, 107–113. ( 10.1242/jeb.106427) [DOI] [PubMed] [Google Scholar]
- 23.Edme A, Zobač P, Korsten P, Albrecht T, Schmoll T, Krist M. 2018. Moderate heritability and low evolvability of sperm morphology in a species with high risk of sperm competition, the collared flycatcher Ficedula albicollis. J. Evol. Biol. 32, 205–217. ( 10.1111/jeb.13404) [DOI] [PubMed] [Google Scholar]
- 24.Schmoll T, Kleven O, Rusche M. 2018. Individual phenotypic plasticity explains seasonal variation in sperm morphology in a passerine bird. Evol. Ecol. Res. 19, 561–574. [Google Scholar]
- 25.Rudolfsen G, Figenschou L, Folstad I, Tveiten H, Figenschou M. 2006. Rapid adjustments of sperm characteristics in relation to social status. Proc. R. Soc. B 273, 325–332. ( 10.1098/rspb.2005.3305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota K, Heg D, Hori M, Kohda M. 2010. Sperm phenotypic plasticity in a cichlid: a territorial male's counterstrategy to spawning takeover. Behav. Ecol. 21, 1293–1300. ( 10.1093/beheco/arq146) [DOI] [Google Scholar]
- 27.Smith CC, Ryan MJ. 2011. Tactic-dependent plasticity in ejaculate traits in the swordtail Xiphophorus nigrensis. Biol. Lett. 7, 733–735. ( 10.1098/rsbl.2011.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizzari T, Cornwallis CK, Froman DP. 2007. Social competitiveness associated with rapid fluctuations in sperm quality in male fowl. Proc. R. Soc. B 274, 853–860. ( 10.1098/rspb.2006.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornwallis CK, O'Connor EA. 2009. Sperm: seminal fluid interactions and the adjustment of sperm quality in relation to female attractiveness. Proc. R. Soc. B 276, 3467–3475. ( 10.1098/rspb.2009.0807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lüpold S, Birkhead TR, Westneat DF. 2012. Seasonal variation in ejaculate traits of male red-winged blackbirds (Agelaius phoeniceus). Behav. Ecol. Sociobiol. 66, 1607–1617. ( 10.1007/s00265-012-1415-3) [DOI] [Google Scholar]
- 31.Purchase CF, Butts IAE, Alonso-Fernandez A, Trippel EA. 2010. Thermal reaction norms in sperm performance of Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 67, 498–510. ( 10.1139/f10-001) [DOI] [Google Scholar]
- 32.Purchase CF, Moreau DTR. 2012. Stressful environments induce novel phenotypic variation: hierarchical reaction norms for sperm performance of a pervasive invader. Ecol. Evol. 2, 2562–2571. ( 10.1002/ece3.364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlegel P, Havenhand JN, Obadia N, Williamson JE. 2014. Sperm swimming in the polychaete Galeolaria caespitosa shows substantial inter-individual variability in response to future ocean acidification. Mar. Pollut. Bull. 78, 213–217. ( 10.1016/j.marpolbul.2013.10.040) [DOI] [PubMed] [Google Scholar]
- 34.Butts IAE, Prokopchuk G, Kaspar V, Cosson J, Pitcher TE. 2017. Ovarian fluid impacts flagellar beating and biomechanical metrics of sperm between alternative reproductive tactics. J. Exp. Biol. 220, 2210–2217. ( 10.1242/jeb.154195) [DOI] [PubMed] [Google Scholar]
- 35.Pérez-Cerezales S, Lopéz-Cardona AP, Gutiérrez-Adán A. 2016. Progesterone effects on mouse sperm kinetics in conditions of viscosity. Reproduction 151, 501–507. ( 10.1530/rep-15-0582) [DOI] [PubMed] [Google Scholar]
- 36.King LM, Donoghue AM. 2000. Adaptation of the sperm mobility test for identification of turkey toms with low fertilizing potential. J. App. Poult. Res. 9, 66–73. ( 10.1093/japr/9.1.66) [DOI] [Google Scholar]
- 37.Westneat DF, Stewart IR.K. 2003. Extra-pair paternity in birds: causes, correlates, and conflict. Annual Review in Ecology , Evol. Sys. 34, 365–396. [Google Scholar]
- 38.Kempenaers B, Schlicht E. 2010. Extra-pair behaviour. In Animal behaviour: evolution and mechanisms (ed. Kappeler P.), pp. 359–412. Berlin, Germany: Springer. [Google Scholar]
- 39.Brouwer L, Griffith SC. 2019. Extra-pair paternity in birds. Mol. Ecol. 28, 4864–4882. ( 10.1111/mec.15259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laskemoen T, Kleven O, Johannessen LE, Fossøy F, Robertson RJ, Lifjeld JT. 2013. Repeatability of sperm size and motility within and between seasons in the Barn Swallow (Hirundo rustica). J. Ornithol. 154, 955–963. ( 10.1007/s10336-013-0961-4) [DOI] [Google Scholar]
- 41.Bjørnstad G, Lifjeld JT. 1997. High frequency of extra-pair paternity in a dense and synchronous population of Willow Warblers Phylloscopus trochilus. J. Avian Biol. 28, 319–324. [Google Scholar]
- 42.Fridolfsson AK, Gyllensten UB, Jakobsson S. 1997. Microsatellite markers for paternity testing in the willow warbler Phylloscopus trochilus: high frequency of extra-pair young in an island population. Hereditas 126, 127–132. [Google Scholar]
- 43.Gil D, Slater PJB, Graves JA. 2007. Extra-pair paternity and song characteristics in the willow warbler Phylloscopus trochilus. J. Avian Biol. 38, 291–297. ( 10.1111/j.2007.0908-8857.03868.x) [DOI] [Google Scholar]
- 44.Via S, Gomulkiewicz R, Dejong G, Scheiner SM, Schlichting CD, Van Tienderen PH.. 1995. Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217. ( 10.1016/s0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- 45.Laskemoen T, Kleven O, Fossøy F, Robertson RJ, Rudolfsen G, Lifjeld JT. 2010. Sperm quantity and quality effects on fertilization success in a highly promiscuous passerine, the tree swallow Tachycineta bicolor. Behav. Ecol. Sociobiol. 64, 1473–1483. ( 10.1007/s00265-010-0962-8) [DOI] [Google Scholar]
- 46.De Jong G. 1990. Quantitative genetics of reaction norms. J. Evol. Biol. 3, 447–468. ( 10.1046/j.1420-9101.1990.3050447.x) [DOI] [Google Scholar]
- 47.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 48.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 49.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. [Google Scholar]
- 50.Gilmour AR, Gogel BJ, Cullis BR, Thompson R. 2009. ASReml user guide, release 3.0. Hemel Hempstead, UK: VSN International. [Google Scholar]
- 51.Jamieson BG.M. 2007. Avian spermatozoa: Structure and phylogeny. In Reproductive biology and phylogeny of birds (ed. Jamieson BGM.), pp. 349–511. Enfield, NH: Science Publishers. [Google Scholar]
- 52.Støstad HN, Johnsen A, Lifjeld JT, Rowe M. 2018. Sperm head morphology is associated with sperm swimming speed: a comparative study of songbirds using electron microscopy. Evolution 72, 1918–1932. ( 10.1111/evo.13555) [DOI] [PubMed] [Google Scholar]
- 53.Lüpold S, Calhim S, Immler S, Birkhead TR. 2009. Sperm morphology and sperm velocity in passerine birds. Proc. R. Soc. B 276, 1175–1181. ( 10.1098/rspb.2008.1645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cramer ERA, Alund M, McFarlane SE, Johnsen A, Qvarnstrom A. 2016. Females discriminate against heterospecific sperm in a natural hybrid zone. Evolution 70, 1844–1855. ( 10.1111/evo.12986) [DOI] [PubMed] [Google Scholar]
- 55.Kvarnemo C, Simmons LW. 2013. Polyandry as a mediator of sexual selection before and after mating. Phil. Trans. R. Soc. B 368, 20120042 ( 10.1098/rstb.2012.0042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Støstad HN, Rekdal SL, Kleven O, Laskemoen T, Marthinsen G, Johnsen A, Lifjeld JT. 2016. Weak geographical structure in sperm morphology across the range of two willow warbler Phylloscopus trochilus subspecies in Scandinavia. J. Avian Biol. 47, 731–741. ( 10.1111/jav.00981) [DOI] [Google Scholar]
- 57.Mossman J, Slate J, Humphries S, Birkhead T. 2009. Sperm morphology and velocity are genetically codetermined in the Zebra Finch. Evolution 63, 2730–2737. ( 10.1111/j.1558-5646.2009.00753.x) [DOI] [PubMed] [Google Scholar]
- 58.Lifjeld JT, Laskemoen T, Kleven O, Pedersen ATM, Lampe HM, Rudolfsen G, Schmoll T, Slagsvold T. 2012. No evidence for pre-copulatory sexual selection on sperm length in a passerine bird. PLoS ONE 7, e32611 ( 10.1371/journal.pone.0032611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saetre CL, Johnsen A, Stensrud E, Cramer ER.A. 2018. Sperm morphology, sperm motility and paternity success in the bluethroat (Luscinia svecica). PLoS ONE 13, e0192644 ( 10.1371/journal.pone.0192644) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data including R and ASReml code are available as electronic supplementary material.