Abstract
Many insects survive internal freezing, but the great complexity of freezing stress hinders progress in understanding the ultimate nature of freezing-induced injury. Here, we use larvae of the drosophilid fly, Chymomyza costata to assess the role of mitochondrial responses to freezing stress. Respiration analysis revealed that fat body mitochondria of the freeze-sensitive (non-diapause) phenotype significantly decrease oxygen consumption upon lethal freezing stress, while mitochondria of the freeze-tolerant (diapausing, cold-acclimated) phenotype do not lose respiratory capacity upon the same stress. Using transmission electron microscopy, we show that fat body and hindgut mitochondria swell, and occasionally burst, upon exposure of the freeze-sensitive phenotype to lethal freezing stress. By contrast, mitochondrial swelling is not observed in the freeze-tolerant phenotype exposed to the same stress. We hypothesize that mitochondrial swelling results from permeability transition of the inner mitochondrial membrane and loss of its barrier function, which causes osmotic influx of cytosolic water into the matrix. We therefore suggest that the phenotypic transition to diapause and cold acclimation could be associated with adaptive changes that include the protection of the inner mitochondrial membrane against permeability transition and subsequent mitochondrial swelling. Accumulation of high concentrations of proline and other cryoprotective substances might be a part of such adaptive changes as we have shown that freezing-induced mitochondrial swelling was abolished by feeding the freeze-sensitive phenotype larvae on a proline-augmented diet.
Keywords: freeze tolerance, mitochondrial morphology, insects
1. Introduction
Many insect species thrive in permanently or seasonally cold habitats. In order to survive when their body temperature falls below 0°C, these insects evolved a suite of biochemical and physiological cold hardiness mechanisms [1,2]. Three major adaptive ‘strategies’ of cold hardiness are distinguished based on the phase behaviour of body water at subzero temperature: supercooling (maintenance of body water in the liquid phase), cryoprotective dehydration (loss of a substantial proportion of the body water by evaporation) and freeze tolerance (conversion of body water to the solid phase—ice crystals) [3]. Freeze tolerance is perhaps the most striking adaptive strategy as it includes responses to a combination of strong abiotic stressors such as low temperature, absence of bulk liquid water and presence of ice crystals. These stressors cause a formidable combination of deleterious effects which act simultaneously on molecules, molecular complexes, metabolism, cells, tissues and integration of systems [4–8]. Despite the challenges associated with internal freezing, freeze tolerance has evolved independently and repeatedly in some ectotherm vertebrates [9], and in many invertebrates and insects [10,11]. The complexity of freezing stress hinders distinguishing between causes and consequences, which is needed for building of integrative models sensu MacMillan [12] to understand both freezing-induced injury and the mechanisms of freeze tolerance.
Recently, Toxopeus & Sinclair [8] reviewed current knowledge on insect freeze tolerance and listed all known and proposed mechanisms underlying freezing injury and freeze tolerance. Toxopeus & Sinclair [8] see freeze tolerance as an ability to survive the whole process of cooling, freezing, being frozen, thawing and subsequent repair of damage to macromolecules and cells. They propose concentrating the effort to obtain holistic characterization of freeze tolerance in a suitable insect model. One such emerging model is the larva of the fly, Chymomyza costata (Diptera: Drosophiliae). The non-diapause, warm- acclimated larvae are relatively freeze sensitive, tolerating freezing only at mild subzero temperatures (−5°C), while the diapausing and cold-acclimated larvae can survive deep freezing in liquid nitrogen (−196°C, LN2) [13]. Entry into diapause results in cessation of development, loss of behavioural activities and deep metabolic suppression, all based on massive alteration of gene expression [14]. Cold acclimation stimulates metabolic reorganization resulting in accumulation of free proline that reaches concentrations higher than 0.3 M [15]. Proline exogenously added to non-diapause larvae, via a proline-augmented diet, increases their freeze tolerance and renders them even cryopreservable in LN2 [13,15]. The cold acclimation is associated with further change in transcriptomic profile suggesting enhanced capacity for protein folding, refolding and processing [16]. Such capacity may help to repair or eliminate proteins damaged by freezing in the process of recovery after freezing. Metabolomic and transcriptomic analysis of recovery after freezing revealed that lethally freeze-injured larvae show perturbations pointing towards a blockade of citric acid cycle metabolism and impaired mitochondrial function [17].
Mitochondria of all eukaryotic cells play crucial functions including energy supply, metabolic conversions, sensing changes in the cell environment and switching between cell survival or death pathways [18]. Mitochondrial morphology (shape, size, internal structure) is highly plastic and influenced by physiological and pathological conditions [19]. Mitochondrial swelling and dilution of the matrix, eventually leading to ruptures of the outer membrane, belong to stereotypically observed abnormalities in different organisms in response to numerous pathologies or stressors: pro-apoptotic signalling in Drosophila [20] and mammals [21]; ageing in Drosophila [22]; ischemia in mammals [23]; anoxia in plants [24], heat stress in Drosophila [25]; and freezing stress in plants [26] and mammals [27,28]. The responses of insect mitochondria to freezing stress are minimally studied so far. To our knowledge, there is just a single anecdotic observation in the insect cold hardiness literature showing occurrence of swollen and rounded mitochondria in Malpighian tubules of the freeze-tolerant, winter phenotype larvae of the gall fly, Eurosta solidaginis exposed to lethal freezing at −55°C, while mitochondria exposed to sub-lethal freezing at −22°C remained normal (rod-shaped) [29]. In addition, no loss of activity upon freezing and thawing was observed in cytochrome c-oxidase in sub-lethally frozen (−16°C) winter larvae [30]. Other work with E. solidaginis indicated reduced mitochondrial function in the winter larvae in comparison to autumn larvae (a freeze-sensitive phenotype). Several mitochondrial enzymes: citrate synthase (CS), glutamate dehydrogenase, NAD-isocitrate dehydrogenase [31] and cytochrome c-oxidase [30] showed approximately 50% reduction in activity. Levin et al. [32] showed that the reduced mitochondrial activity might be caused by reduced mitochondrial counts: the mitochondrial DNA content decreased by approximately half in the winter larvae of E. solidaginis. The remaining winter mitochondria were fully coupled, capable of oxidizing various substrates, and capable of respiration upon transfer to 20°C [33]. It remains unknown to what extent the responses observed in E. solidaginis are general for other freeze-tolerant insects.
Here, we aimed to assess and compare the sensitivity of mitochondria to freezing stress in the freeze-tolerant model insect, C. costata. The relatively freeze-sensitive (non-diapause, warm-acclimated) and freeze-tolerant (either diapause, cold-acclimated; or non-diapause, proline-augmented diet fed) phenotypic/acclimation variants of larvae were exposed to cold (−5°C) and freezing stresses (−5°C and −30°C), and the effects of these stresses on mitochondrial counts, function and morphology were observed in fat body and hindgut tissues. We hypothesized that mitochondrial counts would be reduced upon entry into diapause and cold acclimation. This can be a general response of freeze-tolerant insects, which is dictated by a strong reduction in the need for mitochondrial activity in a state of deep dormancy together with internal freezing [32,34]. Next, we asked whether the mitochondria of freeze-tolerant phenotypes retain respiratory capacity, or whether their function is compromised during winter. Mainly, we asked whether the mitochondria of freeze-sensitive and -tolerant variants differ in their ability to prevent freezing-induced injury displayed as a loss of viable morphology (i.e. swelling and/or bursting) and loss of enzymatic and respiratory function. One specific aim was to find whether the exogenous proline supplied via a proline-augmented diet may protect the mitochondria of non-diapause, warm-acclimated larvae from freezing-induced injury.
2. Material and methods
(a). Insects, phenotypic variants and cold exposures
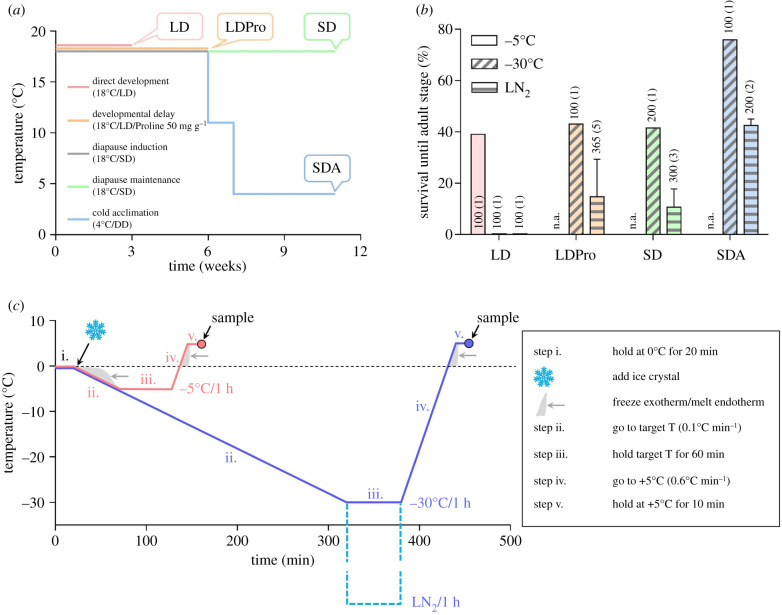
A C. costata colony, Sapporo strain [35] was reared on artificial diet in MIR 154 incubators (Sanyo Electric, Osaka, Japan) as described previously [16]. For experiments, we generated four phenotypic variants of 3rd instar larvae differing in the level of freeze tolerance according to our earlier acclimation protocols [13–16]: (i) non-diapause ‘LD’ larvae; (ii) non-diapause, proline-supplemented ‘LDPro’ larvae; (iii) diapausing, warm-acclimated ‘SD’ larvae; and (iv) diapausing, cold-acclimated ‘SDA’ larvae. Details on acclimation protocols and freeze tolerance are shown in figure 1a,b.
Figure 1.
Phenotypic variants, freeze tolerance and cold/freezing stress. (a) Larvae of C. costata were acclimated at four different temperature/photoperiod regimes in MIR 154 incubators (Sanyo Electric, Osaka, Japan) in order to generate four phenotypic variants of 3rd instar larvae. All larvae were of similar fresh mass of (1.5–2.0 mg). Abbreviations: LD, long days (L16 h : D8 h); SD, short days (L12 h : D12 h); SDA, short days followed by gradual cold acclimation (1 week at 11°C, 4 weeks at 4°C; DD, constant darkness); LDPro, feeding the LD larvae a proline-augmented diet (50 mg of proline added to 1 g of larval diet). Long days promote direct development to pupa and adult, while short days induce diapause in the 3rd instar larva. Gradual cold acclimation and feeding on the proline-augmented diet stimulates cold hardiness. In addition, feeding on the proline-augmented diet causes developmental delay. (b) The phenotypic variants of C. costata larvae differ in the level of freeze tolerance assessed at three different temperatures (−5°C, −30°C, and −196°C (LN2)). The columns show mean ± s.d. survival of frozen larvae through pupation to the adult stage, and the numbers associated with each column show the total number of larvae subjected to the assay with the number of different fly generations assayed given in parentheses (n.a., not assayed). The data presented in part (b) were redrawn from Rozsypal & Koštál [15] and Des Marteaux et al. [16]. (c) Temperature protocols used to expose the larvae to cold/freezing stresses at −5°C and −30°C in this study. Both protocols consisted of five steps (i. to v., see legend) set in a Ministat 240 programmable cryostat (Huber, Offenburg, Germany). Adding a small ice crystal (or not) at the beginning of step ii. induced inoculative internal freezing in larvae (or not, respectively), which allowed us to distinguish between the effects of low temperature per se, i.e. supercooling (−5°C, S) and freezing at the same temperature (−5°C, F). Note that the same protocols (including exposure to LN2, dashed line, not used in this study) were used in our earlier studies to assess larval freeze tolerance. (Online version in colour.)
The acclimated larvae were exposed to cold or freezing stress. Three different stresses were used in LD larvae: supercooling to −5°C (−5°C, S); freezing to −5°C (−5°C, F); and freezing to −30°C (−30°C). While 90% of LD larvae survive the supercooling stress, only 35% survive freezing to −5°C and none survive freezing to −30°C [15]. Other phenotypic variants (LDPro, SD, and SDA) were exposed to the −30°C freezing stress only, which is survived by 43%, 42% and 76% of larvae, respectively [15]. For a schematic depiction of cold/freezing protocols, see figure 1c.
For analyses of mitochondrial counts, shape and function, we dissected larvae in chilled phosphate-buffered saline (PBS) under a dissecting microscope either directly from acclimation protocols (non-cold-exposed controls) or immediately after cold/freezing exposure (at the end of step v. of the protocol, figure 1c). Fat body and hindgut tissues were extracted from each larva and processed as described next.
(b). Mitochondrial counts
We used three different methods to assess the influence of acclimation protocols on mitochondrial counts in the fat body tissue. First, we stained dissected fat body using MitoTracker Green (ThermoFisher Scientific, Waltham, MA, USA), a green-fluorescent mitochondrial stain which localizes mitochondria regardless of mitochondrial membrane potential (for details, see the electronic supplementary material, figure S1). Second, we measured the activity of CS according to [36] (for details, see the electronic supplementary material). CS activity is frequently used to assess the oxidative capacity of mitochondria as well as mitochondria integrity and counts [37]. Third, we measured the ratio of mitochondrial/nuclear DNA using relative quantification (qPCR) of DNA coding for cytochrome c-oxidase I (COX I, mitochondrial gene) and ribosomal protein 19 (RpL19, nuclear gene) [30,38] (for details, see the electronic supplementary material).
(c). Mitochondrial shape
Larval fat body and hindgut tissues were dissected and fixed overnight in PBS containing 2.5% glutaraldehyde. We added proline into the fixation solution for LDPro and SDA tissues (500 mM and 300 mM, respectively) in order to account for osmotic effects of extremely high concentrations of proline in these phenotypic variants [15]. The processing of fixed tissues was described earlier [39] (for details, see the electronic supplementary material).
We analysed the shapes of 3468 fat body mitochondria and 4393 hindgut mitochondria via transmission electron microscopy (TEM) micrographs at 25 000x magnification and ImageJ software (https://imagej.net/) as explained in the electronic supplementary material, figure S2. Two parameters were taken for each mitochondrion: area, surface area of mitochondrial section in µm2 and aspect ratio (AR), ratio of major/minor axis of a fitted ellipse. Next, the median area/AR ratio of mitochondria in each larva was taken to statistically analyse differences in mitochondrial shape between phenotypic variants (considering each single larva as a biological replicate). Rod-like mitochondria (frequently occurring in the non-cold-exposed control larvae) typically showed relatively low median area/AR (less than 0.1), while rounded, swollen mitochondria (frequently occurring in lethally stressed larvae) typically showed relatively high median area/AR (greater than 0.4).
(d). Mitochondrial function
We used two different methods to assess the influence of acclimation protocols and cold/freezing stress on mitochondrial integrity and functionality in the fat body tissue. First, the activity of CS (as described in §2b), was measured in the fat body tissue dissected from larvae immediately after cold/freezing exposure. Second, we measured the oxygen consumption of dissected fat body tissues using the PreSens system (PreSens GmbH, Regensburg, Germany). For more details, see the electronic supplementary material, figure S3.
3. Results and discussion
(a). Acclimation has little impact on mitochondrial counts, morphology and function
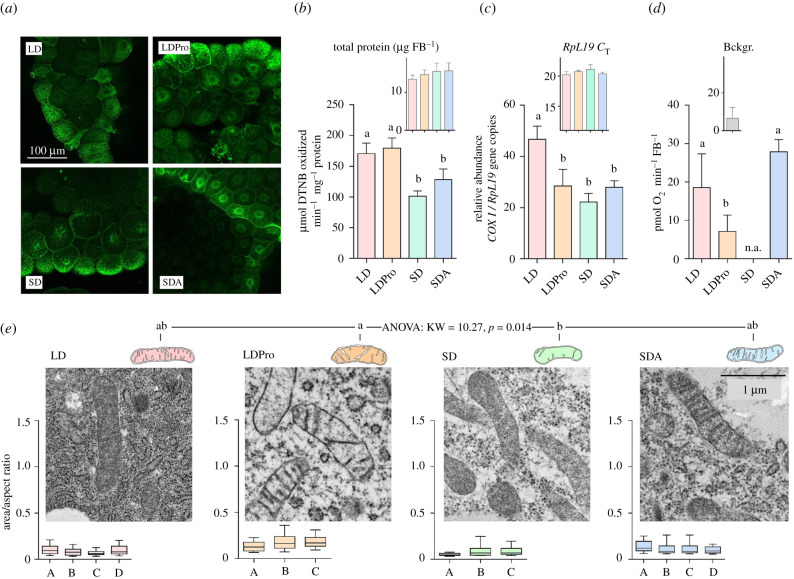
Available literature suggests that mitochondrial counts are reduced in winter phenotypes of freeze-tolerant insects [30,32,34]. We estimated the mitochondrial counts in fat body tissue of freeze-tolerant C. costata larvae using several approaches. The MitoTracker Green staining revealed considerable variation of the signal intensity among different cells. Some cells were brightly stained while the adjacent cells were dim (in all optical sections) (figure 2a). The differences in staining between cells most likely reflect the regional and functional differentiation of the fat body [40]. In addition, mitochondria were not evenly distributed in the cytoplasm but rather formed two distinct populations located around the nucleus and close to the cell periphery (electronic supplementary material, figure S1). Hence, it was technically difficult to count, or even estimate by analysis of staining intensity, mitochondrial numbers in the whole tissue. At least we can conclude that MitoTracker Green staining patterns were generally similar in all larval phenotypic variants (figure 2a; electronic supplementary material, figure S1). Two other analyses, enzyme activity and ratio of mitochondrial/nuclear DNA, suggested that mitochondrial counts in fat body tissue might be slightly reduced during entry into diapause. The activity of CS (figure 2b) was 1.5-fold lower in diapausing (SD) versus active (LD) larvae. The enzyme activities, however, are differentially regulated during complex phenotypic transitions such as insect preparation for the winter season [30,41]. Hence, enzyme activities may indicate changes at the level of enzyme molecular abundance/activity (allosteric regulations, post-translational modifications, etc.) rather than serving as reliable indicators of mitochondrial counts. The ratio of mitochondrial/nuclear DNA is probably the most reliable indicator of mitochondrial counts, although in insects its usage is complicated by very high and variable ploidy of larval cells. For instance, the average ploidy of larval fat body cells is 256C in D. melanogaster [42]. Nevertheless, the ratio of mitochondrial/nuclear DNA (figure 2c) was twofold lower in SD versus LD larvae, which is in a good agreement with data for the winter phenotype of E. solidaginis [32]. No further decreases in CS activity or mitochondrial/nuclear DNA were found during cold acclimation of diapausing larvae (SD versus SDA) (figure 2b,c). The oxygen consumption (measured at a constant temperature of 23°C) did not differ between LD and SDA fat body tissues (figure 2d), which shows that the capacity for respiratory function was influenced neither by entry into diapause and subsequent cold acclimation nor by any potential diapause-linked reduction in mitochondrial counts (see discussion above). Similarly, the mitochondria of winter-acclimated E. solidaginis retained capacity to function at 20°C [33]. Such results indicate that mitochondria of diapausing, cold-acclimated insects retain full capacity for function upon transfer to optimal temperature. The mitochondrial gross morphology and shape were the same for LD, SD and SDA larvae (figure 2e). Collectively, our results indicate that the mitochondrial counts might be slightly (up to twofold) reduced in C. costata fat body upon entry into diapause, but their morphology remains normal and functionality is retained even during deep diapause and metabolic suppression at a low temperature of 4°C.
Figure 2.
The effect of acclimation protocols on C. costata larval fat body mitochondrial counts, shape and function. (a) Example micrographs of fat body tissues dissected from four phenotypic variants differing in freeze tolerance (LD, LDPro, SD and SDA, see §2a and figure 1 for explanations). The tissues were stained using MitoTracker Green (see the electronic supplementary material, figure S1 for more details on staining and microscopy procedures). (b) Citrate synthase (CS) activity in fat body tissues of four freeze tolerance phenotypes. Each column is a mean and s.d. of four biological replicates of CS activity assay (each containing 50 dissected tissues pooled). (c) Ratio of mitochondrial/nuclear (mt nu−1) DNA in larval fat body for four freeze tolerance phenotypes. Each column is a mean and s.d. of four biological replicates (each containing 50 dissected tissues pooled) of the RT-qPCR relative quantitation of mitochondrial (COXI) and nuclear (RpL19) gene copies. (d) Oxygen consumption analysed using the PreSens system (see the electronic supplementary material, figure S3 for details) in larval fat body for three freeze tolerance phenotypes (n.a., SD tissues were not assessed). Each column is a mean and s.d. (after the background (Bckgr.) reading has been subtracted) of four to eight biological replicates (each containing 20 dissected tissues pooled). The differences between phenotypes in (b–d) were assessed by ANOVA followed by Tukey's multiple comparisons test (statistically different variants are flanked by different letters). (e) Representative TEM micrographs of fat body mitochondria in the four phenotypes. All micrographs were taken at 25 000 × magnification. Mitochondrial shape was analysed as explained in the electronic supplementary material, figure S2 and the raw dataset of all mitochondrial parameters can be found in the electronic supplementary material, Dataset S1. The box plots below each micrograph show distributions (median, quartiles, 5% and 95% percentiles) of mitochondrial shapes in individual larvae (A–D). The distributions did not pass D'Agostino & Pearson omnibus normality tests, and therefore, the differences in mitochondrial shape between acclimation variants were analysed using the nonparametric Kruskal–Wallis test (KW statistic and p-value shown) followed by Dunn's multiple comparisons test (statistically different variants are flanked by different letters). (Online version in colour.)
We discuss the larvae reared on a proline-augmented diet (LDPro) separately, as this treatment is toxic: many of the larvae die during early instars, and those which survive are highly variable in size, with development that is slowed or halted, (V. Koštál, many years of unpublished observations). Paradoxically, just these proline-toxified larvae show extremely high freeze tolerance and can even survive cryopreservation in LN2 [13,15]. The LDPro larval variant exhibited normal CS activity (figure 2b), approximately 1.5-fold reduction in mitochondrial count (figure 2c), and twofold reduction in oxygen consumption (figure 2d) when compared to the LD variant. Moreover, LDPro larval mitochondria were slightly enlarged (showed the highest area/AR among all acclimation variants) and their matrix did not contain electron-dense material as in other variants. Similar, or even more pronounced, morphological deviations as observed in LDPro fat body mitochondria were also observed in LDPro hindgut mitochondria (electronic supplementary material, figure S4). We can collectively conclude that feeding C. costata larvae the toxic proline-augmented diet had visible consequences on mitochondrial shape (slightly swollen), morphology (electron-transparent matrix) and functionality (reduced oxygen consumption).
(b). The mitochondria of freeze-sensitive larvae swell and lose function upon freezing stress
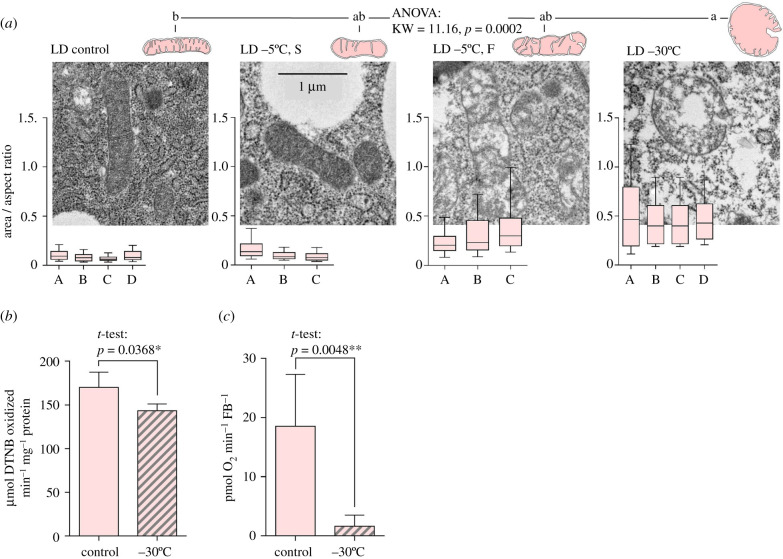
Morphological changes of LD fat body mitochondria in response to cold and freezing stresses are summarized in figure 3a. The mitochondria did not change shape when exposed to supercooling stress (S) at −5°C, which causes practically no mortality in larvae. Exposing LD larvae to the same temperature (−5°C) but combined with internal freezing (F) causes mortality in approximately 65% of larvae [15]. Freezing at −5°C resulted in apparent (though not statistically significant) changes in mitochondrial shape; while some larvae (e.g. A) had area/AR ratios close to that of LD controls, other larvae (e.g. B, C) had enlarged or swollen mitochondria showing partial loss of electron-dense material from the matrix. Freezing at −30°C is invariably lethal for all LD larvae [15]. The fat body mitochondria of lethally stressed larvae were significantly larger, swollen and rounded, showing loss of electron-dense material from the matrix, and in many cases, their membranes were not continuous (mitochondrial bursting). Similar effects of lethal freezing as observed in LD mitochondria of fat body tissue were also observed in LD mitochondria of the hindgut tissue (electronic supplementary material, figure S5A). In addition, the fat body mitochondria of lethally frozen LD larvae showed a relatively modest decrease in CS activity (figure 3b) but had lost almost all capacity for oxygen consumption (figure 3c).
Figure 3.
The effect of cold/freezing stresses on fat body mitochondrial shape and function in freeze-sensitive C. costata larvae. (a) Representative TEM micrographs of fat body mitochondria of larvae (LD phenotype) exposed to different cold/freezing stresses (−5°C, S; −5°C, F; and −30°C) and controls (figure 1 for more explanations). Other descriptions as in figure 2e. (b) Citrate synthase activity and (c) oxygen consumption in fat body tissue of control versus lethally freeze-injured (−30°C) larvae (LD phenotype). Other descriptions as in figure 2b and d, respectively. (Online version in colour.)
(c). The mitochondria of freeze-tolerant larvae maintain shape and function upon freezing stress
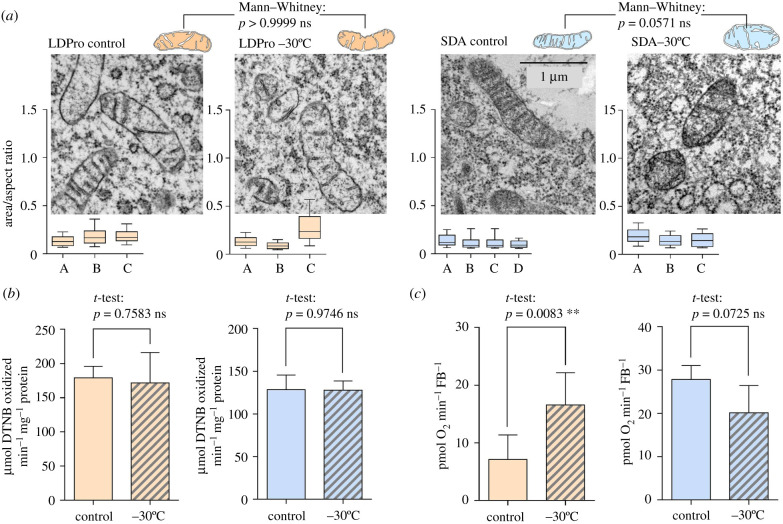
The mitochondria of freeze-tolerant LDPro and SDA larvae exposed to freezing at -30°C did not swell to the same extent as the mitochondria of LD larvae (figure 4a; electronic supplementary material, figure S5B, C). A certain tendency towards enlargement was apparent in some frozen larvae, for instance in the LDPro variant larva C (figure 4a). No loss of CS activity was observed upon freezing in LDPro and SDA larvae (figure 4b). Most importantly, no loss of capacity for oxygen consumption was seen in fat bodies dissected from LDPro and SDA frozen larvae (figure 4c). In fact, in the case of the LDPro variant, we paradoxically observed a statistically significant increase in oxygen consumption for frozen larvae. At least, this result convincingly documents that LDPro mitochondria do not lose functionality upon freezing stress.
Figure 4.
The effect of cold/freezing stresses on fat body mitochondrial shape and function in freeze-tolerant C. costata larvae. (a) Representative TEM micrographs of fat body mitochondria in two freeze-tolerant phenotypes (orange, LDPro; blue, SDA) exposed to sub-lethal freezing stresses (−30°C) and controls (figure 1 for more explanations). Other descriptions as in figure 2e. (b) Citrate synthase activity and (c) oxygen consumption in fat body tissue of control versus frozen (−30°C) larvae (LDPro and SDA phenotypes). Other descriptions as in figure 2b and d, respectively. (Online version in colour.)
A partial conclusion from §§(3b) and (3c) is that mitochondria of freeze-sensitive C. costata larvae (variant LD) exhibited swelling, bursting, and loss of respiratory activity when the larvae were exposed to lethal freezing at −30°C. By contrast, sub-lethal freezing or supercooling at −5°C caused only mild or no changes in mitochondrial morphology, respectively. The mitochondria of freeze-tolerant variants (LDPro and SDA) showed only mild or no changes in mitochondrial shape, and no loss of respiratory activity upon freezing at −30°C. Hence, mitochondrial swelling appears to be intimately linked with freezing-induced injury and mortality in C. costata, similarly as was previously observed in plants [26], mammals [27,28] and the gall midge, E. solidaginis [29].
(d). Potential linkages between mitochondrial swelling and larval mortality
We have seen relatively high activity of CS persisting in the fat body tissue (figure 3b) that lost most of its capacity for oxygen consumption (figure 3c) in lethally freeze-injured LD larvae. Hence, the CS appeared not to be particularly sensitive to freezing stress (at least not when frozen in its natural cellular environment). In general, however, the proteins are considered as important sources of freezing-induced injury in biological systems. The parallel influences of low temperature [6,43,44] and freeze dehydration effects such as low water activity, high ionic concentrations, increasing acidity and molecular crowding [4,5,45,46] may cause protein denaturation and aggregation. In the following discussion, we will purposely, for the sake of conciseness, leave aside damage to proteins and many other potential causes of freezing-induced injury [8]. Instead, we will focus on the major observation of this study—freezing stress-induced mitochondrial swelling in freeze-sensitive larvae—and offer at least speculative explanation of this phenomenon. Our ambition is not to make categorical conclusions but rather to provide hypotheses for further research.
We are unable to precisely determine the exact moment when the initial damage causing mitochondrial swelling occurred (during freeze dehydration or upon rehydration during melting, or even later?). At least two facts suggest that mitochondrial swelling plays an important role in the earlyformation of freezing-induced injury in C. costata: (i) a good correlation between occurrence of mitochondrial swelling and organismal death, not only across phenotypes (LD; LDPro; SD; SDA) but also across stress intensities in a single freeze-sensitive phenotype (−5°C, S; −5°C, F; −30°C in LD larvae); and (ii) timing of mitochondrial swelling occurrence: we have sampled the larvae for TEM analysis of mitochondrial morphology right upon melting (figure 1c).
Whatever the position of mitochondrial swelling in the hierarchy of freezing-induced injury, the most plausible mechanistic explanation for mitochondrial swelling is the loss of barrier function of the inner mitochondrial membrane (IMM). Because the mitochondrial matrix protein concentration is higher than in the cytosol, upon loss of IMM barrier function, the matrix proteins will exert a colloidal osmotic pressure inevitably leading to swelling of the matrix compartment [47]. The phenomenon of sudden permeability transition (PT) of the IMM in response to stressful conditions (such as calcium overload and/or oxidative stress) was observed for the first time in mammalian mitochondria more than 40 years ago. It was shown that PT was a result of the opening of a large non-specific channel in the IMM; the so-called mitochondrial permeability transition pore (mPTP) [48]. The unregulated mPTP opening has a number of deleterious consequences such as equilibration of small molecules across the IMM, disruption of metabolic gradients, loss of ionic gradients including dissipation of proton gradients, resulting in uncoupling of oxphos, and loss of ATP production. The concomitant influx of water from cytosol to matrix causes mitochondrial swelling, unfolding of the cristae, and the outer membrane ruptures [47,49]. This, in turn, leads to release of cytochrome c [50], induction of apoptotic cell death [51] and, in the case of long and massive mPTP opening, cell necrosis [52].
Nevertheless, the existence of mPTP in non-mammalian species is still controversial. Working with an invertebrate model organism, Menze et al. [53] found that all three critical protein components of mPTP (cyclophilin D, adenine nucleotide translocase and a voltage-dependent anion channel) are present in brine shrimp, Artemia franciscana. No PT, however, in response to calcium and phosphate inducers, and no swelling of mitochondria, were observed in encysted embryos or active larval stages of A. franciscana. The authors speculated that the inability to induce opening of mPTP in A. franciscana might play a role in the ability of this animal to survive environmental insults and may hold true for other invertebrates in general [53].
There are at least two alternative hypothetical explanations (to mPTP opening) for PT and mitochondrial swelling observed in C. costata LD larvae: (i) opening of other unregulated pores in the IMM and (ii) IMM bilayer phase transition. (i) In A. franciscana, treatment by mercury opened a non-specific pore in the IMM with the obvious consequences of mitochondrial swelling and cytochrome c release [53]. The authors speculated that interaction of mercury with some of the mitochondrial carriers might be responsible for transformation of the carrier to a channel-like non-specific pore [54]. In another scenario, the non-specific large pores might be created in the membrane from clusters of misfolded integral membrane proteins damaged by oxidants or other stresses [55]. (ii) Unregulated phase transitions readily occur in phospholipid bilayers exposed to low temperature (favouring transition to the lamellar gel phase) and/or low water activity (favouring transition to the reversed hexagonal phase) [7,56]. If such a phase transition occurs in the IMM during freeze dehydration and/or melt rehydration of C. costata cells, the IMM would lose its barrier function, at least temporarily, which may explain influx of water into the matrix and mitochondrial swelling.
Having no certainty about the causes of mitochondrial swelling in LD C. costata, it is even more difficult to speculate about how SDA larvae are protected against swelling. We offer three hypothetical mechanistic explanations. First, the structural component(s) of putative non-specific IMM pore could be downregulated with entry into diapause and cold acclimation, and in this way, the formation of a PT pore upon freezing stress could be avoided. Second, diapause and cold acclimation could induce remodelling of the IMM bilayer composition [7], which may stabilize the liquid-crystalline phase upon freezing stress. The first and second hypothetical mechanisms will need rigorous testing in future. Third, the IMM could be stabilized by cryoprotectants. The stabilizing role of proline was partially supported by our observation that freezing-induced mitochondrial swelling was abolished by feeding freeze-sensitive larvae on a proline-augmented diet. Both LDPro and SDA larvae have extremely high concentrations of proline in their body fluids (487 mM and 339 mM, respectively [15]). The solid theoretical and empirical bases for proline stabilization of membrane structure upon freezing stress were laid out in the 1980s [57,58]. High concentrations of proline alter the temperature of bilayer phase transitions and reduce tendency for fusion in closely packed membranes through hydrophobic interactions of proline imino-groups with the hydrocarbon chains of bilayer phospholipids [59]. In addition to proline, both SDA and LDPro larvae accumulate relatively high concentrations of trehalose: 52 and 51 mM, respectively, compared to 24 mM in LD larvae [15]. Trehalose is famous for its exceptional capacity to stabilize membranes at low water activities [60,61]. Trehalose forms hydrogen bonds with the phospholipid headgroups and, in this way, reduces the temperature of transition to the gel phase while also physically separating closely packed membranes, thereby preventing their fusion [59].
4. Conclusion
We have demonstrated that mitochondrial responses to freezing stress profoundly differ in different phenotypes of C. costata larvae. TEM analysis showed that mitochondria substantially swell, become rounded and occasionally burst upon exposure to lethal freezing stress in freeze-sensitive larvae (non-diapause). By contrast, sub-lethal freezing stress and supercooling stress were not associated with substantial mitochondrial swelling. Respiratory analysis revealed that swollen mitochondria have significantly decreased oxygen consumption rate. These results suggest that biological membranes, including the IMM, are sensitive to freezing stress, which may cause PT, loss of barrier function, osmotic influx of cytosolic water into the matrix and, consequently, mitochondrial swelling. Freeze-tolerant larvae (diapause and cold-acclimated) exhibited no mitochondrial swelling and no loss of respiration capacity at optimum temperature. We suggest that the phenotypic transition to diapause and cold acclimation is associated with adaptive changes that protect the IMM against PT and subsequent mitochondrial swelling. Such changes may hypothetically include a combination of (i) the downregulation of critical component(s) of an unknown, large, non-specific channel that may be generated from some carrier protein upon freezing stress, and the stabilization of the IMM's liquid-crystalline phase via (ii) high concentrations of cryoprotective substances (proline, trehalose) and (iii) restructuring of the IMM's phospholipid bilayer composition.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Jaroslava Korbelová and Irena Vacková for maintainance of C. costata culture and acclimation of larvae, Jitka Pflegerová for sectioning the resin blocks for TEM, Petra Masařová and Martina Tesařová for coating of ultrathin section by carbon film.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
V.K. and T.Š. conceived the study; T.Š. conducted all experiments and analyses; L.E.D.M. performed the confocal microscopic analysis; V.K. drafted the manuscript with contributions from T.Š. and L.E.D.M.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Grantová Agentura České Republiky (grant no. 19-13381S ).
References
- 1.Teets NM, Denlinger DL. 2013. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 38, 105–116. ( 10.1111/phen.12019) [DOI] [Google Scholar]
- 2.Salt R. 1961. Principles of insect cold-hardiness. Annu. Rev. Entomol. 6, 55–74. ( 10.1146/annurev.en.06.010161.000415) [DOI] [Google Scholar]
- 3.Lee RE., Jr 2010. A primer on insect cold-tolerance. In Low temperature biology of insects (eds Denlinger DL, Lee RE), pp. 3–34. New York, NY: Cambridge University Press. [Google Scholar]
- 4.Muldrew K, Acker JP, Elliott JA, McGann LE. 2004. The water to ice transition: implications for living cells. In Life in the frozen state (eds Fuller BJ, Lane N, Benson EE), pp. 93–134. Boca Raton, FL: CRC Press. [Google Scholar]
- 5.Franks F, Hatley RH. 1991. Stability of proteins at subzero temperatures: thermodynamics and some ecological consequences. Pure Appl. Chem. 63, 1367–1380. ( 10.1351/pac199163101367) [DOI] [Google Scholar]
- 6.Privalov PL. 1990. Cold denaturation of protein. Crit. Rev. Biochem. Mol. Biol. 25, 281–306. ( 10.3109/10409239009090612) [DOI] [PubMed] [Google Scholar]
- 7.Koštál V. 2010. Cell structural modifications in insects at low temperature. In Low temperature biology of insects (eds Denlinger DL, Lee R), pp. 116–140. New York, NY: Cambridge University Press. [Google Scholar]
- 8.Toxopeus J, Sinclair BJ. 2018. Mechanisms underlying insect freeze tolerance. Biol. Rev. 93, 1891–1914. ( 10.1111/brv.12425) [DOI] [PubMed] [Google Scholar]
- 9.Storey KB, Storey JM. 1992. Natural freeze tolerance in ectothermic vertebrates. Annu. Rev. Physiol. 54, 619–637. ( 10.1146/annurev.physiol.54.1.619) [DOI] [PubMed] [Google Scholar]
- 10.Storey KB, Storey JM. 1988. Freeze tolerance in animals. Physiol. Rev. 68, 27–84. ( 10.1152/physrev.1988.68.1.27) [DOI] [PubMed] [Google Scholar]
- 11.Chown S., Sinclair B. 2010. The macrophysiology of insect cold hardiness. In Low temperature biology of insects (eds Denlinger DL, Lee RE), pp. 191–222. New York, NY: Cambridge University Press. [Google Scholar]
- 12.MacMillan HA. 2019. Dissecting cause from consequence: a systematic approach to thermal limits. J. Exp. Biol. 222, jeb191593 ( 10.1242/jeb.191593) [DOI] [PubMed] [Google Scholar]
- 13.Koštál V, Zahradníčková H, Šimek P. 2011. Hyperprolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc. Natl Acad. Sci. USA 108, 13041–13046. ( 10.1073/pnas.1107060108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koštál V, Štětina T, Poupardin R, Korbelová J, Bruce AW. 2017. Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomic profiling. Proc. Natl Acad. Sci. USA 114, 8532–8537. ( 10.1073/pnas.1707281114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozsypal J, Moos M, Šimek P, Koštál V. 2018. Thermal analysis of ice and glass transitions in insects that do and do not survive freezing. J. Exp. Biol. 221, 170464 ( 10.1242/jeb.170464) [DOI] [PubMed] [Google Scholar]
- 16.Des Marteaux LE, Hůla P, Koštál V.. 2019. Transcriptional analysis of insect extreme freeze tolerance. Proc. R. Soc. B 286, 20192019 ( 10.1098/rspb.2019.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Štětina T, Hůla P, Moos M, Šimek P, Šmilauer P, Koštál V.. 2018. Recovery from supercooling, freezing, and cryopreservation stress in larvae of the drosophilid fly, Chymomyza costata. Sci. Rep. 8, 4414 ( 10.1038/s41598-018-22757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunnari J, Suomalainen A. 2012. Mitochondria: in sickness and in health. Cell 148, 1145–1159. ( 10.1016/j.cell.2012.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent AE, et al. 2016. The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Sci. Rep. 6, 30610 ( 10.1038/srep30610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K. 2007. Mitochondrial disruption in Drosophila apoptosis. Dev. Cell. 12, 793–806. ( 10.1016/j.devcel.2007.04.004) [DOI] [PubMed] [Google Scholar]
- 21.Sesso A, Belizário JE, Marques MM, Higuchi MDL, Schumacher RI, Colquhoun A, Ito E, Kawakami J. 2012. Mitochondrial swelling and incipient outer membrane rupture in preapoptotic and apoptotic cells. The Anatomical Record: Adv. Integr. Anatomy Evol. Biol. 295, 1647–1659. ( 10.1002/ar.22553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang YF, Lin SS, Chen JM, Tsai HZ, Hsieh TS, Fu CY. 2017. Electron tomographic analysis reveals ultrastructural features of mitochondrial cristae architecture which reflect energetic state and aging. Sci. Rep. 7, 45474 ( 10.1038/srep45474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosetti F, Baracca A, Lenaz G, Solaini G. 2004. Increased state 4 mitochondrial respiration and swelling in early post-ischemic reperfusion of rat heart. FEBS Lett. 563, 161–164. ( 10.1016/S0014-5793(04)00294-7) [DOI] [PubMed] [Google Scholar]
- 24.Virolainen E, Blokhina O, Fagerstedt K. 2002. Ca2+- induced high amplitude swelling and cytochrome c release from wheat (Triticum aestivum L.) mitochondria under anoxic stress. Ann. Bot. 90, 509–516. ( 10.1590/S0100-736X2016000300003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZH, Clark C, Geisbrecht ER. 2016. Analysis of mitochondrial structure and function in the Drosophila larval musculature. Mitochondrion 26, 33–42. ( 10.1016/j.mito.2015.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rurek M. 2014. Plant mitochondria under a variety of temperature stress conditions. Mitochondrion 19(Pt B), 289–294. ( 10.1016/j.mito.2014.02.007) [DOI] [PubMed] [Google Scholar]
- 27.Sherman J. 1971. Correlation in ultrastructural cryoinjury of mitochondria with aspects of their respiratory function. Exp. Cell Res. 66, 378–384. ( 10.1016/0014-4827(71)90691-4) [DOI] [PubMed] [Google Scholar]
- 28.Sherman J. 1972. Comparison of in vitro and in situ ultrastructural cryoinjury and cryoprotection of mitochondria. Cryobiology 9, 112–122. ( 10.1016/0011-2240(72)90018-1) [DOI] [PubMed] [Google Scholar]
- 29.Collins SD, Allenspach AL, Lee RE Jr. 1997. Ultrastructural effects of lethal freezing on brain, muscle and Malpighian tubules from freeze-tolerant larvae of the gall fly, Eurosta solidaginis. J. Insect Physiol. 43, 39–45. ( 10.1016/S0022-1910(96)00073-X) [DOI] [PubMed] [Google Scholar]
- 30.McMullen DC, Storey KB. 2008. Mitochondria of cold hardy insects: responses to cold and hypoxia assessed at enzymatic, mRNA and DNA levels. Insect Biochem. Mol. Biol. 38, 367–373. ( 10.1016/j.ibmb.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 31.Joanisse DR, Storey KB. 1994. Mitochondrial enzymes during overwintering in two species of cold-hardy gall insects. Insect Biochem. Mol. Biol. 24, 145–150. ( 10.1016/0965-1748(94)90080-9) [DOI] [Google Scholar]
- 32.Levin D, Danks H, Barber S. 2003. Variations in mitochondrial DNA and gene transcription in freezing-tolerant larvae of Eurosta solidaginis (Diptera: Tephritidae) and Gynaephora groenlandica (Lepidoptera: Lymantriidae). Insect Mol. Biol. 12, 281–289. ( 10.1046/j.1365-2583.2003.00413.x) [DOI] [PubMed] [Google Scholar]
- 33.Ballantyne JS, Storey KB. 1985. Characterization of mitochondria isolated from the freezing-tolerant larvae of the goldenrod gall fly (Eurosta solidaginis): substrate preferences, salt effects, and pH effects on warm-and cold-acclimated animals. Can. J. Zool. 63, 373–379. ( 10.1139/z85-057) [DOI] [Google Scholar]
- 34.Kukal O, Duman JG, Serianni AS. 1989. Cold-induced mitochondrial degradation and cryoprotectant synthesis in freeze-tolerant arctic caterpillars. J. Comp. Phys. B 158, 661–671. ( 10.1007/bf00693004) [DOI] [PubMed] [Google Scholar]
- 35.Riihimaa AJ, Kimura MT. 1988. A mutant strain of Chymomyza costata (Diptera: Drosophilidae) insensitive to diapause-inducing action of photoperiod. Physiol. Entomol. 13, 441–445. ( 10.1111/j.1365-3032.1988.tb01128.x) [DOI] [Google Scholar]
- 36.Srere P. 1969. Citrate synthase:[EC 4.1. 3.7. Citrate oxaloacetate-lyase (CoA-acetylating)]. Methods Enzymol. 13, 3–11. ( 10.1016/0076-6879(69)13005-0) [DOI] [Google Scholar]
- 37.Larsen S, et al. 2012. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 590, 3349–3360. ( 10.1113/jphysiol.2012.230185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballinger MA, Hess C, Napolitano MW, Bjork JA, Andrews MT. 2016. Seasonal changes in brown adipose tissue mitochondria in a mammalian hibernator: from gene expression to function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R325–R336. ( 10.1152/ajpregu.00463.2015) [DOI] [PubMed] [Google Scholar]
- 39.Des Marteaux LE, Štětina T, Koštál V. 2018. Insect fat body cell morphology and response to cold stress is modulated by acclimation. J. Exp. Biol. 221, jeb189647 ( 10.1242/jeb.189647) [DOI] [PubMed] [Google Scholar]
- 40.Haunerland N, Shirk P. 1995. Regional and functional differentiation in the insect fat body. Annu. Rev. Entomol. 40, 121–145. ( 10.1146/annurev.en.40.010195.001005) [DOI] [Google Scholar]
- 41.Joanisse D, Storey KB. 1994. Enzyme activity profiles in an overwintering population of freeze-tolerant larvae of the gall fly, Eurosta solidaginis. J. Comp. Phys. B 164, 247–255. ( 10.1007/BF00354086) [DOI] [Google Scholar]
- 42.Lee HO, Davidson JM, Duronio RJ. 2009. Endoreplication: polyploidy with purpose. Genes Dev. 23, 2461–2477. ( 10.1101/gad.1829209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dias CL, Ala-Nissila T, Wong-ekkabut J, Vattulainen I, Grant M, Karttunen M. 2010. The hydrophobic effect and its role in cold denaturation. Cryobiology 60, 91–99. ( 10.1016/j.cryobiol.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 44.Sanfelice D, Temussi PA. 2016. Cold denaturation as a tool to measure protein stability. Biophys. Chem. 208, 4–8. ( 10.1016/j.bpc.2015.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamiya T, Okahashi N, Sakuma R, Aoyama T, Akahane T, Matsumoto JJ. 1985. Freeze denaturation of enzymes and its prevention with additives. Cryobiology 22, 446–456. ( 10.1016/0011-2240(85)90156-7) [DOI] [PubMed] [Google Scholar]
- 46.Carpenter JF, Crowe JH. 1988. The mechanism of cryoprotection of proteins by solutes. Cryobiology 25, 244–255. ( 10.1016/0011-2240(88)90032-6) [DOI] [PubMed] [Google Scholar]
- 47.Halestrap AP. 2009. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 46, 821–831. ( 10.1016/j.yjmcc.2009.02.021) [DOI] [PubMed] [Google Scholar]
- 48.Hunter DR, Haworth RA. 1979. The Ca2+-induced membrane transition in mitochondria: I. The protective mechanisms. Arch. Biochem. Biophys. 195, 453–459. ( 10.1016/0003-9861(79)90371-0) [DOI] [PubMed] [Google Scholar]
- 49.Giorgio V, Guo L, Bassot C, Petronilli V, Bernardi P. 2018. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium. 70, 56–63. ( 10.1016/j.ceca.2017.05.004) [DOI] [PubMed] [Google Scholar]
- 50.Doran E, Halestrap AP. 2000. Cytochrome c release from isolated rat liver mitochondria can occur independently of outer-membrane rupture: possible role of contact sites. Biochem. J. 348, 343–350. ( 10.1042/0264-6021:3480343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green DR, Kroemer G. 2004. The pathophysiology of mitochondrial cell death. Science 305, 626–629. ( 10.1126/science.1099320) [DOI] [PubMed] [Google Scholar]
- 52.Halestrap AP, Clarke SJ, Javadov SA. 2004. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc. Res. 61, 372–385. ( 10.1016/S0008-6363(03)00533-9) [DOI] [PubMed] [Google Scholar]
- 53.Menze MA, Hutchinson K, Laborde SM, Hand SC. 2005. Mitochondrial permeability transition in the crustacean Artemia franciscana: absence of a calcium-regulated pore in the face of profound calcium storage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R68–R76. ( 10.1152/ajpregu.00844.2004) [DOI] [PubMed] [Google Scholar]
- 54.Dierks T, Salentin A, Krämer R. 1990. Pore-like and carrier-like properties of the mitochondrial aspartate/glutamate carrier after modification by SH-reagents: evidence for a preformed channel as a structural requirement of carrier-mediated transport. Biochim. Biophys. Acta 1028, 281–288. ( 10.1016/0005-2736(90)90177-p) [DOI] [PubMed] [Google Scholar]
- 55.He L, Lemasters JJ. 2002. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 512, 1–7. ( 10.1016/s0014-5793(01)03314-2) [DOI] [PubMed] [Google Scholar]
- 56.Chapman D. 1975. Phase transitions and fluidity characteristics of lipids and cell membranes. Q. Rev. Biophys. 8, 185–235. ( 10.1017/s0033583500001797) [DOI] [PubMed] [Google Scholar]
- 57.Rudolph AS, Crowe JH. 1985. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 22, 367–377. ( 10.1016/0011-2240(85)90184-1) [DOI] [PubMed] [Google Scholar]
- 58.Rudolph AS, Crowe JH, Crowe LM. 1986. Effects of three stabilizing agents—proline, betaine, and trehalose—on membrane phospholipids. Arch. Biochem. Biophys. 245, 134–143. ( 10.1016/0003-9861(86)90197-9) [DOI] [PubMed] [Google Scholar]
- 59.Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH. 1987. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 24, 324–331. ( 10.1016/0011-2240(87)90036-8) [DOI] [PubMed] [Google Scholar]
- 60.Crowe JH. 2007. Trehalose as a ‘chemical chaperone’. In Molecular aspects of the stress response: chaperones, membranes and networks (eds Csermely P, Vígh L), vol. 594, pp. 143–158. New York, NY: Springer. [Google Scholar]
- 61.Kaushik JK, Bhat R. 2003. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 278, 26 458–26 465. ( 10.1074/jbc.M300815200) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.