Abstract
Colonization of novel habitats can result in marked phenotypic responses to the new environment that include changes in body shape and opportunities for further morphological diversification. Fishes have repeatedly transitioned along the benthic–pelagic axis, with varying degrees of association with the substrate. Previous work focusing on individual lineages shows that these transitions are accompanied by highly predictable changes in body form. Here, we generalize expectations drawn from this literature to study the effects of habitat on body shape diversification across 3344 marine teleost fishes. We compare rates and patterns of evolution in eight linear measurements of body shape among fishes that live in pelagic, demersal and benthic habitats. While average body shape differs between habitats, these differences are subtle compared with the high diversity of shapes found within each habitat. Benthic living increases the rate of body shape evolution and has led to numerous lineages evolving extreme body shapes, including both exceptionally wide bodies and highly elongate, eel-like forms. By contrast, we find that benthic living is associated with the slowest diversification of structures associated with feeding. Though we find that habitat can serve as an impetus for predictable trait changes, we also highlight the diversity of responses in marine teleosts to opportunities presented by major habitats.
Keywords: ecological opportunity, macroevolution, linear morphometrics, fish, body shape
1. Introduction
The invasion of new habitats can affect phenotypic diversification in at least two different ways. A colonization event will typically stimulate an immediate adaptive response of the phenotype to the new selective regime. However, the transition can also serve as an impetus for subsequent diversification, promoting the proliferation of different morphologies as lineages respond to ecological opportunity within the new habitat [1,2]. Though the two processes may act on different temporal scales, both are key to understanding how major habitat shifts have shaped phenotypic diversification.
Fishes have repeatedly transitioned between pelagic (limnetic/open water), demersal (close proximity to the substrate) and fully benthic (in physical contact with substrate) habitats, shifts that are thought to have substantial implications for the evolution of body form. An ecomorphological axis of body shape changes associated with transitions between pelagic and demersal habitats is one of the strongest and most consistently reported patterns in the literature within both freshwater and marine fishes [3–17]. Pelagic species repeatedly evolve a more elongate, slender body shape with a narrow caudal peduncle, thought to be adaptive for steady locomotion in open water [5,17], while demersal fishes evolve wider mouths, as well as deeper bodies, hypothesized to increase manoeuvrability via increased hydrodynamic instability [6,16].
While previous work has focused on specific transitions along the demersal–pelagic axis at the intraspecific and family levels, far less is known about how these habitats shape patterns and rates of subsequent diversification across deep phylogenetic scales (but see [9,14]). One possible expectation is that the physically uniform nature of the pelagic realm results in fewer opportunities to interact with a heterogeneous environment and thus fewer ecological niches to diversify into. By contrast, demersal and particularly benthic habitats provide considerable physical and biological complexity, potentially driving diversification by presenting opportunities to physically interact with the substrate for both feeding and locomotion [18–20]. Here, we study patterns of habitat-mediated body shape diversification across 3344 species of marine teleosts. We explore the effects of the habitat gradient on the average fish shape, overall phenotypic disparity and on the underlying rate of evolution of body shape. Given the consistent morphological trends described by previous studies, we expect these patterns to scale up such that we find convergence on deeper body/head shapes and wider mouths in demersal and benthic fishes and a predominance of comparatively shallow bodies and head shapes with narrow caudal peduncles and smaller mouths in pelagic fishes. We also expect rates of body shape evolution to reflect the physical complexity of the three habitats, mirroring both the ecological opportunity and potential for diversification, with the highest rates in benthic and the slowest in pelagic habitats.
2. Material and methods
(a). Data collection and preparation
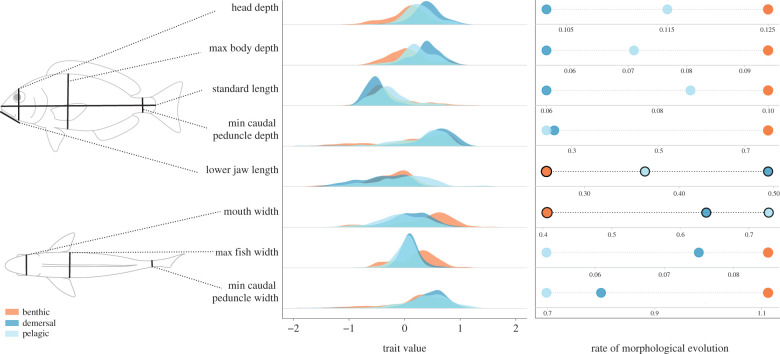
We collected linear measurements of body shape from a maximum of three adult specimens from each of 3344 species (electronic supplementary material, table S1). The sampling includes representatives of 268 families and 1252 genera and spans nearly 20% of all marine teleost species. Using specimens from the National Museum of Natural History we measured eight ecologically and functionally relevant features: standard length, maximum body depth, maximum body width, minimum caudal peduncle depth, minimum caudal peduncle width, lower jaw length, mouth width and head depth (figure 1). Trait values were then averaged across specimens for species means. For further details on data collection, measurements and collation methods see Price et al. [21]. Notably, a large part of the data collection involved undergraduate researchers through a course-based undergraduate research experience [22].
Figure 1.
Generalized fish outline (left) illustrating the eight linear measurements taken on each fish (black lines). Dotted lines connect each measurement to the corresponding density plot (middle), showing the distribution of log-transformed and size-corrected traits from each species in the dataset, grouped by colour coded habitat categories. Habitat-specific rates of morphological evolution determined by the best-fit evolutionary models are visualized in the plot to the right. Rate estimates for feeding-related traits are highlighted, as they show a unique pattern of evolution in this dataset. (Online version in colour.)
For comparative analyses, we used a previously published time-calibrated phylogeny of ray-finned fishes pruned to our species list [23]. All species in this maximum likelihood phylogeny have genetic data determining their placement. We first natural log-transformed and size-corrected the linear traits by taking the residuals of phylogenetic regressions on body size implemented with the phyl.resid function in ‘phytools’ [24]. Across a dataset with substantial body shape diversity, a single anatomical dimension may bias the estimate of body size. For example, though the standard length is a commonly used size metric in fishes, the overall body size of extremely elongate fishes such as eels would be exaggerated, while the size of deep-bodied species such as ocean sunfish would be underestimated. Therefore, we opted to use the geometric mean of the three major body dimensions (cube root of the product of species averages for standard length, maximum body width and maximum body depth) as a composite metric for size [25,26]. All analyses for this study were implemented in R, v. 3.5.0 [27].
Previous literature has focused on the demersal–pelagic axis of diversification, neglecting substrate-dwelling benthic fishes. To comprehensively evaluate how the entirety of the habitat gradient affects morphological diversification, we expanded the classically studied demersal–pelagic axis to include exclusively benthic species. Species were classified into one of three habitat categories: benthic, demersal and pelagic based on their adult habitat preferences and behaviour. Benthic fishes are those that spend the majority of the time with their body physically in contact with the substrate, including fossorial species. For example, frogfishes, flatfishes and moray eels are classified as benthic in this dataset. In order to explicitly test the effect of structural complexity on body shape evolution, open-water species that spend significant time with their body in contact with any substrate, such as sargassum frogfish and remoras, were also classified as benthic. Demersal fishes have some interaction with the benthos but spend little time with their body in contact with the substrate. Here, butterflyfishes, as well as some damselfishes and croakers, are categorized as demersal because they forage on the benthos, but rarely physically rest on the substrate. Finally, pelagic fishes live in the water column, rarely or never coming in contact with the benthos. Tuna, swordfish and needlefishes are representative pelagic fishes. We collected habitat information on each species from FishBase [28] and primary literature sources or our own observations when behavioural or habitat information was unavailable (electronic supplementary material, table S1).
(b). Morphological diversity
We conducted a principal component analysis (PCA) using the correlation matrix to visualize body shape morphospace occupancy by habitat. We also calculated morphological disparity across all eight linear traits using the morphol.disparity function implemented in ‘geomorph’ (v. 3.0.6) [29] to compare the overall variance of forms between habitat regimes. Pairwise disparity estimates for the different habitat groups were assessed with a permutation procedure (1000 iterations). To determine if there were significant differences in average body shape between benthic, demersal, and pelagic fishes, we conducted a phylogenetic ANOVA on each morphological trait as well as a phylogenetic MANOVA, as a more composite comparison of morphology between groups. All ANOVAs and the MANOVA were implemented with 10 000 simulations to assess significance using the function ‘procD.pgls’ in the geomorph package.
Given the phylogenetic scale, we also attempted to determine if habitat itself drives diversification or if the morphological disparity is a result of very different lineages independently transitioning into each habitat. First, assuming a Brownian motion (BM) model of evolution, we determined the maximum likelihood ancestral states using the fastAnc function in ‘phytools’ [24] for each morphological trait and calculated independent contrasts at each node in the phylogeny [30]. We then reconstructed habitat transitions across the phylogeny using stochastic character mapping (simmaps) implemented in the R package ‘phytools’, allowing for asymmetric transition rates between habitat regimes. We determined this was the best-fit model by comparing log-likelihoods of the Q-matrices from models that allowed for equal, symmetric and asymmetric rate transitions between habitat states [31]. For each of the 100 simmaps, we identified the nodes that immediately preceded habitat transitions (transition nodes) and recorded which daughter branch (left or right) the transition occurred on as well as the transition type (e.g. benthic to demersal, demersal to benthic, etc.). We extracted the independent contrasts and ancestral states for only these transition nodes. If the transition occurred on the left branch, we multiplied the independent contrast by −1 to control the contrast direction, as the ‘pic’ function always calculates contrasts as the right branch minus the left. We used a t-test to determine if the independent contrasts for each habitat transition significantly differed from zero. A significant deviation from zero would indicate that particular habitat transitions are associated with directional morphological change. We also implemented a modified version of the rate-by-state test [32], using the ancestral state and independent contrasts for just the transition nodes. For each transition type, we regressed the ancestral state of each trait against the direction-controlled contrast values to determine if the direction of trait change for habitat transitions depended on the ancestral trait at that node. For example, if body depth evolves toward a more slender shape during the transition from demersal to pelagic, we might see this change in deep-bodied taxa but rarely in lineages that are already elongate. A slope that significantly differs from zero indicates that ancestral morphology prior to the habitat transition affects the expected direction of trait change. We repeated these procedures on all eight morphological traits and all 100 simmaps (see [33] for R code).
(c). Rates of morphological evolution
Given computational limitations on a dataset of this size, all evolutionary models were run on 100 simmaps to account for uncertainty in the history of habitat occupation. Using the package ‘OUwie’ [34], we implemented a model-fitting framework on each linear trait to compare rates of morphological evolution between species in different habitats. To test whether rates of evolution vary between habitats, we compared two models: Single rate BM, which does not allow for the rate parameter (σ2) to vary with habitat, and multi-rate Brownian motion (BMS), which fits a different rate parameter to each habitat regime under maximum likelihood. Though more complex evolutionary models exist to infer selective optima (e.g. Ornstein–Uhlenbeck models), here, we are particularly interested in differences in the rate of body shape evolution with respect to habitat. Additionally, these models incorporate an alpha parameter used to infer the strength of selection, which is not necessarily applicable to the questions posed here. As there are concerns of non-identifiability between the alpha and sigma parameters of OU models, coupled with potentially inaccurate alpha estimates [35,36], we have opted to compare only BM models. After the OUwie analyses, we checked our results for positive eigenvalues, which indicate reliable estimates [37]. Model fit was evaluated using a modified Akaike information criterion (AICc), which converges to AIC when samples are large. To determine if we have the power to distinguish between BM and BMS models, we simulated two datasets: one under BMS and one using BM across the phylogeny with the function OUwie.sim. We then recursively ran our model-fitting framework on 100 of each of these simulated datasets to establish if we could recover the original parameters and model, thereby demonstrating statistical power and a lack of bias for the more parameterized model, respectively.
3. Results
(a). Morphological disparity
The first two principal component (PC) axes accounted for more than 68% of the variation in body shape across the dataset (figure 2). PC1 was primarily dominated by elongation−a contrast between deep-bodied and more slender, elongate forms. There appeared to be significant phylogenetic patterning to the morphospace, with Anguilliformes (true eels) having larger values on PC1 while the smallest values were primarily occupied by Pleuronectiformes (flatfishes). Body width loaded highest on PC2 and Lophiiformes (anglerfishes) dominate the larger values along this axis. There was little differentiation of fish body shapes between habitats within the morphospace defined by PC1 and PC2, as species' distributions broadly overlapped between them (figure 2). One notable exception was that only benthic fishes expanded into the region of morphospace corresponding with extremely wide bodies (largest values on PC2).
Figure 2.
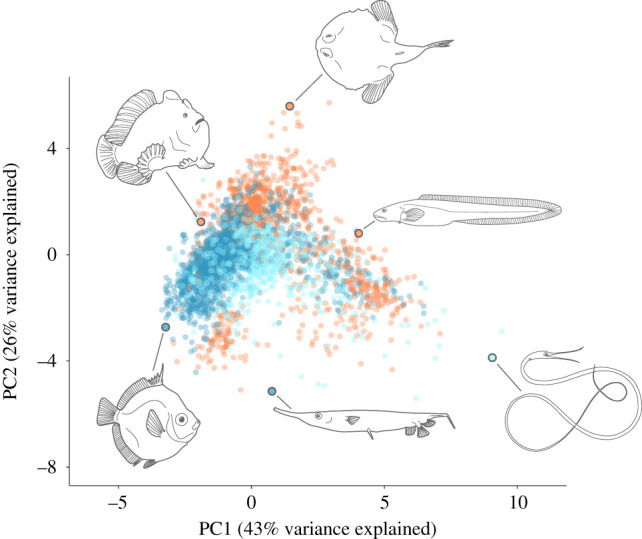
Plot of PC1 and 2 from a principal component analysis of eight variables that characterize body shape for 3344 marine teleost species. Each point represents the average for a single species, with colour corresponding to habitat (orange: benthic; dark blue: demersal; light blue: pelagic). Body shape variation along PC1 is dominated by an increase in standard length and concomitant decrease in maximum body depth (elongation) and PC2 is primarily driven by variation in body width. Six species are illustrated at the extremes to aid in the visualization of morphospace, clockwise from upper left: Antennarius commerson, Halieutichthys aculeatus, Austrolycus laticinctus, Nemichthys scolopaceus, Aeoliscus strigatus and Antigonia combatia.
The multivariate morphological disparity was highest for benthic fishes and lowest for demersal fishes (benthic: 1.94; demersal: 1.30; pelagic: 1.49) and all pairwise comparisons were significant (p < 0.05). Removing Lophiiformes and Pleuronectiformes, which are predominantly benthic and quite morphologically distinct, only slightly affected disparity (benthic: 1.77, demersal: 1.30, pelagic: 1.49) and all comparisons except demersal–pelagic (p = 0.063) remained significantly different (p < 0.05). The overall disparity across the entire dataset was 1.68.
Of the pairwise phylogenetic ANOVAs run on the eight morphological traits across three habitats (24 total comparisons), only five showed significant differences in average trait values. These included maximum body depth, which differed between pelagic–demersal habitats, and minimum caudal peduncle depth (demersal–benthic comparison), as well as head depth for all three habitats (figure 1; electronic supplementary material, table S2). The phylogenetic MANOVA revealed limited differences in average body shape between the habitats (p = 0.034; figure 1). Habitat showed very little explanatory power for body shape differences (r2 = 0.0014), with an effect size (Z-score) of 1.8; habitat appears to have a weak influence on average body shape. In fact, this analysis revealed that the only significant difference in mean body shape appears to be between benthic and demersal communities (p = 0.028). Thus, while some differences in average shape exist between habitats, they are subtle compared to the variation in body shape within each habitat.
Our analysis of the independent contrasts and ancestral states associated with transition nodes for each trait revealed a signal of consistent trait changes associated with habitat transitions. Using the direction-controlled independent contrasts, we found significant evidence for decreases in head depth and caudal peduncle depth in transitions to the benthic realm, increases in head depth for transitions to a demersal habitat, and increasing elongation in pelagic fishes (increasing standard length coupled with reductions in body depth and width; figure 3; electronic supplementary material, table S4). In the linear regressions of ancestral state and independent contrasts, only two traits showed significant results (p < 0.05) in greater than 50% of our stochastic character maps. Caudal peduncle depth increased in demersal–benthic transitions (60/100 simmaps significant) and caudal peduncle width increased in benthic–pelagic transitions (52/100 simmaps significant). These findings imply that the vast majority of traits do not deviate from the BM expectation and that ancestral morphology can influence the direction of certain trait changes associated with a given habitat transition.
Figure 3.
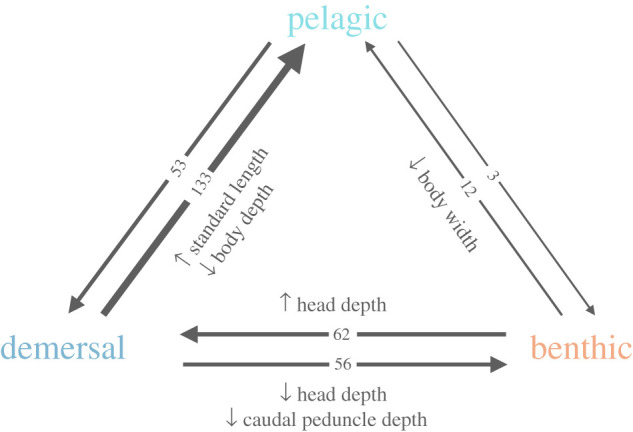
Average number of transitions between habitats estimated across 100 stochastic character maps. Significant morphological shifts and the direction of the trait change as determined by the independent contrasts analysis are detailed next to the associated habitat transition. (Online version in colour.)
(b). Rates of body shape evolution
Across the 100 stochastic character reconstructions, there were, on average, 317 habitat state transitions (figure 3; electronic supplementary material, figure S1). The largest number of transitions were from demersal to pelagic (145.71 on average) and benthic to demersal (86.94 on average). Relatively few transitions were towards the benthic regime (demersal to benthic: 56; pelagic to benthic: 3). From the stochastic character maps, we estimated that around 33% of the evolutionary time was spent in the benthic regime, 41% in the demersal state, while only 25% of the time was spent in the pelagic regime. The root node was reconstructed as demersal in 91 of the 100 simmaps, indicating high probability that it was the ancestral state for marine teleosts.
The model-fitting framework favoured a best-fit model of BMS for all eight of the linear traits (electronic supplementary material, table S3). In other words, we estimated that benthic, demersal and pelagic fishes are diversifying under different rates of morphological evolution. Habitat rate parameters were highly stable and showed little variation across simmaps, indicating that our estimates are robust (electronic supplementary material, figure S2). Benthic fishes showed the fastest rates of evolution in all traits except the jaw measurements (mouth width and lower jaw length, which had the highest rates in the pelagic and demersal realms, respectively). Across the six other (non-jaw-related) linear traits, we estimate benthic fishes evolved 1.8× faster than pelagic fishes and 1.6× faster than demersal fishes, on average (figure 1).
The simulations under our best-fitting model (BMS) indicated that we have substantial power to distinguish between BM, the single rate model, and BMS, the multi-rate model, in our dataset. The AICc estimates for the models share no overlap, unequivocally preferring BMS over BM (electronic supplementary material, table S3 and figure S3). We also recover similar rate estimates to those the dataset was simulated under, though the recovered estimates do not always overlap with the simulated parameters (electronic supplementary material, figure S4). Nevertheless, sigma estimates are on average 0.01–0.03 units different from the original values, indicating that we still have acceptable statistical power. Simulating under a BM model, we find that the BM model is only preferred in 19% of the reconstructions indicating that, at this phylogenetic scale, the model has difficulty favouring the less-parameterized BM model. Nevertheless, our empirical results show no overlap in AICc distribution between the two models, demonstrating that the BMS model is unquestionably preferred.
4. Discussion
We find significant evidence that diversification along the benthic–pelagic axis has a detectable effect on fish body shape at the macroevolutionary scale. We also find that benthic lineages possess the greatest morphological disparity and evolutionary rates of the three habitats as well as unique extremely wide-bodied forms. However, distributions of body shapes are largely overlapping in all three habitats, each roughly mirroring the disparity of all marine fishes combined. These results indicate that, while habitat can drive consistent patterns of trait change, all three habitats house a wide diversity of fish body shapes, producing a complex relationship between habitat and body shape across marine fishes.
Our analyses reveal that habitat imposes predictable selective pressures on body shape evolution, but this effect is relatively subtle compared to the sum of other unaccounted for influences (e.g. phylogenetic conservatism, manifold ecological factors, etc.). Considering the vast ecological and phylogenetic diversity contained in this dataset, it is not surprising that we find such extensive phenotypic diversification within each habitat. However, that we recover consistent morphological trends in specific traits despite this diversity, attests to the influence of the benthic–pelagic paradigm on body shape evolution across teleost fishes. A more slender body shape has been documented in over 40 lineages that have transitioned to the pelagic habitat [6–8,16], creating a strong expectation that these morphological patterns should scale up. Using the direction-controlled independent contrasts, we find evidence that shape changes accompanying these transitions are consistent with the well-established patterns in the literature. These trends are particularly apparent in traits along the depth dimension of body shape, which increase when lineages become demersal and are reduced in transitions to both benthic and pelagic habitats (figure 3; electronic supplementary material, table S4). While our habitat categories are coarse and gloss over much subtlety, we are still able to recover a macroevolutionary signal of the morphological trends anticipated by the literature.
Benthic living appears to be a strong driver of body shape evolution. Extreme and novel body shapes are present here, and rates of evolution are significantly higher in the benthic lineages for most body shape traits compared to demersal or pelagic fishes. Interestingly, we also reconstructed relatively few transitions to the benthic regime across the phylogeny. Thus, while transitions to a fully benthic lifestyle are relatively rare, body shape diversification happens at a higher rate once lineages invade the benthos.
At least two general factors may drive higher rates of body shape diversification in benthic habitats: increased physical contact with sediments and hard surfaces and a reduction in functional constraints associated with swimming. In contrast to midwater habitats where water completely surrounds the organism, benthic habitats offer a heterogeneous environment and the opportunity to interact with the substrate during locomotion and feeding, which has the potential to drive specialization, niche differentiation and speciation [19,20,38]. For example, the complex habitat structure of coral reefs has been shown to enhance the rate of diversification, with studies demonstrating faster cladogenesis and morphological evolution in some coral reef-dwelling lineages [39–41]. Similarly, structurally complex benthic habitats may result in opportunities and a proliferation of forms. From suction cups to cirri, benthic fishes have a variety of adaptations to interact with the substrate upon which they live. Benthic fishes frequently interact with the substrate during both feeding and locomotion, resulting in adaptations for both functions in benthic residents. For example, lie-and-wait predation is associated with diverse secondary adaptations in benthic fish and modifications to the paired fins of sea robins, frogfishes, clingfishes and dragonets that facilitate walking and gripping the substrate [42–44]. In addition to the emergence of unique and novel appendages, physical contact with the substrate appears to be a significant cause of body shape diversification in benthic fishes.
Benthic living may also relax some constraints imparted by a constantly swimming midwater lifestyle. Body shape in aquatic animals is thought to be strongly influenced by the forces that resist movement in a medium that is much denser than air [45]. Drag is a major force resisting movement in water and is strongly affected by body shape [46]. Midwater fishes spend more time swimming than benthic fishes, for whom movement is typically highly periodic and makes extensive use of physical contact with the substrate. Given that many demersal and pelagic fishes use their caudal fins as the major propulsive device, our finding of the fastest rates of evolution in the caudal peduncle measurements of benthic fishes is consistent with a relaxation of constraints on this part of the locomotory apparatus (figure 1). We also find evidence for consistent increases to the caudal peduncle depth upon transitioning to the benthic realm, but that average caudal peduncle depth is reduced in benthic fishes, as shown by our PIC analysis. This suggests that subsequent morphological diversification to the locomotor apparatus may occur once fishes become fully benthic. By providing both a physically complex habitat to interact with and a relaxation from the drag-based constraints on body form, benthic habitats may represent a very different adaptive landscape for the evolution of body shape than demersal or pelagic realms. Interestingly, benthic living only appears to stimulate traits related to locomotion. We find the slowest rates of morphological evolution in traits most functionally relevant to feeding (mouth width and lower jaw length) in benthic fishes (figure 1). While further work is needed, this implies that habitat impacts feeding and locomotor morphology differently and that statements about how habitat affects rates of morphological evolution must be made with specific reference to the functional systems.
Our study reveals that a dorsoventrally depressed, wide body, characterized by species like pancakefishes, clingfishes and flatheads, is a morphotype that only occurs in benthic habitats. We reconstruct at least 12 evolutionarily independent transitions to an extremely wide form (e.g. body depth/body width greater than the 95% quantile across the dataset) in marine benthic fishes, including clingfishes, frogfishes, flatheads, sculpins and dragonets. It has been suggested that this high-lift, low-drag shape is conducive to station-holding when subjected to ambient water flow [47]. By exploiting the boundary layer, a region of reduced flow velocity in close proximity to the substrate, benthic fishes may expend less energy maintaining their position [48]. The benefits of being able to withstand significant water velocities may have been one factor that prompted evolutionary convergence on a dorsoventrally flattened body shape [49]. A similar adaptation is seen in flatfishes but with different anatomical underpinnings. Unlike all other benthic fishes, flatfishes are exceptionally laterally compressed and deep-bodied. But flatfishes functionally mimic the wide bodies of many other benthic fishes by adopting their unique posture of laying on their side. While flattened benthic fishes may enjoy benefits of avoiding flowing water and may also be less conspicuous, an open area of research remains in investigating additional advantages of having a flattened body when lying on or within the substrate.
Another morphotype commonly found in benthic habitats is the extremely elongate form, characterized by eels. This body shape is not restricted to eels, however, as we reconstructed 27 independent origins of body shapes that have achieved a fineness ratio (standard length/maximum body depth) of 14.78 or higher (the top 95% quantile across our dataset). Interestingly, this body shape is also common in pelagic fishes, evolving independently in pelagic eels, needlefishes, oarfishes and cutlassfishes (figure 2). An elongate body is thought to be adaptive for steady swimming efficiency at very low speeds, high maneuverability and flexibility [50]. Energetic efficiency can be paramount in constantly moving pelagic fishes [51]. This may help explain the presence of some extremely elongate forms in pelagic habitats, particularly in slow-swimming deep-water species. Alternatively, in benthic fishes that occupy structurally complex environments, improved ability to navigate this three-dimensional habitat and pass through tight spaces would offer clear advantages. Contact with the substrate can affect the mechanism of locomotion, as midwater fishes swim by pushing their undulating body or fins against the water, while benthic fishes can push against contact points with the substrate. Body elongation may be adaptive for this type of movement in contact with the substrate [52] and may facilitate movement through the tight spaces in reef habitats or beneath the surface of sandy or mud substrates [53]. Furthermore, elongation is a developmentally straightforward way to change form by adding or enlarging existing vertebrae in different modules along the body [54,55] and has already been shown to represent a major axis of morphological variation across fishes [21,50].
At the other extreme of the elongation axis, a deep-bodied form is thought to increase the instability of the moving body and thereby enhance maneuverability [13,56]. A deep-bodied shape, the classic demersal ecomorph of the literature [6,10,12], is indeed commonly found in demersal fishes (figure 2). Consistent with the literature, we find that demersal fishes are significantly more deep-bodied than pelagic fishes on average, (electronic supplementary material, table S2) and evolve deeper heads upon transitioning to demersal habitats (figure 3). As predicted, this suggests that there are features of the deep-bodied form that are functionally adaptive to a lifestyle that requires constant maneuvering.
With over 3300 species, this dataset encompasses broad ecological, behavioural, and morphological diversity, and many of these factors remain unaccounted for in this study. We have also not formally attempted to account for uncertainty in the habitat categorization system here. Although there may be some habitat ambiguity and uncertainty among species, we used an authoritative and diverse set of resources to inform our codings (electronic supplementary material, table S1) and have no reason to believe there are systematic biases in our scheme that would affect the general patterns found in this study. A related concern using comparative methods on a dataset of this size is the tendency to prefer any model that incorporates rate variation over one that assumes constant rates. This may lead to erroneously attributing rate heterogeneity to the discrete trait of interest. While we acknowledge this may present a complication for our study, methods for resolving such issues are limited [57], especially for a dataset of this size. Finally, given that we use a single maximum likelihood phylogeny to account for evolutionary non-independence of species, we are restricted in our ability to account for phylogenetic uncertainty in this study. Yet, our finding of significant and non-overlapping rate differences between our three general habitat categories on such a broad scale points to an overarching effect of the benthic–pelagic axis on body shape diversification.
5. Conclusion
We found repeated transitions along the benthic–pelagic axis in marine fishes, with detectable and predictable consequences for body shape in spite of extensive diversification within each habitat. Benthic habitats have the greatest impact on fish shapes and transitions to the benthos have resulted in some of the most unusual teleost body forms, such as extremely wide-bodied fishes and numerous cases of highly elongate forms. This habitat has also caused the highest rates of body shape evolution, indicating that the physical interactions with soft and hard substrates have been a significant impetus for novel forms that capitalize on the rich resources found in the biologically dynamic interface between water, sediments and hard surfaces.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are very grateful to the skilled and supportive staff at the Smithsonian fish collection and the many FishShapes team members, without whose help it would not have been possible to assemble this dataset. We also thank the members of the Wainwright laboratory for providing stimulating scientific discussions and advice throughout this study.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.25338/B8TG8S [33].
Authors' contributions
S.T.F. and P.C.W. conceived the idea. S.T.F. analysed the data. All authors participated in the study design, collected data and offered feedback during manuscript preparation.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation grant no. DEB-1556953.
References
- 1.Losos JB, Mahler DL. 2010. Adaptive radiation: the interaction of ecological opportunity, adaptation, and speciation. In Evolution since Darwin: the first 150 years (eds Bell MA, Futuyma DJ, Eanes WF, Levinton JS), pp. 382–420. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–639. ( 10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 3.Hulsey CD, Roberts RJ, Loh YHE, Rupp MF, Streelman JT. 2013. Lake Malawi cichlid evolution along a benthic/limnetic axis. Ecol. Evol. 3, 2262–2272. ( 10.1002/ece3.633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muschick M, Indermaur A, Salzburger W. 2012. Convergent evolution within an adaptive radiation of cichlid fishes. Curr. Biol. 22, 2362–2368. ( 10.1016/j.cub.2012.10.048) [DOI] [PubMed] [Google Scholar]
- 5.Cooper WJ, Parsons K, McIntyre A, Kern B, McGee-Moore A, Albertson RC. 2010. Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African Rift-Lakes. PLoS ONE 5, e9551 ( 10.1371/journal.pone.0009551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavera J, Acero P. A, Wainwright PC. 2018. Multilocus phylogeny, divergence times, and a major role for the benthic-to-pelagic axis in the diversification of grunts (Haemulidae). Mol. Phylogenet. Evol. 121, 212–223. ( 10.1016/j.ympev.2017.12.032) [DOI] [PubMed] [Google Scholar]
- 7.Friedman ST, Price SA, Hoey AS, Wainwright PC. 2016. Ecomorphological convergence in planktivorous surgeonfishes. J. Evol. Biol. 29, 965–978. ( 10.1111/jeb.12837) [DOI] [PubMed] [Google Scholar]
- 8.Cooper WJ, Carter CB, Conith AJ, Rice AN, Westneat MW. 2017. The evolution of jaw protrusion mechanics is tightly coupled to bentho-pelagic divergence in damselfishes (Pomacentridae). J. Exp. Biol. 220, 652–666. ( 10.1242/jeb.143115) [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro E, Davis AM, Rivero-Vega RA, Ortí G, Betancur R. 2018. Post-Cretaceous bursts of evolution along the benthic–pelagic axis in marine fishes. Proc. R. Soc. B 285, 20182010 ( 10.1098/rspb.2018.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusche H, Recknagel H, Elmer KR, Meyer A. 2014. Crater lake cichlids individually specialize along the benthic-limnetic axis. Ecol. Evol. 4, 1127–1139. ( 10.1002/ece3.1015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willacker JJ, Von Hippel FA, Wilton PR, Walton KM.. 2010. Classification of threespine stickleback along the benthic-limnetic axis. Biol. J. Linnean Soc. 101, 595–608. ( 10.1111/j.1095-8312.2010.01531.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker JA. 1997. Ecological morphology of lacustrine threespine stickleback Gasterosteus aculeatus (Gasterosteidae) body shape. Biol. J. Linnean Soc. 61, 3–50. ( 10.1111/j.1095-8312.1997.tb01777.x) [DOI] [Google Scholar]
- 13.Svanback R, Eklov P. 2004. Morphology in perch affects habitat specific feeding efficiency. Funct. Ecol. 18, 503–510. ( 10.1111/j.0269-8463.2004.00858.x) [DOI] [Google Scholar]
- 14.Burress ED, Holcomb JM, Tan M, Armbruster JW. 2017. Ecological diversification associated with the benthic-to-pelagic transition by North American minnows. J. Evol. Biol. 30, 549–560. ( 10.1111/jeb.13024) [DOI] [PubMed] [Google Scholar]
- 15.Hollingsworth PR, Simons AM, Fordyce JA, Hulsey CD. 2013. Explosive diversification following a benthic to pelagic shift in freshwater fishes. BMC Evol. Biol. 13, 1 ( 10.1186/1471-2148-13-272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson BW, Wilson DS. 1994. Character release and displacement in fishes: a neglected literature. Am. Nat. 144, 596–627. [Google Scholar]
- 17.Hatfield T, Schluter D. 1999. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution 53, 866–873. ( 10.2307/2640726) [DOI] [PubMed] [Google Scholar]
- 18.Price SA, Tavera J, Near TJ, Wainwright PC. 2012. Elevated rates of morphological and functional diversification in reef-dwelling Haemulid fishes. Evolution 67, 417–428. ( 10.5061/dryad.s049s) [DOI] [PubMed] [Google Scholar]
- 19.Graham NAJ, Nash KL. 2013. The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326. ( 10.1007/s00338-012-0984-y) [DOI] [Google Scholar]
- 20.Gratwicke B, Speight MR. 2005. The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J. Fish Biol. 66, 650–667. ( 10.1111/j.0022-1112.2005.00629.x) [DOI] [Google Scholar]
- 21.Price SA, Friedman ST, Corn KA, Martinez CM, Larouche O, Wainwright PC. 2019. Building a body shape morphospace of teleostean fishes. Integr. Comp. Biol. 59, 716–730. ( 10.1093/icb/icz115) [DOI] [PubMed] [Google Scholar]
- 22.Price SA, Larouche O, Friedman ST, Corn KA, Wainwright PC, Martinez CM.. 2020. A CURE for a major challenge in phenomics: a practical guide to implementing a quantitative specimen-based undergraduate research experience. Integr. Org. Biol. 2, obaa004 ( 10.1093/iob/obaa004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabosky DL, et al. 2018. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559, 392–395. ( 10.1038/s41586-018-0273-1) [DOI] [PubMed] [Google Scholar]
- 24.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 25.Mosimann JE. 1970. Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. J. Am. Stat. Assoc. 65, 930–945. [Google Scholar]
- 26.Klingenberg CP. 2016. Size, shape, and form: concepts of allometry in geometric morphometrics. Dev. Genes Evol. 226, 113–137. ( 10.1007/s00427-016-0539-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. 2014. R: language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Froese R, Pauly D.. 2019. FishBase. See www.fishbase.org.
- 29.Adams DC, Collyer ML, Kaliontzopoulou A. 2019. Geomorph: software for geometric morphometric analyses. R package version 3.1.0.
- 30.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/521238) [DOI] [Google Scholar]
- 31.Bollback JP. 2006. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinf. 7, 88 ( 10.1186/1471-2105-7-88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds RG, Collar DC, Pasachnik SA, Niemiller ML, Puente-rolón AR, Revell LJ. 2016. Ecological specialization and morphological diversification in Greater Antillean boas. Evolution 70, 1882–1895. ( 10.1186/1471-2105-7-88) [DOI] [PubMed] [Google Scholar]
- 33.Friedman ST, Price SA, Corn KA, Larouche O, Martinez CM, Wainwright PC. 2020. Body shape diversification along the benthic-pelagic axis in marine fishes. Dataset, UC Davis. ( 10.25338/B8TG8S) [DOI]
- 34.Beaulieu JM, O'Meara BC. 2015. OUwie: analysis of evolutionary rates in an OU framework. R package 1.57. See https://CRAN.R-project.org/package=OUwie.
- 35.Cooper N, Thomas GH, FitzJohn RG. 2016. Shedding light on the ‘dark side’ of phylogenetic comparative methods. Methods Ecol. Evol. 7, 693–699. ( 10.1111/2041-210X.12533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho LST, Ané C. 2014. Intrinsic inference difficulties for trait evolution with Ornstein-Uhlenbeck models. Methods Ecol. Evol. 5, 1133–1146. ( 10.1111/2041-210X.12285) [DOI] [Google Scholar]
- 37.Beaulieu JM, Jhwueng DC, Boettiger C, O'Meara BC. 2012. Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution 66, 2369–2383. ( 10.1111/j.1558-5646.2012.01619.x) [DOI] [PubMed] [Google Scholar]
- 38.Collar DC, Schulte JA, O'Meara BC, Losos JB. 2010. Habitat use affects morphological diversification in dragon lizards. J. Evol. Biol. 23, 1033–1049. ( 10.1111/j.1420-9101.2010.01971.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfaro ME, Santini F, Brock CD. 2007. Do reefs drive diversification in marine teleosts? Evidence from the pufferfish and their allies (Order Tetraodontiformes). Evolution 61, 2104–2126. ( 10.1111/j.1558-5646.2007.00182.x) [DOI] [PubMed] [Google Scholar]
- 40.Price SA, Holzman RA, Near TJ, Wainwright PC. 2011. Coral reefs promote the evolution of morphological diversity and ecological novelty in labrid fishes. Ecol. Lett. 14, 462–469. ( 10.1111/j.1461-0248.2011.01607.x) [DOI] [PubMed] [Google Scholar]
- 41.Kiessling W, Simpson C, Foote M. 2010. Reefs as cradles of evolution and sources of biodiversity in the phanerozoic. Science 327, 196–198. ( 10.1126/science.1182241) [DOI] [PubMed] [Google Scholar]
- 42.Portnoy DS, Willis SC, Hunt E, Swift DG, Gold JR, Conway KW. 2017. Molecular phylogenetics of New World searobins (Triglidae; Prionotinae). Mol. Phylogenet. Evol. 107, 382–387. ( 10.1016/j.ympev.2016.11.017) [DOI] [PubMed] [Google Scholar]
- 43.Gosline WA. 1994. Function and structure in the paired fins of scorpaeniform fishes. Environ. Biol. Fishes 40, 219–226. ( 10.1007/BF00002508) [DOI] [Google Scholar]
- 44.Yamanoue Y, Setiamarga DHE, Matsuura K. 2010. Pelvic fins in teleosts: structure, function and evolution. J. Fish Biol. 77, 1173–1208. ( 10.1111/j.1095-8649.2010.02674.x) [DOI] [PubMed] [Google Scholar]
- 45.Videler JJ. 1993. Fish swimming New York, NY: Springer; ( 10.1007/978-94-011-1580-3) [DOI] [Google Scholar]
- 46.Denny. 1996. Air and water: the biology and physics of life's media Princeton, NJ: Princeton University Press. [Google Scholar]
- 47.Webb PW. 1989. Station-holding by three species of benthic fishes. J. Exp. Biol. 145, 303–320. [Google Scholar]
- 48.Fletcher T, Altringham J, Peakall J, Wignall P, Dorrell R. 2014. Hydrodynamics of fossil fishes. Proc. R. Soc. B 281, 20140703 ( 10.1098/rspb.2014.0703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnold GP, Weihs D. 1978. The hydrodynamics of rheotaxis in the plaice (Pleuronectes platessa). J. Exp. Biol. 75, 147–169. [Google Scholar]
- 50.Claverie T, Wainwright PC. 2014. A morphospace for reef fishes: elongation is the dominant axis of body shape evolution. PLoS ONE 9, e112732 ( 10.1371/journal.pone.0112732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blake RW. 2004. Fish functional design and swimming performance. J. Fish Biol. 65, 1193–1222. ( 10.1111/j.1095-8649.2004.00568.x) [DOI] [PubMed] [Google Scholar]
- 52.Ward AB, Costa A, Monroe SL, Aluck RJ, Mehta RS. 2015. Locomotion in elongate fishes: a contact sport. Zoology 118, 312–319. ( 10.1016/j.zool.2015.06.002) [DOI] [PubMed] [Google Scholar]
- 53.Gans C. 1975. Tetrapod limblessness: evolution and functional corollaries. Am. Zool. 15, 455–467. [Google Scholar]
- 54.Mehta RS, Ward AB, Alfaro ME, Wainwright PC. 2010. Elongation of the body in eels. Integr. Comp. Biol. 50, 1091–1105. ( 10.1093/icb/icq075) [DOI] [PubMed] [Google Scholar]
- 55.Ward AB, Brainerd EL. 2007. Evolution of axial patterning in elongate fishes. Biol. J. Linnean Soc. 90, 97–116. ( 10.1111/j.1095-8312.2007.00714.x) [DOI] [Google Scholar]
- 56.Webb PW. 1984. Form and function in fish swimming. Sci. Am. 251, 72–82. ( 10.1038/scientificamerican0784-72) [DOI] [Google Scholar]
- 57.Rabosky DL, Goldberg EE. 2015. Model inadequacy and mistaken inferences of trait-dependent speciation. Syst. Biol. 64, 340–355. ( 10.1093/sysbio/syu131) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Friedman ST, Price SA, Corn KA, Larouche O, Martinez CM, Wainwright PC. 2020. Body shape diversification along the benthic-pelagic axis in marine fishes. Dataset, UC Davis. ( 10.25338/B8TG8S) [DOI]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.25338/B8TG8S [33].