Abstract
Background:
Both alcohol and prescription opioid use/misuse are highly prevalent among individuals with chronic pain. Co-use of alcohol and prescription opioids is also common, despite contraindications due to increased risk of negative health effects and mortality. There is evidence that pain-related anxiety (i.e., the tendency to respond to pain with anxiety or fear) may be associated with heavier drinking and prescription opioid use/co-use, and that these associations may be especially salient among men.
Methods:
This study is the first examination of pain-related anxiety in relation to hazardous alcohol use, prescription opioid use/misuse, and alcohol-opioid co-use. Participants included 1812 adults with chronic low back pain (69% female, Mage = 43.95) who completed an online survey assessing health behaviors.
Results:
Pain-related anxiety was positively associated with indices of alcohol (i.e., alcohol-related consequences) and opioid use (i.e., prescription opioid use/misuse, daily opioid consumption). Of note, sex moderated associations between pain-related anxiety and both alcohol-related consequences and prescription opioid misuse In addition to being associated with alcohol and prescription opioid use, independently, pain-related anxiety was also associated with greater likelihood of endorsing co-use of alcohol and opioids and engaging in concurrent hazardous drinking and prescription opioid misuse.
Conclusions:
These findings contribute to a growing literature suggesting that pain-related anxiety is an important transdiagnostic factor in pain and alcohol and prescription opioid use/co-use, perhaps especially among males.
Keywords: Pain, Chronic Pain, Alcohol, Opioids, Co-Use
1. Introduction
Chronic pain affects approximately one-third of American adults and has an annual economic impact nearing $635 billion (Gaskin and Richard, 2012). Pain-related anxiety (i.e., the tendency to respond to pain with anxiety or fear) has been posited to play a critical role in the experience and maintenance of chronic pain and pain-related behaviors (e.g., Larsen et al., 1997; McCracken et al., 1992). Emerging evidence further suggests that pain-related anxiety may be particularly important for understanding alcohol and prescription opioid use/misuse among men with chronic pain (e.g., LaRowe et al., 2018; Zale et al., 2019). For example, hazardous alcohol use (e.g., heavy drinking and binge drinking) is highly prevalent among the 100 million Americans who experience chronic pain (IOM, 2011; Von Korff et al., 2005; Zale et al., 2015), and initial work has demonstrated positive associations between pain-related anxiety and indices of hazardous drinking, including alcohol-related consequences (e.g., injuries from drinking, blackouts) and alcohol dependence symptoms, among males, but not females, with chronic pain (Zale et al., 2019). In addition, opioid medications are commonly prescribed for the treatment of chronic pain, and preliminary evidence suggests that pain-related anxiety may be one factor that maintains opioid misuse. For example, one study of 68 tobacco smokers living with HIV demonstrated that higher levels of pain-related anxiety were associated with current opioid misuse among males, but not females (LaRowe et al., 2018a). A second study of 256 young adults with past four-week pain revealed similar positive associations between pain-related anxiety and prescription opioid misuse, however, potential sex differences were not assessed (Rogers et al., 2018).
Although drinking alcohol is contraindicated in the context of prescription opioid use (e.g., Weathermon and Crabb, 1999), approximately 20% of individuals with opioid prescriptions consume alcohol and opioids in the same day (Peacock et al., 2016), and 12% of daily opioid users consume alcohol within 2 hours of taking their medication (Saunders et al., 2012). Moreover, there is emerging evidence that opioid use disorder (OUD) and alcohol use disorder (AUD) frequently co-occur (Witkiewitz and Vowles, 2018). For example, population-based data have revealed that individuals with OUD (vs. without OUD) are nearly twice as likely to also meet criteria for AUD (Saha et al., 2016). This finding is especially concerning given that alcohol and opioids both act as central nervous system depressants (Weathermon and Crabb, 1999), and that co-use of these substances can substantially increase risk of morbidity and mortality (Witkiewitz and Vowles, 2018). Indeed, interactions between alcohol and opioids can have dangerous (e.g., liver damage, respiratory depression) and potentially fatal health effects (Weathermon and Crabb, 1999). Alcohol and opioid co-use has been related to worse outcomes following treatment for either substance, and may also interfere with treatment for chronic pain (Egli et al., 2012; Witkiewitz and Vowles, 2018). There is a clear need for research focused on assessing and ultimately mitigating hazardous drinking and prescription opioid misuse among persons with chronic pain (e.g., Witkiewitz and Vowles, 2018), however, no research to date has investigated the role of pain-related anxiety in co-use of alcohol and prescription opioids.
Converging evidence indicates that pain can be a potent motivator of substance use in general (e.g., Ditre et al., 2019), and both alcohol (e.g., Moskal et al., 2018) and prescription opioid use (e.g., Barth et al., 2013) in particular, and it is possible that pain-related anxiety may increase the propensity to escape and avoid pain via substance use (e.g., LaRowe et al., 2018b). Indeed, alcohol and opioids have each been shown to confer acute analgesia in experimental pain models (e.g., Staahl et al., 2009; Thompson et al., 2017), and persons with chronic pain consistently report using and misusing these substances for pain-coping (e.g., Goebel et al., 2011; Sheu et al., 2008). For example, among patients attending an outpatient substance use treatment program, more than one-quarter of those with chronic pain cited pain as the impetus for alcohol and drug abuse (Sheu et al., 2008). Individuals with chronic pain and higher levels of pain-related anxiety may also attempt to extend/supplement analgesic effects via co-use of substances (e.g., Powers et al., 2019). In addition, given evidence that associations between anxiety and externalizing behaviors (e.g., substance use) are stronger among males (vs. females; e.g., Altemus et al., 2014; Marmorstein, 2007), there is reason to believe that pain-related anxiety may be more strongly associated with alcohol/opioid use among males.
The goal of the current study was to examine pain-related anxiety in relation to hazardous alcohol use, prescription opioid use/misuse, and alcohol-opioid co-use among individuals with chronic low back pain (CLBP). We focused on CLBP because it is the most common source of chronic pain among American adults, affecting up to 20% of the population (e.g., Meucci et al., 2015). In addition, individuals with CLBP (vs. no pain) are more likely to endorse past-year alcohol use (Shmagel et al., 2016) and meet criteria for AUD (Atkinson et al., 1991), and over one-third of patients with CLBP report prescription opioid use (Gore et al., 2012). We hypothesized that pain-related anxiety would be positively associated with (1) hazardous alcohol use, (2) prescription opioid use and misuse, and (3) alcohol-opioid co-use. We also hypothesized that sex would moderate these relations, such that positive associations would be stronger among male, relative to female respondents. Given that ‘co-use’ has been defined in a variety of ways across the empirical literature (e.g., same day use, concurrent AUD and OUD; Peacock et al., 2016; Saha et al., 2016; Saunders et al., 2012), the current study utilized two approaches to measure alcohol and opioid co-use. First, we assessed for any alcohol-opioid co-use (i.e., current use of both alcohol and prescription opioid medication). Second, we assessed for concurrent hazardous drinking and prescription opioid misuse.
2. Method
2.1. Participants and Survey Procedures
Participants were recruited nationally through Qualtrics, an online survey management system, which has been found to yield valid and reliable data among diverse populations in prior work (Walters et al., 2018). Adults with a Qualtrics Panels account, who endorsed mild to severe CLBP, were sent a survey advertisement. Respondents were screened for eligibility and directed to the online, anonymous, 30-minute survey. To ensure valid responses, a speeding check was included – measured as one-half the median survey completion time (15.2 minutes) – to screen out those who are not responding thoughtfully. Participants could opt to receive compensation in varying forms (e.g., cash-based incentives [i.e., gift cards], rewards miles, rewards points) and the level of compensation was consistent across respondents ($13). The study protocol was approved by the institution where the study was conducted.
2.2. Measures
2.2.1. Pain-Related Anxiety
The Pain Anxiety Symptom Scale - 20 item (PASS-20; McCracken and Dhingra, 2002) assesses the tendency to respond to pain with anxiety or fear (e.g., “When I feel pain, I am afraid that something terrible will happen”; “I avoid important activities when I hurt”), and scores on this measure were the primary independent variable of interest for the current analyses. Items are rated on a 6-point Likert scale ranging from 0 (never) to 5 (always), and responses are summed to generate a total score (range 0–100). Previous work has demonstrated reliability, validity, and measurement invariance of the PASS-20 in chronic pain samples (e.g., McCracken and Dhingra, 2002; Rogers et al., in press-a), and internal consistency was excellent in the current sample (α = .96).
2.2.2. Alcohol Use
The Alcohol Use Disorders Identification Test (AUDIT) is a reliable and validated assessment of alcohol use problems among adults (Babor et al., 1992). The AUDIT includes 10 items that are rated on a 5-point scale ranging from 0 (never) to 4 (4 or more times a week) and summed to generate a total score. Consistent with recommendations (e.g., Bradley et al., 2003), we used a sex-specific modified version of AUDIT question #3, which asks about the frequency of drinking 6 or more drinks on an occasion. Specifically, women were asked to report the frequency of drinking at least 4 (vs. 6) drinks per occasion. A total score cut-off of ≥ 8 is indicative of hazardous drinking behavior (e.g., Babor et al., 1992; Saunders et al., 1993). In addition, factor analytic studies of the AUDIT have supported a two-factor solution representing alcohol consumption (AUDIT-Consumption subscale; e.g., “How often do you have a drink containing alcohol?”) and alcohol-related consequences (AUDIT-Consequences subscale; e.g., “Have you or someone else been injured because of your drinking?”; e.g., Doyle et al., 2007). In the current sample, internal consistency of the AUDIT was excellent (α = .92), and each of the AUDIT domain scales also demonstrated acceptable internal consistency (AUDIT-Consumption: α = .76; AUDIT-Consequences: α = .92).
2.2.3. Prescription Opioid Use and Misuse
Participants indicated whether they are currently taking prescription opioid medication for pain (yes/no). Those who endorsed current opioid use were then asked to specify the types of opioid medication that they are currently prescribed, along with the dose (mg) and frequency (times per day) that each medication is taken. Daily dose of prescription opioid medication in morphine equivalent units (MEUs) was calculated based on CDC guidelines (Dowell et al., 2016). The Current Opioid Misuse Measure (COMM; Butler et al., 2007) was used to assess how often participants have engaged in various aberrant medication related behaviors (e.g., going to someone other than the prescribing physician to get sufficient relief from medications) in the past 30 days. The COMM includes 17 items that are rated on a 5-point scale ranging from 0 (never) to 4 (very often). Items are summed to compute a total score, and scores ≥ 9 are indicative of current opioid misuse. The COMM has evinced strong diagnostic performance characteristics among individuals with pain (Meltzer et al., 2011; Rogers et al., in press-b), and demonstrated excellent internal consistency in the current sample (α = .97).
2.2.4. Alcohol and Opioid Co-Use
Alcohol-opioid co-use was defined as any use (including misuse) of both alcohol and prescription opioid medications. A dichotomous variable was created (0 = no alcohol and opioid co-use; 1 = alcohol-opioid co-use), and participants who endorsed at least occasional alcohol use (response > 0 on first AUDIT item; “How often do you have a drink containing alcohol?”) and current prescription opioid use were coded as engaging in alcohol-opioid co-use.
2.2.5. Concurrent Hazardous Drinking and Prescription Opioid Misuse
A dichotomous variable was created (0 = no concurrent hazardous drinking and prescription opioid misuse, 1 = concurrent hazardous drinking and prescription opioid misuse) to reflect concurrent hazardous drinking and prescription opioid misuse, which was defined as scoring above the AUDIT cutoff for hazardous drinking (total scores ≥ 8) and above the COMM cutoff for opioid misuse (total scores ≥ 9).
2.2.6. Anxiety
The Overall Anxiety Severity and Impairment Scale (OASIS; Norman et al., 2006) is a five-item measure of severity and impairment associated with anxiety, that can be used across anxiety disorders and with subthreshold anxiety symptoms. The OASIS was included as a covariate in these analyses to assess the unique contribution of pain-related anxiety, above and beyond the variance accounted for by generalized anxiety severity. The OASIS has demonstrated excellent test-retest reliability, and convergent and divergent validity (Norman et al., 2006). Internal consistency of the OASIS was excellent in the current sample (α = .95).
2.2.7. Sociodemographic and Pain Characteristics
Participants self-reported age, sex, race, Hispanic ethnicity, education, and income. Consistent with previous work (e.g., Kosiba et al., 2020; LaRowe et al., 2017; LaRowe et al., 2020), a single item from the Short Form Health Survey-12 was used to assess past four-week pain severity (“How much bodily pain have you had during the past four weeks?”; Ware et al., 1996). Response options ranged from 0 (none) to 5 (very severe).
2.3. Data Analytic Strategy
We conducted separate hierarchical linear (for continuous dependent variables) and logistic (for dichotomous dependent variables) regression models to test sex as a moderator of associations between pain-related anxiety and each outcome. Alcohol outcomes included (1) AUDIT-Consumption scores, (2) AUDIT-Consequences scores, and (3) likelihood of scoring above the cut-off for hazardous drinking (AUDIT-Total score ≥ 8). Opioid outcomes included (1) likelihood of endorsing prescription opioid use, (2) daily MEUs, (3) COMM-Total scores (among n = 291 participants who were prescribed opioids), and (4) likelihood of scoring above the cut-off for prescription opioid misuse (COMM-Total scores ≥ 9; among those prescribed opioids). Co-use outcomes included likelihood of (1) co-using alcohol and prescription opioid medications, and (2) endorsing concurrent hazardous drinking and prescription opioid misuse (i.e., scoring above the cut-offs for both the AUDIT and the COMM).
Given previously observed associations with pain-related anxiety and substance use (e.g., LaRowe et al., 2018a; LaRowe et al., 2017; Zale et al., 2019), age, overall anxiety severity/impairment, and past four-week pain severity were included as covariates in each model. For each model, predictors were entered in the following order: Step 1 (age, OASIS scores, past four-week pain severity); Step 2 (sex, pain-related anxiety); Step 3 (sex × pain-related anxiety interaction). Given that running multiple statistical models can increase the likelihood of a Type I error (e.g., Chen et al., 2017), a Bonferroni correction was employed (e.g., Benjamini and Hochberg, 1995) and all models were tested at more stringent significance levels (ps < .006). Significant interactions were probed by testing the conditional effects of pain-related anxiety at each level of sex (i.e., male and female) using the PROCESS Macro for SPSS (Hayes, 2012). If the sex × pain-related anxiety interaction term was not significant, we examined associations between pain-related anxiety and the outcome variable at Step 2 of the model.
3. Results
3.1. Participant Characteristics
Participants included 1812 adults with chronic low back pain (69% female; Mage = 43.95, SD = 11.96; 80.5% White). Over one-third (38.3%) of the sample completed a 4-year college degree or higher education, and nearly three-quarters of participants reported an annual income greater than $25,000. Over 80% of the sample endorsed at least moderate past four-week pain severity, and 16.5% were currently prescribed opioid medication. More than two-thirds (69.5%) of participants reported past-month alcohol use. Additional sociodemographic data are presented in Table 1.
Table 1.
Participant Characteristics
| Total (N=1812) | |
|---|---|
| n (%) | |
| Gender | |
| Male | 561 (31.0%) |
| Female | 1251 (69.0%) |
| Race | |
| White | 1459 (80.5%) |
| Black or African American | 200 (11.0%) |
| Asian/Pacific Islander | 48 (2.6%) |
| Native American/American Indian | 21 (1.2%) |
| Multiracial | 43 (2.4%) |
| Other | 41 (2.3%) |
| Ethnicity | |
| Hispanic | 190 (10.5%) |
| Marital Status | |
| Married/Living with Someone | 1034 (57.1%) |
| Widowed | 58 (3.2%) |
| Divorced/Separated | 267 (14.7%) |
| Single/Never Married | 453 (25.0%) |
| Income | |
| < $10,000 | 162 (8.9%) |
| $10,000 – $34,999 | 559 (30.8%) |
| $35,000 – $74,999 | 679 (37.5%) |
| More than $75,000 | 412 (22.7%) |
| Education | |
| High School or Less | 494 (27.3%) |
| Some college/Associate’s degree | 624 (34.4%) |
| College Degree | 452 (24.9%) |
| School Beyond College | 242 (13.4%) |
| Past Four-Week Pain Severity | |
| None | 1 (0.1%) |
| Very Mild | 24 (1.3%) |
| Mild | 258 (14.2%) |
| Moderate | 683 (37.7%) |
| Severe | 602 (33.2%) |
| Very Severe | 244 (13.5%) |
| Alcohol Use | |
| Yes | 1260 (69.5%) |
| Hazardous Drinkinga | |
| Yes | 395 (21.8%) |
| Prescription Opioid Use | |
| Yes | 299 (16.5%) |
| Prescription Opioid Misuseb | |
| Yes | 165 (9.1%) |
| Co-Use of Alcohol and Prescription Opioids | |
| Yes | 208 (11.5%) |
| Concurrent Hazardous Drinking and Prescription Opioid Misuse | |
| Yes | 62 (3.4%) |
| M (SD) | |
| Age | 43.95 (11.96) |
| AUDIT - Consumptionc | 2.43 (2.67) |
| AUDIT - Consequencesd | 2.12 (4.55) |
| Daily MEUse | 234.02 (674.05) |
| COMM Scoreb | 16.11 (16.27) |
| Pain-Related Anxietyf | 53.11 (24.44) |
Note.
Alcohol Use Disorder Identification Test – Total Score ≥ 8,
Current Opioid Misuse Measure (among those prescribed opioids),
Alcohol Use Disorder Identification Test – Consumption Subscale,
Alcohol Use Disorder Identification Test – Consequences Subscale,
Morphine Equivalent Units (among those prescribed opioids),
Pain Anxiety Symptoms Scale – 20.
3.2. Pain-Related Anxiety, Sex, and Alcohol Use
PASS-20 scores were positively associated with AUDIT-Consequences scores at the second step of the linear regression model (Step 2: β = .02, p < .001; ΔR2 = .046, p = .001; Table 2). Results further indicated that sex moderated the association between PASS-20 and AUDIT – Consequences scores (Step 3: β = .18, p = .001; ΔR2 = .01, p = .001; Table 2), such that the positive association between pain-related anxiety and alcohol-related consequences was stronger among males (B = .04, 95% CI: .03–.06, p < .001), than among females (B = .01, 95% CI: .00–.03, p = .031; Figure 1). Greater pain-related anxiety was also associated with a higher likelihood of scoring above the AUDIT cut-off for hazardous drinking (Step 2: AOR = 1.01, 95% CI: 1.00 – 1.02, p = .004), although this association was not moderated by sex (Step 3: AOR = .99, 95% CI: .98 – 1.01, p = .27; Table 3). Pain-related anxiety was not associated with AUDIT – Consumption scores (Step 2: β = .05, p = .070), and sex did not moderate this association (Step 3: β = .02, p = .79; Table 2).
Table 2.
Linear Regression: Associations between Pain-Related Anxiety, Sex, and Alcohol/Opioid Use
| AUDITa - Consumption | ||||||
|---|---|---|---|---|---|---|
| Step 2 | Step 3 | |||||
| Variable | β | t | p | p | t | p |
| Age | −.114 | −4.814 | <001 | −.114 | −4.802 | <001 |
| Race | −.023 | −1.010 | .313 | −.023 | −.995 | .320 |
| OASISb | .057 | 1.961 | .050 | .057 | 1.954 | .051 |
| Pain Severityc | −.038 | −1.530 | .126 | −.038 | −1.524 | .128 |
| Sex | .223 | 9.833 | <.001 | .210 | 3.934 | <.001 |
| PASS-20d | .053 | 1.813 | .070 | .049 | 1.477 | .140 |
| Sex × PASS-20 | .015 | .264 | .792 | |||
| R2 | .076 | .076 | ||||
| ΔR2 | .052 | .000 | ||||
| F for ΔR2 | 50.724** | .070 | ||||
| AUDIT - Consequences | ||||||
| Step 2 | Step 3 | |||||
| Variable | β | t | p | β | t | p |
| Age | −.047 | −5.352 | <.001 | −.121 | −5.253 | <001 |
| Race | −.061 | −.710 | .478 | −.012 | −.543 | .587 |
| OASIS | .145 | 6.093 | <.001 | .170 | 6.029 | <.001 |
| Pain Severity | −.133 | −1.130 | .259 | −.026 | −1.069 | .285 |
| Sex | 1.859 | 8.561 | <.001 | .029 | .558 | .577 |
| PASS-20 | .022 | 4.248 | <.001 | .069 | 2.161 | .031 |
| Sex × PASS-20 | .184 | 3.409 | .001 | |||
| R2 | .127 | .132 | ||||
| ΔR2 | .046 | .046 | ||||
| F for ΔR2 | 47.099** | 11.622** | ||||
| MEUse | ||||||
| Step 2 | Step 3 | |||||
| Variable | β | t | p | β | t | p |
| Age | −.014 | −.574 | .566 | −.014 | −.571 | .568 |
| Race | −.024 | −1.035 | .301 | −.024 | −1.030 | .303 |
| OASIS | −.011 | −.351 | .725 | −.011 | −.353 | .724 |
| Pain Severity | .055 | 2.103 | .036 | .055 | 2.104 | .036 |
| Sex | .024 | 1.014 | .311 | .020 | .364 | .716 |
| PASS-20 | .084 | 2.780 | .005 | .083 | 2.419 | .016 |
| Sex × PASS-20 | .004 | .074 | .941 | |||
| R2 | .014 | .014 | ||||
| ΔR2 | .005 | .000 | ||||
| F for ΔR2 | 4.490* | .005 | ||||
| COMMf | ||||||
| Step 2 | Step 3 | |||||
| Variable | β | t | p | β | t | p |
| Age | −.244 | −4.107 | <.001 | −.234 | −4.551 | <.001 |
| Race | .053 | 1.116 | .265 | .043 | .927 | .355 |
| OASIS | .271 | 4.590 | <.001 | .258 | 4.404 | <.001 |
| Pain Severity | −.082 | −1.640 | .102 | −.091 | −1.837 | .067 |
| Sex | .171 | 3.595 | <001 | −.267 | −1.653 | .099 |
| PASS-20 | .223 | 3.723 | <.001 | .151 | 2.352 | .019 |
| Sex × PASS-20 | .471 | 2.832 | .005 | |||
| R2 | .368 | .386 | ||||
| ΔR2 | .060 | .017 | ||||
| F for ΔR2 | 13.543** | 8.022** | ||||
Note:
β = standardized beta weights;
F for ΔR2 = F change at each step of the model;
Alcohol Use Disorder Identification Test;
Overall Anxiety Severity and Impairment Scale;
Short-Form Health Survey-12;
Pain Anxiety Symptoms Scale – 20 item;
Morphine Equivalent Units;
Current Opioid Misuse Measure;
p < .05;
p < .01.
Figure 1.
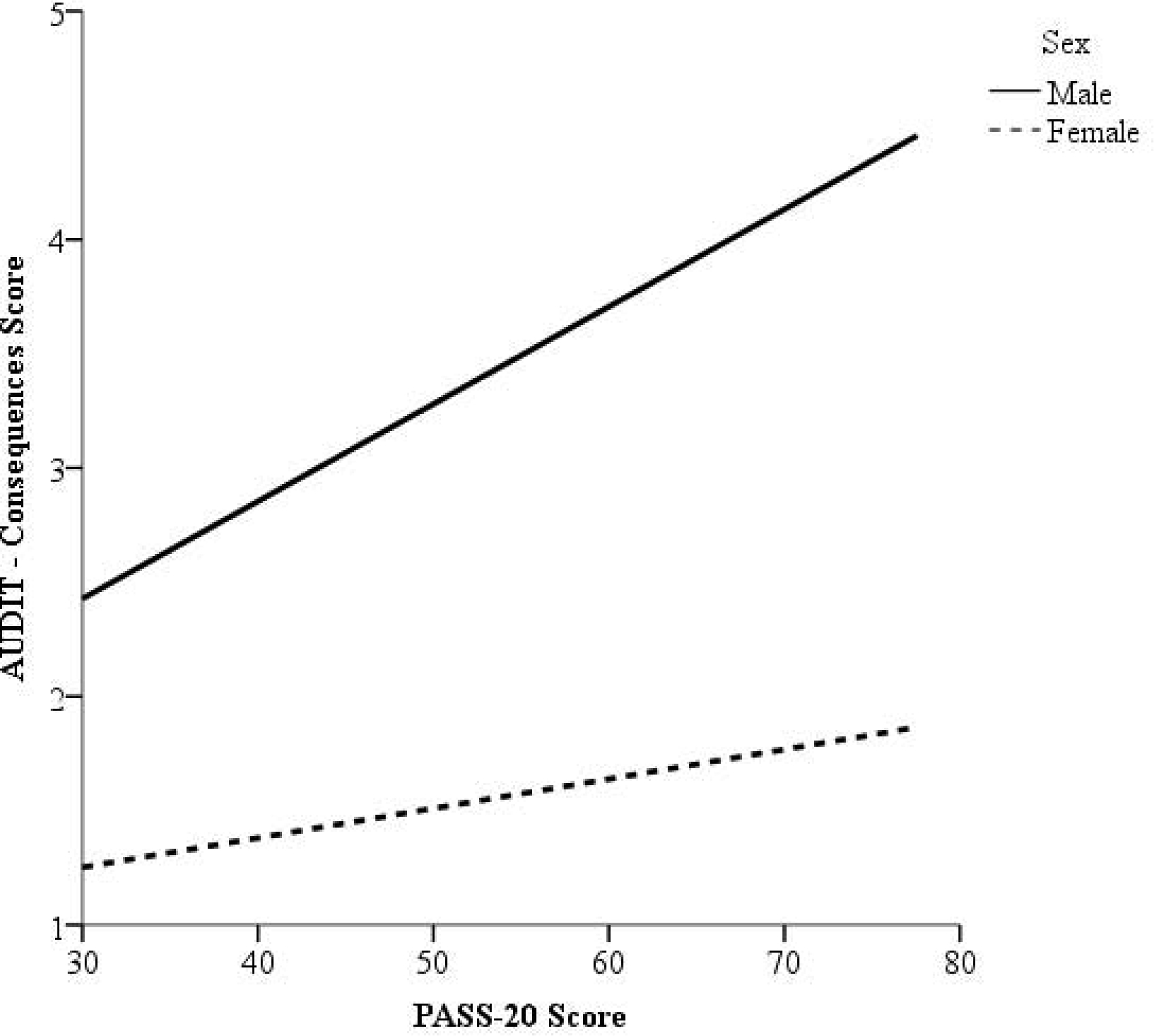
Conditional effects of PASS-20 scores (possible range: 0–100, higher scores reflect greater levels of pain-related anxiety) on AUDIT - Consequences scores (possible range: 0–28; higher scores reflect greater consequences related to alcohol use) as a function of sex.
Table 3.
Logistic Regression: Odds of Alcohol and Opioid Use/Co-Use
| Hazardous Alcohol Usea | ||||||||
|---|---|---|---|---|---|---|---|---|
| Step 2 | Step 3 | |||||||
| Variable | B | SE | OR | p | B | SE | OR | p |
| Age | −.026 | .005 | .974 | <.001 | −.027 | .006 | .974 | <.001 |
| Race | .003 | .051 | 1.003 | .953 | −.001 | .051 | .999 | .991 |
| OASISb | .072 | .015 | 1.074 | <.001 | .072 | .015 | 1.075 | <.001 |
| Pain Severityc | −.155 | .073 | .856 | .033 | −.158 | .073 | .854 | .030 |
| Sex | 1.143 | .126 | 3.136 | <.001 | 1.487 | .335 | 4.423 | <.001 |
| PASS-20d | .010 | .003 | 1.010 | .004 | .012 | .004 | 1.012 | .003 |
| Sex × PASS-20 | −.006 | .005 | .994 | .267 | ||||
| Opioid Use | ||||||||
| Step 2 | Step 3 | |||||||
| Variable | B | SE | OR | p | B | SE | OR | p |
| Age | .023 | .006 | 1.023 | <.001 | .023 | .006 | 1.023 | <.001 |
| Race | −.030 | .061 | .970 | .626 | −.027 | .062 | .974 | .665 |
| OASIS | −.020 | .015 | .981 | .199 | −.020 | .015 | .980 | .192 |
| Pain Severity | .609 | .084 | 1.838 | <.001 | .609 | .084 | 1.838 | <.001 |
| Sex | .063 | .145 | 1.065 | .666 | −.511 | .451 | .600 | .257 |
| PASS-20 | .026 | .004 | 1.026 | <.001 | .023 | .004 | 1.023 | <.001 |
| Sex × PASS-20 | .009 | .007 | 1.009 | .175 | ||||
| Opioid Misusee | ||||||||
| Step 2 | Step 3 | |||||||
| Variable | B | SE | OR | p | B | SE | OR | p |
| Age | −.050 | .014 | .951 | <.001 | −.050 | .014 | .951 | <.001 |
| Race | .112 | .150 | 1.118 | .454 | .108 | .151 | 1.114 | .472 |
| OASIS | .176 | .038 | 1.192 | <.001 | .175 | .038 | 1.191 | <.001 |
| Pain Severity | −.411 | .192 | .663 | .032 | −.413 | .193 | .662 | .032 |
| Sex | .288 | .316 | 1.334 | .363 | .049 | 1.258 | 1.051 | .969 |
| PASS-20 | .024 | .009 | 1.024 | .006 | .023 | .010 | 1.023 | .013 |
| Sex × PASS-20 | .004 | .019 | 1.004 | .845 | ||||
| Alcohol-Opioid Co-Use | ||||||||
| Step 2 | Step 3 | |||||||
| Variable | B | SE | OR | p | B | SE | OR | p |
| Age | .006 | .007 | 1.006 | .398 | .006 | .007 | 1.006 | .381 |
| Race | −.056 | .072 | .946 | .437 | −.053 | .072 | .948 | .457 |
| OASIS | −.006 | .017 | .994 | .741 | −.006 | .017 | .994 | .733 |
| Pain Severity | .478 | .094 | 1.613 | <.001 | .478 | .094 | 1.613 | <.001 |
| Sex | .276 | .161 | 1.318 | .087 | −.076 | .503 | .927 | .880 |
| PASS-20 | .023 | .004 | 1.023 | <.001 | .021 | .005 | 1.021 | <.001 |
| Sex × PASS-20 | .005 | .007 | 1.005 | .458 | ||||
| Concurrent Problematic Alcohol-Opioid Co-Usef | ||||||||
| Step 2 | Step 3 | |||||||
| Variable | B | SE | OR | p | B | SE | OR | p |
| Age | −.021 | .013 | .979 | .099 | −.021 | .013 | .979 | .102 |
| Race | .026 | .108 | 1.026 | .811 | .032 | .108 | 1.033 | .766 |
| OASIS | .069 | .035 | 1.072 | .045 | .069 | .035 | 1.071 | .046 |
| Pain Severity | .015 | .158 | 1.015 | .923 | .021 | .158 | 1.021 | .896 |
| Sex | .904 | .275 | 2.470 | .001 | .031 | 1.199 | 1.031 | .979 |
| PASS-20 | .043 | .009 | 1.044 | <.001 | .038 | .011 | 1.039 | .001 |
| Sex × PASS-20 | .011 | .015 | 1.012 | .454 | ||||
Note:
Alcohol Use Disorder Identification Test;
Overall Anxiety Severity and Impairment Scale;
Short-Form Health Survey-12;
Pain Anxiety Symptoms Scale – 20 item;
Current Opioid Misuse Measure;
Scores above cut-offs on both AUDIT and COMM.
3.3. Pain-Related Anxiety, Sex, and Opioid Use/Misuse
Across the full sample, every one-point increase in PASS-20 score was associated with a 2.6% increased likelihood of endorsing prescription opioid use (Step 2: AOR = 1.03, 95% CI: 1.02–1.03, p < .001; Table 3), and pain-related anxiety was positively associated with total opioid consumption (Step 2: β = .08, p = .005; Table 2). Sex did not moderate these associations (ps > .05). Among participants who were prescribed opioids, PASS-20 scores were positively associated with COMM-Total scores (Step 2: β = .22, p < .001; ΔR2 = .06, p < .001; Table 2). Results further indicated that sex moderated the association between PASS-20 scores and COMM-Total scores (Step 3: β = .47, p = .005; ΔR2 = .02, p = .005; Table 2), such that the positive association between pain-related anxiety and COMM-Total scores was stronger among males (B = .35, 95% CI: .20–.50, p < .001) than females (B = .12, 95% CI: .02–.22, p = .019; Figure 2). Finally, every one-point increase in PASS-20 score was associated with a 2.4% increased likelihood of scoring above the COMM cut-off for prescription opioid misuse (Step 2: AOR = 1.02, 95% CI: 1.01–1.04, p = .006; Table 3). However, sex was not found to moderate this association (Step 3: AOR = 1.00, 95% CI: .97–1.04, p = .85; Table 3).
Figure 2.
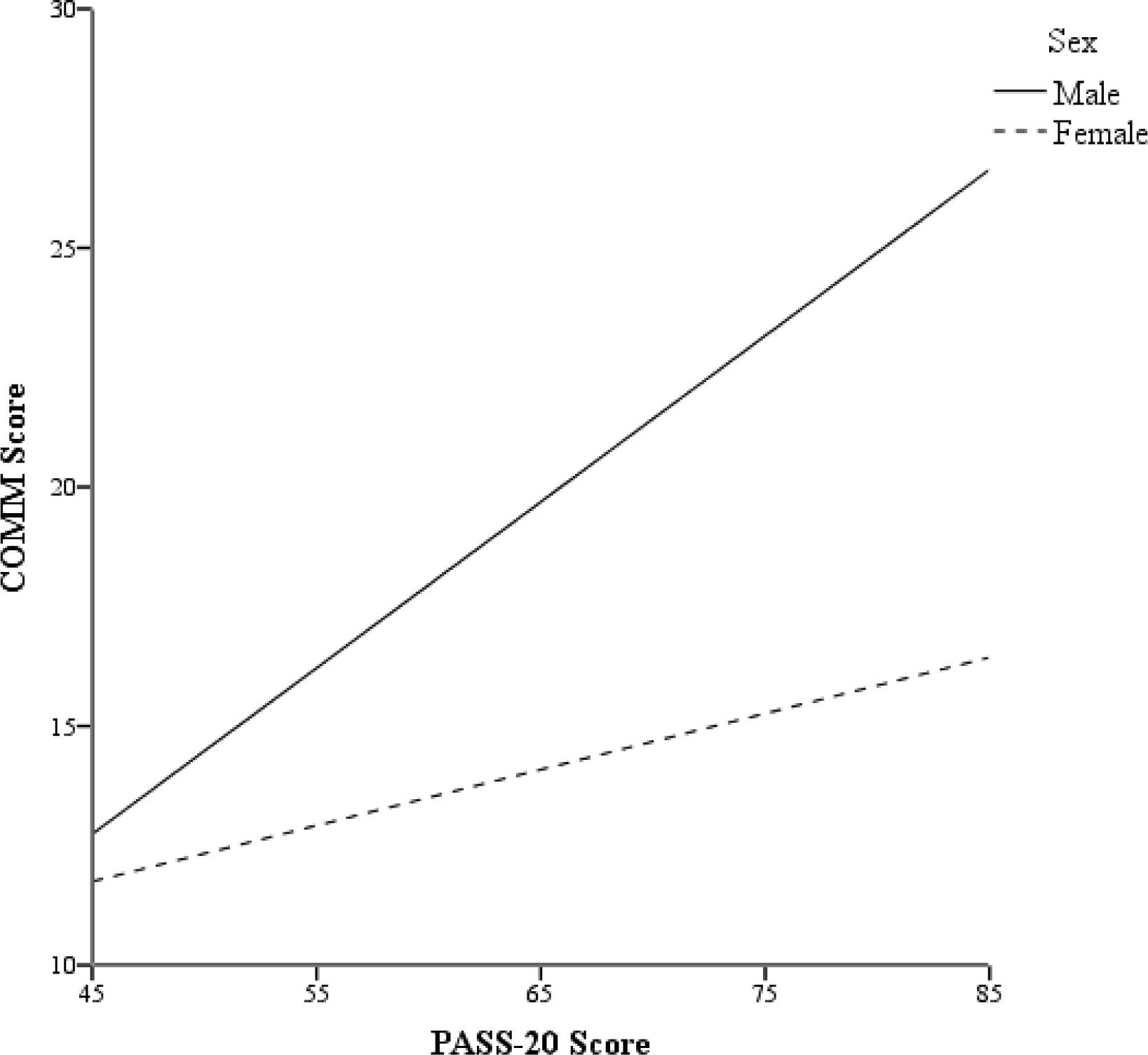
Conditional effects of PASS-20 scores (possible range: 0–100; higher scores reflect greater levels of pain-related anxiety) on COMM scores (possible range: 0–68; higher scores reflect greater aberrant prescription opioid medication related behaviors) as a function of sex.
3.4. Pain-Related Anxiety, Sex, and Alcohol-Opioid Co-Use
Higher PASS-20 scores were associated with an increased risk of endorsing co-use of alcohol and opioids (Step 2: AOR = 1.02, 95% CI: 1.02–1.03, p < .001; Table 3), as well as an increased risk of concurrent hazardous drinking and prescription opioid misuse (Step 2: AOR = 1.04, 95% CI: 1.03–1.06, p < .001; Table 3). Sex did not moderate either of these associations (ps > .05). However, sex was independently associated with the likelihood of scoring above the cut-offs for both hazardous drinking and opioid misuse, such that males were nearly 2.5 times as likely than females to endorse concurrent hazardous drinking and opioid misuse (Step 2: AOR = 2.47, 95% CI: 1.44–4.23, p = .001; Table 3).
4. Discussion
This is the first study to examine pain-related anxiety in relation to use/co-use of alcohol and prescription opioids among persons with chronic pain, and to examine sex as a moderator of these associations. In terms of alcohol use, results indicated that pain-related anxiety was positively associated with alcohol-related consequences (e.g., alcohol-related injuries, blackouts, dependence symptoms), and that this association was stronger among males than females. Pain-related anxiety was also associated with an increased likelihood of scoring above the AUDIT cut-off for hazardous drinking, although no association between pain-related anxiety and quantity/frequency of drinking was observed. In terms of opioid use, participants with greater pain-related anxiety reported taking more total daily morphine equivalent units and were more likely to endorse current use of any prescription opioid, regardless of sex. Of those prescribed opioid medications, pain-related anxiety was positively related with current opioid misuse, and this association was stronger among males than females. Finally, results indicated that pain-related anxiety was associated with a greater likelihood of endorsing co-use of alcohol and opioids, and increased odds of engaging in concurrent hazardous drinking and prescription opioid misuse.
The current results extend previous findings of positive relations between pain-related anxiety and negative consequences of drinking, alcohol dependence symptoms, and current opioid misuse (LaRowe et al., 2018a; Rogers et al., 2018; Zale et al., 2019) to a large sample of adults with CLBP. Consistent with prior research and evidence that men are more likely to use alcohol for pain-coping and increase their pain medication dose without permission from a provider than women (e.g., LaRowe et al., 2018a; Manubay et al., 2015; Riley III and King, 2009; Riley et al., 2002; Zale et al., 2019), we found that relations between pain-related anxiety and alcohol/opioid use were generally more salient among males than females with CLBP. One potential explanation for this observed sex difference may be that males (vs. females) are more likely to cope with pain/anxiety using externalizing strategies (e.g., substance use; e.g., Altemus et al., 2014; Marmorstein, 2007).
Interestingly, sex did not moderate associations between pain-related anxiety and either of the alcohol-opioid co-use variables, and future studies should continue to examine/clarify the role of sex in concurrent alcohol and opioid consumption. Our observation that pain-related anxiety was not related to quantity or frequency of alcohol consumption is consistent with emerging evidence that pain-related anxiety may be more strongly related to the sequelae of alcohol use (e.g., harmful consequences of drinking and alcohol dependence), than levels of consumption, per se (e.g., Zale et al., 2019). In contrast, pain-related anxiety was incrementally associated with total daily opioid consumption, such that higher levels of pain-related anxiety were associated with being prescribed a greater number of MEUs each day.
Alcohol and opioids have both been shown to confer acute analgesia (Niesters et al., 2010; Thompson et al., 2017), and pain-related anxiety may increase the propensity to escape/avoid pain via substance use (LaRowe et al., 2018b). This process could result in the development of problematic patterns of alcohol and prescription opioid use. It is also possible that individuals with higher pain-related anxiety drink alcohol to extend/supplement the analgesia provided by prescription opioid medication. Over time, using alcohol and/or opioids to cope with pain can lead to poorer pain- and substance-related outcomes (e.g., Ditre et al., 2019), and may put individuals at increased risk for other negative health effects (e.g., liver damage; Weathermon and Crabb, 1999).
Several limitations and directions for future research should be noted. First, the cross-sectional nature of these analyses precludes causal interpretation. Prospective designs are needed to examine temporal pathways between pain-related anxiety and the onset/progression of problematic drinking patterns and prescription opioid misuse. Ecological momentary assessment paradigms (e.g., Dhingra et al., 2014) may further enhance understanding of microprocesses underlying associations between the experience of pain-related anxiety and alcohol-opioid consumption. Second, data were collected using online survey methodology, and most participants were White and female. Additional work is needed to generalize these results to larger and more diverse samples that are recruited using a variety of sampling methods (e.g., outpatient pain clinics). Third, the presence of CLBP, alcohol use, and prescription opioid use/misuse were all assessed exclusively via self-report. Future work should examine medical chart data to verify chronic pain status, and include biochemical verification of substance use (e.g., via urine drug screens; Markway and Baker, 2011). Finally, alcohol-opioid co-use was defined as endorsing any alcohol use and a current prescription for opioid analgesics. Future studies should examine whether pain-related anxiety is associated with same day and/or simultaneous (e.g., within 2 hours) alcohol-opioid use (Peacock et al., 2016; Saunders et al., 2012).
Despite these limitations, the current findings add to a growing literature suggesting that pain-related anxiety is a transdiagnostic factor underlying comorbid pain and alcohol/prescription opioid use and co-use (e.g., Ditre et al., 2019; LaRowe et al., 2018a; Rogers et al., 2018; Zale et al., 2019). Identification of transdiagnostic factors can aid in the development of novel treatment protocols, and transdiagnostic approaches to treating comorbid chronic pain and alcohol/prescription opioid use and co-use may offer numerous benefits relative to traditional, more isolated treatment approaches. For example, compared to diagnosis-specific treatment protocols, transdiagnostic treatments are more efficient at treating comorbid conditions (vs. sequentially treating each disorder), facilitate greater generalization of treatment effects across comorbid disorders, and are more cost-effective (e.g., McEvoy et al., 2009). Indeed, transdiagnostic treatments that target pain-related anxiety may simultaneously decrease pain, hazardous drinking, and prescription opioid misuse.
Treatments that incorporate psychoeducation, cognitive restructuring, and interoceptive exposure have been shown to decrease pain-related anxiety among persons with chronic pain (e.g., Watt et al., 2006; Wetherell et al., 2011), and it is possible that these treatments may also reduce hazardous drinking and prescription opioid misuse (particularly among males). In addition, efforts have been made to integrate/deliver treatments for comorbid alcohol and opioid use disorder in primary care settings (e.g., Hunter et al., 2018), and such interventions may offer promise for individuals with chronic pain and high pain-related anxiety. Tailored treatments for individuals with pain-related anxiety could also convey information about the risks of drinking and opioid misuse in the context CLBP, which may aid in the development of discrepancy (Miller and Rollnick, 2012) regarding continued substance use and stated goals for better managing pain. Additionally, coping skills training could emphasize the substitution of maladaptive pain/anxiety coping responses (e.g., alcohol consumption and opioid misuse) with more adaptive strategies (e.g., progressive muscle relaxation, distraction, mindfulness; Fernandez and Turk, 1989; Keefe, 1996; McCracken and Vowles, 2014).
In summary, results of this study suggest that pain-related anxiety may be an important transdiagnostic factor underlying alcohol-prescription opioid use and co-use among individuals with comorbid pain. Further work is needed to clarify the potential moderating role of biological sex in associations between pain-related anxiety and concurrent use/misuse of alcohol and opioids. This and future research has the potential to inform treatments for individuals at-risk for developing problematic patterns of alcohol and prescription opioid use, which may interfere with treatment of chronic pain (Egli et al., 2012; Witkiewitz and Vowles, 2018) and have dangerous and potentially lethal health effects (Weathermon and Crabb, 1999).
Highlights.
Pain-related anxiety was positively associated with alcohol and opioid use/misuse.
Pain-related anxiety was positively associated with co-use of alcohol and opioids.
Future work should continue to investigate sex differences in these associations.
Pain-related anxiety may be a transdiagnostic factor underlying alcohol-opioid use.
Role of Funding Source
This research was supported by NIH Grant No. R01AA024844 awarded to Joseph W. Ditre.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
References
- Altemus M, Sarvaiya N, Epperson CN, 2014. Sex differences in anxiety and depression clinical perspectives. Fron Neuroendocrin 35(3), 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Slater MA, Patterson TL, Grant I, Garfin SR, 1991. Prevalence, onset, and risk of psychiatric disorders in men with chronic low back pain: a controlled study. Pain 45(2), 111–121. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG, 1992. The alcohol use disorders identification test Guidelines for use in primary health care. Geneva: World Health Organization. [Google Scholar]
- Barth KS, Maria MMS, Lawson K, Shaftman S, Brady KT, Back SE, 2013. Pain and motives for use among non-treatment seeking individuals with prescription opioid dependence. Am J Addict 22(5), 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57(1), 289–300. [Google Scholar]
- Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, … Kivlahan DR 2003. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): Validation in a female Veterans Affairs patient population. Arc Intern Med 163(7), 821–829. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN, 2007. Development and validation of the current opioid misuse measure. Pain 130(1), 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-Y, Feng Z, Yi X, 2017. A general introduction to adjustment for multiple comparisons. J Thorac Dis 9(6), 1725–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra LK, Homel P, Grossman B, Chen J, Scharaga E, Calamita S, Shin J, Portenoy R, 2014. Ecological momentary assessment of smoking behavior in persistent pain patients. Clin J Pain 30(3), 205–213. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, LaRowe LR, 2019. A Reciprocal Model of Pain and Substance Use: Transdiagnostic Considerations, Clinical Implications, and Future Directions. Annu Rev Clin 15, 503–528 [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC Guidelines for Prescribing Opioids for Chronic Pain - United States, 2016, in: Control, N.C.f.I.P.a. (Ed.). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Doyle SR, Donovan DM, Kivlahan DR, 2007. The factor structure of the alcohol use disorders identification test (AUDIT). J Stud Alcohol Drugs 68(3), 474–479. [DOI] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S, 2012. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 36(10), 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Turk DC, 1989. The utility of cognitive coping strategies for altering pain perception: a meta-analysis. Pain 38(2), 123–135. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P, 2012. The economic costs of pain in the United States. J Pain 13(8), 715–724. [DOI] [PubMed] [Google Scholar]
- Goebel JR, Compton P, Zubkoff L, Lanto A, Asch SM, Sherbourne CD, Shugarman L, Lorenz KA, 2011. Prescription sharing, alcohol use, and street drug use to manage pain among veterans. J Pain Symptom Manag 41(5), 848–858. [DOI] [PubMed] [Google Scholar]
- Gore M, Sadosky A, Stacey BR, Tai K-S, Leslie D, 2012. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 37(11), E668–E677. [DOI] [PubMed] [Google Scholar]
- Hayes AF, 2012. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. University of Kansas, KS. [Google Scholar]
- IOM, 2011. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington, DC. [PubMed] [Google Scholar]
- Keefe FJ, 1996. Cognitive behavioral therapy for managing pain. Clin Psychol 49(3), 4–5. [Google Scholar]
- Kosiba JD, Mitzel LD, Zale EL, Zvolensky MJ, Ditre JW, 2020. A preliminary study of associations between discomfort intolerance, pain severity/interference, and frequency of cannabis use among individuals with chronic pain. Addict Res Theory 28(1), 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe LR, Chilcott LN, Zvolensky MJ, Vanable PA, Flood K, Ditre JW, 2018a. Associations between Pain-Related Anxiety, Gender, and Prescription Opioid Misuse among Tobacco Smokers Living with HIV/AIDS. Subst Use Misuse, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe LR, Langdon KJ, Zvolensky MJ, Zale EL, Ditre JW, 2017. Pain-Related Anxiety as a Predictor of Early Lapse and Relapse to Cigarette Smoking. Exp Clin Psychopharmacol 25(4), 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe LR, Powers JM, Paladino MB, Ditre JW, 2020. Pain Severity and Alcohol Use Among Daily Tobacco Cigarette Smokers. Am J Addict 29(2), 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe LR, Zvolensky MJ, Ditre JW, 2018b. The Role of Anxiety-Relevant Transdiagnostic Factors in Comorbid Chronic Pain and Tobacco Cigarette Smoking. Cognit Ther Res 43, 102–113. [Google Scholar]
- Larsen DK, Taylor S, Asmundson JG, 1997. Exploratory factor analysis of the Pain Anxiety Symptoms Scale in patients with chronic pain complaints. Pain 69(1–2), 27–34. [DOI] [PubMed] [Google Scholar]
- Manubay J, Davidson J, Vosburg S, Jones J, Comer S, Sullivan M, 2015. Sex differences among opioid-abusing chronic pain patients in a clinical trial. J Addict Med 9(1), 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markway EC, Baker SN, 2011. A review of the methods, interpretation, and limitations of the urine drug screen. Orthopedics 34(11), 877–881. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, 2007. Relationships between anxiety and externalizing disorders in youth: the influences of age and gender. J Anxiety Disord 21(3), 420–432. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Dhingra L, 2002. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag 7(1), 45–50. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Vowles KE, 2014. Acceptance and commitment therapy and mindfulness for chronic pain: model, process, and progress. Am Psychol 69(2), 178–187. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Zayfert C, Gross RT, 1992. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain 50(1), 67–73. [DOI] [PubMed] [Google Scholar]
- Meltzer EC, Rybin D, Saitz R, Samet JH, Schwartz SL, Butler SF, Liebschutz JM, 2011. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM). Pain 152(2), 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci RD, Fassa AG, Faria NMX, 2015. Prevalence of chronic low back pain: systematic review. Rev Saude Publ 49, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S, 2012. Motivational interviewing: Helping people change. Guilford press. [Google Scholar]
- Moskal D, Maisto SA, De Vita M, Ditre JW, 2018. Effects of experimental pain induction on alcohol urge, intention to consume alcohol, and alcohol demand. Exp Clin Psychopharmacol 26(1), 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L, Sarton E, 2010. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain 151(1), 61–68. [DOI] [PubMed] [Google Scholar]
- Norman SB, Hami Cissell S, Means-Christensen AJ, Stein MB, 2006. Development and validation of an overall anxiety severity and impairment scale (OASIS). Depress Anxiety 23(4), 245–249. [DOI] [PubMed] [Google Scholar]
- Peacock A, Bruno R, Larance B, Lintzeris N, Nielsen S, Ali R, Dobbins T, Degenhardt L, 2016. Same-day use of opioids and other central nervous system depressants amongst people who tamper with pharmaceutical opioids: A retrospective 7-day diary study. Drug Alcohol Depend 166, 125–133. [DOI] [PubMed] [Google Scholar]
- Powers J, Heckman B, LaRowe L, Ditre J, 2019. Smokers with pain are more likely to report use of e-cigarettes and other nicotine products. Exp Clin Psychopharmacol, 10.1037/pha0000335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley III JL, King C, 2009. Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain 10(9), 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, Gilbert GH, Heft MW, 2002. Orofacial pain: racial and sex differences among older adults. J Public Health Dent 62(3), 132–139. [DOI] [PubMed] [Google Scholar]
- Rogers AH, Bakhshaie J, Lam H, Langdon KJ, Ditre JW, Zvolensky MJ, 2018. Pain-related anxiety and opioid misuse in a racially/ethnically diverse young adult sample with moderate/severe pain. Cogn Behav Ther 47(5), 372–382. [DOI] [PubMed] [Google Scholar]
- Rogers AH, Gallagher MW, Garey L, Ditre JW, Williams MW, Zvolensky MJ, in press-a. Pain Anxiety Symptoms Scale-20: An empirical evaluation of measurement invariance across race/ethnicity and sex. Psychol Assess. [DOI] [PubMed] [Google Scholar]
- Rogers AH, Gallagher MW, Jamison RN, Zvolensky MJ, in press-b. Exploring the psychometric properties of the Current Opioid Misuse Measure among adults with chronic pain and opioid use. Clin J Pain. [DOI] [PubMed] [Google Scholar]
- Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, 2016. Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the United States. J Clin Psychiat 77(6), 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M, 1993. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Saunders KW, Von Korff M, Campbell CI, Banta-Green CJ, Sullivan MD, Merrill JO, Weisner C, 2012. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J Pain 13(3), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, Portenoy RK, 2008. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Med 9(7), 911–917. [DOI] [PubMed] [Google Scholar]
- Shmagel A, Foley R, Ibrahim H, 2016. Epidemiology of Chronic Low Back Pain in US Adults: Data From the 2009–2010 National Health and Nutrition Examination Survey. Arthrit Care Res 68(11), 1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM, 2009. Assessing analgesic actions of opioids by experimental pain models in healthy volunteers–an updated review. Br J Clin Pharmacol 68(2), 149–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T, Oram C, Correll CU, Tsermentseli S, Stubbs B, 2017. Analgesic effects of alcohol: a systematic review and meta-analysis of controlled experimental studies in healthy participants. J Pain 18(5), 499–510. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R, 2005. Chronic spinal pain and physical–mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 113(3), 331–339. [DOI] [PubMed] [Google Scholar]
- Walters K, Christakis DA, Wright DR, 2018. Are Mechanical Turk worker samples representative of health status and health behaviors in the US? PloS one 13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD, 1996. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34(3), 220–233. [DOI] [PubMed] [Google Scholar]
- Watt MC, Stewart SH, Lefaivre MJ, Uman LS, 2006. A brief cognitive-behavioral approach to reducing anxiety sensitivity decreases pain-related anxiety. Cogn Behav Ther 35(4), 248–256. [DOI] [PubMed] [Google Scholar]
- Weathermon R, Crabb DW, 1999. Alcohol and medication interactions. Alcohol Res Health 23(1), 40–54. [PMC free article] [PubMed] [Google Scholar]
- Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, Solomon BC, Lehman DH, Liu L, Lang AJ, 2011. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain 152(9), 2098–2107. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, 2018. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: A critical review. Alcohol Clin Exp Res 42(3), 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, LaRowe LR, Boissoneault J, Maisto SA, Ditre JW, 2019. Gender differences in associations between pain-related anxiety and alcohol use among adults with chronic pain. Am J Drug Alcohol Abuse 45(5), 479–487. [DOI] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, Ditre JW, 2015. Interrelations between pain and alcohol: an integrative review. Clin Psychol Rev 37, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


