Abstract
Functional neurological (conversion) disorder (FND) is a neuropsychiatric condition whereby individuals present with sensorimotor symptoms incompatible with other neurological disorders. Early-life maltreatment (ELM) is a risk factor for developing FND, yet few studies have investigated brain network-trauma relationships in this population. In this neuroimaging–gene expression study, we used two graph theory approaches to elucidate ELM subtype effects on resting-state functional connectivity architecture in 30 patients with motor FND. 21 individuals with comparable depression, anxiety and ELM scores were used as psychiatric controls. Thereafter, we compared trauma endophenotypes in FND with regional-differences in transcriptional gene expression as measured by the Allen Human Brain Atlas (AHBA). In FND patients only, we found that early-life physical abuse severity, and to a lesser extent physical neglect, correlated with corticolimbic weighted-degree functional connectivity. Connectivity profiles influenced by physical abuse occurred in limbic (amygdalar-hippocampal), paralimbic (cingulo-insular and ventromedial prefrontal) and cognitive control (ventrolateral prefrontal) areas, as well as in sensorimotor and visual cortices. These findings held adjusting for individual-differences in depression/anxiety, PTSD and motor phenotypes. In FND, physical abuse also correlated with amygdala and insula coupling to motor cortices. In exploratory analyses, physical abuse correlated connectivity maps overlapped with the AHBA spatial expression of 3 gene-clusters: i) neuronal morphogenesis and synaptic transmission genes in limbic/paralimbic areas; ii) locomotory behavior and neuronal generation genes in left-lateralized structures; and iii) nervous system development and cell motility genes in right-lateralized structures. These circuit-specific architectural profiles related to individual differences in childhood physical abuse burden advance our understanding of the pathophysiology of FND.
Keywords: conversion disorder, psychogenic, childhood abuse, functional movement disorder, fMRI
Introduction
Functional neurological (conversion) disorder (FND) is a prevalent and disabling condition at the intersection of psychiatry and neurology whereby individuals exhibit sensorimotor symptoms incompatible with other neurological disorders1. While Briquet, Charcot, Freud, Janet and other notable clinicians studied FND, or hysteria as it was then known, this condition has until recently been neglected by researchers2. Adverse life events played a central role in early etiological theories for FND, and evidence supports a high incidence of early-life maltreatment (ELM) in this population3, 4. In early conceptualizations, FND was also closely intertwined with shellshock and the war neuroses5. While the need for an antecedent stressor was removed from the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) criteria for FND6, modern-day conceptual models continue to posit that ELM is an important predisposing vulnerability within a stress-diathesis framework7, 8. Neuroimaging approaches now offer the opportunity to clarify the biological importance of ELM in FND by examining its relationship to brain network architecture.
Interestingly, several brain areas implicated in the pathophysiology of FND also exhibit neuroplastic changes in the context of ELM in non-clinical populations9. Emerging neurobiological themes identified in the FND literature using functional magnetic resonance imaging (fMRI) include: 1) amygdalar hyperreactivity to affectively valenced stimuli10–14; 2) increased amygdalar and cingulo-insular connectivity to motor pathways10, 12, 15–19; 3) altered motor/premotor activity during motor task performance20–22; and 4) hypoactivation and abnormal connectivity of the right temporoparietal junction23–25. In non-clinical populations with ELM, large sample studies and meta-analyses have characterized biased amygdalar responses to emotionally-valenced stimuli26, 27, as well as altered amygdalar connectivity to motor control, paralimbic and limbic areas28–32. Resting-state fMRI studies in traumatized populations show that the prefrontal cortex, insula, cingulate, precuneus, temporal pole and striatum contributions to network architecture is altered in maltreated individuals33. Furthermore, ELM subtypes may have specific biological consequences9. In FND, one study showed that patients who experienced childhood emotional abuse exhibited increased right TPJ - left insula functional connectivity25. The magnitude of early-life physical and sexual abuse has been linked to functional neurological symptom severity, supporting the relevance of ELM subtypes in FND34, 35.
Graph-theory neuroimaging approaches enable the in vivo characterization of brain networks36. Weighted-degree fMRI provides a measure of centrality, quantifying the overall influence of discrete nodes in the network architecture of the brain. Complementary link-level analyses characterize connectivity strength relationships across brain areas. Excitingly, recently constructed brain-wide gene expression atlases such as the Allen Human Brain Atlas allow for the examination of relationships between distinct neuroimaging endophenotypes and the spatial gene expression patterns in the brain37, 38. Our laboratory and others have combined fMRI and gene expression data to characterize novel insights, such as the role of the cAMP Response Element Binding Protein guiding neuroplasticity in blind individuals39 and molecular mechanisms underlying individual differences in network connectivity40. Of relevance to FND and trauma-related disorders, several candidate genes have been implicated through human and animal studies as possibly moderating the effects of ELM on later-life psychopathology, including Brain Derived Neurotropic Factor (BDNF), FK506-binding protein 51 (FKBP5), and corticotropin-releasing hormone receptor type I (CRHR1) among others7, 41. Notably, these genes are implicated in neuroplasticity and stress response systems. To date, little is known regarding relationships between ELM endophenotypes and regional differences in the gene expression profiles across the human brain.
In this study, we investigated neuroimaging endophenotypes linked to ELM subtypes in 30 patients with FND using two graph-theory network approaches (weighted-degree and link-level resting-state functional connectivity analyses). 21 individuals with comparable depression, anxiety and childhood trauma scores were used as psychiatric controls to aid the interpretation of brain-trauma relationships in the FND cohort. In exploratory analyses, we also characterized the association between network consequences of ELM in patients with FND and regional-differences in gene expression as measured by the Allen Human Brain Atlas to identify candidate molecules that may be important in promoting the development of FND. Given the role of the salience network in the pathophysiology of FND and the literature linking trauma subtypes to symptom severity8, 34, 35, 42, we hypothesized that physical and sexual abuse burden in patients with FND would correlate with amygdalar and cingulo-insular functional reorganization and enhanced motor-limbic/paralimbic connectivity. We also hypothesized that the spatial expression of genes implicated in moderating the effects of ELM on later-life psychopathology would overlap with brain areas exhibiting childhood adversity correlated architectural reorganization in FND patients.
Methods
FND cohort and fMRI preprocessing are as previously described19.
Participants and questionnaires
All participants signed informed consent and the Partners Human Research Committee approved this study. Thirty individuals with motor FND (24 women, 6 men; mean age=40.1±12.9; average illness duration=3.0±3.8 years) were recruited from the Massachusetts General Hospital FND Clinic following a “rule-in” FND diagnosis consistent with DSM-5 criteria6. Given the overlap across the motor FND spectrum43, we used a transdiagnostic approach that included clinically-established functional movement disorders (n=16), functional weakness (n=12), and documented (n=12) or clinically-established (n=1) psychogenic nonepileptic seizures (PNES). Ten of the 30 subjects had mixed motor FND. Exclusion criteria included major neurological comorbidities with MRI abnormalities, epilepsy, poorly controlled medical problems with known central nervous system consequences, active substance dependence, a history of mania or psychosis, and/or active suicidality. Comorbid psychiatric diagnoses as assessed using the Structured Clinical Interview (SCID-I) for DSM-IV-TR were present in 27 of 30 participants. Fourteen patients were on selective serotonin reuptake inhibitors (SSRIs) and/or serotonin-norepinephrine reuptake inhibitors (SNRIs). As a psychiatric control group, 21 patients with clinical depression histories (17 women, 4 men; mean age=39.7±14.3; lifetime depression diagnoses based on SCID-I DSM-IV-TR included: n=15, major depression; n=4, depression not-otherwise-specified; n=1, minor depressive episode; and n=1, bipolar disorder II with depression predominant episodes) were recruited through local advertisements. All psychiatric controls reported no history of major neurological co-morbidities (including FND) nor poorly controlled medical problems with known central nervous system consequences. See Supplementary Table 1 and Supplementary Table 2 for additional clinical information for all participants.
As the ELM measure-of-interest, all participants completed the 28-item Childhood Trauma Questionnaire (CTQ)44. The CTQ is a self-report scale of childhood/adolescent maltreatment that can be subdivided into five trauma subtypes: physical, sexual, emotional abuse and physical and emotional neglect; subscale scores range from 5 (no trauma) to 25. Participants also completed the Beck Depression Inventory-II, Spielberger State-Trait Anxiety Inventory (STAI) and the Post-Traumatic Stress Disorder Checklist for DSM-5 (PCL-5)45.
MRI data acquisition and preprocessing
See Supplementary Methods for acquisition and preprocessing procedures including scrubbing head motion correction.
Weighted-degree functional connectivity analysis
To evaluate the importance of each voxel in the overall functional brain architecture of each individual, voxel-level weighted-degree values were computed. First, Pearson correlation coefficients were used to compute the functional connectivity matrices of each subject using the time series of all pairs of cortical-subcortical gray matter voxels. A Fisher transformation was applied to the resulting correlation matrix and negative values were removed due to their controversial interpretation46. To reduce noise, we considered only the most significant links using a false discovery rate (FDR) multiple comparison correction at q-level=0.0001. After obtaining a high-resolution 5,142×5,142 connectivity matrix for each subject, we summed all the connections of each voxel to generate a weighted-degree map showing the extent to which each voxel is functionally connected to the rest of the brain.
In patients with FND, within-group general linear models correlated weighted-degree maps with the 5 CTQ subscales, adjusting for age, gender and SSRI/SNRI use (yes/no). To ensure that results were not overly influenced by single-subject values, a leave-one-out approach was used in which voxel-wise outliers were identified using the 3 interquartile distance from the median value. If an outlier was present, we updated the t-statistic for a given voxel with the value obtained by performing the same analysis removing the outlier subject. Whole-brain correction for multiple comparisons was computed using Monte Carlo simulation with 10,000 iterations to estimate the probability of false positive clusters with a two-tailed p-value<0.05 (3dClustSim, afni.nimh.nih.gov). Post-hoc analyses evaluated if statistically significant within-group findings held adjusting for baseline: 1) mood and anxiety symptoms (BDI-II and STAI-total scores); 2) PCL-5 total scores; and 3) motor FND subtypes (PNES, functional movement disorders and functional weakness). Additionally, for analyses that identified a statistically significant association between CTQ subscale scores and weighted-degree connectivity maps in patients with FND (i.e. for physical abuse and physical neglect, see results), we evaluated the same relationships in psychiatric controls to help determine the potential specificity of these findings.
Link-level functional connectivity strength analysis
For CTQ subtypes that influenced the overall functional brain architecture in the FND cohort as measured by weighted-degree, we subsequently evaluated if these trauma subtypes correlated with link-level connectivity strength values across brain areas. To evaluate link-level functional connectivity strength, we used the previously computed FDR-corrected correlation matrices.
Within-group general linear models in patients with FND correlated link-level functional connectivity strength with either CTQ-physical abuse or physical neglect scores adjusting for age, gender and SSRI/SNRI use. Again, a leave-one-out approach was used to ensure that results were not overly influenced by single-subject values. Whole-brain correction for multiple comparisons was computed adapting the Monte Carlo simulation method to networks. 10,000 random networks were generated with the same smoothing properties, to compute a false positive cluster size with a two-tailed p-value<0.0005. Compared to weighted-degree maps where clusters were defined as contiguous voxels, here clusters were defined as links that connect contiguous voxel groups. Afterwards, we reduced the dimensionality of the surviving links for visualization purposes. The statistically significant links in the 5,142×5,142 matrix were projected onto a connectogram using NeuroMArVL. We specifically tested the hypothesis that ELM subtypes would positively correlate with connectivity strength across motor control and salience networks (insula, anterior cingulate cortex (ACC), amygdala)42. Post-hoc analyses evaluated if statistically significant findings held adjusting for baseline: 1) BDI-II and STAI-total scores; 2) PCL-5 total scores; and 3) motor FND subtypes. Additionally, the same link-level analyses were performed in psychiatric controls to evaluate the specificity of statistically-significant CTQ subtype findings in patients with FND.
Relationship between the spatial distribution of gene expression and trauma-subtype weighted-degree maps
To investigate spatial similarities between regional gene expression profiles and brain areas showing functional reorganization in the context of ELM in patients with FND, we used the statistically significant physical abuse correlated weighted-degree t-statistic maps and microarray gene expression data from the Allen Human Brain Atlas47. (Note: given that weighted-degree results were similar for both physical abuse and physical (though less robust for physical neglect, see results), this exploratory analysis only used the physical abuse weighed-degree maps to limit multiple comparisons). The Allen Human Brain Atlas is the only publicly available database that provides whole-brain, high-resolution genome-wide expression values for six human subjects, quantifying more than 20,000 genes in 3,702 samples spatially distributed throughout the brain47. This database includes MRI images and the coordinates where samples were extracted.
Consistent with recommendations38, brain maps representing the spatial distribution of each gene were created by using the data of the six donors and performing the following steps: i) for each gene, expression values from multiple probes were averaged; ii) each sample was associated with an anatomical label using the 68 cortical regions defined by the Desikan atlas48, 16 subcortical regions of the FreeSurfer segmentation and the 7T probabilistic map of the periaqueductal gray49; iii) for each subject, we computed the median of gene expressions of all the samples within the same region; iv) thereafter, we computed the median gene expression value of each brain area between the six donors with data resampled to follow a gaussian distribution. Pearson correlation assessed the spatial similarity value of each gene with the weighted-degree maps related to the magnitude of previously experienced physical abuse. To compute this similarity value, we used the t-statistic map of the association between weighted-degree and physical abuse scores projected to the 85 regions described above and correlated this with the expression of genes available in Allen Human Brain Atlas. The spatial similarity computation identified those genes where the spatial distribution of their expression closely related to the influence of physical abuse on weighted-degree functional connectivity maps.
As a data-driven strategy, we identified genes with a spatial similarity value higher than 2 standard deviations of the spatial similarity distribution, consistent with published methods39. We subsequently performed a K-means clustering algorithm to group genes with similar spatial distributions in the upper bound of the tail of the distribution. Silhouette identified the optimal number of groups, and a Gene Ontology overrepresentation test evaluated the biological processes associated with the genes located in the upper bound of the tail50. We used PANTHER13.1 software and Fisher’s exact test with Bonferroni correction to perform the statistical testing (p-value<0.05).
Additionally, as a hypothesis-driven strategy, we identified 5 a priori candidate genes from the literature shown to be implicated in risk for developing psychopathology following ELM exposure (See Supplementary Methods): BDNF (Brain Derived Neurotropic Factor), COMT (catechol-O-methyltransferase), CRHR1 (corticotropin-releasing hormone receptor type I), FKBP5 (FK506-binding protein 51), and NR3C1 (Nuclear Receptor Subfamily 3 Group C Member 1 (glucocorticoid receptor gene)). We evaluated if any of these candidates fell in the upper tail of the distribution of spatial similarity values. For candidates located in the upper tail, we tested that the result was not due to chance by generating 10,000 random maps with the same smoothing as our weighted-degree maps and computing the corrected p-value for how likely it was to obtain a comparable similarity value as obtained by chance.
Code Availability
For qualified researchers, the code for the weighted-degree and link-level neuroimaging analyses will be provided upon request.
Results
Questionnaire scores
For patients with FND, CTQ subtype scores were: physical abuse (mean=7.6±3.7; range=5-19); physical neglect (mean=7.6±3.3; range=5-15); sexual abuse (mean=7.5±5.9; range=5-25); emotional abuse (mean=12.2±5.8; range=5-24); and emotional neglect (mean=11.5±5.4; range=5-23). For correlations between trauma subtypes, see Supplementary Table 3. The FND and psychiatric control cohorts showed comparable depression, anxiety and CTQ subtype scores (see Supplementary Table 4).
Weighted-degree findings
Individual differences in CTQ-physical abuse scores in patients with FND positively correlated with increased weighted-degree functional connectivity in the bilateral amygdala, hippocampi, parahippocampi, perigenual ACC, insula, putamen, posterior thalami, ventromedial prefrontal cortices, dorsomedial and lateral prefrontal cortices, dorsal and ventral visual association areas, lateral temporal areas, temporal poles, primary sensorimotor cortices, and supplementary motor/premotor areas (Fig. 1). These findings held adjusting separately for BDI-II/STAI-total scores, PTSD symptom severity (PCL-5), and motor subtypes, except that correlations between physical abuse scores and left lateral prefrontal and premotor weighted-degree functional connectivity did not remain significant adjusting for PNES subtype (see Supplementary Fig. 1–3). Psychiatric controls did not show statistically significant, whole-brain corrected weighted-degree network architectural profiles correlated to physical abuse scores. See Supplementary Fig. 4 for uncorrected weighted-degree findings in the psychiatric controls.
Fig. 1. Physical abuse and physical neglect burden influence the corticolimbic architecture in patients with functional neurological disorder (FND).
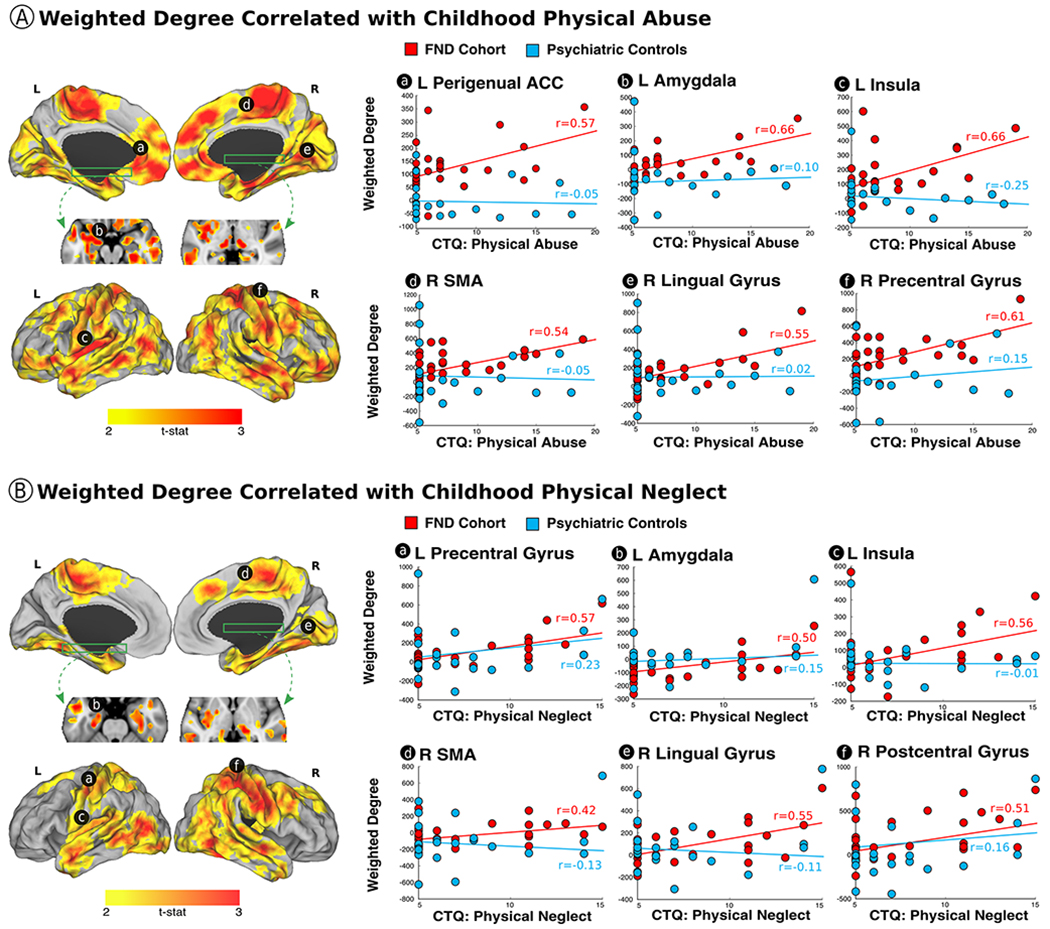
Panel A shows the correlation between the magnitude of previously experienced early-life physical abuse, as measured by the childhood trauma questionnaire (CTQ), and weighted-degree functional connectivity profiles in patients with FND. Note, for comparison descriptive scatterplots are provided for both the FND cohort and psychiatric controls, however, there were no statistically significant, whole-brain corrected physical abuse related weighted-degree correlations in the psychiatric control cohort. Panel B shows the correlation between early-life physical neglect and weighted-degree functional connectivity profiles in patients with FND. Again, for comparison descriptive scatterplots are provided for both the FND cohort and psychiatric controls, however, there were no statistically significant, whole-brain corrected physical neglect related weighted-degree correlations in psychiatric controls. All findings are whole-brain corrected for multiple comparisons, and adjusted for age, gender and antidepressant use. All scatterplots and partial correlations (r-values) reflect data using the leave-one-out approach. L indicates left; R, right; ACC, anterior cingulate cortex; SMA, supplementary motor area.
Correlations between individual differences in CTQ-physical neglect scores in patients with FND and weighted-degree functional connectivity showed similar findings to CTQ-physical abuse, except for the absence of statistically significant findings in the bilateral ventromedial prefrontal cortex, perigenual ACC, inferior frontal gyrus, left premotor and medial occipital cortices. Findings held adjusting separately for BDI-II/STAI-total scores, PCL-5 scores, and motor FND subtypes, except that correlations between physical neglect scores and right dorsomedial prefrontal weighted-degree functional connectivity did not hold adjusting for PTSD symptom severity, PNES or functional weakness. Psychiatric controls did not show statistically significant, whole-brain corrected weighted-degree network architectural profiles correlated to physical neglect scores. See Supplementary Fig. 4 for uncorrected findings.
There were no statistically significant correlations between weighted-degree functional connectivity and sexual abuse, emotional abuse or emotional neglect scores in patients with FND.
Connectivity strength findings
In within-group analyses, left amygdala - bilateral precentral gyri and left insula – bilateral precentral gyri functional connectivity strength each positively correlated with the magnitude of reported physical abuse in patients with FND (Fig. 2). These findings held adjusting separately for BDI-II/STAI-total scores, PCL-5 scores and motor subtypes. ACC – motor cortex connectivity strength did not correlate with physical abuse scores. Furthermore, in psychiatric controls there were no statistically significant relationships between physical abuse scores and link-level functional connectivity profiles correcting for multiple comparisons. Additionally, the above connectivity strength relationships were not found for physical neglect in patients with FND. See Supplementary Fig. 5 and Supplementary Fig. 6 for a complete description of statistically significant physical abuse and physical neglect correlations with link-level connectivity strength values across FND and psychiatric control cohorts.
Fig. 2. The magnitude of early-life physical abuse correlates with amygdala – precentral gyrus and insula – precentral gyrus functional connectivity strength in patients with functional neurological disorder.
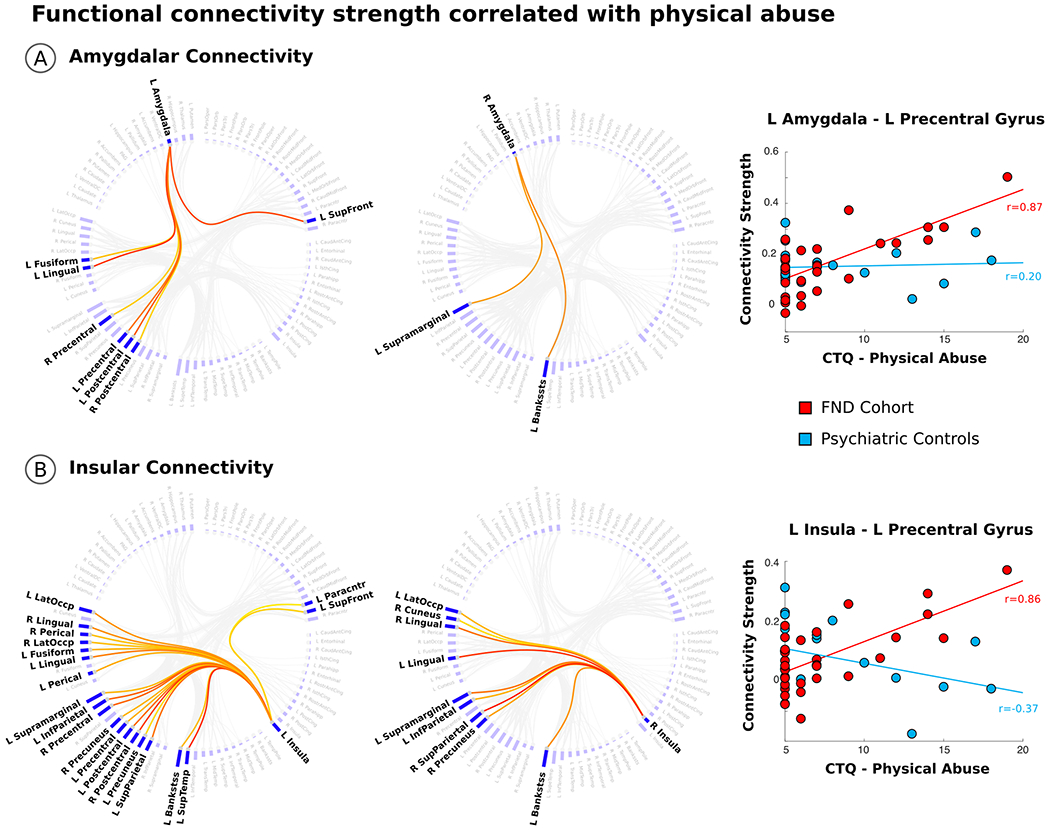
Panel A shows the connectogram of the connectivity strength relationships between the bilateral amygdala and other brain areas that positively correlated with the magnitude of early-life physical abuse, as measured by the childhood trauma questionnaire (CTQ). Consistent with a priori hypotheses, CTQ-physical abuse scores positively correlated with left amygdala – precentral gyrus functional connectivity strength. Note, in psychiatric controls there was no statistically significant relationship between CTQ-physical abuse scores and amygdala - precentral gyrus connectivity strength values. Panel B shows the connectogram of the connectivity strength relationships between the bilateral insula and other brain areas correlated with the magnitude of physical abuse. CTQ-physical abuse scores positively correlated with left insula – precentral gyrus functional connectivity strength. In psychiatric controls there was no statistically significant relationship between CTQ-physical abuse scores and insula - precentral gyrus connectivity strength values. All findings are whole-brain corrected for multiple comparisons, and adjusted for age, gender and antidepressant use. All scatterplots and partial correlations (r-values) reflect data using the leave-one-out approach. Bankstss indicates banks of the Superior Temporal Sulcus; InfParietal, Inferior Parietal; LatOccp, Lateral Occipital; Paracntr, Paracentral; Perical, Pericalcarine; SupFront, Superior Frontal; SupParietal, Superior Parietal; SupeTemp, Superior Temporal.
Exploratory brain organization-gene expression relationships
In the data-driven approach, genes in the upper tail of the spatial similarity values that related to the physical abuse weighted-degree map organized into 3 distinct gene clusters based on the spatial distribution of expression profiles: cluster 1 – bilateral limbic/paralimbic areas; cluster 2 – left hemisphere lateralized cortico-subcortical structures; cluster 3 - right hemisphere lateralized cortico-subcortical structures (see Fig. 3). Using Gene Ontology annotation analysis based on Biological Processes, cluster 1 included genes that were significantly over-represented in neuronal morphogenesis and synaptic transmission; cluster 2 included genes linked to locomotory behavior and neuronal generation; and cluster 3 implicated nervous system development and cell motility genes (see Supplementary Fig. 7).
Fig. 3. Clustering of gene expression profiles associated with physical abuse correlated weighted-degree functional connectivity maps in patients with functional neurological disorder.
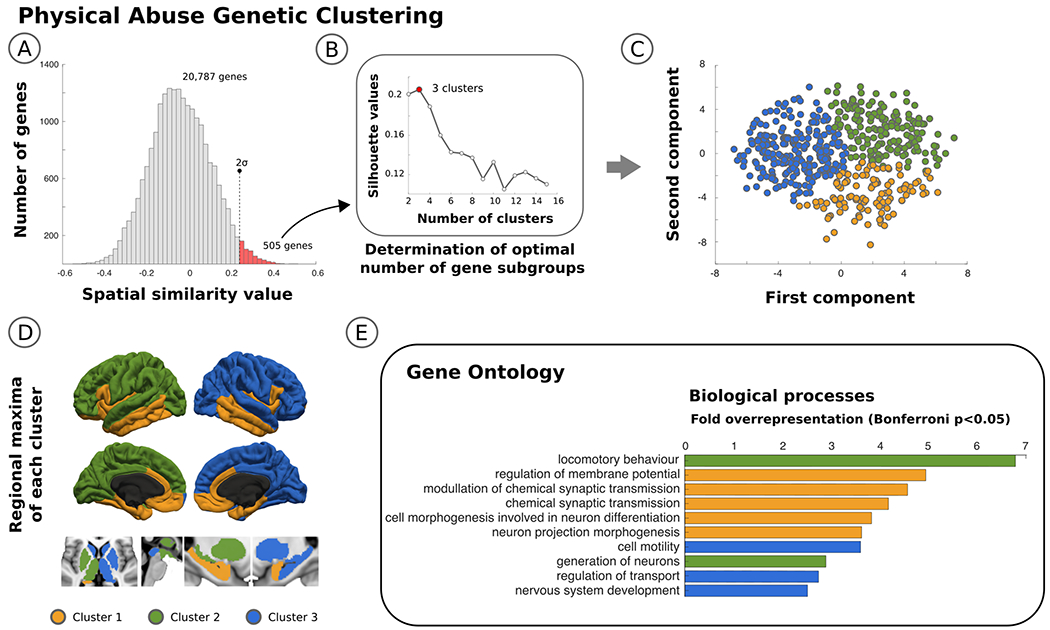
Panel A identifies in the histogram the 505 genes located in the upper tail, representing the subset of regional gene expression profiles that are most similar to the brain areas impacted by physical abuse. Panel B shows the optimal number of gene subgroups computed using silhouette values, and Panel C shows a scatterplot with each of the 505 genes color coded based on the group it belongs to using a principle components analysis. In Panel D, the surface and volume projections are displayed labeling each brain area as part of one of 3 clusters based on peak maxima gene expression profiles. The 3 clusters include: i) bilateral limbic/paralimbic areas; ii) left hemisphere lateralized cortico-subcortical structures; iii) right hemisphere lateralized cortico-subcortical structures. Finally, shown in Panel E are the gene ontology biological process terms organized by cluster using a Bonferroni correction α<0.05.
Thereafter, evaluating spatial similarities between the physical abuse related weighted-degree map in patients with FND and the spatial distribution of 5 a priori hypothesized genes, the topological distribution of cortico-subcortical reorganization positively correlated with regional BDNF gene expression profiles (see Fig. 4). Of note, the BDNF gene was present in cluster 1 as detailed above. The distributions of the other four candidate genes did not correlate with the physical abuse weighted-degree maps.
Fig. 4. Spatial similarity between physical abuse correlated weighted-degree functional connectivity maps in patients with functional neurological disorder (FND) and regional Brain Derived Neurotrophic Factor (BDNF) expression.
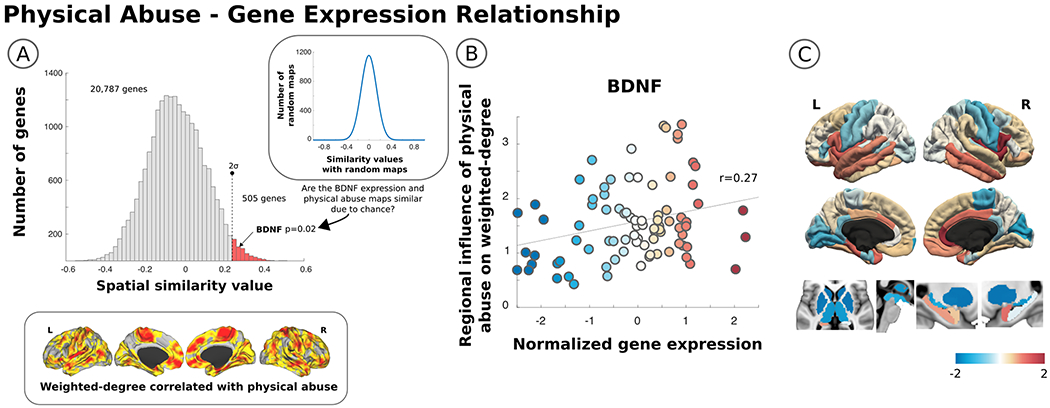
The histogram in Panel A shows the similarity of the physical abuse imaging endophenotype in FND patients (copied below the histogram) with the regional expression of 20,787 genes from the Allen Human Brain Atlas. The red bars in the upper tail of the distribution identify genes with a spatial similarity value above 2 standard deviations from the whole distribution. Regional differences in the expression of BDNF overlapped with brain areas showing functional architectural changes related to the magnitude of previously experienced physical abuse. In Panel B, a scatterplot of the correlation between normalized BDNF expression values and regional differences in the influence of physical abuse on weighted-degree functional connectivity is shown. Also displayed in Panel C are cortical and subcortical images demonstrating the regional differences in BDNF gene expression. Notably, BDNF expression is high in the amygdala, hippocampus, insula, cingulate gyrus, and ventromedial prefrontal cortex, which are the some of the same brain areas exhibiting functional connectivity profiles correlated with physical abuse burden.
Discussion
In this study, the severity of early-life physical abuse, and to a lesser extent the degree of physical neglect, correlated with corticolimbic weighted-degree functional connectivity in patients with FND; the magnitude of reported sexual abuse, emotional abuse and emotional neglect did not relate to individual differences in network architecture profiles. Functional connectivity profiles influenced by physical abuse in patients with FND were found in limbic (amygdalar-hippocampal), paralimbic (cingulo-insular and ventromedial prefrontal) and cognitive control (ventrolateral prefrontal) areas, as well as in sensorimotor and visual cortices. These findings held adjusting for depression/anxiety, PTSD severity and motor phenotypes. Connectivity strength analyses showed that physical abuse severity positively correlated with amygdala and insula coupling to motor cortices. The above identified brain network-trauma subtype relationships were not robustly observed in psychiatric controls with comparable CTQ scores. In exploratory analyses, Allen Human Brain Atlas data identified that the spatial expression of 3 gene-clusters overlapped with the physical abuse correlated weighted-degree maps in patients with FND: i) neuronal morphogenesis and synaptic transmission genes in limbic/paralimbic areas; ii) locomotory behavior and neuronal generation genes in left-lateralized structures; and iii) nervous system development and cell motility genes in right-lateralized structures. Regarding a priori hypothesized genes, regional BDNF expression differences positively correlated with brain areas exhibiting neuroplastic changes linked to physical abuse burden.
Mechanistically, these findings suggest that individual differences in reported early-life physical abuse impact limbic-paralimbic and sensorimotor topology in patients with FND. Consistent with a priori hypotheses, physical abuse severity correlated with motor cortex – limbic/paralimbic (amygdala, insula) functional connectivity strength, suggesting that physical abuse may predispose the central nervous system in some individuals for the development of functional motor symptoms. These brain-trauma relationships advance our understanding of FND and contextualize neuroimaging studies delineating heighted amygdalar connectivity to motor pathways in patients with FND10, 12–14, 17. Voon, Hallett and colleagues suggested that increased amygdala-motor connectivity represented a marker of heightened limbic influence over behavior12, which parallels observations that arousal and emotion can amplify functional symptoms. These amygdalar alterations are also consistent with FND patients displaying a negative attentional bias, hyperarousal and altered stress responses (neuroendocrine and autonomic profiles) linked to ELM8, 51, 52.
Given the lack of direct anatomical connections between the amygdala and primary motor cortices, increased insula weighted-degree functional connectivity and insula – precentral gyrus connectivity strength correlated to physical abuse severity identifies another important mechanistic pathway53. We have previously identified in this cohort that insula-to-primary motor cortex functional connectivity related to symptom severity19, a finding also reported by others18. Resting-state and task-based insular alterations have also been identified across the spectrum of motor FND15, 20, 21, 25, 54. Given that the insula is at the intersection of interoception, multisensory/multimodal integration, salience, and self/emotional awareness55, 56, and impaired interoceptive accuracy has been reported in FND57, this study further supports that the insula is an important node in the neurobiology of FND.
In exploratory analyses using the Allen Human Brain Atlas, brain areas demonstrating physical abuse related functional connectivity changes in patients with FND overlapped with limbic-paralimbic brain areas (cluster 1) that highly expressed genes implicated in neuronal morphogenesis. Within this cluster, BDNF transcript levels were among those genes most highly expressed (See Supplementary Fig. 7). BDNF is important for neuronal development, neurogenesis and memory functions (including extinction and safety learning), and has neuroprotective effects in the setting of adversity58, 59. Two pilot studies in patients with FND showed decreased blood BDNF levels compared to healthy controls60, 61. Importantly, ELM has been linked to changes in BDNF expression levels in several psychiatric populations62. Amongst its functions, BDNF is thought to be important in connecting ELM and later-life psychopathology (through gene x environment interactions). For example, the BDNF Val66Met single nucleotide polymorphism, which influences activity-dependent release of BDNF, intensified the relationship between ELM and development of psychiatric disorders58, 63. Individuals with the Val66Met polymorphism also show amygdalar and cingulo-insular overactivation during fear processing64, 65, impaired amygdalar habituation66 and increased skin conductance responses to threat67. These findings link emotion regulation circuit vulnerabilities to BDNF expression. In convergent data from animal models, ELM alters BDNF expression in prefrontal, amygdalar and hippocampal regions68. We speculate that BDNF may play a role in mediating trauma-related neuroplasticity in FND, and future studies should investigate the presence and impact of the BDNF Val66Met polymorphism in patients with FND.
In addition to BDNF, genes implicated in neural development and locomotory behavior that are highly expressed in sensorimotor regions and the amygdala also correlated with the weighted-degree functional connectivity maps linked to physical abuse burden in patients with FND. While genes associated with locomotory behavior were not part of a priori hypotheses, future work should follow-up on this observation. For example, synaptosomal-associated protein 25 (SNAP-25), a presynaptic plasma membrane protein, has been linked to physical activity phenotypes69. These findings underscore that while genes linking ELM to the later-life development of psychopathology may be important in the pathophysiology of FND, other genes not traditionally considered in trauma-related disorders may also be relevant.
There is debate on how to conceptualize FND, including whether to frame this disorder as trauma-related. For over a century some have supported the etiological importance of ELM, yet not all individuals with FND report early-life abuse or neglect3. The removal of the need for an antecedent stressor from the DSM-5 diagnostic criteria further challenged the importance of adverse life events in the assessment of this population. Our laboratory has demonstrated the utility of characterizing individual differences in FND70, and we contend that for those with severe trauma histories, physical abuse associated corticolimbic architectural changes highlight the need to consider if a subset of individuals with FND have a delayed post-traumatic disorder4. Similar to the inherent variability in developing PTSD following traumatic experiences based on a multiplicity of environmental, genetic and epigenetic resilience and vulnerability factors, we propose that a subset of individuals with FND can be conceptualized using a trauma-related stress-diathesis model7, 8. Additionally, given that overlapping brain-trauma subtype relationships were not identified in our psychiatric controls (particularly within sensorimotor areas and the insula, see Supplementary Fig. 4), we speculate that this may suggest that for a similar magnitude of experienced adverse early-life events, neuroplastic reorganization may occur more robustly in patients with FND. This is based in part on observations that brain-network trauma relationships identified in other published non-FND traumatized populations had sample sizes well above 100 subjects26, 33. In addition to replicating our findings, the neural mechanisms guiding acquisition of “limbic scars” in some patients with FND warrants more inquiry.
Limitations include modest sample sizes, psychiatric comorbidities, psychotropic medication use, phenotypic heterogeneity, and sole reliance on patient-reported scales. The CTQ measures “perceived” ELM that is subject to recall bias, and clinician-rated instruments add complementary information71. While to our knowledge this is the first neuroimaging study in the FND field to include a psychiatric control group, more research using healthy subject trauma controls and other psychiatric populations are needed to further clarify the specificity of the brain-trauma relationships in FND. In addition, physical abuse and physical neglect scores were correlated in patients with FND, suggesting that more research is needed to disentangle brain-trauma subtype relationships in this population. While most of our findings remained significant across motor phenotypes supporting a transdiagnostic approach, more research is needed to identify potentially meaningful subtype differences72. Caution should be taken to not over-interpret negative results, particularly the absence of an association between sexual abuse burden and network architecture as only 8 patients reported sexual abuse. In support of the importance of physical abuse, however, meta-analyses have shown that physical abuse has particularly robust effects on the development of FND3. Future studies should investigate the biological significance of critical (sensitive) periods in modulating the impact of ELM73, as well as characterizing the role of recent life events in the pathophysiology of FND. For the exploratory molecular analyses, a limitation is the comparison of FND connectivity maps with gene expression profiles from the Allen Human Brain Atlas. While the use of this data is highly novel, future data from the post-mortem brains of individuals with FND should seek to replicate our findings. Lastly, to investigate a possible “trauma subtype” in FND, studies should concurrently collect behavioral, autonomic, neuroendocrine, genetic and multimodal neuroimaging data, along with detailed developmental histories74.
In conclusion, individual differences in reported early-life physical abuse severity correlated with motor – limbic/paralimbic connectivity strength in patients with FND. Exploratory ELM neuroimaging endophenotype – gene expression findings suggest that molecules involved in stress-related neuroplasticity, neurodevelopment and locomotory behavior may be important in promoting brain reorganization following physical abuse in this population.
Supplementary Material
Acknowledgements:
Funding:
I.D. was supported by postdoctoral fellowship program from the Basque Country Government. D.L.P. was funded by the National Institute of Mental Health Grant K23MH111983-03, Massachusetts General Hospital Physician-Scientist Development Award and the Sidney R. Baer Jr. Foundation. This study was also supported by the NIH shared instrument grant S10RR023043.
Conflicts of Interest / Disclosures:
T.R.N. is funded by a UK National Institute for Health Research (NIHR) Clinician Scientist Fellowship. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
D.L.P. has received honoraria for continuing medical education lectures in functional neurological disorder.
Footnotes
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Espay AJ, Aybek S, Carson A, Edwards MJ, Goldstein LH, Hallett M et al. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol 2018; 75(9): 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keynejad RC, Carson AJ, David AS, Nicholson TR. Functional neurological disorder: psychiatry’s blind spot. Lancet Psychiatry 2017; 4(3): e2–e3. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 2018; 5(4): 307–320. [DOI] [PubMed] [Google Scholar]
- 4.Kanaan RAA, Craig TKJ. Conversion disorder and the trouble with trauma. Psychol Med 2019; 49(10): 1585–1588. [DOI] [PubMed] [Google Scholar]
- 5.Popkirov S, Wessely S, Nicholson TR, Carson AJ, Stone J. Different shell, same shock. BMJ 2017; 359: j5621. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). 5th edition. edn. American Psychiatric Pub: Washington, DC, 2013, 947pp. [Google Scholar]
- 7.Keynejad RC, Frodl T, Kanaan R, Pariante C, Reuber M, Nicholson TR. Stress and functional neurological disorders: mechanistic insights. J Neurol Neurosurg Psychiatry 2019; 90(7): 813–821. [DOI] [PubMed] [Google Scholar]
- 8.Pick S, Goldstein LH, Perez DL, Nicholson TR. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry 2019; 90(6): 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 2016; 17(10): 652–666. [DOI] [PubMed] [Google Scholar]
- 10.Aybek S, Nicholson TR, Zelaya F, O’Daly OG, Craig TJ, David AS et al. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 2014; 71(1): 52–60. [DOI] [PubMed] [Google Scholar]
- 11.Aybek S, Nicholson TR, O’Daly O, Zelaya F, Kanaan RA, David AS. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One 2015; 10(4): e0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC, Jr. et al. Emotional stimuli and motor conversion disorder. Brain 2010; 133(Pt 5): 1526–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espay AJ, Maloney T, Vannest J, Norris MM, Eliassen JC, Neefus E et al. Impaired emotion processing in functional (psychogenic) tremor: A functional magnetic resonance imaging study. Neuroimage Clin 2018; 17: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassa T, Sebastian A, Liepert J, Weiller C, Schmidt R, Tüscher O. Symptom-specific amygdala hyperactivity modulates motor control network in conversion disorder. Neuroimage Clin 2017; 15: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Kruijs SJ, Bodde NM, Vaessen MJ, Lazeron RH, Vonck K, Boon P et al. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry 2012; 83(3): 239–247. [DOI] [PubMed] [Google Scholar]
- 16.Morris LS, To B, Baek K, Chang-Webb YC, Mitchell S, Strelchuk D et al. Disrupted avoidance learning in functional neurological disorder: Implications for harm avoidance theories. Neuroimage Clin 2017; 16: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wegrzyk J, Kebets V, Richiardi J, Galli S, de Ville DV, Aybek S. Identifying motor functional neurological disorder using resting-state functional connectivity. Neuroimage Clin 2018; 17: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Liu K, Ma X, Li Z, Duan X, An D et al. Altered Functional Connectivity Patterns of the Insular Subregions in Psychogenic Nonepileptic Seizures. Brain Topogr 2015; 28(4): 636–645. [DOI] [PubMed] [Google Scholar]
- 19.Diez I, Ortiz-Teran L, Williams B, Jalilianhasanpour R, Ospina JP, Dickerson BC et al. Corticolimbic fast-tracking: enhanced multimodal integration in functional neurological disorder. J Neurol Neurosurg Psychiatry 2019; 90(8): 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord 2011; 26(13): 2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone J, Zeman A, Simonotto E, Meyer M, Azuma R, Flett S et al. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med 2007; 69(9): 961–969. [DOI] [PubMed] [Google Scholar]
- 22.de Lange FP, Toni I, Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia 2010; 48(6): 1782–1788. [DOI] [PubMed] [Google Scholar]
- 23.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology 2010; 74(3): 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek K, Doñamayor N, Morris LS, Strelchuk D, Mitchell S, Mikheenko Y et al. Impaired awareness of motor intention in functional neurological disorder: Implications for voluntary and functional movement. Psychol Med 2017; 47(9): 1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer CW, LaFaver K, Ameli R, Epstein SA, Hallett M, Horovitz SG. Impaired self-agency in functional movement disorders: A resting-state fMRI study. Neurology 2016; 87(6): 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 2012; 71(4): 286–293. [DOI] [PubMed] [Google Scholar]
- 27.Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp 2013; 34(11): 2899–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA et al. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Development and Psychopathology 2015; 27(4 Pt 2): 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA 2013; 110(47): 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Werff SJ, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, Veltman DJ et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol Med 2013; 43(9): 1825–1836. [DOI] [PubMed] [Google Scholar]
- 31.Ohashi K, Anderson CM, Bolger EA, Khan A, McGreenery CE, Teicher MH. Susceptibility or Resilience to Maltreatment Can Be Explained by Specific Differences in Brain Network Architecture. Biol Psychiatry 2019; 85(8): 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G et al. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol Med 2018; 48(7): 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry 2014; 76(4): 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selkirk M, Duncan R, Oto M, Pelosi A. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia 2008; 49(8): 1446–1450. [DOI] [PubMed] [Google Scholar]
- 35.Spinhoven P, Roelofs K, Moene F, Kuyk J, Nijenhuis E, Hoogduin K et al. Trauma and dissociation in conversion disorder and chronic pelvic pain. International Journal of Psychiatry in Medicine 2004; 34(4): 305–318. [DOI] [PubMed] [Google Scholar]
- 36.Sepulcre J Functional streams and cortical integration in the human brain. Neuroscientist 2014; 20(5): 499–508. [DOI] [PubMed] [Google Scholar]
- 37.Fornito A, Arnatkeviciute A, Fulcher BD. Bridging the Gap between Connectome and Transcriptome. Trends Cogn Sci 2019; 23(1): 34–50. [DOI] [PubMed] [Google Scholar]
- 38.Arnatkevic Iute A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage 2019; 189: 353–367. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz-Teran L, Diez I, Ortiz T, Perez DL, Aragon JI, Costumero V et al. Brain circuit-gene expression relationships and neuroplasticity of multisensory cortices in blind children. Proc Natl Acad Sci USA 2017; 114(26): 6830–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin Q, Ortiz-Teran L, Diez I, Perez DL, Ginsburg J, El Fakhri G et al. Sequence Alterations of Cortical Genes Linked to Individual Connectivity of the Human Brain. Cereb Cortex 2019; 29(9): 3828–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol 2012; 233(1): 102–111. [DOI] [PubMed] [Google Scholar]
- 42.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27(9): 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez DL, Dworetzky BA, Dickerson BC, Leung L, Cohn R, Baslet G et al. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci 2015; 46(1): 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994; 151(8): 1132–1136. [DOI] [PubMed] [Google Scholar]
- 45.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress 2015; 28(6): 489–498. [DOI] [PubMed] [Google Scholar]
- 46.Qian J, Diez I, Ortiz-Teran L, Bonadio C, Liddell T, Goni J et al. Positive Connectivity Predicts the Dynamic Intrinsic Topology of the Human Brain Network. Front Syst Neurosci 2018; 12: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012; 489(7416): 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31(3): 968–980. [DOI] [PubMed] [Google Scholar]
- 49.Keuken MC, Bazin PL, Crown L, Hootsmans J, Laufer A, Muller-Axt C et al. Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. Neuroimage 2014; 94: 40–46. [DOI] [PubMed] [Google Scholar]
- 50.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25(1): 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakvis P, Roelofs K, Kuyk J, Edelbroek PM, Swinkels WA, Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia 2009; 50(5): 1001–1011. [DOI] [PubMed] [Google Scholar]
- 52.Apazoglou K, Mazzola V, Wegrzyk J, Polara GF, Aybek S. Biological and perceived stress in motor functional neurological disorders. Psychoneuroendocrinology 2017; 85: 142–150. [DOI] [PubMed] [Google Scholar]
- 53.Ghaziri J, Tucholka A, Girard G, Houde JC, Boucher O, Gilbert G et al. The Corticocortical Structural Connectivity of the Human Insula. Cereb Cortex 2017; 27(2): 1216–1228. [DOI] [PubMed] [Google Scholar]
- 54.Espay AJ, Maloney T, Vannest J, Norris MM, Eliassen JC, Neefus E et al. Dysfunction in emotion processing underlies functional (psychogenic) dystonia. Mov Disord 2018; 33(1): 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10(1): 59–70. [DOI] [PubMed] [Google Scholar]
- 56.Sepulcre J, Sabuncu MR, Yeo TB, Liu H, Johnson KA. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci 2012; 32(31): 10649–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricciardi L, Demartini B, Crucianelli L, Krahé C, Edwards MJ, Fotopoulou A. Interoceptive awareness in patients with functional neurological symptoms. Biol Psychology 2016; 113: 68–74. [DOI] [PubMed] [Google Scholar]
- 58.Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J et al. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol psychiatry 2012; 17(6): 584–596. [DOI] [PubMed] [Google Scholar]
- 59.Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron 2008; 60(1): 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LaFrance WC Jr., Leaver K, Stopa EG, Papandonatos GD, Blum AS. Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurology 2010; 75(14): 1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deveci A, Aydemir O, Taskin O, Taneli F, Esen-Danaci A. Serum brain-derived neurotrophic factor levels in conversion disorder: Comparative study with depression. Psychiatry Clin Neurosci 2007; 61(5): 571–573. [DOI] [PubMed] [Google Scholar]
- 62.Aas M, Haukvik UK, Djurovic S, Tesli M, Athanasiu L, Bjella T et al. Interplay between childhood trauma and BDNF val66met variants on blood BDNF mRNA levels and on hippocampus subfields volumes in schizophrenia spectrum and bipolar disorders. Journal of Psychiatric Research 2014; 59: 14–21. [DOI] [PubMed] [Google Scholar]
- 63.Gutierrez B, Bellon JA, Rivera M, Molina E, King M, Marston L et al. The risk for major depression conferred by childhood maltreatment is multiplied by BDNF and SERT genetic vulnerability: a replication study. Journal of Psychiatry & Neuroscience : JPN 2015; 40(3): 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee P, Whalley HC, McKirdy JW, McIntosh AM, Johnstone EC, Lawrie SM et al. Effects of the BDNF Val66Met polymorphism on neural responses to facial emotion. Psychiatry Research 2011; 191(3): 182–188. [DOI] [PubMed] [Google Scholar]
- 65.Li A, Jing D, Dellarco DV, Hall BS, Yang R, Heilberg RT et al. Role of BDNF in the development of an OFC-amygdala circuit regulating sociability in mouse and human. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez-Rodriguez MM, New AS, Goldstein KE, Rosell D, Yuan Q, Zhou Z et al. Brain-derived neurotrophic factor Val66Met genotype modulates amygdala habituation. Psychiatry Res Neuroimaging 2017; 263: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young DA, Neylan TC, O’Donovan A, Metzler T, Richards A, Ross JA et al. The interaction of BDNF Val66Met, PTSD, and child abuse on psychophysiological reactivity and HPA axis function in a sample of Gulf War Veterans. J Affect Disord 2018; 235: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth TL, Sweatt JD. Epigenetic marking of the BDNF gene by early-life adverse experiences. Horm Behav 2011; 59(3): 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilkinson AV, Gabriel KP, Wang J, Bondy ML, Dong Q, Wu X et al. Sensation-seeking genes and physical activity in youth. Genes Brain Behav 2013; 12(2): 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez DL, Matin N, Barsky A, Costumero-Ramos V, Makaretz SJ, Young SS et al. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J Neurol Neurosurg Psychiatry 2017; 88(6): 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicholson TR, Aybek S, Craig T, Harris T, Wojcik W, David AS et al. Life events and escape in conversion disorder. Psychol Med 2016; 46(12): 2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomic A, Agosta F, Sarasso E, Petrovic I, Basaia S, Pesic D et al. Are there two different forms of functional dystonia? A multimodal brain structural MRI study. Mol Psychiatry 2018. [DOI] [PubMed] [Google Scholar]
- 73.Dunn EC, Nishimi K, Powers A, Bradley B. Is developmental timing of trauma exposure associated with depressive and post-traumatic stress disorder symptoms in adulthood? J Psychiatr Res 2017; 84: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreano JM, Touroutoglou A, Dickerson B, Barrett LF. Hormonal Cycles, Brain Network Connectivity, and Windows of Vulnerability to Affective Disorder. Trends Neurosci 2018; 41(10): 660–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


