Abstract
BACKGROUND:
Gut microbial imbalance may contribute to endotoxemia, inflammation, and oxidative stress in heart failure (HF). Changes occurring in the intestinal microbiota and inflammatory/oxidative milieu during HF progression and following left ventricular assist device (LVAD) or heart transplantation (HT) are unknown. We aimed to investigate variation in gut microbiota and circulating biomarkers of endotoxemia, inflammation, and oxidative stress in patients with HF (New York Heart Association, Class I–IV), LVAD, and HT.
METHODS:
We enrolled 452 patients. Biomarkers of endotoxemia (lipopolysaccharide and soluble [sCD14]), inflammation (C-reactive protein, interleukin-6, tumor necrosis factor-α, and endothelin-1 adiponectin), and oxidative stress (isoprostane) were measured in 644 blood samples. A total of 304 stool samples were analyzed using 16S rRNA sequencing.
RESULTS:
Gut microbial community measures of alpha diversity were progressively lower across worsening HF class and were similarly reduced in patients with LVAD and HT (p < 0.05). Inflammation and oxidative stress were elevated in patients with Class IV HF vs all other groups (all p < 0.05). Lipopolysaccharide was elevated in patients with Class IV HF (vs Class I–III) as well as in patients with LVAD and HT (p < 0.05). sCD14 was elevated in patients with Class IV HF and LVAD (vs Class I–III, p < 0.05) but not in patients with HT.
CONCLUSIONS:
Reduced gut microbial diversity and increased endotoxemia, inflammation, and oxidative stress are present in patients with Class IV HF. Inflammation and oxidative stress are lower among patients with LVAD and HT relative to patients with Class IV HF, whereas reduced gut diversity and endotoxemia persist in LVAD and HT.
Keywords: heart failure, left ventricular assist device, heart transplantation, inflammation, oxidative stress, gut dysbiosis
Heart failure (HF) is a growing health problem with an estimated prevalence of 6.5 million individuals in the US,1 among whom 5% to 25% of patients will progress to advanced disease2–4 where heart transplantation (HT) and left ventricular assist devices (LVADs) are the only therapies demonstrated to improve prognosis.
HF is recognized as a systemic disease state characterized by progressive increase in congestion, inflammation, and oxidative stress over time. Symptomatic HF from venous congestion is associated with an elevation of circulating biomarkers of inflammation5–9 and oxidative stress,10 which correlate with impairment in functional capacity and predict poor clinical outcomes.11,12 The mechanisms involved in this inflammatory and oxidative response are incompletely understood.
Recently, imbalance of microbial communities in the gut, commonly referred to as gut dysbiosis, has been suggested as a potential contributor to HF progression.13–15 Gut dysbiosis in the context of bowel wall congestion and hypoperfusion from HF, may alter the intestinal barrier function leading to a leaky gut that allows translocation of bacteria and their byproducts into the peripheral circulation. Prior studies have demonstrated that more symptomatic stages of HF are associated with increased bowel wall thickness and increased overgrowth of adherent bacteria in the gut mucosa.16 In addition, increased levels of circulating microbial DNA17 as well as lipopolysaccharides (LPSs), which are produced by gram-negative bacteria, have been described as potential triggers of systemic inflammation.18,19
Presently, we investigate the variation in gut microbial communities, endotoxemia, and established biomarkers of inflammation and oxidative stress across a broad spectrum of HF progression and treatment (LVAD and HT) stages. We tested the following specific hypotheses: (1) HF progression, as reflected by worsened New York Heart Association (NYHA) functional class, is associated with decreased gut microbial diversity and with concurrent increased levels of systemic biomarkers of endotoxemia, inflammation, and oxidative stress; and (2) patients treated with LVAD and after HT have increased microbial diversity and reduced systemic biomarkers of endotoxemia, inflammation, and oxidative stress compared with patients with advanced HF (NYHA Class IV). We additionally explored whether any specific microbial signatures are associated with the advanced HF stage.
Methods
Study population
A total of 452 patients were enrolled from June 2016 to February 2019 at Columbia University Irving Medical Center. Enrolled patients represent multiple distinct stages of HF progression, as defined by HF (NYHA Class I–IV) to LVAD and HT. Inclusion criteria were ≥18 years of age with systolic HF. Exclusion criteria were (1) HF with preserved left ventricular ejection function (LVEF) >40%; (2) infiltrative and hypertrophic cardiomyopathy; (3) advanced renal disease requiring dialysis; (4) liver cirrhosis or active hepatitis; and (5) presence of active malignancy.
Patients were classified into the following 6 categories: HF Class I, II, III, IV, post-LVAD ≥ 3 months, or post-HT ≥ 6 months. Patients were enrolled in the outpatient setting during routine clinical visits or during the index hospitalization for LVAD or HT surgery. NYHA classification was performed by patients’ treating cardiologist and adjudicated by 2 HF specialists (M.Y. and P.C.C.) who were blinded to the results. All patients with HF were treated according to current HF management guidelines and patients with HT were treated with standard immunosuppression per institutional protocol. Demographic and clinical information was extracted from electronic medical records (EMRs). Antibiotic use 1 month before stool and/or blood sample collection was recorded from EMRs and further characterized as peri-procedural antibiotics (those prescribed for prophylaxis before cardiac surgery or other procedures such as dental interventions) vs antibiotics for treatment of confirmed or suspected infection or chronic prophylaxis among patients post-HT. The Columbia University Irving Medical Center Institutional Review Board approved the study protocol.
Measurements of plasma and serum biomarkers
Biomarkers of endotoxemia (LPS and soluble CD14 [sCD14]), inflammation (C-reactive protein [CRP], interleukin-6 [IL-6], tumor necrosis factor-α [TNF-α], endothelin-1 [ET-1], and adiponectin), and oxidative stress (isoprostane) were measured in plasma or serum as detailed in the Supplementary Material online at www.jhltonline.org.
Stool analysis
Patients provided stool samples in sterile stool hats using a protocol similar to the Human Microbiome Project.20 Details on stool collection and DNA extraction and sequencing are provided in the Supplementary Material online. A total of 281 of 304 stool samples had a corresponding blood sample (median difference between blood and stool collection dates was 2 days).
Statistical analysis
Demultiplexed sequence files were processed in R version 3.5.2, using the DADA2 pipeline to identify exact sequence variants.21,22 Reads were truncated at forward and reverse lengths of 260 and 220. After processing, a total of 5,697,709 sequence reads were included with a median library size of 13,625, and 30,959 exact sequence variants were identified. 16S analyses were carried out using the Phyloseq package. Alpha diversity (i.e., number and distribution of bacterial taxa within samples) was defined using the Shannon Index and the number of observed operational taxonomic units (OTUs). Analysis of beta diversity (i.e., microbial community membership similarity between patient categories) utilized principal coordinates analysis of the Bray-Curtis dissimilarity index and principal coordinates analysis (PCoA) plots of the first 2 principal coordinates were plotted according to patient categories. Permutational multivariate analysis of variance analysis was conducted to identify whether patient categories explained microbial variation. The DESeq package23 and Linear discriminant analysis Effect Size24 were used to evaluate whether specific taxa differed by patient categories after multivariable adjustment. A false discovery rate was used to correct for multiple comparisons. Microbial diversity measures as well as biomarkers were regressed on patient categories in multivariable generalized linear mixed-effects models adjusted for age, sex, race/ethnicity, and antibiotic use. A subgroup analysis was performed adjusting for right ventricular (RV) function among patients with RV function assessments available in the EMR. Antibiotic use was defined by indication for treatment of presumed or confirmed infection or chronic prophylaxis among patients with HT within 1 month before stool collection. We did not adjust for short-term use of peri-procedural antibiotics because of the short duration of treatment and our finding that peri-procedural use was not related to gut diversity (data not shown). Mixed-effects models were used to account for repeated measures within patient, as 108 patients entered the model more than once (e.g., as Class IV HF before LVAD or HT and again after implant or post-transplantation).
Results
Among 452 patients, 644 blood samples (12 HF Class I, 55 Class II, 73 Class III, 94 Class IV, 210 LVAD, and 200 HT) were collected. In a subset of 240 patients, 304 stool samples for microbiome assessments were also collected (8 Class I, 31 Class II, 40 Class III, 36 Class IV, 103 LVAD, and 86 HT). Characteristics of patients providing blood samples are reported in Table 1 and characteristics of patients providing stool samples are provided in Supplementary Table S1 (online). All patients studied were predominantly white males, with no difference in body mass index or HF etiology across categories (Table 1). Patients with Class III and IV HF and LVAD were more likely to have a history of smoking. There was a significant difference in history of hypertension and atrial fibrillation across patient categories. In the Class IV HF group, patients were predominantly Interagency Registry for Mechanically Assisted Circulatory Support Profile II or III and had lower sodium, higher liver enzymes, and higher levels of N-terminal prohormone B-type natriuretic peptide and blood urea nitrogen, as well as a greater degree of RV dysfunction and severe tricuspid regurgitation. Class IV patients (vs Classes I, II, and III) were less likely to be treated according to HF guidelines and more likely to receive peri-procedural antibiotics (36%). Of note, the proportion of patients with LVAD or Class I HF treated with statins was significantly lower than HT or Class II–IV (35% and 4%, respectively).
Table 1.
Baseline Characteristics of Patients Providing Blood Samples
| Number of blood samples (N = 644)a | HF NYHA Class I (N = 12) | HF NYHA Class II (N = 55) | HF NYHA Class III (N = 73) | HF NYHA Class IV (N = 94) | LVAD (≥3 months) (N = 210) | HT ≥6 months) (N = 200) | p-value for any difference |
|---|---|---|---|---|---|---|---|
| Demographic and clinical characteristics | |||||||
| Age, years | 53.9 (4.90) | 59.8 (1.77) | 60.5 (1.44) | 58.6 (1.45) | 57.9 (0.97) | 55.3 (0.94) | <0.0001 |
| Sex, male | 9 (75.0) | 39 (70.9) | 60 (82.2) | 79 (84.0) | 186 (88.6) | 166 (83.0) | 0.0492 |
| Race | 0.0168 | ||||||
| White | 3 (25.0) | 25 (45.5) | 44 (60.3) | 55 (58.5) | 120 (57.1) | 109 (54.5) | |
| Black | 3 (25.0) | 14 (25.5) | 19 (26.0) | 16 (17.0) | 53 (25.2) | 59 (29.5) | |
| Hispanic | 3 (25.0) | 5 (9.1) | 7 (9.6) | 7 (7.4) | 17 (8.1) | 19 (9.5) | |
| Other | 3 (25.0) | 11 (20.0) | 3 (4.1) | 16 (17.0) | 20 (9.5) | 13 (6.5) | |
| BMI (kg/m2) | 32.98 (3.36) | 30.39 (1.25) | 31.23 (1.00) | 29.59 (1.42) | 28.64 (0.49) | 30.47 (0.86) | 0.06041 |
| History of smoking | 4 (33.3) | 24 (43.6) | 40 (54.8) | 47 (50.0) | 121 (57.6) | 88 (44.0) | 0.0539 |
| Ischemic etiology | 2 (16.7) | 23 (41.8) | 35 (47.9) | 39 (31.5) | 98 (46.7) | 79 (39.5) | 0.2698 |
| Hypertension | 5 (41.7) | 38 (69.1) | 44 (60.3) | 58 (61.7) | 132 (62.9) | 157 (78.5) | 0.0013 |
| Diabetes | 1 (8.3) | 16 (29.1) | 26 (35.6) | 29 (30.9) | 79 (37.6) | 77 (38.5) | 0.2247 |
| Atrial fibrillation | 3 (25.0) | 17 (30.9) | 35 (47.9) | 47 (50.0) | 94 (44.8) | 71 (35.5) | 0.0206 |
| History of stroke | 0 (0) | 1 (1.8) | 5 (6.8) | 8 (8.5) | 17 (8.1) | 19 (9.5) | 0.4509 |
| INTERMACS profile | |||||||
| 1 | — | — | — | 5 (5.3) | — | — | |
| 2 | — | — | — | 49 (52.1) | — | — | |
| 3 | — | — | — | 21 (22.3) | — | — | |
| 4 | — | — | — | 1 (1.1) | — | — | |
| NA | — | — | — | 18 (19.1) | — | — | |
| Time after procedure, years | — | — | — | — | 1.12 (0.10) | 4.18 (0.37) | |
| Laboratory parameters | |||||||
| eGFR, ml/min/1.73 m2 | 74.57 (7.89) | 62.35 (4.19) | 62.14 (2.62) | 58.67 (3.04) | 61.58 (2.03) | 58.74 (2.77) | 0.1078 |
| BUN, mg/dl | 17.14 (1.42) | 25.73 (2.04) | 23.70 (1.29) | 32.04 (2.50) | 24.14 (0.98) | 27.93 (1.00) | 0.0004 |
| Serum creatinine, mg/dl | 1.09 (0.09) | 1.57 (0.26) | 1.28 (0.04) | 1.42 (0.07) | 1.46 (0.7) | 1.58 (0.09) | 0.0601 |
| NT-ProBNP, pg/ml, median (IQR) | 515.2 (423.45) | 966.7 (1,918.00) | 1,542.0 (3,489.80) | 2,969.0 (2,975.75) | 1,265.0 (1,597.60) | 1,203.5 (2,876.88) | <0.0001 |
| Na, mmol/liter | 141.57 (1.19) | 140.38 (0.53) | 140.16 (0.45) | 136.50 (0.64) | 140.09 (0.26) | 141.52 (0.26) | <0.0001 |
| AST, U/liter | 25.00 (6.01) | 27.60 (3.36) | 25.70 (1.86) | 40.07 (8.59) | 26.22 (1.25) | 25.20 (1.21) | 0.0155 |
| ALT, U/liter | 23.29 (5.82) | 27.03 (3.30) | 29.30 (3.47) | 64.04 (19.19) | 21.99 (1.00) | 23.77 (1.32) | <0.0001 |
| Total bilirubin, mg/dl | 0.51 (0.06) | 0.57 (0.08) | 0.68 (0.05) | 0.95 (0.06) | 0.59 (0.03) | 0.55 (0.03) | <0.0001 |
| Echocardiographic parameters | |||||||
| LVEF, % | 26.85 (4.63) | 25.23 (1.21) | 20.05 (0.91) | 16.87 (0.73) | 18.99 (0.83) | 58.94 (0.50) | <0.0001 |
| TR, ≥2+ | 2 (16.7) | 8 (14.5) | 14 (19.2) | 39 (41.5) | 31 (14.8) | 21 (10.5) | <0.0001 |
| RV function (≥moderately reduced) | 2 (16.7) | 15 (27.3) | 26 (35.6) | 66 (70.2) | 81 (38.6) | 9 (4.5) | <0.0001 |
| Medications | |||||||
| ASA | 7 (58.3) | 40 (72.7) | 38 (52.1) | 53 (56.4) | 151 (71.9) | 177 (88.5) | <0.0001 |
| Coumadin | 0 (0) | 4 (7.3) | 18 (24.7) | 8 (8.5) | 187 (89.0) | 5 (2.5) | <0.0001 |
| ACE inhibitors | 4 (33.3) | 20 (26.4) | 20 (27.4) | 3 (3.2) | 59 (28.1) | 11 (5.5) | <0.0001 |
| ARB | 7 (58.3) | 23 (41.8) | 38 (52.1) | 13 (13.8) | 35 (16.7) | 38 (19.0) | <0.0001 |
| Aldosterone antagonists | 7 (58.3) | 30 (54.5) | 49 (67.1) | 55 (58.5) | 81 (38.6) | 3 (1.5) | <0.0001 |
| β-blockers | 12 (100.0) | 54 (98.2) | 66 (90.4) | 53 (56.4) | 188 (89.5) | 37 (18.5) | <0.0001 |
| Statin | 5 (4.2) | 33 (60.0) | 39 (53.4) | 52 (55.3) | 74 (35.3) | 168 (84.0) | <0.0001 |
| Loop diuretics | 7 (58.3) | 37 (67.3) | 62 (84.9) | 68 (72.3) | 141 (67.1) | 40 (20.0) | <0.0001 |
| Digoxin | 2 (16.7) | 7 (12.7) | 22 (30.1) | 16 (17.0) | 73 (34.8) | 0 (0) | <0.0001 |
| Antibioticsb | 0 (0) | 5 (9.1) | 9 (12.3) | 21 (22.3) | 58 (27.6) | 64 (32.0) | 0.0002 |
| Peri-procedural antibioticsc | 0 (0.0) | 0 (0.0) | 5 (6.8) | 34 (36.2) | 3 (1.4) | 0 (0.0) | <0.0001 |
ACE, angiotensin-converting enzyme; ALT, alanine transaminase; ASA, acetylsalicylic acid; AST, aspartate transaminase; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HF, heart failure; HT, heart transplantation; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; IQR, interquartile range; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; NA, not applicable; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; NYHA, New York Heart Association; RV, right ventricle; TR, tricuspid regurgitation.
Data presented as n (%) or mean (SE) as appropriate, unless otherwise noted.
A total of 452 patients provided a total of 644 blood samples. Repeated measures within LVAD and HT groups are due to longitudinal sampling after LVAD and HT (mean number of samples per patient in the LVAD and HT groups are 1.6 and 1.3).
Antibiotic use is defined as any antibiotic use that was indicated for infection or chronic prophylaxis among post-HT patients and that was estimated to have occurred 1 month before stool collection.
Peri-procedural antibiotic use is defined as antibiotic utilization in relation to cardiac surgery, or for other invasive procedures (e.g., dental interventions).
Variation in circulating biomarkers of endotoxemia, inflammation, and oxidative stress among patients with HF, LVAD, and HT
Figure 1 and Supplementary Figure S1 (online) demonstrate that the patient categories explained variation in all biomarkers (all p-values for any difference < 0.001). All inflammatory biomarkers and isoprostane increased as HF progressed from Class I to IV (Figure 1) but were lower among patients with LVAD and HT compared with Class IV patients. CRP, IL-6, TNF-α, and adiponectin levels were higher in LVAD vs patients with Class I and II HF and among HT vs patients with Class I and II HF. Biomarkers of endotoxemia displayed a different pattern than what was observed for inflammation and oxidative stress. LPS and sCD14 increased in a linear fashion across worsening HF Class (Figure 1f and g, p < 0.01). When compared with patients with Class I, II, and III HF grouped, LPS and sCD14 were elevated among patients with LVAD (both p < 0.001). In contrast, only LPS (p = 0.005) and not sCD14 (p = 0.42) was elevated among patients with HT vs Class I to III HF. Additional adjustment for RV dysfunction did not change these results (Supplementary Figure S2 online).
Figure 1.
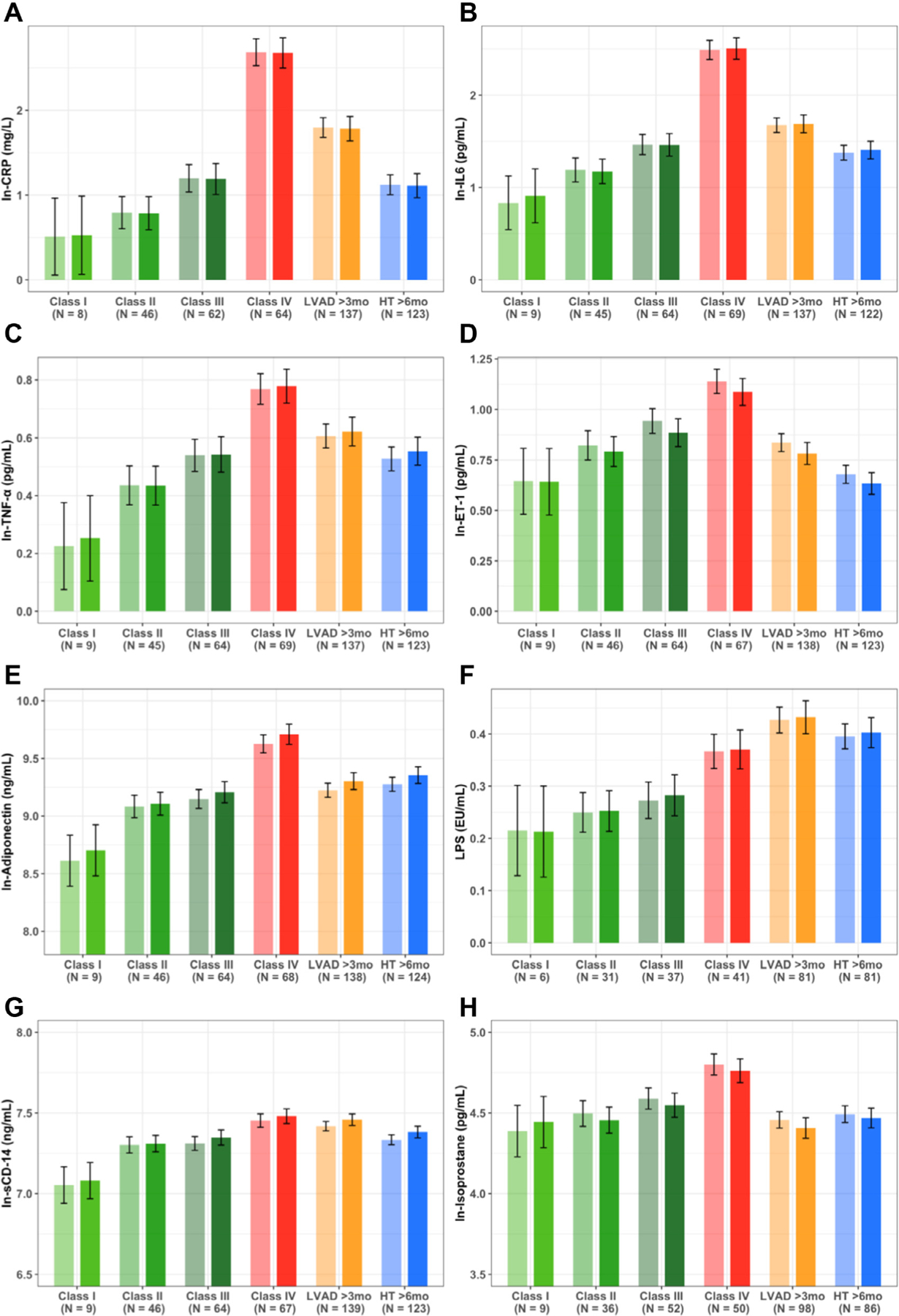
Variation in circulating biomarkers of inflammation, oxidative stress, and endotoxemia across disease categories (HF Class I, II, III, IV, LVAD, and HT). (A) CRP. (B) IL-6. (C) TNF-α. (D) ET-1. (E) Adiponectin. (F) LPS. (G) sCD14. (H) Isoprostane. All p-values for linear trend across HF Class I – IV < 0.01 and all p-values for any difference across all patient categories < 0.001. Light bars: unadjusted. Dark bars: Adjusted for age, sex, and race/ethnicity. CRP, C-reactive protein; ET-1, endothelin-1; HF, heart failure; HT, heart transplantation; IL-6, interleukin-6; LPS, lipopolysaccharide; LVAD, left ventricular assist device; sCD14, soluble CD14; TNF-α, tumor necrosis factor-α.
A subgroup of 45 patients had biomarkers measured longitudinally both pre-LVAD implantation and at least once post-LVAD ≥ 3 months for a total of 137 samples collected (Table 2). Most biomarkers studied were significantly reduced following LVAD but levels of endotoxemia biomarkers (LPS and sCD14) remained similar or increased (p-value for LPS <0.01) (longitudinal stool was not available for analysis).
Table 2.
Mean Biomarker Values Before and After LVAD among Patients with Repeated Longitudinal Measures
| Variables | HF NYHA Class IV (pre-LVAD) | LVAD | p-value |
|---|---|---|---|
| Number of samples | 45 | 92a | |
| Mean number of samples per patient | 1 | 2 | |
| Time after LVAD implant, days | 301.21 (19.09) | ||
| Time before LVAD implant, days | 14.84 (9.66) | ||
| ET-1, pg/ml | 2.96 (2.55, 3.42) | 2.09 (1.82, 2.40) | 0.0003 |
| IL-6, pg/ml | 11.27 (8.60, 14.79) | 5.62 (4.29, 7.35) | <0.0001 |
| TNF-α, pg/ml | 2.17 (1.91, 2.47) | 2.07 (1.81, 2.35) | 0.5373 |
| LPS, EU/ml | 0.29 (0.24, 0.36) | 0.38 (0.31, 0.47) | 0.0091 |
| sCD14, ng/ml | 1,682.6 (1,515.5, 1,868.1) | 1,725.2 (1,569.3, 1,896.5) | 0.7208 |
| Adiponectin, ng/liter | 15,267.6 (12,377.5, 18,832.5) | 10,977.6 (8,861.6, 13,599.0) | 0.0056 |
| CRP, mg/liter | 12.87 (8.74, 18.97) | 6.58 (4.48, 9.64) | 0.0021 |
| Isoprostane, pg/ml | 118.06 (98.32, 141.77) | 75.89 (62.27, 92.48) | <0.0001 |
CRP, C-reactive protein; ET-1, endothelin-1; HF, heart failure; IL-6, interleukin-6; LPS, lipopolysaccharide; LVAD, left ventricular assist device; NYHA, New York Heart Association; sCD14, soluble CD14; TNF-α, tumor necrosis factor-alpha.
Data presented as geometric mean (95% Confidence Interval)
All patients provided 1 pre and at minimum 1 post-LVAD sample; 27 patients provided 2 to 4 post-LVAD samples.
Variation in gut microbiota among patients with HF, LVAD, and HT
Mean values of the Shannon Index and number of observed OTUs were 5.6 ± 0.7 and 520 ± 303, respectively. After multivariable adjustment for for age, sex, race/ethnicity and patient category, antibiotic use was related to lower alpha diversity; mean ± standard error Shannon Index values in those taking vs not taking antibiotics were 5.30 ± 0.10 and 5.86 ± 0.07 (p < 0.0001). Alpha diversity did not differ by age, sex, or race/ethnicity (all p-values were not significant). After multivariable adjustment, mean levels of the Shannon Index decreased across worsening HF Class (p-value for trend across HF Class I–IV = 0.03, Figure 2a); the number of observed OTUs followed the same pattern but was not statistically significant (Figure 2b). Alpha diversity among patients with LVAD or HT was similar to Class IV HF (Figure 2a and b). Additional adjustment for RV function did not change the observed patterns (Supplementary Figure S3 online), although statistical significance became marginal possibly because of the 20% reduction in sample size secondary to missing RV assessment data. Table 3 shows that higher levels of Shannon Index values were associated with lower levels of LPS (p = 0.04), CRP (p = 0.002), IL-6 (p = 0.04), TNF-α (p = 0.0002), and adiponectin (p = 0.03). The addition of Shannon Index to a multivariable model including commonly used clinical variables, marginally improved prediction of HF severity (Class I or II vs Class III or IV), but results were not statistically significant (Supplementary Table S2 online).
Figure 2.

Measures of alpha diversity across patient categories. (A) Shannon Index and (B) number of observed OTUs. Light bars: unadjusted. Dark bars: adjusted for age, sex, race/ethnicity, and antibiotic use. Multivariable adjusted p-values for any difference in Shannon Index = 0.48 and number of observed taxa = 0.66; both p-values derived from 5 df F-test. p-values for linear trend across HF Class I–IV for Shannon Index = 0.03 and number of observed taxa = 0.10. HF, heart failure; HT, heart transplantation; LVAD, left ventricular assist device; OTU, operationalized taxonomic unit.
Table 3.
Multivariable Adjusted Associations Between Shannon Index (Alpha Diversity) and Measures of Inflammation, Endotoxemia, and Oxidative Stress
| Regression coefficient | p-value | |
|---|---|---|
| C-reactive protein | −0.3396 | 0.002 |
| Interleukin-6 | −0.1546 | 0.04 |
| Tumor necrosis factor-alpha | −0.1381 | 0.0002 |
| Endothelin-1 | −0.0490 | 0.19 |
| Adiponectin | −0.1283 | 0.03 |
| Lipopolysaccharide | −0.0405 | 0.04 |
| sCD14 | 0.0005 | 0.98 |
| Isoprostane | 0.0378 | 0.39 |
sCD14, soluble CD14.
All analyses from mixed models adjusted for age, sex, and race/ethnicity. All biomarkers (except lipopolysaccharide) required LN transformation before regression analysis. Regression coefficients represent the difference in biomarker level for every 1 unit increase in Shannon Index. For reference, the standard deviation of the Shannon Index = 0.69 in this patient sample.
Beta diversity findings are presented in Figure 3. PCoA 1 and PCoA 2 explain 5.3% and 3.7% of variation in microbiota, respectively. After adjustment for age, sex, race/ethnicity, and antibiotic use in Permutational multivariate analysis of variance models, differences in microbial community composition by patient categories were not evident (Adonis p-value = 0.09) nor was there any variation explained by age, sex, race/ethnicity, antibiotic use, or RV function (Figure 3a and Supplementary Figure S4 online). However, there was evidence that the first principal coordinate summarizing beta diversity differed strongly by patient category (p < 0.001, Figure 3b and Supplementary Figure S4b online).
Figure 3.
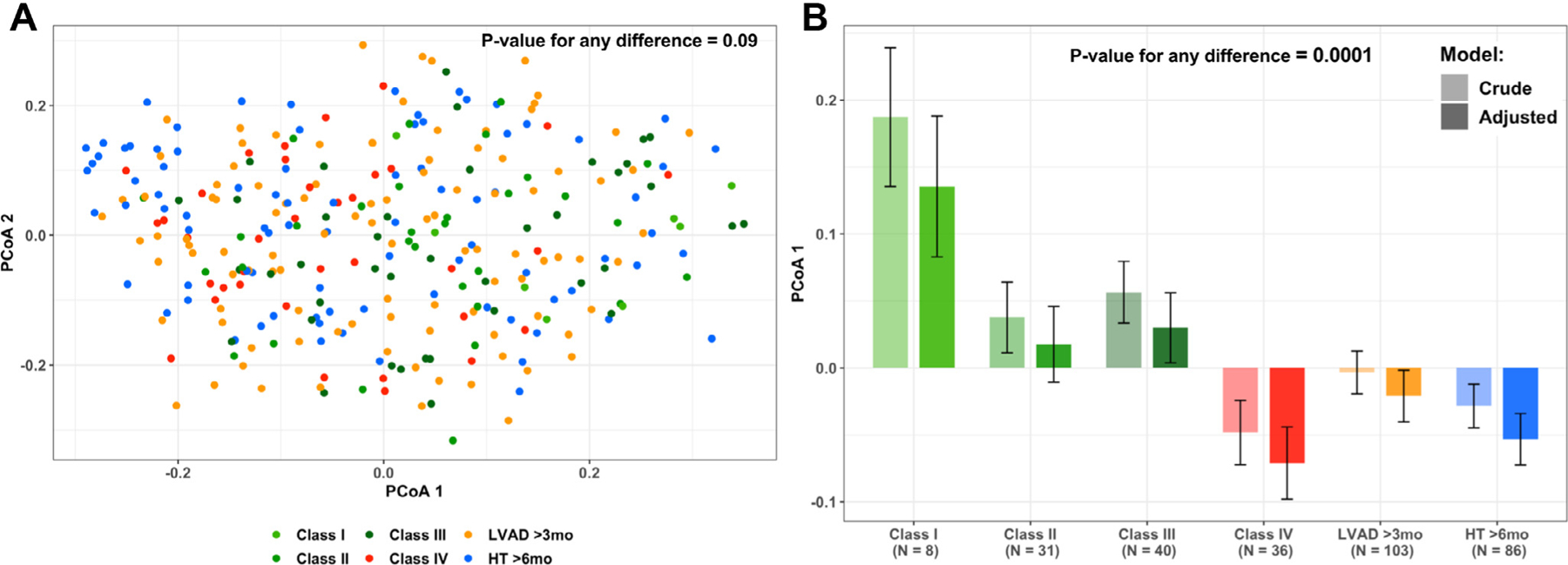
(A) Two principle coordinates are plotted, derived from the Bray-Curtis measure of beta diversity, summarizing similarity of gut microbial communities across patient categories. PCoA 1 explains 5.3% and PCoA 2 explains 3.7% of variation in microbial communities. p-value derived from PERMANOVA Adonis test of any difference across category = 0.09. (B) Means values of the first principle coordinate derived from the Bray-Curtis measure of beta diversity across patient category. Light bars: unadjusted. Dark bars: adjusted for age, sex, race/ethnicity, and antibiotic use. p-value for any difference across patient category <0.0001. The p-value for linear trend among patients with HF < 0.0001. HF, heart failure; HT, heart transplantation, LVAD, left ventricular assist device; PCoA, principal coordinates analysis; PERMANOVA, permutational multivariate analysis of variance.
In phylum level analyses, there was a notable depletion of Bacteroidetes among all patients (Figure 4), although no differences in relative abundance of any phylum were observed across patient categories. Results from linear discriminant analysis for effect size analyses show that several taxa with potential anti-inflammatory effects are enriched in Class I to II patients vs others including taxa from the genus Methanobrevibacter, the family Lachnospiraceae, and the family Ruminococcaceae (Figure 5).
Figure 4.

Stacked bar chart summarizing gut microbiota phylum-level relative abundance across disease categories HF Class I, II, III, IV, LVAD, and HT. HF, heart failure; HT, heart transplantation; LVAD, left ventricular assist device.
Figure 5.

LEfSe results summarizing top taxa differentially abundant between patient categories. Colored circles and shaded branches indicate taxa that are enriched in a particular patient category with p < 0.05. HF, heart failure; HT, heart transplantation; LEfSe, linear discriminant analysis for effect size; LVAD, left ventricular assist device.
Discussion
This study enrolled a cohort of patients with HF at various stages of disease progression (NYHA Class I–IV) and after treatment with LVAD or HT, while concurrently characterizing the intestinal bacterial microbiota along with multiple established biomarkers of endotoxemia, inflammation, and oxidative stress. The main findings of this cross-sectional study are the following:
Patients with advanced HF (Class IV) have significantly elevated levels of endotoxemia (LPS and sCD14), inflammation (CRP, IL-6, TNF-α, and adiponectin), and oxidative stress (isoprostane) when compared with patients with less symptomatic HF;
Most measures of inflammation and oxidative stress levels are lower among patients with LVAD and HT (vs Class IV), but the levels remain similar to those of patients with Class I to III HF;
Biomarkers of endotoxemia remain elevated after LVAD and HT;
Gut diversity decreases across worsening HF Class and diversity appears to remain low among patients with LVAD and HT; and
The observed decline in diversity across HF class is characterized, in part, by a subset of specific microbial taxa with potential anti-inflammatory effects.
Advanced HF is a disease that carries a dismal prognosis, with HT being the gold standard treatment option for these patients. However, the availability of donor hearts remains low, serving only ~0.02% of eligible patients. Recent improvements in LVAD technology have reduced adverse events and improved quality of life, and LVAD now offers 2-year survival rates on par with HT.25,26 However, complications such as RV failure, stroke, and gastro-intestinal bleeding remain25 and might be, in part, driven by the heightened levels of inflammation that persist after LVAD.27–32
Our results are consistent with previous observations that showed non-selective activation of several inflammatory pathways after LVAD or HT. Grosman-Rimon et al.27,28 demonstrated that chemokines and other inflammatory biomarkers including TNF-α are elevated in a small cohort of LVAD patients when compared with patients with HF and healthy subjects using both cross-sectional and longitudinal study designs.27,28 As with the patient population with LVAD, chronic systemic inflammation (despite immunosuppression) has also been observed up to 2 years after HT, as evidenced by increased levels of inflammatory biomarkers such as CRP and TNF-α.33 Inflammation has been linked to HT complications. For example, TNF-α has been shown to suppress myocardial function and contribute to the pathophysiology of rejection and reduced graft survival.34,35 Systemic inflammation has also been implicated in the development of transplant coronary artery disease.36
Our understanding of the factors that contribute to inflammation and oxidative stress in the clinical settings of HF, LVAD, and HT remains incomplete. The gut hypothesis posits that the establishment of a dysbiotic gut microbial community might contribute to adverse outcomes in patients with HF (including LVAD and HT) via chronic activation of inflammatory and/or oxidative stress pathways. Whether dysbiosis is an initial event instigating HF progression or, alternatively, the consequence of RV and left ventricular dysfunction generating gut congestion and ischemia induced-dysbiosis, or both, remains unanswered, and our present cross-sectional study design cannot resolve this question.
The gut hypothesis has been supported by a small number of prior studies.13–16 However, these investigations lack 1 or several of the following: larger sample sizes; inclusion of patients with advanced HF, LVAD, or HT; and microbial community assessments using next generation sequencing methods16 as well as concurrent assessments of the microbiota, endotoxemia, inflammation, and oxidative stress. For example, results from Luedde et al.14 and Kummen et al.13 in 2 separate cohorts suggest depletion of potential butyrate producing taxa (specifically Ruminococcaceae and Lachno-spiraceae) in patients with HF vs controls. However, they included small numbers of patients with HF (20 and 84 patients, respectively) and did not explore whether worsening HF was related to further reductions in gut diversity or whether LVAD or HT improved gut dysbiosis. Similarly, Kamo et al.37 reported older patients with HF to have diminished proportions of taxa from the Bacterioidetes phylum as well as Faecalibacterium spp. Sandek et al.16 elegantly found evidence of increased intestinal wall colonization, endotoxemia, and systemic inflammation among patients with HF vs controls using semi-quantitative assessments that inform bacterial colonization level but not microbial community diversity or differential abundance at the taxa level, thus limiting inference regarding the functional role of microbiota in HF.
Several key observations in our report support and extend previous findings. First, in the largest study to date of gut dysbiosis among patients with HF, LVAD, and HT, we note a striking depletion of taxa from the Bacteroidetes phylum among all patients studied. These findings are in stark contrast to the findings of the Human Microbiome Project (and others) that observed the gut microbiota of healthy individuals to be dominated largely by Firmicutes and Bacteroidetes.38,39 Second, microbial community diversity decreases as HF class worsens, and these alterations are characterized, in part, by depletion of taxa from the family Lachnospiraceae or Ruminococcaceae—consistent with the aforementioned findings.13,14 Third, we have found that LPS levels are increased in advanced HF (consistent with previous reports19) and we demonstrate, for the first time, that LPS is correlated with reduced gut diversity and remains elevated following LVAD or HT.
Our cross-sectional findings suggest that inflammation is lower following LVAD or HT, but it is not fully resolved, and the inflammatory profile of patients with LVAD and HT continues to resemble that of patients with Class II to III HF, raising questions about the source(s) of persistent inflammation. Although previous research has targeted inflammation associated with HF with disappointing results,40,41 these therapies did not address the underlying triggers of inflammation. Herein, we observe that gut diversity remains low following LVAD or HT despite improved hemodynamic profile, and that gut diversity is correlated with endotoxemia and inflammation raising the possibility that unresolved gut dysbiosis contributes to chronic inflammation and immune activation. If demonstrated to be true in future studies, this suggests that once gut diversity is altered, restoration likely requires more than correction of HF hemodynamics. Thus, identification and treatment of gut dysbiosis among patients with HF may have the potential to either prevent HF progression if treated early in the natural history of the disease or to improve outcomes post-LVAD and HT if gut dysbiosis is corrected before these interventions. Fecal microbiota transplantations have been shown to restore gut microflora and treat recurrences of Clostridium difficile infection,42,43 possibly making it a plausible next step. With advancements in technology, there have been promising results using freeze-dried encapsulated forms of fecal microbiota transplantations44 that can be administered orally and can avoid the procedural cost and complication of other routes of administration. However, proof-of-concept clinical studies are necessary to demonstrate biological relevance and safety in patients with HF. This is particularly relevant among patients with Class IV HF or LVAD, many of whom might be destined for HT and the associated immunosuppressive medications.
Some limitations should be acknowledged. We were unable to account for diet or other behavioral variables, and diet in particular has been shown to be associated with gut microbiota.45–47 Future studies informing the contribution of diet to gut microbiota in patients with HF are warranted. The studied cohort was predominantly male, limiting generalizability. Although sampling among patients with LVAD and HT was performed during the chronic phase after treatment, the mean time of blood sampling was 1.1 years after LVAD and 4.2 years after HT, limiting comparability of these 2 groups. There is a clear difference in medication use among the groups. As expected, patients with HT adhere to an immunosuppression regimen, and we observed an uneven distribution of HF medication utilization among patients with Class IV HF, likely owing to reduced tolerance. Steroid use in HT can reduce systemic markers of inflammation and oxidative stress, as it has been shown in other disease states such as emphysema.48 It is also possible that our results are confounded by RV function that varied by patient category. Although our adjustments for RV function did not meaningfully change our findings (Supplementary Figures S2–4 online), assessment of RV function in our clinical echocardiography laboratory was qualitative and therefore subject to measurement error. In addition, larger prospective studies are warranted to address temporality of associations between hemodynamic derangements, gut dysbiosis, endotoxemia, inflammation, and oxidative stress.
In conclusion, we observed that gut microbial communities show reduced diversity among patients with advanced HF compared with less symptomatic patients, and this reduced gut diversity was associated with endotoxemia, inflammation, and oxidative stress. Although inflammation and oxidative stress were lower among patients with LVAD or HT, endotoxemia was elevated and gut diversity was reduced in these 2 groups. These cross-sectional data suggest the possibility that the ongoing inflammatory and oxidative stress phenotype in patients with HF, LVAD, and HT may, in part, stem from dysbiotic gut microbial communities. Longitudinal studies are necessary to address these questions and clarify whether gut dysbiosis is a cause or consequence of the pathogenesis of HF progression.
Supplementary Material
Disclosure statement
This publication was supported by the Susan and Lowell McAdam educational grant, New York, NY, and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. P. C. C., Consultant, Abbott, no honoraria. Research Grant, Abbott. Y.N., Consultant, Abbott, Hourly base reimbursement. R.T. D. and P.C.C. also receive support from R01 DK102932. The sum of payment does not exceed $5,000/year. Consultant, CryoLife, Hourly base reimbursement. The sum of payment does not exceed $5,000/year. The remaining authors have no conflict of interest to declare.
Footnotes
Supplementary data
Supplementary data associated with this article can be found in the online version at www.jhltonline.org/.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2020.02.004.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uretsky BF, Sheahan RG. Primary prevention of sudden cardiac death in heart failure: will the solution be shocking? J Am Coll Cardiol 1997;30:1589–97. [DOI] [PubMed] [Google Scholar]
- 3.Dries DL, Sweitzer NK, Drazner MH, et al. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol 2001;38:421–8. [DOI] [PubMed] [Google Scholar]
- 4.Lee TH, Hamilton MA, Stevenson LW, et al. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol 1993;72:672–6. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Kalman J, Mayer L, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236–41. [DOI] [PubMed] [Google Scholar]
- 6.Kapadia SR, Yakoob K, Nader S, et al. Elevated circulating levels of serum tumor necrosis factor-alpha in patients with hemodynamically significant pressure and volume overload. J Am Coll Cardiol 2000;36:208–12. [DOI] [PubMed] [Google Scholar]
- 7.Deswal A, Petersen NJ, Feldman AM, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the vesnarinone trial (Vest). Circulation 2001;103:2055–9. [DOI] [PubMed] [Google Scholar]
- 8.Anker SD, Egerer KR, Volk HD, et al. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol 1997;79:1426–30. [DOI] [PubMed] [Google Scholar]
- 9.Colombo PC, Doran AC, Onat D, et al. Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect? Curr Heart Fail Rep 2015;12:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol 1998;31: 1352–6. [DOI] [PubMed] [Google Scholar]
- 11.Koller-Strametz J, Pacher R, Frey B, et al. Circulating tumor necrosis factor-alpha levels in chronic heart failure: relation to its soluble receptor II, interleukin-6, and neurohumoral variables. J Heart Lung Transplant 1998;17:356–62. [PubMed] [Google Scholar]
- 12.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart 2004;90:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kummen M, Mayerhofer CCK, Vestad B, et al. Gut microbiota signature in heart failure defined From profiling of 2 independent cohorts. J Am Coll Cardiol 2018;71:1184–6. [DOI] [PubMed] [Google Scholar]
- 14.Luedde M, Winkler T, Heinsen FA, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail 2017;4:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zabell A, Tang WH. Targeting the microbiome in heart failure. Curr Treat Options Cardiovasc Med 2017;19:27. [DOI] [PubMed] [Google Scholar]
- 16.Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1561–9. [DOI] [PubMed] [Google Scholar]
- 17.Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012;485:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda S, Lew WY. Lipopolysaccharide depresses cardiac contractility and beta-adrenergic contractile response by decreasing myofilament response to Ca2+ in cardiac myocytes. Circ Res 1997;81:1011–20. [DOI] [PubMed] [Google Scholar]
- 19.Niebauer J, Volk HD, Kemp M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 1999;353:1838–42. [DOI] [PubMed] [Google Scholar]
- 20.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callahan T, Barnard J, Helmkamp L, et al. Reporting data quality assessment results: identifying individual and organizational barriers and solutions. EGEMS (Wash DC) 2017;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callahan TJ, Bauck AE, Bertoch D, et al. A comparison of data quality assessment checks in six data sharing networks. EGEMS (Wash DC) 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra MR, Goldstein DJ, Uriel N, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018;378:1386–95. [DOI] [PubMed] [Google Scholar]
- 26.Mehra MR, Uriel N, Naka Y, et al. A fully magnetically levitated left ventricular assist device - final report. N Engl J Med 2019;380:1618–27. [DOI] [PubMed] [Google Scholar]
- 27.Grosman-Rimon L, McDonald MA, Jacobs I, et al. Markers of inflammation in recipients of continuous-flow left ventricular assist devices. ASAIO J 2014;60:657–63. [DOI] [PubMed] [Google Scholar]
- 28.Grosman-Rimon L, Jacobs I, Tumiati LC, et al. Longitudinal assessment of inflammation in recipients of continuous-flow left ventricular assist devices. Can J Cardiol 2015;31:348–56. [DOI] [PubMed] [Google Scholar]
- 29.Hennig F, Stepanenko AV, Lehmkuhl HB, et al. Neurohumoral and inflammatory markers for prediction of right ventricular failure after implantation of a left ventricular assist device. Gen Thorac Cardiovasc Surg 2011;59:19–24. [DOI] [PubMed] [Google Scholar]
- 30.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010;87:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 2009;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabit CE, Chen P, Kim GH, et al. Elevated angiopoietin-2 level in patients With continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated With higher nonsurgical bleeding. Circulation 2016;134:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreassen AK, Nordøy I, Simonsen S, et al. Levels of circulating adhesion molecules in congestive heart failure and after heart transplantation. Am J Cardiol 1998;81:604–8. [DOI] [PubMed] [Google Scholar]
- 34.Pagani FD, Baker LS, Hsi C, et al. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest 1992;90:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azzawi M, Hasleton PS, Turner DM, et al. Tumor necrosis factor-alpha gene polymorphism and death due to acute cellular rejection in a subgroup of heart transplant recipients. Hum Immunol 2001;62:140–2. [DOI] [PubMed] [Google Scholar]
- 36.Rahmani M, Cruz RP, Granville DJ, et al. Allograft vasculopathy versus atherosclerosis. Circ Res 2006;99:801–15. [DOI] [PubMed] [Google Scholar]
- 37.Kamo T, Akazawa H, Suda W, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One 2017;12:e0174099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 39.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung ES, Packer M, Lo KH, et al. Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against congestive heart failure (ATTACH) trial. Circulation 2003;107: 3133–40. [DOI] [PubMed] [Google Scholar]
- 41.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248–61. [DOI] [PubMed] [Google Scholar]
- 42.Drekonja D, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection: a systematic review. Ann Intern Med 2015;162:630–8. [DOI] [PubMed] [Google Scholar]
- 43.Staley C, Kaiser T, Vaughn BP, et al. Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation. Microbiome 2018;6:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014;312:1772–8. [DOI] [PubMed] [Google Scholar]
- 45.Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017; 15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shikany JM, Demmer RT, Johnson AJ, et al. Association of dietary patterns with the gut microbiota in older, community-dwelling men. Am J Clin Nutr 2019;110:1003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:760–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


