Abstract
Combination therapy has become a cornerstone in cancer treatment to potentiate therapeutic effectiveness and overcome drug resistance and metastasis. In this work, we explore combination trials in breast cancer brain metastasis (BCBM), highlighting deficiencies in trial design and underlining promising combination strategies. On October 31, 2019, we examined ClinicalTrials.gov for interventional and therapeutic clinical trials involving combination therapy for BCBM, without limiting for date or location. Information on trial characteristics was collected. Combination therapies used in trials were analyzed and explored in line with evidence from the medical literature. Sixty-five combination therapy trials were selected (n=65), constituting less than 0.7% of all breast cancer trials. Most trials (62%) combined ≥2 chemotherapeutic agents. Chemotherapy with radiation was main-stay in 23% of trials. Trastuzumab was mostly used in combination (31%), followed by lapatinib (20%), and capecitabine (15%). Common strategies involved combining tyrosine kinase inhibitors with thymidylate synthase inhibitors (6 trials), dual HER-dimerization inhibitors (3 trials), and microtubule inhibitors and tyrosine kinase inhibitors (3 trials), and HER-dimerization inhibitors and tyrosine kinase inhibitors (3 trials). The combination of tucatinib and capecitabine yielded the highest objective response rate (83%) in early phase trials. The triple combination of trastuzumab, tucatinib, and capecitabine lowered the risk of disease progression or death by 52% in patients with HER2-positive BCBM. Combining therapeutic agents based on biological mechanisms is necessary to increase the effectiveness of available anti-cancer regimens. Significant survival benefit has yet to be achieved in future combination therapy trials. Enhancing drug delivery through blood-brain barrier permeable agents may potentiate the overall therapeutic outcomes.
Keywords: Combination therapy, breast cancer, brain metastasis, clinical trials, systematic review, meta-analysis
INTRODUCTION
Breast cancer is the most frequent neoplasm among women. Its rates are increasing in nearly every region globally, impacting 2.1 million women per year.1 Furthermore, it constitutes the greatest number of cancer-related deaths in women and has one of the highest risks for intracranial spread.2 Breast cancer brain metastases (BCBM) develop when cells spread from the primary breast tumor to the brain. It is estimated that up to 30% of all patients with cancer will eventually develop brain metastases,3 which portends a poor prognosis and a low quality of life.
With close to 10,000 clinical trials investigating breast cancer treatments, less than 1% of trials include patients with brain metastases.4 As such, there has been minimal development of new interventional drugs and diagnostic tools that can target or improve the diagnosis of BCBM.4, 5
In recent years, combining two or more therapeutic agents has become a cornerstone of cancer therapy. The combinatory effect of anti-cancer drugs targets key pathways in a characteristically synergistic or additive manner, which potentially reduces drug resistance, tumor growth and metastatic potential.6, 7 In breast cancer, a number of combinatory regimens have been explored in trials. Nevertheless, no novel therapies have strategically altered the current status quo. For this reason, this work will explore clinical trials that are using combination therapies to treat BCBM. The work will focus on characterizing the inadequacies associated with the clinical trials, and findings will highlight effective strategies and potential areas for improvement.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
On October 1, 2019, we explored ClinicalTrials.gov for all clinical trials involving combination therapies in the treatment of BCBM, without limiting for date or location. ClinicalTrials.gov is a large clinical trial registry that is updated on a weekly basis with new trial entries. It necessitates extensive information on trial profile, protocols and archival history for registration. Analyzing and extrapolating conclusions on clinical trials’ data from the registry has been previously explored.4
Following an elimination schema similar to Fares et al.4 17 trials were removed as they were non-interventional and non-therapeutic studies and/or clinical trials that do not list “breast cancer” or “brain metastasis” in the title or as a condition treated. Twenty trials were further determined to be diagnostic or non-therapeutic despite being listed as “interventional” and were excluded. In addition, as the focus of our analysis is on combination therapy trials, 55 trials did not constitute a combination of therapeutic regimens and were thus excluded (Supplementary Figure 1).
Data Collection
Collection of data included information on trial characteristics, including: phase (I, I/II, II, II/III, III), status (completed, active, recruiting, not recruiting, suspended, terminated, etc.), BCBM classification (newly diagnosed, progressive, recurrent, both), start and end dates, primary endpoints, selection criteria, sample size, study design, combination therapy used, location, results, and publication. Trial history of changes was obtained using the ClinicalTrials.gov archives site. To comply with Section 801 in the Food and Drug Administration Amendments Act of 2007,8 trial duration was defined by the study start date and the primary completion date. Endpoints were classified as based on objective response rate, clinical benefit rate, disease control rate, efficacy, progression-free survival, safety and toxicity, overall survival, and/or pharmacokinetics/pharmacodynamics.
Retrieving Publications
We used the ClinicalTrials.gov registry number (NCTID) to search for relevant publications on the PubMed/Medline and Embase/Scopus records. If the trial had a linked publication, the NCTID identifier was included as part of the original paper and the paper will appear in the search result. Associated publications were collected and reviewed by two investigators (J.F., D.K.) to identify publications reporting on primary results.
Ethical Approval
The epidemiological nature of the study did not necessitate ethical approval or informed consent.
Data Availability
Data supporting the results in the paper that are minimally required to replicate the outcomes of the study will be made available upon reasonable request.
RESULTS
Trial Characteristics
Only 65 trials met our criteria. Table 1 shows trial characteristics by phase. About 34% of the trials (22 trials) were for newly diagnosed or progressive BCBM. Only 1 trial (2%) targeted recurrent BCBM. Ten trials (15%) recruited both newly diagnosed/progressive and recurrent BCBM, and 32 trials (49%) did not specify the classification. Brain metastatic lesions were defined based on the RECIST guideline version 1.1.9 Trials that allowed patients that received prior systemic treatment necessitated a washout period before enrollment. This washout period ranged from one week to six months in some trials.
Table 1:
Clinical trials involving combination therapies for breast cancer brain metastases as found in ClinicalTrials.gov as of October 31, 2019 (n = 65).
| NA | Phase I | Phase I/II | Phase II | Phase III | Total | |
|---|---|---|---|---|---|---|
| Number of Trials | 1 (2%) | 9 (14%) | 8 (12%) | 39 (60%) | 8 (12%) | 65 (100%) |
| Trial Status | ||||||
| Completed | - | 4 | - | 12 | 3 | 19 (29%) |
| Active, not recruiting | 1 | 2 | 1 | 7 | 2 | 13 (20%) |
| Recruiting | - | 2 | 3 | 5 | 3 | 13 (20%) |
| Not yet recruiting | - | 1 | - | 1 | - | 2 (3%) |
| Enrolling by invitation | - | - | - | - | - | - |
| Terminated | - | - | 3 | 9 | - | 12 (18%) |
| Withdrawn | - | - | 1 | 1 | - | 2 (3%) |
| Unknown status | - | - | - | 4 | - | 4 (6%) |
| Estimated Enrollment | ||||||
| 0-10 | - | - | 3 | 6 | - | 9 (14%) |
| 11-50 | 1 | 8 | 2 | 22 | - | 33 (51%) |
| 51-100 | - | 1 | 2 | 4 | - | 7 (11%) |
| >100 | - | - | 1 | 7 | 8 | 16 (25%) |
| BCBM Classification | ||||||
| Newly diagnosed/progressive | - | - | 3 | 17 | 2 | 22 (34%) |
| Recurrent | - | 1 | - | - | - | 1 (2%) |
| Both | - | 3 | 3 | 3 | 1 | 10 (15%) |
| Not specified/other | 1 | 5 | 2 | 19 | 5 | 32 (49%) |
| Breast Cancer Subtype | ||||||
| HER2 – positive | 1 | 6 | 6 | 17 | 5 | 35 (54%) |
| HER2 – negative | - | - | 2 | - | - | 2 (3%) |
| Triple negative (ER-, PR-, HER2-) | - | - | 1 | 4 | 1 | 6 (9%) |
| Unspecified | - | 3 | 1 | 18 | 2 | 24 (37%) |
| Required Prior Therapy | ||||||
| WBRT and/or SRS | - | - | 2 | 6 | - | 8 (12%) |
| Chemotherapy | - | - | 2 | 5 | 2 | 9 (14%) |
| No treatment | - | 1 | - | 6 | 2 | 8 (12%) |
| No Requirement of Prior Therapy | 1 | 8 | 4 | 22 | 4 | 39 (60%) |
| Results Provided | - | 2 | 2 | 11 | 4 | 19 (29%) |
| Related Publication | - | 1 | 1 | 8 | 3 | 13 (20%) |
| Trial Location | ||||||
| North America (US/Canada) | 1 | 7 | 7 | 24 | 4 | 43 (66%) |
| Europe/UK/Russia | - | 2 | - | 7 | - | 9 (14%) |
| Asia/Australia | - | - | - | 6 | - | 6 (9%) |
| Intercontinental | - | - | 1 | 2 | 4 | 7 (11%) |
WBRT: whole brain radiation therapy, SRS: stereotactic radiosurgery.
Trial duration was available for all 9 phase I trials, 8 phase I/II trials, 39 phase II trials, and 8 phase III trials. Three phase I/II trials and 9 phase II trials were terminated. One phase I/II trial and 1 phase II trial were withdrawn. Median trial duration was 36 months for phase I trials and 55 months for phase III trials. Median trial duration including and not including terminated/withdrawn studies was 34 and 61 months for phase I/II trials and 36 and 43 months for phase II trials, respectively. As of October 7, 2019, 22 trials (34%) were yet to reach their primary completion date.
Trials were mostly in the early stages: 14% were phase I, 12% were phase I/II, and 60% were phase II. Phase III trials accounted for the most patients enrolled (80.6%) (Supplementary Figure 2). Although patients enrolled in phase III trials constituted approximately 81% of all patient enrollments, only 12% of the trials were in phase III. No phase IV trial was detected.
More than half of the trials were conducted in North America (43 trials, 66%). Europe, including UK and Russia, was the location of 14% of the trials, whereas Asia, including Australia, constituted 9%. Close to 11% of the trials were conducted intercontinentally. Of the terminated trials, 7 trials were being conducted in North America, 3 in Europe, and 2 were of an intercontinental setting (Table 1).
Twelve trials (23%) were terminated early. The major reason for termination was inadequate recruitment (7 trials). Other reasons included lack of efficacy (1 trial), unavailability of the drug (2 trials), feasibility (1 trial), and operational issues (1 trial). The median trial duration for terminated studies across all phases was 20 months (range 9 to 55 months).
Combination Strategies
Combining 2 or more chemotherapeutic agents was the most common strategy used in trials; 62% of trials followed such regimens (Table 2). Chemotherapy with whole brain radiation was the main-stay strategy in 15 trials (23%). The chemotherapeutic agents mostly used in combination were trastuzumab (19 trials, 29%), lapatinib (13 trials, 20%), and capecitabine (10 trials, 15%) (Table 2). Six trials used combinations of tyrosine kinase inhibitors and thymidylate synthase inhibitors. Different combinations of HER-dimerization inhibitors were used in 3 trials. In addition, tyrosine kinase inhibitors were used in 3 trials with microtubule inhibitors and in 3 trials with HER-dimerization inhibitors (Supplementary Table 1 and Supplementary Table 2).
Table 2:
Combination strategies of therapeutic agents in trials for breast cancer brain metastases as found in ClinicalTrials.gov as of October 9, 2019 (n = 65).
| Drug Description | NA | Phase I | Phase I/II | Phase II | Phase III | Total | |
|---|---|---|---|---|---|---|---|
| Number of Trials | 1 | 9 (14%) | 8 (12%) | 39 (60%) | 8 (12%) | 65 (100%) | |
| Combination Strategy | |||||||
| Chemotherapy Agents (≥2) | - | 4 | 5 | 26 | 5 | 40 (62%) | |
| Chemotherapy + WBRT | 1 | 4 | - | 9 | 1 | 15 (23%) | |
| Chemotherapy + SRS | - | 1 | 2 | 2 | - | 5 (8%) | |
| Chemotherapy + WBRT or SRS | - | - | 1 | - | - | 1 (2%) | |
| Chemotherapy + Surgery | - | - | - | - | 1 | 1 (2%) | |
| WBRT + SRS | - | - | - | 1 | 1 | 2 (3%) | |
| Chemo + Diet | - | - | - | 1 | - | 1 (2%) | |
| Therapeutic Agents | |||||||
| Abemaciclib | CDK inhibitor | - | 1 | - | - | - | 1 (2%) |
| Afatinib | Tyrosine kinase inhibitor | - | - | - | 1 | - | 1 (2%) |
| Atezolizumab | PD-L1 inhibitor | - | - | - | 2 | 2 | 4 (6%) |
| Bevacizumab | VEGF-A inhibitor | - | - | - | 5 | - | 5 (8%) |
| BKM120 | PI3K inhibitor | - | - | 1 | 1 | - | 2 (3%) |
| Cabazitaxel | Taxane, microtubule inhibitor | - | - | - | 1 | - | 1 (2%) |
| Cabozantinib | Tyrosine kinase inhibitor | - | - | - | 1 | - | 1 (2%) |
| Caelyx | Topoisomerase inhibitor | - | - | - | 1 | - | 1 (2%) |
| Capecitabine | Thymidylate synthase inhibitor | - | 1 | - | 8 | 1 | 10 (15%) |
| Carboplatin | Alkylating agent, Cross-links DNA | - | - | 1 | 1 | - | 2 (3%) |
| Cisplatin | Alkylating agent, Cross-links DNA | - | - | - | 4 | - | 4 (6%) |
| Cytarabine | DNA polymerase inhibitor | - | - | - | 1 | - | 1 (2%) |
| Docetaxel | Taxane, microtubule inhibitor | - | - | - | 2 | - | 2 (3%) |
| Durvalumab | PD-1 inhibitor | 1 | - | - | - | - | 1 (2%) |
| Efaproxiral | Propanoic acid | - | - | - | - | 1 | 1 (2%) |
| Etoposide | Topoisomerase inhibitor | - | - | - | 2 | - | 2 (3%) |
| Everolimus | mTOR inhibitor | - | - | 1 | 2 | - | 3 (5%) |
| Galinpepimut-S | WT1 peptide vaccine | - | - | 1 | - | 1 (2%) | |
| GDC-0084 | PI3K inhibitor | - | - | - | 1 | - | 1 (2%) |
| Gemcitabine | Nucleoside analog | - | - | - | 1 | - | 1 (2%) |
| GRN1005 | Taxane, microtubule inhibitor | - | - | - | 1 | - | 1 (2%) |
| GW572016 | Tyrosine kinase inhibitor | - | - | - | - | 1 | 1 (2%) |
| Indinavir+Ritonavir | Protease inhibitors | - | - | - | 1 | - | 1 (2%) |
| Iniparib | PARP inhibitor | - | - | - | 1 | - | 1 (2%) |
| Irinotecan | Topoisomerase inhibitor | - | - | - | 4 | - | 4 (6%) |
| Lapatinib | Tyrosine kinase inhibitor | - | 3 | 1 | 7 | 2 | 13 (20%) |
| Letrozole | Aromatase inhibitor | - | - | - | - | 1 | 1 (2%) |
| Methotrexate | Dihydrofolate reductase inhibitor | - | - | 1 | 1 | - | 2 (3%) |
| Nivolumab | PD-1 inhibitor | - | 1 | - | - | - | 1 (2%) |
| ONC201 | Dopamine receptor antagonist | - | - | - | 1 | - | 1 (2%) |
| Paclitaxel | Taxane, microtubule inhibitor | - | - | - | 1 | 1 | 2 (3%) |
| Peginterferon Alfa-2a | Alpha interferon | - | - | - | 1 | - | 1 (2%) |
| Pembrolizumab | PD-1 inhibitor | - | - | 2 | - | - | 2 (3%) |
| Pertuzumab | HER-dimerization inhibitor | - | - | - | 2 | 1 | 3 (5%) |
| Pyrotinib | Tyrosine kinase inhibitor | - | - | - | 2 | - | 2 (3%) |
| RO4929097 | Gamma secretase inhibitor | - | - | 1 | - | - | 1 (2%) |
| Sorafenib | RAF kinase inhibitor | - | 1 | - | - | - | 1 (2%) |
| Sunitinib | Tyrosine kinase inhibitor | - | - | - | 1 | - | 1 (2%) |
| TDM-1 | HER-dimerization inhibitor/microtubule inhibitor | - | 1 | - | - | - | 1 (2%) |
| Temozolomide | Alkylating agent | - | 1 | 2 | 3 | - | 6 (9%) |
| Tesevatinib | Tyrosine kinase inhibitor | - | - | 1 | - | - | 1 (2%) |
| Topotecan | Topoisomerase inhibitor | - | - | - | 1 | - | 1 (2%) |
| Trastuzumab | HER2 receptor inhibitor | - | 2 | 4 | 10 | 3 | 19 (29%) |
| Tremelimumab | CTLA-4 inhibitor | 1 | - | - | - | - | 1 (2%) |
| Tucatinib | HER2 receptor inhibitor | - | 2 | - | 1 | 1 | 4 (6%) |
| Veliparib | PARP inhibitor | - | - | - | 1 | - | 1 (2%) |
| Vinorelbine | Vinca alkaloid, tubulin inhibitor | - | - | - | 3 | 1 | 4 (6%) |
| Vorinostat | Histone deacetylase inhibitor | - | 1 | - | - | - | 1 (2%) |
WBRT: whole brain radiation therapy, SRS: stereotactic radiosurgery.
Trial Results and Publication
Of all, only 13 trials (20%) had publications linked to their identification number (Table 3).10-22 Nineteen trials (29%) provided results on ClinicalTrials.gov, of which 7 had published their findings. Of the 19 completed trials, only 6 trials (32%) were published, even after 160 months of trial initiation (Supplementary Figure 3). Of the 13 published trials, median time from trial initiation to completion date and from trial initiation to publication date was 38 months and 55 months, respectively.
Table 3:
Clinical findings of published combination therapy trials in breast cancer brain metastases, as of October 31, 2019 (n=13)
| Authors | Year | Trial | NCTID | Phase | Number Enrolled |
Inclusion Criteria |
Primary Outcome | Result |
|---|---|---|---|---|---|---|---|---|
| Morikawa et al.10 | 2017 | Intermittent High-Dose Lapatinib in Tandem With Capecitabine for HER2 Overexpressed/Amplified Metastatic Breast Cancer With Central Nervous System (CNS) Metastases | NCT02650752 | 1 | 11 | HER2+ BCBM | Maximum tolerated dose | No improvement in survival |
| Hurvitz et al.11 | 2018 | Phase 1b/2 Trial Using Lapatinib, Everolimus and Capecitabine for Treatment of HER-2 Positive Breast Cancer With CNS Metastasis | NCT01783756 | 1/2 | 9 | HER2+ BCBM | Objective response rate | The combination of lapatinib, everolimus, and capecitabine is well tolerated and yielded a 27% response rate in the CNS at 12 weeks in heavily pretreated participants |
| Murthy et al.12 | 2018 | A Study of Tucatinib vs. Placebo in Combination With Capecitabine & Trastuzumab in Patients With Advanced HER2+ Breast Cancer (HER2CLIMB) | NCT02614794 | 2 | 612 | HER2+ BCBM | Progression-free survival | Tucatinib in combination with capecitabine and trastuzumab had acceptable toxicity and showed preliminary anti-tumor activity |
| Murthy et al.23 | 2020 | The triple combination lowered the risk of disease progression or death by 52% | ||||||
| Yardley et al.13 | 2018 | Cabazitaxel Plus Lapatinib as Therapy for HER2-Positive Metastatic Breast Cancer Patients With Intracranial Metastases | NCT01934894 | 2 | 11 | HER2+ BCBM | Objective response rate, tolerated dose and toxicity | The combination of cabazitaxel plus lapatinib was not feasible because of toxicity and because no objective CNS activity was seen in the 5 evaluable patients |
| Cortés et al.14 | 2015 | Lux-Breast 3; Afatinib Alone or in Combination With Vinorelbine in Patients With Human Epidermal Growth Factor Receptor 2 (HER2) Positive Breast Cancer Suffering From Brain Metastases | NCT01441596 | 2 | 121 | HER2+ BCBM | Patient benefit rate | Patient benefit with afatinib-containing treatments was not different from that in patients given investigator's choice of treatments, and afatinib-containing treatments were less tolerated |
| Van Swearingen et al.15 | 2018 | A Study Of Everolimus, Trastuzumab And Vinorelbine In HER2-Positive Breast Cancer Brain Metastases | NCT01305941 | 2 | 32 | HER2+ BCBM | Objective response rate | Intracranial response rate to the triple therapy was low and progression-free survival/overall survival was like historical control |
| Wu et al.16 | 2015 | Bevacizumab With Etoposide and Cisplatin in Breast Cancer Patients With Brain and/or Leptomeningeal Metastasis | NCT01281696 | 2 | 40 | Histological confirmed invasive breast cancer | Objective response rate | Bevacizumab combined with etoposide and cisplatin exhibited promising efficacy in breast cancer patients with leptomeningeal carcinomatosis |
| Bachelot et al.17 | 2013 | Lapatinib Ditosylate and Capecitabine in Treating Patients With Stage IV Breast Cancer and Brain Metastases | NCT00967031 | 2 | 45 | HER2+ BCBM | Objective response rate | Objective CNS response was observed in 65·9%of cases; all were partial responses. The combination of lapatinib and capecitabine is active as first-line treatment of HER2+ BCBM |
| Cao et al.18 | 2015 | Radiation Therapy With or Without Temozolomide in Treating Women With Brain Metastases and Breast Cancer | NCT00875355 | 2 | 100 | BCBM | Objective response rate | WBRT combined with TMZ did not significantly improve local control and survival in patients with BMs from breast cancer |
| Lin et al.19 | 2011 | Brain Metastases In ErbB2-Positive Breast Cancer | NCT00437073 | 2 | 22 | HER2+ BCBM | Objective response rate | The study was closed early due to excess toxicity and lack of efficacy in the lapatinib plus topotecan arm. No responses were observed in the lapatinib plus topotecan arm. Promising indications of CNS activity were noted for lapatinib plus capecitabine. The combination of lapatinib plus topotecan was not active and was associated with excess toxicity |
| Pivot et al.20 | 2015 | Lapatinib Plus Capecitabine Versus Trastuzumab Plus Capecitabine in ErbB2 (HER2) Positive Metastatic Breast Cancer | NCT00820222 | 3 | 540 | HER2+ BCBM | CNS relapse | No difference was detected between lapatinib-capecitabine and trastuzumab-capecitabine for the incidence of CNS metastases; serious adverse events were reported in 13% and 17% of patients, respectively |
| Brown et al.21 | 2017 | Stereotactic Radiation Therapy With or Without Whole-Brain Radiation Therapy in Treating Patients With Brain Metastases | NCT00377156 | 3 | 213 | Brain Metastases | Neurocognitive progression | No significant differences in survival according to receipt of WBRT. The use of SRS alone, compared with SRS combined with WBRT, resulted in less cognitive deterioration |
| Prat et al.22 | 2016 | Study Comparing GW572016 And Letrozole Versus Letrozole In Subjects With Advanced Or Metastatic Breast Cancer | NCT00073528 | 3 | 1285 | HER2+ BCBM | Progression-free survival | Patients with HR-positive/HER2-negative disease with a HER2-enriched profile may benefit from lapatinib in combination with endocrine therapy. Patients with luminal A/HER2-negative metastatic breast cancer might be good candidates for letrozole monotherapy in the first-line setting |
Phase I and I/II Trials
There were 9 phase I trials (14%) and 8 phase I/II trials (12%). Of these, 14 trials had a primary outcome related to maximal tolerated dosage and safety/toxicity. Only 1 trial included a secondary pharmacokinetics and/or pharmacodynamics endpoint. Four trials were randomized. There were 12 trials that targeted participants with HER2-positive breast cancer only. Two trials required participants who had received whole brain radiation therapy, another two trials required participants to have received at least one line of chemotherapy, while one trial required patients who had not received any treatment (Table 1).
The average observed sample size for phase I and I/II trials – excluding terminated and withdrawn trials – was 39 patients. Observed enrollment met or exceeded planned sample size in 11 trials. Six trials could not meet the planned sample size. Three trials were terminated. Reasons included: slow enrollment, discontinuation of drug, sponsor revision and assessment of feasibility. One trial was withdrawn for major revision of protocol.
Phase II Trials
Approximately 60% of the conducted BCBM trials were phase II trials (n = 39). Most of the trials followed the single group assignment (67%) and measured objective response rate as the primary outcome (59%). Only 9 trials (23%) were randomized, whereas 36 trials (97%) were open label (Table 4). Seventeen phase II trials targeted patients with HER2-positive breast cancer and 4 trials were for patients with triple negative breast cancer, exclusively (Table 1).
Table 4:
Primary outcomes of Phase II clinical trials (n=39)
| Number | Percent | |
|---|---|---|
| Interventional Model | ||
| Single Group Assignment | 26 | 66.7 |
| Parallel Assignment | 11 | 28.2 |
| Not Specified | 2 | 5.1 |
| Treatment Allocation | ||
| Non-randomized | 30 | 76.9 |
| Randomized | 9 | 23.1 |
| Masking | ||
| Open Label | 36 | 92.3 |
| Masked | 1 | 2.6 |
| Not Specified | 2 | 5.1 |
| Main Primary Endpoint | ||
| Progression-free Survival | 7 | 17.9 |
| Objective Response Rate | 23 | 59.0 |
| Clinical Benefit Rate | 2 | 5.1 |
| Relapse Rate | 2 | 5.1 |
| Safety / Toxicity | 2 | 5.1 |
| Efficacy | 3 | 7.7 |
The average observed sample size for phase II trials – excluding terminated and withdrawn trials – was 82 patients. Observed enrollment met planned sample size in 16 trials and exceeded it in 5 trials. In 16 trials, enrollment number fell short of the planned sample size. Two trials did not specify the planned sample size; thus, comparison could not be drawn.
Eight of the 39 trials had published results related to their primary outcomes (Table 3); 3 of the published studies reported positive outcomes. One trial (NCT02614794) exploring progression-free survival reported that tucatinib in combination with capecitabine and trastuzumab exhibited preliminary anti-tumor activity.12, 23 Objective response rate reached 83% in the combination of tucatinib with capecitabine, 40% in the combination of tucatinib with trastuzumab, and 61% in the combination of tucatinib with both capecitabine and trastuzumab.12 In another trial (NCT01281696), bevacizumab combined with etoposide and cisplatin exhibited promising efficacy in breast cancer patients with metastatic spread.16 A third trial (NCT00967031) concluded that the combination of lapatinib and capecitabine is active as first-line treatment of brain metastases from HER2-positive breast cancer.17
Phase III Trials
All phase III trials (n = 8) were randomized controlled trials. The average observed sample size for phase III trials was 1671 patients. Observed enrollment met planned sample size in two trials and exceeded planned sample size in 3 trials. One trial did not meet the planned sample size and 2 trials did not provide the planned sample size to draw comparisons. Six trials had progression-free survival as their primary outcome. One trial had the primary outcome as the overall survival, while another measured neurocognitive progression.
Three trials had published their results (Table 3). One trial (NCT00073528) reported a positive outcome, whereby patients with HER2-enriched profile may benefit from lapatinib in combination with endocrine therapy. The trial also led to the belief that patients with luminal A/HER2-negative metastatic breast cancer might be good candidates for letrozole monotherapy in the first-line setting regardless of visceral disease and number of metastases.22
As the overall rate of success in phase III trials was low, we queried ClinicalTrials.gov and PubMed/Medline for the phase II trials that preceded using the same drug and indication (Table 5). All trials had antecedent phase II studies: 3 used objective response rate as the primary outcome, 2 used progression-free survival, 1 trial measured overall survival, 1 trial focused on adverse events, and another trial assessed delay in recall. Two of the antecedent trials were randomized, 5 were single-arm trials, and one did not provide study design. The average time from the start of phase II till the completion of phase III averaged 4.6 years (range 1.2 to 8.7 years).
Table 5:
Details of Phase III trials (n = 8) and associated Phase II trials
| Intervention | Phase IIII NCTID |
Indication | Phase III Status |
Phase III Completion Date |
N (III) |
Endpoint (III) | Result | Preceding Phase II? |
Phase II NCTID |
N (II) |
Endpoint (II) | Randomized? | Start of Phase II to End of Phase III (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tucatinib + Trastuzumab | NCT03975647 | HER2+ BCBM | Recruiting | April 2024 | 460 | Progression-Free Survival | - | Yes | NCT02614794 | 612 | Progression-Free Survival | Yes | 56 |
| Atezolizumab + Surgery | NCT03281954 | Triple Negative BCBM | Recruiting | December 2023 | 1520 | Progression-Free Survival | - | Yes | NCT03483012 | 45 | Progression-Free Survival | No | 67 |
| Pertuzumab + Trastuzumab + Paclitaxel + Atezolizumab | NCT03199885 | HER2+ BCBM | Recruiting | December 2020 | 600 | Progression-Free Survival | - | Yes | NCT03125928 | 50 | Adverse Events | No | 42 |
| Lapatinib + Capecitabine vs Trastuzumab + Capecitabine | NCT00820222 | HER2+ BCBM | Completed | June 2012 | 540 | Progression-Free Survival | No difference between regimens | Yes | NCT00967031 | 45 | Objective Response Rate | No | 38 |
| Lapatinib + Trastuzumab | NCT00490139 | HER2+ BCBM | Active, not recruiting | December 2013 | 8382 | Progression-Free Survival | Did not meet anticipations | Yes | NCT00470704 | 116 | Objective Response Rate | No | 79 |
| SRS + WBRT | NCT00377156 | BCBM | Active, not recruiting | October 2014 | 213 | Neurocognitive Progression | Less cognitive deterioration | Yes | NCT01227954 | 113 | Delay in Recall | No | 43 |
| WBRT + Oxygen + Efaproxiral | NCT00083304 | BCBM | Completed | June 2007 | 368 | Overall Survival | - | Yes | NCT00004202 | - | Overall Survival | - | 104 |
| Lapatinib + Letrozole | NCT00073528 | ER+ BCBM | Completed | June 2008 | 1285 | Progression-Free Survival | Increased progression-free survival | Yes | NCT00422903 | 92 | Objective Response Rate | Yes | 14 |
DISCUSSION
We previously discussed the inadequacies in clinical trials in breast cancer. The same trend of trial design error continues to be prevalent in combination therapy trials for BCBM.4 Continuing trials that do not show clinical benefit, poor enrollment strategies, disproportionate distribution of patients among trials, and lack of result publication are major trial mishaps that need to be addressed. The low survival outcomes in BCBM necessitate the adoption of new strategies to target the resistance and adaptation of metastatic cancer cells to current treatments. Combining chemotherapeutic agents with one another and/or with radiotherapy provided an alternative approach that can prevent cancer cell resistance and sensitize the metastatic cells to current regimens. Nonetheless, the effectiveness of novel combination therapies relies on methodically designing clinical trials, efficiently accruing and staying up-to-date with patients, and publishing results in impactful outlets. As such, proper evaluation and analysis of past and current clinical trials using combination therapies is vital so that BCBM loses its ‘end-stage disease’ label.
Inadequacies in Combination Trials
Only 65 combination therapy trials – as of October 11, 2019 – have been found to be interventional. Clinical trials in BCBM constitute less than 1% of the total number of trials in breast cancer.4 Combination trials in BCBM are rare. Out of 9355 registered clinical trials for breast cancer, only 65 were combination trials for BCBM, constituting 0.69% of all trials.
The low number of combination trials targeting BCBM can be attributed to a variety of factors. The lack of mono-therapeutic agents that can be candidates for combination present a major limitation. So far, the development of novel treatments targeting metastatic brain tumors has not been congruent to the dire needs of the patients with BCBM. Furthermore, the fact that diagnosis of brain metastasis is associated with poor prognostic outcomes drives trial coordinators and sponsors to exclude patients with BCBM to avoid affecting the survival and outcome metrics of the trial. Moreover, the blood-brain barrier (BBB) poses its own challenges as it provides metastatic cells in the brain with a safe haven, unreachable by most therapeutic regimens.24
More than one-third of the combination trials in BCBM are set to be completed within the next 10 years. Combination therapy as a strategy against difficult cancers gained a lot of steam in the past decade. The synergistic and/or additive targeting of multiple survival pathways in cancer cells provides a new approach in the fight against BCBM.25 In addition, the fact that the therapeutic agents used in combination are often FDA-approved proves to be cost-effective in terms of trial registration and conduction. In addition, the development of new diagnostic modalities improved the overall detection capabilities of BCBM and allowed the tracking of metastatic progression and clinical tumor benefit.5
Trials using combination therapies continue to be mostly registered from North America and Europe. East Mediterranean, South American and African trials were nil. Lack of resources, collaborative efforts can be possible reasons to the scarcity of trials from these regions.4 In addition, trials need not be registered in clincaltrials.gov in many nations, which can be the reason why we have failed to detect any trials from those regions as well.
Most of the terminated trials suffered from slow or poor accrual. In general, recruitment has been inefficient to amp the numbers of participants in clinical trials.26 Health communication strategies using media portals and advertisement, until now, have failed to improve participant numbers.27 Nevertheless, the proper utilization of electronic health records (EHR) to screen for candidate patients has been shown to increase participation rates in clinical trials.28 With the increasing number of academic medical centers that are adopting EHR systems, recruiting patients can become much more efficient and faster. Moreover, physicians, the care providers to their patients, must be responsible to be acquainted with ongoing trials and recommend adequate patients for recruitment. It has been shown that patients are more likely to join a clinical trial if it is suggested by their physician.29
At the moment, the management system of clinical trials does not halt ineffective therapies in early development, which leads to an imbalance in the recruitment of patients across trials. In BCBM, 12% of the combination therapy trials that were in phase III recruited more than 80% of the total number of participants. As such, a failure at this stage could indicate that accrued patients may have been subjects to inefficient treatments.4
Only 20% of all trials and 32% of completed trials were published. The striking low number of published trials may be potentially be due to the negative trial results and the lack of interest of authors and/or editors in publishing such results.4 Nevertheless, negative results are vital to understand the causes of therapeutic failure and so that combinatorial therapies following similar designs or strategies are not repeated in the future (reviewed in Fares et al.4).
Combination Strategies and Therapeutic Effect
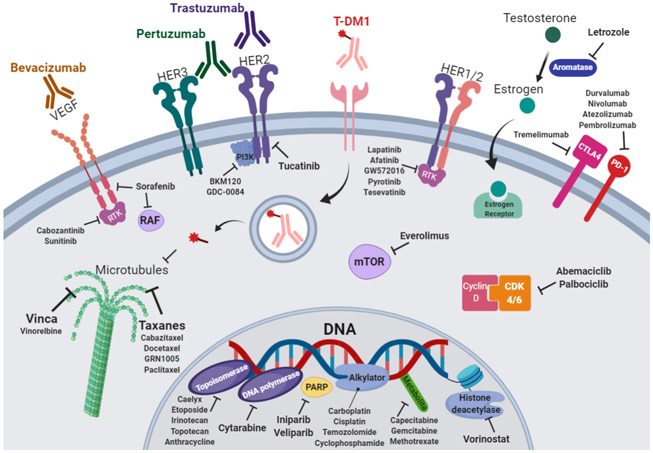
Combination therapy is based on the concept of one drug augmenting the activity of another drug to counter one – or more – of the hallmarks of cancer (Figure 1). The most tested chemotherapeutic agent in combination trials was trastuzumab (19 trials, 29%). The introduction of this anti-HER2 monoclonal antibody therapy revolutionized the treatment of HER2-positive metastatic breast cancer.30 In combination, trastuzumab was shown to increase the objective response rate of chemotherapy agents, like anthracycline and cyclophosphamide, in metastatic breast cancer that overexpresses HER2 by 18%.31, 32 It was also shown to prolong median survival by 5 months and reduce risk of death by 33%.31, 32 Later, dual anti-HER2 blockade strategies gained more steam as the combination of trastuzumab and pertuzumab proved to be more effective than monotherapy in preclinical studies,33, 34 and early breast cancer clinical trials.35 The addition of pertuzumab decreased disease recurrence by 1.6% and increased progression-free survival by 0.9% at the 3-year interval.35 In newly diagnosed patients with advanced HER2-positive breast cancer, it is now recommended to use dual anti-HER2 inhibitors through trastuzumab and pertuzumab in addition to standard chemotherapy.36 Trastuzumab emantasine (T-DM1), a novel dual HER2 and microtubule inhibitor, also decreased the risk of recurrence of invasive breast cancer or death by 50% when added to trastuzumab than when trastuzumab was used alone.37 However, further use of trastuzumab deruxtecan – an antibody-drug conjugate composed of an anti-HER2 antibody, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor – did not significantly improve clinical outcomes.38 The addition of chemotherapeutic agents like docetaxel, a taxane microtubule inhibitor, proved to be safe and showed a 15.7-month survival benefit in patients with advanced breast cancer.39-41 Nevertheless, the combination did not significantly alter the incidence of CNS metastases.42 Sunitinib, a tyrosine kinase inhibitor, plus trastuzumab demonstrated antitumor activity in patients with HER2-positive advanced breast cancer.43 Objective response rate reached 44% and the clinical benefit rate was 59% in patients who were treatment-naïve or had received only adjuvant therapy.43 New investigational drugs that target the HER2 receptor, like tucatinib, have also been deployed against BCBM. In an early phase trial, patients with BCBM receiving a combination of tucatinib and trastuzumab showed 40% response to treatment.12 Nevertheless, the combination of tucatinib and capecitabine, a thymidylate synthase inhibitor, yielded an 83% objective response rate.12 Most recently, the addition of trastuzumab to capecitabine and tucatinib lowered the risk of disease progression or death by 52% in patients with HER2-positive BCBM.23
Figure 1.
Mechanism of action of therapeutic agents used in combination trials for breast cancer brain metastasis.
The combination of thymidylate synthase inhibitors (e.g. capecitabine) with other tyrosine kinase inhibitors (e.g. lapatinib) was the most common combination strategy used in clinical trial (6 trials). Thymidylate synthase inhibitors have been among the most effective chemotherapies used in the treatment of cancer, since the 1940s.44 Thymidylate synthase is a key enzyme for DNA replication in metastatic cancer cells, making it an important target for therapy.45 Lapatinib is a dual tyrosine kinase inhibitor, which interrupts the HER2/neu and epidermal growth factor receptor (EGFR) pathways.46 In phase III clinical trials, the combination of lapatinib and capecitabine was shown to be superior to monotherapy alone in women with breast cancer who had received previous treatments.47 Trial results showed a 51% reduction in disease progression and increase of 4 months in median progression-free survival.47 Two systematic reviews on patients with HER2-positive BCBM have shown that the combination improved both overall survival and progression-free survival in refractory patients to first-line treatment.48, 49 Bachelot et al.17 explored the efficacy of this combination as a first-line therapy in a phase II trial. They found that lapatinib and capecitabine was active in the treatment of brain metastases from HER2-positive breast cancer, whereby partial responses were detected in 66% of recruited patients.17 More recently, a phase I trial exploring this combination showed that patients with HER2-positive BCBM, higher performance status, cranial-only involvement, and the absence of non-CNS disease were independently associated with improved survival.10 The relative success of this combination pushed for the addition of other chemotherapeutic agents in the hopes of increasing the therapeutic efficacy. The addition of everolimus, an mTOR inhibitor, yielded a 27% response rate in the CNS in patients with HER2-positive BCBM.11
The tumor response witnessed as a result of different combinations pushed for trials that compare the efficacy of two combination therapies. A phase III trial compared the combination of lapatinib and capecitabine to trastuzumab and capecitabine and showed no difference in efficacy or clinical benefit; however, patients with the trastuzumab and capecitabine had more serious adverse events reported.20
Still, combination therapy is not always guaranteed to provide clinical benefit as the accumulation of chemotherapeutic agents can lead to more toxicity.50 Microtubule inhibitors were used in combinations with tyrosine kinase inhibitors, HER2-dirmerization inhibitors, and VEGF-A inhibitors. In a dose-finding trial, cabazitaxel, a taxane, was combined with lapatinib for the treatment of BCBM. Unfortunately, the trial was stopped as a result of reported toxicity and the absence of objective response in patients.13 Nonetheless, taxanes showed some efficacy in other combinations. As reported earlier, docetaxel showed benefit when combined with trastuzumab and pertuzumab.39 Beyond taxanes, vinorelbine, a vinca alkaloid and tubulin inhibitor, was combined with afatinib in patients with BCBM. Nevertheless, no patient benefit was obtained and treatment was less tolerated.14 Vinorelbine, unlike docetaxel,51 also failed to show an intracranial response in combination with trastuzumab or everolimus 15. Bevacizumab, a VEGF-A inhibitor, was added to paclitaxel, a taxane, in patients with metastatic breast cancer. Initial therapy prolonged median progression-free survival by 5.9 months but did not alter overall survival.52 This paclitaxel-based combination therapy was compared to another involving sunitinib to determine the best possible matchup. In a phase III, the combination of bevacizumab and paclitaxel was shown to be superior as it was more well-tolerated and led to an increase in progression-free survival by 1.8 months and overall survival by 8%.53 Bevacizumab also showed some benefit in patients with breast cancer leptomeningeal metastasis when combined with etoposide and cisplatin, exhibiting a CNS-specific response rate in 60% of the patients.16
Lately, the combination of endocrine inhibitors with hormone therapy has been favored over chemotherapy for the treatment of metastatic breast cancer. In a phase II trial of premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer, the median progression-free survival increased 6 months with palbociclib plus endocrine therapy group versus the capecitabine group 54. Nonetheless, treatment-related adverse events like neutropenia was worse and more common in the palbociclib plus endocrine therapy arm 54.
The rise of immunotherapy and its demonstrated therapeutic potential with immune checkpoint inhibitors warranted the use of these agents in combination trials to test their efficacy. Dual blockade of PD-1/PD-L1 and CTLA-4 induced long-term responses and increased survival in advanced malignancies, like melanoma and lung cancer.55-58 In metastatic breast cancer, monotherapy with immune checkpoint inhibitors exhibited safe and promising outcomes in early phase trials.59-62 The combination of immune checkpoint inhibitors with T-DM1 has shown survival benefit in preclinical studies for advanced breast cancer.63 In advanced triple negative breast cancer, atezolizumab with nab-paclitaxel increased progression-free survival by 1.7 months and overall survival by 3.7 months among patients in a phase III trial.64 Patients with PD-L1-positive tumors demonstrated a further increase in progression-free survival by 2.5 months and overall survival by 9.5 months.64 In BCBM specifically, combination trials involving PD-1/PD-L1 inhibitors like atezolizumab, durvalumab, nivolumab, and pembrolizumab, and CTLA-4 inhibitors like tremelimumab are ongoing.
Combining chemotherapeutic agents might not always be beneficial. Drug-drug interactions can portend increased toxicity or lesser efficacy to the patients. For this reason, the concept of combining cytotoxic agents to radiation therapy emerged.65 Such combinations exhibit better tolerability and lesser adverse events.65-67 Radiation enhances the immune response against brain metastatic cancer cells through increasing the release of tumor antigens and DNA, and promoting the secretion of cytokines in the tumor microenvironment.68 In premenopausal women with advanced breast cancer, irradiation increased overall survival to 54% at the 10-year interval after undergoing a mastectomy and receiving cyclophosphamide, methotrexate, and fluorouracil.69 In patients with BCBM, whole brain radiation therapy (WBRT) with temozolomide, an alkylating agent, did not significantly improve tumor response and survival.18 Moreover, the combination of WBRT with stereotactic radiosurgery (SRS) did not show significant differences in survival.21 Eventually, monotherapy with SRS was found to lead to less cognitive deterioration.21
Limitations
This study focused on combination therapy trials that treated patients with metastatic breast cancer to the brain. To avoid selection bias, we had two authors (J.F. and D.K.) revise all trials identified and the trial selection steps. The incorrect registration of data in ClinicalTrials.gov has been reported before.70 Still, this study is impactful and unique in the way it provides an overview on the landscape of combination trials in BCBM.
CONCLUSION
Comprehending the full extent of effectiveness of targeted cancer treatments depends on discovering the best combination therapies. Current combination therapy trials in BCBM suffer from lack of trials, slow accrual, inadequate trial design, scarcity of published results, and positive therapeutic outcomes. As the BBB poses a challenge to drug delivery, using drugs that are known to cross the BBB in combination with effective anti-cancer agents may potentiate the overall therapeutic outcomes. In addition, elucidating the mechanism of action of combination therapy targets will allow better understanding of the therapeutic effect and the development of innovative targeted strategies.
Supplementary Material
Novelty & Impact Statement.
Advances in combination therapy have created new opportunities in cancer therapeutics, especially with regard to combatting drug resistance and tumor metastasis. Here, the authors surveyed data from clinical trials registered in clinicaltrials.gov to identify combination strategies effective specifically against breast cancer brain metastases (BCBM). Of multiple combination strategies identified, those employing thymidylate synthase inhibitors, HER2 inhibitors, and microtubule inhibitors were notably more common. The combination of trastuzumab, tucatinib, and capecitabine cut risk of disease progression or death from BCBM by more than half. The findings warrant investigation of mechanisms underlying effective therapeutic combinations to better understand their actions.
Acknowledgments
Funding: This work was supported by: NIH grants R35CA197725 (M.S.L.), R01NS87990 (M.S.L.), and R01NS093903 (M.S.L.).
List of Abbreviations
- BBB
blood-brain barrier
- BCBM
breast cancer brain metastases
- CNS
central nervous system
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- EGFR
epidermal growth factor receptor
- FDA
Food and Drug Administration
- HER
electronic health records
- HER
human epidermal growth factor receptor
- PD-1/PD-L1
programmed cell death protein 1/ programmed death-ligand 1
- SRS
stereotactic radiosurgery
- T-DM1
trastuzumab emantasine
- VEGF-A
vascular endothelial growth factor A
- WBRT
whole brain radiation therapy
Footnotes
Conflict of Interest: None declared
REFERENCES
- 1.Organization WH. Breast cancer, vol. 2019, 2019. [Google Scholar]
- 2.Surgeons AAoN. Metastatic Brain Tumors, 2019.
- 3.Loeffler JS, Wen P. Epidemiology, clinical manifestations, and diagnosis of brain metastases. Wolters Kluwer Health 2018. [Google Scholar]
- 4.Fares J, Kanojia D, Cordero A, Rashidi A, Miska J, Schwartz CW, Savchuk S, Ahmed AU, Balyasnikova IV, Cristofanilli M, Gradishar WJ, Lesniak MS. Current state of clinical trials in breast cancer brain metastases. Neuro-Oncology Practice 2019;6: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fares J, Kanojia D, Rashidi A, Ahmed AU, Balyasnikova IV, Lesniak MS. Diagnostic Clinical Trials in Breast Cancer Brain Metastases: Barriers and Innovations. Clin Breast Cancer 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, De P, Krie A, Piccioni DE, Miller VA, Ross JS, Benson A, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med 2019;25: 744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduction and Targeted Therapy 2020;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Congress t . Food and Drug Administration Amendments Act of 2007 2007.
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45: 228–47. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa A, Jordan L, Rozner R, Patil S, Boire A, Pentsova E, Seidman AD. Characteristics and Outcomes of Patients With Breast Cancer With Leptomeningeal Metastasis. Clin Breast Cancer 2017;17: 23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurvitz S, Singh R, Adams B, Taguchi JA, Chan D, Dichmann RA, Castrellon A, Hu E, Berkowitz J, Mani A, DiCarlo B, Callahan R, et al. Phase Ib/II single-arm trial evaluating the combination of everolimus, lapatinib and capecitabine for the treatment of HER2-positive breast cancer with brain metastases (TRIO-US B-09). Ther Adv Med Oncol 2018;10: 1758835918807339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy R, Borges VF, Conlin A, Chaves J, Chamberlain M, Gray T, Vo A, Hamilton E. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018;19: 880–8. [DOI] [PubMed] [Google Scholar]
- 13.Yardley DA, Hart LL, Ward PJ, Wright GL, Shastry M, Finney L, DeBusk LM, Hainsworth JD. Cabazitaxel Plus Lapatinib as Therapy for HER2 + Metastatic Breast Cancer With Intracranial Metastases: Results of a Dose-finding Study. Clinical breast cancer 2018;18: e781–e7. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, Le Rhun E, Espie M, Kim S-B, Schneeweiss A, Sohn JH, Nabholtz J-M, et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol 2015;16: 1700–10. [DOI] [PubMed] [Google Scholar]
- 15.Van Swearingen AED, Siegel MB, Deal AM, Sambade MJ, Hoyle A, Hayes DN, Jo H, Little P, Dees EC, Muss H, Jolly T, Zagar TM, et al. LCCC 1025: a phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2-positive breast cancer brain metastases. Breast Cancer Res Treat 2018;171: 637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu P-F, Lin C-H, Kuo C-H, Chen W-W, Yeh D-C, Liao H-W, Huang S-M, Cheng A-L, Lu Y-S. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer 2015;15: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga J-Y, Goncalves A, Leheurteur M, Domont J, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14: 64–71. [DOI] [PubMed] [Google Scholar]
- 18.Cao KI, Lebas N, Gerber S, Levy C, Le Scodan R, Bourgier C, Pierga JY, Gobillion A, Savignoni A, Kirova YM. Phase II randomized study of whole-brain radiation therapy with or without concurrent temozolomide for brain metastases from breast cancer. Ann Oncol 2015;26: 89–94. [DOI] [PubMed] [Google Scholar]
- 19.Lin NU, Eierman W, Greil R, Campone M, Kaufman B, Steplewski K, Lane SR, Zembryki D, Rubin SD, Winer EP. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol 2011;105: 613–20. [DOI] [PubMed] [Google Scholar]
- 20.Pivot X, Manikhas A, Zurawski B, Chmielowska E, Karaszewska B, Allerton R, Chan S, Fabi A, Bidoli P, Gori S, Ciruelos E, Dank M, et al. CEREBEL (EGF111438): A Phase III, Randomized, Open-Label Study of Lapatinib Plus Capecitabine Versus Trastuzumab Plus Capecitabine in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol 2015;33: 1564–73. [DOI] [PubMed] [Google Scholar]
- 21.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG 2nd, Deming R, Burri SH, Menard C, Chung C, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316: 401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prat A, Cheang MCU, Galvan P, Nuciforo P, Pare L, Adamo B, Munoz M, Viladot M, Press MF, Gagnon R, Ellis C, Johnston S. Prognostic Value of Intrinsic Subtypes in Hormone Receptor-Positive Metastatic Breast Cancer Treated With Letrozole With or Without Lapatinib. JAMA Oncol 2016;2: 1287–94. [DOI] [PubMed] [Google Scholar]
- 23.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 24.Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nature Reviews Cancer 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. Combination therapy in combating cancer. Oncotarget 2017;8: 38022–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds T. Clinical trials: can technology solve the problem of low recruitment? Bmj 2011;342: d3662. [DOI] [PubMed] [Google Scholar]
- 27.Unger JM, Cook E, Tai E, Bleyer A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ Book 2016: 185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Embi PJ, Jain A, Clark J, Bizjack S, Hornung R, Harris CM. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med 2005;165: 2272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siminoff LA, Zhang A, Colabianchi N, Sturm CM, Shen Q. Factors that predict the referral of breast cancer patients onto clinical trials by their surgeons and medical oncologists. J Clin Oncol 2000;18: 1203–11. [DOI] [PubMed] [Google Scholar]
- 30.Hayes DF. HER2 and Breast Cancer - A Phenomenal Success Story. N Engl J Med 2019;381: 1284–6. [DOI] [PubMed] [Google Scholar]
- 31.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine 2001;344: 783–92. [DOI] [PubMed] [Google Scholar]
- 32.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr., Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. The New England journal of medicine 2005;353: 1673–84. [DOI] [PubMed] [Google Scholar]
- 33.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 2004;64: 2343–6. [DOI] [PubMed] [Google Scholar]
- 34.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69: 9330–6. [DOI] [PubMed] [Google Scholar]
- 35.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N, Clark E, Knott A, Lang I, et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. The New England journal of medicine 2017;377: 122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann Oncol 2018;29: 1634–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A, Redondo A, Fischer HH, Jacot W, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380: 617–28. [DOI] [PubMed] [Google Scholar]
- 38.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2019. [Google Scholar]
- 39.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366: 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372: 724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, Clark E, Ross G, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14: 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swain SM, Baselga J, Miles D, Im YH, Quah C, Lee LF, Cortes J. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol 2014;25: 1116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachelot T, Garcia-Saenz JA, Verma S, Gutierrez M, Pivot X, Kozloff MF, Prady C, Huang X, Khosravan R, Wang Z, Cesari R, Tassell V, et al. Sunitinib in combination with trastuzumab for the treatment of advanced breast cancer: activity and safety results from a phase II study. BMC Cancer 2014;14: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol 2014;11: 282–98. [DOI] [PubMed] [Google Scholar]
- 45.Rose MG, Farrell MP, Schmitz JC. Thymidylate synthase: a critical target for cancer chemotherapy. Clin Colorectal Cancer 2002;1: 220–9. [DOI] [PubMed] [Google Scholar]
- 46.Higa GM, Abraham J. Lapatinib in the treatment of breast cancer. Expert Rev Anticancer Ther 2007;7: 1183–92. [DOI] [PubMed] [Google Scholar]
- 47.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355: 2733–43. [DOI] [PubMed] [Google Scholar]
- 48.Petrelli F, Ghidini M, Lonati V, Tomasello G, Borgonovo K, Ghilardi M, Cabiddu M, Barni S. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur J Cancer 2017;84: 141–8. [DOI] [PubMed] [Google Scholar]
- 49.Madden R, Kosari S, Peterson GM, Bagheri N, Thomas J. Lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer: A systematic review. Int J Clin Pharmacol Ther 2018;56: 72–80. [DOI] [PubMed] [Google Scholar]
- 50.Fisusi FA, Akala EO. Drug Combinations in Breast Cancer Therapy. Pharm Nanotechnol 2019;7: 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joensuu H, Kellokumpu-Lehtinen P-L, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi-Hujanen T, Jyrkkio S, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. The New England journal of medicine 2006;354: 809–20. [DOI] [PubMed] [Google Scholar]
- 52.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357: 2666–76. [DOI] [PubMed] [Google Scholar]
- 53.Robert NJ, Saleh MN, Paul D, Generali D, Gressot L, Copur MS, Brufsky AM, Minton SE, Giguere JK, Smith JW 2nd, Richards PD, Gernhardt D, et al. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer 2011;11: 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park YH, Kim T-Y, Kim GM, Kang SY, Park IH, Kim JH, Lee KE, Ahn HK, Lee MH, Kim H-J, Kim HJ, Lee JI, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15–10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 2019. [DOI] [PubMed] [Google Scholar]
- 55.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, Atkins MB, Ernstoff MS, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med 2018;379: 722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp H-G, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20: 924–37. [DOI] [PubMed] [Google Scholar]
- 57.Fares J, Fares MY, Fares Y. Immune checkpoint inhibitors: Advances and impact in neuro-oncology. Surg Neurol Int 2019;10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fares J, Fares MY, Fares Y. Natural killer cells in the brain tumor microenvironment: Defining a new era in neuro-oncology. Surg Neurol Int 2019;10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau H-T, Forero-Torres A, Boccia R, Lippman ME, Somer R, Smakal M, Emens LA, Hrinczenko B, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018;167: 671–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, Kuter I, Nanda R, Cassier PA, Delord J-P, Gordon MS, ElGabry E, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol 2019;5: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nanda R, Chow LQM, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34: 2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rugo HS, Delord J-P, Im S-A, Ott PA, Piha-Paul SA, Bedard PL, Sachdev J, Tourneau CL, van Brummelen EMJ, Varga A, Salgado R, Loi S, et al. Safety and Antitumor Activity of Pembrolizumab in Patients with Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer. Clin Cancer Res 2018;24: 2804–11. [DOI] [PubMed] [Google Scholar]
- 63.Muller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, von Bergwelt-Baildon M, Kreipe H, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med 2015;7: 315ra188. [DOI] [PubMed] [Google Scholar]
- 64.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im S-A, Shaw Wright G, Henschel V, Molinero L, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. The New England journal of medicine 2018;379: 2108–21. [DOI] [PubMed] [Google Scholar]
- 65.Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol 2012;30: 679–92. [DOI] [PubMed] [Google Scholar]
- 66.Jackman AL, Kaye S, Workman P. The combination of cytotoxic and molecularly targeted therapies–can it be done? Drug Discovery Today: Therapeutic Strategies 2004;1: 445–54. [Google Scholar]
- 67.Rodon J, Perez J, Kurzrock R. Combining targeted therapies: practical issues to consider at the bench and bedside. Oncologist 2010;15: 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jutzy JMS, Lemons JM, Luke JJ, Chmura SJ. The Evolution of Radiation Therapy in Metastatic Breast Cancer: From Local Therapy to Systemic Agent. Int J Breast Cancer 2018;2018: 4786819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337: 949–55. [DOI] [PubMed] [Google Scholar]
- 70.Cihoric N, Tsikkinis A, Minniti G, Lagerwaard FJ, Herrlinger U, Mathier E, Soldatovic I, Jeremic B, Ghadjar P, Elicin O, Lossl K, Aebersold DM, et al. Current status and perspectives of interventional clinical trials for glioblastoma - analysis of ClinicalTrials.gov. Radiat Oncol 2017;12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the results in the paper that are minimally required to replicate the outcomes of the study will be made available upon reasonable request.