Abstract
Serrated polyps and conventional adenomas represent two distinct groups of colorectal premalignancy. The influence of early-life adiposity on risk of these precursors remains unclear. Within the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals Follow-up Study, we assessed body fatness during childhood using 9-level somatotype and obtained weight and body mass index (BMI) in adulthood. We used multivariable-adjusted logistic regressions to examine the association of serrated polyps and conventional adenomas with body fatness in early childhood (age 5), late childhood (age 10), early adulthood (age 18/21), and middle adulthood (baseline) and weight change during early-to-middle adulthood. During 18–20 years of follow-up, we documented 8,697 serrated polyps and 10,219 conventional adenomas in 132,514 women; 2,403 serrated polyps and 4,495 conventional adenomas in 29,207 men. We found a modest positive association of adiposity in early and late childhood with risk of serrated polyps and conventional adenomas, with odds ratios ranging from 1.12 to 1.18 for comparison of extreme somatotypes groups. The associations were attenuated after adjusting for adulthood BMI but remained significant for conventional adenomas. No association was found in men. Adulthood body fatness and weight change during early-to-middle adulthood showed positive relationships with serrated polyps and conventional adenomas in both women and men, with stronger associations observed for serrated polyps (Pheterogeneity <0.0001). Our findings indicated a potential role in development of colorectal cancer precursors of childhood body fatness in women, and early-to-middle adulthood weight gain and attained adiposity in both sexes.
Keywords: obesity, colorectal cancer, serrated polyp, adenomatous polyp
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer mortality in the world1. Evidence suggests a pivotal role of obesity in CRC carcinogenesis and a link between early-life obesity and adulthood risk of CRC and alterations in metabolic markers, such as insulin, that have been implicated in CRC2. The long latency for CRC development makes it plausible that the anthropometric exposures most relevant to risk might have occurred early in life. To date, there have been studies that showed a positive association between early life body fatness and CRC3–5, whereas only few investigations focused on precursor lesions of CRC that represent early carcinogenic changes. Although endoscopic removal of precursor lesions has established benefit for CRC prevention, the effectiveness of screening is limited for proximal colorectal neoplasms due to incomplete colonoscopies, poor bowel preparation, and a larger portion of serrated polyps in the right colon6. Therefore, identifying environmental risk factors for prevention of precursor lesions remains a priority to reduce CRC incidence and mortality.
Serrated polyp (SP) and conventional adenoma are the two well-recognized CRC precursor lesions, each taking on distinct pathways and contributing to 25–30% and 60–70% of CRC cases, respectively7,8. SPs undergo the microsatellite instability pathway which is characterized by BRAF mutation, CpG island methylator phyenotype, and epigenetic inactivation of the mismatch repair gene MLH19. Conventional adenoma develops into CRC through the chromosomal instability pathway that features K-Ras activation, inactivation of tumor suppression genes APC and p53, and loss of heterozygosity for the long arm of chromosome 18 (18q LOH)10. Similar to CRC, obesity has been associated with an increased risk of SP and conventional adenoma11,12. However, in contrast to the well-documented positive associations between overweight/obesity in adulthood and polyp risk13,14, the effect of body fatness during childhood, adolescence, and young adulthood remains unclear. Because of the potential contribution of early life obesity to the development of CRC later on in adulthood, a detailed examination of adiposity throughout the lifespan is vital to better understand the influence of adiposity on CRC.
Therefore, we conducted a prospective study within three large US-based cohorts of women and men to comprehensively investigate the association of obesity at different points in life with risk of SPs and conventional adenomas. We also performed detailed analysis by polyp features, including size, histology, and subsite, which predict the malignant potential of polyps15.
MATERIALS AND METHODS
Study population
We included participants from three nationwide prospective cohort studies, the Nurses’ Health Study (NHS), Nurses’ Health Study 2 (NHS2) and Health Professionals Follow-up Study (HPFS). Briefly, the NHS enrolled 121,700 registered US female nurses aged 30 to 55 years in 1976. The NHS2 included 116,429 registered US female nurses aged 25 to 42 years at enrollment in 1989. The HPFS enrolled 51,529 male health professionals between the ages of 40 to 75 in 198616,17. Questionnaires were mailed to participants at enrollment and every two years thereafter, inquiring health and lifestyle information. Diet was assessed via validated food frequency questionnaires (FFQ) every four years. The average follow-up rates for the three cohorts have been greater than 90%. The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, and those of participating registries as required. Informed consent was indicated by questionnaire return.
Anthropometric measurement
In 1988, participants in the NHS and HPFS were asked to choose one of the nine pictorial body diagrams (somatotypes) developed by Stunkard et al18 that best depicted their body shape at ages 5, 10, 20, 30, and 40 (Figure 1). Participants in the NHS2 provided the same information in 1989. Level 1 represents the leanest and level 9 represents the heaviest. The validity of this measure was assessed previously in the Third Harvard Growth Study19.
Figure 1.
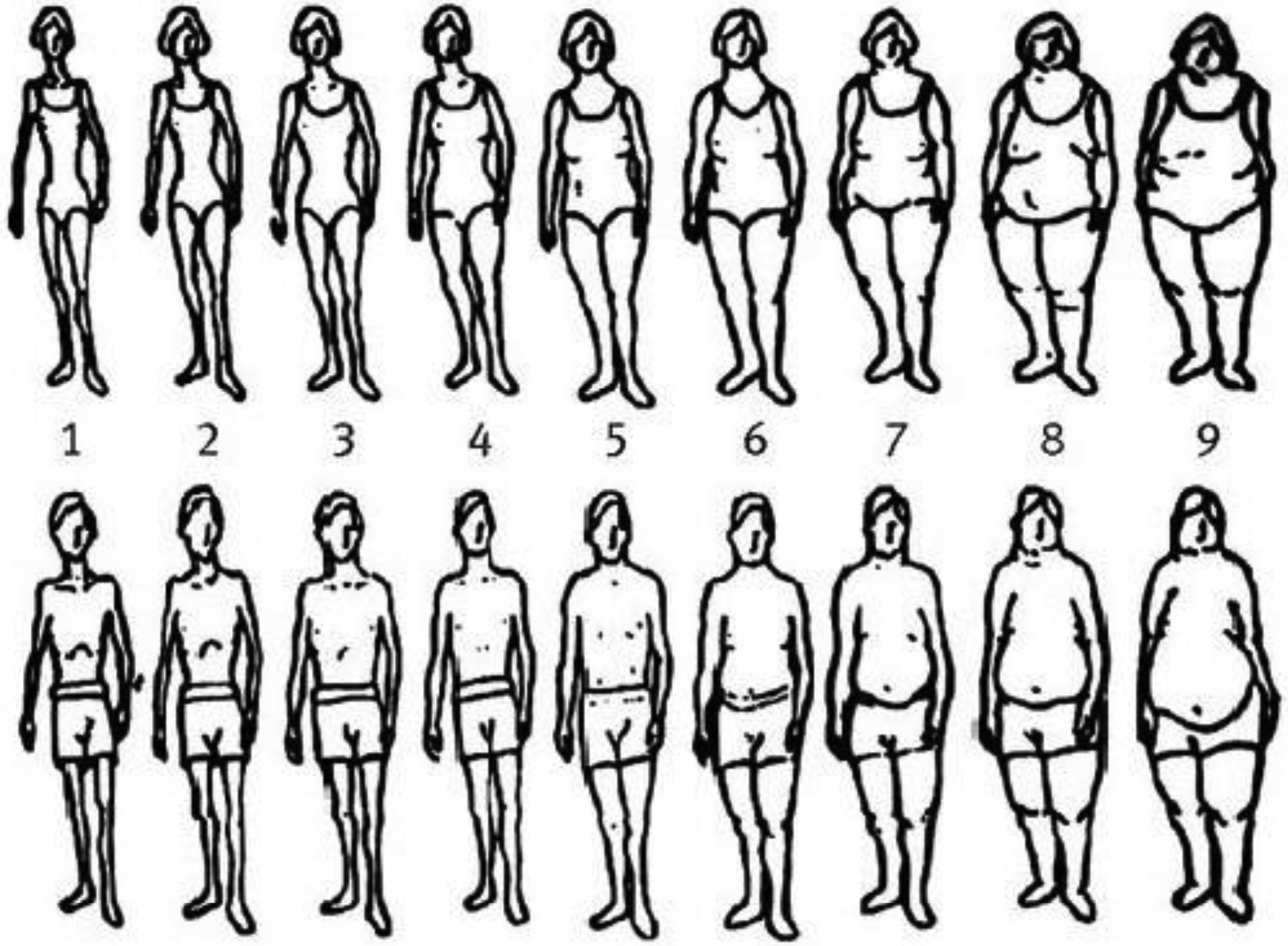
Pictorial diagrams by Stunkard et al. used for estimating somatotype at ages 5 and 10 in women and men.
Participants in the NHS and NHS2 recalled their weight at age 18 years in 1988 and 1989, respectively; participants in the HPFS recalled their weight at age 21 years in 1986. In the three cohorts, current height and weight were inquired at enrollment and updated weight was collected biennially. We calculated participants’ body mass index (BMI) at age 18/21 and at study baseline by dividing their respective weight in kilograms by height squared in meters. We also assessed weight change from early adulthood (age 18 years for women, age 21 years for men) to baseline. Self-reported weight and measured weight were highly correlated in a validation study within the NHS and HPFS20. Recalled weight at age 18 years also showed high validity within the NHS2 cohort21.
We assessed early and late childhood body fatness with somatotype at age 5 and age 10, respectively. Due to the low number of individuals in higher somatotype categories, we collapsed level 3 and level 4 into one category and all levels above 5 into one category. We therefore categorized early and late childhood body fatness as: somatotype 1, 2, 3–4, and ≥53. Early adulthood body fatness was assessed using BMI at age 18 years for women and age 21 years for men. For adulthood body fatness, we examined BMI at study baseline and weight change from early adulthood to baseline. BMI at age 18 years for women and age 21 for men and baseline BMI were categorized as <22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9 and ≥30.0 kg/m2. To balance sample size and clinical meaningfulness, we created six categories for weight change based on the observed distribution in the study population: weight loss ≥3.0 kg; loss or gain<3.0 kg; gain 3.0–5.9 kg; gain 6.0–9.9 kg; gain 10.0–19.9 kg; and gain ≥20.0 kg.
Ascertainment of colorectal polyp cases and subtypes
In each follow-up questionnaire, we asked the participants if they have undergone a lower endoscopy and if they have been diagnosed with colorectal polyp in the past two years. For those who reported a polyp diagnosis, we asked for their permission to acquire their endoscopic and pathologic records. Study investigators blinded to exposure information reviewed all records and extracted detailed clinicopathologic data. Because detailed histologic information of polyps was not collected until 1992 for the NHS/HPFS and 1991 for the NHS2, we used these years as the baseline for the respective cohorts in this study. We considered hyperplastic polyps as SPs before 2002 and included both hyperplastic polyps and mixed/serrated adenomas as SPs since 2002 to reflect the advances in terminology and clinical practice. Mixed/serrated adenomas included polyps with both adenomatous and hyperplastic changes in histology and polyps with any serrated diagnosis (e.g., serrated adenoma, serrated polyp, and sessile serrated polyp/adenoma). Conventional adenomas included tubular, tubulovillous, and villous adenomas and adenomas with high-grade dysplasia. Advanced conventional adenomas were defined as having at least one conventional adenoma of 10 mm or greater in diameter or with advanced histology (tubulovillous/villous histologic features or high-grade or severe dysplasia)22.
Assessment of lifestyle and dietary factors
On the baseline and biennial questionnaires, we inquired information on family history of CRC, physical activity, aspirin use, and smoking status. We used the FFQs to asesss dietary factors, including alcohol, folate, calcium, vitamin D, and processed red meat. The validity of FFQs in assessing food and nutrient intake has been documented previously23,24. To handle missing data of the covariates that occurred in the follow-up questionnaires, we carried forward the most recent available information from prior questionnaires.
Statistical analysis
For the present study, participants were included if they had at least one endoscopy from baseline until the end of follow-up (June 1, 2012 for the NHS, June 1, 2011 for the NHS2, and January 1, 2010 for the HPFS). To account for possible multiple records per participant and to handle time-varying exposure and covariates efficiently, we used an Andersen-Gill data structure with a new record for each two-year follow-up period during which a participant underwent an endoscopy. At baseline, we excluded participants who had a history of cancer (except non-melanoma skin cancer), colorectal polyp, or inflammatory bowel disease. Participants were censored at the diagnosis of colorectal polyp, CRC, death, or the end of the follow-up, whichever occurred first. We pooled the two female cohorts (NHS and NHS2; Supplementary Table 1) to maximize statistical power.
We used multivariable-adjusted logistic regressions for clustered data (PROC GENMOD) to account for repeated observations per individual and to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). To test for linear trend, we used somatotype at ages 5 and 10 as ordinal score variables and BMI and weight change as continuous variables. Multivariable models were adjusted for age at present (continuous), race (white, nonwhite), family history of CRC (no, yes), smoking (never smokers, past smokers <30 pack-years, past smokers ≥30 pack-years, current smokers <30 pack-years, current smokers ≥30 pack-years), BMI at ages 18/21 years (weight change only; <22.5 kg/m2, 22.5–24.9 kg/m2, 25.0–27.4 kg/m2, 27.5–29.9 kg/m2, ≥30.0 kg/m2), height (somatotype in childhood and weight change only; continuous, cm), physical activity (<7.5 MET-hr/wk, 7.5–14.9 MET-hr/wk, 15.0–29.9 MET-hr/wk, 30.0–59.9 MET-hr/wk, and ≥60 MET-hr/wk), alcohol use (women: never, <3.5 g/d, 3.5–6.9 g/d, and ≥7.0 g/d; men: never, <7.0 g/d, 7.0–13.9 g/d, and ≥14.0 g/d), folate (in quartiles, μg/d), calcium (in quartiles, mg/d), vitamin D (in quartiles, IU/d), processed red meat intake (in quartiles, servings/wk); regular aspirin use (no, yes), as well as endoscopy-related factors, including time period of endoscopy (in two-year intervals), number of prior endoscopies (continuous), time since the most recent endoscopy (continuous, year). Analyses for women were further adjusted for menopausal hormone use (premenopausal/missing menopause, no history of menopausal hormone use, current menopausal hormone use, past menopausal hormone use). We allowed covariates to be time-varying to account for changes in these covariates over time. To assess the independent association of early life body fatness, we further adjusted for biennially updated adult BMI for somatotype at ages 5 and 10 and BMI at ages 18 and 21.
In secondary analysis, we examined the associations of body fatness with SPs by polyp size (small, large) and with conventional adenomas by risk classification (nonadvanced, advanced). We also examined whether the relationship between body fatness and polyps differed according to anatomic subsite (proximal colon, distal colon, rectum). Pheterogeneity between case groups was calculated by case-only analysis12.
We conducted all analyses using the SAS software (SAS Institute, Inc., Version 9.4, Cary, NC). All statistical analyses were two-sided with a p-value less than 0.05 indicating statistical significance.
DATA AVAILABILITY
The data that support the findings of this study are available on request at https://www.nurseshealthstudy.org/researchers (nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/. The data are not publicly available due to privacy or ethical restrictions.
RESULTS
This study included 132,514 women from the NHS and NHS2 and 29,207 men from the HPFS. Among 308,871 endoscopies in women, we observed 8,697 cases of SPs and 10,219 cases of conventional adenomas. In men, 2,403 SPs and 4,495 conventional adenomas were diagnosed among 77,406 endoscopies. Table 1 showed the baseline characteristics of study participants and Supplementary Figures 1 & 2 showed the prevalence of SPs and conventional adenoams over the study period. The mean age at baseline was 45.1 years in women and 55.9 years in men. This age difference was mainly driven by the younger population in the NHS2. Lifestyle factors were strongly related to adulthood BMI and weight change but minimally to early life somatotype. Comparing participants in extreme BMI and weight change groups, those in the highest category were more likely to consume processed red meat and less likey to be physically active and consume folate, calcium, and vitamin D.
Table 1.
Basic characteristics of study participants according to measures of body fatness in women (NHS and NHS2) and men (HPFS)a
| Somatotype at age 10 | BMI at baseline | Weight change | ||||
|---|---|---|---|---|---|---|
| Variable | Somatotype 1 | Somatotype ≥5 | BMI <22.5 | BMI ≥30 | Loss ≥3.0 kg | Gain ≥20.0 kg |
| Women (NHS, NHS2) | ||||||
| No. of participants | 28,418 | 14,110 | 44,423 | 20,803 | 7,995 | 22,617 |
| Age, years, mean (SD) | 44.53 (11.52) | 44.01 (11.06) | 44.63 (11.77) | 45.39 (11.05) | 44.29 (12.04) | 45.00 (10.91) |
| White, % | 95 | 98 | 97 | 96 | 98 | 95 |
| Family history of CRC, % | 18 | 18 | 17 | 18 | 17 | 18 |
| Smoking, pack-year, mean (SD) | 7.55 (14.25) | 10.37 (16.67) | 8.25 (15.53) | 8.70 (15.43) | 11.80 (18.40) | 8.00 (14.56) |
| Body mass index, kg/m2, mean (SD) | ||||||
| Age 18 | 19.48 (2.11) | 24.06 (4.15) | 19.79 (1.99) | 24.31 (4.22) | 24.80 (4.33) | 21.88 (3.46) |
| Baseline | 23.57 (3.97) | 27.81 (6.38) | 20.76 (1.24) | 34.66 (4.49) | 21.90 (3.38) | 32.77 (5.36) |
| Height, cm, mean (SD) | 164.56 (6.54) | 164.71 (6.41) | 164.69 (6.36) | 163.84 (6.78) | 164.24 (6.61) | 165.35 (6.46) |
| Physical activity, MET-h/wk, mean (SD)b | 20.21 (21.50) | 19.32 (20.31) | 23.29 (23.05) | 13.51 (15.04) | 25.09 (25.07) | 14.08 (16.01) |
| Alcohol intake, g/d, mean (SD) | 4.73 (7.36) | 4.71 (7.64) | 5.81 (8.21) | 2.74 (5.81) | 5.71 (8.31) | 3.15 (6.29) |
| Total folate intake, mg/d, mean (SD) | 501 (208) | 518 (214) | 531 (219) | 484 (202) | 535 (228) | 480 (200) |
| Calcium intake, mg/d, mean (SD) | 1089 (406) | 1135 (410) | 1151 (422) | 1053 (390) | 1166 (436) | 1046 (383) |
| Vitamin D intake, IU/d, mean (SD) | 390 (209) | 404 (210) | 414 (222) | 381 (207) | 417 (228) | 377 (202) |
| Processed red meat intake, serving/wk, mean (SD) | 1.70 (1.58) | 1.61 (1.56) | 1.44 (1.43) | 2.08 (2.00) | 1.40 (1.49) | 2.10 (2.00) |
| Regular aspirin use, %c | 26 | 29 | 24 | 32 | 26 | 31 |
| Menopausal hormone use, % | ||||||
| Premenopausal/missing menopause | 61 | 63 | 63 | 58 | 62 | 59 |
| No history of menopausal hormone use | 16 | 16 | 14 | 21 | 16 | 19 |
| Current menopausal hormone use | 16 | 14 | 17 | 13 | 15 | 14 |
| Past menopausal hormone use | 7 | 7 | 6 | 8 | 7 | 8 |
| Men (HPFS) | ||||||
| No. of participants | 5,241 | 3,987 | 3,610 | 2,849 | 2,040 | 3,046 |
| Age, years, mean (SD) | 56.49 (8.74) | 55.57 (8.99) | 56.19 (9.45) | 55.51 (8.48) | 56.14 (9.22) | 56.07 (8.61) |
| White, % | 91 | 92 | 90 | 92 | 91 | 92 |
| Family history of CRC, % | 17 | 16 | 16 | 17 | 16 | 16 |
| Smoking, pack-year, mean (SD) | 11.55 (17.33) | 13.51 (18.60) | 9.22 (16.61) | 15.23 (19.74) | 11.03 (17.44) | 15.65 (19.86) |
| Body mass index, kg/m2, mean (SD) | ||||||
| Age 18 | 21.66 (2.45) | 24.99 (2.93) | 20.90 (2.04) | 26.17 (4.11) | 26.11 (3.19) | 22.13 (3.42) |
| Baseline | 25.01 (2.88) | 26.81 (3.87) | 21.36 (0.99) | 32.91 (4.02) | 23.72 (2.69) | 30.35 (4.25) |
| Height, cm, mean (SD) | 178.77 (6.78) | 178.28 (6.60) | 178.77 (6.86) | 177.74 (8.98) | 178.59 (6.57) | 179.97 (7.59) |
| Physical activity, MET-h/wk, mean (SD)b | 29.02 (23.25) | 30.67 (23.19) | 33.14 (27.24) | 21.93 (19.01) | 37.51 (28.05) | 20.67 (17.89) |
| Alcohol intake, g/d, mean (SD) | 11.11 (12.87) | 10.79 (12.79) | 10.30 (12.63) | 9.70 (13.57) | 10.28 (12.53) | 10.64 (13.99) |
| Total folate intake, mg/d, mean (SD) | 561 (231) | 584 (237) | 598 (245) | 534 (226) | 608 (249) | 523 (220) |
| Calcium intake, mg/d, mean (SD) | 930 (339) | 960 (357) | 962 (354) | 950 (351) | 993 (367) | 925 (348) |
| Vitamin D intake, IU/d, mean (SD) | 444 (234) | 457 (246) | 471 (255) | 425 (240) | 486 (266) | 416 (230) |
| Processed red meat intake, serving/wk, mean (SD) | 1.95 (1.92) | 1.71 (1.87) | 1.56 (1.95) | 2.48 (2.42) | 1.44 (2.00) | 2.61 (2.49) |
| Regular aspirin use, %c | 50 | 51 | 42 | 54 | 48 | 52 |
Abbreviations: NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; BMI, body mass index; CRC, colorectal cancer; SD, standard deviation; MET; metabolic equivalent of task.
The basic characteristics are presented separately for women (NHS, NHS2) and men (HPFS) by body fatness levels. All variables are adjusted for age except for age. Cumulative average values at baseline are presented. Mean (SD) is presented for continuous variables and percentage of participants for categorical variables.
Physical activity is represented by the product sum of the MET of each specific recreational activity and hours spent on that activity per week.
Regular aspirin users were defined as those who used at least two standard tablets (325 mg) per week.
Tables 2 & 3 shows the associations of adiposity at different ages with risk of SPs and conventional adenomas in women and men. We found a positive association of body fatness at ages 5 and 10 with risks of SPs and conventional adenomas in women but not in men. Compared to women with somatotype 1, the ORs of SPs and conventional adenomas for those with somatotype ≥5 at age 5 were 1.12 (95% CI, 1.02–1.22; Ptrend = 0.0007) and 1.18 (95% CI, 1.09–1.28; Ptrend <0.0001), respectively, while the ORs for those with somatotype ≥5 at age 10 were 1.18 (95% CI, 1.10–1.28; Ptrend <0.0001) and 1.16 (95% CI, 1.08–1.24; Ptrend <0.0001), respectively. These results were attenuated upon adjustment for adulthood BMI, leading to null associations for SPs and significantly stronger association with somatotype at age 5 for conventional adenomas compared with SPs. Higher body fatness in early adulthood as represented by increased BMI at age 18 was weakly associated with higher risks of both types of CRC precursor lesions in women (Ptrend of SPs: 0.0002; conventional adenomas: <0.0001). Further adjustment for adulthood BMI substantially attenuated the associations. Overall, there was no association between early life body fatness and polyp risk in men.
Table 2.
Multivariable associations of body fatness over the life course with serrated polyps and conventional adenomas in women (NHS and NHS2)
| Non-polyp | Serrated polyps | Conventional adenomas | |||||
|---|---|---|---|---|---|---|---|
| Person-endoscopies | n | MVa | MV + adulthood BMIb | n | MVa | MV + adulthood BMIb | |
| Body fatness at age 5 | |||||||
| Somatotype 1 | 85061 | 2492 | 1 (ref) | 1 (ref) | 2996 | 1 (ref) | 1 (ref) |
| Somatotype 2 | 70633 | 2200 | 1.03(0.97–1.09) | 1.02(0.96–1.08) | 2562 | 1.07(1.01–1.13) | 1.07(1.01–1.13) |
| Somatotype 3–4 | 83295 | 2792 | 1.09(1.03–1.15) | 1.04(0.98–1.10) | 3162 | 1.11(1.06–1.17) | 1.09(1.03–1.15) |
| Somatotype ≥5 | 18055 | 633 | 1.12(1.02–1.22) | 1.04(0.95–1.14) | 744 | 1.18(1.09–1.28) | 1.14(1.05–1.24) |
| P for trendc | 0.0007 | 0.24 | <0.0001 | <0.0001 | |||
| P for heterogeneitye | 0.19 | 0.03 | |||||
| Body fatness at age 10 | |||||||
| Somatotype 1 | 65351 | 1835 | 1 (ref) | 1 (ref) | 2316 | 1 (ref) | 1 (ref) |
| Somatotype 2 | 73710 | 2278 | 1.07(1.00–1.14) | 1.05(0.99–1.12) | 2587 | 1.02(0.96–1.08) | 1.01(0.96–1.07) |
| Somatotype 3–4 | 89386 | 2961 | 1.12(1.06–1.19) | 1.05(0.99–1.12) | 3403 | 1.11(1.05–1.18) | 1.08(1.02–1.14) |
| Somatotype ≥5 | 30502 | 1094 | 1.18(1.10–1.28) | 1.08(1.00–1.17) | 1221 | 1.16(1.08–1.24) | 1.11(1.03–1.19) |
| P for trendc | <0.0001 | 0.13 | <0.0001 | 0.0006 | |||
| P for heterogeneitye | 0.73 | 0.11 | |||||
| Body fatness at age 18 | |||||||
| BMI <22.5 kg/m2 | 203038 | 6409 | 1 (ref) | 1 (ref) | 7537 | 1 (ref) | 1 (ref) |
| BMI 22.5–24.9 kg/m2 | 39926 | 1397 | 1.09(1.03–1.16) | 0.99(0.93–1.05) | 1602 | 1.07(1.01–1.13) | 1.02(0.97–1.08) |
| BMI 25.0–27.4 kg/m2 | 14870 | 507 | 1.05(0.96–1.15) | 0.91(0.83–1.00) | 619 | 1.12(1.03–1.22) | 1.05(0.97–1.15) |
| BMI 27.5–29.9 kg/m2 | 4624 | 156 | 1.01(0.86–1.19) | 0.85(0.72–1.01) | 212 | 1.24(1.08–1.43) | 1.15(1.00–1.33) |
| BMI ≥30 kg/m2 | 4806 | 176 | 1.12(0.96–1.31) | 0.92(0.79–1.08) | 179 | 1.06(0.91–1.23) | 0.97(0.83–1.14) |
| P for trendd | 0.0002 | 0.05 | <0.0001 | 0.14 | |||
| P for heterogeneitye | 0.75 | 0.005 | |||||
| Body fatness at baseline | |||||||
| BMI <22.5 kg/m2 | 94533 | 2505 | 1 (ref) | 3036 | 1 (ref) | ||
| BMI 22.5–24.9 kg/m2 | 72201 | 2114 | 1.15(1.08–1.22) | 2476 | 1.05(1.00–1.11) | ||
| BMI 25.0–27.4 kg/m2 | 51822 | 1634 | 1.28(1.20–1.37) | 1954 | 1.16(1.09–1.23) | ||
| BMI 27.5–29.9 kg/m2 | 27306 | 918 | 1.41(1.30–1.52) | 1051 | 1.19(1.10–1.28) | ||
| BMI ≥30 kg/m2 | 45716 | 1526 | 1.42(1.32–1.52) | 1702 | 1.18(1.11–1.26) | ||
| P for trendd | <0.0001 | <0.0001 | |||||
| P for heterogeneitye | <0.0001 | ||||||
| Weight change from age 18 to baseline | |||||||
| Loss ≥3.0 kg | 16936 | 489 | 0.91(0.81–1.01) | 606 | 0.93(0.84–1.03) | ||
| Loss or gain <3.0 kg | 43562 | 1272 | 1 (ref) | 1560 | 1 (ref) | ||
| Gain 3.0–5.9 kg | 36947 | 1068 | 1.02(0.94–1.11) | 1256 | 0.97(0.90–1.05) | ||
| Gain 6.0–9.9 kg | 47485 | 1557 | 1.16(1.08–1.25) | 1824 | 1.07(1.00–1.15) | ||
| Gain 10.0–19.9 kg | 72437 | 2475 | 1.25(1.17–1.34) | 2850 | 1.08(1.01–1.15) | ||
| Gain ≥20.0 kg | 50150 | 1790 | 1.35(1.25–1.46) | 2064 | 1.13(1.05–1.21) | ||
| P for trendd | <0.0001 | <0.0001 | |||||
| P for heterogeneitye | <0.0001 | ||||||
Abbreviations: ref, reference; NHS, Nurses’ Health Study; BMI, body mass index; MV, multivariable model; MET, metabolic equivalent of task.
Multivariable logistic regression model was adjusted for time period of endoscopy (in two-year intervals), number of prior endoscopies (continuous), time since the most recent endoscopy (continuous, year), age (continuous, year), race (white, nonwhite), family history of colorectal cancer (no, yes), smoking (never smoker, past smoker <30 pack-years, past smoker ≥30 pack-years, current smoker <30 pack-years, current smoker ≥30 pack-years), height (only for somatotype and weight change; continuous, cm), physical activity (<7.5 MET-h/wk, 7.5–14.9 MET-h/wk, 15.0–29.9 MET-h/wk, 30.0–59.9 MET-h/wk, ≥60.0 MET-h/wk), alcohol intake (never, <3.5 g/d, 3.5–6.9 g/d, ≥7.0 g/d, dietary factors (folate, vitamin D, calcium, processed red meat; in quartiles), regular aspirin use (no, yes), and menopausal hormone use (premenopausal/missing menopause, no history of menopausal hormone use, current menopausal hormone use, past menopausal hormone use). Weight change was also adjusted for BMI at age 18 (<22.5 kg/m2, 22.5–24.9 kg/m2, 25.0–27.4 kg/m2, 27.5–29.9 kg/m2, ≥30.0 kg/m2).
Cumulative updated BMI collected at the current questionnaire cycle was further adjusted in the model to examine the independent effect of early life body fatness.
P for trend was calculated using somatotype as an ordinal score variable.
P for trend was calculated using BMI and weight change as continuous variables.
P for heterogeneity was calculated in case-only analysis.
Table 3.
Multivariable associations of body fatness over the life course with serrated polyps and conventional adenomas in men (HPFS)
| Non-polyp | Serrated polyps | Conventional adenomas | |||||
|---|---|---|---|---|---|---|---|
| Person-endoscopies | n | MVa | MV + adulthood BMIb | n | MVa | MV + adulthood BMIb | |
| Body fatness at age 5 | |||||||
| Somatotype 1 | 20017 | 680 | 1 (ref) | 1 (ref) | 1358 | 1 (ref) | 1 (ref) |
| Somatotype 2 | 12889 | 436 | 0.98(0.87–1.11) | 0.98(0.86–1.11) | 816 | 0.94(0.86–1.03) | 0.94(0.86–1.03) |
| Somatotype 3–4 | 14825 | 520 | 1.03(0.91–1.16) | 1.01(0.90–1.14) | 1002 | 1.03(0.94–1.12) | 1.02(0.93–1.11) |
| Somatotype ≥5 | 8002 | 289 | 1.04(0.90–1.20) | 1.00(0.86–1.15) | 502 | 0.97(0.87–1.08) | 0.95(0.85–1.06) |
| P for trendc | 0.54 | 0.99 | 0.92 | 0.74 | |||
| P for heterogeneitye | 0.81 | 0.68 | |||||
| Body fatness at age 10 | |||||||
| Somatotype 1 | 13018 | 431 | 1 (ref) | 1 (ref) | 888 | 1 (ref) | 1 (ref) |
| Somatotype 2 | 17307 | 623 | 1.07(0.94–1.21) | 1.06(0.93–1.20) | 1138 | 0.96(0.88–1.05) | 0.96(0.87–1.05) |
| Somatotype 3–4 | 15784 | 511 | 0.97(0.85–1.11) | 0.94(0.82–1.07) | 1039 | 0.98(0.89–1.08) | 0.96(0.87–1.06) |
| Somatotype ≥5 | 10128 | 380 | 1.10(0.96–1.27) | 1.04(0.90–1.20) | 644 | 0.98(0.88–1.09) | 0.95(0.85–1.06) |
| P for trendc | 0.48 | 0.88 | 0.72 | 0.35 | |||
| P for heterogeneitye | 0.93 | 0.87 | |||||
| Body fatness at age 21 | |||||||
| BMI <22.5 kg/m2 | 29872 | 1002 | 1 (ref) | 1 (ref) | 1941 | 1 (ref) | 1 (ref) |
| BMI 22.5–24.9 kg/m2 | 23134 | 754 | 0.96(0.87–1.06) | 0.90(0.81–1.00) | 1409 | 0.96(0.89–1.03) | 0.93(0.86–1.00) |
| BMI 25.0–27.4 kg/m2 | 11969 | 423 | 1.04(0.92–1.17) | 0.91(0.80–1.03) | 721 | 0.94(0.86–1.03) | 0.89(0.81–0.98) |
| BMI 27.5–29.9 kg/m2 | 2437 | 91 | 1.04(0.83–1.30) | 0.87(0.69–1.10) | 182 | 1.16(0.98–1.36) | 1.08(0.91–1.27) |
| BMI ≥30 kg/m2 | 1158 | 43 | 1.05(0.76–1.45) | 0.86(0.62–1.19) | 80 | 1.07(0.84–1.36) | 0.99(0.78–1.27) |
| P for trendd | 0.66 | 0.04 | 0.60 | 0.40 | |||
| P for heterogeneitye | 0.57 | 0.07 | |||||
| Body fatness at baseline | |||||||
| BMI <22.5 kg/m2 | 9115 | 238 | 1 (ref) | 483 | 1 (ref) | ||
| BMI 22.5–24.9 kg/m2 | 22559 | 683 | 1.13(0.97–1.31) | 1356 | 1.12(1.01–1.25) | ||
| BMI 25.0–27.4 kg/m2 | 23303 | 795 | 1.21(1.04–1.41) | 1504 | 1.16(1.04–1.29) | ||
| BMI 27.5–29.9 kg/m2 | 9906 | 411 | 1.39(1.18–1.64) | 663 | 1.16(1.02–1.31) | ||
| BMI ≥30 kg/m2 | 6452 | 276 | 1.40(1.16–1.68) | 489 | 1.27(1.11–1.46) | ||
| P for trendd | <0.0001 | 0.001 | |||||
| P for heterogeneitye | 0.15 | ||||||
| Weight change from age 21 to baseline | |||||||
| Loss ≥3.0 kg | 5318 | 147 | 1.00(0.81–1.22) | 284 | 0.95(0.82–1.10) | ||
| Loss or gain <3.0 kg | 13200 | 361 | 1 (ref) | 742 | 1 (ref) | ||
| Gain 3.0–5.9 kg | 10737 | 333 | 1.13(0.97–1.31) | 639 | 1.04(0.93–1.16) | ||
| Gain 6.0–9.9 kg | 14033 | 475 | 1.20(1.04–1.38) | 883 | 1.07(0.96–1.18) | ||
| Gain 10.0–19.9 kg | 18433 | 689 | 1.27(1.11–1.46) | 1266 | 1.12(1.01–1.23) | ||
| Gain ≥20.0 kg | 6867 | 308 | 1.45(1.23–1.71) | 520 | 1.17(1.04–1.33) | ||
| P for trendd | <0.0001 | 0.0009 | |||||
| P for heterogeneitye | 0.02 | ||||||
Abbreviations: ref, reference; HPFS, Helath Professionals Follow-up Study; BMI, body mass index; MV, multivariable model; MET, metabolic equivalent of task.
Multivariable logistic regression model was adjusted for time period of endoscopy (in two-year intervals), number of prior endoscopies (continuous), time since the most recent endoscopy (continuous, year), age (continuous, year), race (white, nonwhite), family history of colorectal cancer (no, yes), smoking (never smoker, past smoker <30 pack-years, past smoker ≥30 pack-years, current smoker <30 pack-years, current smoker ≥30 pack-years), height (only for somatotype and weight change; continuous, cm), physical activity (<7.5 MET-h/wk, 7.5–14.9 MET-h/wk, 15.0–29.9 MET-h/wk, 30.0–59.9 MET-h/wk, ≥60.0 MET-h/wk), alcohol intake (never, <7.0 g/d, 7.0–13.9 g/d, ≥14.0 g/d), dietary factors (folate, vitamin D, calcium, processed red meat; in quartiles), and regular aspirin use (no, yes). Weight change was also adjusted for BMI at age 21 (<22.5 kg/m2, 22.5–24.9 kg/m2, 25.0–27.4 kg/m2, 27.5–29.9 kg/m2, ≥30.0 kg/m2).
Cumulative updated BMI collected at the current questionnaire cycle was further adjusted in the model to examine the independent effect of early life body fatness.
P for trend was calculated using somatotype as an ordinal score variable.
P for trend was calculated using BMI and weight change as continuous variables.
P for heterogeneity was calculated in case-only analysis.
Higher BMI at baseline was associated with increased risks of SPs and conventional adenomas in both women and men, with the ORs comparing BMI ≥30 kg/m2 and BMI <22.5 kg/m2 ranging from 1.18 to 1.42. Similar associations were found for weight change from early adulthood to baseline, with the ORs comparing weight gain ≥20 kg and weight loss or gain within 3 kg ranging from 1.13 to 1.45. For BMI at baseline and weight change, we found stronger associations for SPs than conventional adenomas in women (Pheterogeneity <0.0001). In men, this pattern was observed for weight change only (Pheterogeneity = 0.02).
We then examined the associations according to polyp size for SPs and risk classification for conventional adenomas (Tables 4 & 5). Although stronger associations were observed for a number of subgroups, no statistically significant heterogeneity was detected between small and large SPs and between nonadvanced and advanced conventional adenomas (Pheterogeneity >0.05).
Table 4.
Multivariable associations of body fatness over the life course with serrated polyps by size and conventional adenomas by risk classification in women (NHS and NHS2)
| Serrated polyps | Conventional adenomas | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-polyp | Small serrated polyps | Large serrated polyps | Nonadvanced conventional adenomas | Advanced conventional adenomas | |||||
| Person-endoscopy | n | OR (95% CI)a | n | OR (95% CI)a | n | OR (95% CI)a | n | OR (95% CI)a | |
| Body fatness at age 5 | |||||||||
| Somatotype 1 | 85061 | 2171 | 1 (ref) | 215 | 1 (ref) | 2013 | 1 (ref) | 983 | 1 (ref) |
| Somatotype 2 | 70633 | 1887 | 1.00(0.94–1.07) | 217 | 1.18(0.97–1.43) | 1759 | 1.05(0.98–1.12) | 803 | 1.11(1.01–1.22) |
| Somatotype 3–4 | 83295 | 2411 | 1.07(1.01–1.14) | 251 | 1.13(0.93–1.36) | 2182 | 1.09(1.03–1.17) | 980 | 1.13(1.03–1.24) |
| Somatotype ≥5 | 18055 | 542 | 1.09(0.99–1.21) | 58 | 1.16(0.87–1.56) | 520 | 1.20(1.09–1.33) | 224 | 1.13(0.97–1.31) |
| P for trendb | 0.008 | 0.19 | <0.0001 | 0.005 | |||||
| P for heterogeneitye | 0.77 | 0.96 | |||||||
| Body fatness at age 10 | |||||||||
| Somatotype 1 | 65351 | 1586 | 1 (ref) | 164 | 1 (ref) | 1559 | 1 (ref) | 757 | 1 (ref) |
| Somatotype 2 | 73710 | 1963 | 1.06(0.99–1.13) | 224 | 1.18(0.97–1.45) | 1770 | 1.00(0.93–1.07) | 817 | 1.05(0.95–1.16) |
| Somatotype 3–4 | 89386 | 2558 | 1.11(1.04–1.19) | 267 | 1.14(0.93–1.39) | 2348 | 1.09(1.02–1.17) | 1055 | 1.14(1.04–1.25) |
| Somatotype ≥5 | 30502 | 945 | 1.18(1.09–1.28) | 96 | 1.15(0.89–1.48) | 842 | 1.15(1.05–1.25) | 379 | 1.16(1.03–1.32) |
| P for trendb | <0.0001 | 0.43 | 0.0001 | 0.002 | |||||
| P for heterogeneitye | 0.64 | 0.56 | |||||||
| Body fatness at age 18 | |||||||||
| BMI <22.5 kg/m2 | 203038 | 5521 | 1 (ref) | 612 | 1 (ref) | 5135 | 1 (ref) | 2402 | 1 (ref) |
| BMI 22.5–24.9 kg/m2 | 39926 | 1214 | 1.10(1.03–1.17) | 117 | 0.96(0.79–1.17) | 1052 | 1.04(0.97–1.12) | 550 | 1.13(1.03–1.24) |
| BMI 25.0–27.4 kg/m2 | 14870 | 443 | 1.07(0.97–1.18) | 40 | 0.87(0.63–1.20) | 409 | 1.09(0.98–1.21) | 210 | 1.17(1.01–1.35) |
| BMI 27.5–29.9 kg/m2 | 4624 | 124 | 0.91(0.76–1.10) | 17 | 1.14(0.70–1.84) | 150 | 1.30(1.10–1.54) | 62 | 1.12(0.86–1.44) |
| BMI ≥30 kg/m2 | 4806 | 148 | 1.10(0.93–1.30) | 15 | 1.06(0.63–1.77) | 133 | 1.15(0.96–1.37) | 46 | 0.86(0.64–1.16) |
| P for trendc | 0.003 | 0.48 | <0.0001 | 0.05 | |||||
| P for heterogeneityd | 0.70 | 0.56 | |||||||
| Body fatness at baseline | |||||||||
| BMI <22.5 kg/m2 | 94533 | 2137 | 1 (ref) | 249 | 1 (ref) | 2106 | 1 (ref) | 930 | 1 (ref) |
| BMI 22.5–24.9 kg/m2 | 72201 | 1841 | 1.18(1.10–1.25) | 184 | 1.01(0.83–1.23) | 1696 | 1.08(1.01–1.15) | 780 | 1.02(0.93–1.13) |
| BMI 25.0–27.4 kg/m2 | 51822 | 1415 | 1.31(1.22–1.40) | 156 | 1.25(1.02–1.53) | 1298 | 1.17(1.09–1.25) | 656 | 1.15(1.04–1.28) |
| BMI 27.5–29.9 kg/m2 | 27306 | 793 | 1.41(1.29–1.53) | 84 | 1.33(1.03–1.70) | 684 | 1.18(1.08–1.29) | 367 | 1.20(1.06–1.36) |
| BMI ≥30 kg/m2 | 45716 | 1304 | 1.42(1.32–1.53) | 137 | 1.35(1.08–1.68) | 1136 | 1.20(1.11–1.30) | 566 | 1.15(1.03–1.29) |
| P for trendc | <0.0001 | 0.002 | <0.0001 | 0.003 | |||||
| P for heterogeneityd | 0.65 | 0.32 | |||||||
| Weight change from age 18 to baseline | |||||||||
| Loss ≥3.0 kg | 16936 | 416 | 0.92(0.81–1.04) | 47 | 0.92(0.65–1.31) | 422 | 0.95(0.84–1.06) | 184 | 0.89(0.74–1.07) |
| Loss or gain <3.0 kg | 43562 | 1082 | 1 (ref) | 121 | 1 (ref) | 1078 | 1 (ref) | 482 | 1 (ref) |
| Gain 3.0–5.9 kg | 36947 | 922 | 1.04(0.95–1.13) | 92 | 0.91(0.69–1.19) | 861 | 0.96(0.88–1.05) | 395 | 1.00(0.87–1.14) |
| Gain 6.0–9.9 kg | 47485 | 1349 | 1.19(1.10–1.30) | 144 | 1.12(0.88–1.43) | 1259 | 1.09(1.00–1.18) | 565 | 1.05(0.92–1.19) |
| Gain 10.0–19.9 kg | 72437 | 2136 | 1.27(1.18–1.38) | 242 | 1.28(1.02–1.60) | 1910 | 1.09(1.01–1.18) | 940 | 1.07(0.95–1.20) |
| Gain ≥20.0 kg | 50150 | 1550 | 1.39(1.28–1.51) | 156 | 1.27(0.99–1.62) | 1357 | 1.13(1.04–1.24) | 707 | 1.13(1.00–1.28) |
| P for trendc | <0.0001 | 0.002 | <0.0001 | 0.02 | |||||
| P for heterogeneityd | 0.83 | 0.28 | |||||||
Abbreviations: ref, reference; NHS, Nurses’ Health Study; BMI, body mass index; OR, odds ratio; CI, confidence interval; MET, metabolic equivalent of task.
Multivariable logistic regression model was adjusted for time period of endoscopy (in two-year intervals), number of prior endoscopies (continuous), time since the most recent endoscopy (continuous, year), age (continuous, year), race (white, nonwhite), family history of colorectal cancer (no, yes), smoking (never smoker, past smoker <30 pack-years, past smoker ≥30 pack-years, current smoker <30 pack-years, current smoker ≥30 pack-years), height (only for somatotype and weight change; continuous, cm), physical activity (<7.5 MET-h/wk, 7.5–14.9 MET-h/wk, 15.0–29.9 MET-h/wk, 30.0–59.9 MET-h/wk, ≥60.0 MET-h/wk), alcohol intake (never, <3.5 g/d, 3.5–6.9 g/d, ≥7.0 g/d), dietary factors (folate, vitamin D, calcium, processed red meat; in quartiles), regular aspirin use (no, yes), and menopausal hormone use (premenopausal/missing menopause, no history of menopausal hormone use, current menopausal hormone use, past menopausal hormone use). Weight change was also adjusted for BMI at age 18 (<22.5 kg/m2, 22.5–24.9 kg/m2, 25.0–27.4 kg/m2, 27.5–29.9 kg/m2, ≥30.0 kg/m2).
P for trend was calculated using somatotype as an ordinal score variable.
P for trend was calculated using BMI and weight change as continuous variables.
P for heterogeneity was calculated in case-only analysis.
Table 5.
Multivariable associations of body fatness over the life course with serrated polyps by size and conventional adenomas by risk classification in men (HPFS)
| Serrated polyps | Conventional adenomas | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-polyp | Small serrated polyps | Large serrated polyps | Nonadvanced conventional adenomas | Advanced conventional adenomas | |||||
| Person-endoscopy | n | OR (95% CI)a | n | OR (95% CI)a | n | OR (95% CI)a | n | OR (95% CI)a | |
| Body fatness at age 5 | |||||||||
| Somatotype 1 | 20017 | 581 | 1 (ref) | 53 | 1 (ref) | 855 | 1 (ref) | 503 | 1 (ref) |
| Somatotype 2 | 12889 | 364 | 0.95(0.83–1.09) | 37 | 1.06(0.69–1.63) | 547 | 0.98(0.87–1.09) | 269 | 0.88(0.75–1.02) |
| Somatotype 3–4 | 14825 | 428 | 0.98(0.86–1.12) | 52 | 1.30(0.87–1.94) | 638 | 1.01(0.91–1.12) | 364 | 1.07(0.93–1.23) |
| Somatotype ≥5 | 8002 | 243 | 1.01(0.87–1.18) | 20 | 0.93(0.55–1.56) | 309 | 0.91(0.80–1.05) | 193 | 1.06(0.89–1.26) |
| P for trendb | 0.88 | 0.89 | 0.28 | 0.11 | |||||
| P for heterogeneityd | 0.96 | 0.06 | |||||||
| Body fatness at age 10 | |||||||||
| Somatotype 1 | 13018 | 362 | 1 (ref) | 39 | 1 (ref) | 549 | 1 (ref) | 339 | 1 (ref) |
| Somatotype 2 | 17307 | 528 | 1.07(0.94–1.23) | 48 | 0.91(0.59–1.40) | 747 | 1.01(0.90–1.13) | 391 | 0.88(0.76–1.02) |
| Somatotype 3–4 | 15784 | 417 | 0.94(0.81–1.08) | 54 | 1.10(0.72–1.68) | 652 | 0.98(0.87–1.10) | 387 | 0.99(0.85–1.15) |
| Somatotype ≥5 | 10128 | 325 | 1.11(0.95–1.30) | 23 | 0.73(0.43–1.24) | 419 | 0.99(0.87–1.13) | 225 | 0.96(0.80–1.14) |
| P for trendb | 0.47 | 0.27 | 0.50 | 0.78 | |||||
| P for heterogeneityd | 0.21 | 0.47 | |||||||
| Body fatness at age 21 | |||||||||
| BMI <22.5 kg/m2 | 29872 | 820 | 1 (ref) | 93 | 1 (ref) | 1219 | 1 (ref) | 722 | 1 (ref) |
| BMI 22.5–24.9 kg/m2 | 23134 | 642 | 0.99(0.89–1.10) | 62 | 0.82(0.59–1.14) | 930 | 0.99(0.90–1.08) | 479 | 0.92(0.81–1.03) |
| BMI 25.0–27.4 kg/m2 | 11969 | 365 | 1.09(0.96–1.24) | 32 | 0.81(0.54–1.22) | 438 | 0.90(0.80–1.00) | 283 | 1.03(0.89–1.18) |
| BMI 27.5–29.9 kg/m2 | 2437 | 75 | 1.04(0.81–1.32) | 10 | 1.24(0.63–2.41) | 127 | 1.26(1.04–1.52) | 55 | 0.97(0.73–1.29) |
| BMI ≥30 kg/m2 | 1158 | 33 | 0.99(0.69–1.41) | 4 | 1.03(0.37–2.85) | 48 | 1.01(0.75–1.36) | 32 | 1.18(0.82–1.71) |
| P for trendc | 0.46 | 0.84 | 0.86 | 0.53 | |||||
| P for heterogeneityd | 0.68 | 0.50 | |||||||
| Body fatness at baseline | |||||||||
| BMI <22.5 kg/m2 | 9115 | 194 | 1 (ref) | 21 | 1 (ref) | 334 | 1 (ref) | 149 | 1 (ref) |
| BMI 22.5–24.9 kg/m2 | 22559 | 561 | 1.14(0.96–1.35) | 68 | 1.24(0.75–2.03) | 872 | 1.05(0.92–1.19) | 484 | 1.30(1.08–1.57) |
| BMI 25.0–27.4 kg/m2 | 23303 | 668 | 1.25(1.06–1.48) | 72 | 1.22(0.74–2.00) | 924 | 1.05(0.92–1.19) | 580 | 1.42(1.18–1.71) |
| BMI 27.5–29.9 kg/m2 | 9906 | 349 | 1.46(1.21–1.75) | 28 | 1.03(0.58–1.84) | 423 | 1.08(0.93–1.26) | 240 | 1.33(1.08–1.64) |
| BMI ≥30 kg/m2 | 6452 | 238 | 1.49(1.22–1.81) | 23 | 1.36(0.74–2.49) | 310 | 1.19(1.01–1.40) | 179 | 1.47(1.17–1.85) |
| P for trendc | <0.0001 | 0.25 | 0.03 | 0.007 | |||||
| P for heterogeneityd | 0.80 | 0.60 | |||||||
| Weight change from age 21 to baseline | |||||||||
| Loss ≥3.0 kg | 5318 | 122 | 1.02(0.81–1.27) | 11 | 0.69(0.34–1.37) | 197 | 1.01(0.85–1.20) | 87 | 0.84(0.66–1.09) |
| Loss or gain <3.0 kg | 13200 | 295 | 1 (ref) | 39 | 1 (ref) | 493 | 1 (ref) | 249 | 1 (ref) |
| Gain 3.0–5.9 kg | 10737 | 286 | 1.19(1.00–1.40) | 29 | 0.89(0.55–1.45) | 409 | 1.00(0.87–1.14) | 230 | 1.11(0.93–1.34) |
| Gain 6.0–9.9 kg | 14033 | 398 | 1.24(1.06–1.44) | 42 | 0.97(0.62–1.51) | 545 | 1.00(0.88–1.14) | 338 | 1.19(1.01–1.41) |
| Gain 10.0–19.9 kg | 18433 | 568 | 1.31(1.13–1.51) | 54 | 0.90(0.58–1.39) | 787 | 1.07(0.95–1.21) | 479 | 1.21(1.03–1.42) |
| Gain ≥20.0 kg | 6867 | 266 | 1.58(1.32–1.89) | 26 | 1.16(0.69–1.96) | 332 | 1.18(1.01–1.37) | 188 | 1.17(0.96–1.44) |
| P for trendc | <0.0001 | 0.16 | 0.01 | 0.01 | |||||
| P for heterogeneityd | 0.99 | 0.88 | |||||||
Abbreviations: ref, reference; HPFS, Health Professionals Follow-up Study; BMI, body mass index; OR, odds ratio; CI, confidence interval; MET, metabolic equivalent of task.
Multivariable logistic regression model was adjusted for time period of endoscopy (in two-year intervals), number of prior endoscopies (continuous), time since the most recent endoscopy (continuous, year), age (continuous, year), race (white, nonwhite), family history of colorectal cancer (no, yes), smoking (never smoker, past smoker <30 pack-years, past smoker ≥30 pack-years, current smoker <30 pack-years, current smoker ≥30 pack-years), height (only for somatotype and weight change; continuous, year), physical activity (<7.5 MET-h/wk, 7.5–14.9 MET-h/wk, 15.0–29.9 MET-h/wk, 30.0–59.9 MET-h/wk, ≥60.0 MET-h/wk), alcohol intake (never, <7.0 g/d, 7.0–13.9 g/d, ≥14.0 g/d), dietary factors (folate, vitamin D, calcium, processed red meat; in quartiles), and regular aspirin use (no, yes). Weight change was also adjusted for BMI at age 21 (<22.5 kg/m2, 22.5–24.9 kg/m2, 25.0–27.4 kg/m2, 27.5–29.9 kg/m2, ≥30.0 kg/m2).
P for trend was calculated using somatotype as an ordinal score variable.
P for trend was calculated using BMI and weight change as continuous variables.
P for heterogeneity was calculated in case-only analysis.
Finally, we performed subgroup analysis according to polyp subsite (Supplementary Tables 2 & 3). No subsite heterogeneity was found for measures of childhood body fatness. For SPs in women, adulthood body fatness and weight change showed the strongest positive associations with rectal SPs, followed in order by distal and proximal SPs (Pheterogeneity comparing proximal and rectal SPs = 0.004 and 0.007). Interestingly, an opposite pattern was found for conventional adenomas; the associations with body fatness at baseline seemed to increase from rectum, distal colon, to proximal colon (Pheterogeneity comparing proximal and rectal adenomas = 0.02 in women, 0.005 in men). Body fatness in early adulthood in women and weight change in men showed a similar pattern (Pheterogeneity comparing proximal and rectal adenomas = 0.006 and 0.01).
DISCUSSION
In this large prospective cohort study, we found that women and men with increased body fatness in adulthood and weight gain from early adulthood were more likely to develop SPs and conventional adenomas, while women with increased body fatness in early and late childhood experienced greater risk of conventional adenomas. These results indicate the importance of attained adiposity in adulthood in both sexes and a potential role of early-life body fatness in women. Our findings provide further rationale for maintaining a healthy weight throughout the life course for CRC prevention.
Given that SPs underwent major discoveries and changes in terimology in the past two decades, conventional adenomas were studied more extensively than SPs. A number of observational studies reported a positive association between adiposity and conventional adenomas25,26. These results were supported by two meta-analyses studying the risk of conventional adenomas in relation to BMI and waist circumference, both of which found a significant positive relationship27,28. With regards to recent studies investigating body fatness and the risk of SPs, positive associations were reported in some observational studies29–31 while null results in others32–34. Previous analysis showed a stronger association between adulthood BMI and SPs compared with conventional adenomas12. In the present study, we extended this finding by reporting a greater risk of SPs associated with weight gain since early adulthood compared with conventional adenomas. Compared to attained BMI, weight change may better capture the effect of excess body fat during adulthood. Increased body fatness creates a chronic subclinical inflammatory environment35, which, through a variety of mechanisms, downregulates DNA repair pathways. The resulting mismatch repair induces microsatellite instability36, which is closely linked to the serrated pathway37. Evidence also suggests that obesity, in addition to altering the regional inflammatory status, increases the abundance of specific microbes such as Fusobacterium nucleatum38,39, a bacterium strongly associated with the serrated pathway and CRC37. Taken together, these growing data indicate a role of obesity-induced inflammation and dysbiosis in the serrated pathway for CRC.
To date, studies on conventional adenomas and SPs have largely focused on body fatness during adulthood. Evidence is limited regarding how early-life body fatness influences the development of CRC precursors40. To our knowledge, our study is the first prospective analysis to comprehensively assess the association beween body fatness during childhood and adolescence and the risk of CRC precursors. In the present study, women who were obese in early and late childhood had a greater risk of SPs and conventional adenomas. These positive associations were attenuated after adjustment for adult BMI, particularly for SPs, suggesting that the increased risk was at least partly mediated by attained adiposity later in life since childhood and adolescence obesity often tracks to adulthood. These findings are in line with our prior report in the NHS240 that women with higher body fatness at age 5 years had slightly higher risk of developing conventional adenomas. In addition, our data further demonstrated, for the first time, that women who were obese in childhood were at higher risk for conventional adenomas than SPs. This finding is in constrast to adulthood body fatness which had stronger association with SPs. Collectively, our results indicate that early life body fatness may have a greater impact on the development of conventional adenomas while the risk of SPs is increased primarily due to attained body fatness later in life.
The difference in strength of association with early life body fatness between the two colorectal precursors can be linked to the distinct features of CRCs that arise from heterogenenous pathways. Serrated CRCs generally have an older age of onset compared with their conventional counterpart. Assuming lifestyle has a cumulative effect on the risk of disease, the lag of age of onset between serrated CRC and conventional CRC may be explained in part by the difference in contribution of lifestyle to early colorectal carcinogenesis at different ages. Studies also suggest other lifestyle factors during adolescent such as physical activity and diet to be pertinent to the development of conventional adenomas41,42. Nonetheless, future investigations should focus on the biologic mechanism of the age-specific effect of lifestyle on the two colorectal precursors.
Interestingly, we did not find any association in men between early-life body fatness and risk of polyps. These findings are consistent with those of CRC3,43, in which a positive association between body fatness in early life and CRC risk was found in women but not in men. A potential explanation for these differential findings by sex is the role of sex hormones, which have been implicated in CRC44,45. In men and postmenopausal women, obesity increases the estrogen/testosterone ratio (E/T ratio) by elevating the production of estrogen from androstenedione in the fat tissue44. Higher E/T ratio has been associated with greater CRC risk in men and a lower CRC risk in postmenopausal wome45. However, it remains largely unknown how early-life adiposity may influence adulthood sex hormones and CRC development46. Further studies are needed to better understand the sex-dependent role of adiposity over the life course in CRC.
Our study has several strengths. First, we utilized three large prospective cohorts equipped with long-term follow-up; comprehensive longitudinal assessment of adiposity, lifestyle, and dietary risk factors; and confirmation of polyp diagnosis with detailed recording of histopathologic information based on pathology reports. These features enabled us to comprehensively examine measures of adiposity over the life course while properly controlling for confounding factors. Several limitations of our study should be noted as well. First, body shape assessed by recalled somatotype in early life and self-reported BMI in adulthood is subject to measurement error. However, good validity of recalled early life body fatness had been established by previous studies19, therefore we do not expect such error to have substantial influence on our findings. Moreover, given the prospective design, any error in exposure assessment would have likely attenuated the observed association. Second, because of the evolving nature and lack of consensus regarding the diagnostic criteria of specific subtypes of SPs, we were unable to distinguish hyperplastic polyps from sessile serrated adenomas/polyps and traditional serrated adenomas. However, large SPs have been established as a good proxy for sessile serrated adenoma/polyp47 and predict the likelihood of progression into advanced neoplasia15. Third, our results need to be interpreted cautiously as multiple comparisons were performed and some of the findings may be due to chance. However, we interpreted our results in a holistic way, prioritizing coherence and consistency rather than only statistical significance. Finally, our study participants were mostly Caucasians and therefore our results need to be confirmed in other racial/ethnic populations.
In conclusion, we found that weight gain during early adulthood and attained adiposity were associated with increased risk of CRC presurcors in both women and men, while increased body fatness in childhood showed a positive association in women. Given the strong correlation between obesity early and later in life, our findings provide further support for the importance of weight management across the lifespan for CRC prevention.
Supplementary Material
Novelty and Impact.
Adulthood obesity is associated with increased risk of serrated and conventional colorectal precursors but the influence of early-life adiposity remains unclear. This work represents the first effort to assess the association of adiposity from childhood to adulthood with risk of serrated polyps and conventional adenomas. Our findings provide evidence for a role of early life adiposity in colorectal tumorigenesis and highlight the importance of weight management across the lifespan for colorectal cancer prevention.
ACKNOWLEDGEMENT
We would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
FUNDING
This work was supported by the American Cancer Society Mentored Research Scholar Grant (MRSG-17-220-01 - NEC to M.S.); by the U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S. Fuchs; K24 DK098311, R01 CA137178, R01 CA202704, R01 CA176726 to A.T.C.; R01 CA151993, R35 CA197735 to S.O.; R03 CA197879 to KW; R21 CA230873 to K.W. and S.O.; R00 CA215314 to M.S.]; and by grants from the American Institute for Cancer Research (K.W.), the Project P Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. Dr. Chan is a Stuart and Suzanne Steele MGH Research Scholar. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- NHS
Nurses Health Study
- HPFS
Health Professionals Follow-up Study
- BMI
body mass index
- CRC
colorectal cancer
- SP
serrated polyp
- HP
hyperplastic polyp
- FFQ
food frequency questionnaire
- MET
metabolic equivalent of task
- OR
odds ratio
- CI
confidence interval
- MV
multivariable model
Footnotes
CONFLICT OF INTEREST
Andrew T. Chan previously served as a consultant for Bayer Healthcare and Pfizer Inc. for work unrelated to the topic of this manuscript. This study was not funded by Bayer Healthcare or Pfizer Inc. No other conflict of interest exists.
REFERENCES
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–53. [DOI] [PubMed] [Google Scholar]
- 2.Caprio S, Hyman LD, Limb C, McCarthy S, Lange R, Sherwin RS, Shulman G, Tamborlane WV. Central adiposity and its metabolic correlates in obese adolescent girls. American Journal of Physiology-Endocrinology and Metabolism 1995;269:E118–26. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Wu K, Giovannucci EL, Ma J, Colditz GA, Fuchs CS, Willett WC, Stampfer MJ, Nimptsch K, Ogino S, Wei EK. Early life body fatness and risk of colorectal cancer in U.S. women and men--results from two large cohort studies. Cancer Epidemiology Biomarkers & Prevention 2015;24:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantor ED, Udumyan R, Signorello LB, Giovannucci EL, Montgomery S, Fall K. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut 2016;65:1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidayat K, Yang C-M, Shi B-M. Body fatness at an early age and risk of colorectal cancer. International Journal of Cancer 2018;142:729–40. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. JNCI Journal of the National Cancer Institute 2010;102:89–95. [DOI] [PubMed] [Google Scholar]
- 7.East JE, Vieth M, Rex DK. Serrated lesions in colorectal cancer screening: detection, resection, pathology and surveillance. Gut 2015;64:991–1000. [DOI] [PubMed] [Google Scholar]
- 8.Snover DC. Update on the serrated pathway to colorectal carcinoma. Human Pathology 2011;42:1–10. [DOI] [PubMed] [Google Scholar]
- 9.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138:2088–100. [DOI] [PubMed] [Google Scholar]
- 10.Leslie A, Carey FA, Pratt NR, Steele RJC. The colorectal adenoma-carcinoma sequence: the colorectal adenoma-carcinoma sequence. Br J Surg 2002;89:845–60. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JC, Rangasamy P, Rustagi T, Myers M, Sanders M, Vaziri H, Wu G, Birk JW, Protiva P. Risk factors for sessile serrated adenomas. Journal of Clinical Gastroenterology 2011;45:694–9. [DOI] [PubMed] [Google Scholar]
- 12.He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology 2018;155:355–373.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace K, Grau MV, Ahnen D, Snover DC, Robertson DJ, Mahnke D, Gui J, Barry EL, Summers RW, McKeown-Eyssen G, Haile RW, Baron JA. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiology Biomarkers & Prevention 2009;18:2310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995;122:327–34. [DOI] [PubMed] [Google Scholar]
- 15.He X, Hang D, Wu K, Nayor J, Drew DA, Giovannucci EL, Ogino S, Chan AT, Song M. Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology 2019;S001650851941086X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. The Lancet 1991;338:464–8. [DOI] [PubMed] [Google Scholar]
- 18.Stunkard AJ, Sørensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983;60:115–20. [PubMed] [Google Scholar]
- 19.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol 1993;138:56–64. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women: Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 21.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570–2. [PubMed] [Google Scholar]
- 22.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 23.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Rood JC, Harnack LJ, Sampson LK, Willett WC. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. American Journal of Epidemiology 2018;187:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. American Journal of Epidemiology 2017;185:570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States). Cancer Causes Control 1996;7:253–63. [DOI] [PubMed] [Google Scholar]
- 26.Huang L, Wang X, Gong W, Huang Y, Jiang B. The comparison of the clinical manifestations and risk factors of colorectal cancer and adenomas: results from a colonoscopy-based study in southern Chinese. Int J Colorectal Dis 2010;25:1343–51. [DOI] [PubMed] [Google Scholar]
- 27.Omata F, Deshpande GA, Ohde S, Mine T, Fukui T. The association between obesity and colorectal adenoma: systematic review and meta-analysis. Scandinavian Journal of Gastroenterology 2013;48:136–46. [DOI] [PubMed] [Google Scholar]
- 28.Hong S, Cai Q, Chen D, Zhu W, Huang W, Li Z. Abdominal obesity and the risk of colorectal adenoma: a meta-analysis of observational studies. European Journal of Cancer Prevention 2012;21:523–31. [DOI] [PubMed] [Google Scholar]
- 29.Martinez M, McPherson R, Levin B, Glober G. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology 1997;113:423–9. [DOI] [PubMed] [Google Scholar]
- 30.Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev 2002;11:1012–8. [PubMed] [Google Scholar]
- 31.Fu Z, Shrubsole MJ, Smalley WE, Wu H, Chen Z, Shyr Y, Ness RM, Zheng W. Lifestyle factors and their combined impact on the risk of colorectal polyps. American Journal of Epidemiology 2012;176:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omata F, Brown WR, Tokuda Y, Takahashi O, Fukui T, Ueno F, Mine T. Modifiable risk factors for colorectal neoplasms and hyperplastic polyps. Intern Med 2009;48:123–8. [DOI] [PubMed] [Google Scholar]
- 33.Burnett-Hartman AN, Passarelli MN, Adams SV, Upton MP, Zhu L-C, Potter JD, Newcomb PA. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. American Journal of Epidemiology 2013;177:625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fliss-Isakov N, Zelber-Sagi S, Webb M, Halpern Z, Shibolet O, Kariv R. Distinct metabolic profiles are associated with colorectal adenomas and serrated polyps: metabolic alterations and polyps. Obesity 2017;25:S72–80. [DOI] [PubMed] [Google Scholar]
- 35.John BJ, Abulafi AM, Poullis A, Mendall MA. Chronic subclinical bowel inflammation may explain increased risk of colorectal cancer in obese people. Gut 2007;56:1034–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–81. [DOI] [PubMed] [Google Scholar]
- 37.Bettington M, Walker N, Rosty C, Brown I, Clouston A, Wockner L, Whitehall V, Leggett B. Critical appraisal of the diagnosis of the sessile serrated adenoma. The American Journal of Surgical Pathology 2014;38:158–66. [DOI] [PubMed] [Google Scholar]
- 38.Maciel SS, Feres M, Gonçalves TED, Zimmermann GS, da Silva HDP, Figueiredo LC, Duarte PM. Does obesity influence the subgingival microbiota composition in periodontal health and disease? J Clin Periodontol 2016;43:1003–12. [DOI] [PubMed] [Google Scholar]
- 39.Andoh A, Nishida A, Takahashi K, Inatomi O, Imaeda H, Bamba S, Kito K, Sugimoto M, Kobayashi T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr 2016;59:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimptsch K, Giovannucci E, Willett WC, Fuchs CS, Wei EK, Wu K. Body fatness during childhood and adolescence, adult height, and risk of colorectal adenoma in women. Cancer Prev Res (Phila) 2011;4:1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Rezende LFM, Lee DH, Keum N, Nimptsch K, Song M, Lee I-M, Eluf-Neto J, Ogino S, Fuchs C, Meyerhardt J, Chan AT, Willett W, et al. Physical activity during adolescence and risk of colorectal adenoma later in life: results from the Nurses’ Health Study II. Br J Cancer 2019;121:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nimptsch K, Malik VS, Fung TT, Pischon T, Hu FB, Willett WC, Fuchs CS, Ogino S, Chan AT, Giovannucci E, Wu K. Dietary patterns during high school and risk of colorectal adenoma in a cohort of middle-aged women: high school dietary patterns and adenoma risk. Int J Cancer 2014;134:2458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo A, Franceschi S, La Vecchia C, Dal Maso L, Montella M, Conti E, Giacosa A, Falcini F, Negri E. Body size and colorectal-cancer risk. Int J Cancer 1998;78:161–5. [DOI] [PubMed] [Google Scholar]
- 44.Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, Wu K, Tworoger SS, Hankinson SE, Fuchs C, Gaziano JM, Buring JE, Giovannucci E. Association between sex hormones and colorectal cancer risk in men and women. Clinical Gastroenterology and Hepatology 2013;11:419–424.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H, Giovannucci EL. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control 2017;28:1–4. [DOI] [PubMed] [Google Scholar]
- 46.Tworoger SS, Eliassen AH, Missmer SA, Baer H, Rich-Edwards J, Michels KB, Barbieri RL, Dowsett M, Hankinson SE. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiology Biomarkers & Prevention 2006;15:2494–501. [DOI] [PubMed] [Google Scholar]
- 47.Lu F-I, van Niekerk DW, Owen D, Tha SPL, Turbin DA, Webber DL. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma: The American Journal of Surgical Pathology 2010;34:927–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request at https://www.nurseshealthstudy.org/researchers (nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/. The data are not publicly available due to privacy or ethical restrictions.


