Abstract
Objective
To report sex-specific changes in CVD risk following Roux-en-Y gastric bypass surgery (RYGB).
Background:
Long-term changes in cardiovascular disease (CVD) risk following bariatric surgery are not well characterized.
Methods:
Between 2006–2009 1770 adults enrolled in a prospective cohort study underwent Roux-en-Y gastric bypass (RYGB) at 1 of 10 U.S. hospitals. Research assessments were conducted pre-surgery and annually post-surgery over 7 years. Sex-specific predicted 10-year and lifetime CVD risk were calculated using the Framingham-lipid, Framingham-body mass index (BMI) and Atherosclerotic (ASCVD) scoring algorithms among participants with no history of CVD. Of 1566 eligible participants, 1234 (75.9%) with CVD risk determination pre- and post-surgery were included (1013 females, 221 males).
Results:
Based on the Framingham-lipid, the percentage of females with predicted high (>20%) 10-year CVD risk declined from pre-surgery (6.5% [95% CI:6.7–7.5]) to 1 year post-surgery (1.0% [95% CI:0.8–1.2]; p<0.001), then increased 1 to 7 years post-surgery (to 2.8% [95% CI:1.6–3.3]; p=0.003), but was lower 7 years post-surgery versus pre-surgery (p<0.001). Time trends for percentage of high-risk participants and mean CVD risk scores were similar for both sexes and other evaluated CVD risk scores. For example, among males mean lifetime ASCVD score declined from pre-surgery to 1 year post-surgery, then increased 1 to 7 years post-surgery. However, there was a net decline from pre-surgery (p<0.001).
Conclusion:
Among both females and males, predicted 10-year and lifetime CVD risk was substantially lower 7 years post-RYGB than pre-surgery, suggesting RYGB surgery can lead to sustained improvements in short- and long-term CVD risk.
Keywords: bariatric surgery, gastric bypass, severe obesity, cardiovascular disease
Introduction
As body mass index (BMI) increases, the risk of developing cardiovascular disease (CVD) also increases1. The association is thought to be primarily a function of an increase in CVD risk factors (i.e., hypertension, dyslipidemia, and insulin resistance2) related to increasing BMI3. Obesity also directly increases risk of CVD by impacting cardiovascular structure and function. For example, obesity increases the risk of left ventricular structural abnormalities which may lead to remodeling and left ventricle hypertrophy, left atrial enlargement, and impairment of systolic and diastolic function4. Those with severe obesity (BMI ≥ 40 kg/m2) are an especially high risk population for specific types of CVD including coronary artery disease, myocardial infarction and heart arrhythmias, compared to those with less severe obesity5.
Roux-en-Y gastric bypass (RYGB) surgery, which was the most common modern-day bariatric surgical procedure prior to 2013 and is currently the second most common procedure6,7, is an effective treatment for severe obesity8. Surgical changes to the gastrointestinal tract result in substantial weight loss and impact metabolic disorders including the remission of Type 2 diabetes (T2D)9. There is also evidence that CVD risk and CVD-related mortality decline in the first few years following RYGB surgery10–12. However, associations between post-surgery weight regain with worsening of T2D, hyperlipidemia, and hypertension13, suggest initial improvements in CVD risk may diminish over time. Studies of currently performed bariatric surgical procedures with repeated measures over long-term follow-up are needed to evaluate the sustainability of the reduction in CVD risk12,14. Furthermore, given evidence that biological sex may moderate the effect of interventions on CVD risk (i.e., due to general biological differences, as well as differences in environment, lifestyle and attitudes that can affect CVD risk and CVD outcomes)15,16, there is a need to evaluate sex-specific changes in CVD17,18.
Since CVD events (e.g. stroke, heart attack, angina) usually occur later in life, risk assessment for future CVD events is critical to monitoring health status19. This can be done by evaluating individual risk factors (e.g. systolic pressure, T2D, treatment for hypertension), or with CVD risk scores, which use an array of CVD risk factors to estimate the likelihood of an individual having a CVD event within a specified time frame (i.e. 10-years or lifetime)20,21.
The primary aim of this study was to report sex-specific changes in CVD risk following RYGB in a large multisite prospective cohort study with long-term follow-up. Changes from pre-surgery to 7 years post-surgery in predicted 10-year and lifetime CVD risk (based on the Framingham Risk Score (FRS)22 and the Atherosclerotic CVD (ASCVD)21 risk score), as well as individual CVD risk factors, were evaluated. Secondary aims were to evaluate changes in predicted CVD risk in relation to post-surgery weight regain, as well as to report short- and long-term rates of post-surgery non-fatal CVD events and CVD-related mortality.
Materials and Methods
Design and Participants
The Longitudinal Assessment of Bariatric Surgery (LABS)-2 was a prospective cohort study of 2,458 adults who were at least age 18 at time of enrollment23. Participants who underwent their first bariatric surgery between April 2006 and April 2009 were recruited between February 2005 and February 2009 at one of ten hospitals at six clinical centers throughout the United States. Research assessments are described in Supplementary Appendix 1.
Participants were eligible for evaluation of the secondary aim of CVD event reporting if they underwent RYGB (N=1770) regardless of CVD status at pre-surgery assessment. Per recommended exclusion criteria19,21,22, CVD risk scores were not calculated for participants with a history of CVD (n=204. For the primary aim of reporting change in CVD risk after RYGB, all data components to calculate CVD risk scores at the pre-surgery assessment and at least one follow-up assessment were required for participants to be included in the analysis of CVD risk (n=1234 of 1566 without a history of CVD prior to the first post-surgery assessment, 78.7%) (Supplementary Figure 1).
Measures
Assessment of sociodemographics, anthropometrics and individual CVD risk score components has been described previously13 and is provided in Supplementary Appendix 1.
CVD risk scores
The Framingham and the ASCVD 10-year and lifetime CVD risk scores were calculated using published algorithms19,21,22. All four scores utilize age, sex, total cholesterol, systolic blood pressure, blood pressure treatment, smoking status, and T2D and hyperlipidemia treatment. Race is also used in ASCVD scoring algorithms. Both risk scores both predict coronary heart disease (CHD) death, nonfatal myocardial infarction (MI) and stroke, but the Framingham also predicts coronary insufficiency, transient ischemic attack, intermittent claudication, heart failure and angina pectoris. Alternative versions of the Framingham 10-year and lifetime risk scores (hereafter referred to as “Framingham-lipid”) replace lipid variables (total cholesterol and hyperlipidemia treatment) with BMI22 which allows for calculation of risk with less clinical data (hereafter referred to as “Framingham-BMI”). Pre-surgery age was used to calculate risk scores at all assessments to eliminate the effect of aging on change in risk24. CVD scores were not calculated for participants following a CVD event19,21,22.
To describe CVD risk and to help guide statin therapy decisions in the clinical setting, both the Framingham and ASCVD 10-year risk scores are categorized25,26. The Framingham 10-year risk categories are defined as: low (score <10%), intermediate (10–20%), and high (>20% or more) 10-year risk27. The ASCVD 10-year categories are defined as low (score <7.5%) and high (≥7.5%) 10-year risk28. The Framingham 10-year intermediate and high risk categories29 and the ASCVD high risk category indicate it may be appropriate to initiate statin therapy30. Lifetime scores are intended to motivate patients to make lifestyle changes rather than to guide pharmacological interventions19. The Framingham-lipid, Framingham-BMI and ASCVD lifetime scores utilize the same cut point to indicate low (<39%) and high (≥39%) lifetime CVD risk.
CVD events
A LABS-certified clinical researcher used medical records, physical examination, and patient interviews to determine history of CVD prior to surgery, and CVD events annually post-surgery23. CVD-related mortality was determined using the annual study follow-up and the National Death Index31 through December 31, 2014. For this study, CVD was defined as having a nonfatal myocardial infarction (MI), stroke, ischemic heart disease, congestive heart failure, angina, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG) or death attributed to CVD. Additional information on CVD assessment and identification of CVD-related mortality is available in Supplementary Appendix 1.
To be able to compare the observed CVD-related mortality in the 7 years following RYGB to the CVD-related mortality rate in the general population matched on participants’ sex, age and race, and calendar year, sex-, age-, race-, and year-specific crude mortality rates from the U.S. general population for each calendar year from 2006–2014 were downloaded from the death certificate database collected by the Centers for Disease Control and Prevention (CDC) Wonder Underlying Cause of Death32.
Statistical analysis
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). All reported p values are two-sided; p values less than 0.05 are reported to guide interpretation of findings. All further analyses were stratified by sex. Descriptive statistics were used to summarize participant characteristics. The observed CVD risk scores, as well as individual CVD risk components [i.e., systolic blood pressure/diastolic blood pressure/treatment for hypertension, total cholesterol /treatment for hypercholesterolemia, high-density lipoproteins (HDL-C), T2D and current cigarette smoking], were reported by time point in relation to surgery through 7 years of follow-up. Both continuous and categorical versions of the risk scores and components are reported. Because the Framingham 10-year intermediate and high risk categories29 can both be used to initiate statin therapies, these groups were combined for some analyses, while the three category version (i.e., low, intermediate and high) was used to describe the more detailed change in risk score category over time.
Binary mixed models were used for dichotomous CVD risk categories and CVD risk score components, and mixed-effects ordinal logistic regression models were used for three-level Framingham 10-year CVD risk categories. Likewise, linear mixed models were used to estimate the mean of continuous CVD risk scores and CVD risk score components by time point. Mixed models used all available data via maximum likelihood, with a person-level random intercept, and controlled for pre-surgery factors related to missing data (i.e., site, age and pre-surgery smoking status), with time since surgery entered as a discrete fixed effect. Pairwise comparisons were made between pre-surgery and year 1 (to assess short-term change), pre-surgery and 7 years (to assess long-term change), as well as year 1 and 7 years (to assess the durability of the short-term change). P values were adjusted by simulation for multiple comparisons33. The modeling was repeated for estimating the distributions of categorical risk groups and the mean of continuous CVD risk scores in relation to weight regain, with time since participants’ lowest recorded weight entered as a discrete fixed effect.
Event rates were calculated for non-fatal CVD events and CVD-related mortality by dividing the number of events by the person-years of observation per 1000 person-years, overall and by short (<5 year) and long-term follow-up (≥ 5 years)34. The matched mortality rate in the general population was calculated by multiplying the sex-, age-, race-, and year-specific crude mortality rate per 1,000 from the general population by the number of LABS-2 RYGB participants with each characteristic. Standardized mortality ratios (SMRs) were calculated as the ratio of observed mortality rate in the RYGB sample to the calculated mortality rate in the matched-general population. The 95% confidence intervals (CI) for the event rates and the SMRs were constructed using the Poisson distribution.
Results
Within the CVD risk score sample (1013 females, 221 males), the median age of females was 45 years (IQR: 36–53), 84.9% were white, and median BMI was 46.1 kg/m2 (42.2–51.3). Among males, the median age was 47 years (IQR: 38–55), 92.3% were white, and median BMI was 47.4 kg/m2 (IQR: 43.2–53.0). Additional characteristics by sex are reported in Table 1.
Table 1.
Characteristics of adults prior to undergoing Roux-en-Y gastric bypass in the CVD risk score sample and CVD event sample, stratified by sex.
| CVD risk score sample (N=12345)a No. (%)b | CVD event sample (N=1770) No. (%)b | |||
|---|---|---|---|---|
| Female (N=1013) | Male (N=221) | Female (N=1413) | Male (N=357) | |
| Age, years | ||||
| Median (25th −75th %-ile) | 45 (36, 53) | 47 (38, 55) | 45 (36, 53) | 47 (39, 56) |
| Range | 19–70 | 19–75 | 19–73 | 19–75 |
| Race | N=1397 | N=354 | ||
| White | 860 (84.9) | 204 (92.3) | 1177 (84.3) | 317 (89.5) |
| Black | 115 (11.4) | 14 (6.3) | 168 (12.0) | 28 (7.9) |
| Other | 38 (3.8) | 3 (1.4) | 52 (3.7) | 9 (2.5) |
| Ethnicity | N=356 | |||
| Hispanic | 39 (3.8) | 7 (3.2) | 72 (5.1) | 15 (4.2) |
| Non-Hispanic | 974 (96.2) | 214 (96.8) | 1341 (94.9) | 341 (95.8) |
| Married or living as marriedc | 788/1302 (60.6) | 231/332 (69.6) | 788/1301 (60.6) | 231/332 69.6 |
| Education | N=970 | N=209 | N=1303 | N=332 |
| High school or less | 231 (23.8) | 45 (21.5) | 312 (23.9) | 73 (22.0) |
| Some college | 419 (43.2) | 87 (41.6) | 559 (42.9) | 141 (42.5) |
| College degree | 320 (33.0) | 77 (36.8) | 432 (33.2) | 118 (35.5) |
| Employed for payc | 680/967 (70.3) | 150/206 (72.8) | 894/1298 (68.9) | 231/328 (70.4) |
| Household income, US $ | N=939 | N=205 | N=1263 | N=326 |
| Less than 25,000 | 181 (19.3) | 29 (14.2) | 262 (20.7) | 54 (16.6) |
| 25,000–49,999 | 278 (29.6) | 58 (28.3) | 368 (29.1) | 79 (24.2) |
| 50,000–74,999 | 216 (23.0) | 42 (20.5) | 299 (23.7) | 79 (24.2) |
| 75,000–99,999 | 141 (15.0) | 30 (14.6) | 188 (14.9) | 49 (15.0) |
| >100,000 | 123 (13.1) | 46 (22.4) | 146 (11.6) | 65 (19.9) |
| Current smoker | 18 (1.8) | 5 (2.3) | 32 (2.3) | 11 (3.1) |
| N=1011 | N=1410 | N=356 | ||
| Body mass index, kg/m2 | ||||
| Median (25th −75th %-ile) | 46.1 (42.2, 51.3) | 47.4 (43.2, 53.0) | 46.3 (42.1, 51.5) | 47.8 (43.4, 53.3) |
| Range | 34.1–81.0 | 33.7–76.0 | 33.8–81.0 | 33.7–76.0 |
| Hypertension medicationc | 560 (55.3) | 140 (63.3) | 777/1394 (55.7) | 239/353 (67.7) |
| Hyperlipidemia medicationc | 266/1007 (26.4) | 75/221 (33.9) | 380/1379 (27.6) | 147/352 (41.8) |
| Total cholesterol (mg/dl) | N=1357 | N=344 | ||
| Median (25th −75th %-ile) | 189.0 (162.0, 215.0) | 175.0 (153.0, 198.0) | 188.0 (162.0, 215.0) | 170.5 (148.0, 197.0) |
| Range | 84.0–384.0 | 96.0–298.0 | 84.0–384.0 | 96.0–469.0 |
| High-density lipoproteins (mg/dl) | N=1357 | N=344 | ||
| Median (25th −75th %-ile) | 44.0 (38.0, 53.0) | 37.0 (32.0, 43.0) | 44.0 (38.0, 52.0) | 36.0 (31.0, 42.0) |
| Range | 21.0–107.0 | 21.0–83.0 | 21.0–107.0 | 15.0–83.0 |
| Low-density lipoprotein (mg/dl) | N=866 | N=192 | N=1160 | N=299 |
| Median (25th −75th %-ile) | 112.0 (89.0, 134.0) | 105.0 (82.0, 124.0) | 112.0 (89.0, 134.0) | 101.0 (78.0, 124.0) |
| Range | 27.0–308.0 | 23.0–223.0 | 27.0–308.0 | 23.0–223.0 |
| Triglycerides (mg/dl) | N=863 | N=192 | N=1155 | N=299 |
| Median (25th −75th %-ile) | 137.0 (101.0, 191.0) | 141.0 (106.0, 199.5) | 138.0 (100.0, 193.0) | 142.0 (107.0, 208.0) |
| Range | 37.0–1584.0 | 43.0–675.0 | 30.0–1584.0 | 43.0–1677.0 |
| Systolic blood pressure (mmHg) | N=1383 | N=354 | ||
| Median (25th −75th %-ile) | 129.0 (120.0, 138.0) | 133.0 (120.0, 142.0) | 128.0 (119.0, 138.0) | 130.0 (120.0, 142.0) |
| Range | 83.0–189.0 | 90.0–186.0 | 83.0–189.0 | 90.0–192.0 |
| Type 2 Diabetes | 314 (31.0) | 90 (40.7) | 455/1385 (32.9) | 160/352 (45.5) |
| Hypertension | 667 (65.8) | 164 (74.2) | 926/1387 (66.8) | 281/353 (79.6) |
Abbreviations: CVD, cardiovascular disease.
Participants free of pre-surgery history of cardiovascular disease and have cardiovascular disease risk score at pre-surgery and at least one follow-up assessment.
Denominator shifts between variables due to missing data. Data are reported as No. (%) unless otherwise indicated.
No./total (%).
Data completeness of CVD scores among the sex-specific analysis samples by time period is provided in supplemental material (Supplementary Table 1). Across follow-up, 64.1% of potential CVD risk scores were determined among females and 60.9% among males.
CVD risk.
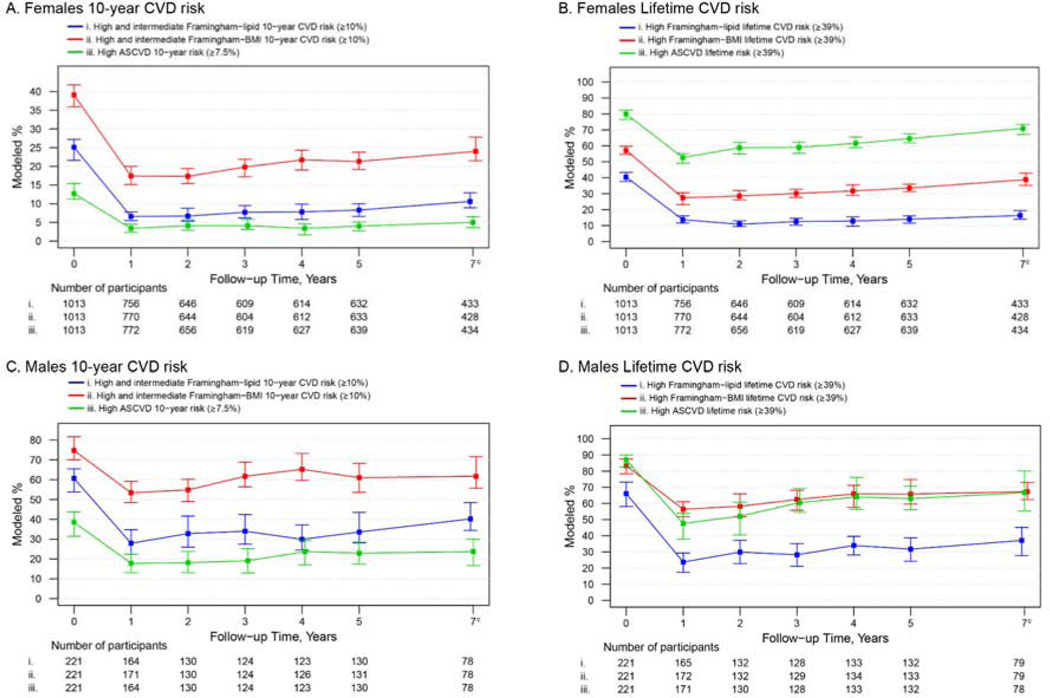
Figure 1 shows the modeled percentages (95% CI) of females and males categorized as intermediate/high or high risk of having a CVD event within 10-years or lifetime based on the Framingham-lipid, Framingham-BMI and ASCVD scores. The estimated percentage of females and males with predicted intermediate/high or high 10-year risk was lower 1 year post-surgery vs pre-surgery (p for all <0.001), then appeared to increase from 1 year to 7 years post-surgery (p for both Framinghams <0.01; for ASCVD females p=0.14, males p=0.08). Still, the percentage with predicted intermediate/high or high 10-year risk was lower at 7 years compared to pre-surgery (p for all <0.001). Similar to the 10-year results, both Framinghams and ASCVD categorized fewer female and male participants with high lifetime risk at 1 year post-surgery compared to pre-surgery (p for all <0.001, Figure 1; values provided in Supplementary Table 2). Though the percentage of females and males with high risk increased from 1 year to 7 years post-surgery (p for all ≤0.01 with exception of female Framingham-lipid, p=0.12), the percentage with high risk was lower at 7 years compared to pre-surgery (p for all <0.001). A comparison of the modeled means of the 10-year and lifetime CVD risk scores by time point revealed similar time trends (Supplementary Table 3, Supplementary Figure 2).
Figure 1.
Modeleda percentage and 95% confidence intervals of adults categorized as intermediate/highb or high CVD risk by time in relation to Roux-en-Y gastric bypass, stratified by sex.
Abbreviations: ASCVD, Atherosclerotic cardiovascular disease; BMI, Body Mass Index (kg/m2); CVD, cardiovascular disease.
a Adjusted for factors related to missing follow-up data (i.e. site, age and current smoking status at pre-surgery). All pairwise comparison tests (for pre-surgery vs 1 year, 1 year vs 7 years and pre-surgery vs 7 years) were significant (p ≤0.01) with the exception of 1 year vs 7 years 10-year ASCVD (p=0.14) and lifetime Framingham-lipids (p=0.12) in females, and the 10-year ASCVD in males (p=0.08).
b Because the Framingham intermediate risk category can be used to initiate statin therapy, the Framingham intermediate and high risk groups were combined.
c Data collection ended before the 7 year assessment of 316 females and 71 males.
Although time trends were similar across 10-year and lifetime CVD risk scores for both sexes, the percentage identified as high risk varied by score and sex (e.g., among females, the percentage with high 10-year CVD risk, was highest with the Framingham-BMI and lowest with the ASCVD; the percentage with predicted high lifetime CVD risk was highest with ASCVD and lowest with Framingham-lipid). Likewise, the magnitude of change over time varied by score and sex (e.g., among males, there was a greater absolute and relative decrease in the percentage with predicted high lifetime risk 1 year and 7 years post-surgery based on the Framingham-lipid vs. the Framingham-BMI or ASCVD).
CVD risk score components.
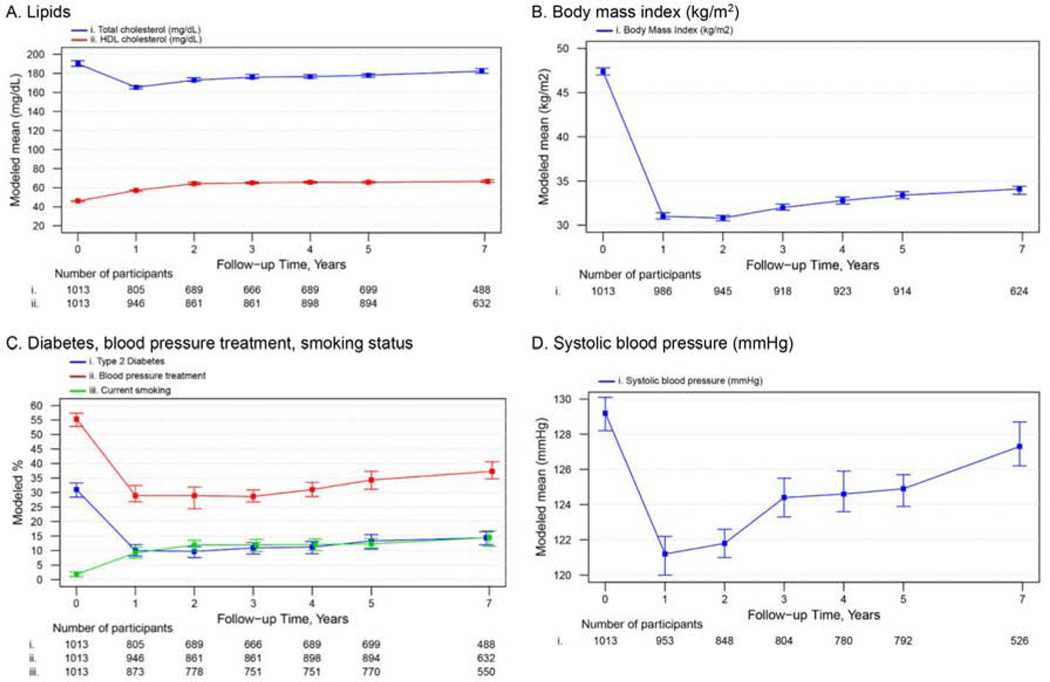
Figure 2 shows the modeled values of CVD risk score components (i.e. total cholesterol, HDL-C, systolic blood pressure, blood pressure treatment, smoking prevalence, T2D and BMI) by time in relation to RYGB among females. Although most CVD risk score components were different (i.e. worse) in year 7 compared year 1 (Figure 2, values provided in Supplementary Table 4), both year 1 and year 7 values were different (i.e. better) compared pre-surgery (p for all <.01). An exception was smoking, the prevalence of which was higher (i.e., worse) 1 year and 7 years post-surgery vs pre-surgery (p for both <.001). Time comparisons were similar among males compared to females (i.e. worse at year 7 compared to year 1 but better at year 7 compared to pre-surgery) (Supplementary Figure 3, Supplementary Table 4).
Figure 2.
Modeleda mean or percentage and 95% confidence intervals of CVD risk components among females by timepoint in relation to Roux-en-Y gastric bypass.
Abbreviations: HDL, high-density lipoprotein.
a Adjusted for factors related to missing follow-up data (i.e. site, age and current smoking status at pre-surgery). All pairwise comparison tests (for pre-surgery vs 1 year, 1 year vs 7 years and pre-surgery vs 7 years) were significant (p ≤0.01).
Changes in CVD risk in relation to weight regain.
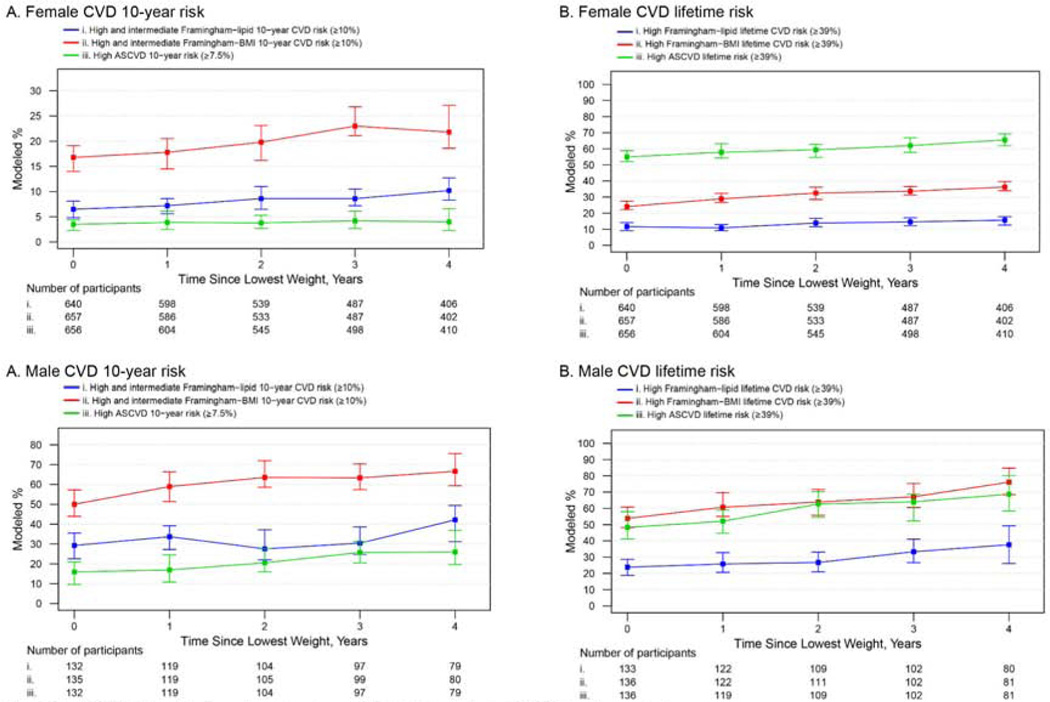
From the CVD risk sample of 1234 participants, 1102 (911 females and 191 males; Supplementary Figure 1) had CVD risk determination following their lowest recorded weight (median time since surgery=4.4 [IQR=1.6–7.5] years; median follow-up after lowest weight=2.7 [IQR=0.4–6.5] years). The percentage of participants with predicted intermediate/high or high 10-year and lifetime risk increased across time as a linear function of weight regain for both females and males (p for all ≤0.01) except for the ASCVD 10-year, which showed a similar trend in males (p=0.06) but did not appear to increase among females (p=0.92) (Figure 3).
Figure 3.
Modeleda percentage and 95% confidence intervals of participants categorized as intermediate/highb or high CVD risk by time since lowest weight, stratified by sex.
Abbreviations: ASCVD, Atherosclerotic cardiovascular disease; BMI, Body Mass Index (kg/m2); CVD, cardiovascular disease.
a Adjusted for factors related to missing follow-up data (i.e. site, age and current smoking status at pre-surgery). All pairwise comparison tests (for pre-surgery vs 1 year, 1 year vs 7 years and pre-surgery vs 7 years) were significant (p ≤0.01) with the exception of 1 year vs 7 years.
b Because the Framingham intermediate risk category can be used to initiate statin therapy, the Framingham intermediate and high risk groups were combined.
c A linear term for time since lowest weight recorded was significant (p ≤0.05) for all CVD scores with the exception of 10-year ASCVD in females (p=0.92) and males (p=0.06).
CVD events.
The frequency and the rates of non-fatal CVD events and CVD mortality are reported in Table 2 among all participants who underwent RYGB (N=1770); the frequency of these outcomes by time in relation to RYGB is available in supplemental material (Supplementary Table 5). For both females and males, atherosclerotic cardiovascular disease was the most common ICD-10 coded CVD-related mortality (Supplementary table 6).
Table 2.
CVD event rates following Roux-en-Y Gastric Bypass, stratified by sex (N=1770 a).
| Total Events, No. | Total Person-Years | Event rate per 1000 person-year |
||||
|---|---|---|---|---|---|---|
| Overall (≤7 years) | Short-term (<5 years) | Long-term (5–7 years) | p-valueb | |||
| Females (N=1413)c | ||||||
| Total non-fatal CVD events | 499 | 9891 | 50.4 (41.6–61.2) | 42.5 (33.8–53.3) | 61.1 (48.5–77.0) | 0.004 |
| Nonfatal myocardial infarction | 37 | 7065 | 5.3 (3.6–7.8) | 4.1 (2.6–6.6) | 7.0 (4.3–11.4) | 0.07 |
| Stroke | 22 | 5182 | 4.2 (2.5–6.9) | 2.7 (1.3– 5.5) | 7.4 (3.9– 13.8) | 0.02 |
| Ischemic heart disease | 206 | 6278 | 33.6 (27.7–40.7) | 30.5 (24.3–38.3) | 37.4 (29.7–47.1) | 0.11 |
| Congestive heart failure | 25 | 5182 | 4.8 (2.8–8.1) | 4.0 (2.1–7.5) | 6.5 (3.3–12.7) | 0.21 |
| Angina | 181 | 6738 | 27.1 (22.1–33.4) | 22.6 (17.5–29.1) | 33.4 (26.2–42.5) | 0.005 |
| Revascularization | 28 | 6749 | 3.9 (2.2–6.7) | 2.0 (1.0–4.3) | 6.3 (3.6–11.1) | 0.001 |
| Percutaneous coronary intervention | 22 | 6749 | 3.3 (1.9–5.8) | 1.3 (0.5–3.2) | 6.0 (3.3–10.9) | 0.002 |
| Coronary artery bypass grafting | 6 | 6748 | 0.9 (0.3–2.1) | 1.0 (0.4–2.7) | 0.6 (0.2–2.6) | 0.50 |
| CVD Mortality | 8 | 8452 | 0.9 (0.5–1.9) | 0.8 (0.3–2.1) | 1.2 (0.5–3.3) | 0.52 |
| Males (N=357)d | ||||||
| Total non-fatal CVD events | 179 | 2499 | 71.6 (53.7–95.5) | 76.3 (53.9–108.1) | 65.4 (45.7–93.6) | 0.47 |
| Nonfatal myocardial infarction | 15 | 1727 | 8.6 (4.9–15.1) | 9.6 (4.9–18.8) | 7.3 (3.0–17.6) | 0.62 |
| Stroke | 10 | 1297 | 6.7 (3.2–14.4) | 5.5 (2.2–13.3) | 9.4 (3.7–23.6) | 0.29 |
| Ischemic heart disease | 63 | 1552 | 39.6 (29.0–54.1) | 46.7 (32.0–68.3) | 31.0 (19.5–49.4) | 0.16 |
| Congestive heart failure | 21 | 1293 | 15.3 (8.7–26.9) | 12.3 (6.0–25.2) | 22.2 (11.6–42.3) | 0.14 |
| Angina | 42 | 1684 | 24.1 (16.0–36.2) | 25.5 (15.3–42.5) | 22.2 (12.7–39.0) | 0.70 |
| Revascularization | 28 | 1682 | 14.6 (9.0–23.6) | 11.5 (5.8–22.7) | 19.2 (10.4–35.4) | 0.24 |
| Percutaneous coronary intervention | 22 | 1682 | 12.6 (7.6–20.9) | 8.7 (4.6–16.5) | 18.3 (9.8–34.1) | 0.057 |
| Coronary artery bypass grafting | 6 | 1680 | 3.3 (1.1–10.0) | 5.0 (1.4–17.6) | 1.2 (0.1–11.3) | 0.28 |
| CVD Mortality | 9 | 2087 | 4.3 (2.3–8.2) | 1.6 (0.4–6.2) | 8.8 (4.2–18.3) | 0.03 |
Abbreviations: CVD, cardiovascular disease; No., number.
Includes participants with pre-surgery history of CVD (n=145).
Comparing short and long term rates
Number of events for females with a pre-surgery history of CVD (N=82): myocardial infarction:13; stroke: 9; ischemic heart disease: 51; congestive heart failure: 16; angina: 41; revascularization: 16; CVD mortality: 2
Number of events for males with a pre-surgery history of CVD (N=63): myocardial infarction: 9; stroke: 4; ischemic heart disease: 42; congestive heart failure: 15; angina:27; revascularization: 21; CVD mortality: 3
Among females, there were 499 non-fatal CVD events and 8 CVD deaths within 7 years following RYGB, corresponding to a non-fatal event rate of 50.4 (95% CI:41.6–61.2) per 1000 person-years and CVD mortality rate of 0.9 (95% CI:0.5–1.9) per 1000 person-years.The SMR for females was 1.18 (95% CI:0.51, 2.32; p=0.74). The non-fatal event rate was higher during long-term versus short-term follow-up (61.1 [95% CI:48.5–77.0] vs 42.5 [33.8–53.3] per 1000 person-year; p<0.001). The mortality rate appeared higher during the long-term versus short term follow-up but given the low frequency of the outcome, statistical power to evaluate this comparison was limited (1.2 [95% CI:0.5–3.3] vs 0.8 [95% CI:0.3–2.1] per 1000 person-years; p=0.52).
Among males, there were 179 non-fatal CVD events within 7 years following RYGB, corresponding to an event rate of 71.6 (95% CI:53.7–95.5) per 1000 person-years and CVD mortality rate of 4.3 (95% CI:2.3–8.2) per 1000 person-years. The SMR for males was 1.96 (95% CI:0.89, 3.71; p=0.09). There was not clear evidence that the non-fatal event rates among males differed during long-term versus short-term follow-up (65.4 [95% CI:45.7–93.6] vs 76.3 [95% CI:53.9–108.1] per 1000 person-year; p=0.47). The mortality rate was higher during the long-term versus short term follow-up (8.8 [95% CI:4.2–18.3] vs 1.6 [95% CI:0.4–6.2] per 1000 person-years; p=0.03).
Discussion
Among a large cohort of adults with severe obesity, discounting age, predicted 10-year and lifetime CVD risk was lower throughout 7 years following RYGB surgery versus pre-surgery whether assessed with the Framingham-lipid, the Framingham-BMI or the ASCVD scoring algorithm. This was true whether considering the percentage of participants with elevated risk or the sample’s mean risk. Although the magnitude of improvement in CVD risk varied by score and timeframe, improvement was substantially larger 1 through 7 years following RYGB than what is typically achieved from diet, exercise or lifestyle interventions aimed at weight loss or CVD risk reduction in overweight and obese adults35–37. For example, 1 year post-RYGB, the percentage of participants with high CVD risk decreased by 85% in females and 89% in males with the Framingham-lipid algorithm, or by 73% in females and 61% in males with the ASCVD algorithm. Seven years post-RYGB these values were still striking, with 57% and 79% decreases in females and males, respectively, with the Framingham-lipid algorithm, or 61% and 38% decreases in females and males, respectively, with the ASCVD algorithm.
While there is limited data on long-term changes in CVD risk with multiple assessments following bariatric surgery12, the reductions in overall CVD risk found in this study are supported by a retrospective cohort study with 1724 RYGB participants and 1724 matched non-surgical controls which reported 63 CVD events (e.g. myocardial infarction, congestive heart failure, or stroke) in the RYGB group versus 110 in the control group across 12 years of follow-up14. In addition, the findings of this study support the durability of effect reported in studies with short-term follow-up12.
Multiple short term (10-year) and long-term (lifetime) CVD risk scores were employed due to the difference between the usage of the scores in clinical care and lack of a consensus on which CVD risk score is best for the bariatric surgery population. The selected CVD risk scores were chosen due to the ability of each score to be used in younger populations in which CVD events are less likely to occur. Additionally, the 2008 Framingham score was chosen due to its prevalence, recognizability, and high external validity. However, the Framingham was developed among only Caucasians, and includes CVD outcomes without proven statin therapy benefit (e.g. heart failure). Thus, the ASCVD risk score, which addresses these limitations and is recommended by the American College of Cardiology/American Heart Association (ACC/AHA)21, was also used.
While both Framingham scores and the ASCVD score showed similar trends of change in 10-year and lifetime CVD risk over time, the scores differed in the percentage of participants identified as having high 10-year and lifetime CVD risk. For example, the Framingham-BMI 10-year algorithm categorized more participants as high risk across follow-up compared to the lipid version. This difference in categorization may be due to the high levels of obesity among bariatric surgery patients both before and after surgery, compared to the general population in which the scores were developed. Compared to the ASCVD score, both Framingham scores identified more women and men as having intermediate or high 10-year risk, perhaps because the Framingham predicts more outcomes. Given these risk designations can be used for the initiation of statin therapy, and the lifetime risk scores can be used to motivate patients to make behavioral changes, score selection has important clinical implications. For example, more females and males would be identified as potential candidates for initiation of statins if the Framingham-BMI was used versus the Framingham-lipid, or either Framingham versus the ASCVD.
Similar to a previous study showing select CVD risk factors (i.e. increases in SBP, HDL-C) increased as a function of weight regain38, the current study demonstrated that the percentage of participants with predicted intermediate/high or high for 10-year and lifetime CVD risk (with the exception of the 10-year ASCVD in women) increased as a function of time since lowest weight. Future work should investigate additional factors that contribute to change in CVD risk over time among adults who undergo bariatric surgery and interventions that may mitigate weight regain and associated CVD risk factor changes.
The non-fatal CVD event rates within the current study revealed females had a higher rate of non-fatal CVD events in the long-term (i.e., 5 or more years) versus short-term (i.e., fewer than 5 years) post-surgery. In contrast, the short- vs. long-term non-fatal CVD event rates were similar among males (and similar to the female long-term rate). There are several reasons why females, but not males, may have lower rates in the short-term. Females may be physically healthier prior to surgery. Specifically, men undergoing bariatric surgery tend to be older, have higher BMI, and more obesity-related comorbidities compared to women39. Additionally, women develop CVD, on average, 7 to 10 years after men. Thus, the higher rate among women 5 years after surgery may be a reflection of aging40.
Our post-RYGB sample had a SMR higher than 1 (females: 1.18; males: 1.96), indicating a higher mortality rate compared to the general population, adjusted for age, sex and race. This could reflect that despite improvements in weight and comorbidities following surgery, post-surgical patients still have higher levels of obesity and other comorbidities including T2D, respiratory disorders and non-alcoholic fatty liver disease41. However, the 95% CI of these SMR included 1. Thus, additional research is needed to determine whether these findings reflect low power to detect a difference or no actual difference.
Strengths and limitations
Major strengths of this study were the longer-term follow-up, which allowed for continued measurement of CVD risk through maximum weight loss and several years of weight regain in adults undergoing RYGB, and the large geographically diverse cohort. Additionally, multiple common CVD risk algorithms were compared.
Several limitations should be considered when interpreting the data, including the lack of representation of the gastric sleeve procedure which was uncommon during the study’s time period (2006–2009), but has surpassed RYGB as the most common procedure performed in the United States42. Also, this study lacked a non-surgical comparison group. Thus, the impact surgery had on reductions in risk, events and mortality cannot be commented on directly. Similarly, because CVD risk was calculated independent of age (i.e. participant age at pre-surgery was used for all CVD risk score calculations), the impact of age on CVD risk is lost. Additionally, our cohort was 86% Caucasian. While the Framingham scoring algorithm was developed among a largely Caucasian sample, and the ASCVD scoring algorithm takes race into account, applying the findings of this study to other racial groups may not be appropriate. Finally, due to the lack of CVD events in our cohort, there was a lack of statistical power for some short-term versus long-term comparisons and for a precise SMR estimate.
Conclusions
Among a large cohort of adults who underwent RYGB surgery, predicted 10-year and lifetime CVD risk improved after surgery. In general, discounting age, CVD risk declined from pre-surgery to 1 year, then increased between 1 and 7 years post-surgery. However, after 7 years, CVD risk was substantially lower among both females and males compared to pre-surgery. While similar time trends were evident using all three CVD risk scores, the percentage of patients identified as having high 10-year and lifetime CVD risk and the magnitude of change over time, differed by score and sex. These findings help inform sex-specific short and long-term improvements in CVD risk after RYGB surgery and demonstrate that CVD risk score selection has important clinical implications.
Supplementary Material
Highlights.
Sex-specific predicted 10-year and lifetime cardiovascular disease (CVD) risk were calculated using the Framingham-lipid, Framingham-body mass index (BMI) and Atherosclerotic (ASCVD) scoring algorithms among participants with no history of CVD.
The percentage of patients identified as having high 10-year and lifetime CVD risk pre- and post-surgery, and the magnitude of change over time, differed by score and sex.
The mean improvement in short- and long-term CVD risk was substantially larger 1 through 7 years following RYGB than what is typically achieved from diet, exercise or lifestyle interventions aimed at weight loss or CVD risk reduction in overweight and obese adults.
Acknowledgments
Funding: The Longitudinal Assessment of Bariatric Surgery-2 was funded by a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: DCC-U01 DK066557; Columbia - U01-DK66667 (in collaboration with Cornell University Medical Center CTRC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Footnotes
Conflicts of interest statement
Authors have no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work other than the following: Dr. Couroulas reports grant from Allurion Inc.,
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in cardiovascular diseases. 2014;56(4):369–381. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(5):968–976. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. Journal of the American College of Cardiology. 2009;53(21):1925–1932. [DOI] [PubMed] [Google Scholar]
- 4.Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circulation research. 2016;118(11):1752–1770. [DOI] [PubMed] [Google Scholar]
- 5.The Impact of Obesity on Your Body and Health. https://asmbs.org/patients/impact-of-obesity, 2019.
- 6.Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nature reviews Gastroenterology & hepatology. 2017;14(3):160–169. [DOI] [PubMed] [Google Scholar]
- 7.Ponce J, Nguyen NT, Hutter M, Sudan R, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in the United States, 2011–2014. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2015;11(6):1199–1200. [DOI] [PubMed] [Google Scholar]
- 8.Kissler HJ, Settmacher U. Bariatric surgery to treat obesity. Seminars in nephrology. 2013;33(1):75–89. [DOI] [PubMed] [Google Scholar]
- 9.Koliaki C, Liatis S, le Roux CW, Kokkinos A. The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC endocrine disorders. 2017;17(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. Jama. 2012;307(1):56–65. [DOI] [PubMed] [Google Scholar]
- 11.Lee GK, Cha YM. Cardiovascular benefits of bariatric surgery. Trends in cardiovascular medicine. 2016;26(3):280–289. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Yu J, Li L, et al. Effects of Bariatric Surgery on Mortality, Cardiovascular Events, and Cancer Outcomes in Obese Patients: Systematic Review and Meta-analysis. Obesity surgery. 2016;26(11):2590–2601. [DOI] [PubMed] [Google Scholar]
- 13.King WCBS, Hinerman AS, Mitchell JE, Steffen KJ, Courcoulas AP. Precision bariatric surgery: patient behaviors and characteristics related to weight regain following Roux-en-Y gastric bypass In review. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benotti PN, Wood GC, Carey DJ, et al. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. Journal of the American Heart Association. 2017;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. European heart journal. 2016;37(1):24–34. [DOI] [PubMed] [Google Scholar]
- 16.Harvey RE, Coffman KE, Miller VM. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Women’s health (London, England). 2015;11(2):239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min T, Prior SL, Caplin S, Barry JD, Stephens JW. Temporal Effect of Bariatric Surgery on Predicted 10-Year and Lifetime Cardiovascular Risk at 1 Month, 6 Months, and 5 Years Following Surgery: A Pilot Study. Metabolic syndrome and related disorders. 2017;15(3):130–136. [DOI] [PubMed] [Google Scholar]
- 18.Domienik-Karlowicz J, Dzikowska-Diduch O, Lisik W, Chmura A, Pruszczyk P. Short-term cardiometabolic risk reduction after bariatric surgery. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese. 2015;56(1):61–65. [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. [DOI] [PubMed] [Google Scholar]
- 20.Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circulation Cardiovascular quality and outcomes. 2010;3(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino RB Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 23.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2013;9(6):926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batsis JA, Sarr MG, Collazo-Clavell ML, et al. Cardiovascular risk after bariatric surgery for obesity. The American journal of cardiology. 2008;102(7):930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firnhaber JM. Estimating Cardiovascular Risk. American family physician. 2017;95(9):580–581. [PubMed] [Google Scholar]
- 26.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. Jama. 2016;316(19):1997–2007. [DOI] [PubMed] [Google Scholar]
- 27.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2010;56(25):e50–103. [DOI] [PubMed] [Google Scholar]
- 28.Garg N, Muduli SK, Kapoor A, et al. Comparison of different cardiovascular risk score calculators for cardiovascular risk prediction and guideline recommended statin uses. Indian heart journal. 2017;69(4):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosomworth NJ. Practical use of the Framingham risk score in primary prevention: Canadian perspective. Canadian family physician Medecin de famille canadien. 2011;57(4):417–423. [PMC free article] [PubMed] [Google Scholar]
- 30.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(25 Pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 31.U.S.Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics. 2017; https://www.cdc.gov/nchs/ndi/index.htm.
- 32.Multiple Cause of Death 1999–2014 on CDC WONDER Online Database. 2019; https://wonder.cdc.gov/mcd.html.
- 33.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43(4):913–928. [PubMed] [Google Scholar]
- 34.Thereaux J, Lesuffleur T, Paita M, et al. Long-term follow-up after bariatric surgery in a national cohort. The British journal of surgery. 2017;104(10):1362–1371. [DOI] [PubMed] [Google Scholar]
- 35.Sackner-Bernstein J, Kanter D, Kaul S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PloS one. 2015;10(10):e0139817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zomer E, Leach R, Trimmer C, et al. Effectiveness and cost-effectiveness of interventions that cause weight loss and reduce the risk of cardiovascular disease. Diabetes, obesity & metabolism. 2017;19(1):118–124. [DOI] [PubMed] [Google Scholar]
- 37.Yadav R, Yadav RK, Sarvottam K, Netam R. Framingham Risk Score and Estimated 10-Year Cardiovascular Disease Risk Reduction by a Short-Term Yoga-Based LifeStyle Intervention. Journal of alternative and complementary medicine (New York, NY). 2017;23(9):730–737. [DOI] [PubMed] [Google Scholar]
- 38.Akil L, Ahmad HA. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. Journal of health care for the poor and underserved. 2011;22(4 Suppl):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natvik E, Gjengedal E, Moltu C, Raheim M. Translating weight loss into agency: Men’s experiences 5 years after bariatric surgery. International journal of qualitative studies on health and well-being. 2015;10:27729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maas AH, Appelman YE. Gender differences in coronary heart disease. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2010;18(12):598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Who is a Candidate for Bariatric Surgery? 2018; https://asmbs.org/patients/who-is-a-candidate-for-bariatric-surgery.
- 42.Chaar ME, Lundberg P, Stoltzfus J. Thirty-day outcomes of sleeve gastrectomy versus Roux-en-Y gastric bypass: first report based on Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2018;14(5):545–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.