Abstract
Background:
Preclinical data implicate the endocannabinoid system in the pathology underlying obsessive-compulsive disorder (OCD), while survey data have linked OCD symptoms to increased cannabis use. Cannabis products are increasingly marketed as treatments for anxiety and other OCD-related symptoms. Yet, few studies have tested the acute effects of cannabis on psychiatric symptoms in humans.
Methods:
We recruited 14 adults with OCD and prior experience using cannabis to enter a randomized, placebo-controlled, human laboratory study to compare the effects on OCD symptoms of cannabis containing varying concentrations of Δ−9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on OCD symptoms to placebo. We used a within-subjects design to increase statistical power. Across three laboratory sessions, participants smoked three cannabis varietals in random order: placebo (0% THC/0% CBD); THC (7.0% THC/0.18% CBD); and CBD (0.4% THC/10.4% CBD). We analyzed acute changes in OCD symptoms, state anxiety, cardiovascular measures, and drug-related effects (e.g. euphoria) as a function of varietal.
Results:
12 participants completed the study. THC increased heart rate, blood pressure, and intoxication compared to CBD and placebo. Self-reported OCD symptoms and anxiety decreased over time in all three conditions. Although OCD symptoms did not vary as a function of cannabis varietal, state anxiety was significantly lower immediately after placebo administration relative to both THC and CBD.
Conclusions:
This is the first placebo-controlled investigation of cannabis in adults with OCD. The data suggest that smoked cannabis, whether containing primarily THC or CBD, has little acute impact on OCD symptoms and yields smaller reductions in anxiety compared to placebo.
Keywords: Obsessive-Compulsive Disorder, Anxiety, Cannabis, Marijuana, Cannabinoids, THC, Cannabidiol
Introduction
The endocannabinoid system (ECS) has increasingly garnered interest as a potential new target for treating various psychiatric conditions. A lipid-based, regulatory neurotransmitter system, the ECS is comprised of two receptors (CB1R and CB2R), endogenous ligands (“endocannabinoids”), and synthetic/metabolic enzymes (Lu & MacKie, 2016). It is broadly-distributed throughout the brain, where it modulates the activity of other neurotransmitter systems including glutamate (Cohen, Weizman, & Weinstein, 2019), gamma-aminobutyric acid (GABA) (Cohen et al., 2019), serotonin (Cohen et al., 2019; Morales & Reggio, 2017) and dopamine (Covey, Mateo, Sulzer, Cheer, & Lovinger, 2017). A major function of the ECS appears to be regulating neurophysiological processes including sleep (Pava, Makriyannis, & Lovinger, 2016), memory (Lupica, Hu, Devinsky, & Hoffman, 2017), affective state (Lutz, Marsicano, Maldonado, & Hillard, 2015), and response to stress (Hill, Campolongo, Yehuda, & Patel, 2018).
These findings have spurred greater research focused on the role of the ECS in psychiatric illness. For example, data from both human and animal studies suggest that modulators of the ECS may improve symptoms of anxiety disorders (Korem, Zer-Aviv, Ganon-Elazar, Abush, & Akirav, 2016), PTSD (Hill et al., 2018), and psychosis (Minichino et al., 2019). In particular, increasing evidence links the ECS to anxiety, fear, and repetitive behavior (Kayser, Snorrason, Haney, Lee, & Simpson, 2019). As a result, it has been hypothesized that targeting the ECS could affect symptoms of obsessive-compulsive disorder (OCD), a disabling condition marked by recurrent anxiety-producing thoughts and repetitive behaviors (American Psychiatric Association, 2014; Kayser et al., 2019; Kessler et al., 2005; Murray & Lopez, 1996).
Preclinical data suggest that ECS activity may regulate compulsive-like behaviors. For example, CB1R in projections from orbitofrontal cortex to striatum appears to regulate appropriate shifts between goal-directed and habitual behaviors in rodents (Gremel et al., 2016). Patients with OCD have abnormal functionality in this circuit (Milad & Rauch, 2012), and compulsive behavior has been linked to an over-reliance on habit (Voon et al., 2015). Other rodent studies have found that CB1R agonists reduce marble-burying behavior, a preclinical model of compulsions (Gomes, Casarotto, Resstel, & Guimarães, 2011; Umathe, Manna, & Jain, 2011).
ECS activity may also affect anxiety, fear, and/or obsessions. Both preclinical and human studies show that these agents can mitigate responsiveness to threat (Fusar-Poli, 2009; Phan et al., 2008) and enhance fear extinction learning (Dincheva et al., 2015; Hammoud et al., 2019; Mayo et al., 2019; Rabinak et al., 2014, 2013; Rabinak, Peters, Marusak, Ghosh, & Phan, 2018), apparently by influencing top-down control by frontal cortex over the limbic system. In patients with OCD, anxiety and obsessions are thought to arise in part from excessive responsiveness to threatening stimuli (Apergis-Schoute et al., 2017; Rouhani et al., 2019) and deficits in fear extinction (Dougherty et al., 2018; McLaughlin et al., 2015; Milad et al., 2013). Indeed, recent data from our group (Kayser et al., 2020) suggest that nabilone, a synthetic analogue of Δ−9-tetrahydrocannabinol (THC) and CB1R agonist, enhances the effectiveness of cognitive-behavioral therapy consisting of exposure and response/ritual prevention (EX/RP), the primary evidence-based psychotherapy for OCD. This effect may result from THC’s capacity to facilitate fear extinction learning, which is thought to underlie in part the effects of exposure-based treatment (Dougherty et al., 2018).
Though the findings above are promising, there are few controlled studies of cannabinoids in patients with psychiatric conditions, and none in those with OCD. At the same time, shifting cultural and political attitudes in the United States have been associated with a surge in interest in cannabis and related substances for potential therapeutic use (Haney & Evins, 2016). From 2008 to 2018, the number of adults reporting near-daily cannabis use increased by 4.8 million (Lipari & Park-Lee, 2019), and marketing of cannabis-related products has exploded (Richter & Levy, 2014; Subritzky, Lenton, & Pettigrew, 2016). A recent survey of college students also found that OCD symptom severity, and obsessions specifically, predicted cannabis misuse and suggested that some individuals with OCD use cannabis to control their symptoms (Spradlin, Mauzay, & Cuttler, 2017). In addition, a residential treatment facility recently reported that over 6 months, nearly 30% of patients inquiring about treatment for OCD (51 out of 174) reported prior cannabis use (Storch & Kay, 2019). Anecdotally, increasing numbers of our clinic patients report using cannabinoids to relieve anxiety, obsessions, or compulsions. Conversely, others describe that cannabinoids worsen their symptoms.
Interpreting these studies is challenging given that cannabis is a pharmacologically complex substance comprising over 100 constituent agents (phytocannabinoids), each with unique properties and mechanisms of action (Russo & Marcu, 2017). Human studies show that individual cannabinoids can have vastly different and even competing effects on psychiatric symptoms (Fusar-Poli, 2009; Fusar-Poli et al., 2010; Hindocha et al., 2015; Morgan et al., 2018). Among the phytocannabinoids, THC and cannabidiol (CBD) have been the most studied. THC binds to CB1R and is the primary mediator of the intoxicating effects of cannabis (Cooper & Haney, 2008). In contrast, CBD has few psychoactive effects (Zhornitsky & Potvin, 2012), low CB1R affinity (Pertwee et al., 2010), binds other receptor targets (e.g. TRPV1,2, GPR55, and 5HT1A receptors) and inhibits the metabolism of endogenous cannabinoids (Grotenhermen, 2005; Mechoulam, Parker, & Gallily, 2002; Ryberg et al., 2007). Both THC and CBD are hypothesized to have anxiolytic, anti-compulsive, and anti-obsessional properties (Kayser et al., 2019), but these putative effects have never before been directly tested in individuals with OCD. To address this knowledge gap, we conducted a within-subject, placebo-controlled, human laboratory study to assess the acute effects of smoked cannabis varying in THC and CBD concentrations in adults with OCD. We hypothesized that both THC and CBD would acutely reduce anxiety and OCD symptoms relative to placebo.
Methods
Overview
This study was a joint effort between the Center for OCD Research and Cannabis Research Laboratory at the New York State Psychiatric Institute/Columbia University. The study was approved by the Institutional Review Board (Protocol #7405) and registered at ClinicalTrials.gov (NCT03274440). All participants provided written informed consent and were enrolled between December 2017 and February 2019.
Participants
Potential participants were recruited via an online recruitment portal and flyers and underwent initial phone screening and, if eligible, an on-site psychiatric and medical evaluation (including physical exam, electrocardiogram, and urine/blood chemistries) with a trained clinician. The Structured Clinical Interview for DSM-5-RV (Research Version) (First, Williams, Karg, & Spitzer, 2015) was used to diagnose OCD and other psychiatric illnesses. OCD symptom severity and depressive severity were assessed respectively using the Yale-Brown Obsessive-Compulsive Scale (YBOCS) (Goodman et al., 1989) and the Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960), while trait anxiety was assessed using the Spielberger State-Trait Anxiety, trait subscale (STAI-T) (Spielberger, Gorssuch, & Lushene, 1983). Participants taking serotonin reuptake inhibitors (SRIs, including clomipramine and the selective SRIs, which are the first-line medications for treating OCD) were eligible for inclusion provided that their dose had not changed in the six weeks prior to enrollment. Participants taking other psychoactive medications, including over-the-counter supplements or nutraceuticals, were not included. Inclusion and exclusion criteria are provided in Table 1. Eligible participants were told the study objective was to determine whether different combinations of THC and CBD in the cannabis cigarette affect OCD symptoms, and that in each of three sessions, they would smoke a portion of a cannabis cigarette containing different THC and CBD concentrations. Participants were not told the specific concentrations in each condition, or that they would receive placebo cannabis in one session.
Table 1.
Inclusion/Exclusion Criteria
| Inclusion | Exclusion |
|---|---|
| 1. Male or female aged 21–55 | 1. Lifetime history of bipolar disorder or psychosis, or first-degree relative with these conditions |
| 2. Physically healthy | 2. HDRS-17 > 25 or current suicidal ideation |
| 3. Principal DSM-5 diagnosis of OCD with illness duration ≥ 1 year and near-constant symptoms (e.g. >8 hours per day or maximum symptom-free interval <1 hour) | 3. History of significant medical condition that could increase risk of cannabis side effects |
| 4. YBOCS ≥ 16 | 4. Currently enrolled in or planning to begin EX/RP |
| 5. No psychotropic medication in last 6 weeks other than SRIs, no change to SRI dose in past 6 weeks | 5. Substance use disorder within the last year (including cannabis use disorder) or positive urine toxicology (for substances other than cannabis) at screening |
| 6. Prior experience using cannabis without significant adverse effects | 6. Seeking treatment for substance use |
| 7. Capable of providing informed consent | 7. Pregnant or nursing female |
Abbreviations: HDRS-17, Hamilton Depression Rating Scale, 17-Item; DSM-5, Diagnostic and Statistical Manual for Psychiatric Disorders, 5th Edition; OCD, Obsessive-Compulsive Disorder; YBOCS, Yale-Brown Obsessive Compulsive Scale; SRI, Serotonin Reuptake Inhibitor
Study Design
To mitigate between-subject effects and maximize statistical power, we used a within-subjects design in which each participant received all three cannabis varietals over the course of three laboratory sessions, each lasting 3–4 hours. A repeated measures, within-subjects design is a powerful statistical approach that reduces inter-subject variability and yields substantial correlations between levels (Haney et al., 2013). Participants completed no more than one session per calendar week (mean days between sessions=12.5, SD=11.7, range=5–56) to avoid potential carryover effects. The National Institute on Drug Abuse (NIDA) provided cannabis cigarettes (IND#131,990). Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 hours before sessions. During each session, participants smoked 50% of a cigarette according to a cued-dosing procedure (described below). Each cigarette contained approximately 800mg of cannabis that was either primarily THC (7.0% THC/0.18% CBD), CBD (0.4% THC/10.4% CBD) or placebo (0% THC/0% CBD). Of note, a study examining the contents of 39,000 illicit cannabis preparations seized by the US Drug Enforcement Agency indicated that average THC percentage increased from approximately 4% to 12% between 1995 and 2014, while CBD percentage decreased from 0.28% to <0.15% between 2001 and 2014 (ElSohly et al., 2016). Thus, the NIDA cannabis used in this study likely differed substantially in THC/CBD content from illicit cannabis. Placebo-controlled studies on cannabis conducted in the US are required to use NIDA cannabis (Haney, 2020, under review). Yet, we and others have demonstrated that cannabis users adjust their inhalation patterns as a function of THC content (i.e., increase inhalation as THC levels decrease and vice versa) (Cooper & Haney, 2009; Ramesh, Haney, & Cooper, 2013), and even regular cannabis users become intoxicated from a single low-potency NIDA cannabis cigarette (e.g., Haney et al., 2016), demonstrating the clinical relevance of the study design and cued-dosing procedure.
Randomization
Participants received three cannabis varietals in randomized, double-blind dosing order. Randomization was determined by a research assistant who had no other interaction with the investigators using a random number generator in Excel 2017 (Excel for Windows, 2017 Edition, Microsoft Corp., Redmond, WA). Cannabis was administered by an investigator who had no other interaction with the participant.
Clinical Assessments
The study clinician assessed OCD symptoms at the beginning of each session using the YBOCS. Participants also provided self-ratings of their OCD symptoms using the Yale-Brown Obsessive-Compulsive Challenge Scale (YBOCCS) and the Obsessive Compulsive Visual Analogue Scale (OCD-VAS); both of which have been used in prior pharmacological challenge studies to detect acute changes in OCD symptoms (Rodriguez, Kegeles, Flood, & Simpson, 2011; Rodriguez et al., 2013). Participants also self-reported anxiety symptoms using the Spielberger State-Trait Anxiety Inventory, state subscale (STAI-S) (Spielberger et al., 1983), which has also been used to detect changes in state anxiety following drug administration (Liechti & Vollenweider, 2000). We used a modified version of the Marijuana Rating Form (MRF) to assess subjective drug-related effects, where participants rated their experience of cannabis-related effects on a scale from 0 (no effect) to 4 (extreme effect) across several categories including “Strength of Marijuana Effect”, “Felt High”, “Good Effect”, “Bad Effect”, and “Desire to Take Again” (Ramesh et al., 2013).
Experimental Sessions
Table 2 presents the schedule for experimental sessions. Participants were instructed not to use any substances including cannabis or alcohol in the 12 hours before each session. No participants reported nicotine use. At the beginning of each session, carbon monoxide, breath alcohol, and urine toxicology were assessed to detect recent use of substances. Female participants underwent urine pregnancy testing prior to each session.
Table 2.
Time Course of Sessions
| Time (min) | Event |
|---|---|
| −50 | CO, urine toxicology and pregnancy test, breathalyzer |
| −30 | Balance, TLFB, Vitals (BP/HR), self-report scales (OCD-VAS, YBOCCS, STAI-S, MRF) |
| 0 | Cannabis administration |
| 20, 40, 60, 90, 120 | Vitals, self-report scales |
| 180 | Vitals, self-report scales, field sobriety test, participant discharge |
Abbreviations: Balance, number of seconds balancing for a maximum of 30s on each foot; TLFB, Marijuana Timeline Follow-back; CO, carbon monoxide; BP, blood pressure; HR, heart rate; OCD-VAS, OCD Visual Analogue Scale; YBOCCS, Yale-Brown Obsessive-Compulsive Challenge Scale; STAI-S, State/Trait Anxiety Inventory – state subscale; MRF, Marijuana Rating Form
Participants smoked 50% of a cannabis cigarette using a cued-smoking procedure: From a separate room with a two-way mirror, the investigator instructed participants to ‘inhale’ (5 seconds), ‘hold smoke in lungs’ (10 seconds), and ‘exhale’ with a 40-second interval between puffs. Cued dosing has been shown to produce reliable increases in heart rate (HR) and plasma THC levels (Foltin, Fischman, Pedroso, & Pearlson, 1987). Participants followed this procedure until 50% of the cigarette was pyrolyzed. The cannabis was put in a plastic mouthpiece, rolled at the tip, and smoked only to a line indicating 50% of the cigarette to improve blinding of participants and research staff by preventing them from seeing the color of the contents (which might differ across dose conditions). After cannabis administration, we assessed HR, systolic/diastolic blood pressure (SBP/DBP), self-ratings of anxiety, obsessions, and compulsions, and drug effects at set timepoints (20, 40, 60, 90, 120, 180 minutes after smoking).
Data Analysis
A random-effects linear mixed model evaluated changes in each continuous outcome (YBOCCS, OCD-VAS, STAI-S, drug-related effects, cardiovascular measures) as a function of condition (THC, CBD, placebo [PBO]), time point, and their interaction. Mixed-effects modeling was chosen because it accounts for clustering of repeated measures within subjects, allows modeling of time as a continuous rather than a categorical variable, and has been used in similar acute pharmacological challenge studies with repeated assessments (Gibbons et al., 1993; Papolos, Teicher, Faedda, Murphy, & Mattis, 2013; Phillips et al., 2019; Rodriguez et al., 2013).
The model included participants as a random effect, condition and time as fixed within-subjects factors, the interaction between condition and time, and a random intercept. An autoregressive heterogeneous (ARH1) variance-covariance matrix best fit the data using Akaike’s information criterion, and restricted maximum likelihood estimation was used. Time was modeled as a continuous variable as the interval between assessments increased over the course of each session (i.e. every 20 minutes for the first hour, then every 30 minutes, then hourly). Contrasts within each mixed-effects linear regression model were used to estimate differences in outcomes between both active cannabis varietals and placebo at different time points. All statistical tests were conducted using an alpha level of 0.05. Analyses were conducted using SPSS25 (SPSS Statistics for Windows, Version 25.0, IBM Corp. Armonk, NY).
Results
Sample
Figure 1 portrays participant recruitment and flow. Twenty-three potential participants were screened. Seven did not meet inclusion criteria; two were eligible but chose not to participate (one sought treatment for OCD instead, another did not enter because of the time commitment). Fourteen participants were randomized. Two dropped after the first session due to the time commitment. Twelve participants completed all three sessions and were included in the final analysis.
Figure 1. Recruitment and Participant Flow.
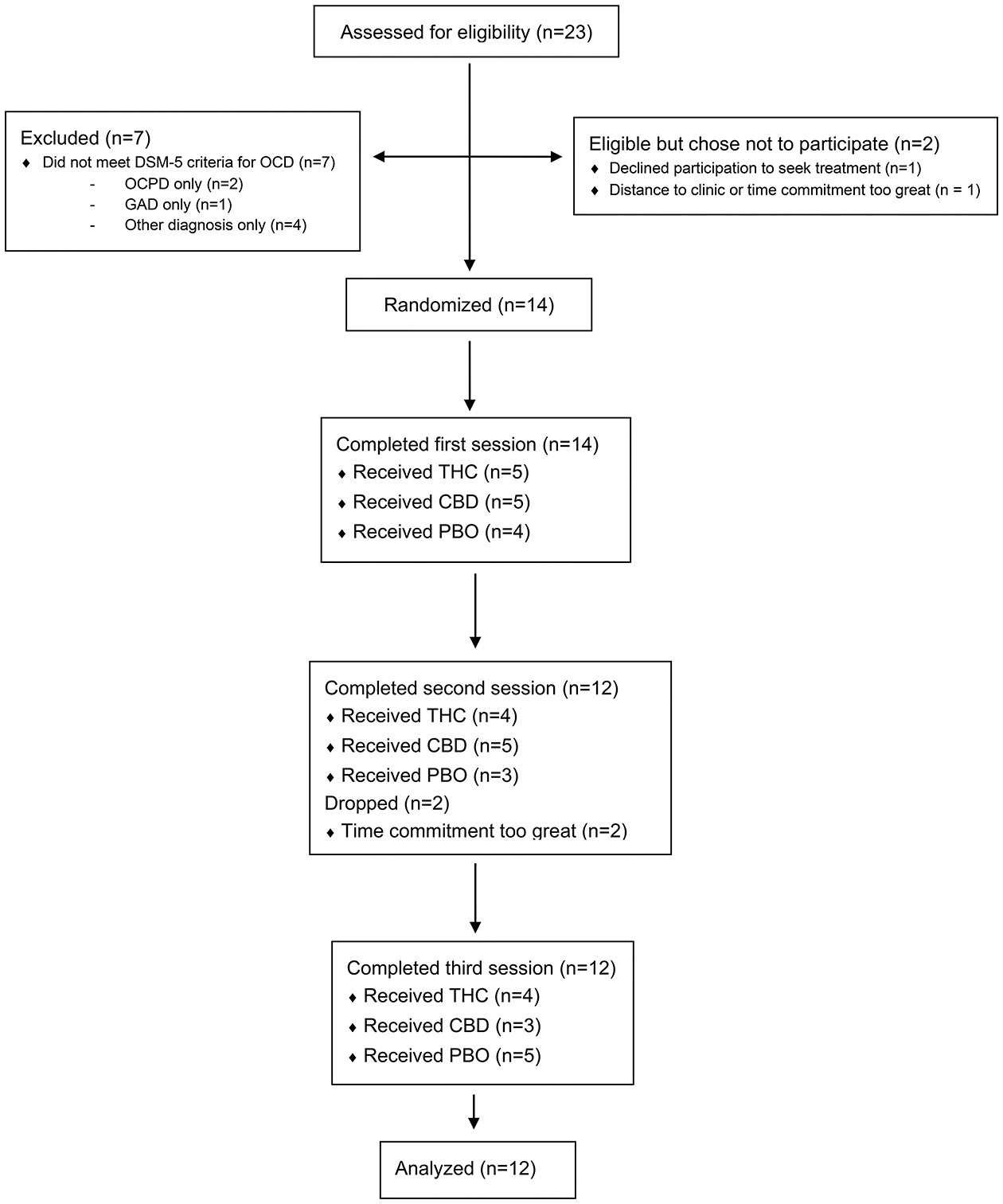
Abbreviations: THC = 7.0% THC/0.18% CBD; CBD = 0.4% THC/10.4% CBD; PBO = 0% THC/0% CBD; OCPD = Obsessive-Compulsive Personality Disorder; GAD = Generalized Anxiety Disorder
Table 3 describes sample demographic characteristics (N=12). The sample was heterogeneous in both sex and race. Consistent with the inclusion criteria (Table 1), all participants had severe, near-constant OCD symptoms, with specific symptoms covering all DSM-5 domains (e.g. contamination, harm, symmetry, taboo thoughts, hoarding). Eight participants had no psychiatric comorbidities. Two participants met criteria for generalized anxiety disorder (GAD), one of whom also met criteria for panic disorder. Mean STAI-T scores were more than one SD above the established adult norm (34.9±9.2) (Spielberger et al., 1983), indicating that participants overall had greater-than-average trait anxiety. Baseline depression symptoms were low; two participants (17%) met criteria for comorbid major depression (with HDRS-17 scores of 13 and 15, both within the “mild” range). Only two participants were taking psychotropic medications; of these, one had been on sertraline 50mg for two years, and one had been on fluoxetine 20mg for six weeks prior to enrolling.
Table 3.
Demographic Characteristics of Participantsa
| Variable | Value |
|---|---|
| Age | 26.8±7.4, 21–48 |
| Male | 8 (67) |
| Single | 12 (100) |
| Hispanic | 2 (17) |
| Race | |
| White | 9 (75) |
| Black | 2 (17) |
| Asian | 1 (8) |
| Other | 0 (0) |
| Psychiatric Comorbidity | |
| None | 8 (67) |
| MDD | 2 (17) |
| GAD | 2 (17) |
| Panic Disorder | 1 (8) |
| Medication Status | |
| Unmedicated | 10 (83) |
| SSRI | 2 (17) |
| Cannabis use (#days/week) | 3.8±2.9, 0–7 |
| Baseline YBOCS score | 20.1±4.9, 16–25 |
| Baseline STAI-T score | 48.5±13.9, 25–77 |
| Baseline HDRS-17 score | 4.9±4.7, 0–15 |
Values shown as means (±SD), range; for frequencies, as n (%)
Abbreviations: MDD = Major Depressive Disorder; GAD = Generalized Anxiety Disorder; SSRI = Selective Serotonin Reuptake Inhibitor; YBOCS = Yale-Brown Obsessive-Compulsive Scale; STAI-T = State Trait Anxiety Inventory, Trait Version; HDRS-17 = 17-item Hamilton Depression Rating Scale
OCD Symptom Ratings
Figure 2 illustrates self-rated OCD symptoms (YBOCCS, OCD-VAS), state anxiety (STAI-S), and drug-related effects as a function of cannabis varietal and time. Results from the linear mixed-effects models found no interaction effect between cannabis varietal and time (YBOCCS, p=.577, OCD-VAS, p=.818, STAI-S, p=.740). There was a main effect of time on all three symptom self-report measures, with significant decreases for YBOCCS (F=10.50; df=6, 10; p<.001), OCD-VAS (F=8.93; df=6, 10; p<.001), and STAI-S scores (F=7.00; df=6, 10; p<.001).
Figure 2. Self-report Ratings and Heart Rate, mean values.
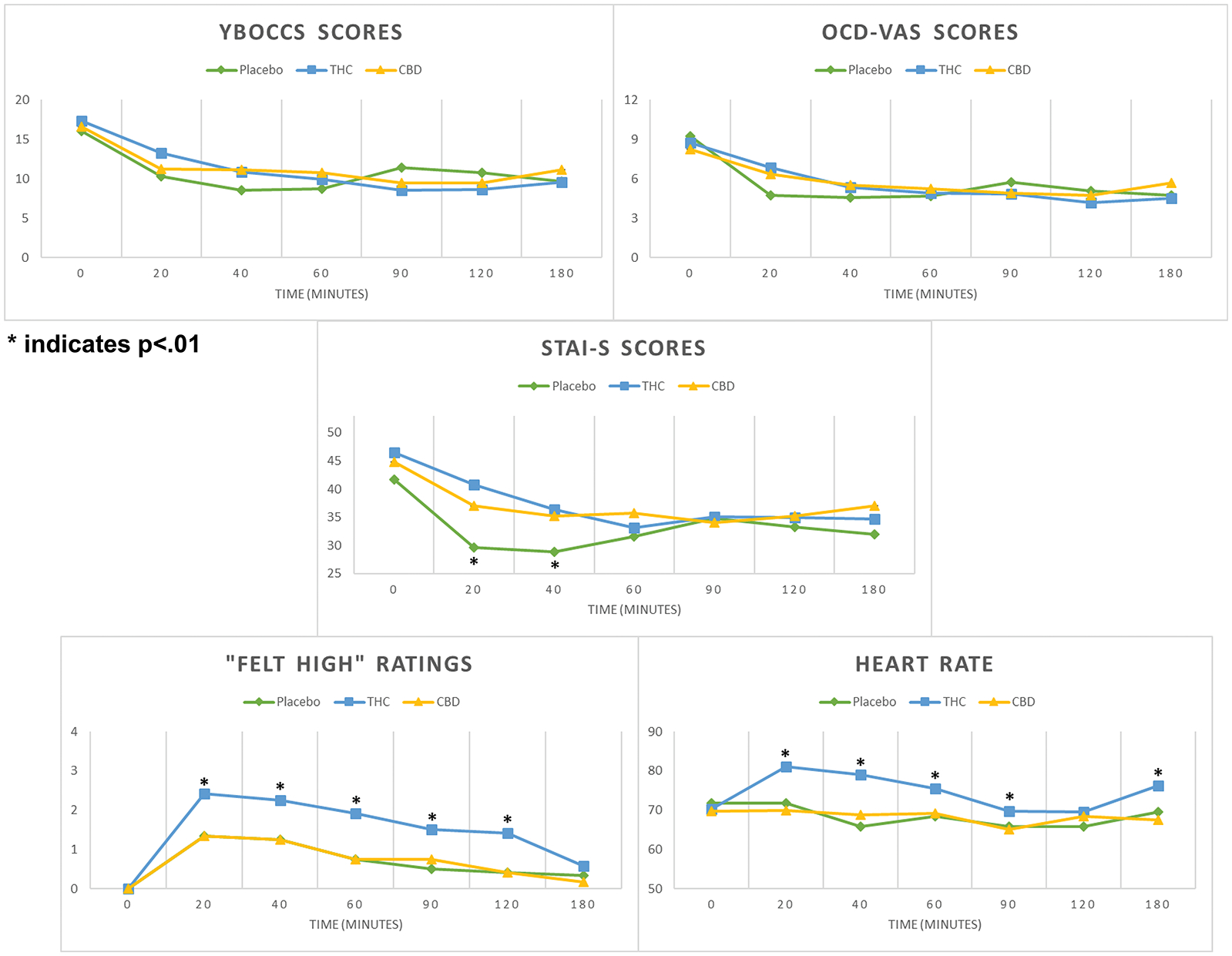
Abbreviations: THC = 7.0% THC/0.18% CBD; CBD = 0.4% THC/10.4% CBD; PBO = 0% THC/0% CBD
Main effect of time was observed for YBOCCS, OCD-VAS, STAI-S, and “Felt High” scores (all p values <.001).
Main effect of cannabis varietal was observed only for STAI-S scores (p=.002).
There was a significant main effect of cannabis varietal on STAI-S (F=6.26; df=2, 10; p=.002), but not on YBOCCS (F=.33; df=2, 10; p=.72) or OCD-VAS (F=.10; df=2, 10; p=.90). Though mean STAI-S scores decreased in all three conditions, they were significantly lower for PBO relative to both THC (mean difference=−4.31, SE=1.34, p=.001) and CBD (mean difference=−3.85, SE=1.34, p=.004). Post-hoc analyses revealed participants had significantly lower STAI-S scores 20 minutes after PBO administration compared to both THC (mean difference=−11.25, SE=3.54, p=.002) and CBD cannabis (mean difference=−7.33, SE=3.54, p=.039). At minute 40, STAI-S scores remained significantly lower for PBO compared to THC (mean difference=−7.58, SE=3.54, p=.033) and trended towards significance when comparing PBO and CBD (mean difference=−6.33, SE=3.54, p=.075). There were no between-group differences in STAI-S at minute 60 and subsequent time points.
Drug Effect Ratings
There was no cannabis varietal-by-time interaction for self-reported drug-related effects (“Felt High”), but there were significant main effects of time (F=31.11; df=6, 10; p<.001) and cannabis varietal (F=26.43; df=2, 10; p<.001). In all 3 conditions, scores were increased at all follow-up time points compared to minute 0 (all p values<.001). At all time points from 20 minutes to 120 minutes, THC significantly increased self-ratings of “Felt High” compared to both CBD and PBO (all p values <.05). At 180 minutes, there were no significant differences in drug effect ratings between groups.
Cardiovascular Effects and Safety Outcomes
There were no interaction effects between condition and time on cardiovascular outcomes, nor was there a main effect of time. There was a main effect of cannabis varietal on HR (F=11.88, df=2,10, p<.001), SBP (F=4.24, df=2,10, p=.016), and DBP (F=3.67, df-2,10, p=.027). Post-hoc contrasts revealed greater mean values for all cardiovascular endpoints with THC relative to CBD (HR, F=4.73, SE=1.2, p<.001; SBP, F=4.77, SE=1.86, p=.011; DBP, F=3.94, SE=1.47, p=.008). In addition, mean HR and SBP increased for THC relative to PBO (HR, F=5.29, SE=1.2, p<.001; SBP, F=4.60, SE=1.86, p=.014).
No serious adverse events were reported during the study. The most common self-reported side effects across all conditions were nervousness and dry mouth. One participant, a daily cannabis user diagnosed with comorbid panic disorder, reported panic symptoms about 20 minutes after administration of THC. The participant was given the opportunity to end the session but elected to complete it and reported that the symptoms resolved within 40 minutes.
Discussion
This is the first randomized, within-subjects, placebo-controlled human laboratory study to assess the effects of smoked cannabis on OCD symptoms and anxiety in adults with a DSM-5 diagnosis of OCD. The study capitalized on a unique collaboration between the Center for OCD Research to the Cannabis Research Laboratory at New York State Psychiatric Institute/Columbia University which presented a rare opportunity to test the acute effects of cannabinoids in patients with OCD. There were four main findings. First, THC produced the expected increase in self-ratings of “Felt High” and in cardiovascular outcomes (HR, SBP/DBP) compared to CBD and placebo. This increase in self-rated intoxication parallels findings from similar studies of smoked cannabis (Haney et al., 2016; Vadhan, Corcoran, Bedi, Keilp, & Haney, 2017), and suggests that the cued-dosing procedure generated sufficient cannabis exposure to enable observation of acute effects. Second, in this within-subject design, self-reported ratings of both OCD symptoms and anxiety decreased over time compared to baseline across all three conditions. Third, neither active cannabis varietal (THC or CBD) significantly affected OCD symptoms relative to placebo. Fourth, participants who received placebo experienced greater state anxiety reduction in the first 40 minutes after administration compared to those who received either active cannabis varietal.
That self-reported OCD symptoms and anxiety decreased over time could be an effect of time within the session. Alternatively, it might also reflect expectancy effects in individuals with OCD. Cannabis users have been shown to have significant expectancy effects in response to cannabis-associated cues (cannabis cigarette appearance and smell, the act of smoking) (Chait et al., 1988; Fillmore, Mulvihill, & Vogel-Sprott, 1994; Kirk, Doty, & De Wit, 1998). Moreover, when they anticipate receiving active cannabis but receive placebo cannabis instead, they still report cannabis-like effects. (Metrik et al., 2009). Our finding that placebo cannabis reduced anxiety and OCD symptoms in this study highlights the critical importance of including a placebo condition when evaluating the potential therapeutic utility of cannabis or cannabinoids.
Neither active cannabis varietal significantly affected obsessions or compulsions relative to placebo cannabis. Moreover, immediately after administration, placebo cannabis was associated with greater reductions in self-reported state anxiety than either active varietal, a difference which persisted for at least 40 minutes in the THC condition. We recruited participants with OCD who reported prior neutral or positive experiences using cannabis, excluding those who reported prior adverse reactions. Even among this selected group, when compared to placebo active cannabis varietals containing either primarily THC or CBD performed similarly (for OCD symptoms) or worse (for state anxiety).
Though THC is known to have both anxiolytic and anxiogenic effects (thought to result partly from its dose-dependent effects on TRPV1 receptors) (Lutz et al., 2015), it is noteworthy that CBD produced less reduction in anxiety than placebo, as prior studies have suggested it may have anxiolytic properties (Bergamaschi et al., 2011; Crippa et al., 2011). Whereas those studies examined the effects of a single dose of oral CBD (400–600mg) on symptoms of social anxiety, our study focused on the influence of a single smoked cannabis cigarette on anxiety symptoms in the context of OCD. Thus, our contrasting findings may result from differences in patient sample or route of administration (smoked vs. oral). The CBD cannabis we used contained approximately 0.4% THC, but there was no evidence that this low level of THC produced intoxication or increased heart rate compared to placebo, suggesting that THC effects were likely minimal. Nonetheless, our findings align with an expanding literature indicating that, like THC, CBD may not be a purely anxiolytic substance. Rather, it may have complex interactions with anxiety symptoms (Solowij et al., 2019).
Three limitations should be considered. First, this pilot study had a small sample size, and as a result Type II error could possibly explain the lack of effect on obsessions or compulsions that we observed. Though a larger replication study is needed, the issue of sample size is mitigated by our use of a within-subjects design, which significantly increases statistical power by reducing inter-subject variability. Second, measures to assess rapid changes in OCD symptoms are limited. The measures used in this study, the YBOCCS and OCD-VAS, have successfully detected drug effects in prior pharmacological challenge studies (Rodriguez et al., 2011, 2013), but the need remains for validated measures to characterize changes in obsessions and compulsions at short intervals. Finally, this study measured the effects of an acute dose of smoked cannabis. The THC condition yielded intoxication relative to both CBD and placebo, indicating that the dose and type of cannabis used was appropriate to achieve clinically meaningful effects. Long-term dosing or a different route of administration (for example, oral capsules) may have yielded different effects on symptoms. However, this preliminary study was not designed to assess for these possibilities.
Our findings suggest important directions for future research. This study found that the acute effects of smoked THC on OCD symptoms did not differ from placebo, highlighting the importance of placebo-controlled designs in studying the clinical effects of cannabis. In parallel, our recent trial of nabilone (a synthetic THC analogue) suggested that daily nabilone did not affect OCD symptoms on its own but enhanced the therapeutic impact of EX/RP when both treatments were delivered simultaneously (Kayser et al., 2020). Future studies should explore how the effects of THC vary depending on the route (oral vs. smoked), frequency, and context of administration (monotherapy vs. combined with EX/RP). Second, cannabis is a complex substance composed of multiple active constituents of diverse pharmacology. This preliminary study of smoked cannabis containing varied amounts of the best-studied of these constituents (THC and CBD) does not suggest that it is an effective acute treatment for anxiety or OCD symptoms. However, further research is needed to determine whether other cannabis formulations or individual cannabis constituents may have acute anxiolytic or anticompulsive effects in individuals with OCD. Whether THC, CBD, or other cannabinoids can influence fear extinction learning or other OCD-relevant neurocognitive processes to yield clinically meaningful benefits also warrants further study. Finally, individual differences in several factors including gender (Cooper & Craft, 2018), age (Gorey, Kuhns, Smaragdi, Kroon, & Cousijn, 2019), and genetics (Palmer, McGeary, Knopik, Bidwell, & Metrik, 2019) have been shown to influence the clinical response to cannabinoids. Future studies would benefit from investigating more extensively how these factors mediate the response to cannabis among individuals with OCD.
Acknowledgments
The authors would like to thank Dr. Shanghong Xie, PhD for statistical support, and Drs. John Markowitz, MD, Franklin Schneier, MD and Dianne Hezel, PhD for their assistance in editing an earlier draft of this manuscript.
Funding and Disclosures
This work was supported by a National Institute of Mental Health T32 Training Grant in Mood, Anxiety and Related Disorders (Grant No. T32MH15144, to RK) and also in part by generous donations to the New York Presbyterian Youth Anxiety Center (New York Presbyterian Hospital, Columbia University Vagelos College of Physicians and Surgeons, Weill Cornell Medical College, New York, NY, to BS). The authors declare no conflict of interest
Footnotes
Data Sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- American Psychiatric Association. (2014). Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Association In DSM. 10.1176/appi.books.9780890425596.744053 [DOI] [Google Scholar]
- Apergis-Schoute AM, Gillan CM, Fineberg NA, Fernandez-Egea E, Sahakian BJ, & Robbins TW (2017). Neural basis of impaired safety signaling in Obsessive Compulsive Disorder. Proceedings of the National Academy of Sciences, 114(12), 3216–3221. 10.1073/pnas.1609194114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RHC, Chagas MHN, de Oliveira DCG, De Martinis BS, Kapczinski F, … Crippa JAS (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 36(6), 1219–1226. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, & Schuster CR (1988). Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology, 94(2), 206–212. 10.1007/bf00176846 [DOI] [PubMed] [Google Scholar]
- Cohen K, Weizman A, & Weinstein A (2019). Modulatory effects of cannabinoids on brain neurotransmission. The European Journal of Neuroscience, 50(3), 2322–2345. 10.1111/ejn.14407 [DOI] [PubMed] [Google Scholar]
- Cooper ZD, & Craft RM (2018). Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology, 43(1), 34–51. 10.1038/npp.2017.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, & Haney M (2008). Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor PREVALENCE OF MARIJUANA USE AND DEPENDENCE. 10.1111/j.1369-1600.2007.00095.x [DOI] [PMC free article] [PubMed]
- Cooper ZD, & Haney M (2009). Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug and Alcohol Dependence, 103(3), 107–113. 10.1016/j.drugalcdep.2009.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Mateo Y, Sulzer D, Cheer JF, & Lovinger DM (2017). Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology, 124, 52–61. 10.1016/j.neuropharm.2017.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FLS, Martin-Santos R, … Hallak JEC (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. Journal of Psychopharmacology (Oxford, England), 25(1), 121–130. 10.1177/0269881110379283 [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC, … Lee FS (2015). FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nature Communications, 6, 1–9. 10.1038/ncomms7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Brennan BP, Stewart SE, Wilhelm S, Widge AS, & Rauch SL (2018). Neuroscientifically Informed Formulation and Treatment Planning for Patients With Obsessive-Compulsive Disorder. JAMA Psychiatry, 75(10), 1081 10.1001/jamapsychiatry.2018.0930 [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, & Church JC (2016). Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biological Psychiatry, 79(7), 613–619. 10.1016/j.biopsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Mulvihill LE, & Vogel-Sprott M (1994). The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacology, 115(3), 383–388. 10.1007/BF02245081 [DOI] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, & Spitzer R (2015). Structured Clinical Interview for DSM-5: Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV, Version 1.0.0). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, & Pearlson GD (1987). Marijuana and cocaine interactions in humans: Cardiovascular consequences. Pharmacology Biochemistry and Behavior, 28(4), 459–464. 10.1016/0091-3057(87)90506-5 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P (2009). Distinct Effects of Δ9-Tetrahydrocannabinol and Cannabidiol on Neural Activation During Emotional Processing. Archives of General Psychiatry, 66(1), 95 10.1001/archgenpsychiatry.2008.519 [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, … McGuire P (2010). Modulation of effective connectivity during emotional processing by Δ9-tetrahydrocannabinol and cannabidiol. International Journal of Neuropsychopharmacology, 13(4), 421–432. 10.1017/S1461145709990617 [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, … Watkins JT (1993). Some Conceptual and Statistical Issues in Analysis of Longitudinal Psychiatric Data: Application to the NIMH Treatment of Depression Collaborative Research Program Dataset. Archives of General Psychiatry, 50(9), 739–750. 10.1001/archpsyc.1993.01820210073009 [DOI] [PubMed] [Google Scholar]
- Gomes FV, Casarotto PC, Resstel LBM, & Guimarães FS (2011). Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(2), 434–438. 10.1016/j.pnpbp.2010.11.027 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, & Charney DS (1989). The Yale-Brown Obsessive Compulsive Scale: II. Validity. Archives of General Psychiatry, 46(11), 1012–1016. 10.1001/archpsyc.1989.01810110054008 [DOI] [PubMed] [Google Scholar]
- Gorey C, Kuhns L, Smaragdi E, Kroon E, & Cousijn J (2019, February 1). Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. European Archives of Psychiatry and Clinical Neuroscience, Vol. 269, pp. 37–58. 10.1007/s00406-019-00981-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, … Costa RM (2016). Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron, 90(6), 1312–1324. 10.1016/j.neuron.2016.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F (2005). Cannabinoids. Current Drug Targets. CNS and Neurological Disorders, 4(5), 507–530. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16266285 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud MZ, Peters C, Hatfield JRB, Gorka SM, Luan Phan K, Milad MR, & Rabinak CA (2019). Influence of Δ9-tetrahydrocannabinol on long-term neural correlates of threat extinction memory retention in humans. 10.1038/s41386-019-0416-6 [DOI] [PMC free article] [PubMed]
- Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, & Foltin RW (2013). Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 38(8), 1557–1565. 10.1038/npp.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, & Evins AE (2016). Does Cannabis Cause, Exacerbate or Ameliorate Psychiatric Disorders? An Oversimplified Debate Discussed. Neuropsychopharmacology, 41(2), 393–401. 10.1038/npp.2015.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, … Walsh SL (2016). Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology, 41(8), 1974–1982. 10.1038/npp.2015.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Campolongo P, Yehuda R, & Patel S (2018). Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology, 43(1), 80–102. 10.1038/npp.2017.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJA, & Curran HV (2015). Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: A randomised, double-blind, placebo-controlled study in cannabis users. European Neuropsychopharmacology, 25(3). 10.1016/j.euroneuro.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser RR, Raskin M, Snorrason I, Hezel DM, Haney M, & Simpson HB (2020). Cannabinoid Augmentation of Exposure-Based Psychotherapy for Obsessive-Compulsive Disorder. Journal of Clinical Psychopharmacology, 40(2), 1 10.1097/JCP.0000000000001179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser RR, Snorrason I, Haney M, Lee FS, & Simpson HB (2019). The Endocannabinoid System: A New Treatment Target for Obsessive Compulsive Disorder? Cannabis and Cannabinoid Research, 4(2). 10.1089/can.2018.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, Vol. 62, pp. 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kirk JM, Doty P, & De Wit H (1998). Effects of expectancies on subjective responses to oral delta9-tetrahydrocannabinol. Pharmacology, Biochemistry, and Behavior, 59(2), 287–293. 10.1016/s0091-3057(97)00414-0 [DOI] [PubMed] [Google Scholar]
- Korem N, Zer-Aviv TM, Ganon-Elazar E, Abush H, & Akirav I (2016). Targeting the endocannabinoid system to treat anxiety-related disorders. Journal of Basic and Clinical Physiology and Pharmacology, 27(3), 193–202. 10.1515/jbcpp-2015-0058 [DOI] [PubMed] [Google Scholar]
- Liechti ME, & Vollenweider FX (2000). Acute psychological and physiological effects of MDMA (“Ecstasy”) after haloperidol pretreatment in healthy humans. European Neuropsychopharmacology : The Journal of the European College of Neuropsychopharmacology, 10(4), 289–295. 10.1016/s0924-977x(00)00086-9 [DOI] [PubMed] [Google Scholar]
- Lipari RN, & Park-Lee E (2019). Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. Retrieved from https://www.samhsa.gov/data/
- Lu HC, & MacKie K (2016). An introduction to the endogenous cannabinoid system. Biological Psychiatry, 79(7), 516–525. 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Hu Y, Devinsky O, & Hoffman AF (2017). Cannabinoids as hippocampal network administrators. Neuropharmacology, 124, 25–37. 10.1016/j.neuropharm.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Lutz B, Marsicano G, Maldonado R, & Hillard CJ (2015). The endocannabinoid system in guarding against fear, anxiety and stress. Nature Reviews Neuroscience, 16(12), 705–718. 10.1038/nrn4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo LM, Asratian A, Lindé J, Morena M, Haataja R, Hammar V, … Heilig M (2019). Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase (FAAH): a randomized, controlled experimental medicine trial. Biological Psychiatry. 10.1016/j.biopsych.2019.07.034 [DOI] [PubMed] [Google Scholar]
- McLaughlin NCR, Strong D, Abrantes A, Garnaat S, Cerny A, O’Connell C, … Greenberg BD (2015). Extinction retention and fear renewal in a lifetime obsessive-compulsive disorder sample. Behavioural Brain Research. 10.1016/j.bbr.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, & Gallily R (2002). Cannabidiol: An Overview of Some Pharmacological Aspects. The Journal of Clinical Pharmacology, 42(S1), 11S–19S. 10.1002/j.1552-4604.2002.tb05998.x [DOI] [PubMed] [Google Scholar]
- Metrik J, Rohsenow DJ, Monti PM, McGeary J, Cook TAR, de Wit H, … Kahler CW (2009). Effectiveness of a marijuana expectancy manipulation: Piloting the balanced-placebo design for marijuana. Experimental and Clinical Psychopharmacology, 17(4), 217–225. 10.1037/a0016502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, … Wilhelm S (2013). Deficits in Conditioned Fear Extinction in Obsessive-Compulsive Disorder and Neurobiological Changes in the Fear Circuit. JAMA Psychiatry, 70(6), 608 10.1001/jamapsychiatry.2013.914 [DOI] [PubMed] [Google Scholar]
- Milad MR, & Rauch SL (2012). Obsessive Compulsive Disorder: Beyond Segregated Cortico- striatal Pathways. Trends Cogn Sci, 16(1), 43–51. 10.1016/j.tics.2011.11.003.Obsessive [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichino A, Senior M, Brondino N, Zhang SH, Godwlewska BR, Burnet PWJ, … Lennox BR (2019). Measuring Disturbance of the Endocannabinoid System in Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 10.1001/jamapsychiatry.2019.0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P, & Reggio PH (2017). An Update on Non-CB 1, Non-CB 2 Cannabinoid Related G-Protein-Coupled Receptors. Cannabis and Cannabinoid Research, 2(1), 265–273. 10.1089/can.2017.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJA, Freeman TP, Hindocha C, Schafer G, Gardner C, & Curran HV (2018). Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Translational Psychiatry, 8, 181 10.1038/s41398-018-0191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, & Lopez AD (1996). The global burden of disease: a comprehensive assessment of mortality and morbidity from disease, injuries, and risk factors in 1990 and projected to 2020. Harvard: World Health Organization. [Google Scholar]
- Palmer RHC, McGeary JE, Knopik VS, Bidwell LC, & Metrik JM (2019). CNR1 and FAAH variation and affective states induced by marijuana smoking. The American Journal of Drug and Alcohol Abuse, 45(5), 514–526. 10.1080/00952990.2019.1614596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papolos DF, Teicher MH, Faedda GL, Murphy P, & Mattis S (2013). Clinical experience using intranasal ketamine in the treatment of pediatric bipolar disorder/fear of harm phenotype. Journal of Affective Disorders, 147(1–3), 431–436. 10.1016/J.JAD.2012.08.040 [DOI] [PubMed] [Google Scholar]
- Pava MJ, Makriyannis A, & Lovinger DM (2016). Endocannabinoid Signaling Regulates Sleep Stability. PloS One, 11(3), e0152473 10.1371/journal.pone.0152473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, … Ross RA (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB 1 and CB 2. 10.1124/pr.110.003004 [DOI] [PMC free article] [PubMed]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, & de Wit H (2008). Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(10), 2313–2319. 10.1523/JNEUROSCI.5603-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, … Blier P (2019). Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. American Journal of Psychiatry, 176(5), 401–409. 10.1176/appi.ajp.2018.18070834 [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, & Luan Phan K (2014). Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiology of Learning and Memory, 113, 125–134. 10.1016/j.nlm.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, & Phan KL (2013). Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology, 64, 396–402. 10.1016/j.neuropharm.2012.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Peters C, Marusak HA, Ghosh S, & Phan KL (2018). Effects of acute Δ9-tetrahydrocannabinol on next-day extinction recall is mediated by post-extinction resting-state brain dynamics. Neuropharmacology. 10.1016/j.neuropharm.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Haney M, & Cooper ZD (2013). Marijuana’s dose-dependent effects in daily marijuana smokers. Experimental and Clinical Psychopharmacology, 21(4), 287–293. 10.1037/a0033661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, & Levy S (2014). Big Marijuana — Lessons from Big Tobacco. New England Journal of Medicine, 371(5), 399–401. 10.1056/nejmp1406074 [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Flood P, & Simpson HB (2011). Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. The Journal of Clinical Psychiatry, 72(4), 567–569. 10.4088/JCP.10l06653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, … Simpson HB (2013). Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 38(12), 2475–2483. 10.1038/npp.2013.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhani N, Wimmer GE, Schneier FR, Fyer AJ, Shohamy D, & Simpson HB (2019). Impaired generalization of reward but not loss in obsessive–compulsive disorder. Depression and Anxiety. 10.1002/da.22857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, & Marcu J (2017). Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Advances in Pharmacology, 80, 67–134. 10.1016/BS.APHA.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson N-O, Leonova J, … Greasley PJ (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. British Journal of Pharmacology, 152, 1092–1101. 10.1038/sj.bjp.0707460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Broyd S, Greenwood L-M, van Hell H, Martelozzo D, Rueb K, … Croft R (2019). A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. European Archives of Psychiatry and Clinical Neuroscience, 269(1), 17–35. 10.1007/s00406-019-00978-2 [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorssuch R, & Lushene P (1983). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press. [Google Scholar]
- Spradlin A, Mauzay D, & Cuttler C (2017). Symptoms of obsessive-compulsive disorder predict cannabis misuse. Addictive Behaviors, 72, 159–164. 10.1016/j.addbeh.2017.03.023 [DOI] [PubMed] [Google Scholar]
- Storch EA, & Kay BC (2019). Commentary on Spradlin et al.: Is marijuana use common in OCD? Addictive Behaviors. 10.1016/j.addbeh.2017.07.028 [DOI] [PubMed] [Google Scholar]
- Subritzky T, Lenton S, & Pettigrew S (2016). Legal cannabis industry adopting strategies of the tobacco industry. Drug and Alcohol Review, 35(5), 511–513. 10.1111/dar.12459 [DOI] [PubMed] [Google Scholar]
- Umathe SN, Manna SSS, & Jain NS (2011). Involvement of endocannabinoids in antidepressant and anti-compulsive effect of fluoxetine in mice. Behavioural Brain Research, 223(1), 125–134. 10.1016/j.bbr.2011.04.031 [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Corcoran CM, Bedi G, Keilp JG, & Haney M (2017). Acute effects of smoked marijuana in marijuana smokers at clinical high-risk for psychosis: A preliminary study. Psychiatry Research, 257, 372–374. 10.1016/j.psychres.2017.07.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, … Bullmore ET (2015). Disorders of compulsivity: A common bias towards learning habits. Molecular Psychiatry, 20(3), 345–352. 10.1038/mp.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, & Potvin S (2012). Cannabidiol in Humans-The Quest for Therapeutic Targets. Pharmaceuticals, 5, 529–552. 10.3390/ph5050529 [DOI] [PMC free article] [PubMed] [Google Scholar]


