Abstract
Objective.
To examine how primary care physicians define placebo concepts, use placebos in clinical practice, and view open-label placebos (OLPs).
Design.
Semi-structured focus groups that were audio-recorded and content-coded.
Methods.
Two focus groups with a total of 15 primary care physicians occurred at medical centres in the New England region of the United States. Prior experience using placebo treatments and attitudes towards open-label placebos were explored. Themes were analysed using an inductive data-driven approach.
Results.
Physicians displayed a nuanced understanding of placebos and placebo effects in clinical contexts which sometimes focused on relational factors. Some respondents reported that they prescribed treatments with no known pharmacological effect for certain conditions and symptoms (‘impure placebos’) and that such prescriptions were more common for pain disorders, functional disorders, and medically unexplained symptoms. Opinions about OLP were mixed: Some viewed OLPs favourably or considered them ‘harmless’; however, others strongly rejected OLPs as disrespectful to patients. Other issues in relation to OLPs included the following: lack of guidelines, legal and reputational concerns, and the notion that such treatments would run counter to customary medical practice.
Conclusions.
A number of physicians reported prescribing impure placebos in clinical care. Although some primary care physicians were resistant to the possibility of recommending OLPs, others regarded OLPs more favourably, viewing them as potential treatments, albeit with restricted potential.
In the past two decades, placebo studies have evolved into a mature scientific field (Blease, 2018; Blease & Annoni, 2019; Evers et al., 2018; Kaptchuk & Miller, 2015). Researchers have elucidated neurobiological mechanisms of the placebo effect (Benedetti, 2014; Wager & Atlas, 2015), found emerging genetic biomarkers that predict placebo responsiveness (Hall, Loscalzo, & Kaptchuk, 2015; Hall, Loscalzo, & Kaptchuk, 2018), and identified cultural factors that moderate placebo efficacy (Moerman, 2002). There is now a growing understanding that clinicians can make use of the placebo effect via factors such as mindset (Zion & Crum, 2018), rituals (Bernstein & Brown, 2017), and classical conditioning models (Benedetti, Pollo, & Colloca, 2007; Kirchhof et al., 2018; Perlis et al., 2015; Schafer, Colloca, & Wager, 2015). However, scholars continue to discuss how to ethically and effectively implement placebo effects in clinical settings (Blease, Bernstein, & Locher, 2019; Blease, Colloca, & Kaptchuk, 2016; Kaptchuk, 2018; Ongaro & Kaptchuk, 2019). Addressing this issue, much recent attention in the field has focused on the possibility of using ‘open-label placebos’ (OLPs), or placebos administered without deception or concealment (Blease et al., 2019; Charlesworth et al., 2017; Kaptchuk & Miller, 2015). Randomized controlled trials reporting encouraging findings on the effectiveness of open-label placebos for irritable bowel syndrome, cancer-related fatigue, and chronic lower back pain (Carvalho et al., 2016; Hoenemeyer, Kaptchuk, Mehta, & Fontaine, 2018; Kaptchuk et al., 2010) have been published in medical journals and widely disseminated across major international news outlets.
The acceptability of OLPs depends on the views of both patients and clinicians. To date, some studies have begun to examine patients’ attitudes about deceptive placebos and OLPs. In 2014, in the United Kingdom, Bishop, Aizlewood, and Adams (2014) conducted focus groups with the general public and reported that most participants believed that placebos necessitated deception to be effective, with individuals divided over whether this deception was ever justified. These findings echo results from the United States: In a telephone survey conducted with a 53% response rate (853/1,598), Hull et al. (2013) reported that 80% of patients believed that placebos necessitate deception to be effective. Respondents judged deceptive placebos to be objectionable, with nearly twice as many patients believing placebo use would have negative effects rather than positive effects on patient-physician relationships (53.9% vs. 28.5%). In contrast, when presented with a scenario involving non-deceptive, open-label placebos (‘OLPs’) nearly 85% considered their use to be acceptable.
Aside from ongoing research aimed at investigating patients’ opinions about placebo use, it is important to examine clinicians’ views. Physicians, in particular, are the primary gatekeepers of how – if at all – placebo research informs clinical practice. To that end, there is considerable value in examining the attitudes and experiences of practicing primary care physicians about placebos and placebo effects. So far, research aimed at investigating physicians’ opinions has overwhelmingly employed closed-ended surveys. In one exception, Hróbjartsson and Norup (2003) asked 286 Danish doctors about their frequency of placebo use (closed-ended items) and examples for when they used placebos (open-ended). Among general practitioners, 86% reported placebo use in the past year; responses to the open-ended item suggested that placebos were most widely used to treat pain and viral infections. According to a recent review, over half (10/16) of all published surveys on this topic with primary care physicians have been conducted in European countries (Linde et al., 2018), and the most recent study was based on an Australian GP population (Faasse & Colagiuri, 2019). The few studies with US-based physicians reveal that around half of those surveyed reported prescribing placebos (Kermen, Hickner, Brody, &Hasham, 2010; Tilburt, Emanuel, Kaptchuk, Curlin, &Miller, 2008). In one crosscountry comparison, Harris, Campbell, and Raz (2015) examined placebo prescribing among internists and rheumatologists in Canada and the United States; while participants in both groups reported comparable rates of prescribing treatments without a proven efficacy, placebo use was almost twice as high in the United States versus Canada. In a 2010 survey, Kermen et al. (2010) found that, among family physicians in the United States, 92% said it is ethical to sometimes use placebos, 85% reported that placebos can have physical and psychological benefits, and 61% would recommend a placebo instead of no treatment.
Rationale and aims
Given the inherently complex nature of this topic, qualitative research is important to obtain a more nuanced understanding of physicians’ attitudes, understanding, and practices with respect to placebos in clinical contexts. To date, qualitative research has been limited to investigations in the United Kingdom (Bishop et al., 2014), Denmark (Hróbjartsson & Norup, 2003), and Switzerland (Fent, Rosemann, Fässler, Senn, & Huber, 2011). Two studies, one with patients (Bishop et al., 2014) and one with physicians (Hróbjartsson & Norup, 2003), utilized written responses in a questionnaire. We are not aware of any qualitative research among US-based physicians. Furthermore, no qualitative research, aside from one recent study (Ratnapalan et al., 2020), has explored physicians’ views about OLPs, a topic that is especially important in the light of a growing body of research on these interventions (Blease et al., 2019; Charlesworth et al., 2017; Kaptchuk & Miller, 2015). To address these gaps, the goal of the present study was to conduct focus groups with US primary care physicians with the aim of exploring experiences of prescribing placebos and physicians’ opinions about using OLPs in primary care practice.
Method
Design
In the present study, focus groups were used as a method of discussing physicians’ understanding about placebo concepts, their opinions about the role of placebo effects in clinical contexts, the acceptability of OLPs, and their experiences – if any – of using placebos in primary care. This focus group methodology enabled the research team to generate a large volume of initial data that might also inform further surveys using closed-ended questions. Focus groups, rather than qualitative interviews, were chosen because they offer participants the chance to compare their responses with one another (Morgan, 2012). Focus groups are unique insofar as the interaction between participants is itself a method of gathering data (McLafferty, 2004). Given the topic, we expected that discussion among physicians would yield interesting information and chose focus groups to capitalize on this rich source of data. Since many of our questions requested that participants reflect on conceptual or general practice issues, rather than personal questions, focus groups provided a more suitable methodology to encourage open dialogue (see Table 1). Although there is no consensus about the ideal number of participants in focus groups, our goal was to recruit between 6 and 10 participants per group (Carlsen & Glenton, 2011). All study procedures were deemed exempt by the Institutional Review Board. Prior to attending focus groups, participants were informed about the purpose and nature of the study. As part of the informed consent process, participants were informed that their contributions would remain anonymous in written reports, that they could refuse to answer any question, and that they could withdraw from the study at any time.
Table 1.
Focus group question guide
| Questions | Allotted time (min) |
|---|---|
| 1. What is your understanding of placebos and placebo effects in clinical practice? | 10 |
| Follow-up question: How should we define placebos/placebo effects? | |
| Follow-up question: For which conditions or symptoms – if any – do you believe placebo effects may be therapeutic? | |
| 2. Should physicians strive to maximize placebo effects in clinical practice? | 10 |
| Follow-up question: Why or why not? | |
| 3. Participants read the following brief vignette: | 15 |
| A patient visits their primary care physician suffering from symptoms of chronic back pain. The physician advises the patient that placebo pills made of an inert substance, like sugar, have been shown in clinical studies to significantly reduce pain through mind-body self-healing processes. | |
| The physician prescribes placebo pills and mentions the following points during the dialogue: ‘(1) the placebo effect is powerful; (2) that the body can automatically respond to taking placebo pills like Pavlov’s dogs who salivated when they heard a bell; (3) a positive attitude helps but is not necessary; and (4) taking placebo pills faithfully is critical’. | |
| The physician then gives the patient placebo pills, advising them to take thepills twice per dayfor 14 days, and to call if symptoms worsen or if they are not experiencing adequate relief. Follow-up question: What are your thoughts on this scenario? | |
| Follow-up question: Would you ever consider doing this in your own practice? | |
| Follow-up question: Do you envisage open-label placebos being routinely used in primary care? | |
| 4. Do you have any experiences of placebo use in your own practice? | 10 |
| Follow-up question: In your opinion, are there any circumstances when it is acceptable to use placebos in primary care? |
Recruitment and participants
Participants were recruited via email flyers distributed during March and April 2019, to primary care physicians based at medical centres affiliated with a medical school in the New England region of the United States. The theme of the study was described as ‘primary care physicians opinions about placebos in clinical care’, and participants were advised they would receive $150 for participating. While our hope was to conduct focus groups until data saturation, this population was difficult to recruit, and only 15 physicians indicated willingness to participate. We conducted the two focus groups on different days of the week at 6:30 pm to increase the pool of potential physicians who could attend. Recruitment was also constrained by funding limitations.
Setting
Focus groups were conducted in a conference room at a large hospital and facilitated by two researchers (CB and MHB). Neither facilitator was personally known to the participants. In the first focus group, CB served as the primary facilitator and MHB served as the secondary facilitator; this arrangement was reversed for the second focus group. CB is an interdisciplinary health researcher who has published on ethical and conceptual issues in placebo studies; MHB is an experimental health psychologist who is interested in harnessing placebo effects to improve treatment outcomes. The study specifically aimed to investigate conceptual considerations in relation to placebo terminology, and clinical practice issues with consequences for ethical practice. Therefore, it was important that the discussions be led with individuals who had expertise in these issues. In addition, we were cautious not to include physicians or practitioners in guiding the focus groups, to better encourage honest reporting among participants. Focus groups were conducted in May and June 2019, and both sessions were audio-recorded.
Procedure
After the group assembled, participants were reminded by the primary facilitator of the purpose of the study, who ensured that participants fully understood and demonstrated their consent prior to commencing the focus groups and beginning the audio recordings. To encourage open and transparent dialogue, participants were asked to keep the conversation confidential. A semi-structured questionnaire (designed by MHB, CB, CD) was used to maintain consistency between focus group sessions (see Table 1). Question 1 was designed to assess knowledge of placebos and the placebo effect; Question 2 was designed to assess the perceived utility of placebo effects in primary care; Question 3 was geared towards determining physicians’ attitudes about open-label placebos, specifically. Participants each read a very brief, fictitious vignette of OLP administration based on prior experiments (Carvalho et al., 2016; Kaptchuk et al., 2010) and were asked several questions related to their perception of the acceptability of this scenario in primary care. The final topic was designed to elicit primary care physicians’ personal experiences – if any – of using placebos in clinical practice. The focus groups closed by requesting participants to add any further comments they might have on the discussion. The question guide was created in this manner to try and generate discussion that comprised a mix of knowledge, attitudes, and personal experience. Also, this allowed for us to examine viewpoints related to both OLPs, and placebos as typically conceived. In total, the focus groups lasted 45–50 min, and full study participation was 60 min.
Analysing the focus groups
A structuring content analysis after Mayring (2014) was used to evaluate the data from the semi-structured interviews (Berger, Braehler, & Ernst, 2012; Gensichen et al., 2012; Goetz et al. ,2012; Hertenstein et al. ,2012). The transcribed interviews were imported into QCAmap (Qualitative Content Analysis software) for analysis. We applied an inductive data-driven approach because it allows an examination of core topics for a phenomenon with limited existing theory or research findings (Hsieh & Shannon, 2005; Johansson & Eklund, 2003). In line with the principles of Mayring (2014), a multistage analytic process was performed:
First, eight questions were formulated (Box 1), focusing on PCPs’ attitudes of placebos, experiences with placebos, and their opinion of using OLPs in practice. Second, and in line with the principles of content analysis, we defined the category definition and level of abstraction a priori, referring to theoretical considerations. Coders worked through the text material line by line. As soon as a text passage fitted the research question, a category was constructed. The next time a text passage fitted the research question, we checked whether it could be subsumed under the previous category or whether a new category had to be formulated. Fourth, after working through a significant amount of the material (i.e., 10–15%), we reviewed the whole category system. This led to some minor adjustments. Fifth, after working through the whole text material inter-coder agreement was conducted. Coders discussed disagreements and reached consensus. Finally, the final list of categories was grouped into a hierarchical structure, which entailed: superordinate themes, main categories, and categories (Table 2). For this process, standard rules for summarizing qualitative content analysis were applied (Mayring, 2014). An example of the coding process is shown in Figure 1.
Box 1.
Research questions for focus group interpretation
How do primary care physicians define placebos?
What are the reasons primary care physicians think placebos work?
What are primary care physicians’ experiences of using impure or pure placebos?
For what patients do primary care physicians currently prescribe placebos, and what are their reasons for doing so?
What are primary care physicians’ general opinions of OLPs?
What are primary care physicians’ opinions about the OLP vignette?
What do primary care physicians’ consider as barriers to OLP use?
What is the medical community’s general outlook on prescribing placebos?
Table 2.
Superordinate themes and main categories
| Superordinate themes | Main categories |
|---|---|
| 1. Primary care physicians’ understanding about placebo concepts | Placebos Placebo effects |
| 2. Primary care physicians’ experiences of prescribing placebos | Symptoms and conditions relevant to placebo use Reasons for prescribing placebos |
| 3. Opinions about open-label placebos | Support for using OLPs Rejection of OLPs Ambiguous OLP attitudes |
| 4. Perceived barriers to using open-label placebos | Lack of OLP guidelines Physician factors Patient factors Institutional and cultural barriers |
Figure 1.
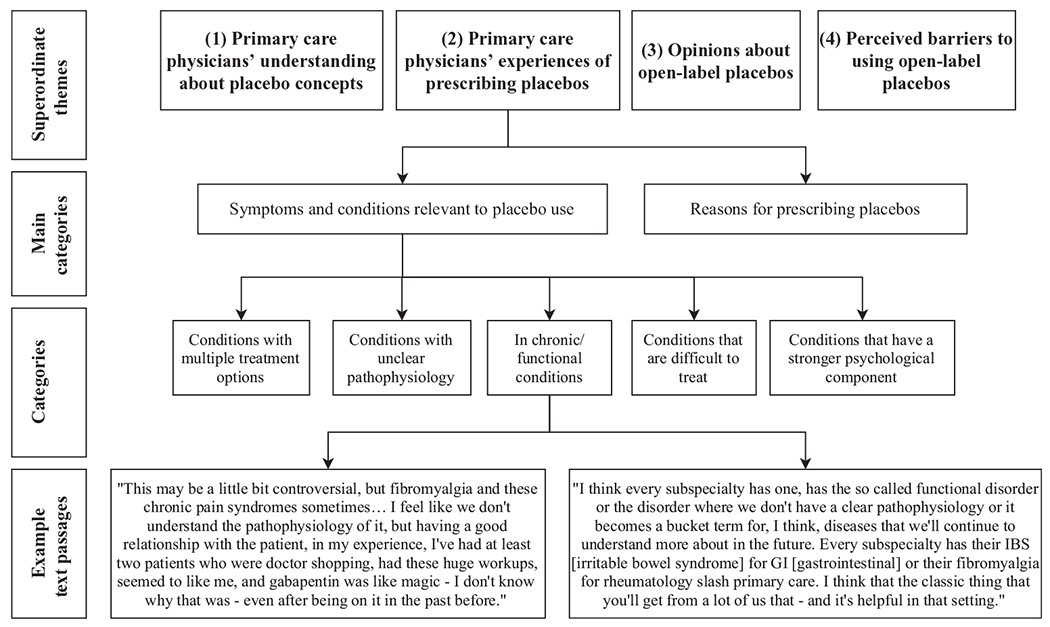
Coding procedure.
Results
Overview
We enrolled a total of 15 participants, nine in the first group and six in the second (participation rate = 100%). Participants were 40% (6/15) female and their age ranged from 28 to 67 (M = 40.9, SD = 13.8). All participants were primary care physicians working in an urban area of New England.
As a result of the iterative coding process, four superordinate themes were identified. Each of these was composed of 2–4 main categories. Superordinate themes, which are discussed in greater depth below, included the following: (1) How Primary care physicians understand placebo concepts, (2) Primary care physicians’ experiences of prescribing placebos, (3) Opinions about open-label placebos, and (4) Perceived barriers to using open-label placebos. Numbers in parentheses refer to participant IDs.
Primary care physicians’ understanding about placebo concepts
Multiple comments reflected participants’ understanding about the nature of placebos, and placebo effects, and many physicians drew conceptual distinctions between these terms, as described below:
Placebos
When it came to describing ‘placebos’, two categories emerged. Specifically, physicians differed according to whether they defined placebos in terms of an inactive treatment, or contextual aspects of healing. Regarding the former, some participants defined placebos as medical interventions without any specific effect, such as ‘sugar pills’ and ‘saline injections’. When defined in this manner, placebos were described as a type of treatment that was offered without full transparency, frequently with the assumption that some degree of deception was imbedded within the concept of placebos; for example:
We think of [placebos] as being something fake, basically. (Participant 2)
A placebo is an inert substance, but we’re imbuing it with power by virtue of saying ‘this may help you’. (Participant 1)
Some participants’ definitions of placebos focused on the patient–physician relationship, such as practitioners’ behaviour and dispositions to help; for example:
Reassurance is a huge placebo. (Participant 2)
Maybe just having people be heard [is the] strongest placebo. (Participant 6)
However, one participant was undecided as to whether relational factors constituted a placebo:
Some would argue that [the therapeutic relationship] is not a placebo. Either that is one or it’s not, but we do that all the time. (Participant 6)
Several comments also referred to fundamental aspects of the practice of medicine as in some sense synonymous with placebos; for example:
The test is a placebo. There will be tests that I will definitely order just because the patient [thinks she] has a brain tumor once a week because she has this headache. (Participant 2)
I think we use it all the time, and don’t label it as such. It’s basically framing…trying to encourage the patient to have positive expectations, and try to frame those as positively as possible. (Participant 11)
Placebo effects
Some participants suggested that placebo effects are related to physiologic changes:
We use [the placebo effect] all the time because we’re tapping into the… emotional physiologic realm in all kinds of ways, we’re doing that. (Participant 2)
Some participants described methods of clinical communication that are not physiologic but may tap physiologic processes:
It reflects all of the non-physiologic ways we try to encourage people. I think framing the expectations is part of it, to heal. Whether it’s from an acute problem or an ongoing problem. (Participant 11)
Many comments suggested that placebo effects were related to relational aspects of patient–physician relationships and particularly focused on the influence of practioner’s behaviour; for example:
I also think of a placebo effect [as] what doctoring is, like making and facilitating a relationship that we can’t really put words to or scientific meaning to why that would help someone, and it does. (Participant 6)
The patient/provider relationship is a very, very important piece. I think that oftentimes we talk about harnessing placebo in that therapeutic relationship. (Participant 13)
We have to be confident what we’re giving our patients… We can’t oversell it, but we have to sell it. We’re obligated to. That’s why people come to us. They’re looking for help. (Participant 7)
Primary care physicians’ experiences of prescribing placebos
The majority of physicians talked openly about prescribing placebos to patients. Participants reported using what placebo researchers describe as ‘impure placebos,’ which are interventions that have pharmacological effects but not for the disease or symptoms being treated at the dose prescribed. Examples of impure placebos include prescribing antibiotics for viral infections, or vitamin supplements that are not indicated for the patient’s particular ailments. ‘Impure placebos’ stand in contrast to ‘pure placebos’: The latter are usually interpreted as referring to interventions that have no pharmacological effects such as microcrystalline cellulose (‘sugar pills’) or saline injections. Sometimes the placebos described resided in a grey zone between impure placebo and a possibly active drug.
That we use pharmaceuticals as placebos all the time… We use gabapentin. It’s only FDA- approved for seizure disorder and shingles, and we use it—it’s a drug and it affects the neurologic system, but it’s never been proven, at least to the FDA’s satisfaction, to be good for nerve pain yet…I think we use a lot of pharmaceuticals as placebo, basically. Secretly, we’re thinking, I don’t think this is gonna help them. (Participant 2)
You can only tell someone to go to physical therapy so many times, and maybe that’s a placebo too. [So that is why I think] we do things like gabapentin. We know that [it is] probably not gonna help them that much, and that [it is an] expensive placebo. (Participant 1)
We do it. We do it. We don’t prescribe an inert pill, but we say, “You’ve got this cough. Take this guaifenesin.” Studies show it’s not really better than placebo. I’m not gonna tell my patients that. They’re looking for help, and I don’t have anything. (Participant 7)
Physicians also elaborated on the circumstances under which they might offer placebos in clinical contexts. Two categories emerged: The particular symptoms and conditions under which physicians would consider prescribing placebos, and specific justifications or reasons for prescribing placebos. In all cases, placebos were conceived of as being prescribed in a covert manner.
Symptoms and conditions relevant to placebo use
Many participants identified patients with chronic diseases or conditions with no known aetiology as prevalent targets for the use of placebos. There was widespread agreement that functional disorders and pain disorders, in particular, were common candidates for placebo usage, including chronic fatigue syndrome and fibromyalgia; for example:
This may be a little bit controversial, but fibromyalgia and these chronic pain syndromes sometimes… I feel like we don’t understand the pathophysiology of it, but having a good relationship with the patient, in my experience, I’ve had at least two patients who were doctor shopping, had these huge workups, seemed to like me, and gabapentin was like magic —I don’t know why that was—even after being on it in the past before. (Participant 8)
I think every subspecialty has one, has the so-called functional disorder or the disorder where we don’t have a clear pathophysiology or it becomes a bucket term for, I think, diseases that we’ll continue to understand more about in the future. Every subspecialty has their IBS [irritable bowel syndrome] for GI [gastrointestinal] or their fibromyalgia for rheumatology slash primary care. I think that the classic thing that you’ll get from a lot of us that—and it’s helpful in that setting. (Participant 7)
In addition, there was agreement among many participants that placebos were prescribed commonly to treat patients with medically unexplained symptoms; for example:
I think the areas that we reach for it and hope for the placebo effect is in areas that are poorly understood physiologically. (Participant 11)
But the things that I think of it as being most useful for in my panel are patients who have a weird neurologic something, whether that be pain or something along those lines, and there’s no great treatment and they’ve tried a thousand different things. It’s a little bit of a wish and a prayer that maybe if we just add Tums, that will do it. Sometimes if you have a good relationship, sometimes it works, at least temporarily, but there’s no real reason that it should. (Participant 4)
Reasons for prescribing placebos
Physicians identified two primary reasons for prescribing placebos: first, to appease demanding or difficult patients; and, second, because of a perceived risk/benefit trade off, that some physicians argued justified placebo use. With respect to the first reason, a few participants reported that placebos were given to difficult patients who were adamant about receiving treatment. In these cases, it was acknowledged that the treatment did not have a direct therapeutic benefit but might still placate the patient, and furthermore, that such prescribing was commonplace. For example:
When primary care physicians prescribe antibiotics for your eye symptoms and things like that. In some ways, we’re also trying to—in some ways they’re really acquiescing to patient’s demands, when physiologically it doesn’t necessarily seem to make sense. Then also, going for that effect of—I guess maintaining that patient/provider relationship, even though we know that it has harmful effects with antibiotic resistance and whatnot. (Participant 14)
I prescribe antibiotics sometimes when I know they’re not necessary… I tell them, ‘I don’t think you need antibiotics, here you have a viral illness, it’s going to get better in 7 to 10 days.’ If they push hard, sometimes yes, we do. We prescribe stuff we know is not effective. … We do that. Try to minimize it, but it gets done all the time. (Participant 11)
Second, some comments focused on the potential benefit of placebos, and/or the fact that some placebos are low risk in terms of side effects; for example:
A lot of times we check [vitamin D, if] it’s low, you’re like I don’t know, maybe I’ll just prescribe it. It’s probably not harmful. Probably good for them, but unclear if that’s helping whatever symptom. (Participant 12)
However, not all participants agreed that placebo use was risk-free; for example:
I tend to agree, but I think the part ofthere not being harm in it is the part that I might disagree with in certain cases. Even with certain supplements or vitamins, like we’re seeing evidence like that… too much of certain supplements can be harmful. (Participant 14)
Another participant suggested that placebos can be beneficial as an experimental tool facilitating diagnoses on the basis of trial and error:
I think we have a lot of drugs, like guaifenesin, gabapentin, whatever, whatever, that don’t work well, and we know they don’t work well, but we give it to patients almost as a pseudoplacebo, but can also help with your diagnosis, right? If you have someone with a chronic cough, guaifenesin does not work. Benzonatate does not work. Maybe it’s not a J receptor. Maybe it’s asthma. Maybe it’s GERD. Maybe it’s an anatomical structure. It actually helps in your doctoring along the way. (Participant 8)
Opinions about open-label placebos
There was considerable disagreement among physicians about whether open-label placebos should be used in clinical care. OLPs involve recommending an openly described pill with no medication in it, with the aim of promoting benefit via the placebo effect. Some were supportive of the approach, some were against it, and many were undecided.
Support for using OLPs
Participants who indicated support for OLPs often anticipated few negative outcomes, offering this as a rationale for using such interventions; for example:
[I have a patient with] nerve compression from being hit by a car… and he is chronically uncomfortable. I would love to treat his depression and anxiety… I’m not saying that every patient with pain I would do this for, but if I could say to him, “Have a Tic Tac once a day, and I believe that that will help you if you believe that will help you,” what’s the worst that can happen? (Participant 4)
If the patients were open to it, I feel like why not? (Participant 13)
One physician supported using OLPs as another potential treatment option that might be offered alongside other potential treatments.
I could see myself offering it as one of a number of options to a patient. We have this option of trying this, or this, and having them decide in a way that you wanna pursue this. I suppose I could see that. We could try this, or we could try more traditional treatment. You have that conversation. (Participant 6)
Rejection of OLPs
A number of physicians were adamant that OLPs should never be offered to patients. Some described OLPs as likely to offend some patients who might consider their use disrespectful; for example:
It’s like a joke. It means you disrespect your patient. (Participant 3)
More strongly, one participant suggested that OLPs contravened social conventions in patient–physician interactions, where patients held particular expectations of the treatments that would be offered:
It’s very silly. I think using the placebo effect is one thing, but having someone come to you, to my fine dining restaurant and give them pork liver instead of a filet mignon is just not alright. (Participant 8)
Ambiguous OLP attitudes
Many participants did not have a clear opinion about OLPs. Some were unsure about whether they should be offered; others expressed moderate optimism that they could be helpful. A number of physicians commented that they would support prescribing OLPs if there were strong evidence to support their effectiveness; for example:
If there was some data that showed that patients knew they were getting the placebo and still showed improvements in pain despite knowing that—and I’d be very interested to see if that data exists—then I would feel 100 percent comfortable with doing that, but I just don’t think that that is something that exists, and it seems like a deviation. (Participant 5)
I don’t think it’s particularly my style, but if I saw people doing it and it worked, if I saw some studies and it worked, I could try it. (Participant 1)
If some research were done to show that that particular technique were [sic] efficacious, then I would [use it]. (Participant 2)
Perceived barriers to using open-label placebos
Even physicians who had positive attitudes about the potential of using OLPs did not express unmitigated optimism, and a number of barriers were identified.
Lack of OLP guidelines
Most physicians agreed that some type of clear professional guidelines would be needed for prescribing OLPs. Comments frequently described the necessity of guidelines on appropriate use of this in patient care; for example:
I do think when we practice, a lot of our motivation is we wanna do the right thing for the patient. Sometimes that’s based on evidence. Sometimes it’s a consensus among the peers and whatnot. I think having guidelines at least allow for that. (Participant 14)
There needs to be some support within the medical community that this is part, this should be part of what we provide and meets the community standard of practice. It’s not out there. Probably the quickest way to do that would be to have some authoritative guidelines. (Participant 11)
It can kind of take a dark road. I think that we don’t have any clear guidelines for these kind of practices and I think maybe they would be useful. (Participant 7)
[Medical guidelines] would provide at least some guidance from experts in the field about what sort of reasonable standard of care for administering placebos. I think it would make me more comfortable. (Participant 11)
Physician factors
Some participants discussed ways in which prescribing placebos might lead to professional liability if there were unintended consequences.
Legal concerns.
Two physicians flagged potential legal issues as a barrier to prescribing OLPs:
Somebody starts freelancing with this, the first few people that start doing it, and there’s one bad outcome… I occasional[ly] serve [as a] witness for legal cases. I could just see what the opposing attorney would do. They gave the patient what? What? (Participant 11)
Inevitably, there’s gonna be a patient that’s gonna have an adverse effect, right? Yeah, how is that handled [from a] legal perspective? (Participant 13)
Doctor–patient relationship.
Other physicians were concerned that prescribing OLPs would cause reputational damage with their patients, and concerns about potential harms. Although this was depicted as less severe than legal entanglements, the concern was identified by multiple participants; for example:
I think there’s that concern… if I gave them a sugar pill and then I documented [it] this way, and then it doesn’t really help, and this person goes elsewhere, or just overall how would [they] view me? How would the patient view me? (Participant 14)
My concern is less about the ethics of this and more about alienating my patients. (Participant 8)
Unless you pick the patient and pick the circumstance. They might construe it as you trivializing their complaint. (Participant 11)
OLPs as a second-line treatment.
Participants generally agreed that OLPs should not be used as an initial treatment option. Instead, there was a greater willingness to consider using this intervention only after other treatment options have been tried; for example:
I can’t envision doing this if there are other alternatives. I can picture myself doing this in situations where we’ve tried everything, and it hasn’t worked. (Participant 15)
If the placebo worked, if there’s no harm, I’m fine with it, but if someone comes to me with a complaint, and I’m offering them something that is not real, then I would not feel comfortable doing that up front. (Participant 8)
Patient factors
Some physicians indicated that OLPs maybe acceptable to some patients, suggesting that such decisions would be based on the physician’s clinical intuitions; for example:
A patient coming in who I don’t know, it’s like, your patient or something like that, and I’m just meeting them for the first time, and I’m seeing them for their back pain, I’d probably be reluctant to do this, but someone who I know, someone who you and I know, for example, right, he’d come in, I would feel comfortable doing that, and it would probably work, quite honestly. It is different. You’ve got to decide who you’re gonna use this for. (Participant 7)
I mean I certainly can name patients I’ve had who would totally buy into this, and absolutely others who would be, ‘What are you talking about?’ (Participant 12)
Institutional and cultural barriers
Multiple comments indicated concern about institutional or cultural barriers of OLP use. Physicians suggested that this form of treatment would be ‘counter-cultural’ to current medical practice in the US; for example:
There’s a huge amount of inertia in the practice of medicine. It takes years to get really, really well scientific based guidelines out into clinical practice. I have no doubt that this would face similar types. Not just inertia, but active resistance from some people. (Participant 11)
I think in general the barriers are gonna be higher than any other new treatment coming on, because it just requires a whole paradigm shift. (Participant 13)
There’s a certain percentage of health professionals and patients that are gonna be skeptical about it, which you don’t have so, so much with new therapies. It’s just you have a lot of inertia. (Participant 11)
Discussion
Our exploratory study builds on previous findings in two ways. First, no previous survey has examined physicians’ opinions about OLPs. Second, the present study examines the opinions of US-based physicians on placebo use, which has only been previously explored once using qualitative methodology (Ratnapalan et al., 2020). If OLPs are to find a role within primary care, it is first important to develop a rich and nuanced understanding of how physicians perceive their usefulness in clinical practice.
Physicians expressed a range of definitions of placebos, and placebo effects. While these encompassed pure placebos (e.g., sugar pills), participants also commonly viewed placebo effects as entwined with the patient–physician relationship. The idea that placebo effects are related to relational aspects ofhealth care, and can be invoked without the administration of a placebo, is consistent with the way some experts working in placebo studies discuss the term (Blease, 2018; Blease & Annoni, 2019; Brown, 2013; Evers et al., 2018; Ongaro & Kaptchuk, 2019).
Some participants openly agreed that placebos were frequently prescribed (‘it gets done all the time’). This finding supports previous evidence that use of placebos may be common in primary care (Linde et al., 2018). Placebos prescribed were typically those that have been categorized as ‘impure,’ or in a grey zone between impure and active drug, and physicians reported that such prescribing was especially used in the treatment of functional disorders, coughs, and medically unexplained symptoms. Rationales behind these practices included: recognition that sometimes placebo can be helpful/beneficial (and those potential benefits may outweigh risks), realization that the physician had nothing else to offer the patient combined with a felt obligation to offer something, acquiescence to patient demand for a treatment, and as a way of dealing with uncertainty in medicine (e.g., the prescription might be helpful diagnostically).
Notably, a number of participants’ views and routine experiences with deceptive (impure) placebos differed from their opinions about OLPs. Although some physicians expressed cautious support for the possibility of prescribing OLPs, many opposed their use as ‘disrespectful’ towards patients. The divergent opinions observedhere is consistent with the one other study we are aware of that has investigated open placebo willingness among physicians (Ratnapalan et al., 2020). Reasons for scepticism included lack of clinical guidelines about when to use OLPs, perceptions of cultural or institutional resistance within the medical community, and concerns about the potential harms of OLPs, including patients’ perceptions that their complaint would be trivialized. Indeed, placebo researchers have recently emphasized that further consideration must be given to OLPs among different patient groups, and that risks of self-stigmatization may be acute for some conditions, or individuals (Blease, 2019; Blease et al., 2019).
The focus groups revealed an interesting and ethically complex dichotomy between many participants’ hesitancy around prescribing OLPs, yet common use of impure placebos. Central to many participants’ rationale is a desire to maintain positive patient- physician relationships and a motivation to ‘do the right thing for the patient’. There appears to be an implicit assumption that some degree of lack of transparency is necessary for placebos to be beneficial [a belief that recent studies are questioning (Kaptchuk, 2018)]. These practices should also be understood in the context of modern clinical practice in the United States where physicians have little time or training in how to effectively counsel patients around the nuances of placebo effects, and where patient satisfaction scores are taken into account as part of physician evaluations.
Strengths, limitations, and future directions
The major strength of the present study is that it offers qualitative data on the attitudes of U.S. physicians regarding placebo treatments and comprises the first attempt to examine how physicians view open placebos. As OLP research mounts, it is imperative that placebo experts work alongside practicing physicians to consider how this burgeoning body of research might ethically and effectively translate into clinical care. More broadly, a limited number of qualitative studies related to placebo usage (though not OLPs) have been conducted previously; typically these samples are comprised of European physicians (Linde et al., 2018), whereas we focus on doctors in the United States (for relevant exceptions, see Harris et al., 2015; Kermen et al., 2010; Tilburt et al., 2008).
A primary limitation was the small number of focus groups. It would have been preferable to conduct additional groups until saturation of themes was obtained. Also, while we believe that on balance focus groups was a superior methodology to qualitative interviews, one inherent limitation is that less experienced physicians may have felt pressure to defer to their more senior counterparts. Another limitation is that because the study was advertised as a placebo project, participants may have had strong views about the topic, and the extent to which results generalize beyond the sample is unclear.
Future studies should include investigating attitudes of physicians to OLP when they are explicitly informed about randomized trials that have evaluated this form oftreatment, and they could examine physician perspectives of OLPs in other specialties (e.g., gastroenterology) where this approach might be used. The qualitative data obtained in the present study might usefully lay the groundwork for quantitative surveys with larger sample sizes of physicians to develop a more complete understanding of perceptions of OLPs. In addition, it will be important to examine the attitudes of other medical specialists, including nurse practitioners, psychiatrists, as well as clinical psychologists, and psychotherapists, about openly prescribed placebos and to examine whether their views differ from our participants’.
A better understanding of the credibility and acceptability of OLPs among patients is crucial. In the light of the findings of the current study, we suggest that the views of patients with pain disorders, functional disorders, and medically unexplained symptoms, in particular, should be sought. These groups include highly vulnerable patients who may be distrustful of physicians and/or have changed physicians multiple times (Blease, 2019; Blease, Carel, & Geraghty, 2017; Locher, Gaab, & Blease, 2018). In addition, it would be worthwhile surveying the views of patients from vulnerable demographic groups, such as minority patients (Friesen & Blease, 2018), who – an extensive body of research suggests – are often distrustful of medical practitioners. Finally, aspects of the US health system (for example, the fee for service model, and relatively high rates of patient litigation) may have influenced some of the participants’ responses. We therefore caution against generalizing these findings to PCPs in other countries.
Conclusion and implications
Primary care physicians exhibited advanced understanding about definitions of placebo concepts, and empirical knowledge about placebo effects. A number of physicians reported using impure placebos in primary care with some participants describing such prescribing as more common among patients with functional disorders (such as fibromyalgia), chronic pain, coughs, and those with medically unexplained symptoms. Opinions about honestly described placebos were mixed with many physicians questioning their effectiveness and acceptability among patients. Other barriers, such as cultural and institutional resistance to OLPs, were identified. The findings of these focus groups provide new insight into placebo use, and opinions about placebos (including OLPs), in primary care contexts.
Statement of contribution.
What is already known?
Many physicians report prescribing drugs for the purposes of eliciting a placebo effect.
Initial evidence for the efficacy of open-label placebos is promising.
What does this study add?
A more nuanced description of the circumstances under which primary care physicians report placebo prescribing.
A qualitative account of physician attitudes about using open-label placebos in clinical practice.
Acknowledgements
This study was supported by K01DA048087 (Bernstein), the Irish Research Council-Marie Skłodowska-Curie Fellowship (CRB), the Swiss National Science Foundation (SNSF P400PS_180730) (CL), K23 AT009218 (MLD), and the Foundation for the Science of the Therapeutic Encounter. The study design, and collection, analysis, and interpretation of data were not influenced by the funding sources. The authors thank the 15 primary care physicians who consented to participate in this study. We also thank Dr Lisa Conboy and Dr Leonor Fernandez for advice and feedback at early stages of the project.
Footnotes
Conflicts of interest
All authors declare no conflict of interest.
Data availability statement
Given the nature of the data reported in this study, it is not available to be shared publicly.
References
- Benedetti F (2014). Placebo effects. New York, NY: Oxford University Press. [Google Scholar]
- Benedetti F, Pollo A, & Colloca L (2007). Opioid-mediated placebo responses boost pain endurance and physical performance: Is it doping in sport competitions? Journal of Neuroscience, 27, 11934–11939. 10.1523/JNEUROSCI.3330-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Braehler E, & Ernst J (2012). The health professional-patient-relationship in conventional versus complementary and alternative medicine. A qualitative study comparing the perceived use of medical shared decision-making between two different approaches of medicine. Patient Education and Counseling, 88(1), 129–137. [DOI] [PubMed] [Google Scholar]
- Bernstein MH, & Brown WA (2017). The placebo effect in psychiatric practice. Current Psychiatry, 16(11), 29–34. [PMC free article] [PubMed] [Google Scholar]
- Bishop FL, Aizlewood L, & Adams AE (2014). When and why placebo-prescribing is acceptable and unacceptable: A focus group study of patients’ views. PLoS ONE, 9(7), e101822 10.1371/journal.pone.0101822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blease C (2018). Consensus in placebo studies: Lessons from the philosophy of science. Perspectives in Biology and Medicine, 61, 412–429. 10.1353/pbm.2018.0053 [DOI] [PubMed] [Google Scholar]
- Blease CR (2019). The role of placebos in family medicine: Implications of evidence and ethics for general practitioners. Australian Journal of General Practice, 48, 700–705. 10.31128/AJGP-05-19-4939 [DOI] [PubMed] [Google Scholar]
- Blease C, & Annoni M (2019). Overcoming disagreement: A roadmap for placebo studies. Biology and Philosophy, 34(2), 18 10.1007/s10539-019-9671-5 [DOI] [Google Scholar]
- Blease CR, Bernstein MH, & Locher C (2019). Open-label placebo clinical trials: Is it the rationale, the interaction or the pill? BMJ Evidence-Based Medicine. 10.1136/bmjebm-2019-111209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blease C, Carel H, & Geraghty K (2017). Epistemic injustice in healthcare encounters: Evidence from chronic fatigue syndrome. Journal of Medical Ethics, 43, 549–557. 10.1136/medethics-2016-103691 [DOI] [PubMed] [Google Scholar]
- Blease C, Colloca L, & Kaptchuk TJ (2016). Are open-label placebos ethical? Informed consent and ethical equivocations. Bioethics, 30 407–414. 10.1111/bioe.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WA (2013). The placebo effect in clinical practice. New York, NY: Oxford University Press. [Google Scholar]
- Carlsen B, & Glenton C (2011). What about N? A methodological study of sample-size reporting in focus group studies. BMC Medical Research Methodology, 11(1), 26 10.1186/1471-2288-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, & Kirsch I (2016). Open-label placebo treatment in chronic low back pain: A randomized controlled trial. Pain, 157, 2766–2772. 10.1097/j.pain.0000000000000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth JE, Petkovic G, Kelley JM, Hunter M, Onakpoya I, Roberts N, … Howick J (2017). Effects of placebos without deception compared with no treatment: A systematic review and meta-analysis. Journal of Evidence-Based Medicine, 10(2), 97–107. 10.1111/jebm.12251 [DOI] [PubMed] [Google Scholar]
- Evers A, Colloca L, Blease C, Annoni M, Atlas LY, Benedetti F, … Kelley JM (2018). Implications of placebo and nocebo effects for clinical practice: Expert consensus. Psychotherapy andpsychosomatics, 87, 204–210. 10.1159/000490354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faasse K, & Colagiuri B (2019). Placebos in Australian general practice: A national survey of physician use, beliefs and attitudes. Australian Journal of General Practice, 48(12), 876–882. [DOI] [PubMed] [Google Scholar]
- Fent R, Rosemann T, Fassler M, Senn O, & Huber CA (2011). The use of pure and impure placebo interventions in primary care - A qualitative approach. BMC Family Practice, 12(1), 11 10.1186/1471-2296-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P, & Blease C (2018). Placebo effects and racial and ethnic health disparities: An unjust and underexplored connection. Journal of Medical Ethics, 44, 774–781. 10.1136/medethics-2018-104811 [DOI] [PubMed] [Google Scholar]
- Gensichen J, Guethlin C, Sarmand N, Sivakumaran D, Jager C, Mergenthal K,… Petersen JJ (2012). Patients’ perspectives on depression case management in general practice-A qualitative study. Patient Education and Counseling, 86(1), 114–119. 10.1016/j.pec.2011.02.020 [DOI] [PubMed] [Google Scholar]
- Goetz K, Szecsenyi J, Campbell S, Rosemann T, Rueter G, Raum E,… Miksch A (2012). The importance of social support for people with type 2 diabetes-a qualitative study with general practitioners, practice nurses and patients. GMSPsycho-Social-Medicine, 9, Doc02 10.3205/psm000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KT, Loscalzo J, & Kaptchuk TJ (2015). Genetics and the placebo effect: The placebome. Trends in Molecular Medicine, 21, 285–294. 10.1016/j.molmed.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KT, Loscalzo J, & Kaptchuk T (2018). Pharmacogenomics and the placebo response. ACS Chemical Neuroscience, 9(4), 633–635. 10.1021/acschemneuro.8b00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CS, Campbell NK, & Raz A (2015). Placebo trends across the border: US versus Canada. PloSOne, 10(11), e0142804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertenstein E, Rose N, Voderholzer U, Heidenreich T, Nissen C, Thiel N, … Kulz AK (2012). Mindfulness-based cognitive therapy in obsessive-compulsive disorder-A qualitative study on patients’ experiences. BMC Psychiatry, 12(1), 185 10.1186/1471-244X-12-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenemeyer TW, Kaptchuk TJ, Mehta TS, & Fontaine KR (2018). Open-label placebo treatment for cancer-related fatigue: A randomized-controlled clinical trial. Scientific Reports, 8 (1), 2784–2790. 10.1038/s41598-018-20993-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hróbjartsson A, & Norup M (2003). The use of placebo interventions in medical practice—A national questionnaire survey of Danish clinicians. Evaluation and the Health Professions, 26 (2), 153–165. 10.1177/0163278703026002002 [DOI] [PubMed] [Google Scholar]
- Hsieh H-F, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15, 1277–1288. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Hull SC, Colloca L, Avins A, Gordon NP, Somkin CP, Kaptchuk TJ, & Miller FG (2013). Patients’ attitudes about the use of placebo treatments: Telephone survey. BMJ, 347, f3757 10.1136/bmj.f3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H, & Eklund M (2003). Patients’ opinion on what constitutes good psychiatric care. Scandinavian Journal of Caring Sciences, 17, 339–346. https://doi.org/10.1046Zj.0283-9318.2003.00233.x [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ (2018). Open-label placebo: Reflections on a research agenda. Perspectives in Biology and Medicine, 61(3), 311–334. 10.1353/pbm.2018.0045 [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, … Lembo AJ (2010). Placebos without deception: A randomized controlled trial in irritable bowel syndrome. PLoS ONE, 5(12), e15591 10.1371/journal.pone.0015591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, & Miller FG (2015). Placebo effects in medicine. New England Journal of Medicine, 373(1), 8–9. 10.1056/NEJMp1504023 [DOI] [PubMed] [Google Scholar]
- Kermen R, Hickner J, Brody H, & Hasham I (2010). Family physicians believe the placebo effect is therapeutic but often use real drugs as placebos. Family Medicine, 42, 636–642. [PubMed] [Google Scholar]
- Kirchhof J, Petrakova L, Brinkhoff A, Benson S, Schmidt J, Unteroberdorster M, … Schedlowski M (2018). Learned immunosuppressive placebo responses in renal transplant patients. Proceedings of the National Academy of Sciences of the United States of America, 115, 4223–4227. 10.1073/pnas.1720548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde K, Atmann O, Meissner K, Schneider A, Meister R, Kriston L, & Werner C (2018). How often do general practitioners use placebos and non-specific interventions? Systematic review and meta-analysis of surveys. PLoS ONE, 13(8), e0202211 10.1371/journal.pone.0202211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Gaab J, & Blease C (2018). When a placebo is not a placebo: Problems and solutions to the gold standard in psychotherapy research. Frontiers in Psychology, 9, 2317 10.3389/fpsyg.2018.02317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayring P (2014). Qualitative content analysis: Theoretical foundation, basic procedures and software solution.
- McLafferty I (2004). Focus group interviews as a data collecting strategy. Journal of Advanced Nursing, 48, 187–194. 10.1111/j.1365-2648.2004.03186.x [DOI] [PubMed] [Google Scholar]
- Moerman DE (2002). Meaning, medicine, and the” placebo effect”. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Morgan DL (2012). Focus groups and social interaction In Gubrium JF, Holstein JA, Marvasti AB, & McKinney KD (Eds.), The Sage handbook of interview research: The complexity of the craft (p. 2). Thousand Oaks, CA: Sage. [Google Scholar]
- Ongaro G, & Kaptchuk TJ (2019). Symptom perception, placebo effects, and the Bayesian brain. Pain, 160(1), 1–4. 10.1097/j.pain.0000000000001367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis M,Grandner M, Zee J, Bremer E, Whinnery J, Barilla H,.. .Ader R (2015). Durability of treatment response to zolpidem with three different maintenance regimens: A preliminary study. Sleep Medicine, 16, 1160–1168. https://doi.org/10.1016Zj.sleep.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnapalan M, Coghlan B, Tan M, Everitt H, Geraghty AWA, Little P,… Bishop FL (2020). Placebos in primary care? A nominal group study explicating UK GP and patient views of six theoretically plausible models of placebo practice. British Medical Journal Open, 10(2), e032524 10.1136/bmjopen-2019-032524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer SM, Colloca L, & Wager TD (2015). Conditioned placebo analgesia persists when subjects know they are receiving a placebo. The Journal of Pain, 16, 412–420. 10.1016/j.jpain.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, & Miller FG (2008). Prescribing “placebo treatments”: Results of national survey of US internists and rheumatologists. BMJ, 337, a1938 10.1136/bmj.a1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, & Atlas LY (2015). The neuroscience of placebo effects: Connecting context, learning and health. Nature Reviews Neuroscience, 16, 403–418. 10.1038/nrn3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion SR, & Crum AJ (2018). Mindsets matter: A new framework for harnessing the placebo effect in modern medicine. International Review ofNeurobiology, 138,137–160. 10.1016/bs.irn.2018.02.002 [DOI] [PubMed] [Google Scholar]


