Abstract
Background:
Cixutumumab, a monoclonal antibody targeting insulin-like growth factor I receptor, did not improve undetectable PSA rate at 28 weeks when combined with androgen deprivation in the randomized phase II SWOG S0925 trial for patients with new metastatic hormone-sensitive prostate cancer. We now present mature survival analyses, along with pre-specified secondary and exploratory endpoints.
Methods:
We randomized 210 patients to androgen deprivation with or without cixutumumab, 105 per treatment arm. We used Kaplan-Meier curves to analyze overall survival, radiographic progression-free survival, and castration resistance-free survival by treatment arm, disease volume, and risk group. We explored differences in survival by treatment arm via covariate-adjusted Cox proportional hazards models adjusted for disease volume and risk.
Results:
No difference was seen between treatment arms in overall survival (HR 1.01 [0.70-1.45]; p=0.97), radiographic progression-free survival (HR 1.17 [0.85-1.60]; p=0.35), or castration resistance-free survival (HR 1.02 [0.75-1.41]; p=0.88). At baseline, 105/198 (53.0%) patients had high risk features and 119/210 (56.7%) had high volume disease; 16.7% of patients had discordant classifications of high or low category for risk and volume. Adjusting for risk or volume yielded no differences in overall survival between arms. Inferior survival was observed in high risk (HR 1.89 [1.29-2.80]; p=0.001) and high volume (HR 2.75 [1.84-4.10]; p<0.0001) disease. Disease volume was a better fit to survival data than risk group (AIC 878.3 vs. 889.2). Compared to patients achieving undetectable PSA at 28 weeks, inferior survival was observed in patients whose PSA was >0.2 to ≤4.0 ng/mL (HR 3.72 [1.99-6.95]; p<0.0001) or >4.0 ng/mL (HR 7.13 [4.24-11.9]; p<0.0001).
Conclusions:
In new metastatic hormone-sensitive prostate cancer, addition of cixutumumab to androgen deprivation did not improve survival. Baseline risk and disease volume carried prognostic value for this distinct trial population, although disease volume added more prognostic information. PSA treatment response was a strong intermediate endpoint for survival.
Introduction
Cixutumumab, or IMC-A12, is a recombinant human monoclonal immunoglobulin G1 antibody targeting insulin-like growth factor I receptor (IGF-IR). IGF-IR signaling is a candidate factor in prostate cancer progression as it leads to nuclear translocation of androgen receptors and androgen receptor-mediated signaling in the absence of androgens.1 Cixutumumab induces IGF-IR internalization, and in preclinical studies, led to apoptosis and G1 cell-cycle arrest in hormone-sensitive prostate cancer xenograft models and G2 arrest in castration-resistant prostate cancer murine models.2 A prior phase II trial of cixutumumab in metastatic castration-resistant prostate cancer (mCRPC) yielded a 29% radiographic stabilization rate at ≥6 months,3, 4 and a neoadjuvant trial of cixutumumab with androgen deprivation (AD) showed synergistic pharmacodynamic effects.5 Another neoadjuvant trial with figitumumab, a different monoclonal antibody targeting IGF-IR, induced prostate-specific antigen (PSA) declines of ≥25% in 94% of patients and ≥50% in 31% of patients without concurrent AD.6
SWOG S0925 was a randomized phase II trial of cixutumumab with AD versus AD alone in patients with new metastatic hormone-sensitive prostate cancer (mHSPC). As previously reported, cixutumumab was generally well tolerated with a small increase in grade 3 adverse events, most notably hyperglycemia.7 The primary endpoint of SWOG S0925 was rate of undetectable PSA (≤0.2 ng/mL) after 28 weeks of treatment, based on the randomized SWOG 9346 trial of intermittent versus continuous AD in mHSPC, which demonstrated that PSA response after 28 weeks was strongly associated with overall survival (OS).8 The primary outcome of SWOG S0925 was negative, with no difference seen in undetectable PSA rate between trial arms.7 We now report long-term results of SWOG S0925 with mature progression and survival outcomes, including pre-specified secondary validation of the intermediate endpoint of PSA response at 28 weeks and its prognostic value for subsequent survival. Given that SWOG 9346 was conducted prior to the current era of survival-prolonging therapies for mCRPC, study of this intermediate endpoint in a more modern population of patients is of great interest.
Other criteria have emerged to help assess pre-treatment prognosis and guide treatment selection in the setting of several landmark clinical trials which changed the treatment landscape for mHSPC. In the CHAARTED trial, docetaxel combined with AD improved OS in patients with mHSPC compared to AD alone, with similar results found by Arm C of the STAMPEDE trial studying AD with docetaxel and prednisolone.9-11 In CHAARTED, only patients with high volume disease, defined by visceral metastasis and/or ≥4 bone metastases with at least one outside the axial skeleton, were observed to have significant benefit with the addition of docetaxel to AD.10, 12 Of note, post-hoc analyses of CHAARTED also supported the prognostic value of undetectable PSA at 28 weeks, concordant with the findings of SWOG 9346.8, 13 Abiraterone acetate was also approved for use in mHSPC after OS benefit was seen with the LATITUDE trial, which randomized patients with mHSPC and high risk disease, defined as having ≥2 of 3 prognostic factors of Gleason score ≥8, visceral disease, and ≥3 bone lesions, to abiraterone acetate plus AD versus AD alone.14, 15 Arm G of the STAMPEDE trial also showed OS benefit with the addition of abiraterone acetate to AD, but this arm included patients with a variety of different baseline characteristics.16 Post-hoc analyses of STAMPEDE demonstrated that the OS benefit with abiraterone acetate was seen for all disease volume and risk groups, using the definitions from CHAARTED and LATITUDE, respectively.17
With the developments above, disease volume and risk as defined in the CHAARTED and LATITUDE trials are increasingly being used to inform prognosis for patients with mHSPC, and in the case of docetaxel, to guide treatment selection. SWOG S0925 is unique in that it provides information prior to the utilization of docetaxel, abiraterone acetate, enzalutamide, and apalutamide for the mHSPC disease state, yet the patients likely received several of these therapies for mCRPC. Final data from SWOG S0925 thus serves as an independent dataset to not only validate the prognostic value of undetectable PSA at 28 weeks of treatment in the modern era, but also to examine the correlation of disease volume and risk with OS for patients with new mHSPC. Therefore, we present survival of our cohort stratified by disease risk and volume as secondary, post-hoc analyses.
Materials and Methods
The details of the SWOG S0925 study protocol (ClinicalTrials.gov Identifier: NCT01120236) have previously been published.7 Briefly, this was a multicenter randomized phase II trial designed and conducted within SWOG, approved by the Cancer Therapy Evaluation Program of the National Cancer Institute and the independent institutional review board of each participating center, with all study patients providing written informed consent. Eligibility requirements included a diagnosis of prostate cancer (pathologically confirmed), PSA ≥5 ng/mL, at least one site of metastasis on imaging (including at minimum a bone scan and CT or MRI of the abdomen and pelvis), and Zubrod performance status of 0 to 2 (or 3 if resulting from bone pain only). More than 2 years must have elapsed from completion of remote AD in the neoadjuvant, concurrent, and/or adjuvant settings, and prior AD for metastatic disease was allowed if the first luteinizing hormone-releasing hormone (LHRH) agonist injection was within 30 days of enrollment.
The two treatment arms consisted of AD with LHRH agonist and daily oral bicalutamide, or AD with LHRH agonist and daily oral bicalutamide with the addition of cixutumumab (10 mg/kg intravenously over 1 hour every 2 weeks for seven cycles, each cycle comprising of two treatments in 28 days) (Supplemental Figure 1). The accrual goal was 180 eligible patients, with an additional 10% (n=198) to account for potential ineligibility; this was designed to have 90% statistical power to detect an absolute difference of 20% in the primary endpoint of undetectable PSA rate at 28 weeks using Fisher’s exact test, using a one-sided type I error rate of 0.10 and assuming an undetectable PSA rate of 45% in the control arm.7 Patients were randomized in a 1:1 ratio using a dynamic balancing algorithm with stratification based on Zubrod Performance Status (0-1 vs. 2-3) and baseline PSA (<20 ng/mL vs. ≥20 ng/mL) to either treatment arm, resulting in 105 patients per treatment arm.18 The National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) were used for assessment of adverse events, and treatment on protocol continued until completion of seven cycles (28 weeks), early disease progression (by symptoms, imaging, or PSA), unacceptable toxicity, or patient desire to withdraw from the trial.
Statistical Analysis
Imaging with the same modalities used for baseline disease assessment was performed after 28 weeks of treatment, and subsequently bone scan and CT or MRI of the chest, abdomen, and pelvis as well as survival assessments were performed every 6 months for the first 2 years and then annually until progression or death. Progression by radiographic criteria was defined as a 20% increase in the sum of the diameters of target measurable lesions over the smallest sum of diameters observed during study protocol, with an absolute increase of at least 0.5 cm; patients were also considered to have progressed if they had unequivocal progression of non-measurable disease in the opinion of the treating physician (written explanation was required), any new metastatic lesion, or if they died.
PSA was assessed every 4 weeks during study protocol and after the completion of seven cycles (28 weeks) of treatment, and subsequently at least every 6 months for the first 2 years and then at least annually until progression by radiographic criteria or death. PSA progression was defined as two consecutive increases in PSA at least 2 weeks apart with a total testosterone level of <50 ng/dL, which we defined as the development of castration resistance. Although not a standard endpoint, we did perform analyses of castration resistance-free survival defined as the development as castrate resistance or death, as we felt this was of clinical relevance to mHSPC. Two patients who only had PSA assessment prior to registration were excluded from the castration resistance-free survival analysis. For patients not meeting criteria for PSA progression, last date of PSA assessment was used for censoring. PSA response at 28 weeks was divided into three pre-specified categories of 0.2, >0.2 to 4.0, and >4.0 ng/mL.8 Patients without a PSA value after the completion of 28 weeks were assumed to not have achieved a PSA of ≤4.0 ng/mL.
Time to event curves were estimated using methods of Kaplan-Meier. Cox’s proportional hazards models were used to compare OS, radiographic progression-free survival (rPFS), and castration resistance-free survival by treatment arm, as well as to evaluate the effect of treatment arm, disease volume, and risk group either alone or in combination. Akaike’s information criterion (AIC) was used to determine whether disease volume or risk was a better predictor of OS. Residual Chi-square values were calculated to determine whether disease volume contributed to survival in a model of risk group and OS, and vice versa. A landmark analysis was used to assess OS by PSA response at 28 weeks (pre-specified secondary endpoint of trial).
Disease volume and risk prior to protocol treatment were defined as per the CHAARTED and LATITUDE trials, respectively, with central review of Gleason score and sites of disease from pre-registration pathology and radiology reports.9, 10, 14, 15 To compare PSA response by disease risk and volume, χ2 analysis was used.
All analyses were conducted using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
The intention-to-treat population included 210 patients (Supplemental Figure 1). Baseline demographics and disease characteristics were generally balanced between treatment groups (Table 1). Median follow-up time was 5.3 years.
Table 1.
Baseline demographic and clinical characteristics
| AD plus cixutumumab (N=105) | AD alone (N=105) | |||
|---|---|---|---|---|
| Characteristic | N | % | N | % |
| Age, years | ||||
| Median | 65 | 66 | ||
| Interquartile range | 60-72 | 58-73 | ||
| PSA, ng/mL | ||||
| Median | 31 | 37 | ||
| Interquartile range | 12-74 | 10-200 | ||
| Gleason score* | ||||
| <7 | 5 | 4.8 | 5 | 4.8 |
| 7 | 28 | 26.7 | 14 | 13.3 |
| ≥7 | 66 | 62.9 | 80 | 76.2 |
| Race | ||||
| Black | 4 | 3.8 | 10 | 9.5 |
| White | 94 | 89.5 | 88 | 83.8 |
| Other | 7 | 6.7 | 7 | 6.7 |
| Zubrod PS | ||||
| 0 | 62 | 59.0 | 65 | 61.9 |
| 1 | 41 | 39.1 | 38 | 36.2 |
| 2 | 2 | 1.9 | 2 | 1.9 |
| Site of metastasis | ||||
| Lymph node only | 17 | 16.2 | 11 | 10.5 |
| Bone only | 43 | 41.0 | 37 | 35.2 |
| Lymph node and bone | 33 | 31.4 | 50 | 47.6 |
| Visceral | 12 | 11.4 | 7 | 6.7 |
| Bone pain | 28 | 26.6 | 35 | 33.3 |
Abbreviations: AD, androgen deprivation; PSA, prostate-specific antigen; PS, performance score.
Gleason score missing for 12 patients.
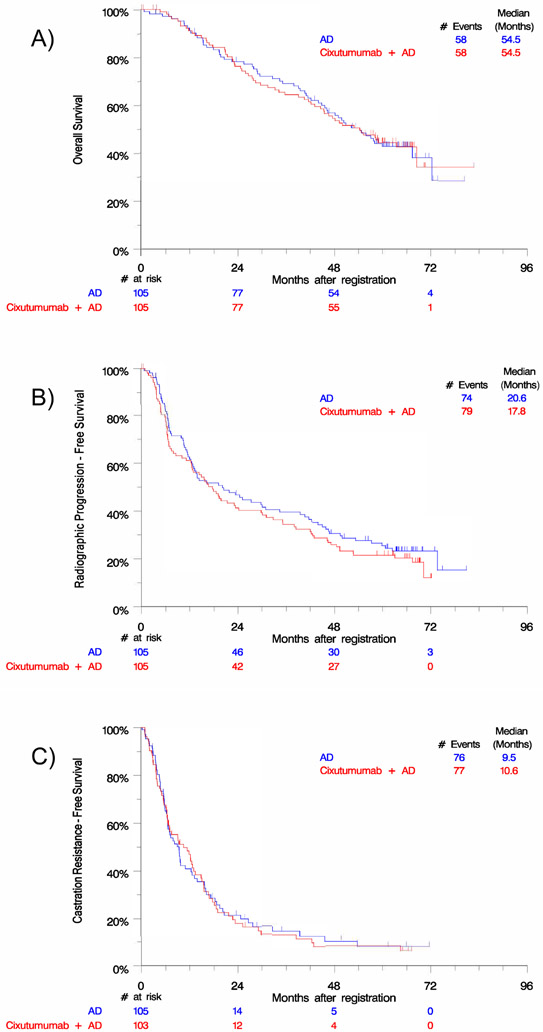
There was no statistical difference seen between treatment arms in OS (cixutumumab arm HR 1.01 [0.70-1.45]; p=0.97), rPFS (cixutumumab arm HR 1.17 [0.85-1.60]; p=0.35), or castration resistance-free survival (cixutumumab arm HR 1.02 [0.75-1.41]; p=0.88) (Figure 1).
Figure 1.
Kaplan-Meier analysis by treatment arm reveals no difference in A) overall survival, B) radiographic progression-free survival, or C) castration resistance-free survival. Abbreviations: AD, androgen deprivation.
Prior to protocol treatment, 105/198 (53.0%) patients had high risk disease and 119/210 (56.7%) had high volume disease as determined by central review (Table 2). Twelve patients did not have a Gleason score recorded and so risk group could not be assessed. Of note, 17.3% of patients with high volume disease were classified as low risk, and 15.9% of patients with low volume disease were classified as high risk; 83.3% of patients had concordant classification of high or low category for both risk and volume. When OS was adjusted for risk group, there remained no statistical difference between the two treatment arms (cixutumumab arm HR 1.00 [0.69-1.47]; p=0.99). Similar results were seen when OS was adjusted for disease volume (cixutumumab arm HR 0.99 [0.69-1.43]; p=0.95). Thus, for the remainder of our analyses, we combined both treatment arms into a single pooled cohort for study.
Table 2.
Baseline risk and volume status*
| High volume N (%) |
Low volume N (%) |
|
|---|---|---|
| High risk | 91 (82.7) | 14 (15.9) |
| Low risk | 19 (17.3) | 74 (84.1) |
Table contains N=198 patients (risk not assessed in 12 patients due to missing Gleason scores)
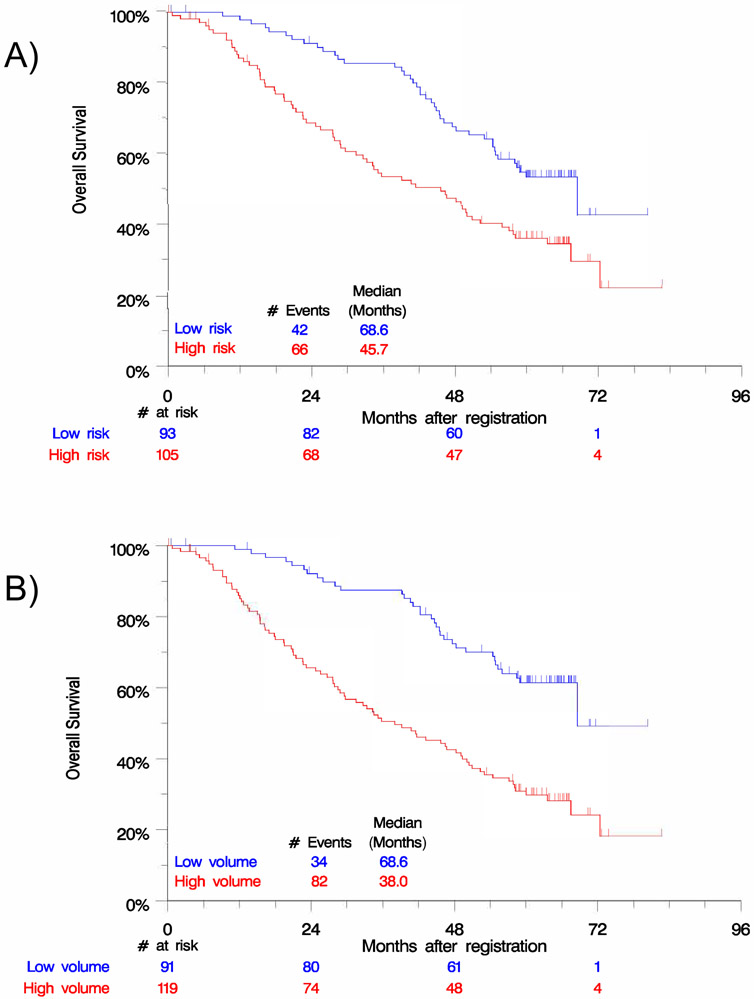
Inferior OS was seen for patients with high risk disease (HR 1.89 [1.29-2.80]; p=0.001) and high volume disease (HR 2.75 [1.84-4.10]; p<0.0001) (Figure 2). Disease volume was a better fit to the survival data than risk group (AIC 878.3 vs. 889.2). When disease volume was in the model, risk group did not provide a significant contribution to predicting OS (p=0.82 residual Chi-square). However, disease volume did provide a significant contribution to prediction of OS when risk group was in the model (p=0.001 residual Chi-square).
Figure 2.
Kaplan-Meier analysis by A) risk group and B) disease volume reveals significant differences in overall survival.
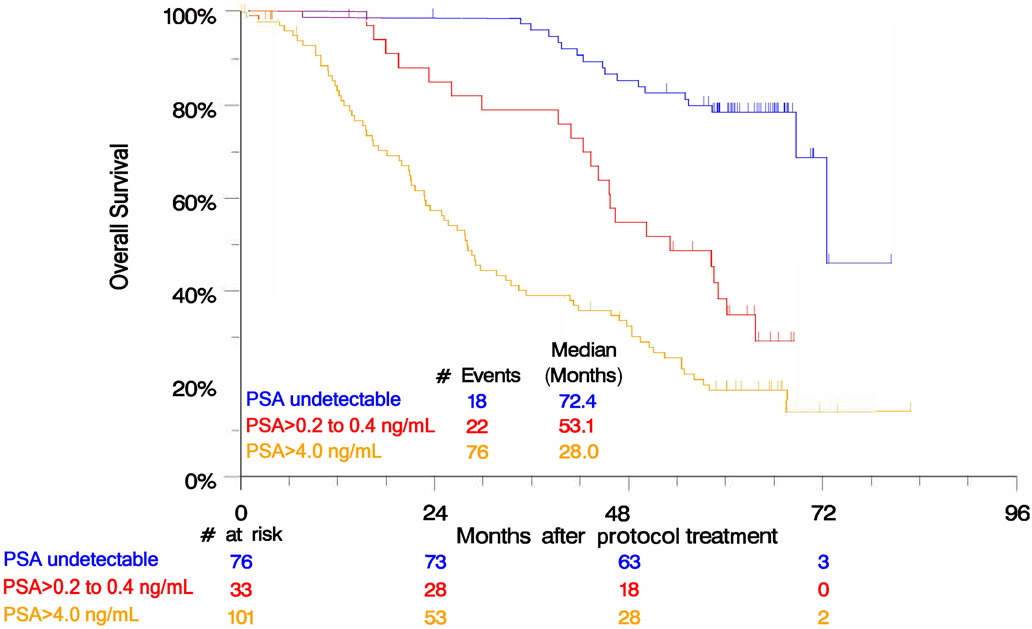
Compared to patients with undetectable PSA after 28 weeks of treatment, inferior OS was seen for patients with PSA >0.2 to ≤4.0 ng/mL (HR 3.72 [1.99-6.95]; p<0.0001) and PSA >4.0 ng/mL (HR 7.13 [4.24-11.9]; p<0.0001) (Figure 3). When PSA response was stratified by disease risk and volume, high risk patients were more likely to have PSA >4.0 ng/mL (61.0% vs. 29.0%; p<0.0001) as were high volume patients (63.0% vs. 28.6%; p<0.0001) (Supplemental Table 1).
Figure 3.
Kaplan-Meier analysis by PSA response at 28 weeks of treatment reveals significant differences in overall survival.
Discussion
Previously, the SWOG S0925 trial for patients with new mHSPC reported no difference in the primary endpoint of undetectable PSA rate at 28 weeks between the two arms of cixutumumab plus AD versus AD alone.7 The long-term survival analyses of SWOG S0925 definitively demonstrate no benefit to adding cixutumumab to AD for patients with mHSPC; fortunately, thanks to recent advances, patients now have docetaxel, abiraterone acetate, enzalutamide, and apalutamide as options with evidence for OS benefit when combined with AD in the mHSPC setting.9-11, 14-16, 19-21
The SWOG S0925 data also confirms utility of the intermediate PSA endpoint first described in SWOG 9346, but report better survival for patients not achieving undetectable PSA, in concordance with other more recent data.8, 22 SWOG 9346 reported a median OS of 13 months for patients with PSA >4.0 ng/mL and 44 months for patients with PSA >0.2 to ≤4.0 ng/mL at 28 weeks of treatment, while SWOG S0925 found a median OS of 28.0 months and 53.1 months for patients in those PSA groups, respectively. Baseline patient characteristics in SWOG S0925 differed from those in SWOG 9346 in that patients in SWOG S0925 had a lower median age and starting PSA and a larger proportion were white; however, more had higher Gleason scores and high volume disease. Despite differences in patient characteristics, it is likely that some of the improved survival in this more modern dataset is attributable to the current era of available therapies for mCRPC. Interestingly, in SWOG S0925 the median survival of patients with undetectable PSA at 28 weeks was 72.4 months, not better than the median survival of 75 months seen for the same PSA group in SWOG 9346.8
The CHAARTED trial utilized SWOG criteria for high versus low volume disease, in part based on an earlier clinical trial investigating the addition of flutamide to AD in patients with mHSPC which found overall and progression-free survival benefit to flutamide, particularly in patients with good performance status and/or minimal disease (defined as absence of disease in the ribs, long bones, skull, or soft tissue, other than lymph node involvement).9, 10, 23 Similarly, the LATITUDE trial introduced criteria for high versus low risk disease.14, 15 These risk and volume criteria are increasingly being used to assess prognosis and guide treatment decisions, and the final outcomes from SWOG S0925, with central review of baseline disease volume and risk, provide an independent dataset collected before the era of the new mHSPC agents (abiraterone acetate, enzalutamide, docetaxel, apalutamide) for validation of the prognostic significance of these criteria.10, 12 Our data confirm that disease volume and risk conferred prognostic significance in this group of patients with new mHSPC, and as a novel finding, that disease volume was a better predictor of OS than risk group.
Limitations
Although SWOG S0925 was a well-performed randomized trial conducted within the National Clinical Trials Network mechanism, limitations exist. Although we reviewed radiology reports centrally, images and tumor measurements were provided by independent site investigators with no central review of images. Assessments of rPFS were limited by the interval for scans outlined by the protocol (every 6 months for the first 2 years, then annually). Study definition of rPFS did not mandate confirmation of soft tissue progression or the 2+2 new bone scan lesion rules defined by Prostate Cancer Working Group (PCWG) 2 criteria.24 SWOG S0925 defined PSA progression as two consecutive increases in PSA at least 2 weeks apart, while PCWG2 defines PSA progression as a ≥25% increase and absolute increase of ≥2 ng/mL from nadir confirmed by a second value obtained ≥3 weeks later;24 the latter criteria is generally applied to mCRPC, while SWOG S0925 studied treatment in the mHSPC setting. Thus, we chose criteria to match clinical practice patterns where clinicians and patients would likely be motivated to identify and address castration resistance early with subsequent interventions.
Conclusions
The findings from the SWOG S0925 trial with cixutumumab support that newer agents for mHSPC may require more extensive preclinical testing in multiple models and need to demonstrate greater synergy with standard of care agents before introduction into clinical trials. Fortunately, patients with mHSPC now have a number of treatment options, reducing pressure for new drug development and allowing time for more comprehensive pre-clinical testing of novel agents in the future. We validated the prognostic significance of disease volume and risk classifications in patients with new mHSPC, with disease volume being a better predictor of overall survival than risk group, and our data strengthens the correlation between undetectable PSA at 28 weeks of treatment and OS in the current era of mCRPC therapies. This earlier intermediate endpoint should be considered for future screening or phase II clinical trials in mHSPC, as it can potentially reduce commitment of resources and minimize the risk of patients exposed to ineffective investigational therapies.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health, the Clinical Research Division of the Fred Hutchinson Cancer Research Center, and in part by ImClone Systems (subsidiary of Eli Lilly and Company).
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers CA180888, CA180819, CA180818, CA180828, CA233328, CA46368, CA180801, CA180835, CA35421, CA180834, CA142559, CA35281, CA35090, CA37981, CA45807, CA46282, CA180846, CA180830, CA35431, CA58416, CA63848, CA63844, CA12644, CA11083, CA35178, CA67575, CA45808, by the Clinical Research Division of the Fred Hutchinson Cancer Research Center, and in part by ImClone Systems (subsidiary of Eli Lilly and Company). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
Dr. Yu has received research support from Dendreon, Merck, and Seattle Genetics and consulted for Bayer, Clovis, Dendreon, Janssen, and Merck. Dr. Agarwal has consulted for Astellas, AstraZeneca, BMS, Bayer, Clovis, Eisai, Exelixis, EMD Serono, Eli Lilly, Foundation Medicine, Genentech, Janssen, Merck, Novartis, Nektar, Pfizer, and Pharmacyclics. Dr. Cheng has received research support from Clovis, Janssen, Medivation, and Sanofi. Dr. Hussain has received honoraria from Aptitude Health, Astellas, Epics, Genentech, PER, Research to Practice, and Sanofi/Genzyme, consulted for AstraZeneca, Bayer, and Pfizer, received research support from AstraZeneca, Bayer, Genentech, and Pfizer, and received travel and accommodation support from Astellas, AstraZeneca, Bayer, Genentech, and Pfizer. Dr. Quinn has consulted for Astellas, Bayer, Janssen, Pfizer, Sanofi, and AstraZeneca. Dr. Wong, Mai Duong, Dr. Tangen, Dr. Vogelzang, and Dr. Thompson have no conflicts of interest to report.
References
- 1.Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem 2006; 99: 392–401. [DOI] [PubMed] [Google Scholar]
- 2.Wu JD, Odman A, Higgins LM, Haugk K, Vessella R, Ludwig DL et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res 2005; 11: 3065–3074. [DOI] [PubMed] [Google Scholar]
- 3.Higano CS, Alumkal J, Ryan CJ, Yu EY, Beer TM, Chandrawansa K et al. A phase II study evaluating the efficacy and safety of single agent IMC A12, a monoclonal antibody, against the insulin-like growth factor-1 receptor, as monotherapy in patients with metastatic, asymptomatic castration-resistant prostate cancer. J Clin Oncol 2009; 27: 269s (suppl; abstr 5142). [Google Scholar]
- 4.Higano CS, Alumkal J, Ryan CJ, Yu EY, Beer TM, Fox FE et al. A phase II study of cixutumumab (IMC-A12), a monoclonal antibody against the insulin-like growth factor 1 receptor (IGF-IR), monotherapy in metastatic castration-resistant prostate cancer: feasibility of every 3-week dosing and updated results. J Clin Oncol 2010; 28 (suppl; abstr 189). [Google Scholar]
- 5.Dean JP, Sprenger CC, Wan J, Haugk K, Ellis WJ, Lin DW et al. Response of the insulin-like growth factor (IGF) system to IGF-IR inhibition and androgen deprivation in a neoadjuvant prostate cancer trial: effects of obesity and androgen deprivation. J Clin Endocrinol Metab 2013; 98: E820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi KN, Gleave ME, Fazli L, Goldenberg SL, So A, Kollsmannsberger C et al. A phase II pharmacodynamic study of preoperative figitumumab in patients with localized prostate cancer. Clin Cancer Res 2012; 18: 3407–3413. [DOI] [PubMed] [Google Scholar]
- 7.Yu EY, Li H, Higano CS, Agarwal N, Pal SK, Alva A et al. SWOG S0925: a randomized phase II study of androgen deprivation combined with cixutumumab versus androgen deprivation alone in patients with new metastatic hormone-sensitive prostate cancer. J Clin Oncol 2015; 33: 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 2006; 24: 3984–3990. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015; 373: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018; 36: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016; 387: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravis G, Boher JM, Chen YH, Liu G, Fizazi K, Carducci MA et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 studies. Eur Urol 2018; 73: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harshman LC, Chen YH, Liu G, Carducci MA, Jarrard D, Dreicer R et al. Seven-month prostate-specific antigen is prognostic in metastatic hormone-sensitive prostate cancer treated with androgen deprivation with or without docetaxel. J Clin Oncol 2018; 36: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017; 377: 352–360. [DOI] [PubMed] [Google Scholar]
- 15.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019; 20: 686–700. [DOI] [PubMed] [Google Scholar]
- 16.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017; 377: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyle AP, Ali SA, James ND, Parker CC, Cook AD, Attard G et al. Effects of abiraterone acetate plus prednisone/prednisolone in high and low risk metastatic hormone sensitive prostate cancer ESMO 2018 Congress. Munich, Germany, October 21, 2018. [Google Scholar]
- 18.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31:103–115. [PubMed] [Google Scholar]
- 19.Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019; 381: 121–131. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong AJ, Szmulewitz RZ, Petrylak DP, Villers A, Azad A, Alcaraz A et al. Phase 3 study of androgen deprivation therapy (ADT) with enzalutamide (ENZA) or placebo (PBO) in metastatic hormone-sensitive prostate cancer (mHSPC): the ARCHES trial (abstract). J Clin Oncol 2019; 37: (suppl 7S: abstr 687). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019; 381: 13–24. [DOI] [PubMed] [Google Scholar]
- 22.Flaig TW, Plets M, Hussain MHA, Agarwal N, Mitsiades N, Deshpande HA et al. Abiraterone acetate for metastatic prostate cancer in patients with suboptimal biochemical response to hormone induction. JAMA Oncol 2017; 3: e170231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989; 321: 419–424. [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.