Abstract
Atopic dermatitis (AD) lesional skin is often colonized with S. aureus, and the load of S. aureus correlates with disease severity. However, a causative and mechanistic link between S. aureus skin colonization and severity of AD is not well established. We made use of well-established mouse model of AD elicited by epicutaneous sensitization of tape stripped skin with ovalbumin to investigate the relationship between allergic skin inflammation and cutaneous S. aureus colonization. Topical application of S aureus exacerbated allergic skin inflammation induced by epicutaneous sensitization with ovalbumin, whereas allergic skin inflammation generated a permissive environment for S. aureus persistence. Our results establish a mutually reinforcing role of allergic skin inflammation and S. aureus skin colonization.
1. INTRODUCCION.
Atopic dermatitis (AD) is characterized by a defective skin barrier function and a type 2 cytokine dominated local and systemic response to antigens encountered through the skin [1]. AD lesional skin is often colonized with S. aureus, and the load of S. aureus correlates with disease severity [1, 2], suggesting that the microenvironment in AD skin lesions promotes the persistence/growth of S. aureus, which in turn may aggravate skin inflammation. Indeed, some, but not all studies, report clinical improvement of AD following control of S. aureus infection [3], and a decreased AD severity following vigorous S. aureus decolonization of the skin [4]. However, although believed to exist, a bidirectional causative link between S. aureus skin colonization and AD severity has not been formally established.
We previously demonstrated that epicutaneous (EC) sensitization with ovalbumin (OVA) elicits allergic skin inflammation that shares many features with AD skin lesions [5, 6]. These include epidermal hyperplasia, infiltration with CD4+ T cells and eosinophils and increased local Il4, Il13 and Il17a mRNA levels [5, 6]. In addition, OVA sensitized mice developed a systemic response with OVA specific IgE antibodies and cytokine secretion by splenocytes in response to OVA stimulation in vitro [5, 6]. We made use of this well-established mouse model of AD to investigate the relationship between allergic skin inflammation and cutaneous S. aureus colonization.
2. MATERIAL AND METHODS.
2.1. Mice.
Il4−/−, Il13−/− and Il4/13−/− mice on BALB/c background were previously described [7–9]. BALB/c mice were purchased from Charles River Laboratory. All mice were kept in a pathogen-free environment and fed an OVA-free diet. All procedures were performed in accordance with the Animal Care and Use Committee of the Children's Hospital Boston.
2.2. S. aureus preparation and quantification.
S. aureus inoculum was streaked onto tryptic soy agar plate and grown overnight at 37°C. Single colonies were picked and inoculated into a 5 ml tube containing tryptic soy broth and cultured overnight in a shaking incubator. The following morning 1:50 dilution of bacterial suspension was inoculated in 5 ml of tryptic soy broth and cultured for another 2 hrs. Bacterial concentrations were estimated by measuring absorbance at 600 nm. The bacteria were concentrated to 108 CFU/50 μl of PBS, and used for cutaneous infection. CFUs were verified by overnight culturing of inoculum on Chrom-agar plates. To enumerate the bacterial load in vivo, S. aureus was labeled with PSVue794 reagent kit (LI-COR), following manufacturer’s instructions. Then, PSVue794 fluorescence was quantified at different time points using Pearl® Trilogy Small Animal Imaging System (LI-COR). To enumerate the bacterial load from the skin, two 8 mm2 skin biopsies were obtained. After mechanical homogenization, serial dilutions of skin homogenates were cultured on Chrom-agar plates. The growth of USA300 strain was quantified by counting only pink colonies after overnight incubation.
2.3. Epicutaneous (EC) sensitization and S. aureus application.
Female mice 6-8-weeks old were epicutaneously sensitized for 8 days as described previously [5, 6], Briefly, Mice were anesthetized, and their back skin was shaved and tape-stripped with a film dressing (TegadermTM, 3M) followed by the application of 200 μg OVA (Sigma-Aldrich) or saline every other day. At day 9, 108 S. aureus CFU in 50 ml of PBS were superficially applied on epicutaneous sensitized skin with the help of a cotton swab. Analyses were done at D12.
2.4. Histology and measurement of epidermal thickness.
Skin specimens were fixed in 4% paraformaldehyde embedded in paraffin and H&E stained. ImageJ was used for the quantification of the epidermal thickness.
2.5. Skin cell preparation, and flow cytometry.
1cm2 skin pieces from unmanipulated or tape stripped mice, were obtained. Skin pieces were finely chopped using scissors after fat removal and digested for 90 minutes in the media containing Liberase (0.2mg/mlRoche) and DNAse II (Sigma), with continuous shaking at 37° C. Digested skin homogenates were filtered, washed and resuspended in PBS and used for flow cytometry. Cells were preincubated with FcγR-specific blocking mAb (2.4G2) and washed before staining with the following monoclonal antibodies (mAbs): CD3 (17A2), CD45 (30F11), Gr1 (RB6-8C5) from eBioscience, CD11b (M1/70) from Biolegend and anti-Siglec-F (E50-2440) from BD Biosciences. Cells were analyzed by flow cytometry using an LSRFortessa machine (BD Biosciences).
2.6. mRNA expression analyses.
Total skin RNA was extracted with Total RNA Isolation Kit (Ambion). cDNA was prepared with iscript cDNA synthesis kit (Biorad). PCR reactions were run on ABI Prism 7300 (Applied Biosystems) sequence detection system platform. Taqman primers and probes were obtained from Life technologies. The housekeeping gene β2-microglobulin was used as an internal control. Relative mRNA expression was quantified using the 2−ΔΔCt method.
2.7. OVA-specific IgE measurement.
anti-OVA IgE concentrations were measured in sera collected at day 12 by means of ELISA, using a homemade sandwich ELISA for anti-OVA IgE. The capture detection antibody (rat anti-mouse IgE clone R35–72) was obtained from BD Bioscience and purified mouse anti-OVA IgE (clone TOe) standard antibody was produced in the lab. OVA was biotinylated using a kit (Pierce) and used for detection.
2.8. Cell culture and in vitro cytokine expression.
Single cell suspensions of splenocytes were cultured and stimulated with OVA and their supernatants analyzed for cytokines by ELISA as previously described [10].
2.9. Statistical analysis.
Statistical significance was determined by the two-tailed Student’s t test. A p value <0.05 was considered statistically significant.
3. RESULTS AND DISCUSSION.
3.1. Application of S. aureus exacerbates allergic skin inflammation caused by EC sensitization with OVA.
Shaved skin of wild-type (WT) Balb/c mice was tape stripped followed by topical application of OVA or saline, with or without subsequent application of S. aureus (Fig. 1A). As previously reported [5, 6], EC sensitization with OVA caused epidermal thickening, accumulation of CD3+ T cells and eosinophils, and local upregulation of Il4, Il13, and Il17a, but not Ifng expression (Fig. 1 B–D and data not shown), compared to EC sensitization with saline. In addition, mice EC sensitized with OVA had serum OVA specific IgE antibodies (Fig. 1E), and their splenocytes secreted IL-4, IL-13, and IL-17A in response to in vitro restimulation with OVA (Fig. 1F). Topical application of 1×108 CFUs of methicillin resistant S. aureus (MRSA) strain SF8300 (USA300) on skin EC sensitized with OVA significantly increased the local accumulation of T cells and eosinophils (Fig. 1B–C), as well as Il4 and Il13 expression compared to EC sensitized skin with OVA (Fig. 1D). S. aureus application also abolished Il17a upregulation caused by OVA sensitization (Fig. 1D), possibly due to downregulation of Il17a expression by the increased Th2 cytokines. The effects of S. aureus on cytokine expression at sites of cutaneous antigen sensitization application was not due to an enhanced systemic response to EC sensitization, as there was no detectable effect on serum levels of OVA-specific IgE or cytokine secretion by splenocytes in response to in vitro restimulation with OVA (Fig 1E–F). Application of S. aureus to skin EC sensitized with saline caused increased cutaneous accumulation of CD3+ T cells, but had no detectable effect on eosinophil accumulation or local expression of Il4, Il13 and Il17a. Of note S. aureus application to saline sensitized skin causes cutaneous upregulation of l17a on D9 and D10 that wanes by D12 (data not shown and Fig.1D). Altogether the above results indicate that S. aureus colonization aggravates allergic skin inflammation.
Figure 1. Application of S. aureus exacerbates allergic skin inflammation caused by EC sensitization with OVA.
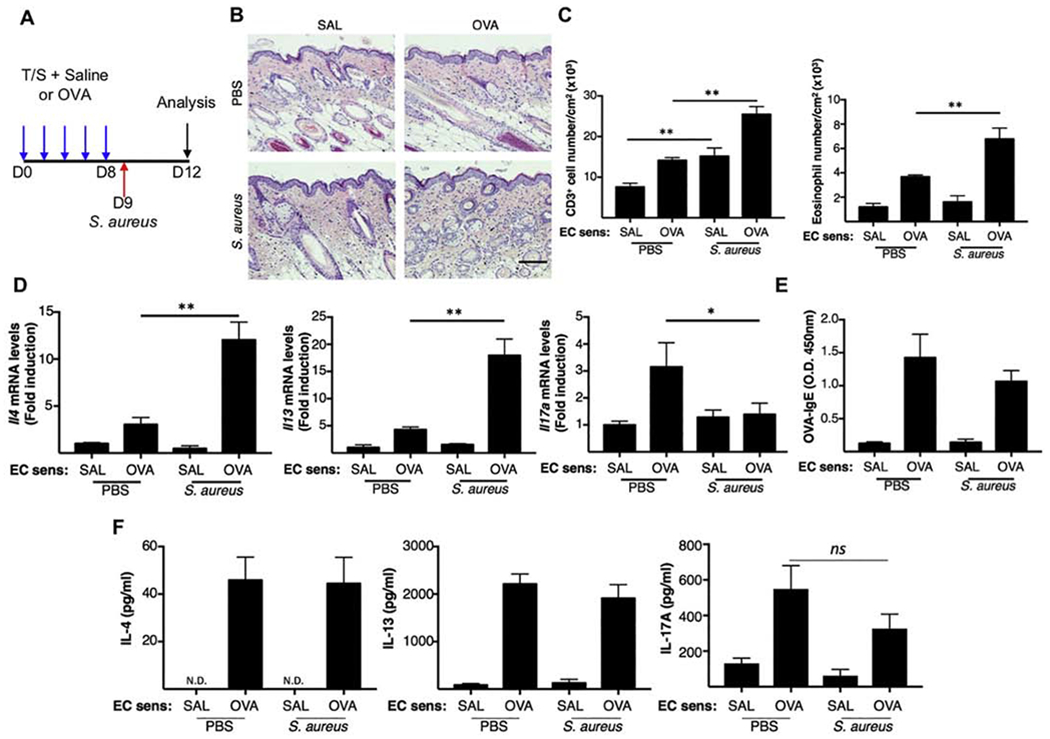
A. Experimental protocol. Mice were EC sensitized mice with OVA or saline on tape stripped skin with or without application of S. aureus. B. Representative H&E sections. Scale bar: 100 mm. C. Skin CD3+ T cell (left) and eosinophil (right) numbers. D. Skin Il4, Il13 and Il17a mRNA levels E. OVA-specific IgE. F. Cytokine production by OVA stimulated splenocytes. Results in A-E are representative of 2 independent experiments with 4-5 mice/group. Bars represent means±SEM. * = p<0.05, ** =p<0.005. Significance was calculated by using two-tailed Student’s t test.
3.2. IL-4 and IL-13 impair S. aureus clearance from sites of allergic skin inflammation.
Type 2 cytokines promote S. aureus skin infection by suppressing the production of antimicrobial peptides (AMPs) by keratinocytes, impairing skin barrier integrity and increasing adherence of S. aureus to the skin [11]. In contrast, IL-17A promotes the clearance of S. aureus from skin by inducing production AMPs and neutrophil chemoattractant chemokines by keratinocytes [12]. In a previous study, we reported that antigen sensitization renders the skin of flaky tail mice susceptible to S. aureus skin colonization [13]. However, these mice have mutations in both filaggrin and ma (matted hair) genes, and their skin demonstrates type 17 cytokine dominated inflammation, which is not the case in either Flg−/− mice or patients with AD. To analyze whether type 2 cytokine dominated allergic skin inflammation promotes S. aureus skin colonization, mice were EC sensitized with OVA or saline for 8 days and PSVue 794 labeled S. aureus was applied the following day according to the protocol in Fig. 1A. S. aureus load was examined by in vivo whole animal imaging system, as well as by measuring the numbers of colony forming units (CFUs) in skin homogenates plated on Chromagar. In vivo imaging revealed an increase in PSVue 794 fluorescence in mice EC sensitized with OVA compared to saline sensitized controls (Fig. 2A). Moreover, significantly higher numbers of CFUs were recovered from homogenates of OVA sensitized skin compared to saline sensitized skin (Fig. 2B). To investigate the role of type 2 cytokines in S. aureus colonization at sites of allergic inflammation, we made use of Il4−/−, Il13−/− and Il4/Il13−/− mice. Topical S. aureus application to OVA-EC sensitized skin of WT, Il4−/−, Il13−/−' and Il4/Il13−/− mice promoted similar epidermal hyperplasia (Fig. 2C). Accumulation of eosinophils, but not T cells, was decreased in OVA sensitized skin of Il4−/−, Il13−/− and Il4/Il13−/− mice compared with WT controls (Fig. 2D). Moreover, Il4−/−, Il13−/− and Il4/Il13−/− mice exhibited a significantly increase in Il17a mRNA expression compared with WT controls (Fig. 2E), and importantly, demonstrated a significantly decreased S. aureus load in OVA sensitized skin compared with WT control (Fig. 2F). Our results indicate that the type 2 cytokines IL-4 and IL-13 promote S. aureus persistence at sites of allergic skin inflammation. This effect could be mediated by suppressing local IL-17A production.
Figure 2. IL-4 and IL-13 impair S. aureus clearance from sites of allergic skin inflammation.
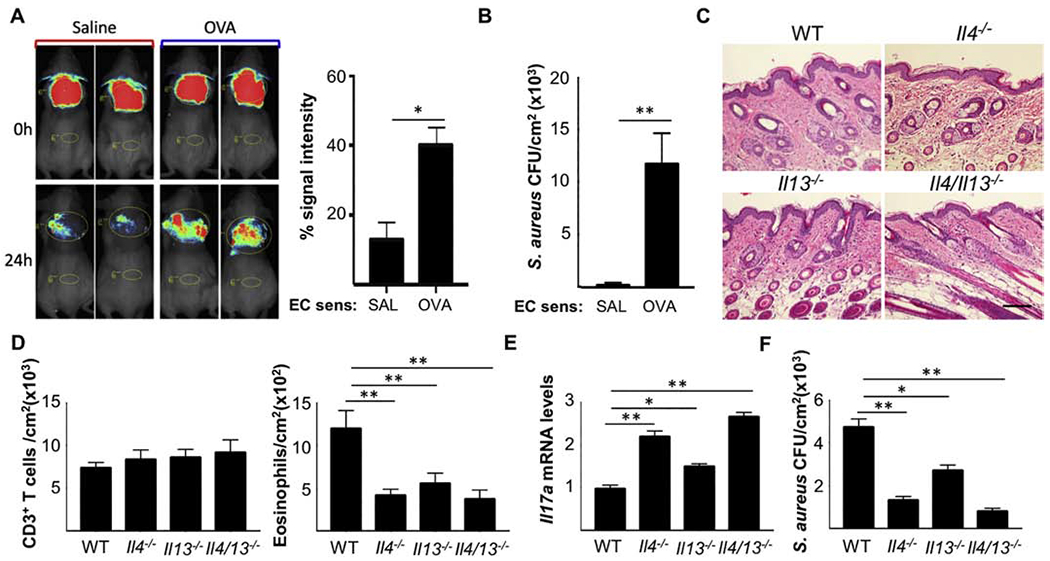
A. Representative in vivo imaging (left) and quantitation (right) of S. aureus fluorescence. B. S. aureus CFUs in skin homogenates. C. Representative H&E sections. Scale bar: 100 mm. D. Numbers of CD3+ T cells (left) and eosinophils (right) in skin. E. Skin Il17a mRNA levels F. S. aureus CFUs in the OVA sensitized skin of Il4−/−, Il13−/− and Il4/13−/− mice and WT controls. Results in B-F are representative of 2 independent experiments with 4-5 mice/group. Bars represent means±SEM. * = p<0.05, ** =p<0.005. Significance was calculated by using two-tailed Student’s t test.
Our results establish a mutually reinforcing role of allergic skin inflammation and S. aureus skin colonization. Furthermore, they provide a mechanistic explanation for the recent observation that clinical improvement and amelioration of Th2 biomarkers in AD patients following IL-4Rα blockade correlate with reduced abundance of S. aureus in the skin [14].
Highlights.
Topical application of S aureus exacerbated allergic skin inflammation.
Allergic skin inflammation generated a permissive environment for S. aureus persistence.
IL-4 and IL-13 impair S. aureus clearance from sites of allergic skin inflammation.
ACKNOWLEDGMENTS.
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIAID) Atopic Dermatitis Research Network grant U19AI117673 and NIAID T32 training grant 5T32AI007512-32.
ABBREVIATIONS
- AD
Atopic dermatitis
- EC
epicutaneous
- OVA
ovalbumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES.
- [1].Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD, Atopic dermatitis, Nat Rev Dis Primers, 4 (2018) 1. [DOI] [PubMed] [Google Scholar]
- [2].Simpson EL, Villarreal M, Jepson B, Rafaels N, David G, Hanifin J, Taylor P, Boguniewicz M, Yoshida T, De Benedetto A, Barnes KC, Leung DYM, Beck LA, Patients with Atopic Dermatitis Colonized with Staphylococcus aureus Have a Distinct Phenotype and Endotype, J Invest Dermatol, 138 (2018) 2224–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, Yi D, Zhao B, Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial, Br J Dermatol, 155 (2006) 680–687. [DOI] [PubMed] [Google Scholar]
- [4].Breuer K, S HA, Kapp A, Werfel T, Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis, Br J Dermatol, 147 (2002) 55–61. [DOI] [PubMed] [Google Scholar]
- [5].Leyva-Castillo JM, Galand C, Mashiko S, Bissonnette R, McGurk A, Ziegler SF, Dong C, McKenzie ANJ, Sarfati M, Geha RS, ILC2 activation by keratinocyte-derived IL-25 drives IL-13 production at sites of allergic skin inflammation, J Allergy Clin Immunol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS, Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice, J Clin Invest, 101 (1998) 1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN, Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses, J Exp Med, 189 (1999) 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN, Impaired development of Th2 cells in IL-13-deficient mice, Immunity, 9 (1998) 423–432. [DOI] [PubMed] [Google Scholar]
- [9].Kuhn R, Rajewsky K, Muller W, Generation and analysis of interleukin-4 deficient mice, Science, 254 (1991) 707–710. [DOI] [PubMed] [Google Scholar]
- [10].He R, Oyoshi MK, Jin H, Geha RS, Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge, Proc Natl Acad Sci U S A, 104 (2007) 15817–15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leung DY, Guttman-Yassky E, Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches, J Allergy Clin Immunol, 134 (2014) 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS, IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice, J Clin Invest, 120 (2010) 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, Hata TR, Gallo RL, Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression, J Invest Dermatol, 136 (2016) 2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P, Ardeleanu M, Hultsch T, Guttman-Yassky E, Bissonnette R, Silverberg JI, Krueger J, Menter A, Graham NMH, Pirozzi G, Hamilton JD, Gallo RL, IL-4Ralpha Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis, J Invest Dermatol, 140 (2020) 191–202 e197. [DOI] [PMC free article] [PubMed] [Google Scholar]


