Abstract
Trait heritability is necessary for evolution by both natural and artificial selection, yet we know little about the heritability of cognitive traits. Domestic dogs are a valuable study system for questions regarding the evolution of phenotypic diversity due to their extraordinary intraspecific variation. While previous studies have investigated morphological and behavioral variation across dog breeds, few studies have systematically assessed breed differences in cognition. We integrated data from Dognition.com—a citizen science project on dog cognition—with breed-averaged genetic data from published sources to estimate the among-breed heritability of cognitive traits using mixed models. The resulting dataset included 11 cognitive measures for 1508 adult dogs across 36 breeds. A factor analysis yielded four factors interpreted as reflecting inhibitory control, communication, memory, and physical reasoning. Narrow-sense among-breed heritability estimates—reflecting the proportion of cognitive variance attributable to additive genetic variation—revealed that scores on the inhibitory control and communication factors were highly heritable (inhibitory control: h2 = 0.70; communication: h2 = 0.39), while memory and physical reasoning were less heritable (memory: h2 = 0.17; physical reasoning: h2 = 0.21). Although the heritability of inhibitory control is partially explained by body weight, controlling for breed-average weight still yields a high heritability estimate (h2 = 0.50), while other factors are minimally affected. Our results indicate that cognitive phenotypes in dogs covary with breed relatedness and suggest that cognitive traits have strong potential to undergo selection. The highest heritabilities were observed for inhibitory control and communication, both of which are hypothesized to have been altered by domestication.
Keywords: Cognitive evolution, Breed differences, Test battery, Citizen science, Domestication, Canine cognition
Introduction
In order for cognition to evolve by natural or artificial selection, cognitive processes must vary between individuals, these differences must affect reproductive fitness, and the variation must be heritable (Darwin 1859). Addressing the first criterion, many studies have documented variation in cognitive traits both across (Herrmann et al. 2007; Rosati et al. 2014; MacLean et al. 2014, 2017) and within species (Dukas 2004). A smaller set of studies, mostly in birds, have also linked certain cognitive abilities to reproductive fitness (Keagy et al. 2009; Boogert et al. 2011; Cole et al. 2012; Sonnenberg et al. 2019). Relatively few studies, however, have investigated the heritability of cognitive traits, and the majority of these studies have focused on a small set of cognitive measures (Drent et al. 2003; Persson et al. 2015). Thus, despite its theoretical importance, we still know relatively little about this important criterion for cognitive evolution.
The extreme variation amongst dog breeds presents a unique opportunity to study phenotypic evolution. Studies of dogs have already contributed to our understanding of many morphological and disease phenotypes such as pigmentation, dwarfism, and cancer (Sutter et al. 2007; Karlsson et al. 2007; Hayward et al. 2016a). Surprisingly, although both the history of dog breeding (American Kennel Club 1938) and the intuitions of people working and living with purebred dogs suggest the existence of breed differences in cognitive and behavioral traits (Hart and Hart 1985; Hart and Miller 1985), there have been relatively few empirical studies on these topics. In part, the study of breed differences has posed challenges due to high levels of intra-breed variation, small sample sizes in experimental studies, and limited breed coverage (Pongrácz et al. 2005; Dorey et al. 2009; Mehrkam and Wynne 2014; Arden et al. 2016). Nonetheless, recent studies using large samples have discovered robust and highly heritable breed differences in behavior (Saetre et al. 2006; MacLean et al. 2019).
Less work has been done on cognitive traits, most likely because cognitive phenotypes are best measured through experiments, rather than the observational methods typically employed in behavioral studies (Tomasello and Call 2008, 2011). Few studies have systematically assessed breed differences in cognition, and the most common approach has involved a limited set of pairwise comparisons among a few breeds. Early work by Scott and Fuller (1965) on dog behavior and cognition revealed some breed differences, including that Basenjis were superior to four other breeds at solving a physical manipulation problem. More recently, Wobber et al. (2009) found that working dog breeds (shepherds and huskies) were better and more flexible at using social cues, such as pointing, than non-working breeds (toy poodles and Basenjis). Using three breeds, each representing hunting, herding, or guarding dogs, Udell et al. (2014) also found breed differences on a pointing social cue task; however, the worst-performing breed showed considerable improvement with more experience, indicating a role for non-genetic factors such as learning. In a human-oriented gaze task, Jakovcevic et al. (2010) found that Labrador and golden retrievers gazed towards human faces more than German shepherds or poodles.
A few studies have aggregated data across breeds to enable comparisons at the breed-group level, based on genetically defined breed groups (Parker et al. 2004; VonHoldt et al. 2010). Using 11 breeds that represented herding, mastiff, working, and retriever groups, with individuals in each group that were both highly trained and untrained, Marshall-Pescini et al. (2016) found that herding dogs were most likely to look at a person when interacting with a puzzle box. However, training was found to be an important explanatory variable on a wider variety of measures and across multiple tasks, again indicating the importance of experience. Konno et al. (2016) tested 26 breeds representing five groups and did not find breed group differences on Jakovcevic’s et al. (2010) visual contact task, but did for an unsolvable task (Miklósi et al. 2003), with ancient breeds taking longer to look at the human and looking for shorter durations than herding, hound, retriever-mastiff, and working breed groups. Heberlein et al. (2017) also reported breed-group differences in dogs’ sensitivity to their owner’s perception in a task involving taking food from behind transparent or opaque barriers; ancient and hunting dogs preferentially retrieved food from behind the opaque barrier, while shepherds and mastiffs showed a statistically insignificant difference between the conditions.
While all of these studies demonstrated some form of breed difference, the results are not entirely consistent in terms of which tasks revealed differences or how certain breeds and breed-groups performed. Furthermore, other studies have found an absence of statistically significant breed-group differences for both pointing (Dorey et al. 2009) and object permanence (Pongrácz et al. 2005), although the latter used functionally rather than genetically defined breed groupings. While certain tasks may not exhibit breed differences, it is also possible that null results are due to small sample sizes, focus on a small number of common breeds, and a high level of intra-breed variation. Arden et al. (2016) note that most studies on dog cognition have been underpowered and emphasize the importance of studying individual differences. An alternative to pairwise comparisons between breeds or breed groups—and one that benefits, rather than suffers from relatively high levels of individual variation—is to examine heritability, the extent to which genetic relatedness within a population explains trait variation. This method also enables the use of data from a large number of breeds, shifting the emphasis from pairwise tests of mean differences to phenotypic and genetic covariance.
Most studies of cognitive heritability have been conducted in humans, in which cognitive traits are often highly heritable (Deary et al. 2009; Wilmer et al. 2010; Davies et al. 2011); one meta-analysis of twin studies found that 1507 cognitive traits, measured across 292 studies, displayed an average narrow-sense heritability of 0.47 (Polderman et al. 2015), indicating that approximately half of the observed cognitive variation was attributable to additive genetic factors. It should be noted, however, that non-independence of genetic and environmental variables in humans may inflate these estimates (Visscher et al. 2008). Less is known about the heritability of cognitive traits in other animals (reviewed in Croston et al. 2015).
Although the majority of cognitive experiments focus on a single task or ability, a set of complementary tests—such as the Primate (Herrmann et al. 2007) or Dog Cognition Test Batteries (MacLean et al. 2017)—has multiple advantages (Shaw and Schmelz 2017). First, it assesses cognitive abilities in a range of different contexts, including tasks that presumably rely on different neural structures and networks (Yeo et al. 2011); second, since most tasks rely on more than one cognitive process, the use of multiple measures may provide a more reliable indicator of underlying cognitive abilities (Thornton and Lukas 2012; Olsen 2018); third, a test battery enables the exploration of patterns of individual differences across tasks, allowing inferences about latent cognitive variables (Herrmann et al. 2010). Although this test battery approach has been less common, a recent comparative study of individual differences in cognition across chimpanzees, human children, and domestic dogs revealed a common factor related to communicative processes in domestic dogs and human children, but not in chimpanzees (MacLean et al. 2017). Together with other studies that have been interpreted as evidence for evolutionary convergence between humans and dogs (Hare et al. 2002, 2010; Hare and Tomasello 2005; Cieri et al. 2014; Hare 2017; although see Udell et al. 2010; Range and Viranyi 2015; Wynne 2016 for contrasting interpretations), these findings suggest that in addition to addressing fundamental questions about cognitive evolution, understanding the evolution of cognition in dogs may also yield important insights regarding human cognitive evolution.
This study therefore examined individual differences in a large sample of dogs and breeds across a battery of cognitive tasks, using citizen science data from Dognition.com that has been validated by laboratory testing (Stewart et al. 2015). These individual differences were characterized using factor analysis to identify latent variables underlying cognitive variation, and the resultant factors were combined with breed-average genetic data to estimate the narrow-sense heritability of cognitive traits across dog breeds.
Methods
Data
The cognitive data were collected through Dognition.com, a citizen science website that guides owners through a series of experiments that they can complete at home with their own dogs. Previous analyses with these data have replicated findings from similar protocols implemented by researchers in traditional laboratory settings, supporting the validity of this citizen science approach (Stewart et al. 2015). We used data from nine of the ten core Dognition tasks, excluding the contagious yawning task, which as a binary outcome is not well suited to factor analysis; the 11 measures from these tasks are summarized in Table 1. The task measuring time to eat a forbidden treat when a person was or was not watching was originally designed to assess a dog’s sensitivity to cues about a person’s visual perspective, indexed via differences in latencies between conditions (termed ‘cunning’ in the original publication describing this battery Stewart et al. 2015). However, all conditions in this task also involve delaying gratification by inhibiting the consumption of an accessible food reward, which may be assessed by the latency to consume the forbidden food (Horschler et al. 2019; Watowich et al. 2020). Here we analyzed the latencies because we were particularly interested in inhibitory control—an aspect of cognition that is highly heritable in humans—but the alternative approach, which produces a factor interpreted as “cunning” or gaze-sensitivity, is included in the supplementary information (Figure S2).
Table 1.
Summary of measures used from Dognition
| Task | Description | Trials | Dependent measure |
|---|---|---|---|
| Eye contact | The participant holds food near their face and records how long the dog makes eye contact without looking away for more than 2 s | 3 | Mean latency until eye contact broken, up to 90 s |
| Arm pointing | The participant places two treats on the ground—one to the left and one to the right—and gestures towards one of them using an extended arm and index finger | 6 | Proportion of first approaches to the indicated treat |
| Foot pointing | Identical to arm pointing, except the method of indication is the participant’s foot | 6 | Proportion of first approaches to the indicated treat |
| Inhibitory control: watching mean | The participant places a treat on the floor in front of dog, verbally forbids the dog from taking it, and watches their dog | 2 | Mean latency to eat the treat, up to 90 s |
| Inhibitory control: back turned | Identical to the watching condition, but the participant turns their back to the dog after placing the food on the ground | 2 | Mean latency to eat the treat, up to 90 s |
| Inhibitory control: eyes covered | Identical to the watching condition, but the participant covers their eyes after placing the food on the ground | 2 | Mean latency to eat the treat, up to 90 s |
| Memory vs. pointing | The participant visibly places food under one of two cups and points with their arm and index finger to the other cup | 6 | Proportion of first approaches to the visible (remembered) cup |
| Memory vs. smell | The participant visibly places food under one of two cups, but then blocks the dogs view and moves the food to under the other cup | 4 | Proportion of first approaches to the visible (remembered) cup |
| Delayed memory | The participant visibly places food under one of two cups but waits for a delay (60, 90, 120, 180 s) before the dog is allowed to approach the cups | 1 each 4 total | Proportion of first approaches to the correct (remembered) cup |
| Inferential reasoning | Out of view of the dog, the participant hides food under one of two cups. The participant then lifts the empty cup, revealing that there is no food underneath | 4 | Proportion of first approaches to the correct (not shown) cup |
| Physical reasoning | The owner places food under one of two folded sheets of paper, such that the food props up the one piece of paper, while the other lies flat on the ground | 4 | Proportion of first approaches to the correct (displaced) side |
For more information on the battery, including the order of trials and familiarizations, see Stewart et al. (2015). Eye contact was ultimately excluded from the analyses, as it did not load significantly on any factor
Genetic data were obtained from a publicly available data set (Parker et al. 2017) that combined newly analysed data with previously published data (Vaysse et al. 2011; Hayward et al. 2016a, b), all of which was collected using the Illumina CanineHD bead array, supplemented with three publicly available genome sequences. The full dataset includes 150,067 single-nucleotide polymorphisms (SNPs) from 1346 dogs representing 161 breeds.
Since puppies are still developing, and may not yet have reached stable cognitive phenotypes (Davidson et al. 2006; Watowich et al. 2020), we restricted analyses to data obtained from dogs that were at least 1-year of age at the time of testing. Due to our use of breed-average genetic data, we also restricted our analyses to purebred dogs (owner report). Because factor analysis does not allow for missing data, we limited our analyses to individuals who had completed the entire set of Dognition tasks. Factors were calculated with 2044 individuals representing 172 breeds (Tables S1 and S2). Subsequent analyses were further limited to breeds represented in the genetic data. Lastly, to ensure representative samples, we restricted heritability analyses to breeds including at least 15 individuals in the cognitive dataset (see supplementary information for sensitivity analyses using other thresholds as inclusionary criteria, Figures S3 and S4). Our final dataset included 1508 individuals representing 36 breeds (see Table S1).
Factor analysis
We performed exploratory factor analysis using the psych package (Revelle 2018) in R version 3.5.2 (R Core Team 2018). Although Stewart et al. (Stewart et al. 2015) conducted initial exploratory factor analyses on the first 522 dogs to participate in Dognition, our current sample size is approximately four times larger, we restricted our analysis to purebred dogs only due to the subsequent genetic analyses, and we used latencies rather than differences scores for the cunning measures (see Data section above); an exploratory factor analysis was therefore deemed the most appropriate method. In our initial analyses, the eye contact measure was found to load poorly across factors and so was dropped from further analyses. Bartlett’s test of sphericity (X2 (45) = 5702, p < 10−200) indicated that there were sufficient correlations between variables to make factor analysis appropriate. Sampling adequacy for all traits was met, with an overall Kaiser–Meyer–Olkin index of 0.76 and all individual indices ≥ 0.5. Four factors were extracted, based on a parallel analysis of simulated and resampled data (Figure S1). As most of the underlying variables were not normally distributed, the minimizing residuals method (minres) was used (Harman and Jones 1966). To accommodate potential correlation between cognitive factors, we used an oblique rotation (oblimin). Based on our sample size, we interpreted factor loadings greater than 0.20 as a practical significance threshold for identifying variables contributing saliently to a factor (Stevens 2002).
Genetic relatedness
An identity-by-state (IBS) matrix, representing the proportion of SNPs shared by each pair of individuals, was calculated using PLINK (Purcell and Chang 2018; Purcell et al. 2015). These values were then averaged for every pair of breeds to generate a breed-average IBS matrix. This breed-level IBS matrix was extrapolated to an individual-level IBS matrix by assuming breed-average similarity between each pair of individuals: for individuals of different breeds, the IBS value was set to the average similarity between those two breeds; for individuals of the same breed, the average similarity of individuals within that breed was used (see SI).
Heritability
Narrow-sense heritability—the proportion of phenotypic variation explained by additive genetic effects—was estimated by partitioning the cognitive variance between genetic and residual effects with linear mixed models using the ‘animal model’ (Wilson et al. 2010), which includes a random effect for relatedness (see SI). We employed Efficient Mixed-Model Analysis (EMMA) (Kang et al. 2008; Zhou and Stephens 2012), which uses restricted maximum likelihood estimation of the variance components, as implemented in the NAM (Xavier et al. 2015) and EMMREML (Akdemir and Godfrey 2015) packages with R version 3.5.1 (R Core Team 2018). To incorporate all individual-level cognitive data without biasing the estimates towards the most common breeds (see Table S1), we used a resampling approach. Across 1000 iterations, we extracted a random sample of 15 individuals per breed (without replacement) and calculated heritability estimates for each factor. We report the mean heritability estimate across iterations. For null hypothesis testing, we used a permutation test in which the correspondence between cognitive and genetic data was randomly permuted prior to calculating heritability. Paired two-sided t tests were conducted on the pairs of real and permuted heritability estimates (each of which used the same sample of individuals) to produce the reported p-values. The null distributions of heritability estimates are shown in Figure S5.
Controlling for breed-average weight
To control for potential effects of body and brain mass, which have been found to correlate with behavior and cognition in dogs (McGreevy et al. 2013; Horschler et al. 2019) and other species (Kotrschal et al. 2013; Benson-Amram et al. 2016), we ran additional models controlling for breed-average weight as a fixed effect; breed-averages were calculated from reported body weights on the Canine Behavioral Assessment and Research Questionnaire (C-BARQ) (Hsu and Serpell 2003; McGreevy et al. 2013; Horschler et al. 2019).
Controlling for training history
Since training is often hypothesized to affect performance on cognitive tasks, we also performed a sensitivity analysis investigating how controlling for training history affected heritability estimates. This analysis was limited to the subset of dogs for whom training history data were available (n = 489) and is detailed in the supplemental information.
Results
Exploratory factor analysis reveals four latent variables
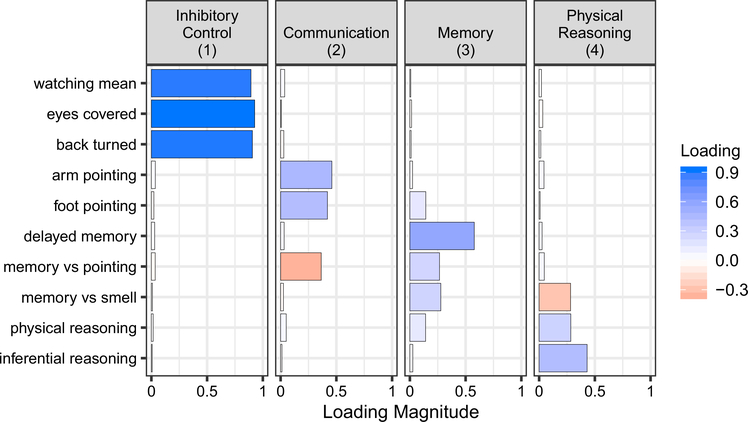
A four-factor model described the cognitive data well (Tucker Lewis Index = 0.998; RMSEA = 0.012) and explained 39% of the common variance across measures. The first factor explained 25% of the common variance; it was positively loaded by all three measures in the inhibitory control task (see Table 1), indicating that higher scores on factor one reflected an increased tendency to wait before taking the prohibited food, across experimental conditions. We therefore interpreted this factor as reflecting inhibitory control, albeit in a social context. The second factor explained 5% of the common variance and was positively loaded by both gesture-following tasks (arm and foot pointing) and negatively loaded by the memory vs. pointing task. Dogs with higher scores on the second factor were thus more likely to follow human gestures, both when these gestures were presented as the only cue and also when pitted against a dog’s own memory. Based on the combination of loadings, we interpreted this factor as reflecting individual differences in sensitivity to human communication. The third factor explained 5% of the common variance and was loaded by all three memory-related tasks (memory vs. pointing, memory vs. smell, delayed memory); we therefore interpreted this factor as reflecting individual differences in memory. The fourth factor explained 4% of the common variance and was loaded positively by the physical and inferential reasoning tasks, as well as negatively by the memory vs. smell task. Since the inferential reasoning task requires dogs to make inferences based on physical properties of the world, and a negative loading of memory vs. smell implies reliance on a sensory cue over a subject’s own memory, we interpreted this factor as reflecting physical reasoning. Inter-factor correlations were generally low, except for between factors one and three (r = 0.28; see Table S3) (Fig. 1).
Fig. 1.
Factor loadings from the factor model performed using the minimizing residuals method and an oblimin rotation. The first factor is loaded by measures related to inhibitory control, the second by tasks involving communication, the third by memory-related tasks, and the fourth by tasks requiring physical reasoning. The first factor explained 25% of the common variance, the second and third factors each explained 5%, and the fourth factor explained 4%
Breed differences in social inhibitory control and communication are highly heritable
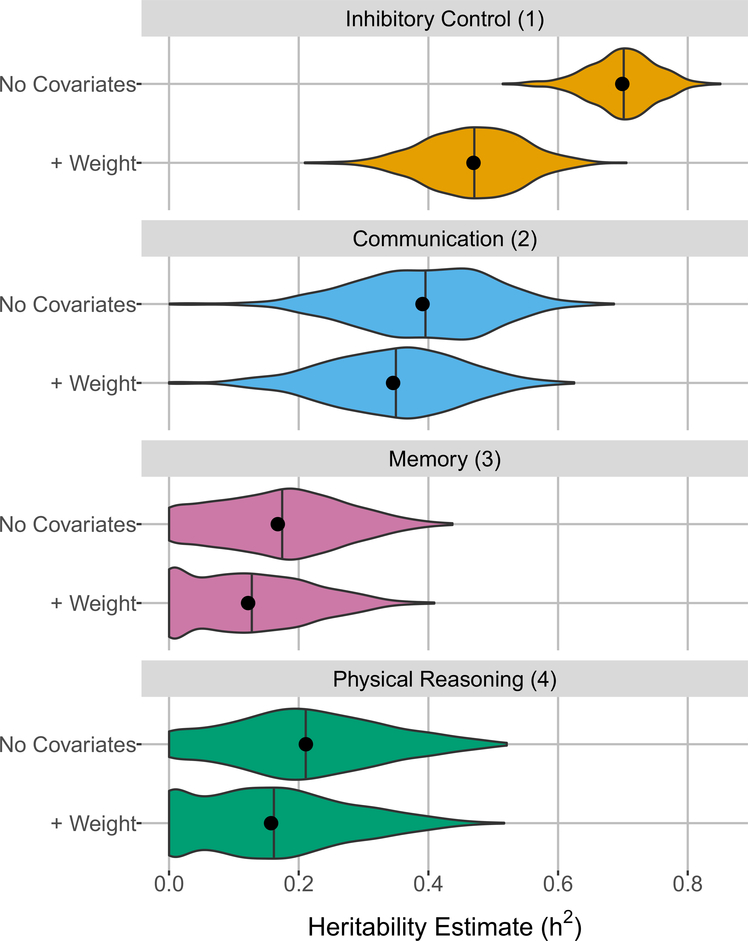
Heritability analysis revealed that scores on the inhibitory control factor were highly heritable (h2 = 0.70, p < 10−300), as were scores on the communication factor (h2 = 0.39, p = 10−300). Scores on the memory factor had a lower heritability estimate (h2 = 0.17, p = 6.42 × 10−150), while scores on the physical reasoning factor were only marginally more heritable (h2 = 0.21, p = 1.35 × 10−65) (Fig. 2). All heritability scores were significantly higher than those generated by random permutation of the data.
Fig. 2.
Distribution of narrow-sense heritability estimates for each factor, both without covariates (“No Covariates”) and controlling for breed-average weight as a fixed effect (“ + Weight”). Each model was run with resampled cognitive data across 1000 iterations, using 15 individuals per breed at each iteration. The black points represent the mean and the vertical black lines represent the median heritability estimate over these 1000 runs. Scores on the inhibitory control factor have the highest heritability, followed by scores on the communication factor. Scores on the memory and physical reasoning factors had lower heritability estimates. Controlling for breed-average weight reduced the heritability estimates across all factors, but the effect was minimal except for the inhibitory control factor
Heritability estimates controlling for weight and training history
Given the demonstrated associations between body weight and both behavior (McGreevy et al. 2013) and cognition (Horschler et al. 2019) in dogs, we performed the same analyses while controlling for breed-average weight. Although the heritabilities for all factors were reduced after controlling for weight, this reduction was generally minimal, with the exception of the inhibitory control factor (Fig. 2). Despite a decrease in the magnitude of heritability estimates, the overall pattern of results remained consistent, with inhibitory control being the most heritable (h2 = 0.47, p < 10−300), followed by communication (h2 = 0.35, p < 10−300). The heritabilities for memory (h2 = 0.12, p = 2.18 × 10−56) and physical reasoning (h2 = 0.16 p = 1.36 × 10−114) remained small but statistically significant in these analyses.
Our sensitivity analysis of the role of training history (for the subset of dogs for whom this information was available) revealed that while controlling for training history did decrease heritability estimates across all factors (h2Inhibitory 0.45, h2Communication = 0.41, h2Memory = 0.01, h2Reasoning = 0.16), the effect was generally less than that of controlling for weight, and the overall pattern of results remained consistent (Figure S6). In particular, the inhibitory control and communicative factors both remain relatively highly heritable (h2 > 0.3) when controlling for both weight and training history in the same statistical model.
Discussion
Using factor analysis, we quantified dimensions of individual differences in dogs’ cognitive abilities and found four underlying cognitive constructs that we interpreted as inhibitory control, communication, memory, and physical reasoning. Interestingly, the highest inter-factor correlation was between the inhibitory control and memory factors; inhibitory control and working memory are both components of executive function, and given the short delays used in Dognition, our memory factor is likely to be most reflective of working memory. Narrow-sense heritability estimates further revealed variable heritability amongst traits, with the highest heritability for inhibitory control, followed by communication. Although we interpret the first factor—loaded by the latencies to eat a forbidden treat across conditions—as inhibitory control, it is possible that these measures also reflect training or obedience, which may vary systematically by breed. Controlling for training history in the subset of individuals for whom training history was known reduced these heritability estimates somewhat but did not considerably alter the overall pattern of results (Figure S6). It should also be noted that inhibitory control is increasingly recognized to be a complex construct (Olsen 2018) that is context-specific (Bray et al. 2014); thus, this factor—which represents multiple conditions, but only one paradigm, in a social context involving forbidden food—is likely an incomplete measure of inhibitory control that should be supplemented by additional tasks in future work.
Our results are consistent with the first article published with Dognition data, which also conducted a factor analysis on the first 522 dogs in this dataset, including both pure and mixed breed dogs; Stewart et al. also found four factors, with a similar pattern of loadings (Stewart et al. 2015). In addition, MacLean’s et al. (2017) Dog Cognition Test Battery—which involved a partially overlapping set of 15 tasks conducted on pet, assistance, and detection dogs—revealed six factors, including factors for inhibitory control, communication, and memory; other factors involved time looking to human faces, affect and visual discrimination, and sensory bias and retrieval. The concordance of our findings with other factor analyses using different individuals, breeds, and methods highlights the utility of cognitive test batteries in providing composite measures for cognitive traits and corroborates the main findings from earlier studies.
In contrast to human studies (Davies et al. 2011), our results do not support a single highly heritable general intelligence factor in dogs. This difference may reflect a limitation in human intelligence tests, which typically do not include direct measures of social cognition or inhibitory control (Carpenter et al. 1990; Deary et al. 2010). This is particularly problematic given that multiple hypotheses of human evolution emphasize the importance of social cognition and inhibitory control in human cognitive evolution (Leach 2003; Herrmann et al. 2007; Moll and Tomasello 2007; Cieri et al. 2014; MacLean 2016; Hare 2017). Claims of a general learning ability in mice also tend to rely heavily on physical or spatial tasks and lack social measures entirely (Matzel et al. 2003; Galsworthy et al. 2005). Evidence for general intelligence in non-human primates is mixed (Herrmann et al. 2007; Banerjee et al. 2009; Hopkins et al. 2014); interestingly, Hopkins et al. (2014) found evidence in chimpanzees for both heritable general intelligence and heritable social cognition using the Primate Cognition Test Battery. Although one study reports evidence for general intelligence in dogs (Arden and Adams 2016), there are several reasons to be cautious about this interpretation: first, only three tasks were administered and only one was social; second, they used confirmatory factor analysis (with pre-specified factor structures) that mixed social and physical tasks; third, only 68 individuals of a single breed were tested; and fourth, the purported g-factor only explained 17% of the variance in performance. In contrast, we found that measures related to inhibitory control and communication loaded on separate factors, each of which was highly heritable, in addition to less-heritable factors for memory and physical reasoning.
Similar to previous studies of cognitive heritability in other species (Croston et al. 2015), we found heritable variation in cognitive traits amongst dog breeds. Since heritability is necessary for selection to operate, estimated trait heritability is one indication of how much scope there is for selection on a given trait. Our results suggest that all of our cognitive factors may have sufficient heritability to be selectable, while the inhibitory control and communication factors are most likely to have the scope for considerable and rapid response to either natural or artificial selection.
Horschler et al. (2019) recently documented breed differences in the Dognition dataset that were associated with brain size, as estimated from body weight, particularly in the tasks that contribute to the inhibitory control factor. Consistent with their findings, the heritability of the inhibitory control factor is partially dependent on breed-average body weight, with a 23% reduction in the proportion of cognitive variance accounted for by genetic factors after controlling for weight; however, even after controlling for weight, inhibitory control remained the most heritable factor. This robust heritability of inhibitory control in dogs is consistent with studies of self-control in humans; a meta-analysis of twin-studies found that self-control measures were 60% heritable (Willems et al. 2019), although it should be noted that the Dognition measures of inhibitory control are in a social context, and performance may depend in part on training. Our results are also consistent with a broad phylogenetic study of self-control, which found not only that absolute brain size was a major predictor of self-control task performance, but also that scores were similar in closely related species (i.e., phylogenetic signal), suggesting a strong biological basis for variation in these processes (MacLean et al. 2014). However, our results are also consistent with work in hyaenas showing that inhibitory control is developmentally plastic (Johnson-Ulrich and Holekamp 2020), as a considerable proportion of the variance in this trait is not explained by breed-average genetics and may instead be explained in part by environmental factors. It should also be noted that the finding that the inhibitory control factor was more heritable than the other factors may be due to the large amount of phenotypic variation observed in these measures.
There are indications across taxa that brain size correlates with certain cognitive abilities, including problem solving in carnivores (Benson-Amram et al. 2016), numerical learning in guppies (Kotrschal et al. 2013), and self-control in birds and mammals (MacLean et al. 2014). While it therefore seems quite plausible that observed effects of body size could be attributable to the correlation with brain weight (Bronson 1979; Carreira 2016; Horschler and MacLean 2019), perhaps due to an increased number of neurons in larger brains within a given lineage (Herculano-Houzel 2017; Jardim-Messeder et al. 2017), there are several alternative explanations for these effects. In particular, body size may be confounded with training or other behavioral traits that may partially explain associations with body mass. For example, McGreevy et al. (2013) found that a range of undesirable behaviors are negatively associated with body-size in dogs, and suggested a variety of explanations, including relaxed selection in small dogs, increased selection in large dogs, genetically-based neurological or physiological differences, and human-mediated differences in training and the environment.
Our findings are of particular interest in the context of dog-wolf differences, as previous studies suggest that communication, inhibitory control, and physical reasoning are cognitive domains in which dog and wolf cognition may differ. With respect to communication, many studies suggest that dogs are biologically prepared (Cummins and Cummins 1999) to communicate with humans cooperatively and flexibly in ways that wolves are not (Hare et al. 2002, 2010; Miklósi et al. 2003; Riedel et al. 2008; Virányi et al. 2008). In contrast, a growing body of evidence suggests that wolves outperform dogs on a variety of tasks related to reasoning about causal properties of the physical world (Frank and Frank 1982; Hiestand 2011; Range et al. 2014; Lampe et al. 2017). Lastly, Marshall-Pescini et al. (2015) found that two different tests of inhibitory control in dogs and wolves demonstrated divergent effects across tasks—with dogs outperforming wolves on the cylinder task, but wolves outperforming dogs on a fence-detour task. It is therefore particularly interesting that we see the highest heritability estimates for the inhibitory control and communication factors, with some heritability for the physical reasoning factor, as these variables align with cognitive systems that are hypothesized to have undergone important changes during domestication. Our findings suggest that a substantial fraction of variability in these cognitive processes can be attributed to genetic factors, making it likely that they were affected by the domestication process, whether by direct selection, as a by-product of other selective pressures, or through the relaxation of selection in domestic dogs. It should be noted that heritability can change over time, especially as a response to selection, which can alter both phenotypic and genotypic variation (Visscher et al. 2008). If the heritable variation in extant dog populations was also present in ancestral wolf populations, selection could have acted on this variation during domestication. However, it is also possible that cognitive variance has increased much more recently in dog evolution, perhaps in association with the proliferation of modern breeds, many of which were selected for functional roles.
The use of citizen science data presents both strengths and limitations associated with the current work. On the one hand, citizen science enabled the collection of the vast quantities of data necessary for comparisons across breeds at this scale. On the other hand, lacking paired cognitive and genetic data on the same individuals necessitated a breed-average approach for the incorporation of genetic data, relying on owner-reported breed status. Thus, future work will benefit from paired cognitive and genetic data from the same subjects, which will not only improve our ability to estimate heritability but also to fine-map genetic loci associated with variance in cognitive phenotypes. Given that highly heritable traits present powerful opportunities for genome-wide association studies (Visscher et al. 2008), we expect that cognitive traits related to inhibitory control and communication present good candidates for future genetic research.
Supplementary Material
Acknowledgements
We thank David Ivy, Eliot Cohen, Kip Frey, and everyone else who helped create Dognition.com, as well as the members of the advisory board: Josep Call, Juliane Kaminski, Ádám Miklósi, Laurie R. Santos, and Richard Wrangham. We thank Daniel J. Horschler for discussions of the Dognition data and sharing already-tabulated breed-average body weight data, as well as Stacey R. Tecot, Ivy L. Pike, and two anonymous reviewers for comments on previous versions of this manuscript. Lastly, we thank all the dogs and people who participated in Dognition and made this work possible. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. (DGE-1746060). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Funding G.E.G. was funded by the University of Arizona’s University Fellows Program and the NSF Graduate Research Fellowship Program (DGE-1746060). B.H. is supported in part by the National Institute of Health (Grant 1R01HD097732-01).
Footnotes
Compliance with ethical standards
Conflict of interest BH is a founder of Dognition.com and a member of its Scientific Advisory Board. The authors declare no other competing interests.
Ethical approval All animals included in this study were pet dogs tested by citizen scientists in their own homes. The use of third-party data from Dognition.com was approved by Duke University IACUC protocol A138-11-06 and data were collected in accordance with relevant guidelines and regulations.
Data and code Genetic data used in these analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.266k4 (Hayward et al. 2016b) and GEO accession nos. GSE90441, GSE83160, GSE70454 and GSE96736. A subset of the Dognition data and the code for our linear mixed models are available at https://github.com/GGnanadesikan/dognition_heritability/. The remaining Dognition data used in these analyses are available from Brian Hare at b.hare@duke.edu.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10071-020-01400-4) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akdemir D, Godfrey OU (2015) EMMREML: fitting mixed models with known covariance structures. https://cran.r-project.org/package=EMMREML
- American Kennel Club (1938) The complete dog book. Halcyon House, New York [Google Scholar]
- Arden R, Adams MJ (2016) A general intelligence factor in dogs. Intelligence 55:79–85. 10.1016/j.intell.2016.01.008 [DOI] [Google Scholar]
- Arden R, Bensky MK, Adams MJ (2016) A review of cognitive abilities in dogs, 1911 through 2016: more individual differences, please! Curr Dir Psychol Sci 25:307–312. 10.1177/0963721416667718 [DOI] [Google Scholar]
- Banerjee K, Chabris CF, Johnson VE et al. (2009) General intelligence in another primate: individual differences across cognitive task performance in a new world monkey (Saguinus oedipus). PLoS ONE 4:e5883 10.1371/journal.pone.0005883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson-Amram S, Dantzer B, Stricker G et al. (2016) Brain size predicts problem-solving ability in mammalian carnivores. Proc Natl Acad Sci 113:2532–2537. 10.1073/pnas.1505913113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogert NJ, Anderson RC, Peters S et al. (2011) Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim Behav 81:1209–1216. 10.1016/j.anbehav.2011.03.004 [DOI] [Google Scholar]
- Bray EE, MacLean EL, Hare BA (2014) Context specificity of inhibitory control in dogs. Anim Cogn 17:15–31. 10.1007/s10071-013-0633-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson RT (1979) Brain weight-body weight scaling in breeds of dogs and cats. Brain Behav Evol 16:227–236. 10.1159/000121839 [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Shell P (1990) What one intelligence test measures: a theoretical account of the processing in the Raven progressive matrices test. Psychol Rev 97:404–431. 10.1037/0033-295X.97.3.404 [DOI] [PubMed] [Google Scholar]
- Carreira LM (2016) Using Bronson equation to accurately predict the dog brain weight based on body weight parameter. Vet Sci 3:25–27. 10.3390/vetsci3040036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieri RL, Churchill SE, Franciscus RG et al. (2014) Craniofacial feminization, social tolerance, and the origins of behavioral modernity. Curr Anthropol 55:419–443. 10.1086/677209 [DOI] [Google Scholar]
- Cole EF, Morand-Ferron J, Hinks AE, Quinn JL (2012) Cognitive ability influences reproductive life history variation in the wild. Curr Biol 22:1808–1812. 10.1016/j.cub.2012.07.051 [DOI] [PubMed] [Google Scholar]
- Croston R, Branch CL, Kozlovsky DY et al. (2015) Heritability and the evolution of cognitive traits. Behav Ecol 26:1447–1459. 10.1093/beheco/arv088 [DOI] [Google Scholar]
- Cummins DD, Cummins R (1999) Biological preparedness and evolutionary explanation. Cognition 73:37–53 [DOI] [PubMed] [Google Scholar]
- Darwin C (1859) On the origin of species by means of natural selection, or preservation of favoured races in the struggle for life. John Murray, London: [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A (2006) Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 44:2037–2078. 10.1016/j.neuropsychologia.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Tenesa A, Payton A et al. (2011) Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol Psychiatry 16:996–1005. 10.1038/mp.2011.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Johnson W, Houlihan LM (2009) Genetic foundations of human intelligence. Hum Genet 126:215–232. 10.1007/s00439-009-0655-4 [DOI] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W (2010) The neuroscience of human intelligence differences. Nat Rev Neurosci 11:201–211. 10.1038/nrn2793 [DOI] [PubMed] [Google Scholar]
- Dorey NR, Udell MAR, Wynne CDL (2009) Breed differences in dogs sensitivity to human points: a meta-analysis. Behav Processes 81:409–415. 10.1016/j.beproc.2009.03.011 [DOI] [PubMed] [Google Scholar]
- Drent PJ, Van Oers K, Van Noordwijk AJ (2003) Realized heritability of personalities in the great tit (Parus major). Proc R Soc B Biol Sci 270:45–51. 10.1098/rspb.2002.2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukas R (2004) Evolutionary biology of animal cognition. Annu Rev Ecol Evol Syst 35:347–374. 10.1146/annurev.ecolsys.35.112202.130152 [DOI] [Google Scholar]
- Frank H, Frank MG (1982) Comparison of problem-solving performance in six-week-old wolves and dogs. Anim Behav 30:95–98. 10.1016/S0003-3472(82)80241-8 [DOI] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Liu L et al. (2005) Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav Genet 35:675–692. 10.1007/s10519-005-3423-9 [DOI] [PubMed] [Google Scholar]
- Hare B (2017) Survival of the friendliest: homo sapiens evolved via selection for prosociality. Annu Rev Psychol 68:155–186. 10.1146/annurev-psych-010416-044201 [DOI] [PubMed] [Google Scholar]
- Hare B, Tomasello M (2005) Human-like social skills in dogs? Trends Cogn Sci 9:439–444. 10.1016/j.tics.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Hare B, Brown M, Williamson C, Tomasello M (2002) The domestication of social cognition in dogs. Science 298:1634–1636. 10.1126/science.1072702(80-) [DOI] [PubMed] [Google Scholar]
- Hare B, Rosati A, Kaminski J et al. (2010) The domestication hypothesis for dogs’ skills with human communication: a response to Udell et al. (2008) and Wynne al. (2008). Anim Behav 79:1–6. 10.1016/j.anbehav.2009.06.031 [DOI] [Google Scholar]
- Harman HH, Jones WH (1966) Factor analysis by minimizing residuals (minres). Psychometrika 31:351–368 [DOI] [PubMed] [Google Scholar]
- Hart BL, Hart LA (1985) Selecting pet dogs on the basis of cluster analysis of breed behavior profiles and gender. J Am Vet Med Assoc 186:1181–1185 [PubMed] [Google Scholar]
- Hart BL, Miller MF (1985) Behavioral profiles of dog breeds. J Am Vet Med Assoc 186:1175–1180 [PubMed] [Google Scholar]
- Hayward JJ, Castelhano MG, Oliveira KC et al. (2016a) Complex disease and phenotype mapping in the domestic dog. Nat Commun 7:10460 10.1038/ncomms10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward JJ, Castelhano MG, Oliveira KC et al. (2016b) Data from: complex disease and phenotype mapping in the domestic dog. 10.5061/dryad.266k4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein MTE, Turner DC, Manser MB (2017) Dogs ‘ (Canis familiaris) attention to human perception: influence of breed groups and life experiences. J Comp Psychol 131:19–29 [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S (2017) Numbers of neurons as biological correlates of cognitive capability. Curr Opin Behav Sci 16:1–7. 10.1016/j.cobeha.2017.02.004 [DOI] [Google Scholar]
- Herrmann E, Call J, Hernandez-Lloreda MV et al. (2007) Humans have evolved specialised skills of social cognition: the cultural intelligence hypothesis. Science 317:1360–1366. 10.1126/science.1146282(80-) [DOI] [PubMed] [Google Scholar]
- Herrmann E, Hernández-Lloreda MV, Call J et al. (2010) The structure of individual differences in the cognitive abilities of children and chimpanzees. Psychol Sci 21:102–110. 10.1177/0956797609356511 [DOI] [PubMed] [Google Scholar]
- Hiestand L (2011) A comparison of problem-solving and spatial orientation in the wolf (Canis lupus) and dog (Canis familiaris). Behav Genet 41:840–857. 10.1007/s10519-011-9455-4 [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Schaeffer J (2014) Chimpanzee intelligence is heritable. Curr Biol 24:1649–1652. 10.1016/j.cub.2014.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horschler DJ, MacLean EL (2019) Leveraging brain-body scaling relationships for comparative studies. Anim Cogn 22:1197–1202. 10.1007/s10071-019-01316-8 [DOI] [PubMed] [Google Scholar]
- Horschler DJ, Hare B, Call J et al. (2019) Absolute brain size predicts dog breed differences in executive function. Anim Cogn 22:187–198. 10.1007/s10071-018-01234-1 [DOI] [PubMed] [Google Scholar]
- Hsu Y, Serpell JA (2003) Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. J Am Vet Med Assoc 223:1293–1300 [DOI] [PubMed] [Google Scholar]
- Jakovcevic A, Elgier AM, Mustaca AE, Bentosela M (2010) Breed differences in dogs’ (Canis familiaris) gaze to the human face. Behav Processes 84:602–607. 10.1016/j.beproc.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Jardim-Messeder D, Lambert K, Noctor S et al. (2017) Dogs Have the Most Neurons, Though Not the Largest Brain: Trade-Off between Body Mass and Number of Neurons in the Cerebral Cortex of Large Carnivoran Species. Front Neuroanat 11:1–18. 10.3389/fnana.2017.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Ulrich L, Holekamp KE (2020) Group size and social rank predict inhibitory control in spotted hyaenas. Anim Behav 160:157–168. 10.1016/j.anbehav.2019.11.020 [DOI] [Google Scholar]
- Kang HM, Zaitlen NA, Wade CM et al. (2008) Efficient control of population structure in model organism association mapping. Genetics 178:1709–1723. 10.1534/genetics.107.080101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EK, Baranowska I, Wade CM et al. (2007) Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet 39:1321–1328. 10.1038/ng.2007.10 [DOI] [PubMed] [Google Scholar]
- Keagy J, Savard JF, Borgia G (2009) Male satin bowerbird problem-solving ability predicts mating success. Anim Behav 78:809–817. 10.1016/j.anbehav.2009.07.011 [DOI] [Google Scholar]
- Konno A, Romero T, Inoue-Murayama M et al. (2016) Dog breed differences in visual communication with humans. PLoS ONE 11:1–14. 10.1371/journal.pone.0164760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrschal A, Rogell B, Bundsen A et al. (2013) Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr Biol 23:168–171. 10.1016/j.cub.2012.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M, Bräuer J, Kaminski J, Virányi Z (2017) The effects of domestication and ontogeny on cognition in dogs and wolves. Sci Rep 7:1–8. 10.1038/s41598-017-12055-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach HM (2003) Human domestication reconsidered. Curr Anthropol 44:349–368. 10.1086/368119 [DOI] [Google Scholar]
- MacLean EL (2016) Unraveling the evolution of uniquely human cognition. Proc Natl Acad Sci 113:201521270 10.1073/pnas.1521270113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Hare B, Nunn CL et al. (2014) The evolution of self-control. Proc Natl Acad Sci 111:E2140–E2148. 10.1073/pnas.1323533111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean EL, Herrmann E, Suchindran S, Hare B (2017) Individual differences in cooperative communicative skills are more similar between dogs and humans than chimpanzees. Anim Behav 126:41–51. 10.1016/j.anbehav.2017.01.005 [DOI] [Google Scholar]
- MacLean EL, Snyder-Mackler N, vonHoldt BM, Serpell JA (2019) Highly heritable and functionally relevant breed differences in dog behaviour. Proc R Soc B Biol Sci 286:1–9. 10.1098/rspb.2019.0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Pescini S, Virányi Z, Range F (2015) The effect of domestication on inhibitory control: wolves and dogs compared. PLoS ONE 10:1–16. 10.1371/journal.pone.0118469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Pescini S, Frazzi C, Valsecchi P (2016) The effect of training and breed group on problem-solving behaviours in dogs. Anim Cogn 19:571–579. 10.1007/s10071-016-0960-y [DOI] [PubMed] [Google Scholar]
- Matzel LD, Han YR, Grossman H et al. (2003) Individual differences in the expression of a “general” learning ability in mice. J Neu- rosci 23:6423–6433. 10.1523/JNEUROSCI.23-16-06423.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy PD, Georgevsky D, Carrasco J et al. (2013) Dog behavior co-varies with height, bodyweight and skull shape. PLoS ONE 8:e80529 10.1371/journal.pone.0080529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrkam LR, Wynne CDL (2014) Behavioral differences among breeds of domestic dogs (Canis lupus familiaris): current status of the science. Appl Anim Behav Sci 155:12–27. 10.1016/j.applanim.2014.03.005 [DOI] [Google Scholar]
- Miklósi Á, Kubinyi EE, Topál J et al. (2003) A simple reason for a big difference: wolves do not look back at humans, but dogs do. Curr Biol 13:763–766. 10.1016/S0960-9822(03)00263-X [DOI] [PubMed] [Google Scholar]
- Moll H, Tomasello M (2007) Cooperation and human cognition: the Vygotskian intelligence hypothesis. Philos Trans R Soc Lond B Biol Sci 362:639–648. 10.1098/rstb.2006.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen MR (2018) A case for methodological overhaul and increased study of executive function in the domestic dog (Canis lupus familiaris). Anim Cogn. 10.1007/s10071-018-1162-6 [DOI] [PubMed] [Google Scholar]
- Parker HG, Kim LV, Sutter NB et al. (2004) Genetic structure of the purebred domestic dog. Science 304:1160–1164. 10.1126/science.1097406 [DOI] [PubMed] [Google Scholar]
- Parker HG, Dreger DL, Rimbault M et al. (2017) Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell Rep 19:697–708. 10.1016/j.celrep.2017.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson ME, Roth LSV, Johnsson M et al. (2015) Human-directed social behaviour in dogs shows significant heritability. Genes, Brain Behav 14:337–344. 10.1111/gbb.12194 [DOI] [PubMed] [Google Scholar]
- Polderman TJC, Benyamin B, De Leeuw CA et al. (2015) Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 47:702–709. 10.1038/ng.3285 [DOI] [PubMed] [Google Scholar]
- Pongrácz P, Miklósi Á, Vida V, Csányi V (2005) The pet dogs ability for learning from a human demonstrator in a detour task is independent from the breed and age. Appl Anim Behav Sci 90:309–323. 10.1016/j.applanim.2004.08.004 [DOI] [Google Scholar]
- Purcell SM, Chang C (2018) PLINK [1.90]. www.cog-genomics.org/plink/1.9/
- Purcell SM, Chang CC, Chow CC et al. (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:1–16. 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018) R: a language and environment for statistical computing. Vienna, Austria: https://www.r-project.org/ [Google Scholar]
- Range F, Virányi Z (2015) Tracking the evolutionary origins of dog-human cooperation: the “canine cooperation hypothesis”. Front Psychol 6:1–10. 10.3389/fpsyg.2015.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F, Jenikejew J, Schröder I, Virányi Z (2014) Difference in quantity discrimination in dogs and wolves. Front Psychol 5:1–10. 10.3389/fpsyg.2014.01299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W (2018) Psych: procedures for psychological, psychometric, and personality research. Evanston, Illinois: https://cran.r-project.org/package=psych [Google Scholar]
- Riedel J, Schumann K, Kaminski J et al. (2008) The early ontogeny of human-dog communication. Anim Behav 75:1003–1014. 10.1016/j.anbehav.2007.08.010 [DOI] [Google Scholar]
- Rosati AG, Rodriguez K, Hare B (2014) The ecology of spatial memory in four lemur species. Anim Cogn. 10.1007/s10071-014-0727-2 [DOI] [PubMed] [Google Scholar]
- Saetre P, Strandberg E, Sundgren PE et al. (2006) The genetic contribution to canine personality. Genes, Brain Behav 5:240–248. 10.1111/j.1601-183X.2005.00155.x [DOI] [PubMed] [Google Scholar]
- Scott JP, Fuller JL (1965) Genetics and the social behavior of the dog. Univ ChicagoPress, Chicago, p 111 [Google Scholar]
- Shaw RC, Schmelz M (2017) Cognitive test batteries in animal cognition research: evaluating the past, present and future of comparative psychometrics. Anim Cogn 20:1003–1018. 10.1007/s10071-017-1135-1 [DOI] [PubMed] [Google Scholar]
- Sonnenberg BR, Branch CL, Pitera AM et al. (2019) Natural selection and spatial cognition in wild food-caching mountain chickadees. Curr Biol 29:670–676.e3. 10.1016/j.cub.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Stevens J (2002) Applied multivariate statistics for the social sciences. Lawrence Erlbaum Associates, Inc [Google Scholar]
- Stewart L, MacLean EL, Ivy D et al. (2015) Citizen science as a new tool in dog cognition research. PLoS ONE 10:1–16. 10.1371/journal.pone.0135176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter NB, Bustamante CD, Chase K et al. (2007) A single IGF1 allele is a major determinant of small size in dogs. Science 316:112–115. 10.1126/science.1137045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A, Lukas D (2012) Individual variation in cognitive performance: developmental and evolutionary perspectives. Philos Trans R Soc B Biol Sci 367:2773–2783. 10.1098/rstb.2012.0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Call J (2008) Assessing the validity of ape-human comparisons: a reply to Boesch (2007). J Comp Psychol 122:449452 10.1037/0735-7036.122.4.449 [DOI] [PubMed] [Google Scholar]
- Tomasello M, Call J (2011) Methodological challenges in the study of primate cognition. Science 334(6060):1227–1228. 10.1126/science.1213443 [DOI] [PubMed] [Google Scholar]
- Udell MAR, Dorey NR, Wynne CDL (2010) What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol Rev 85:327–345. 10.1111/j.1469-185X.2009.00104.x [DOI] [PubMed] [Google Scholar]
- Udell MAR, Ewald M, Dorey NR, Wynne CDL (2014) Exploring breed differences in dogs (Canis familiaris): does exaggeration or inhibition of predatory response predict performance on human-guided tasks? Anim Behav 89:99–105. 10.1016/j.anbehav.2013.12.012 [DOI] [Google Scholar]
- Vaysse A, Ratnakumar A, Derrien T et al. (2011) Identification of genomic regions associated with phenotypic variation between dog breeds using selection mapping. PLoS Genet 7:1–21. 10.1371/journal.pgen.1002316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virányi Z, Gácsi M, Kubinyi E et al. (2008) Comprehension of human pointing gestures in young human-reared wolves (Canis lupus) and dogs (Canis familiaris). Anim Cogn 11:373–387. 10.1007/s10071-007-0127-y [DOI] [PubMed] [Google Scholar]
- Visscher PM, Hill WG, Wray NR (2008) Heritability in the genomics era—concepts and misconceptions. Nat Rev Genet 9:255–266. 10.1038/nrg2322 [DOI] [PubMed] [Google Scholar]
- VonHoldt BM, Pollinger JP, Lohmueller KE et al. (2010) Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464:898–902. 10.1038/nature08837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich MM, MacLean EL, Hare B et al. (2020) Age influences domestic dog cognitive performance independent of average breed lifespan. Anim Cogn. 10.1007/s10071-020-01385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems YE, Boesen N, Li J et al. (2019) The heritability of self-control: a meta-analysis. Neurosci Biobehav Rev 100:324–334. 10.1016/j.neubiorev.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Wilmer JB, Germine L, Chabris CF et al. (2010) Human face recognition ability is specific and highly heritable. Proc Natl Acad Sci 107:5238–5241. 10.1073/pnas.0913053107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Réale D, Clements MN et al. (2010) An ecologist’s guide to the animal model. J Anim Ecol 79:13–26. 10.1111/j.1365-2656.2009.01639.X [DOI] [PubMed] [Google Scholar]
- Wobber V, Hare B, Koler-Matznick J et al. (2009) Breed differences in domestic dogs’ (Canis familiaris) comprehension of human communicative signals. Interact Stud 10:206–224. 10.1075/is.10.2.06wob [DOI] [Google Scholar]
- Wynne CDL (2016) What is special about dog cognition? Curr Dir Psychol Sci 25:345–350. 10.1177/0963721416657540 [DOI] [Google Scholar]
- Xavier A, Xu S, Muir W, Rainey K (2015) {NAM}: association studies in multiple populations. Bioinformatics 31:3862–3864 [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Stephens M (2012) Genome-wide efficient mixed-model analysis for association studies. Nat Genet 44:821–824. 10.1038/ng.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.