Abstract
Microtubules (MTs) are a fundamental cytoskeletal component that give neurons structure and are the primary polymer system for long distance transport of cargo throughout the cytoplasm. Although neurons are highly polarized and their structure is often maintained throughout the life of an organism, MTs can remain dynamic in axons and dendrites, undergoing bouts of polymerization and depolymerization, referred to as dynamic instability. Furthermore, MTs can be nucleated outside of the centrosome or MT organizing center (MTOC) that is located in the cell body, allowing dynamic formation and branching of MT polymers throughout the neuron. Together, these recent findings point to a much more dynamic landscape of microtubules in developing and mature neurons than was previously appreciated. Here we will focus on recent studies that show MT dynamics are playing a role at the synapse, both post-synaptically in dendrites and pre-synaptically in axons.
Introduction
Microtubules (MTs) are one of four polymer systems present in neurons. The other three include actin filaments, intermediate/neurofilaments and septin polymers. All of these polymers are capable of polymerizing and depolymerizing. Moreover, MTs are polarized polymers composed of alpha/beta tubulin dimers that assemble in a head to tail fashion, resulting in a polymer with a plus and minus end. The minus end is fairly stable [1], but recent work indicates that free MT minus ends are capable of growth in neurons [2]. However, at the plus ends MTs can be very dynamic, polymerizing and depolymerizing over tens of micrometers through a stochastic process termed dynamic instability [3,4]. This stochastic polymerization and depolymerization allows MTs to explore the entire intracellular landscape of cells [5].
In addition to exhibiting plus end dynamics, MTs in neurons are generally not attached to the centrosome as in many other cell types. Instead, they are cut by severing enzymes [6] and are transported by motor proteins as polymers throughout axonal and dendritic arbors [7,8]. Additionally, the more recently described local nucleation of MTs in both axons and dendrites is likely to play critical roles in the development and maintenance of the neuronal MT cytoskeleton [9–12]. It was long thought that MTs, unlike actin filaments, were incapable of branching. However, recent studies have shown that MTs are indeed capable of branching in neurons [13,14]. One of the primary functions of MTs is to transport material both anterogradely and retrogradely throughout both axons and dendrites through rapid movement of both kinesin and dynein motor proteins. Kinesins generally transport cargo toward the plus end of MTs, while cytoplasmic dynein transports toward the minus end. Recent studies indicate that motor proteins are sensitive to posttranslational modifications of tubulin and bound MT associated proteins (MAPs) as they transport material throughout the neuron [15,16]. Such modifications to MTs direct motors to bind and unbind in distinctive regions of the neuron, allowing for targeted transport of cargoes.
Most cell types have their MTs positioned with plus ends oriented toward the cell membrane. However, neurons develop long, branched axons and dendrites. In axons, MTs are positioned with their plus ends oriented distally, either toward the extending growth cone in developing neurons or toward the distal process in mature neurons. In contrast, MTs develop an anti-parallel orientation in dendrites where individual MTs are either positioned plus end distal or minus end distal. The percentage of minus end distal MTs can vary from approximately 50–95% depending on the location in the dendrite or the organism studied [17,18]. It should be noted that MTs of both orientations appear to be capable of undergoing dynamic instability although a recent study suggests that minus-end distal MTs are more stable than plus-end distal MTs [19]. Although it is still unclear why dendrites develop such a MT orientation it obviously affects transport properties in the dendrite. Together, MT dynamic instability, branching, orientation, posttranslational modifications and MAP associations all affect how MTs function at synapses. For this review we will focus on new studies that illuminate the increasingly important function MTs play both postsynaptically at the dendritic spine and presynaptically in the axonal bouton.
Postsynaptic Microtubules
For many years it was not appreciated how dynamic MTs were in mature neurons. Labeling tubulin with fluorescent compounds or through genetically encoded fluorescent proteins labeled all of the MTs in neurons, resulting in a highly fluorescent outline of the entire neuron. It was not until growing MT ends were labeled with end binding (EB) proteins that MT dynamics in mature neurons were first appreciated [20]. When only growing ends of MTs were visualized with EB3 it was clear that MTs maintained dynamic instability throughout the life of the neuron [21]. Several groups discovered that MTs were capable of polymerizing into and out of all regions of neurons, including dendritic spines [21–23] (Figure 1). These studies also showed that MT entry into dendritic spines was activity-dependent, occurring after NMDA receptor activation and calcium influx [24,25]. Chemically induced, long-term potentiation (cLTP) increased MT invasions of spines, while chemically induced long-term depression (cLTD) decreased MT invasions [24–26]. These results suggest that MTs are sensitive to the intrinsic activity of neurons and target dendritic spines undergoing synaptic plasticity.
Figure 1. Dynamic microrubules play important roles both presynaptically and postsynaptically.
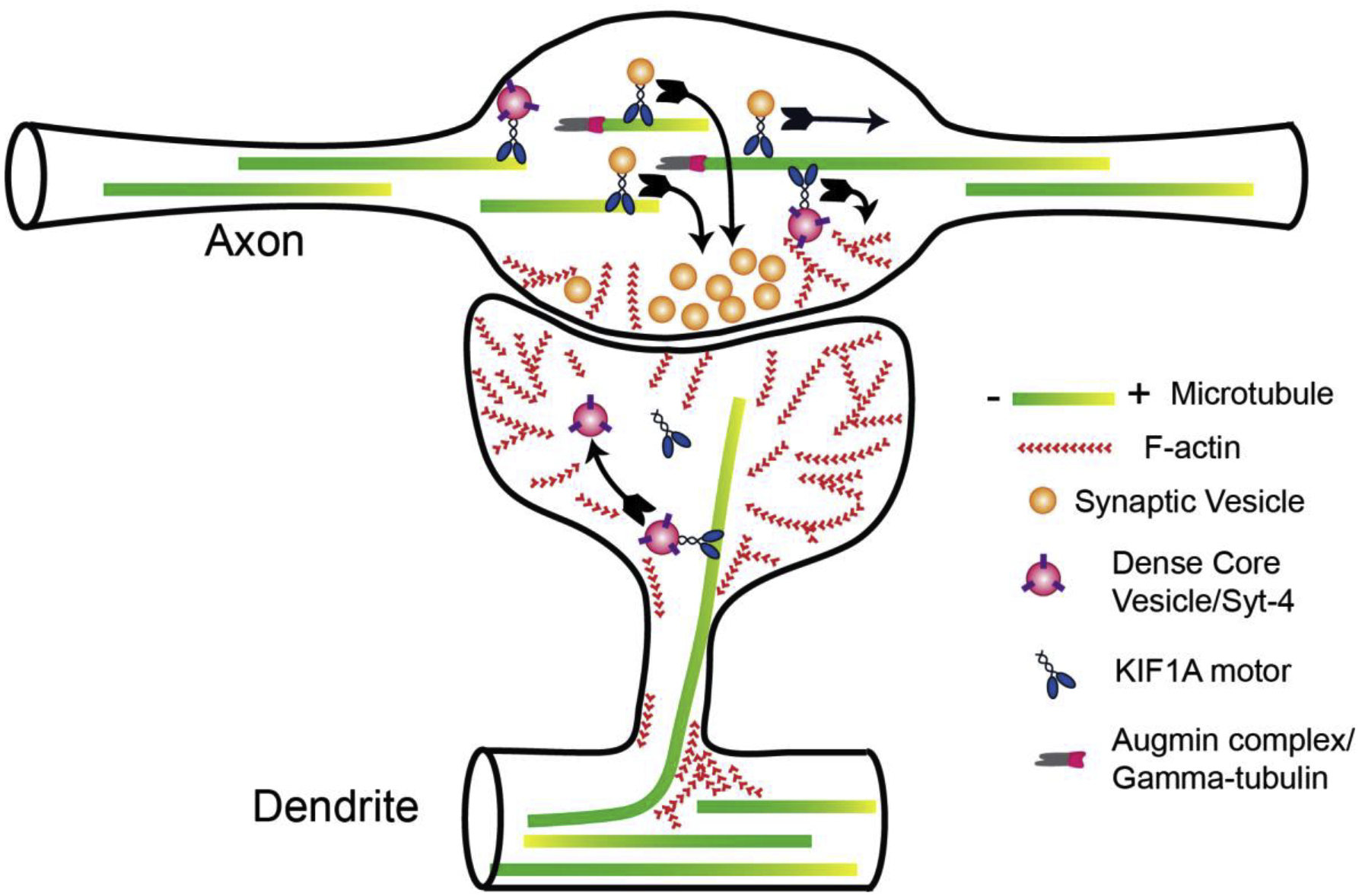
Presynaptically in the axon, microtubules preferentially nucleate via the augmin/gamma-tubulin complex as well as concentrate their plus ends in the en passant dialation termed the synaptic bouton. Both synaptic vesicles and dense core vesicles are transported along microtubules by the motor protein KIF1A and concentrate at boutons. Postsynaptically in the dendrite, microtubules polymerize directly into the dendritic spine and transport vesicles via KIF1A as well. Microtubule polymerization into the dendritic spine is dependent on filamentous actin (F-actin) concentrating at the base of spines.
Since dendritic spines are oriented perpendicular to the dendrite it was curious that MTs polymerizing anterogradely or retrogradely throughout the dendritic arbor would suddenly change their direction of polymerization from parallel to the dendritic shaft to perpendicular and enter dendritic spines. This behavior suggested that MTs were being directed to enter specific spines, but not others, along the dendritic arbor. As it turned out, MT entry into dendritic spines is entirely dependent on actin filaments [25]. More recent studies confirmed that MT entry of dendritic spines requires actin filaments in the dendritic shaft [27*]. One group has suggested that the developmentally regulated brain protein (drebrin) is important for MT entry of spines by showing that knockdown of drebrin decreased MT invasions, while overexpression of drebrin increased invasions [25]. However, recent work from another group suggests that knockdown of drebrin and several other actin-associated proteins do not affect MT invasion of spines [27*]. This group showed that only knockdown of the actin-associated protein cortactin or inhibition of the Arp2/3 complex inhibited MT entry of spines [27*]. Although these two studies came to different conclusions in regard to the importance of drebrin in MT entry of dendritic spines it is clear that actin filaments in the dendrite shaft play an instrumental role in guiding MTs into dendritic spines. Mechanical deflection of MTs from the dendritic shaft into the spine is the most parsimonious explanation for how MTs are guided to enter spines. However, these studies cannot rule out direct interactions between MT- and actin-associated proteins.
When MTs enter spines generally they are only present in spine for seconds to minutes before depolymerizing out of spines [21,23]. However, MTs can target individual spines a number of times over extended time periods. Because one of the primary functions of MTs is to transport material throughout cells these transient excursions into dendritic spines suggest they may be transporting cargo into spines. Since MT-based transport is generally 5–10 times faster than MT polymerization, even transient MT invasion of spines would allow transport of cargo into spines. To determine if MTs could transport material into dendritic spines McVicker and colleagues imaged the motor protein KIF1A and one of its cargoes, synaptotagmin IV (SytIV), in addition to MTs, and discovered that MTs were capable of transporting this motor/cargo pair into dendritic spines [28**]. By using pHlourin-labeled SytIV they were able to show that SytIV-containing vesicles exocytosed in dendritic spines after delivery by MTs. Paradoxically, this study also showed that knockdown of KIF1A resulted in more exocytosis of SytIV in dendritic spines and in the dendrite shaft, and increased mobility of SytIV puncta in the plane of the plasma membrane. These data suggest that KIF1A, in addition to serving to transport cargo, is also important for sequestering cargo along MTs so that unregulated exocytosis does not occur throughout the dendritic arbor. Further studies have shown that the actin-associated proteins TANC2 and liprin-alpha recruit KIF1A-associated vesicles into dendritic spines [29].
Taken together, the studies outlined above indicate that there are dynamic MTs in mature dendrites and they appear to target dendritic spines undergoing plasticity in an NMDA, calcium and actin-dependent manner. Although the exact function of MT entry into dendritic spines is still unclear, they target synaptically active spines and are capable of transporting cargo into those spines. Thus, in addition to diffusion of material in the plane of the membrane [30] and actomyosin-based transport of material into spines [31], MTs provide an additional mechanism by which dendritic cargo can be targeted to specific spines.
Presynaptic Microtubules
Axonal MTs, like dendritic MTs, are important for motor-based transport of material and delivery of cargo. Early electron microscopy studies from George Gray implicated MT involvement in presynaptic architecture. Specifically, he showed that MTs were often associated with synaptic vesicles at presynaptic sites [32,33]. These studies suggested that presynaptic MTs may be functioning in synaptic vesicle release, but there has been a relative dearth of studies on how MTs may be functioning presynaptically in mammalian neurons.
Much of the research on MT involvement in presynaptic function has focused on the Drosophila neuromuscular junction (NMJ) [34] (Figure 2). In the Drosophila NMJ, MTs form a looped structure in the terminal boutons, that dynamically reorganize to form new boutons [35–37]. The MT-associated protein Futsch/MAP1B is especially important in presynaptic MT organization [38] and acts as a link between MTs and the active zone [39]. This work also showed that Futsch/MAP1B may link MTs to active zones to regulate neurotransmitter release and active zone density [39]. Further studies implicated giant Drosophila Ankyrin2 (Ank2) isoforms working synergistically with Futsch/MAP1B to regulate synaptic geometry and release properties [40]. A recent study has implicated another member of this MTs/Futsch/Ank2 complex. Migh and colleagues discovered that a MT- and actin-associated formin family protein, Disheveled associated activator of morphogenesis (DAAM), functions together with MTs/Futsch/Ank2 in the active zone scaffold at the Drosophila NMJ [41*]. Based on electrophysiological data the authors suggest that DAAM plays a role in synaptic vesicle release. Interestingly, it is mostly through its MT-related, rather than actin-related, interactions that DAAM acts in developing presynaptic boutons. Together these studies provide ample data that MTs, functioning through a number of MT-associated proteins, play important roles in presynaptic physiology at the Drosophila NMJ. Do MTs play similar roles in mammalian presynaptic function in central synapses?
Figure 2. Microtubules associate with multiple components at the Drosophila neuromuscular junction.
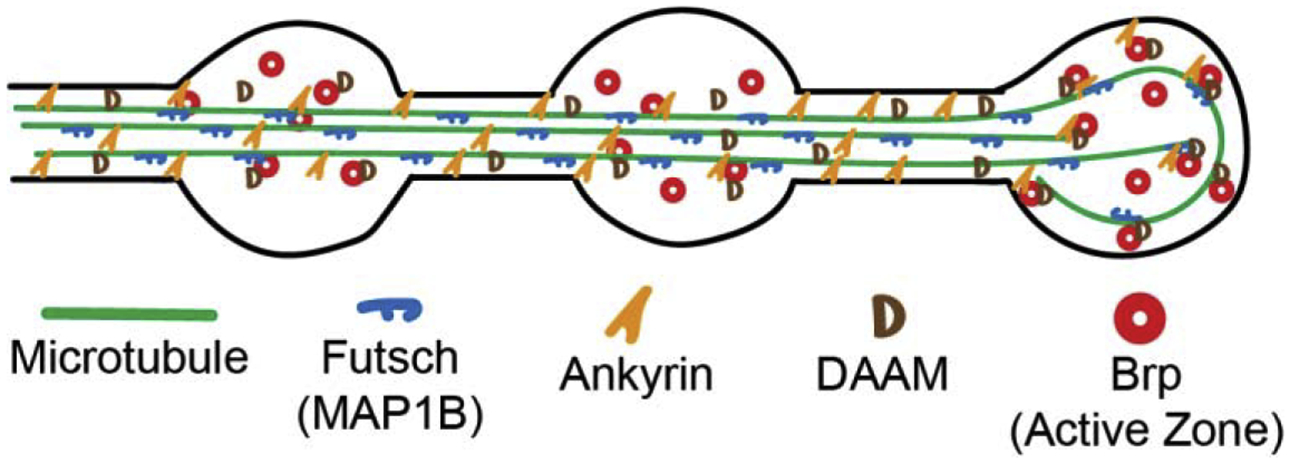
A number of recent studies have shown that there is a complex of proteins, including Futsch/MAP1B, Ankyrin proteins (Ankyrin2-L, Ankyrin2-XL), and formin proteins (DAAM shown, Dia not shown) that associate with microtubules and with active zones (shown as red circles of bruchpilot (Brp)). These proteins appear to be important for organizing synaptic architecture, regulating synaptic microtubules and potentially guiding the delivery of synaptic vesicles to active zones. Synaptic vesicles and actin are not shown for simplicity. Microtubules are shown as continuous structures but are likely bundles of shorter dynamic microtubules.
Interestingly, a recent study indicates that local axonal MT dynamics and kinesin-based transport is important for delivery of synaptic vesicle precursors (SVPs) to en passant synapses in cultured mammalian hippocampal neurons [42**] (Figure 1). This work showed that MT polymerization often initiates and terminates at synapses, suggesting there are localized regions of MT dynamics in the axon and pharmacologically blocking MT dynamics was sufficient to inhibit anterograde delivery of SVPs to synapses. Moreover, they discovered that the motor protein KIF1A was important for delivery of SVPs through a mechanism whereby KIF1A unloaded cargo at synapses because of its decreased affinity for the GTP-rich tubulin lattice that comprises the plus end of growing MTs. As the authors point out, this mechanism of KIF1A-dependent transport and release of SVPs is likely working in coordination with other mechanisms that regulate KIF1A activity. Previous work in C. elegans has shown that the ARF-like small GTPase Arl8 promotes SVP movement by relieving KIF1A from an autoinhibited state [43*]. Thus, coordination of kinesin motor activity and the ability of KIF1A to detach from growing MT ends that are positioned close to synapses helps replenish synaptic vesicles to presynaptic sites.
A similar system appears to guide dense core vesicles (DCVs) to synapses. DCVs were shown to bind KIF1A through SytIV and phosphorylation of SytIV by JNK destabilizes this interaction, allowing capture of DCVs at synapses by actin [44*] (Figure 1). Interestingly, these authors showed synaptic activity increases DCV capture at presynaptic sites. Although Bharat and colleagues did not study MT dynamics, it is likely release of DCVs occurs at MT plus ends presynaptically, as has been shown postsynaptically [28**]. How DCVs and SVPs possibly coordinate their delivery and capture at boutons awaits further research.
The idea that MT plus ends, localized to synapses, allow for the unloading of cargo in the form of synaptic vesicle precursors is a compelling model. However, very recent work suggests there is an additional MT-dependent mechanism upon which synaptic vesicles rely as they make their way to synapses. This work demonstrated that MT nucleation occurs preferentially at en passant synapses in mature hippocampal neurons in both culture and slices [45**] (Figure 1). Non-centrosomal MT nucleation is a relatively newly discovered phenomenon in neurons that allow MTs to locally nucleate de novo or nucleate a new MT branch from an existing MT [11,13]. Interestingly, Qu and colleagues [45**] revealed that nucleation of MTs at synapses is activity-dependent and requires gamma-tubulin to nucleate MTs and the augmin complex to direct MT growth to the distal axon. These nucleated MTs at synapses serve as tracks for bidirectional movement of synaptic vesicles between boutons. Importantly, loss of de novo nucleated MTs at boutons impairs high-frequency evoked neurotransmitter release, suggesting these nucleated MTs serve an important role in synapse function.
Together, these recent studies suggest that MTs are playing a heretofore unappreciated role in SVP and DCV transport and synaptic function at en passant synapses in hippocampal neurons [42**,44*,45**]. Results from these studies are complementary and suggest that MT nucleation, polymerization and motor protein-dependent transport and release at MT plus ends serve important roles in SVP and DCV targeting to presynaptic regions of the axon.
Concluding Remarks
Within the last ten years great strides have been made in determining the function of MTs in both the pre and post-synaptic compartments, but questions remain. Does MT nucleation and branching function post-synaptically, as it has been shown to function pre-synaptically [45**]? Are these MT dynamics disrupted in disease states, such as Alzheimer’s or epilepsy? How exactly are MT- and actin-based polymerization and transport linked at synapses? It is difficult to study MT dynamics at synapses because temporal and spatial control of polymerization and depolymerization is not trivial and MTs and actin filaments contain an entire host of associated proteins, that are likely to play important roles at the synapse as well. Continued developments in high/super-resolution live-cell microscopy will likely play an important role in answering these and other important questions regarding cytoskeletal function at the synapse.
Highlights:
Microtubules remain dynamic in mature axons and dendrites
Microtubule nucleation and polymerization concentrates at presynaptic boutons
Synaptic and dense core vesicles are transported by KIF1A at presynaptic boutons
Polymerizing microtubules transport vesicles into spines via KIF1A
Acknowledgements:
Apologies for all of the excellent work that could not be included in this article due to space constraints. This work was supported by NIH grant NS098372 to E.W.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Nothing declared.
References and recommended reading
- 1.Akhmanova A, Steinmetz MO: Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol 2015, 16:711–726. [DOI] [PubMed] [Google Scholar]
- 2.Feng C, Thyagarajan P, Shorey M, Seebold DY, Weiner AT, Albertson RM, Rao KS, Sagasti A, Goetschius DJ, Rolls MM: Patronin-mediated minus end growth is required for dendritic microtubule polarity. J Cell Biol 2019, 218:2309–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchison T, Kirschner M: Dynamic instability of microtubule growth. Nature 1984, 312:237–242. [DOI] [PubMed] [Google Scholar]
- 4.Goodson HV, Jonasson EM: Microtubules and Microtubule-Associated Proteins. Cold Spring Harb Perspect Biol 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meiring JCM, Shneyer BI, Akhmanova A: Generation and regulation of microtubule network asymmetry to drive cell polarity. Curr Opin Cell Biol 2019, 62:86–95. [DOI] [PubMed] [Google Scholar]
- 6.McNally FJ, Roll-Mecak A: Microtubule-severing enzymes: From cellular functions to molecular mechanism. J Cell Biol 2018, 217:4057–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Castillo U, Norkett R, Gelfand VI: Unconventional Roles of Cytoskeletal Mitotic Machinery in Neurodevelopment. Trends Cell Biol 2019, 29:901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao AN, Baas PW: Polarity Sorting of Microtubules in the Axon. Trends Neurosci 2018, 41:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WS, Chen YJ, Huang YA, Hsieh BY, Chiu HC, Kao PY, Chao CY, Hwang E: Ran-dependent TPX2 activation promotes acentrosomal microtubule nucleation in neurons. Sci Rep 2017, 7:42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ori-McKenney KM, Jan LY, Jan YN: Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 2012, 76:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunha-Ferreira I, Chazeau A, Buijs RR, Stucchi R, Will L, Pan X, Adolfs Y, van der Meer C, Wolthuis JC, Kahn OI, et al. : The HAUS Complex Is a Key Regulator of Non-centrosomal Microtubule Organization during Neuronal Development. Cell Rep 2018, 24:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen MM, Stone MC, Rolls MM: Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev 2011, 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Huertas C, Freixo F, Viais R, Lacasa C, Soriano E, Luders J: Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat Commun 2016, 7:12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basnet N, Nedozralova H, Crevenna AH, Bodakuntla S, Schlichthaerle T, Taschner M, Cardone G, Janke C, Jungmann R, Magiera MM, et al. : Direct induction of microtubule branching by microtubule nucleation factor SSNA1. Nat Cell Biol 2018, 20:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelliher MT, Saunders HA, Wildonger J: Microtubule control of functional architecture in neurons. Curr Opin Neurobiol 2019, 57:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guedes-Dias P, Holzbaur ELF: Axonal transport: Driving synaptic function. Science 2019, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yau KW, Schatzle P, Tortosa E, Pages S, Holtmaat A, Kapitein LC, Hoogenraad CC: Dendrites In Vitro and In Vivo Contain Microtubules of Opposite Polarity and Axon Formation Correlates with Uniform Plus-End-Out Microtubule Orientation. J Neurosci 2016, 36:1071–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN: Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol 2008, 10:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tas RP, Chazeau A, Cloin BMC, Lambers MLA, Hoogenraad CC, Kapitein LC: Differentiation between Oppositely Oriented Microtubules Controls Polarized Neuronal Transport. Neuron 2017, 96:1264–1271 e1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N: Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J Neurosci 2003, 23:2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, Viesselmann C, Nam S, Merriam E, Dent EW: Activity-dependent dynamic microtubule invasion of dendritic spines. The Journal of neuroscience 2008, 28:13094–13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu J, Firestein BL, Zheng JQ: Microtubules in dendritic spine development. The Journal of neuroscience 2008, 28:12120–12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, et al. : Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 2009, 61:85–100. [DOI] [PubMed] [Google Scholar]
- 24.Merriam EB, Lumbard DC, Viesselmann C, Ballweg J, Stevenson M, Pietila L, Hu X, Dent EW: Dynamic microtubules promote synaptic NMDA receptor-dependent spine enlargement. PLoS One 2011, 6:e27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, Ferhat L, Dent EW: Synaptic regulation of microtubule dynamics in dendritic spines by calcium, f-actin, and drebrin. The Journal of neuroscience 2013, 33:16471–16482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapitein LC, Yau KW, Gouveia SM, van der Zwan WA, Wulf PS, Keijzer N, Demmers J, Jaworski J, Akhmanova A, Hoogenraad CC: NMDA receptor activation suppresses microtubule growth and spine entry. J Neurosci 2011, 31:8194–8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.*.Schatzle P, Esteves da Silva M, Tas RP, Katrukha EA, Hu HY, Wierenga CJ, Kapitein LC, Hoogenraad CC: Activity-Dependent Actin Remodeling at the Base of Dendritic Spines Promotes Microtubule Entry. Curr Biol 2018, 28:2081–2093 e2086. [DOI] [PubMed] [Google Scholar]; Using live cell imaging, local glutamate uncaging and super-resolution microscopy this study confirms previous work [24, 25] that microtubule entry into dendritic spines is dependent on NMDA activity, calcium influx and filamentous actin polymerization in the spine neck. In contrast to [25] they show that cortactin, rather than drebrin, affects microtubule polymerization into spines, and posit, based on mutant analysis of the microtubule plus end-binding protein EB3, that EB3/actin-associated protein interactions may not be necessary for microtubule invasion of spines.
- 28.**.McVicker DP, Awe AM, Richters KE, Wilson RL, Cowdrey DA, Hu X, Chapman ER, Dent EW: Transport of a kinesin-cargo pair along microtubules into dendritic spines undergoing synaptic plasticity. Nat Commun 2016, 7:12741. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that microtubule invasion of spines can result in the transport of a motor (KIF1A)/cargo (synaptotagmin-4) pair into spines and exocytosis of syt-4-containing vesicles inside dendritic spines. They also show that mitochondria do not transport into spines along microtubules, using myosin-V for spine entry instead. Surprisingly, knockdown of KIF1A increases syt-4 entry and exocytosis into spines and movement of syt-4 puncta in the plasma membrane, suggesting that KIF1A both transports and sequesters cargo that might otherwise randomly exocytose along the dendritic shaft. Along with [44], this study shows KIF1A and syt-4 function both pre- and postsynaptically in the delivery of vesicles to synapses.
- 29.Stucchi R, Plucinska G, Hummel JJA, Zahavi EE, Guerra San Juan I, Klykov O, Scheltema RA, Altelaar AFM, Hoogenraad CC: Regulation of KIF1A-Driven Dense Core Vesicle Transport: Ca(2+)/CaM Controls DCV Binding and Liprin-alpha/TANC2 Recruits DCVs to Postsynaptic Sites. Cell Rep 2018, 24:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huganir RL, Nicoll RA: AMPARs and synaptic plasticity: the last 25 years. Neuron 2013, 80:704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner W, Brenowitz SD, Hammer JA 3rd: Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nature cell biology 2011, 13:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon-Weeks PR, Burgoyne RD, Gray EG: Presynaptic microtubules: organisation and assembly/disassembly. Neuroscience 1982, 7:739–749. [DOI] [PubMed] [Google Scholar]
- 33.Gray EG: Presynaptic microtubules and their association with synaptic vesicles. Proc R Soc Lond B Biol Sci 1975, 190:367–372. [PubMed] [Google Scholar]
- 34.Bodaleo FJ, Gonzalez-Billault C: The Presynaptic Microtubule Cytoskeleton in Physiological and Pathological Conditions: Lessons from Drosophila Fragile X Syndrome and Hereditary Spastic Paraplegias. Front Mol Neurosci 2016, 9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawson C, Eaton BA, Davis GW: Formin-dependent synaptic growth: evidence that Dlar signals via Diaphanous to modulate synaptic actin and dynamic pioneer microtubules. J Neurosci 2008, 28:11111–11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roos J, Hummel T, Ng N, Klambt C, Davis GW: Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron 2000, 26:371–382. [DOI] [PubMed] [Google Scholar]
- 37.Yan Y, Broadie K: In vivo assay of presynaptic microtubule cytoskeleton dynamics in Drosophila. J Neurosci Methods 2007, 162:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Canada C, Ashley J, Moeckel-Cole S, Drier E, Yin J, Budnik V: New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron 2004, 42:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lepicard S, Franco B, de Bock F, Parmentier ML: A presynaptic role of microtubule-associated protein 1/Futsch in Drosophila: regulation of active zone number and neurotransmitter release. J Neurosci 2014, 34:6759–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephan R, Goellner B, Moreno E, Frank CA, Hugenschmidt T, Genoud C, Aberle H, Pielage J: Hierarchical microtubule organization controls axon caliber and transport and determines synaptic structure and stability. Dev Cell 2015, 33:5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.*.Migh E, Gotz T, Foldi I, Szikora S, Gombos R, Darula Z, Medzihradszky KF, Maleth J, Hegyi P, Sigrist S, et al. : Microtubule organization in presynaptic boutons relies on the formin DAAM. Development 2018, 145. [DOI] [PubMed] [Google Scholar]; Using genetic interactions, physiology and EM analysis this study shows that the formin DAAM functions in NMJ development in Drosophila. Interestingly, DAAM is important for microtubule organization, rather than its role in actin assembly. Moreover, DAAM is shown to function in the Wg/Ank2/Futsch pathway.
- 42.**.Guedes-Dias P, Nirschl JJ, Abreu N, Tokito MK, Janke C, Magiera MM, Holzbaur ELF: Kinesin-3 Responds to Local Microtubule Dynamics to Target Synaptic Cargo Delivery to the Presynapse. Curr Biol 2019, 29:268–282 e268. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nice combination of live in vivo and in vitro imaging shows that the kinesin-3 motor protein KIF1A delivers synaptic vesicles to presynaptic sites. This delivery is accomplished because microtubule plus ends are concentrated at presynaptic sites and KIF1A has low affinity for GTP-tubulin at the plus ends. This paper provides a mechanism for the transport and release of synaptic vesicles at synapses along the axon.
- 43.*.Niwa S, Lipton DM, Morikawa M, Zhao C, Hirokawa N, Lu H, Shen K: Autoinhibition of a Neuronal Kinesin UNC-104/KIF1A Regulates the Size and Density of Synapses. Cell Rep 2016, 16:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the size and density of synapses in C. elegans DA9 neurons is regulated by the activity of the motor protein UNC104/KIF1A. Overactivation of UNC104/KIF1A results in smaller synapses, decreased density of synapses and ectopic synapse formation. Additionally, they show that the small GTPase ARL-8, which binds to synaptic vesicles in its active state is responsible for releasing the autoinhibition of UNC104/KIF1A.
- 44.*.Bharat V, Siebrecht M, Burk K, Ahmed S, Reissner C, Kohansal-Nodehi M, Steubler V, Zweckstetter M, Ting JT, Dean C: Capture of Dense Core Vesicles at Synapses by JNK-Dependent Phosphorylation of Synaptotagmin-4. Cell Rep 2017, 21:2118–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a variety of techniques this study shows that synaptotagmin-4 (Syt-4) binds to dense core vesicles (DCVs), which are trafficked in axons by the motor protein KIF1A. Activity-dependent phosphorylation of Syt-4 at presynaptic sites by JNK disrupts Syt-4/KIF1A association, leading to release of DCVs from microtubules and capture by presynaptic actin. These data, along with results from [28], show that Syt-4 and KIF1A are functioning in similar ways both presynaptically and postsynaptically.
- 45.**.Qu X, Kumar A, Blockus H, Waites C, Bartolini F: Activity-Dependent Nucleation of Dynamic Microtubules at Presynaptic Boutons Controls Neurotransmission. Curr Biol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides an additional, complementary mechanism to that outlined in [42] by which microtubules function to deliver synaptic vesicles to excitatory en passant synapses. Here they show that microtubules are preferentially nucleated at synapses via gamma-tubulin and augmin. This nucleation and polymerization of microtubules is critical for release of vesicles at presynaptic sites and is regulated by synaptic activity.


