Abstract
Clinicians who manage glaucoma patients carefully monitor the visual field to determine if treatments are effective or interventions are needed. Visual field tests may reflect disease progression or variability among examinations. We describe the approaches and perimetric tests used to evaluate glaucomatous visual field progression and factors that are important for identifying progression. These include stimulus size, which area of the visual field to assess (central versus peripheral), and the testing frequency, which is important to detect change early while minimizing patient testing burden. We also review the different statistical methods developed to identify change. These include trend- and event-based analyses, parametric and non-parametric tests, population-based versus individualized approaches, as well as pointwise and global analyses. We hope this information will prove useful and important to enhance the management of glaucoma patients. Overall, analysis procedures based on series of at least 5 to 6 examinations that require confirmation and persistence of changes, that are guided by the pattern and shape of the glaucomatous visual field deficits, and that are consistent with structural defects provide the best clinical performance.
Keywords: Glaucoma, visual field progression, perimetric tests, analysis methods, clinical considerations
1. Introduction
The evaluation of patients with glaucoma and those at risk of developing glaucoma typically consists of determining intraocular pressure (IOP), structural properties (e.g. appearance of the optic nerve head and thickness of the retinal nerve fiber layer and ganglion cell complex) and functional characteristics (e.g. visual field sensitivity) of the visual pathway. Regular follow-up assessments are then performed throughout the lifespan of the patient to identify change in any of these key parameters. While detecting the disease is important, the initiation and escalation of therapy often hinges on the determination of progression. Early detection of glaucoma progression is therefore critical to determine whether the current treatment is effective or when additional interventions are needed. The detection of worsening in visual function is particularly important because of its close association with activities of daily living, quality of life, the likelihood of falls and related navigational tasks (e.g., driving), as well as job-related skills and personal task performance.156
2. Clinical judgment
Clinical judgment is commonly used to assess visual field progression within busy clinical settings. Clinicians usually compare the gray scale representation, the decibel sensitivity values, the global indices, or the total and pattern deviation plots across a series of visual field tests. This approach is subjective, relies on one’s knowledge and experience, and results in variable assessments even among glaucoma specialists who are highly trained and experienced in identifying progression (i.e. experts). The reported level of agreement among experts in identifying visual field progression varies across studies, from fair to substantial agreement. In a study by Viswanathan and coworkers, five expert clinicians were asked to grade 27 visual field series as either definitely stable, probably stable, probably progressing, or definitely progressing.186 Each series had at least 19 visual field tests performed on the Humphrey Field Analyzer (HFA) (Carl Zeiss Meditec, Inc., Dublin, USA). All visual fields were reliable and met relatively strict criteria concerning macular threshold and patient age. The experts were asked to use their clinical judgment based on the perimetric outputs and were not given any additional guidelines. Using the kappa statistics, only fair interobserver agreement was found (κ = 0.32).
There is a lack of standardized criteria to identify progression. It is therefore possible that the modest interobserver agreement is due to poor intraobserver agreement. Tanna and coworkers, however, reported good to excellent intraobserver agreement.178 Five glaucoma specialists were asked to grade 5 visual field tests from each of 100 eyes of 83 patients as having either no, questionable, probable, or definite progression. While only moderate interobserver agreement (κ = 0.45) was observed, the kappa values for intraobserver determinations ranged from 0.62 to 0.78. Although the graders were not aware that they would be asked to reevaluate the visual field series at a later time, most of their assessments remained the same. Therefore, the modest interobserver agreement is likely not due to intraobserver variability.
Another possible reason for the modest interobserver agreement may be that the large quantity of data within longitudinal visual field can be difficult to integrate. Fair to moderate interobserver agreement persists, however, even when clinicians have the opportunity to review automated indicators of progression such as visual field indices, Bebie curves (cumulative defect curves), HFA overview printouts, Guided Progression Analysis printouts, STATPAC2, and PROGRESSOR.90,91,115,134,179 For example, Lin and coworkers asked 8 glaucoma experts and 8 comprehensive ophthalmologists to review 40 visual field series and determine whether they were progressing or stable.115 For glaucoma experts, the median values for interobserver agreement were 0.47, 0.60 and 0.43 when HFA printouts, STATPAC2 and PROGRESSOR were used, respectively. For comprehensive ophthalmologists, the values were 0.43, 0.43 and 0.35, respectively. These results suggest that access to automated methods does not dramatically improve interobserver agreement.
In summary, there is overall only modest agreement among clinicians in identifying progression from visual field series, either alone or in conjunction with software packages. The disagreement present among experts highlights the challenge in determining whether true progression is present, given that perimetric tests are subjective and that variability is always embedded within the data.
3. Event analysis
Event analysis determines whether progression (the event) is present or not. It does so by comparing the results of a given visual field test to the average of two baseline tests. Progression is identified if there is sufficient worsening at each visual field location and if this change persists over multiple visual field tests. One drawback of this method is that only the test of interest and the baseline tests are considered in the determination of progression (i.e. the interim test results are not considered). Progression can therefore be identified on one test, but not on the next due to the test-retest variability. Another drawback is that the determination of progression is highly dependent on the quality of the baseline tests. Without reliable and accurate baseline tests, the determination of progression will be questionable.
3.1. Guided Progression Analysis
The Guided Progression Analysis (GPA) (Carl Zeiss Meditec, Inc., Dublin, USA) is a pointwise event analysis program based on the pattern deviation (PD) data from the Swedish Interactive Thresholding Algorithm (SITA) tests or Full-Threshold tests and is based on the Early Manifest Glaucoma Trial progression criteria (described in the next section).80 To establish a baseline, the procedure averages the patient’s first 2 reliable visual fields. The GPA compares each consecutive test to this baseline, point by point, to find any points that deviate beyond the 95% confidence interval for expected test-retest variability obtained from a group of stable glaucoma patients.24 If 3 or more locations are confirmed (2 consecutive tests), the GPA outcome is “possible progression” and if 3 or more are persistent (3 consecutive tests), the GPA outcome is “likely progression”. Figure 1 presents an example of “possible” and “likely” progression. The diagnosis of progression using the GPA was found to closely correlate with clinical assessments. Arnalich-Montiel and coworkers reported that the “possible progression” criterion achieved a sensitivity and specificity of 93% and 95% respectively, and a positive likelihood ratio of 20 based on a thorough objective clinical assessment.10
Figure 1.
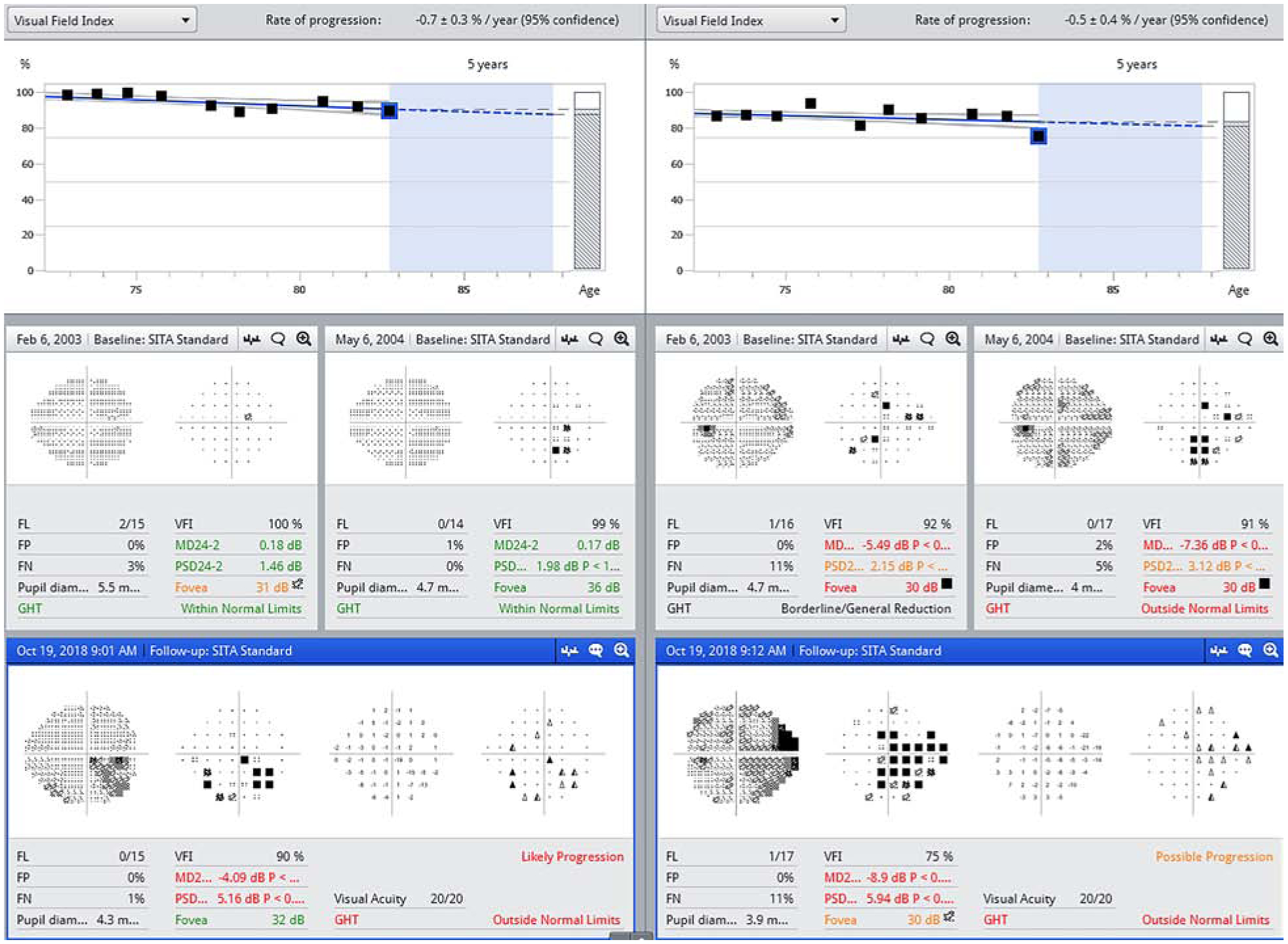
The GPA summary report is shown for the right eye (left panel) and left eye (right panel) of a patient with glaucoma. Two baseline tests were obtained in 2003 and 2004 and the GPA was performed on the tests through 2018. Open triangles represent locations for which change from baseline is observed, but unconfirmed. The half-filled triangles represent locations at which change from baseline was observed and confirmed on one additional test. Filled triangles represent locations at which change from baseline was observed and confirmed on two additional tests (persistence). When the same three (or more) half-filled circles are present on two consecutive tests, the GPA outcome will be “possible progression”. When the same three (or more) filled circles are present on three consecutive tests, the GPA outcome will be “likely progression”. FL: Fixation Loss; FP: False Positive; FN: False Negative; GHT: Glaucoma Hemifield Test; VFI: Visual Field Index; MD: Mean Deviation; PSD: Pattern Standard Deviation
The GPA is clinically useful because it presents both the statistical significance of the changes compared with baseline tests, as well as whether these changes are confirmed and persistent on subsequent tests. This allows clinicians to distinguish true pathologic changes from test-retest variability. Nevertheless, a drawback of the GPA is the potential for false positive outcomes. By randomly reordering 12 visual field tests over a 3-month period, Artes and coworkers found false-positive rates of 18.5% and 2.6%, respectively for the GPA “possible progression” and “likely progression” alerts.18 Based on a simulated dataset of stable glaucoma eyes, Wu and coworkers observed 5-year cumulative false-positive rates of 34% and 7%, respectively, for the “possible progression” and “likely progression” outcomes when the long-term variability within visual field series was considered.200 The GPA is more likely to make false-positive decisions on progression in patients with large test-retest variability and frequent response errors. This should be considered when the GPA is used in clinical practice.
In routine clinical practice, an advantage of the GPA is that it is not greatly affected by diffuse or widespread changes caused by media opacities or refractive errors.24,111 As a result, however, it may not identify progression in glaucoma patients with diffuse loss only.12,15,82 The usefulness of the GPA is also limited in advanced glaucoma where severely depressed points (i.e. from around −15 to −20 dB) are outside the range of analysis.65,142,195 Additionally, the GPA criteria overlook cases of progression where the presence of only one or two worsening points indicates true progression. This is of particular concern when these points are in the paracentral region. Another drawback of the GPA is that additional software is needed and all visual fields have to be stored in a single perimeter, which may be challenging in some clinics. The use of the FORUM software (Carl Zeiss Meditec, Dublin, California, USA), which is proprietary, may be useful as it allows access to visual field data from multiple HFA devices.
3.2. Early Manifest Glaucoma Trial
The Early Manifest Glaucoma Trial (EMGT) defined visual field progression as significant change from baseline on at least 3 visual field locations on 3 consecutive visual fields. This criterion is the Glaucoma Change Probability (GCP).113 It is based on the PD and compares the average of results from two baseline EMGT Pattern Change Probability Maps with the current test on a point-by-point basis.81 This model takes into account initial defect depth, test point location, and general level of visual field damage.80 These criteria were subsequently incorporated into the GPA specifications.
Since the EMGT included an untreated control group of patients with glaucoma, the clinical trial needed to identify progression in participants as early as possible even at the expense of specificity. In the untreated control group it took an average of 44.8 and 61.1 months to detect early signs of visual field progression for high-tension and normal-tension glaucoma, respectively.78 In a follow-up study Heijl and coworkers showed that definite progression required a mean change in mean deviation (MD) from baseline of −1.93 dB (standard error ± 0.2 dB) and a mean change of +4.85 (standard error ± 0.35) in number of significant points.80 Katz and coworkers determined that the proportion of patients showing progression by the EMGT criteria was comparable with that of the Collaborative Initial Glaucoma Treatment Study (CIGTS) criteria and was twice that of the Advanced Glaucoma Intervention Study (AGIS) criteria (described below).101 The EMGT criteria may detect progression 1 year earlier than the other 2 criteria, however, only fair to moderate agreement was observed among these criteria and clinical assessment.
3.3. Severity classification systems used to identify progression
Although they were not designed to assess progression, some severity classification systems have been used to identify progression as an event analysis in large clinical trials. The two most prominent examples are the Advanced Glaucoma Intervention Study (AGIS) severity scoring system50 and the Collaborative Initial Glaucoma Treatment Study (CIGTS) visual field classification.137 The AGIS trial was designed to identify progression for advanced glaucoma patients who are not controlled well by medications and are in need of surgery. The CIGTS trial was designed to determine whether the better initial treatment for newly diagnosed glaucoma patients was medications or immediate filtration surgery. Both scoring systems are scaled ranging from 0 to 20. Katz and coworkers compared these two systems and showed that the CIGTS scores were systematically slightly higher than the AGIS scores.100 In another study, Katz’s group showed that the proportion of patients progressing with CIGTS was twice as large as that of the AGIS.101 The CIGTS scores identified a similar proportion of patients as progressing as a clinical assessment, but only half of the patients were identified by both methods. Vesti and coworkers analyzed progression in 76 patients with open-angle glaucoma and found that the AGIS and CIGTS methods had high specificity, but classified fewer cases of progression compared to 3 criteria based on the GCP analysis, and 2 criteria based on pointwise linear regression analysis (described in section 4.2).185 Heijl and coworkers compared the AGIS and CIGTS criteria with the visual field progression system used in the EMGT.77 While good specificity was reported for all 3 criteria, the EMGT identified progression earlier and in more patients compared to AGIS and CIGTS. Essentially these approaches represent various tradeoffs between sensitivity and specificity.
4. Trend analysis
Trend analysis determines whether progression is present in a series of visual field tests by applying linear regression or some of its variants (e.g. three-omitting,58 lasso56) to a series of visual fields.23 An important advantage of trend analysis is that it provides information not only about the presence of change, but also about the rate of change over time. Information about the rate of change is crucial to identify patients who are progressing rapidly (fast progressors) and need more intensive intervention. Trend analysis assumes that the changes are linear (i.e. they occur at a fixed rate over time) and requires a minimum of 5 or 6 visual fields to achieve good performance (high sensitivity and specificity). Section 7.1 provides an in-depth discussion of the relevance of testing frequency.
Linear regression can be applied to global indices, to clusters, or to individual points. Because progression may occur either through an expansion of an existing scotoma or through a general depression across the entire visual field, it is clinically relevant to assess change with global and local measurements.13,15,26 Linear regression applied to global indices and individual locations, and event-based analyses may each identify progression in different eyes. While their overall performance is similar for monitoring progression, only fair to moderate agreement was found among these analyses.32,44,139,144 De Moraes and coworkers reported good spatial agreement was achieved between the pointwise event-based GPA-EMGT criteria and the pointwise trend-based PROGRESSOR in eyes identified as progressing by both analyses.44 The degree of agreement between these analyses is dependent on various factors, including the status of disease, the extent and type of the subsequent visual field progression, the quality of the collected data, the temporal position of the progressing fields within the test series, and the strictness of selected criteria.
4.1. Trend analyses - Global indices
Global indices routinely used in clinical practice to assess glaucomatous defects include the mean deviation (MD), the pattern standard deviation (PSD) and the visual field index (VFI). MD is the average of the differences from the mean of age-adjusted normal values of all visual field locations tested. Positive values reflect visual field sensitivity that is better than the average normal, and negative values indicate sensitivity that is below the average normal values for a given age. The overall rate of visual field loss in glaucoma (in dB/year) can be estimated by applying linear regression to the MD obtained over a series of visual fields. A visual field is classified as progressing if a negative slope is significant with a P < 0.05. The MD is affected by glaucomatous progression, but also by other factors such as media opacities and poor refraction.109 The trend analyses of MD ignore the detailed spatial information within the field and were shown to be poorly correlated with clinical judgment. Furthermore, it is worthwhile to note that, for a similar rate of progression in dB/year, eyes with mild glaucoma lose more linear sensitivity over a given period of time than those with more severe damage.114 This is because a change of 3 units at one end of the scale does not reflect the same amount of change as a difference of 3 units at the other end of the scale due to the nonlinear nature of the dB scale.
PSD measures the irregularity in the visual field of a patient by averaging the absolute value of the difference between the threshold sensitivity value for each point and the average visual field sensitivity within the field. Large PSD values indicate the presence of localized damage, while normal visual fields and those with severe or diffuse loss will have low PSD. Trend analysis of PSD may fail to detect progression in cases of advanced glaucoma because the slopes tend to be flat due to a floor effect.
The VFI is a trend analysis that scales the overall visual field status from 100% (normal) to 0% (end stage glaucoma). The GPA II presents the linear regression of the VFI. The VFI estimates the rate of visual field deterioration based on the HFA PD values and weighs central loss more heavily than peripheral loss.23 When severe visual field damage is present, total deviation (TD) values are used instead of PD values. Based on the projection of the linear regression line, clinicians can use the VFI in conjunction with other clinical information to determine the extent of treatment intervention and to provide a prediction of future visual field status. The VFI detects progression that is most likely to affect visual function but has lower sensitivity to detect progression in early-stage glaucoma and provides variable results for cases with advanced glaucomatous visual field loss due to its reliance on PD probability plots.17,32
A number of studies have compared the ability of these indices to detect glaucomatous visual field progression. Gros-Otero and coworkers reported that the diagnosis of progression using the VFI was not significantly associated with IOP levels, which is a known risk factor for progression.74 The rates of change obtained with the VFI and the MD were found to correlate well and both detected a similar proportion of eyes with progression in different stages of glaucoma, although without perfect agreement.17,38 Since the VFI and the MD measure localized and diffuse damage, respectively, these two indices can be complementary to each other in monitoring glaucomatous progression.38 Gardiner and coworkers recently reported that MD may detect significant progression sooner than PSD or the VFI in patients with early or moderate glaucoma.60 In the first 5 years following diagnosis, the MD detected a larger proportion of patients with progression compared to the PSD or the VFI (138, 107, and 104 progressing eyes by MD, PSD, and VFI respectively in 508 glaucoma eyes). No single index, however, was the first to detect every case of progression.
Studies have also compared global trend-based analysis using VFI or MD with the event-based GPA analysis. Casas-Llera and coworkers found that the GPA II detected progression in 16.7% of eyes in which the trend analysis using VFI failed, and the GPA detected progression 6.8 months earlier than the VFI.32 In a recent study by Wu and colleagues, the two methods showed similar sensitivity to detect visual field progression when rigorously matched for specificity.200 Overall, moderate agreement was found between these methods.32,157,200 Since each method provides unique but complementary information, using global trend analyses and pointwise event analysis jointly may improve the detection of glaucoma progression. A Bayesian method combining the event analysis GPA and the trend analysis VFI of visual field progression was found to perform better than either method alone.127
Global analyses based on summary indices may not be as sensitive as pointwise analyses for early damage because small changes may not be reflected when averaging data across the entire field.120 In early disease, global analyses are less helpful to detect progression in view of the wide range of variability in normal subjects and the small effect of early visual field loss, especially when using the VFI.17,32 On the other hand, purely focal visual field changes occur relatively rarely in glaucoma patients. Artes and coworkers investigated longitudinal changes of the MD and general height of the visual field (defined by the 85th percentile of the TD values) in 168 glaucoma eyes with early to moderately advanced visual field loss at baseline and no clinical evidence of media opacity.15 Most glaucoma eyes showed negative general height slopes as well as negative MD slopes, indicating that glaucomatous progression almost always includes a diffuse component. Artes and colleagues further reported a significant diffuse component of progressive glaucomatous visual field loss in the Ocular Hypertension Treatment Study (OHTS) data.13 The magnitude of the diffuse loss was small (approximately 1–2 dB) in most participants, but was readily apparent from an assessment of more than 3,000 eyes of greater than 1,600 patients with a mean of 15 visual fields over more than 6 years. This small diffuse glaucomatous loss may not be evident for individual patients because other factors such as small pupils, refractive errors, and cataract development can also produce diffuse visual field loss. In the OHTS trial, however, proper refraction was performed at the perimeter bowl, pupil diameter was ≥ 3 mm and measurement of the Lens Opacity Classification System II revealed no indication of cataract development. The diffuse progressive loss was therefore clearly due to glaucomatous damage. The presence of a diffuse component to progression highlights the value of global trend analyses.
4.2. Trend analyses - Pointwise linear regression
Pointwise linear regression (PLR) evaluates longitudinal changes at individual visual field locations. PLR provides estimates of slope (dB/year) for each location based on threshold sensitivities, as well as a level of significance. This method provides an estimate of the rate of progression at each test location. The criteria for overall change have commonly been based on a fixed number of significantly changing test locations. The PROGRESSOR software (Medisoft, Ltd, Leeds, United Kingdom) performs this analysis and has been widely used in research settings to test the effectiveness of treatment and risk factors for glaucoma progression.45,54,68 Early studies have shown that PROGRESSOR has good agreement with the STATPAC GCP analysis in detecting progressing locations within the field,54 and may detect progression earlier than STATPAC.187 De Moraes and colleagues reported that PROGRESSOR detected a similar number of cases of glaucomatous visual field progression compared to the GPA procedure and that there was moderate agreement between PROGRESSOR and GPA.44 The time to detect progression, however, was reported to be longer with the PLR methods compared to the GCP and CIGTS methods.185
A potential shortcoming of the conventional PLR is that confounding factors, such as the aging effect and media opacities, are not teased out when using absolute threshold sensitivity. An alternative PLR developed to circumvent this limitation, used TD and PD values, but did not perform as well as the conventional PLR, especially in advanced glaucoma.119 Another potential limitation of PLR is that outlier values can have a large impact on the slope estimates. Gardiner and coworkers reported that truncating testing at unreliable locations (defined as below 15 to 19 dB) did not adversely affect the ability of the PLR to detect and monitor progression.64 Similarly, Wall and coworkers reported that censoring sensitivity values < 20 dB has relatively little impact on the pointwise progression rates in patients with mild to moderate glaucoma.193 Limiting testing to the range of sensitivities with good repeatability was therefore recommended to shorten the test and save the time to assess more clinically useful locations. De Moraes and coworkers reported an alternative truncated analysis, which uses the first and last 3 tests, offers better specificity than applying PLR to the entire series.47 While both approaches detected a similar number of progressing eyes and provided similar rate of change estimates, the truncated analysis showed fewer eyes presenting visual field improvement compared to PLR applied to the entire series of tests. This approach is similar in principle to the wait-and-see method proposed by Crabb and Garway-Heath (described in more details in Section 7.1).41
PLR provides useful spatial information to monitor glaucoma progression. There is, however, no consensus on what clinical standard should be adopted to classify progression at any given location in terms of slope magnitude, significance level, and confirmation on subsequent fields.110,185 Fitzke and coworkers proposed the criterion of a slope of less than −1.0 dB/year with an associated P < 0.01 significance level, which later was validated by Viswanathan and coworkers.187 Kummett and colleagues assessed the performance of various combinations of negative slope values and significance levels and found that a slope less than −1.2 dB/year with an associated P < 0.04 maximized the performance of PLR in a group of early to moderate glaucoma patients.110 Regardless of the criteria, using a single test location to determine progression may result in high false-positive rates, requiring longer follow-up periods to ensure high specificity.58,167,198
Given the anatomy of the retinal nerve fiber layer, criteria requiring at least 2 progressing locations along the same retinal nerve fiber bundle significantly enhance the specificity of PLR.46,48,198 Based on this information, spatially customized approaches utilizing individually derived test grids were developed to enhance the detection rate of progression.141 Alternatively, Gardiner and coworkers implemented the notion of “confirmation tests” to improve the specificity of PLR, and proposed a “three-omitting” method which required two confirmation fields to determine progression when omitting the field flagged as progression at first.58 Similarly, Vesti and colleagues proposed that a significant (P<0.01) slope of −1.0 dB/year or less in the same 2 or 3 test locations in 3 of 4 consecutive fields should be required to generate high specificity with PLR.185
Because focal visual field loss is a characteristic component of glaucomatous damage, pointwise analyses may be advantageous particularly when looking at locations that may be more predictive of future progression.166,184 Figure 2 presents an example of focal progression accompanied by the development of a paracentral scotoma. The detailed spatial information offered by PLR provides an opportunity to learn more about the focal nature of glaucomatous progression, allowing the development of more sophisticated strategies to detect progression.48,141,145,173 Pointwise analyses, however, overlook the diffuse component of glaucomatous visual field loss and are not well suited to assess this aspect of progression.15,72
Figure 2.
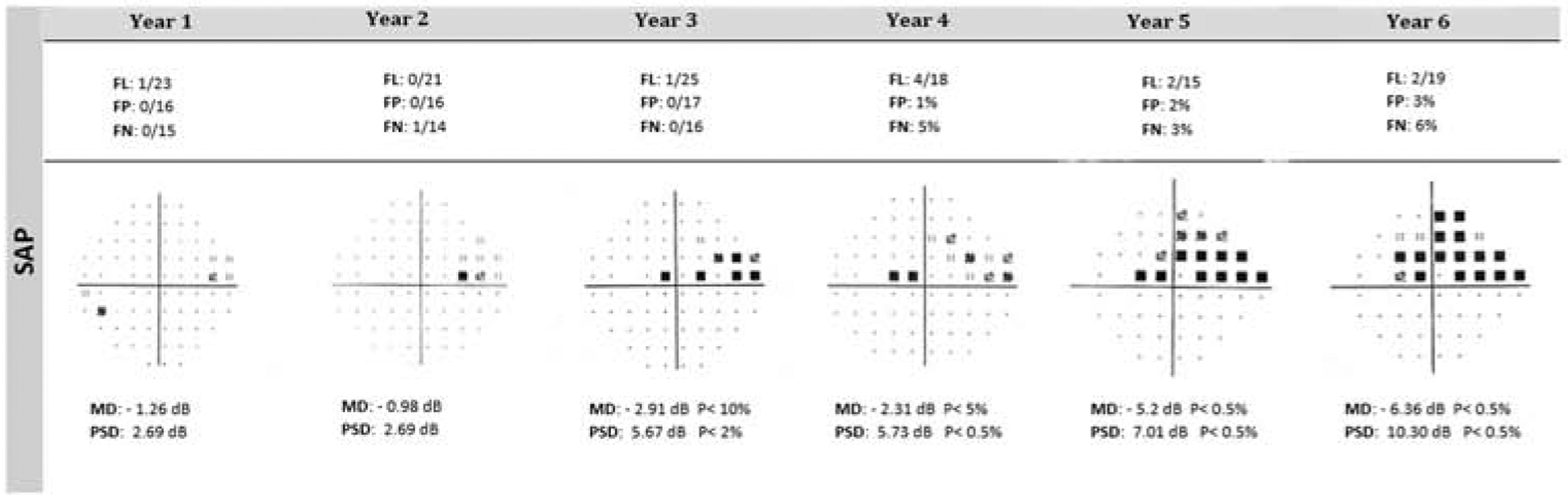
An example of focal progression is presented for the left eye, using the pattern deviation plot on SAP. Of note, is the development of a paracentral scotoma in the superior temporal field at Year 3. Paracentral scotomas need vigilant attention as they can significantly impair activities of daily living such as reading. The presence of even one significantly abnormal point in this area warrants longitudinal scrutiny. Static Automated Perimetry (SAP)
4.3. Trend analysis - Clusters
The inherent test-retest variability present at individual locations can lead to a high false-positive rate for PLR.14,46 A possible approach to reduce variability is to divide the visual field into clusters (or sectors) and monitor progression based on the average of the test locations within these clusters.174 Mayama and coworkers reported that regression analysis of the mean TD by sectors provided a good combination of sensitivity and specificity to determine progression compared with the global MD analysis, pointwise TD analysis or AGIS scores.120 The Octopus 900 EyeSuite software (Haag-Streit, Inc., Köniz, Switzerland) provides a cluster trend analysis (CTA), which investigates longitudinal change in the absolute cluster defect without correction. It also provides the Corrected Cluster Trend Analysis (CCTA), which investigates longitudinal change in the cluster defect corrected for diffuse change of the global visual field sensitivity. Using the predefined clusters in the EyeSuite software, Gardiner and coworkers showed that the CTA procedure detected and then confirmed progression sooner than either the global or pointwise analysis in early glaucoma.63 Naghizadeh and coworkers have also reported that the Octopus CTA and CCTA procedures provide clinically useful information regarding glaucomatous visual field progression.138 The CCTA detected about 2.9 times more progressing perimetric glaucoma eyes than event analysis and agreed well with the event analysis in the spatial characteristics. Aoki and coworkers showed that the CTA provides similar results as performing trend-analysis on the mean of the TD values.8 The CTA may also be more sensitive to focal visual field progression compared with global trend analysis. Their work suggests that defining progression as the presence of progression in 2 or more clusters may provide a good compromise between pointwise and global progression analyses.
Key points related to the cluster trend analysis are the methodology used to cluster test locations and the determination of the optimum number of clusters. Early studies established clusters based on cross-sectional correlations of visual field test locations.20,174 Alternatively, Nouri-Mahdavi and coworkers divided the HFA 24–2 field into 10 clusters based on pointwise rates of progression, which were, in addition, consistent with retinal nerve fiber layer bundle patterns.145 Using the minimum mean/medium split silhouette criteria, a method that objectively identifies fine structure in data, Hirasawa and coworkers divided the 24–2 field into 23 small clusters of test locations with similar rates of progression, named as “progression sectors”.83 They validated the usefulness of the “progression sectors” in predicting future visual field progression in both short (1–10 visual fields) and long (>10 visual fields) series compared to pointwise analysis and the cluster analysis based on the 10 clusters identified by Nouri-Mahdavi and coworkers.145
5. Sophisticated approaches
Researchers continue to develop new approaches to identify glaucoma progression. Some of these methods use sophisticated statistical and mathematical approaches. While they are promising, they are also limited by the inherent variability associated with visual field data. From a clinical standpoint, these sophisticated models are limited by their computationally intensive analysis procedures, extensive learning curve, and the lack of familiarity that clinicians have with this approach. An inadequate understanding of the underlying assumptions of the models, of their applicability and limitations, may compromise the use of these procedures for the appropriate management of glaucoma patients.
5.1. Bayesian analyses
Bayesian statistics are based on the notion that probabilities derived from the known data under investigation can be used to modify the estimates of unknown variables. Bayesian analysis specifies this modification statistically using a method known as Bayes’ theorem, which calculates the posterior probability (the revised probability of an event occurring after taking into consideration new information) by updating the prior probability (the probability of an event based on current knowledge). Bayesian analysis is essentially a procedure similar to weather forecasting, which adjusts the probability of events from previous determinations from population-based findings with new individual information. Typically, Bayesian approaches provide a data framework in which population-based prior information and empirical data from an individual patient could be combined to estimate a particular variable, for example, the rate of progression for this patient. This methodology offers a desirable alternative to the conventional ordinary least squares linear regression (OLSLR) used in visual field trend analyses, which assumes that the signal is distinguishable from noise in the data if there are sufficient and reliable measurements available for analysis. In fact, there is often large measurement variability in individual visual field data and also insufficient visits in clinical practice due to either patient burden or medical capacity. If the visual fields show large variability, or only a few measurements are available over time, the progression rate would be poorly estimated with OLSLR. In this situation, the precision of the estimates can be improved by borrowing strength from the population-based prior information. It is important to note, however, that this approach relies on high-quality prior information. As a surrogate for population-based prior information, the sample used needs to be similar to the individual patient of interest, and must be sufficiently large.
Several Bayesian (forecasting) models have been developed to improve the detection of glaucoma visual field progression. Medeiros and coworkers built a hierarchical Bayesian model to incorporate the GPA results from event analysis as the prior distribution into the estimates of VFI slopes in trend analysis.127 In eyes with confirmed progression by GPA, Bayesian slopes of VFI change detected more eyes as progressing than the OLSLR (63/64 eyes vs. 32/64 eyes, respectively), while both methods showed the same specificity of 96% in a stable glaucoma group. Bayesian slopes further detected an additional group of eyes as progressing but not by GPA. Empirical Bayesian estimates of slopes derived from growth mixture models were also found to be more accurate compared with the OLSLR method in predicting future VFI using the first 5 visual fields.130 Medeiros and coworkers further successfully developed a joint Bayesian hierarchical modeling approach for combining functional and structural measurements that takes into account the correlation between these tests and allows the prior information derived from structural measurement to influence the inferences on the significance of functional change and vice versa.123,128 In 405 glaucomatous and suspect eyes, the joint Bayesian approach detected a significantly larger proportion of eyes as having progressed over time compared with conventional OLSLR (22.7% vs. 12.8%), while having the same specificity of 100% in 29 healthy eyes. In a separate longitudinal study, the follow-up time was divided into two halves: the first half was used to calculate the slopes of change and the second period was used to test the predictions. The slopes obtained with the Bayesian joint regression modeling approach were more accurate than those of OLSLR in predicting the future MD and rim area values.
In conventional Bayesian methods, the prior distribution must first be specified. It quantifies the frequency with which various progression rates are expected to occur in the population, and then the information from the prior is combined with a patient’s data to produce estimates of progression rate for this patient. Since the true rate of progression of the population can never be fully known, Bayesian analyses commonly use large samples3,123,127,128 or the data itself in the case of empirical Bayesian methods.130 Based on statistical bootstrapping analysis, Anderson reported that decreasing sample size increases the variability in estimating the prior distribution.3 Variability in the distribution tails was estimated to produce a 3.5-fold variation in fast progressors for a cohort size of 200, while falling to a 1.8- and 1.3-fold for cohort sizes of 800 and 3200, respectively. Hence, care should be taken in interpreting Bayesian estimates when the prior information is derived from a small sample of noisy data. Furthermore, it is also important to note that a Bayesian estimate of rate may not be ideal for all individual patients in all situations even when the prior information is derived from a large sample.6 Efforts have been made to incorporate various risk factors associated with glaucoma progression, such as higher IOP, thinner central corneal thickness, and presence of progressive optic disc damage into the Bayesian decision framework to provide better estimates for individual patients.129 Medeiros and coworkers showed the estimates of individual slopes of MD change can be improved by incorporating structural and other risk factor information into a Bayesian regression model.129 Alternatively, Russell and coworkers developed a Bayesian normal error linear regression approach, which integrates individual structural measurement separately as the informative prior for each patient.161 In a group of ocular hypertension patients, the linear regression of rim area was computed as the prior and more accurate estimates of the rate of change in mean sensitivity were obtained compared with the OLSLR, especially in short time series of up to 8 tests.
The Markov chain Monte Carlo method has been commonly used to calculate the posterior distributions of the parameters of interest in Bayesian approaches.123,127,128,161,194 This method can be computationally cumbersome, however, and rather time-consuming for fitting complex models to relatively large datasets. Considering the computational efficiency, Murata and coworkers developed a variational Bayesian linear regression approach using the variational approximation instead of the Markov chain Monte Carlo method.135 Using this method, they showed that it would take only 0.2 seconds to predict the future status of a patient based on a visual field series of 9 tests. This advantage in computational efficiency would be beneficial for its future implementation in clinical settings. The prediction accuracy of the variational Bayesian model was shown to surpass that of the OLSLR in predicting visual field TD or mean TD values. For the TD of the 11th visual field, the prediction error with the variational Bayesian model using only the initial two visual field tests was smaller than that with the conventional OLSLR using 7 visual field tests. Murata and coworkers further successfully validated their model with two datasets external to the training dataset.136
The powerful Bayesian frameworks also provide the opportunity to jointly estimate longitudinal changes at each test location by considering the spatial correlation present within the visual field. Betz-Stablein and coworkers fitted conditional autoregressive priors, weighted by spatial and spatiotemporal correlations over visual fields, within a Bayesian framework using Metropolis-Hastings algorithms.25 The model was designed to account for several physiologic features, such as the nerve fibers serving adjacent test locations, the presence of the blind spot, and large measurement fluctuation. By pooling spatial correlations within and between proximate sectors, the model was shown to be robust to outlier measurements at individual test locations and provide better receiver operating characteristics compared with the conventional PLR methods. Furthermore, the model was designed to present heat maps showing the slopes at each location in a manner that is intuitive to clinicians. Likewise, Warren and colleagues developed a spatial probit regression model in the Bayesian setting, which jointly consider highly correlated changes across the entire visual field when determining the progression status based on clinician expert consensus.194 VanBuren and coworkers built a Bayesian hierarchical modeling framework, which incorporated a dimension reduction matrix and individual baseline covariates, to analyze the direction and rate of visual field progression individually and epidemiologically.183
5.2. Machine learning classifiers
Machine learning classifiers (MLC) are designed to allow computers to extract information from a training dataset using iterative processes. With the strength of unconstrained statistical assumptions, MLCs are more adaptable to complex data such as longitudinal pointwise visual field data.29 These classifiers can be supervised (specific prior information given to them) or unsupervised (guided by patterns within the data). Once trained, MLCs are applied to a different dataset in which they identify progression. One drawback of this approach is that MLCs are somewhat of a black box, making it difficult to understand the factors that drive their results.
Sample and coworkers first validated the ability of a variational Bayesian independent component analysis mixture model (VIM), a form of unsupervised machine learning, to quantitatively identify areas of progression in full-threshold visual field data.162 In their model, patterns of glaucomatous visual field defects were generated, and then changes in one pattern of visual field defect were assessed with changes in other areas of the same visual field serving as a control for variability. This approach was based on the assumption that progression often occurs within or adjacent to the existing baseline defect and new scotomas rarely occur during follow-up.26 The VIM model identified a higher percentage of progressing eyes compared with the AGIS and the EMGT criteria (32, 10 and 11 eyes, respectively out of 191 eyes). This study group further validated the usefulness of the VIM model in SITA visual field data by separating them into distinctly different yet recognizable patterns of glaucomatous field defects as a preliminary process for detecting progression.69 To maximize the chance to detect true progression, the progression of patterns (POP) was then developed on the basis of the patterns of glaucomatous visual field defects generated by the VIM.70 Since not all areas of visual field are changing over time, POP concentrates on the particular areas of the visual field with the most change and eliminates noisy areas that have little or no real change, thereby improving the signal to noise ratio. Goldbaum and coworkers reported that POP detected more progressing eyes than the conventional GPA procedure in glaucoma eyes (16.0% vs. 7.3%, respectively) and in a group of eyes with stereophotographic evidence of progressive glaucomatous optic neuropathy (26.3% vs. 14.5%, respectively).70 The POP and the linear regression of VFI or MD detected similar proportion of progressing cases in eyes with progressive glaucomatous optic neuropathy, however, the average rate of progression was 1.7 and 1.6 times faster when estimated with POP compared to VFI or MD, respectively.
Computational efficiency is an important factor that must be taken into consideration for future application in clinical settings. The VIM is a semiautomatic approach as the user manually selects the model with the best average of sensitivity and specificity among a large number of VIM models and the optimal model is retrained to further improve its diagnostic accuracy. Alternatively, Yousefi and coworkers built a Gaussian mixture-model with expectation maximization (GEM), which is fully automatic and produces similar outputs, but learns 50 times faster than the VIM approach.204 Both the VIM and the GEM approaches were designed to provide clinicians with localized progression information in specific patterns of glaucomatous visual field defects. In contrast to the simultaneously converging VIM environment, GEM uses a modular approach to first generate the clusters of disease severity and then identifies defect patterns, which could be more adaptable to clinical data. Yousefi and coworkers reported that the GEM-POP provided better receiver operating characteristics to identify progression compared with the linear regression of MD or VFI, and the permutation of pointwise linear regression approach (PoPLR, described below), with receiver operating characteristic curve areas of 0.86, 0.69, 0.76, and 0.81 respectively. The GEM-POP may therefore outperform the linear regression of MD and VFI, and PoPLR in detecting glaucomatous progression.202
5.3. PoPLR
A drawback of the conventional PLR is that the criteria can be somewhat arbitrary based on different definitions of slope and associated P value for each test location, and number of changing locations. In view of this, O’Leary and coworkers developed the PoPLR approach that combines the significance of progression at each location into a single statistic using the Truncated Product method, and then calculates the P value for overall change through permutation analysis.147 PoPLR is an individualized approach based on the assumption that the order of the tests is irrelevant unless a real change has taken place, allowing a better control in the specificity to detect progression. PoPLR makes no assumptions about the distribution of measurement variability. Since the specificity of PoPLR is independent of factors such as baseline visual field damage, length of follow-up, number of test locations, and dB scale, it may be used to compare visual field progression across different types of follow-up protocols and tests.147,158 In a cohort study of 944 glaucoma eyes, PoPLR detected 12%, 29%, and 42% of cases as progression at the fifth, eighth, and final examinations, respectively, at P < 0.05, performing consistently better than PLR at matched specificity.
5.4. ANSWERS
Analysis with Non-Stationary Weibull Error Regression and Spatial Enhancement (ANSWERS) is a trend analysis approach that incorporates the nonstationary variability at different levels of visual field severity in contrast to the conventional OLSLR, which assumes fixed and normally distributed errors.207 It also takes into account the spatial correlation among test locations using a Bayesian framework and outputs the estimates of the rates of change for both a global index and each location. Zhu and coworkers reported that ANSWERS is more sensitive to detect visual field progression and predicts future visual field loss better than linear regression of MD and PoPLR, especially in short time series.207 In their study, 75% of visual field series were better predicted by the ANSWERS compared with PoPLR, and the prediction error of ANSWERS was 15% lower than that of PoPLR. Garway-Heath and coworkers further developed ANSWERS to combine visual field and optical coherence tomography (OCT) measurements to detect glaucomatous progression and found that this method outperformed the field-only approach.66,67 At a specificity of 95%, the combined ANSWERS approach achieved a sensitivity of 70% to detect progression, while the field-only ANSWERS and PoPLR showed the similar sensitivity of 35%. Nevertheless, the present version of combined method failed to differentiate eyes on treatment from those not on treatment, which may therefore limit its use to evaluate treatment effects in clinical trials.
5.5. Non-linear models
OLSLR, the most commonly used method for trend analysis, assumes that change occurs at a constant rate over time. If the rate of change over time is not constant, then non-linear models may be better-suited to monitor progression. Several non-linear models have been investigated to fit longitudinal visual field data and predict future damage. The exponential regression was initially proposed based on the notion that the rate of change of the threshold sensitivity values slows as they approach 0 dB in a way that is consistent with an exponential curve.30 Evidence has shown that exponential models can accurately track visual field change at the majority of test locations across a wide range of glaucoma severity, and may predict future status better than, or at least as well as OLSLR.21,30,112,151,152 The pointwise exponential model also allows the isolation of faster and slower regions of visual field deterioration for a given individual.30 To address the fact that there is a slow approach towards 0 dB in visual field sensitivities, Russell and Crabb alternatively proposed a linear regression model (Tobit) which censors values that are at or close to 0 dB sensitivity.159
The logistic model is another non-linear model in which there is an asymptote at both ends of the visual field data series.36 This model may provide the best fit for longitudinal visual field data over a long time period, but was not found to outperform exponential models in predicting future visual field status.36 Controversy remains as to whether a model’s goodness of fit for visual field data is indicative of its predictive accuracy for future status. Taketani and coworkers reported that neither exponential nor logistic models exhibit predictive accuracy superior to that of linear regression.177
6. Perimetric Tests Used to Identify Visual Field Progression
Many different approaches have been developed to evaluate various properties of visual function.95 While most of them have not been incorporated into conventional clinical diagnostic testing, several procedures have been found to be clinically useful in detecting and monitoring visual field progression. In this section, we review static automated perimetry (SAP), which is the clinical standard to identify progression. We also review two function-specific tests, short-wavelength automated perimetry and frequency-doubling technology perimetry, which have been assessed to monitor glaucomatous progression. Other procedures are not available commercially and do not have an accepted normative database or a standardized statistical analysis procedure, and will not be discussed in this paper.
6.1. Static Automated Perimetry
SAP is the most common procedure used for evaluating the visual field. Most assessments of progression are based on this test, with either event- or trend-based analyses, or more sophisticated approaches. SAP typically presents a small white target, superimposed on a uniform white background. Static perimetry is performed by placing the target at fixed locations and varying the intensity of light to determine the threshold sensitivity.154 Depending on which test is used, the number of locations tested and their spatial distribution vary. A pseudorandom procedure is used to present stimuli at various locations throughout the test procedure. Patients are asked to fixate and click a response button when they see a flash of light. Threshold values can be estimated in different ways.154 In some tests, the intensity of the light is increased when patients do not respond and decreased when they respond. The amount by which the light is increased or dimmed varies, becoming smaller as the threshold is neared (i.e. staircase procedure). To reduce the duration of the test, more recent algorithms use a Bayesian approach to determine thresholds. These algorithms take into account, for example, sensitivities at nearby locations to quickly narrow in on the threshold values.
6.2. Short-Wavelength Automated Perimetry
The sensitivity to different wavelengths of light (color perimetry) has been evaluated in glaucoma patients. These tests have not gained popularity clinically, however, because they are difficult for the patients to perform, and adequate equipment calibration can be difficult to achieve and maintain in a busy clinical setting.155 In order to isolate and measure the sensitivity of individual color vision mechanisms, specific background and stimulus conditions (luminance, wavelength distribution, stimulus size, etc) are used. Many years ago, Stiles developed the two-color increment threshold procedure to accomplish this task.170 This technique allowed investigators to independently measure the sensitivity profiles of rod mechanisms and the short (“blue”), middle (“green”) and long (“red”) wavelength sensitive cone mechanisms. By utilizing an intense broad band yellow background to suppress the sensitivity of middle and long wavelength mechanisms in conjunction with presentation of a large short wavelength stimulus, it is possible to target the short-wavelength sensitive pathway and measure its sensitivity.170,171 This procedure was applied to automated perimetry and is generally referred to as Short-Wavelength Automated Perimetry (SWAP), which consists of a 100 cd/m2 broadband yellow background upon which a size V narrowband short wavelength (440 nm) stimulus is superimposed.163 Using these stimulus conditions, it is possible to achieve at least 10 dB (1 log unit) of short wavelength sensitivity isolation. The isolation of short-wavelength mechanisms can be achieved in visual field regions with moderate to advanced visual field sensitivity loss.49
Early studies reported that SWAP was able to detect visual field progression 1 to 3 years before SAP and that the rate of change was shown to be faster for SWAP compared to SAP.96 SWAP defects were shown to predict future progression on SAP95 and to identify more patients with change on structure. Recent investigations from independent laboratories, however, have reported that SWAP and SAP demonstrate similar properties in the ability to detect glaucomatous visual field progression.22,181 This may be due, among other factors, to improvements in the algorithms used for SAP. In the follow-up of 416 ocular hypertension subjects, van der Schoot and coworkers reported that SWAP detected earlier glaucomatous damage than SAP in only 2 eyes (up to 18 months), whereas SAP detected earlier glaucomatous damage than SWAP in 15 eyes.181 Many patients also feel that SWAP is a more difficult visual field test to perform compared to SAP. Figure 3 presents an example of progression on SWAP.
Figure 3.
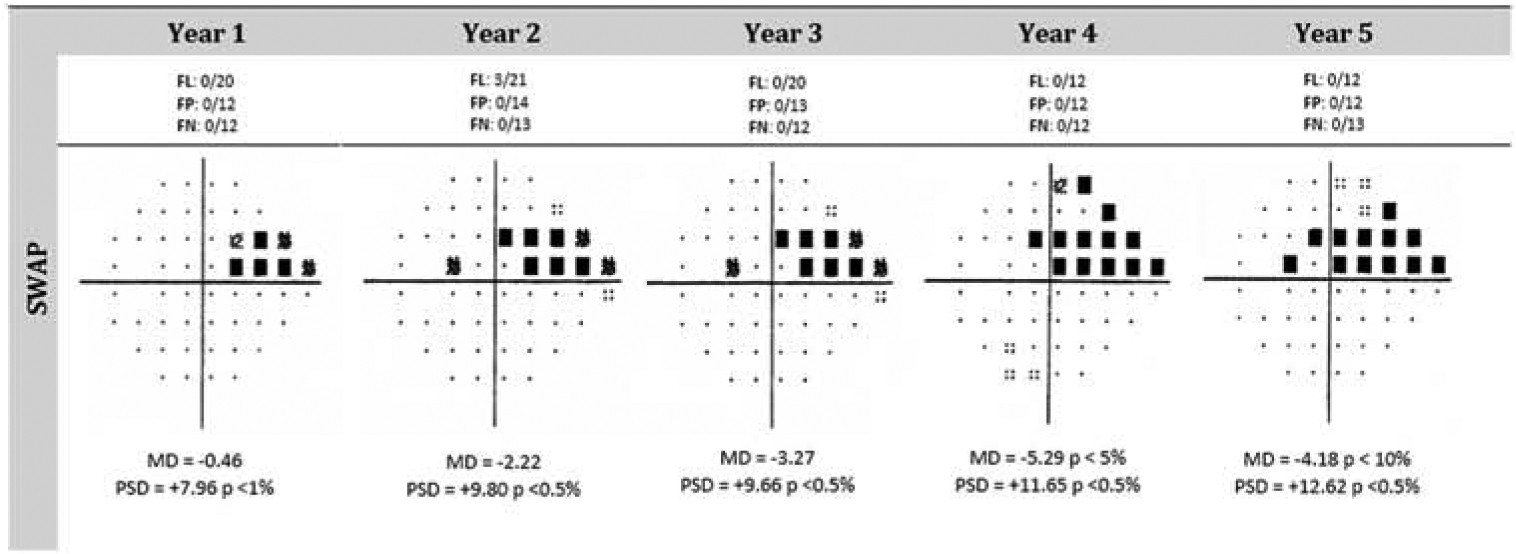
Progression can be observed over a five-year period as represented by the SWAP pattern deviation plots. Both the MD and PSD values worsen over time. Furthermore, an inspection of the plots shows that the defect worsens at several locations over time and that new defects appear. The large nasal step deepened over time and elongated along the anatomy of retinal nerve fibers. Short-Wavelength Automated Perimetry (SWAP)
6.3. Frequency-Doubling Technology and Humphrey Matrix Perimetry
When a low spatial frequency (less than 1 cycle per degree) sinusoidal grating undergoes high temporal frequency (greater than 15 hertz) counterphase flicker, the display appears to have approximately twice as many light and dark bars as are physically present, which forms the basis for referring to this procedure as frequency-doubling technology (FDT) perimetry.4 It was originally described by Kelly103,104 and was later attributed to nonlinear magnocellular (My cell) retinal ganglion cell (RGC) mechanisms that perform a rectification of the sinusoidal stimulus.118 Subsequent psychophysical and electrophysical investigations have revealed, however, that this effect is not determined by rectification, but is rather produced by a combined response of different types of RGCs that are processed by higher visual mechanisms.175,197,205 The FDT, which targets the magnocellular pathway, was developed as a function-specific test for early detection of glaucomatous visual field loss. While glaucoma does not selectively affect this pathway, the function-specific nature of the FDT stimulus reduces the redundancy within the visual system, which may explain why this test performs better than SAP in some patients.
The FDT procedure and its more recent version, Humphrey Matrix Perimetry (Carl Zeiss Meditec Inc., Dublin, California) using Welch-Allyn technology, are commercially available.4,7,53 FDT has been reported to have lower test-retest variability and to be less affected by blur and defocus than SAP.4,16,34,40,168,169 Conversion to glaucoma as well as visual field progression can be detected with FDT in glaucoma suspects89,176 and diagnosed glaucoma patients.76,88,196 Examples of progression on the original and Matrix FDT test are shown in Figure 4. One study reported that prediction of conversion to glaucoma is better achieved by FDT compared to SAP, but this was accomplished at the expense of slightly lower specificity.176 Overall, studies show that FDT identifies progression in a similar proportion of patients as SAP,88,89,196 and the two tests have comparable rates of change.89 The rate of change on FDT was shown to be highly predictive of the development of visual field loss on SAP in glaucoma suspects.132 Some studies reported that FDT was able to detect and monitor progressive visual field changes that are not revealed by SAP.89,117,126,132 Finally, SAP and FDT are consistently shown to identify progression in different patients, and the reasons for this are not clear.88,196
Figure 4.
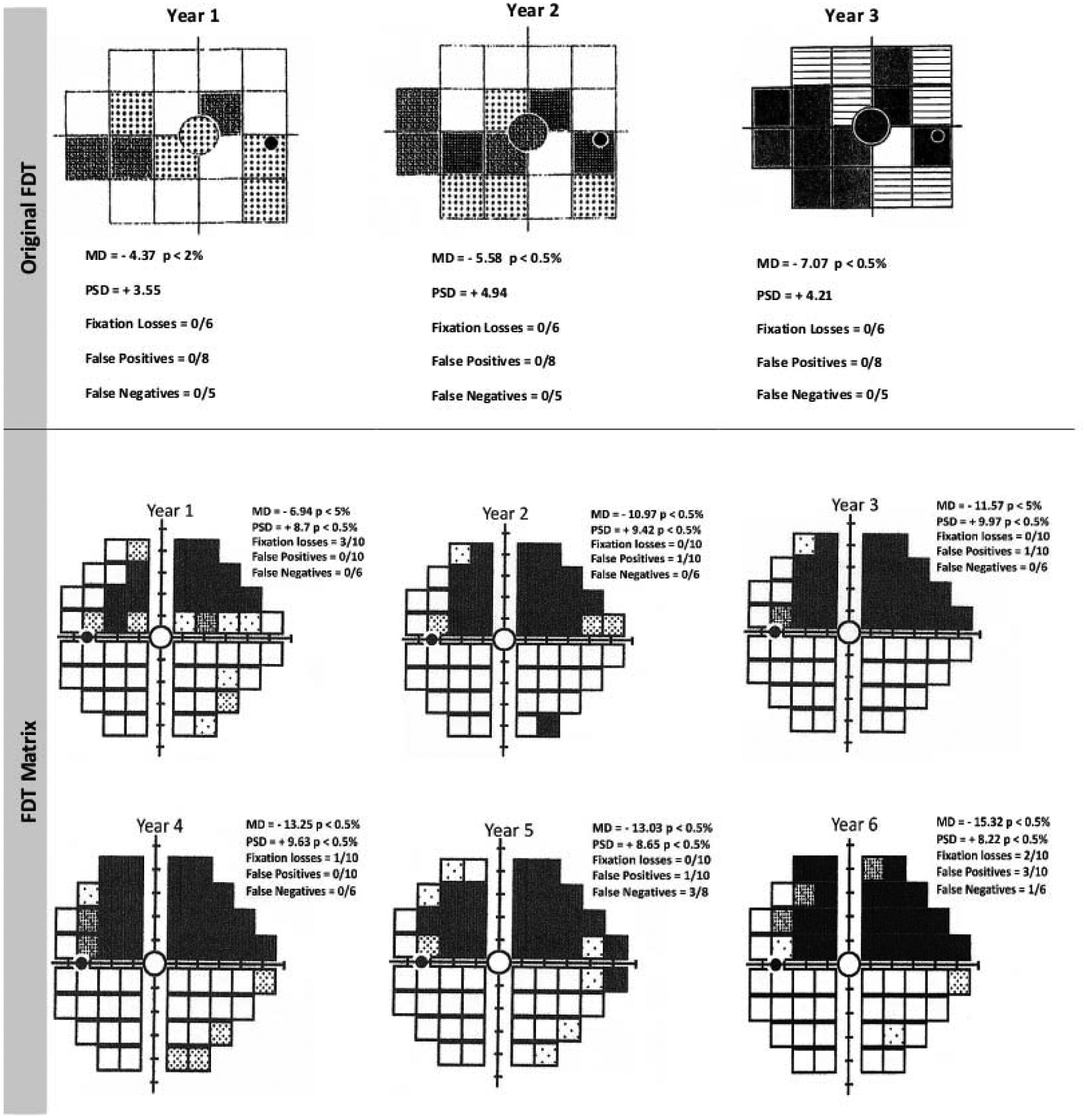
The longitudinal data for this patient show progression on the original Frequency-Doubling Technology (FDT) Perimetry test for the right eye (upper panel). The pattern deviation plots show an expansion of the scotoma as well as deepening of the defects in several locations. Worsening is also observed on the Mean Deviation (MD) and Pattern Standard Deviation (PSD) across this visual field series. An example of progression of a superior arcuate deficit for the left eye of a glaucoma patient obtained through six years of testing with the 24–2 test pattern on the Humphrey Matrix FDT perimeter. Note that there are also changes in MD and PSD during this time period. FL: Fixation Loss; FP: False Positive; FN: False Negative; MD: Mean Deviation; PSD: Pattern Standard Deviation
7. Other considerations to identify visual field progression
7.1. Testing frequency
Testing frequency is a major factor to be considered in monitoring glaucomatous progression. Testing glaucoma patients at an optimal frequency helps eye care specialists detect progression and determine treatment correctly and in a timely fashion.146,153,201 An inadequate testing frequency may delay the detection of progression and increase the likelihood of adverse disease outcomes.5,108 In routine clinical practice, visual field tests are commonly performed approximately once35,79,107,160 or twice per year.9,45,55 Based on simulated statistical power calculations, Chauhan and coworkers suggested that newly diagnosed glaucoma patients should be tested with SAP 3 times per year during the first 2 years following diagnosis.33 This recommendation has been adopted in the European Glaucoma Society guidelines,52 but is not consistently met in multicenter cross-sectional investigations, in which most patients only undergo 2 to 3 visual field tests in the first 2 years after diagnosis.42,57 Wu and coworkers reported that increasing the number of tests does not decrease the time required to detect progression in a proportional manner.201 By incorporating population-based variability data in computer simulations, they showed that testing performed at a frequency of once and twice per year identified 80% of eyes with a loss of 2 dB/year on MD after 3.3 (once/year) and 2.4 (twice/year) years, respectively. Increasing the testing frequency to 3 tests per year yielded only a small improvement to 2.1 years. They therefore suggested that testing at a frequency of twice per year after 2 reliable baseline tests may provide a good compromise for detecting progression and controlling the burden that frequent testing places on health care resources in clinical practice.
Instead of testing at fixed regular intervals, Crabb and coworkers proposed a wait-and-see approach in which visual field tests were clustered at baseline and then again at the end of a 2-year period based on computer simulations.41 No tests were performed between the baseline and the 2-year end. They hypothesized that the wait-and-see approach would improve the detection of progression compared to testing performed at regular intervals (e.g. at 4 or 6 month-intervals). A significantly larger proportion of rapidly progressing simulated patients was detected by the wait-and-see approach compared with testing at regular intervals. The lower rate of false positive obtained with the wait-and-see approach (0.4% versus up to 5.9%) is an advantage that outweighs the slightly longer overall average detection time. A likely explanation for the benefits of the wait-and-see approach is that data points near the beginning and end of a time period may have greater influence than intermediate data points for trend analysis (linear regression).
Flexible testing schedules have also been investigated to improve the detection of progression. Jansonius and coworkers found that testing frequency can be optimized from fixed-space to adaptive intervals. When a patient is apparently stable, the next test could be postponed; but the next test should be performed at a shorter interval in cases of suspected progression.93,94 This approach would allow clinicians to optimize the limited clinical resources at their disposal by testing stable patients less frequently and testing patients at risk of progression at shorter intervals. Schell and coworkers, using Kalman filters, developed a dynamic forecasting model to determine the optimal timing for future tests to monitor progression by incorporating most recent visual field and IOP measurements.165 They showed the dynamic approach detected glaucoma progression 57% earlier than following a fixed yearly testing schedule, without increasing the total number of visual field tests and IOP measurements required.
Some investigators assessed the optimal testing frequency to identify progression when using pointwise analyses. Spry and coworkers applied simulations to PLR and showed that it can detect a 1 dB/year rate of visual field loss.167 To maintain high specificity, however, the slope of the regression line must be significantly different from zero, and a minimum of 7 to 8 annual visual field test results is recommended. Gardiner and coworkers found that, as the test frequency increased, the sensitivity of PLR increased, while the specificity decreased. Based on simulations, a frequency of 3 tests per year was then recommended to achieve a good compromise between sensitivity and specificity for PLR.59
In clinical settings, multiple factors must be taken into consideration to determine the optimum testing frequency to monitor progression in individual patients. These include intra-individual measurement variability, stage of disease, age and life expectancy.27,28,42 A patient with high test- retest variability would require more frequent tests to rule out the “noise” than those with low test-retest variability. Given that glaucoma leads to irreversible visual field loss, patients with more advanced visual field loss at diagnosis may require more frequent testing to avoid significant impairment over time. Saunders and coworkers combined the residual life expectancy derived from the UK National Statistics with the linear regression of MD to predict the MD values at the end of the expected lifetime for each patient in a large retrospective study.164 The likelihood of patients suffering visual impairment in their lifetime was linked to the visual field damage severity at diagnosis, as more than 90% of the patients predicted to progress to statutory blindness (MD of −22 dB or worse) had a MD worse than −6 dB in at least one eye at presentation. More frequent testing might also be required for patients presenting with the disease at a younger age. Younger patients are likely to cope with the disease for many years to come and visual loss, if not detected early, can progress to more severe stages in later years and also to a loss of productivity in the working years, particularly in cases of fast progression. In contrast, less frequent testing could be considered for an 85-year-old patient with early glaucoma who has a variety of systemic diseases. Less frequent testing would be needed in such patients because it is unlikely that the disease would progress to meaningful impairment because of the expected lifespan. Furthermore, given the presence of comorbidities, it may be better to focus on those diseases that inconvenience the patient the most.
From a societal perspective, there is a trade-off between the frequency of visual field testing and cost. Based on computer simulations, van Gestel and coworkers showed that testing every 12 months may be more cost-effective compared to every 6 or 24 months in the long run.182 Using a health economic model, Crabb and coworkers suggested that increased early visual field monitoring (3 tests per year in the first 2 years after diagnosis) may be cost-effective.42 The same group further examined the cost-effectiveness of increasing frequency of visual field tests on groups of patients stratified by age and severity of glaucoma, and found that increasing visual field monitoring at the earliest stages of follow-up would be more cost-effective than a yearly test schedule, especially for younger patients.27
7.2. Minimum length of follow-up
In order to obtain a reliable assessment of visual field progression, a minimum number of visual field tests are required. An advantage of trend analysis, in contrast to event analysis, is that it allows one to use all the data available in a follow-up series to quantify progression. The rates of change, however, can be underestimated when the entire series is used, as patients may be stable for a long time before progression begins.17 In this view, it may be useful to have different fits for different sections of the longitudinal data199 or to use a nonlinear analysis procedure.36,152 Using the Ocular Hypertension Treatment Study (OHTS) data, Gardiner and coworkers reported that linear trend analysis detected more progression using shorter recent subseries (between 6 and 9 tests) rather than using the entire series.61
Multiple factors need to be taken into consideration to determine the optimum series length to detect progression in trend analysis, such as test frequency, measurement variability, and disease severity. For example, longer series of visual field tests or more frequent tests may be needed to obtain more accurate estimates of rates of change in advanced cases with high measurement variability. In addition, the optimum series length also varies with different global visual field indices. In a cohort tested every 6 months, Gardiner and coworker showed the MD may detect progression more than 1 year earlier than the VFI and more than 3 years earlier than the PSD.60 Increased odds of detecting progression with PLR analysis were also associated with longer duration of follow-up.143,185
7.3. Population versus individualized approaches
The determination of visual field progression is complicated by the substantial amount of variability present in visual field tests. In addition, it is often not possible to obtain an adequate number of visual field tests due to either limited time or economic burden in a clinical setting. In such circumstances, it would be helpful to identify progression by leveraging information from population data. Based on this rationale, various population-based approaches have been developed to monitor visual field progression, such as Bayesian analyses,123,127–129,135,136,183,194 machine learning classifiers,69,70,162,202–204 and Kalman filters.102,165 Bayesian analyses typically use information from a large sample to update the estimate in an individual patient. Machine learning classifiers are unconstrained by statistical assumptions and exploit the patterns in data using iterative processes. Kalman filters combine multiple relevant measurements for optimal noise reduction (e.g. visual field test and IOP measurement) and integrate population-based understanding of the natural history of the disease with the individual patient’s disease dynamics. The prospects of these sophisticated population-based approaches were shown to be promising, however, none of them has been widely accepted and further implemented into clinical practice yet. Furthermore, controversy remains in considering the benefits of population-based approaches because of the complexity of the prior information from population data.3,6
On the other hand, efforts have been made to improve the ability of individual approaches to assess progression. For example, PoPLR used a statistical significance derived from repeated reordering of individual visual field series to replace the conventional P value for visual field progression.147 ANSWERS took into account the increasing measurement variability present as glaucoma progresses and the spatial correlation among test locations within the visual field in contrast to the assumed fixed and normally distributed variability used in the conventional OLSLR approach.207
7.4. Parametric versus non-parametric approaches
Parametric analysis procedures can be performed when the data is consistent with a normal distribution (bell-shaped curve) of values, whereas nonparametric methods can be used when the data are not normally distributed. The HFA and Octopus perimeters provide both parametric and nonparametric analysis methods. Several parametric algorithms for progression analysis have been developed for SAP, often as part of large glaucoma trials. To date, only the EMGT algorithm (the GPA) has been implemented into commercially available software (Carl Zeiss Meditec Inc., Dublin, California). These approaches efficiently summarize progression distributions and offer metrics beyond the mode and interquartile range.
The rate of visual field change is a key prognostic factor in monitoring glaucoma progression. The population distribution of glaucoma progression rates can be skewed, which has a longer tail for negative rates of progression than for positive rates.35,78 One way to efficiently quantify distributions is by fitting them with parametric models. Anderson and Johnson reported a modified hyperbolic secant model fits the general shape of the distribution well in simulated data and can specify the mode and slopes of the upper and lower tails.6 Anderson further tested the ability of three parametric models to fit three independent datasets with different rate of change distributions.2 The parametric models investigated were either a modified hyperbolic secant model, a modified Gaussian model, or a modified Cauchy model. Summing the evidence across all datasets, the modified hyperbolic secant model was the best-fitting and performed well for all distributions investigated.
Alternatively, a non-parametric approach (NPA) was designed to imitate routine clinical evaluation to rank visual fields based on the MD values.92,148,195 With the NPA, suspected progression, possible progression, and likely progression are determined respectively if one, two, and three consecutive visual fields show an MD lower than the lowest MD of baseline visual fields. This approach is simple to use and to understand, and not dependent on software, which makes its use possible in a wide range of clinical settings. It can be applied to all disease stages and used with visual fields from perimeters in different locations. Wesselink and coworkers reported that the NPA reached a fairly good agreement with the GPA195 and in advanced cases, NPA labeled more progressing eyes than the GPA.195 The specificity of NPA can be improved when more follow-up confirmations are included.92 In an early glaucoma cohort, Pantalon and coworkers found that the NPA may tend to overestimate progression compared to the GPA; however, the authors suggested that it can be of value to identify more fast progressors at an early stage.148
7.5. Peripheral versus central change
Considerable evidence suggests that glaucoma affects the central area of the visual field, which has the highest density of RGCs within the retina and is most important for visual function.1,86,150,173 Clinicians should be aware that progression in the macula is common and can be missed and/or underestimated with standard visual field tests that use a 6 degrees grid, such as the 24–2 test. Previous studies found that glaucoma patients can have a normal 24–2 test result, but an abnormal 10–2 test result, and 10–2 test may detect more progressing eyes than 24–2 test in patients with the macula involved, such as early parafoveal scotoma.86,150 An example of progression observed on the 10–2 without change on the 24–2 is shown in Figure 5. In a group of glaucoma eyes with initial parafoveal scotoma, Park and coworkers reported that the 10–2 test detected more progressing eyes than did the 24–2 test (48% vs. 22%) based on pointwise analyses, and about two thirds of progressing eyes in the 10–2 analysis were missed in the 24–2 analysis.150 This difference is likely due to the fact that the 24–2 SAP test only has 12 test points located within the area tested by the 10–2 test, while the 10–2 test has 68 test points arranged in a closely spaced grid. Therefore, it could be more sensitive to use tests that assess the central area of the visual field to monitor central changes rather than using tests that extend into the periphery. Based on sensitivity/specificity consideration, De Moraes and coworkers suggested a pointwise progression criterion for the 10–2 test that requires progression faster than −1.0 dB/year at P < 0.01 in at least 3 spatially correlated test points.48
Figure 5.
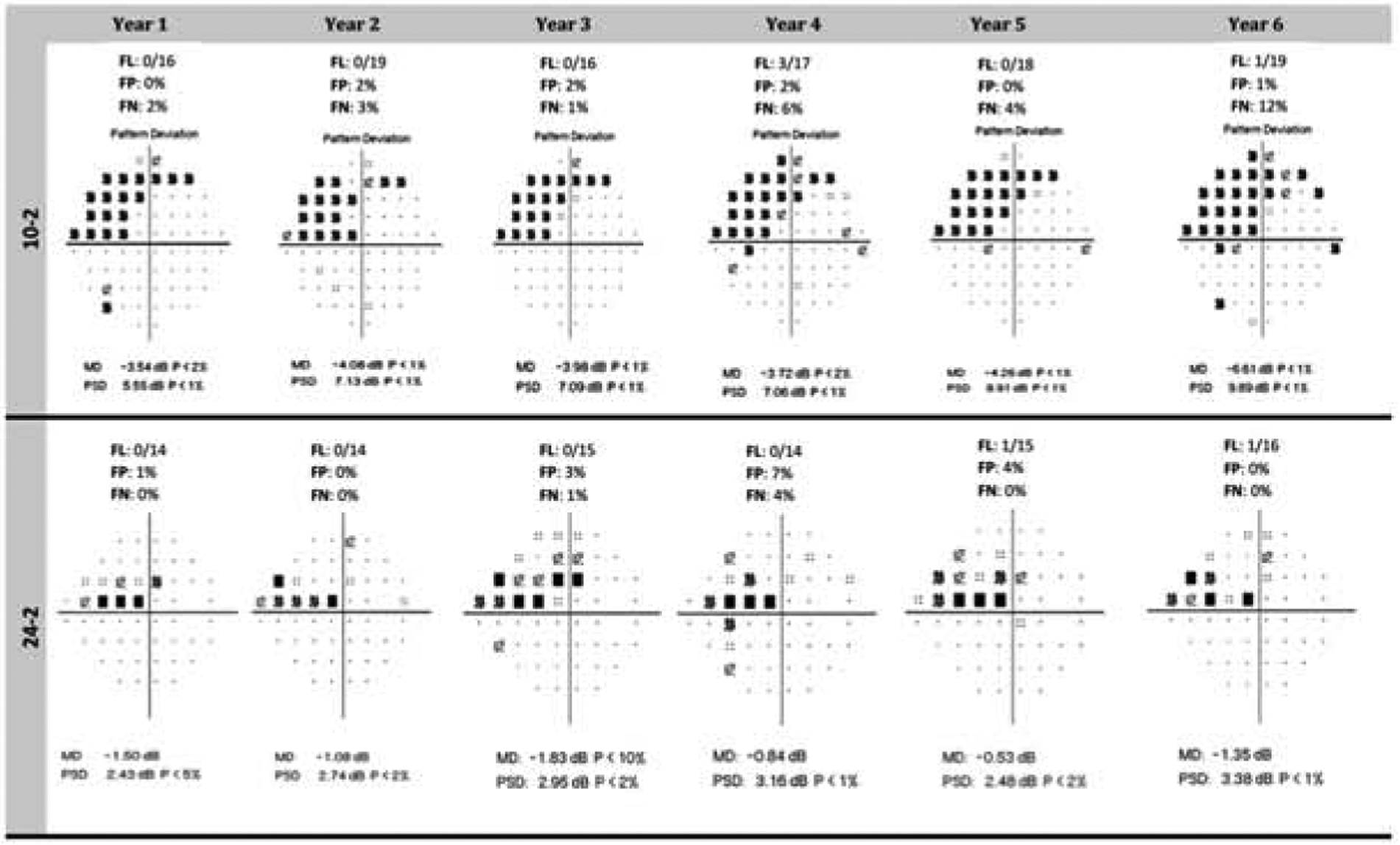
The longitudinal data for this patient shows progression on the 10–2 test (upper panel) but not on the 24–2 procedure (lower panel). The Pattern Standard Deviation (PSD) of the 10–2 is 5.55 dB on the first visual field and 9.89 dB on the last test. The Mean Deviation also worsens across the test series. Worsening can also be seen on the Pattern Deviation plot. An inspection of the 24–2 tests does not show progression.
It has been speculated that scotomas tend to be detectable earlier in more peripheral locations due to the increasing size of receptive fields with the eccentricity.31 Nevertheless, various factors, ocular or systemic, may affect the central deterioration versus peripheral deterioration in the natural course of the disease.106,149 Compared with an initial nasal step, an initial parafoveal scotoma is more closely associated with normal tension glaucoma and systemic vascular risk factors, such as systemic hypotension, migraine, Raynaud’s phenomenon, and sleep apnea.149 Kim and coworkers further reported that systemic factors were closely associated with superior paracentral defects, such as more migraine and less hypertension, while ocular factors were more closely associated with inferior paracentral defects, such as steeper cup and longer axial length.106
A typical parafoveal scotoma develops an arcuate pattern initially, later deepens and then elongates toward the physiologic blind spot and the nasal periphery respectively.173 Interestingly, Cho and coworkers found that the rates of change of either central or peripheral zones did not significantly differ between the initial central scotomas and the initial peripheral scotomas in a group of normal-tension glaucoma patients under treatment.37 Shon and coworkers found the superior paracentral arcuate and superior and inferior nasal locations showed the relatively consistent rates of change over time, while the central and edge locations progressed at faster rates in the second half of the follow-up period.166 A separate study by Nassiri and coworkers showed that the rate of change can be faster within the central 10 degrees compared to the 20 degree and 30 degree areas in glaucoma eyes.139 These findings highlight the importance of closely monitoring glaucomatous progression in the central locations.
7.6. Stimulus size
A key factor of contrast sensitivity is target size and the Goldmann size III stimulus (0.43 degrees in diameter) has almost exclusively been used in conventional automated perimetry.71 The size V stimulus, however, has been shown to have lower test-retest variability and possibly a greater dynamic range compared to size III. While stimulus size III and V both show increasing variability with more advanced visual field loss, the size V stimulus shows comparatively smaller change.191 Wall and coworkers tested participants over a 5-week period and reported that the repeatability of MD was slightly better with size V than with size III. There was a 15% (P = 0.10) and 12% (P = 0.08) reduction in the standard deviation following quantile regression with size V compared to size III, respectively, in glaucoma patients and healthy subjects.190 For SAP using a size III stimulus, the effective dynamic range, that is physiologically meaningful and clinically useful, is substantially less than its physically tested limits by the device. Compared to size III, size V has a greater effective dynamic range by about 1 log unit, and has a lower floor and more discriminable steps.192 In a separate study, no significant difference was observed for the lower limit of the effective stimulus range between the two sizes, however, more locations remained within the range and higher sensitivity was reported at the same location when using the size V stimulus.62 This suggests that these locations will not reach the lower limit of the reliable measurement until later in the disease process, thus potentially resulting in better assessment of visual field damage in these areas.
The benefits of using larger stimuli for visual field testing in terms of disease severity have been assessed. In a group of glaucoma patients with an average MD of −6.67 dB, Wall et al reported that size V full-threshold testing identified a similar number of abnormal test locations in comparison with size III SITA testing across severity.188 Wall et al further showed that size V stimulus and the size threshold perimetry, a method that finds threshold by changing stimulus size while maintaining the contrast constant, were able to detect early visual field loss as well as size III stimulus.189 This ability decreased, however, when the stimulus size was further increased to a size VI. These findings challenged the previous conception that larger stimuli are less sensitive for early visual field loss. In a recent longitudinal study, size V stimulus was reported to perform at least as well as the size III based on PLR, PoPLR, and linear regression of MD in detecting progression.193 Since the reliability of the conventional size III stimulus may be poor in advanced disease, further longitudinal studies are needed to investigate alternative test strategies to monitor progression for this stage.64,65,98 Using larger stimuli may be a promising approach. One of the current limitations associated with the size V target is that a normative database and a statistical analysis package are not yet available for commercial perimetric devices. It is crucial that these items become available so that they can be applied to the results of patients with profound visual impairment. This could then be used as a clinical tool for determining a patient’s ability to perform certain tasks, and the impact of degraded vision on quality of life and activities of daily living.
7.7. Binocular visual field assessment
In naturalistic conditions, patients perceive the world binocularly. Assessing visual field progression using binocular visual field assessments may therefore be desirable. The Esterman test is a suprathreshold test taken with both eyes open that provides an assessment of the binocular visual field.51 The Esterman test is typically performed to determine whether patients meet the vision standard required for driving and is rarely performed in clinical settings to monitor ocular conditions. Methods have been developed to integrate monocular visual field results to predict the binocular visual field.43,140 A recent study showed that the distributions of glaucomatous defects can be different between the Esterman test and the integrated binocular visual field for the same patient.75 With the Esterman test, defects were more frequently found around the bitemporal and Bjerrum areas, while with the integrated binocular visual field, defects were more frequently found around the Mariotte blind spots and the Bjerrum areas and extended to the periphery.
Studies have investigated the usefulness of binocular visual field assessment in monitoring glaucoma progression. Chun and coworkers reported that the rate of change in the integrated binocular visual fields can be significantly faster than that of the slower-changing eyes and significantly slower than that of the faster-changing eyes defined based on monocular visual fields.39 The precision of the VFI forecasts was reported to be better with binocular measures than with monocular measures. The average 5-year forecasts of the binocular VFI were significantly higher than that of either monocular VFI, suggesting that a better visual prognosis might be obtained with the binocular visual field assessment compared to the conventional monocular assessment.19 On the other hand, patients with faster rates of binocular visual field change have been shown to be at higher risk of experiencing abnormalities in vision-related quality of life, even after adjusting for demographic and socioeconomic factors.116 Lisboa and colleagues analyzed a cohort of 398 patients with diagnosed or suspected glaucoma and found that the rate of binocular change on MD was significantly faster with patients reporting worse vision-related quality of life than those who did not (0.18 versus −0.06 dB/year, P < 0.001).116 Baseline severity, magnitude and rates of change in binocular visual field sensitivity were also reported to be significantly associated with longitudinal changes in quality of life of glaucoma patients.122 It was estimated that a change of 1dB in binocular SAP mean sensitivity per year may result in a deteriorating change of approximately 2.9 units per year in the NEI VFQ-25 Rasch scores. These findings suggest that longitudinal assessment of the binocular visual field is useful as a predictor of future functional impairment in glaucoma patients. Assessment of longitudinal binocular visual field changes may help identify patients at higher risk for developing real world disability associated with glaucoma.
7.8. Combining structural and functional information
Results from large clinical trials have shown that different patients reach the structural or the functional endpoint first, although the majority of patients reveal structural changes as the initial damage from glaucoma.11,99,105,121,133,172 This finding has been reported for more than 100 years in spite of major advancements in technology, with various proposals offered to explain this result.97 Hood and Kardon have provided a model that accounts for structural glaucomatous changes becoming noticeable prior to functional loss in the majority of patients.85 Given that structural and functional measurements may each provide unique information about disease progression, there may be value in combining both types of information to monitor glaucoma progression. Because SAP and OCT have limitations in estimating rates of change in early and late disease, respectively, Medeiros et al combined information from structural and functional measurements into a single intuitive metric which can be used to assess rates of change throughout the whole spectrum of the disease.124 Studies have demonstrated the ability of this index to determine disease status and measure rates of change compared to either structural or functional measurements alone.73,125,131,180 A recent study further showed that the combined index of structure and function identified a group of fast progressors that were missed by the GPA event analysis.206
The Octopus Field Analysis software provides the Polar Trend Analysis (PTA) (Haag-Streit, Inc., Köniz, Switzerland) to combine OCT measurements with visual field data. This analysis provides a graphical representation of the pointwise linear regression analysis of focal defects at the corresponding nerve fiber angle at the disc margin. PTA progression is defined as more than 1 significantly progressing test point location per sector. Holló and coworkers found that the PTA may identify glaucomatous visual field progression at an early stage when the only corresponding change in structural measurements is increased long-term variability.84 In their study, the frequency of statistically significant progression with OCT measurements did not differ between the progressor and non-progressor groups classified by the PTA.
Bayesian approaches combining structural and functional measurements have also been investigated to monitor glaucoma progression and show improved estimates of glaucoma progression.128,161 MLCs provide better diagnostic accuracy to differentiate healthy and glaucomatous eyes based on either structural or functional data, however, MLCs combining structural and functional measurements have not obtained better performance to assess progression than including structural measurements alone.203 Using an intuitive two-dimensional space, Hu and coworkers proposed a dynamic structure-function model that presents centroids as estimates of the disease status and velocity vectors as estimates of direction and rate of change over time.87 Similar prediction accuracy was observed compared to conventional linear regression and the dynamic structure-function model performed better in shorter time series, which may be of value in clinical settings.
8. Conclusions
While being able to identify glaucomatous visual field progression is critical, the lack of a ground-truth test poses a significant challenge. Several analyses have been developed, each having advantages and drawbacks, and no consensus currently exists. The best approach for providers in busy clinical settings may be to use procedures based on tests that are consistently used in their clinics. Monitoring change with event-based analysis such as the GPA will alert clinicians to the onset of progression, but will not provide information about the rate of change. Trend-based analyses can be performed to assess the rate at which visual function is worsening. Researchers continue to develop and evaluate more novel analysis procedures that will eventually be translated into the clinical realm. Challenges associated with these more sophisticated procedures include the ability to provide accurate assessments based on computationally intensive evaluations, presentation of findings that can be quickly determined in a busy clinical setting, and developing methods that can directly relate to how patients experience the glaucomatous disease process. The current uncertainty as to the optimal procedure for evaluating glaucomatous progression provides a wonderful opportunity for new and established investigators to continue to pursue this issue. It is hoped that the information provided in this paper will be useful to clinicians who are concerned with the management of patients with or at risk of developing glaucoma. Although we have made significant progress in the detection, analysis and interpretation of visual fields in glaucoma, there is also much that remains to be done. We encourage clinical researchers who are interested in this topic to pursue new avenues and refine existing procedures to enhance our ability to assess the integrity and status of visual function in glaucoma patients and glaucoma suspects.
9. Method of Literature Search
A search of the PubMed database was conducted for “glaucoma visual field progression” and each of the following keywords: AGIS, ANSWERS, Bayesian, Binocular, CIGTS, EMGT, Event-based analysis, Frequency-Doubling Technology, GPA, Length of follow-up, Machine Learning, Number of Visual Fields, Parametric, Perimetry, Peripheral, Pointwise Linear Regression, PoPLR, Short-Wavelength Automated Perimetry, Standard Automated Perimetry, Static Automated Perimetry, Stimulus Size, Testing Frequency, and Trend-based analysis. We limited our search to articles published from 1998 to present day that were written in English. The search was conducted in July 2018. All abstracts were screened, and relevant articles were included in this review. Seminal papers published prior to 1998 were also included.
12. Acknowledgements
The authors wish to thank Cindy Albert and Mahmoud Tawfik KhalafAllah for their assistance with the figures.
Supported by the National Natural Science Foundation of China Grant 81900854 and the Zhejiang Provincial Natural Science Foundation of China Grant LQ17H120003 (RH), grants R01EY025756 (LR), T35HL007473-37 Summer Research Training Program (KC) from the National Institutes of Health, and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
None of the authors have a conflict of interest.
13. References
- 1.Abe RY, Diniz-Filho A, Costa VP, et al. The Impact of Location of Progressive Visual Field Loss on Longitudinal Changes in Quality of Life of Patients with Glaucoma. Ophthalmology. 2016;123(3):552–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson AJ. Comparison of Three Parametric Models for Glaucomatous Visual Field Progression Rate Distributions. Transl Vis Sci Technol. 2015;4(4):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson AJ. Estimating the true distribution of visual field progression rates in glaucoma. Invest Ophthalmol Vis Sci. 2015;56(3):1603–8 [DOI] [PubMed] [Google Scholar]
- 4.Anderson AJ, Johnson CA. Frequency-doubling technology perimetry, in Sample PA and Girkin CA (eds): Ophthalmology Clinics of North America. Philadelphia, W.B. Saunders, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Anderson AJ, Asokan R, Murata H, Asaoka R. Detecting glaucomatous progression with infrequent visual field testing. Ophthalmic Physiol Opt. 2018;38(2):174–82 [DOI] [PubMed] [Google Scholar]
- 6.Anderson AJ, Johnson CA. How useful is population data for informing visual field progression rate estimation? Invest Ophthalmol Vis Sci. 2013;54(3):2198–206 [DOI] [PubMed] [Google Scholar]
- 7.Anderson AJ, Johnson CA, Fingeret M, et al. Characteristics of the normative database for the Humphrey matrix perimeter. Invest Ophthalmol Vis Sci. 2005;46(4):1540–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki S, Murata H, Fujino Y, et al. Investigating the usefulness of a cluster-based trend analysis to detect visual field progression in patients with open-angle glaucoma. Br J Ophthalmol. 2017;101(12):1658–65 [DOI] [PubMed] [Google Scholar]
- 9.Aptel F, Aryal-Charles N, Giraud JM, et al. Progression of visual field in patients with primary open-angle glaucoma - ProgF study 1. Acta Ophthalmol. 2015;93(8):e615–20 [DOI] [PubMed] [Google Scholar]
- 10.Arnalich-Montiel F, Casas-Llera P, Munoz-Negrete FJ, Rebolleda G. Performance of glaucoma progression analysis software in a glaucoma population. Graefes Arch Clin Exp Ophthalmol. 2009;247(3):391–7 [DOI] [PubMed] [Google Scholar]
- 11.Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24(3):333–54 [DOI] [PubMed] [Google Scholar]
- 12.Artes PH, Chauhan BC, Keltner JL, et al. Longitudinal and cross-sectional analyses of visual field progression in participants of the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128(12):1528–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artes PH, Chauhan BC, Keltner JL, et al. Longitudinal and cross-sectional analyses of visual field progression in participants of the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128(12):1528–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artes PH, Iwase A, Ohno Y, et al. Properties of perimetric threshold estimates from Full Threshold, SITA Standard, and SITA Fast strategies. Invest Ophthalmol Vis Sci. 2002;43(8):2654–9 [PubMed] [Google Scholar]
- 15.Artes PH, Nicolela MT, LeBlanc RP, Chauhan BC. Visual field progression in glaucoma: total versus pattern deviation analyses. Invest Ophthalmol Vis Sci. 2005;46(12):4600–6 [DOI] [PubMed] [Google Scholar]
- 16.Artes PH, Nicolela MT, McCormick TA, et al. Effects of blur and repeated testing on sensitivity estimates with frequency doubling perimetry. Invest Ophthalmol Vis Sci. 2003;44(2):646–52 [DOI] [PubMed] [Google Scholar]
- 17.Artes PH, O’Leary N, Hutchison DM, et al. Properties of the statpac visual field index. Invest Ophthalmol Vis Sci. 2011;52(7):4030–8 [DOI] [PubMed] [Google Scholar]
- 18.Artes PH, O’Leary N, Nicolela MT, et al. Visual field progression in glaucoma: what is the specificity of the Guided Progression Analysis? Ophthalmology. 2014;121(10):2023–7 [DOI] [PubMed] [Google Scholar]
- 19.Asaoka R, Russell RA, Malik R, et al. Five-year forecasts of the Visual Field Index (VFI) with binocular and monocular visual fields. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1335–41 [DOI] [PubMed] [Google Scholar]
- 20.Asman P, Heijl A. Arcuate cluster analysis in glaucoma perimetry. J Glaucoma. 1993;2(1):13–20 [PubMed] [Google Scholar]
- 21.Azarbod P, Mock D, Bitrian E, et al. Validation of point-wise exponential regression to measure the decay rates of glaucomatous visual fields. Invest Ophthalmol Vis Sci. 2012;53(9):5403–9 [DOI] [PubMed] [Google Scholar]
- 22.Bengtsson B, Heijl A. Diagnostic sensitivity of fast blue-yellow and standard automated perimetry in early glaucoma: a comparison between different test programs. Ophthalmology. 2006;113(7):1092–7 [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145(2):343–53 [DOI] [PubMed] [Google Scholar]
- 24.Bengtsson B, Lindgren A, Heijl A, et al. Perimetric probability maps to separate change caused by glaucoma from that caused by cataract. Acta Ophthalmol Scand. 1997;75(2):184–8 [DOI] [PubMed] [Google Scholar]
- 25.Betz-Stablein BD, Morgan WH, House PH, Hazelton ML. Spatial modeling of visual field data for assessing glaucoma progression. Invest Ophthalmol Vis Sci. 2013;54(2):1544–53 [DOI] [PubMed] [Google Scholar]
- 26.Boden C, Blumenthal EZ, Pascual J, et al. Patterns of glaucomatous visual field progression identified by three progression criteria. Am J Ophthalmol. 2004;138(6):1029–36 [DOI] [PubMed] [Google Scholar]
- 27.Boodhna T, Crabb DP. More frequent, more costly? Health economic modelling aspects of monitoring glaucoma patients in England. BMC Health Serv Res. 2016;16(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boodhna T, Saunders LJ, Crabb DP. Are rates of vision loss in patients in English glaucoma clinics slowing down over time? Trends from a decade of data. Eye (Lond). 2015;29(12):1613–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowd C, Goldbaum MH. Machine learning classifiers in glaucoma. Optom Vis Sci. 2008;85(6):396–405 [DOI] [PubMed] [Google Scholar]
- 30.Caprioli J, Mock D, Bitrian E, et al. A method to measure and predict rates of regional visual field decay in glaucoma. Invest Ophthalmol Vis Sci. 2011;52(7):4765–73 [DOI] [PubMed] [Google Scholar]
- 31.Carreras FJ, Rica R, Delgado AV. Modeling the patterns of visual field loss in glaucoma. Optom Vis Sci. 2011;88(1):E63–79 [DOI] [PubMed] [Google Scholar]
- 32.Casas-Llera P, Rebolleda G, Munoz-Negrete FJ, et al. Visual field index rate and event-based glaucoma progression analysis: comparison in a glaucoma population. Br J Ophthalmol. 2009;93(12):1576–9 [DOI] [PubMed] [Google Scholar]
- 33.Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92(4):569–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chauhan BC, Johnson CA. Test-retest variability of frequency-doubling perimetry and conventional perimetry in glaucoma patients and normal subjects. Invest Ophthalmol Vis Sci. 1999;40(3):648–56 [PubMed] [Google Scholar]
- 35.Chauhan BC, Malik R, Shuba LM, et al. Rates of glaucomatous visual field change in a large clinical population. Invest Ophthalmol Vis Sci. 2014;55(7):4135–43 [DOI] [PubMed] [Google Scholar]
- 36.Chen A, Nouri-Mahdavi K, Otarola FJ, et al. Models of glaucomatous visual field loss. Invest Ophthalmol Vis Sci. 2014;55(12):7881–7 [DOI] [PubMed] [Google Scholar]
- 37.Cho HK, Lee J, Lee M, Kee C. Initial central scotomas vs peripheral scotomas in normal-tension glaucoma: clinical characteristics and progression rates. Eye (Lond). 2014;28(3):303–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho JW, Sung KR, Yun SC, et al. Progression detection in different stages of glaucoma: mean deviation versus visual field index. Jpn J Ophthalmol. 2012;56(2):128–33 [DOI] [PubMed] [Google Scholar]
- 39.Chun YS, Shin JH, Park IK. Comparison of rates of change between binocular and monocular visual fields. Invest Ophthalmol Vis Sci. 2015;56(1):451–7 [DOI] [PubMed] [Google Scholar]
- 40.Contestabile MT, Perdicchi A, Amodeo S, et al. Effect of refractive correction on the accuracy of frequency-doubling technology Matrix. J Glaucoma. 2013;22(5):413–5 [DOI] [PubMed] [Google Scholar]
- 41.Crabb DP, Garway-Heath DF. Intervals between visual field tests when monitoring the glaucomatous patient: wait-and-see approach. Invest Ophthalmol Vis Sci. 2012;53(6):2770–6 [DOI] [PubMed] [Google Scholar]
- 42.Crabb DP, Russell RA, Malik R, et al. : Health Services and Delivery Research: Frequency of visual field testing when monitoring patients newly diagnosed with glaucoma: mixed methods and modelling. Southampton (UK), NIHR Journals Library, 2014 [PubMed] [Google Scholar]
- 43.Crabb DP, Viswanathan AC. Integrated visual fields: a new approach to measuring the binocular field of view and visual disability. Graefes Arch Clin Exp Ophthalmol. 2005;243(3):210–6 [DOI] [PubMed] [Google Scholar]
- 44.De Moraes CG, Ghobraiel SR, Ritch R, Liebmann JM. Comparison of PROGRESSOR and Glaucoma Progression Analysis 2 to Detect Visual Field Progression in Treated Glaucoma Patients. Asia Pac J Ophthalmol (Phila). 2012;1(3):135–9 [DOI] [PubMed] [Google Scholar]
- 45.De Moraes CG, Juthani VJ, Liebmann JM, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011;129(5):562–8 [DOI] [PubMed] [Google Scholar]
- 46.De Moraes CG, Liebmann CA, Susanna R Jr., et al. Examination of the performance of different pointwise linear regression progression criteria to detect glaucomatous visual field change. Clin Exp Ophthalmol. 2012;40(4):e190–6 [DOI] [PubMed] [Google Scholar]
- 47.De Moraes CG, Ritch R, Tello C, Liebmann JM. Modified visual field trend analysis. J Glaucoma. 2011;20(4):203–6 [DOI] [PubMed] [Google Scholar]
- 48.de Moraes CG, Song C, Liebmann JM, et al. Defining 10–2 visual field progression criteria: exploratory and confirmatory factor analysis using pointwise linear regression. Ophthalmology. 2014;121(3):741–9 [DOI] [PubMed] [Google Scholar]
- 49.Demirel S, Johnson CA. Isolation of short-wavelength sensitive mechanisms in normal and glaucomatous visual field regions. J Glaucoma. 2000;9(1):63–73 [DOI] [PubMed] [Google Scholar]
- 50.Ederer F, Gaasterland DE, Sullivan EK. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials. 1994;15(4):299–325 [DOI] [PubMed] [Google Scholar]
- 51.Esterman B Functional scoring of the binocular field. Ophthalmology. 1982;89(11):1226–34 [DOI] [PubMed] [Google Scholar]
- 52.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Part 1. EGS Foundation British Journal of Ophthalmology, 2017 [Google Scholar]
- 53.Fingeret M, Johnson CA. The new Humphrey Matrix FDT. Optometric Management, 2003 [Google Scholar]
- 54.Fitzke FW, Hitchings RA, Poinoosawmy D, et al. Analysis of visual field progression in glaucoma. Br J Ophthalmol. 1996;80(1):40–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujino Y, Asaoka R, Murata H, et al. Evaluation of Glaucoma Progression in Large-Scale Clinical Data: The Japanese Archive of Multicentral Databases in Glaucoma (JAMDIG). Invest Ophthalmol Vis Sci. 2016;57(4):2012–20 [DOI] [PubMed] [Google Scholar]
- 56.Fujino Y, Murata H, Mayama C, Asaoka R. Applying “Lasso” Regression to Predict Future Visual Field Progression in Glaucoma Patients. Invest Ophthalmol Vis Sci. 2015;56(4):2334–9 [DOI] [PubMed] [Google Scholar]
- 57.Fung SS, Lemer C, Russell RA, et al. Are practical recommendations practiced? A national multi-centre cross-sectional study on frequency of visual field testing in glaucoma. Br J Ophthalmol. 2013;97(7):843–7 [DOI] [PubMed] [Google Scholar]
- 58.Gardiner SK, Crabb DP. Examination of different pointwise linear regression methods for determining visual field progression. Invest Ophthalmol Vis Sci. 2002;43(5):1400–7 [PubMed] [Google Scholar]
- 59.Gardiner SK, Crabb DP. Frequency of testing for detecting visual field progression. Br J Ophthalmol. 2002;86(5):560–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardiner SK, Demirel S. Detecting Change Using Standard Global Perimetric Indices in Glaucoma. Am J Ophthalmol. 2017;176(148–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardiner SK, Demirel S, De Moraes CG, et al. Series length used during trend analysis affects sensitivity to changes in progression rate in the ocular hypertension treatment study. Invest Ophthalmol Vis Sci. 2013;54(2):1252–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gardiner SK, Demirel S, Goren D, et al. The Effect of Stimulus Size on the Reliable Stimulus Range of Perimetry. Transl Vis Sci Technol. 2015;4(2):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardiner SK, Mansberger SL, Demirel S. Detection of Functional Change Using Cluster Trend Analysis in Glaucoma. Invest Ophthalmol Vis Sci. 2017;58(6):Bio180–bio90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardiner SK, Swanson WH, Demirel S. The Effect of Limiting the Range of Perimetric Sensitivities on Pointwise Assessment of Visual Field Progression in Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(1):288–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gardiner SK, Swanson WH, Goren D, et al. Assessment of the reliability of standard automated perimetry in regions of glaucomatous damage. Ophthalmology. 2014;121(7):1359–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garway-Heath DF, Quartilho A, Prah P, et al. Evaluation of Visual Field and Imaging Outcomes for Glaucoma Clinical Trials (An American Ophthalomological Society Thesis). Trans Am Ophthalmol Soc. 2017;115(T4. [PMC free article] [PubMed] [Google Scholar]
- 67.Garway-Heath DF, Zhu H, Cheng Q, et al. Combining optical coherence tomography with visual field data to rapidly detect disease progression in glaucoma: a diagnostic accuracy study. Health Technol Assess. 2018;22(4):1–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Girkin CA. Relationship between structure of optic nerve/nerve fiber layer and functional measurements in glaucoma. Curr Opin Ophthalmol. 2004;15(2):96–101 [DOI] [PubMed] [Google Scholar]
- 69.Goldbaum MH, Jang GJ, Bowd C, et al. Patterns of glaucomatous visual field loss in sita fields automatically identified using independent component analysis. Trans Am Ophthalmol Soc. 2009;107(136–44 [PMC free article] [PubMed] [Google Scholar]
- 70.Goldbaum MH, Lee I, Jang G, et al. Progression of patterns (POP): a machine classifier algorithm to identify glaucoma progression in visual fields. Invest Ophthalmol Vis Sci. 2012;53(10):6557–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldmann H Fundamentals of exact perimetry. 1945. Optom Vis Sci. 1999;76(8):599–604 [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez de la Rosa M, Gonzalez-Hernandez M, Sanchez-Mendez M, et al. Detection of morphological and functional progression in initial glaucoma. Br J Ophthalmol. 2010;94(4):414–8 [DOI] [PubMed] [Google Scholar]
- 73.Gracitelli CP, Tatham AJ, Zangwill LM, et al. Estimated rates of retinal ganglion cell loss in glaucomatous eyes with and without optic disc hemorrhages. PLoS One. 2014;9(8):e105611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gros-Otero J, Castejon M, Paz-Moreno J, et al. Perimetric progression using the Visual Field Index and the Advanced Glaucoma Intervention Study score and its clinical correlations. J Optom. 2015;8(4):232–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashimoto S, Matsumoto C, Eura M, et al. Distribution and Progression of Visual Field Defects With Binocular Vision in Glaucoma. J Glaucoma. 2018;27(6):519–24 [DOI] [PubMed] [Google Scholar]
- 76.Haymes SA, Hutchison DM, McCormick TA, et al. Glaucomatous visual field progression with frequency-doubling technology and standard automated perimetry in a longitudinal prospective study. Invest Ophthalmol Vis Sci. 2005;46(2):547–54 [DOI] [PubMed] [Google Scholar]
- 77.Heijl A, Bengtsson B, Chauhan BC, et al. A comparison of visual field progression criteria of 3 major glaucoma trials in early manifest glaucoma trial patients. Ophthalmology. 2008;115(9):1557–65 [DOI] [PubMed] [Google Scholar]
- 78.Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology. 2009;116(12):2271–6 [DOI] [PubMed] [Google Scholar]
- 79.Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol. 2013;91(5):406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heijl A, Leske MC, Bengtsson B, et al. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmol Scand. 2003;81(3):286–93 [DOI] [PubMed] [Google Scholar]
- 81.Heijl A, Lindgren G, Olsson J, Asman P. Visual field interpretation with empiric probability maps. Arch Ophthalmol. 1989;107(2):204–8 [DOI] [PubMed] [Google Scholar]
- 82.Henson DB, Artes PH, Chauhan BC. Diffuse loss of sensitivity in early glaucoma. Invest Ophthalmol Vis Sci. 1999;40(13):3147–51 [PubMed] [Google Scholar]
- 83.Hirasawa K, Murata H, Hirasawa H, et al. Clustering visual field test points based on rates of progression to improve the prediction of future damage. Invest Ophthalmol Vis Sci. 2014;55(11):7681–5 [DOI] [PubMed] [Google Scholar]
- 84.Hollo G, Naghizadeh F. Evaluation of Octopus Polar Trend Analysis for detection of glaucomatous progression. Eur J Ophthalmol. 2014;24(6):862–8 [DOI] [PubMed] [Google Scholar]
- 85.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hood DC, Raza AS, de Moraes CG, et al. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32(1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu R, Marin-Franch I, Racette L. Prediction accuracy of a novel dynamic structure-function model for glaucoma progression. Invest Ophthalmol Vis Sci. 2014;55(12):8086–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu R, Wang C, Gu Y, Racette L. Comparison of Standard Automated Perimetry, Short-Wavelength Automated Perimetry, and Frequency-Doubling Technology Perimetry to Monitor Glaucoma Progression. Medicine (Baltimore). 2016;95(7):e2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu R, Wang C, Racette L. Comparison of matrix frequency-doubling technology perimetry and standard automated perimetry in monitoring the development of visual field defects for glaucoma suspect eyes. PLoS One. 2017;12(5):e0178079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iester M, Capris E, De Feo F, et al. Agreement to detect glaucomatous visual field progression by using three different methods: a multicentre study. Br J Ophthalmol. 2011;95(9):1276–83 [DOI] [PubMed] [Google Scholar]
- 91.Iester M, Corallo G, Capris E, Capris P. Agreement in detecting glaucomatous visual field progression by using guided progression analysis and Humphrey overview printout. Eur J Ophthalmol. 2011;21(5):573–9 [DOI] [PubMed] [Google Scholar]
- 92.Jansonius NM. Bayes’ theorem applied to perimetric progression detection in glaucoma: from specificity to positive predictive value. Graefes Arch Clin Exp Ophthalmol. 2005;243(5):433–7 [DOI] [PubMed] [Google Scholar]
- 93.Jansonius NM. Towards an optimal perimetric strategy for progression detection in glaucoma: from fixed-space to adaptive inter-test intervals. Graefes Arch Clin Exp Ophthalmol. 2006;244(3):390–3 [DOI] [PubMed] [Google Scholar]
- 94.Jansonius NM. Progression detection in glaucoma can be made more efficient by using a variable interval between successive visual field tests. Graefes Arch Clin Exp Ophthalmol. 2007;245(11):1647–51 [DOI] [PubMed] [Google Scholar]
- 95.Johnson CA. Selective versus nonselective losses in glaucoma. J Glaucoma. 1994;3 Suppl 1(S32–44 [PubMed] [Google Scholar]
- 96.Johnson CA, Adams AJ, Casson EJ, Brandt JD. Progression of early glaucomatous visual field loss as detected by blue-on-yellow and standard white-on-white automated perimetry. Arch Ophthalmol. 1993;111(5):651–6 [DOI] [PubMed] [Google Scholar]
- 97.Johnson CA, Cioffi GA, Liebmann JR, et al. The relationship between structural and functional alterations in glaucoma: a review. Semin Ophthalmol. 2000;15(4):221–33 [DOI] [PubMed] [Google Scholar]
- 98.Junoy Montolio FG, Wesselink C, Jansonius NM. Persistence, spatial distribution and implications for progression detection of blind parts of the visual field in glaucoma: a clinical cohort study. PLoS One. 2012;7(7):e41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13; discussion 829–30 [DOI] [PubMed] [Google Scholar]
- 100.Katz J Scoring systems for measuring progression of visual field loss in clinical trials of glaucoma treatment. Ophthalmology. 1999;106(2):391–5 [DOI] [PubMed] [Google Scholar]
- 101.Katz J, Congdon N, Friedman DS. Methodological variations in estimating apparent progressive visual field loss in clinical trials of glaucoma treatment. Arch Ophthalmol. 1999;117(9):1137–42 [DOI] [PubMed] [Google Scholar]
- 102.Kazemian P, Lavieri MS, Van Oyen MP, et al. Personalized Prediction of Glaucoma Progression Under Different Target Intraocular Pressure Levels Using Filtered Forecasting Methods. Ophthalmology. 2018;125(4):569–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelly DH. Frequency doubling in visual responses. J Opt Soc Am. 1966;56(1628–32 [Google Scholar]
- 104.Kelly DH. Nonlinear visual responses to flickering sinusoidal gratings. J Opt Soc Am. 1981;71(9):1051–5 [DOI] [PubMed] [Google Scholar]
- 105.Keltner JL, Johnson CA, Anderson DR, et al. The association between glaucomatous visual fields and optic nerve head features in the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113(9):1603–12 [DOI] [PubMed] [Google Scholar]
- 106.Kim JM, Kyung H, Shim SH, et al. Location of Initial Visual Field Defects in Glaucoma and Their Modes of Deterioration. Invest Ophthalmol Vis Sci. 2015;56(13):7956–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kirwan JF, Hustler A, Bobat H, et al. Portsmouth visual field database: an audit of glaucoma progression. Eye (Lond). 2014;28(8):974–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kosoko O, Quigley HA, Vitale S, et al. Risk factors for noncompliance with glaucoma follow-up visits in a residents’ eye clinic. Ophthalmology. 1998;105(11):2105–11 [DOI] [PubMed] [Google Scholar]
- 109.Koucheki B, Nouri-Mahdavi K, Patel G, et al. Visual field changes after cataract extraction: the AGIS experience. Am J Ophthalmol. 2004;138(6):1022–8 [DOI] [PubMed] [Google Scholar]
- 110.Kummet CM, Zamba KD, Doyle CK, et al. Refinement of pointwise linear regression criteria for determining glaucoma progression. Invest Ophthalmol Vis Sci. 2013;54(9):6234–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lam BL, Alward WL, Kolder HE. Effect of cataract on automated perimetry. Ophthalmology. 1991;98(7):1066–70 [DOI] [PubMed] [Google Scholar]
- 112.Lee JM, Nouri-Mahdavi K, Morales E, et al. Comparison of regression models for serial visual field analysis. Jpn J Ophthalmol. 2014;58(6):504–14 [DOI] [PubMed] [Google Scholar]
- 113.Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999;106(11):2144–53 [DOI] [PubMed] [Google Scholar]
- 114.Liebmann K, De Moraes CG, Liebmann JM. Measuring Rates of Visual Field Progression in Linear Versus Nonlinear Scales: Implications for Understanding the Relationship Between Baseline Damage and Target Rates of Glaucoma Progression. J Glaucoma. 2017;26(8):721–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin AP, Katz LJ, Spaeth GL, et al. Agreement of visual field interpretation among glaucoma specialists and comprehensive ophthalmologists: comparison of time and methods. Br J Ophthalmol. 2011;95(6):828–31 [DOI] [PubMed] [Google Scholar]
- 116.Lisboa R, Chun YS, Zangwill LM, et al. Association between rates of binocular visual field loss and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol. 2013;131(4):486–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu S, Yu M, Weinreb RN, et al. Frequency-doubling technology perimetry for detection of the development of visual field defects in glaucoma suspect eyes: a prospective study. JAMA Ophthalmol. 2014;132(1):77–83 [DOI] [PubMed] [Google Scholar]
- 118.Maddess T, Henry GH. Performance of nonlinear visual units in ocular hypertension and glaucoma. Clin Vision Sci. 1992;7(371–83 [Google Scholar]
- 119.Manassakorn A, Nouri-Mahdavi K, Koucheki B, et al. Pointwise linear regression analysis for detection of visual field progression with absolute versus corrected threshold sensitivities. Invest Ophthalmol Vis Sci. 2006;47(7):2896–903 [DOI] [PubMed] [Google Scholar]
- 120.Mayama C, Araie M, Suzuki Y, et al. Statistical evaluation of the diagnostic accuracy of methods used to determine the progression of visual field defects in glaucoma. Ophthalmology. 2004;111(11):2117–25 [DOI] [PubMed] [Google Scholar]
- 121.Medeiros FA, Alencar LM, Zangwill LM, et al. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127(10):1250–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Medeiros FA, Gracitelli CP, Boer ER, et al. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology. 2015;122(2):293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci. 2011;52(8):5794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Medeiros FA, Lisboa R, Weinreb RN, et al. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol. 2012;130(9):1107–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Medeiros FA, Lisboa R, Weinreb RN, et al. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology. 2013;120(4):736–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol. 2004;137(5):863–71 [DOI] [PubMed] [Google Scholar]
- 127.Medeiros FA, Weinreb RN, Moore G, et al. Integrating event- and trend-based analyses to improve detection of glaucomatous visual field progression. Ophthalmology. 2012;119(3):458–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Medeiros FA, Zangwill LM, Girkin CA, et al. Combining structural and functional measurements to improve estimates of rates of glaucomatous progression. Am J Ophthalmol. 2012;153(6):1197–205.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Medeiros FA, Zangwill LM, Mansouri K, et al. Incorporating risk factors to improve the assessment of rates of glaucomatous progression. Invest Ophthalmol Vis Sci. 2012;53(4):2199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. J Glaucoma. 2012;21(3):147–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meira-Freitas D, Lisboa R, Tatham A, et al. Predicting progression in glaucoma suspects with longitudinal estimates of retinal ganglion cell counts. Invest Ophthalmol Vis Sci. 2013;54(6):4174–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meira-Freitas D, Tatham AJ, Lisboa R, et al. Predicting progression of glaucoma from rates of frequency doubling technology perimetry change. Ophthalmology. 2014;121(2):498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112(3):366–75 [DOI] [PubMed] [Google Scholar]
- 134.Moreno-Montanes J, Anton V, Anton A, et al. Intraobserver and Interobserver Agreement of Structural and Functional Software Programs for Measuring Glaucoma Progression. JAMA Ophthalmol. 2017;135(4):313–9 [DOI] [PubMed] [Google Scholar]
- 135.Murata H, Araie M, Asaoka R. A new approach to measure visual field progression in glaucoma patients using variational bayes linear regression. Invest Ophthalmol Vis Sci. 2014;55(12):8386–92 [DOI] [PubMed] [Google Scholar]
- 136.Murata H, Zangwill LM, Fujino Y, et al. Validating Variational Bayes Linear Regression Method With Multi-Central Datasets. Invest Ophthalmol Vis Sci. 2018;59(5):1897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Musch DC, Lichter PR, Guire KE, Standardi CL. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106(4):653–62 [DOI] [PubMed] [Google Scholar]
- 138.Naghizadeh F, Hollo G. Detection of early glaucomatous progression with octopus cluster trend analysis. J Glaucoma. 2014;23(5):269–75 [DOI] [PubMed] [Google Scholar]
- 139.Nassiri N, Moghimi S, Coleman AL, et al. Global and pointwise rates of decay in glaucoma eyes deteriorating according to pointwise event analysis. Invest Ophthalmol Vis Sci. 2013;54(2):1208–13 [DOI] [PubMed] [Google Scholar]
- 140.Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41(8):2212–21 [PubMed] [Google Scholar]
- 141.Nevalainen J, Paetzold J, Papageorgiou E, et al. Specification of progression in glaucomatous visual field loss, applying locally condensed stimulus arrangements. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1659–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nouri-Mahdavi K Selecting visual field tests and assessing visual field deterioration in glaucoma. Can J Ophthalmol. 2014;49(6):497–505 [DOI] [PubMed] [Google Scholar]
- 143.Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111(9):1627–35 [DOI] [PubMed] [Google Scholar]
- 144.Nouri-Mahdavi K, Hoffman D, Ralli M, Caprioli J. Comparison of methods to predict visual field progression in glaucoma. Arch Ophthalmol. 2007;125(9):1176–81 [DOI] [PubMed] [Google Scholar]
- 145.Nouri-Mahdavi K, Mock D, Hosseini H, et al. Pointwise rates of visual field progression cluster according to retinal nerve fiber layer bundles. Invest Ophthalmol Vis Sci. 2012;53(4):2390–4 [DOI] [PubMed] [Google Scholar]
- 146.Nouri-Mahdavi K, Zarei R, Caprioli J. Influence of visual field testing frequency on detection of glaucoma progression with trend analyses. Arch Ophthalmol. 2011;129(12):1521–7 [DOI] [PubMed] [Google Scholar]
- 147.O’Leary N, Chauhan BC, Artes PH. Visual field progression in glaucoma: estimating the overall significance of deterioration with permutation analyses of pointwise linear regression (PoPLR). Invest Ophthalmol Vis Sci. 2012;53(11):6776–84 [DOI] [PubMed] [Google Scholar]
- 148.Pantalon A, Feraru C. Non-parametric tests in detecting glaucoma progression. Rom J Ophthalmol. 2017;61(3):212–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park SC, De Moraes CG, Teng CC, et al. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology. 2011;118(9):1782–9 [DOI] [PubMed] [Google Scholar]
- 150.Park SC, Kung Y, Su D, et al. Parafoveal scotoma progression in glaucoma: humphrey 10–2 versus 24–2 visual field analysis. Ophthalmology. 2013;120(8):1546–50 [DOI] [PubMed] [Google Scholar]
- 151.Pathak M, Demirel S, Gardiner SK. Nonlinear, multilevel mixed-effects approach for modeling longitudinal standard automated perimetry data in glaucoma. Invest Ophthalmol Vis Sci. 2013;54(8):5505–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pathak M, Demirel S, Gardiner SK. Nonlinear Trend Analysis of Longitudinal Pointwise Visual Field Sensitivity in Suspected and Early Glaucoma. Transl Vis Sci Technol. 2015;4(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Perdicchi A, Abdolrahimzadeh S, Cutini A, et al. Evaluation of the progression of visual field damage in patients suffering from early manifest glaucoma. Clin Ophthalmol. 2016;10(1647–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Racette L, Fischer M, Bebie H, et al. Visual Field Digest: A guide to perimetry and the Octopus perimeter. Koeniz, Haag-Streit AG, 2018 [Google Scholar]
- 155.Racette L, Sample PA. Short-wavelength automated perimetry. Ophthalmol Clin North Am. 2003;16(2):227–36, vi-vii [DOI] [PubMed] [Google Scholar]
- 156.Glaucoma Ramulu P. and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20(2):92–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rao HL, Kumbar T, Kumar AU, et al. Agreement between event-based and trend-based glaucoma progression analyses. Eye (Lond). 2013;27(7):803–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Redmond T, O’Leary N, Hutchison DM, et al. Visual field progression with frequency-doubling matrix perimetry and standard automated perimetry in patients with glaucoma and in healthy controls. JAMA Ophthalmol. 2013;131(12):1565–72 [DOI] [PubMed] [Google Scholar]
- 159.Russell RA, Crabb DP. On alternative methods for measuring visual field decay: Tobit linear regression. Invest Ophthalmol Vis Sci. 2011;52(13):9539–40 [DOI] [PubMed] [Google Scholar]
- 160.Russell RA, Crabb DP, Malik R, Garway-Heath DF. The relationship between variability and sensitivity in large-scale longitudinal visual field data. Invest Ophthalmol Vis Sci. 2012;53(10):5985–90 [DOI] [PubMed] [Google Scholar]
- 161.Russell RA, Malik R, Chauhan BC, et al. Improved estimates of visual field progression using bayesian linear regression to integrate structural information in patients with ocular hypertension. Invest Ophthalmol Vis Sci. 2012;53(6):2760–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sample PA, Boden C, Zhang Z, et al. Unsupervised machine learning with independent component analysis to identify areas of progression in glaucomatous visual fields. Invest Ophthalmol Vis Sci. 2005;46(10):3684–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sample PA, Johnson CA, Haegerstrom-Portnoy G, Adams AJ. Optimum parameters for short-wavelength automated perimetry. J Glaucoma. 1996;5(6):375–83 [PubMed] [Google Scholar]
- 164.Saunders LJ, Russell RA, Kirwan JF, et al. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci. 2014;55(1):102–9 [DOI] [PubMed] [Google Scholar]
- 165.Schell GJ, Lavieri MS, Helm JE, et al. Using filtered forecasting techniques to determine personalized monitoring schedules for patients with open-angle glaucoma. Ophthalmology. 2014;121(8):1539–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shon K, Wollstein G, Schuman JS, Sung KR. Prediction of glaucomatous visual field progression: pointwise analysis. Curr Eye Res. 2014;39(7):705–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Spry PG, Bates AB, Johnson CA, Chauhan BC. Simulation of longitudinal threshold visual field data. Invest Ophthalmol Vis Sci. 2000;41(8):2192–200 [PubMed] [Google Scholar]
- 168.Spry PG, Johnson CA. Within-test variability of frequency-doubling perimetry using a 24–2 test pattern. J Glaucoma. 2002;11(4):315–20 [DOI] [PubMed] [Google Scholar]
- 169.Spry PG, Johnson CA, McKendrick AM, Turpin A. Variability components of standard automated perimetry and frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci. 2001;42(6):1404–10 [PubMed] [Google Scholar]
- 170.Stiles WS. Separation of the ‘blue’ and ‘green’ mechanisms of foveal vision by measurements of increment thresholds. Proc R Soc Med. 1946;134(873):418–34 [DOI] [PubMed] [Google Scholar]
- 171.Stiles WS. The approach through increment-threshold sensitivity. London, Academic Press, 1978 [Google Scholar]
- 172.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47(7):2904–10 [DOI] [PubMed] [Google Scholar]
- 173.Su D, Park SC, Simonson JL, et al. Progression pattern of initial parafoveal scotomas in glaucoma. Ophthalmology. 2013;120(3):520–7 [DOI] [PubMed] [Google Scholar]
- 174.Suzuki Y, Kitazawa Y, Araie M, et al. Mathematical and optimal clustering of test points of the central 30-degree visual field of glaucoma. J Glaucoma. 2001;10(2):121–8 [DOI] [PubMed] [Google Scholar]
- 175.Swanson WH, Sun H, Lee BB, Cao D. Responses of primate retinal ganglion cells to perimetric stimuli. Invest Ophthalmol Vis Sci. 2011;52(2):764–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Takahashi G, Demirel S, Johnson CA. Predicting conversion to glaucoma using standard automated perimetry and frequency doubling technology. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):797–803 [DOI] [PubMed] [Google Scholar]
- 177.Taketani Y, Murata H, Fujino Y, et al. How Many Visual Fields Are Required to Precisely Predict Future Test Results in Glaucoma Patients When Using Different Trend Analyses? Invest Ophthalmol Vis Sci. 2015;56(6):4076–82 [DOI] [PubMed] [Google Scholar]
- 178.Tanna AP, Bandi JR, Budenz DL, et al. Interobserver agreement and intraobserver reproducibility of the subjective determination of glaucomatous visual field progression. Ophthalmology. 2011;118(1):60–5 [DOI] [PubMed] [Google Scholar]
- 179.Tanna AP, Budenz DL, Bandi J, et al. Glaucoma Progression Analysis software compared with expert consensus opinion in the detection of visual field progression in glaucoma. Ophthalmology. 2012;119(3):468–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tatham AJ, Weinreb RN, Zangwill LM, et al. The relationship between cup-to-disc ratio and estimated number of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2013;54(5):3205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van der Schoot J, Reus NJ, Colen TP, Lemij HG. The ability of short-wavelength automated perimetry to predict conversion to glaucoma. Ophthalmology. 2010;117(1):30–4 [DOI] [PubMed] [Google Scholar]
- 182.van Gestel A, Webers CA, Severens JL, et al. The long-term outcomes of four alternative treatment strategies for primary open-angle glaucoma. Acta Ophthalmol. 2012;90(1):20–31 [DOI] [PubMed] [Google Scholar]
- 183.VanBuren J, Oleson JJ, Zamba GK, Wall M. Integrating independent spatio-temporal replications to assess population trends in disease spread. Stat Med. 2016;35(28):5210–21 [DOI] [PubMed] [Google Scholar]
- 184.Verma S, Nongpiur ME, Atalay E, et al. Visual Field Progression in Patients with Primary Angle-Closure Glaucoma Using Pointwise Linear Regression Analysis. Ophthalmology. 2017;124(7):1065–71 [DOI] [PubMed] [Google Scholar]
- 185.Vesti E, Johnson CA, Chauhan BC. Comparison of different methods for detecting glaucomatous visual field progression. Invest Ophthalmol Vis Sci. 2003;44(9):3873–9 [DOI] [PubMed] [Google Scholar]
- 186.Viswanathan AC, Crabb DP, McNaught AI, et al. Interobserver agreement on visual field progression in glaucoma: a comparison of methods. Br J Ophthalmol. 2003;87(6):726–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Viswanathan AC, Fitzke FW, Hitchings RA. Early detection of visual field progression in glaucoma: a comparison of PROGRESSOR and STATPAC 2. Br J Ophthalmol. 1997;81(12):1037–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wall M, Brito CF, Woodward KR, et al. Total deviation probability plots for stimulus size v perimetry: a comparison with size III stimuli. Arch Ophthalmol. 2008;126(4):473–9 [DOI] [PubMed] [Google Scholar]
- 189.Wall M, Doyle CK, Eden T, et al. Size threshold perimetry performs as well as conventional automated perimetry with stimulus sizes III, V, and VI for glaucomatous loss. Invest Ophthalmol Vis Sci. 2013;54(6):3975–83 [DOI] [PubMed] [Google Scholar]
- 190.Wall M, Doyle CK, Zamba KD, et al. The repeatability of mean defect with size III and size V standard automated perimetry. Invest Ophthalmol Vis Sci. 2013;54(2):1345–51 [DOI] [PubMed] [Google Scholar]
- 191.Wall M, Woodward KR, Doyle CK, Artes PH. Repeatability of automated perimetry: a comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Invest Ophthalmol Vis Sci. 2009;50(2):974–9 [DOI] [PubMed] [Google Scholar]
- 192.Wall M, Woodward KR, Doyle CK, Zamba G. The effective dynamic ranges of standard automated perimetry sizes III and V and motion and matrix perimetry. Arch Ophthalmol. 2010;128(5):570–6 [DOI] [PubMed] [Google Scholar]
- 193.Wall M, Zamba GKD, Artes PH. The Effective Dynamic Ranges for Glaucomatous Visual Field Progression With Standard Automated Perimetry and Stimulus Sizes III and V. Invest Ophthalmol Vis Sci. 2018;59(1):439–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Warren JL, Mwanza JC, Tanna AP, Budenz DL. A Statistical Model to Analyze Clinician Expert Consensus on Glaucoma Progression using Spatially Correlated Visual Field Data. Transl Vis Sci Technol. 2016;5(4):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wesselink C, Heeg GP, Jansonius NM. Glaucoma monitoring in a clinical setting: glaucoma progression analysis vs nonparametric progression analysis in the Groningen Longitudinal Glaucoma Study. Arch Ophthalmol. 2009;127(3):270–4 [DOI] [PubMed] [Google Scholar]
- 196.Wesselink C, Jansonius NM. Glaucoma progression detection with frequency doubling technology (FDT) compared to standard automated perimetry (SAP) in the Groningen Longitudinal Glaucoma Study. Ophthalmic Physiol Opt. 2017;37(5):594–601 [DOI] [PubMed] [Google Scholar]
- 197.White AJ, Sun H, Swanson WH, Lee BB. An examination of physiological mechanisms underlying the frequency-doubling illusion. Invest Ophthalmol Vis Sci. 2002;43(11):3590–9 [PubMed] [Google Scholar]
- 198.Wilkins MR, Fitzke FW, Khaw PT. Pointwise linear progression criteria and the detection of visual field change in a glaucoma trial. Eye (Lond). 2006;20(1):98–106 [DOI] [PubMed] [Google Scholar]
- 199.Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012;96(1):47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu Z, Medeiros FA. Comparison of Visual Field Point-Wise Event-Based and Global Trend-Based Analysis for Detecting Glaucomatous Progression. Transl Vis Sci Technol. 2018;7(4):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu Z, Saunders LJ, Daga FB, et al. Frequency of Testing to Detect Visual Field Progression Derived Using a Longitudinal Cohort of Glaucoma Patients. Ophthalmology. 2017;124(6):786–92 [DOI] [PubMed] [Google Scholar]
- 202.Yousefi S, Balasubramanian M, Goldbaum MH, et al. Unsupervised Gaussian Mixture-Model With Expectation Maximization for Detecting Glaucomatous Progression in Standard Automated Perimetry Visual Fields. Transl Vis Sci Technol. 2016;5(3):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yousefi S, Goldbaum MH, Balasubramanian M, et al. Glaucoma progression detection using structural retinal nerve fiber layer measurements and functional visual field points. IEEE Trans Biomed Eng. 2014;61(4):1143–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yousefi S, Goldbaum MH, Balasubramanian M, et al. Learning from data: recognizing glaucomatous defect patterns and detecting progression from visual field measurements. IEEE Trans Biomed Eng. 2014;61(7):2112–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zeppieri M, Demirel S, Kent K, Johnson CA. Perceived spatial frequency of sinusoidal gratings. Optom Vis Sci. 2008;85(5):318–29 [DOI] [PubMed] [Google Scholar]
- 206.Zhang C, Tatham AJ, Daga FB, et al. Event-based analysis of visual field change can miss fast glaucoma progression detected by a combined structure and function index. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1227–34 [DOI] [PubMed] [Google Scholar]
- 207.Zhu H, Crabb DP, Ho T, Garway-Heath DF. More Accurate Modeling of Visual Field Progression in Glaucoma: ANSWERS. Invest Ophthalmol Vis Sci. 2015;56(10):6077–83 [DOI] [PubMed] [Google Scholar]


