Abstract
Goals:
To quantify the association between demographic factors and advanced colorectal cancer in patients under age 50.
Background:
Colorectal cancer (CRC) incidence in the US has declined in older individuals but increased in those under age 50 (early-onset). More than 60% of early-onset CRC patients present with advanced disease (Stage III/IV), but predictors of stage in this population are poorly defined.
Study:
We analyzed CRC cases diagnosed between age 20–49 in the US Surveillance, Epidemiology, and End Results (SEER) 18 database during 2004–2015. Logistic regression models were fit to assess the impact of age, sex, race, ethnicity, marital status, and cancer site on the probability of advanced disease.
Results:
The analysis included 37,044 cases. On multivariable regression, age was inversely associated with advanced disease. Relative to 45–49-year-olds, 40–44-year-olds had 8% greater odds of having advanced CRC, and 20–24-year-olds had 53% greater odds. Asians, blacks, and Pacific Islanders had 10%, 12%, and 45% greater odds of advanced disease compared to whites. Compared to non-partnered individuals, those with partners had 11% lower odds of advanced CRC. Both right- and left-sided colon cancer were more likely to be diagnosed at Stage IV compared to rectal cancer.
Conclusions:
Among individuals with early-onset CRC, younger age, Asian, black, or Pacific Islander race, and being non-partnered were predictors of advanced disease at presentation. Colon cancer was more likely to be diagnosed at Stage IV than rectal cancer. Patient characteristics associated with advanced CRC may indicate both differences in tumor biology and disparities in health care access.
Keywords: Colorectal cancer, early-onset, SEER, health disparities
Introduction
Colorectal cancer (CRC) is the second most common malignancy diagnosed in women and the third most common in men. In 2018, it claimed more than 880,000 lives and was the second leading cause of cancer death worldwide1.
Overall, CRC incidence in the US decreased by 2–3% per year from 2005 to 20142. This is partially attributed to increased utilization of screening, including with colonoscopy and stool-based tests3. However, as incidence in older adults declined, the opposite trend has been observed for adults younger than age 50 (early-onset CRC). From the early 1970’s to 1999, the incidence of early-onset CRC increased by approximately 0.75% per year4. From 1992 to 2013, US Surveillance, Epidemiology, and End Results Reporting (SEER) data showed that the incidence of early-onset CRC increased by 2% annually5.
Additionally, younger adults tend to present with advanced disease, with more than 60% of patients under the age of 50 diagnosed with Stage III or IV disease6. This is reflected by a parallel rise in CRC mortality, which increased by 1% annually from 2004 to 2014 in persons diagnosed before age 547.
Prior studies of early-onset CRC have focused on examining population-level trends. However, it is unclear which patient characteristics predict advanced disease at diagnosis in individuals with early-onset CRC. This has potentially important implications for risk stratification and targeted screening. In this study, we assessed predictors of Stage III/IV early-onset CRC using the SEER database.
Materials and Methods
The SEER program provides high-quality epidemiological data collected from regional and state cancer registries that currently cover 35% of the US population8. The SEER 18 registry includes 10 states (Connecticut, Georgia, California, Hawaii, Iowa, Kentucky, Louisiana, New Mexico, New Jersey, and Utah), the city of Detroit, the Seattle-Puget Sound area, and the Alaska Native Tumor Registry. For this analysis, we queried the SEER 18 dataset for all CRC cases diagnosed between 20 and 49 years of age from 2004 to 2015. This was the period for which derived American Joint Committee on Cancer (AJCC) staging using Collaborative Stage was available. While the majority of prior research using SEER data has categorized disease based on the SEER summary staging system (localized, regional, distant), using the AJCC staging system (I-IV) provides an advantage since it is used to determine clinical prognosis and treatment. CRC was defined by the International Classification of Diseases for Oncology (ICD-O-3). Tumor site was categorized as right colon (cecum, ascending colon, hepatic flexure, and transverse colon; C180, C182-C184), left colon (splenic flexure, descending colon, and sigmoid colon; C185-C187), and rectum (rectosigmoid junction and rectum; C199, C209). Appendiceal cancer (C181) was excluded, as it is considered a separate entity. Large intestine, not otherwise specified (C188-C189, C260) was omitted because primary site could not be determined.
R (Vienna, Austria) was used for all analysis9. Packages used included Base for regression and ggplot2 for graphics10,11. We fit univariable and multivariable logistic regression models to calculate the odds of being diagnosed with advanced (Stage III/IV) relative to early-stage (Stage I/II) CRC for the following six variables: age (in 5-year groups), sex, race, ethnicity, marital status, and cancer site (right colon, left colon, and rectum). Marital status was categorized as partnered (married, domestic partner) or non-partnered (single, divorced, separated, and widowed). Those with unknown marital status or race were excluded. Two multivariable regression models were constructed. Model 1 included the five demographic variables that could be used to risk stratify individuals younger than age 50. Model 2 included demographic variables as well as tumor site. We performed a post hoc sensitivity analysis to assess whether predictors of Stage III and Stage IV disease differed compared to early-stage disease. Additionally, we compared risk estimates from Models 1 and 2 for patients under age 50 to those aged 50 and older. Statistical significance was set at 0.05 and all tests were two-sided.
Results
A total of 37,044 patients diagnosed with CRC between age 20–49 were identified during the study period, of whom 14,560 (39%) had early-stage cancer and 22,484 (61%) had advanced disease (Table 1). The number of individuals with early-onset CRC approximately doubled with each 5-year increment in age, such that 20–24-year-olds accounted for only 1% of all cases, whereas 45–49-year-olds accounted for 49%. Distribution by race was 75% white, 14% black, 9% Asian, 1% American Indian, and 1% Pacific Islander; 16% identified as Hispanic. The most common cancer site was rectum (40%), followed by left colon (33%) and right colon (27%).
Table 1.
Predictors of advanced stage, early-onset colorectal cancer. Model 1 includes only demographic variables (age, sex, race, Hispanic ethnicity, marital status), while model 2 also includes tumor site. Both univariable and multivariable regression models exclude missing data.
| Variable | Stage I/II (n=14560) |
Stage III/IV (n=22484) | Odds Ratio - Univariable (95% CI) | Adjusted Odds Ratio - Multivariable Model 1 (95% CI) |
Adjusted Odds Ratio - Multivariable Model 2 (95% CI) |
|---|---|---|---|---|---|
| Age, n (%) | |||||
| 20 – 24 | 138 (0.9) | 300 (1.3) | 1.62 (1.31–2.01) | 1.53 (1.23–1.89) | 1.53 (1.23–1.89) |
| 25 – 29 | 359 (2.5) | 778 (3.5) | 1.57 (1.38–1.79) | 1.52 (1.33–1.74) | 1.52 (1.33–1.74) |
| 30 – 34 | 883 (6.1) | 1634 (7.3) | 1.31 (1.20–1.43) | 1.30 (1.18–1.42) | 1.30 (1.19–1.42) |
| 35 – 39 | 1775 (12.2) | 3166 (14.1) | 1.28 (1.19–1.36) | 1.27 (1.19–1.36) | 1.27 (1.19–1.36) |
| 40 – 44 | 3895 (26.8) | 5961 (26.5) | 1.08 (1.03–1.14) | 1.08 (1.03–1.14) | 1.09 (1.03–1.14) |
| 45 – 49 | 7510 (51.6) | 10645 (47.3) | 1.00 | 1.00 | 1.00 |
| Male sex, n (%) | 7651 (52.5) | 12012 (53.4) | 1.03 (0.99–1.07) | 1.03 (0.99–1.08) | 1.03 (0.99–1.08) |
| Race, n (%) | |||||
| American Indian | 165 (1.1) | 246 (1.1) | 1.07 (0.87–1.33) | 1.05 (0.85–1.31) | 1.05 (0.84–1.30) |
| Asian | 1239 (8.5) | 2035 (9.1) | 1.08 (1.00–1.16) | 1.10 (1.02–1.19) | 1.09 (1.01–1.18) |
| Black | 1914 (13.1) | 3277 (14.6) | 1.13 (1.06–1.20) | 1.12 (1.05–1.19) | 1.13 (1.06–1.20) |
| Pacific Islander | 92 (0.6) | 214 (1.0) | 1.47 (1.15–1.89) | 1.45 (1.13–1.87) | 1.45 (1.13–1.86) |
| White | 11004 (75.6) | 16642 (74.0) | 1.00 | 1.00 | 1.00 |
| Missing | 146 (1.0) | 70 (0.3) | - | - | - |
| Hispanic ethnicity, n (%) | 2278 (15.6) | 3626 (16.1) | 1.05 (0.99–1.11) | 1.05 (0.99–1.11) | 1.05 (0.99–1.12) |
| Marital status, n (%) | |||||
| Partnered | 8588 (59.0) | 12740 (56.7) | 0.86 (0.83–0.90) | 0.89 (0.85–0.93) | 0.89 (0.85–0.93) |
| Non-partnered | 5161 (35.4) | 8873 (39.5) | 1.00 | 1.00 | 1.00 |
| Missing | 811 (5.6) | 871 (3.9) | - | - | - |
| Site, n (%) | |||||
| Left Colon | 4731 (32.5) | 7409 (33.0) | 1.02 (0.97–1.07) | - | 1.01 (0.96–1.07) |
| Right Colon | 4037 (27.7) | 5990 (26.6) | 0.94 (0.90–1.00) | - | 0.93 (0.88–0.98) |
| Rectum | 5792 (39.8) | 9085 (40.4) | 1.00 | - | 1.00 |
The direction and magnitude of associations were similar in the univariable and two multivariable regression models. Sex was not a significant predictor while age was inversely associated with the odds of advanced disease. In Model 1 of the multivariable analysis, compared to patients aged 45–49, those aged 20–24 had an odds ratio (OR) of 1.53 (95% CI 1.23 – 1.89; Figure 1) of being diagnosed with advanced CRC. The OR decreased to 1.08 (95% CI 1.03 – 1.14) in the 40–44 age group. Asians, blacks, and Pacific Islanders had respective ORs of 1.10 (95% CI 1.02 – 1.19), 1.12(95% CI 1.05 – 1.19), and 1.45 (95% CI 1.13 – 1.87) to be diagnosed with advanced CRC compared to whites. With respect to marital status, individuals with a partner had an OR of 0.89 (95% CI 0.85 – 0.93) of advanced CRC compared to those without.
Figure 1.
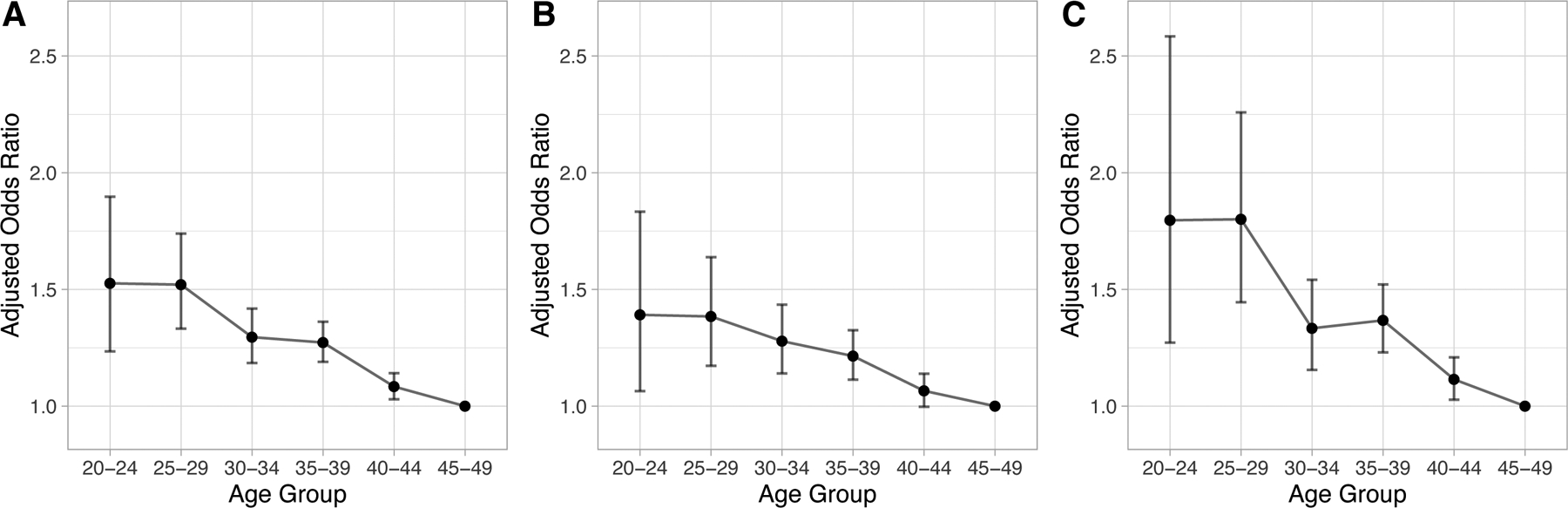
Adjusted odds ratios of diagnosis with Stage III/IV colorectal cancer relative to Stage I/II by age group, with age 45 – 49 as the reference group. Odds ratios are adjusted for sex, race, Hispanic ethnicity, and marital status. Panel A: colorectal cancer, Panel B: colon cancer, Panel C: rectal cancer.
Model 2, which included tumor site, showed that compared to individuals with rectal cancer, those with right-sided colon cancer had an OR of 0.93 (95% CI 0.88 – 0.98) of having advanced CRC. There was no difference between rectal and left-sided colon cancer. In the sensitivity analysis comparing Stage III vs. early-stage CRC, the OR of having advanced disease was lower for both right-sided (OR 0.83, 95% CI 0.78–0.89) and left-sided (OR 0.91, 95% CI 0.86–0.96) colon cancer compared to rectal cancer (Table 2). In contrast, when comparing Stage IV vs. early-stage CRC, the odds of advanced disease was higher for both right-sided (OR 1.07, 95% CI 1.00–1.15) and left-sided (OR 1.17, 95% CI 1.09–1.24) colon cancer compared to rectal cancer (Table 2). Distribution of tumor stage by site showed that rectal cancer contributed 40% of early-stage, 43% of Stage III, and 37% of Stage IV CRC (Figure 2). Conversely, both left-sided and right-sided colon cancer made up a lower proportion of Stage III and higher proportion of Stage IV CRC compared to early-stage disease.
Table 2.
Post hoc sensitivity analysis assessing whether predictors of Stage III and Stage IV disease differ compared to early-stage disease. Odds ratios were obtained using multivariable logistic regression
| Variable | Stage I/II (N=13659) |
Stage III (N=12047) |
Stage IV (N=9503) |
Adjusted Odds Ratio - Multivariable Stage III vs I/II (95% CI) | Adjusted Odds Ratio - Multivariable Stage IV vs I/II (95% CI) |
|---|---|---|---|---|---|
| Age, n (%) | |||||
| 20 – 24 | 123 (0.9) | 162 (1.3) | 126 (1.3) | 1.62 (1.28–2.06) | 1.44 (1.12–1.85) |
| 25 – 29 | 332 (2.4) | 402 (3.3) | 350 (3.7) | 1.51 (1.30–1.76) | 1.54 (1.31–1.79) |
| 30 – 34 | 830 (6.1) | 902 (7.5) | 665 (7.0) | 1.36 (1.23–1.51) | 1.21 (1.09–1.35) |
| 35 – 39 | 1653 (12.1) | 1731 (14.4) | 1311 (13.8) | 1.32 (1.22–1.42) | 1.22 (1.13–1.33) |
| 40 – 44 | 3672 (26.9) | 3241 (26.9) | 2493 (26.2) | 1.11 (1.05–1.18) | 1.05 (0.99–1.12) |
| 45 – 49 | 7049 (51.6) | 5609 (46.6) | 4558 (48.0) | 1.00 | 1.00 |
| Male sex, n (%) | 7192 (52.7) | 6403 (53.2) | 5101 (53.7) | 1.02 (0.97–1.07) | 1.05 (01.00–1.11) |
| Race, n (%) | |||||
| American Indian | 134 (1.0) | 118 (1.0) | 103 (1.1) | 0.99 (0.77–1.27) | 1.13 (0.87–1.46) |
| Asian | 1191 (8.7) | 1128 (9.4) | 843 (8.9) | 1.09 (1.00–1.19) | 1.10 (1.00–1.21) |
| Black | 1805 (13.2) | 1591 (13.2) | 1528 (16.1) | 1.04 (0.96–1.12) | 1.24 (1.15–1.34) |
| Pacific Islander | 91 (0.7) | 108 (0.9) | 98 (1.0) | 1.32 (1.00–1.75) | 1.61 (1.21–2.15) |
| White | 10438 (76.4) | 9102 (75.6) | 6931 (72.9) | 1.00 | 1.00 |
| Hispanic ethnicity, n (%) | 2121 (15.5) | 1934 (16.1) | 1545 (16.3) | 1.03 (0.96–1.11) | 1.08 (1.00–1.16) |
| Marital status, n (%) | |||||
| Partnered | 8531 (62.5) | 7355 (61.1) | 5349 (56.3) | 0.96 (0.91–1.01) | 0.81 (0.76–0.85) |
| Non-partnered | 5128 (37.5) | 4692 (38.9) | 4154 (43.7) | 1.00 | 1.00 |
| Site, n (%) | |||||
| Left Colon | 4364 (31.9) | 3787 (31.4) | 3290 (34.6) | 0.91 (0.86–0.96) | 1.17 (1.09–1.24) |
| Right Colon | 3821 (28.0) | 3057 (25.4) | 2691 (28.3) | 0.83 (0.78–0.89) | 1.07 (1.00–1.15) |
| Rectum | 5474 (40.1) | 5203 (43.2) | 3522 (37.1) | 1.00 | 1.00 |
Figure 2.
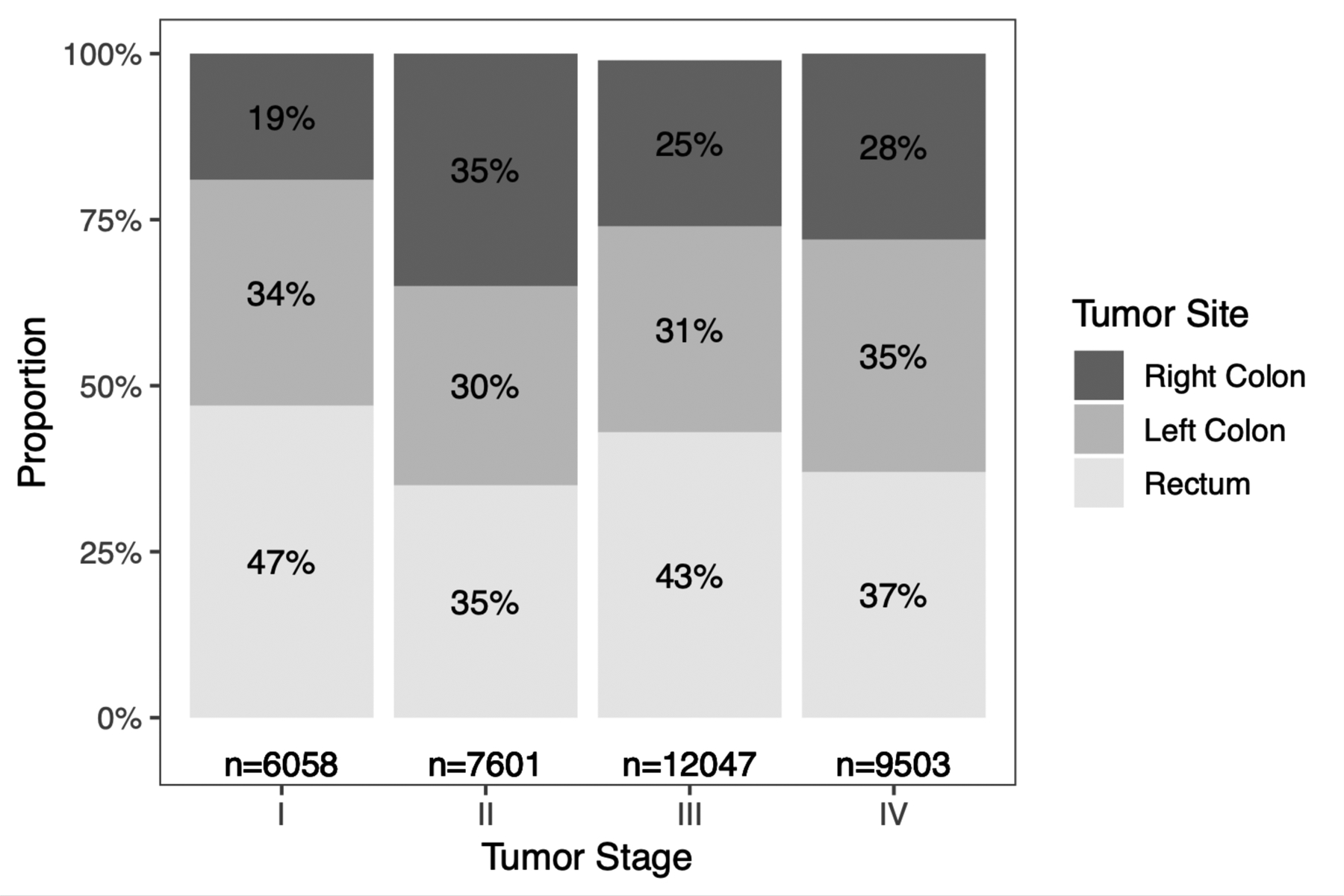
Frequency and proportion of early-onset colorectal cancers by tumor stage and site.
The risk estimates from Models 1 and 2 for early-onset CRC were similar to those for patients aged 50 and older (Table 3). The association with advanced CRC was slightly stronger for American Indians, Asians, and blacks in older compared to younger patients. Pacific Islanders also had the strongest association with advanced disease of any racial group among older patients, although the risk estimate was attenuated compared to that of younger patients.
Table 3.
Predictors of advanced stage colorectal cancer in younger (< 50 years) and older patients (≥ 50 years). Multivariable model 1 includes only demographic variables (age, sex, race, Hispanic ethnicity, marital status), while model 2 includes also includes tumor site.
| Variable | Younger Patients - Model 1 Odds Ratio (95% CI) | Older Patients - Model 1 Odds Ratio (95% CI) | Younger Patients - Model 2 Odds Ratio (95% CI) | Older Patients - Model 2 Odds Ratio (95% CI) |
|---|---|---|---|---|
| Male sex, n (%) | 1.03 (0.99–1.08) | 1.02 (1.00 – 1.03) | 1.03 (0.99–1.08) | 1.02 (1.00 – 1.03) |
| Race, n (%) | ||||
| American Indian | 1.05 (0.85–1.31) | 1.10 (1.00 – 1.21) | 1.05 (0.84–1.30) | 1.10 (1.00 – 1.21) |
| Asian | 1.10 (1.02–1.19) | 1.15 (1.12 – 1.18) | 1.09 (1.01–1.18) | 1.16 (1.12 – 1.19) |
| Black | 1.12 (1.05–1.19) | 1.18 (1.15 – 1.21) | 1.13 (1.06–1.20) | 1.18 (1.15 – 1.21) |
| Pacific Islander | 1.45 (1.13–1.87) | 1.23 (1.10 – 1.37) | 1.45 (1.13–1.86) | 1.24 (1.11 – 1.38) |
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Hispanic ethnicity, n (%) | 1.05 (0.99–1.11) | 1.09 (1.07 – 1.12) | 1.05 (0.99–1.12) | 1.09 (1.07 – 1.12) |
| Marital Status, n (%) | ||||
| Partnered | 0.89 (0.85–0.93) | 0.89 (0.87 – 0.90) | 0.89 (0.85–0.93) | 0.89 (0.87 – 0.90) |
| Non-partnered | 1.00 | 1.00 | 1.00 | 1.00 |
| Site, n (%) | ||||
| Left Colon | - | - | 1.01 (0.96–1.07) | 0.95 (0.93 – 0.96) |
| Right Colon | - | - | 0.93 (0.88–0.98) | 1.01 (0.99 – 1.03) |
| Rectum | - | - | 1.00 | 1.00 |
Discussion
In this large, population-based study of individuals diagnosed with CRC before age 50 in the US, younger age, Asian, black, or Pacific Islander race, and not having a partner were all independent predictors of advanced stage at presentation. Both right- and left-sided colon cancer was more likely to be diagnosed at Stage IV than rectal cancer. These epidemiological findings help to characterize important biologic and social determinants of early-onset CRC and may be especially useful for risk stratification.
The association between young age and late-stage disease may be attributed to both biologic and social factors. Prior studies have demonstrated that younger individuals tend to have tumors with worse clinicopathological features, including later stage at presentation and higher rates of mucinous or poorly differentiated histopathology12,13. A similar trend is observed in patients greater than age 50, with increasing age associated with earlier stage, greater tumor differentiation, and less mucin production at time of diagnosis14. Younger patients are also less likely to have insurance, seek care, and receive appropriate diagnostic testing. Lower rates of health insurance are partially due to employment at temporary jobs offering inadequate healthcare coverage15,16. Individuals may also lose coverage from parental insurance during young adulthood, leaving them vulnerable to lapses in medical care17. In addition, studies have shown that younger individuals are less likely to seek medical care17,18. When younger adults do seek care, clinicians are less inclined to suspect malignancy, which may result in a delayed diagnosis19.
Similarly, the association between race and late-stage disease may also be attributed to both biologic and social factors. A number of unique genetic and epigenetic characteristics have been identified in CRC among black patients20. One study sequencing 103 CRCs in blacks and 129 in whites identified three somatic mutations (EPHA6, FLCN, HTRIF) exclusively in black patients21. One of these mutations, EPHA6, has been associated with late-stage, metastatic disease22. Another population-based study demonstrated lower rates of CRC microsatellite instability among blacks compared to whites (7% vs. 14%), which portends a poorer prognosis23. Finally, CRC in blacks have been shown to have decreased immunogenicity, which is associated with poor prognosis and early metastasis23. Likewise, Asians have been shown to have unique genetic features that impart an elevated risk for CRC. A large genome-wide association study revealed 13 loci associated with risk for CRC in East Asian populations. These loci were used to calculate a polygenic risk score, and individuals in the highest risk score quintile had a 3.2-fold increase in CRC risk compared to those in the lowest quintile24. Although less is known about the genetic factors driving CRC in Pacific Islanders, there is evidence that tumors in this population tend to arise proximally when compared to white and black patients25. Proximal tumors, in turn, are associated with mutations in BRAF and KRAS, which are associated with a poorer prognosis26,27.
In addition to genetic influences, socioeconomic factors also likely contribute to the late presentation of early-onset CRC in minority populations. It is well documented that racial minorities have less access to medical resources and subsequently worse medical outcomes28,29. Blacks, in particular, face significant barriers to healthcare access stemming from provider bias, distrust of the healthcare system, and paucity of community healthcare resources30,31. Blacks are also more likely to be uninsured and socioeconomically disadvantaged, limiting the affordability of medical care32,33. These disparities are reflected in cancer screening, with non-white Medicare beneficiaries being 48% less likely to undergo CRC screening than their white counterparts34. Asian Americans similarly have lower CRC screening rates when compared with non-Hispanic whites, with Filipinos, Koreans, and South Asians having the lowest screening rates35. Reasons for low CRC screening rates among Asian Americans includes fear of testing, absence of healthcare coverage, limited access to physicians, limited English proficiency, and low health literacy36,37.
Our results suggest Pacific Islanders may have the highest odds of late-stage CRC of any racial group, although the smaller sample size makes the risk estimate less reliable than for larger groups. Similar to blacks and Asians, Pacific Islanders face barriers to healthcare access38. Small-scale studies have found lower screening uptake among Pacific Islanders, with only 38.7% of native Hawaiians completing stool testing and 58.9% receiving endoscopy39,40. Pacific Islanders also report higher prevalence of unhealthy behaviors such as tobacco use, physical inactivity, and low fruit/vegetable consumption, all of which increase CRC risk41. Although we did not find a statistically significant difference between American Indians compared to whites, American Indians also face well-documented socioeconomic barriers to healthcare, including rural residence, lower income, and poor healthcare literacy42,43. This is illustrated by a study which surveyed American Indians between the ages of 30–49 regarding their perceptions of CRC screening44. The majority of participants demonstrated a poor understanding of CRC and associated healthcare knowledge. Certain American Indian groups, such as Pima Indians, are also at increased risk for CRC due to higher rates of obesity, metabolic syndrome, and excessive alcohol consumption45,46.
The protective effect of having a partner is inherently social. Analysis of the SEER database has previously demonstrated that marriage is correlated with lower-stage CRC and improved survival47. Similarly, married patients are more likely to comply with CRC screening48.
Our sensitivity analysis showed that compared to rectal cancer, colon cancer was less frequently diagnosed at Stage III but more frequently diagnosed at Stage IV. The reason for this may be related to the timing of symptoms. Rectal cancer tends to present with overt symptoms such as bleeding and changes in bowel habits49, which tend to prompt earlier clinical consultation and diagnosis. Prior analyses of the SEER database confirm that rectal cancers tend to be diagnosed at earlier stages than colon cancers3,50. Conversely, colon cancers tend to present with more insidious symptoms, such as anemia and weight loss49, which may delay diagnosis.
Our study is among the first to evaluate demographic risk factors associated with advanced disease in early-onset CRC. This provides valuable information for potential risk stratification and targeted screening in younger adults. The American Cancer Society recommended lowering the general screening age to 45 in 2018, but it remains to be seen whether insurers and policymakers will follow this recommendation51. There are no recommendations to screen average-risk individuals below age 45, but it is important to recognize that younger adults can develop CRC and clinical symptoms must be appropriately addressed in a timely manner. Our results may be helpful for organizations interested in either offering selective screening in individuals younger than age 50 or prioritizing diagnostic colonoscopy for those with symptoms.
A strength of our study is the use of the SEER database, which is representative of the US population and one of the only data sources with sufficient sample size to explore understudied racial groups, such as Pacific Islanders. Additionally, the use of AJCC staging allows for more clinically relevant interpretation than the SEER summary staging conventionally used to describe this dataset. A key limitation is the lack of data on a number of well-established factors that are relevant for CRC stage at presentation, including medical comorbidities, socioeconomic status, and the availability of local healthcare resources. Information regarding family history and genetic syndromes such as familial adenomatous polyposis are also not available. However, the variables in our model are readily available in most clinical settings and can form the basis for more comprehensive models and risk-stratification tools.
In summary, age, race, and marital status are independent predictors of advanced stage CRC in individuals diagnosed under age 50. These results show the importance of both biologic and social determinants in early-onset CRC and help to identify high-risk groups for potential intervention.
Grant Support:
PSL is supported by grant K08CA230162 from the National Cancer Institute.
Abbreviations:
- AJCC
American Joint Committee on Cancer
- CRC
colorectal cancer
- ICD-O
International Classification of Diseases for Oncology
- SEER
US Surveillance, Epidemiology, and End Results
Footnotes
Disclosures: The authors declare no potential conflicts of interest
Writing Assistance: no writing assistance to disclose
References
- 1.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. https://gco.iarc.fr/today. Published 2018. Accessed June 3, 2019, 2018. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Ward EM, Jemal A. Trends in Colorectal Cancer Incidence Rates in the United States by Tumor Location and Stage, 1992–2008. Cancer Epidemiology Biomarkers & Prevention. 2012;21(3):411–416. [DOI] [PubMed] [Google Scholar]
- 4.Davis DM, Marcet JE, Frattini JC, et al. Is It Time to Lower the Recommended Screening Age for Colorectal Cancer? Journal of the American College of Surgeons. 2011;213(3):352–361. [DOI] [PubMed] [Google Scholar]
- 5.Gyawali CP, Fass R. Management of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):302–318. [DOI] [PubMed] [Google Scholar]
- 6.Dozois EJ, Boardman LA, Suwanthanma W, et al. Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine. 2008;87(5):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. Jama. 2017;318(6):572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1975–2016), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
- 9.R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 10.Base: R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 11.Ggplot2: H. Wickham. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016. [Google Scholar]
- 12.Schellerer VS, Merkel S, Schumann SC, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer. International Journal of Colorectal Disease. 2012;27(1):71–79. [DOI] [PubMed] [Google Scholar]
- 13.Mitry E, Benhamiche AM, Jouve JL, et al. Colorectal adenocarcinoma in patients under 45 years of age: comparison with older patients in a well-defined French population. Dis Colon Rectum. 2001;44(3):380–387. [DOI] [PubMed] [Google Scholar]
- 14.Chiang J-M, Chen M-C, Changchien CR, et al. Favorable Influence of Age on Tumor Characteristics of Sporadic Colorectal Adenocarcinoma. Diseases of the Colon & Rectum. 2003;46(7):904–910. [DOI] [PubMed] [Google Scholar]
- 15.Spencer DL, McManus M, Call KT, et al. Health Care Coverage and Access Among Children, Adolescents, and Young Adults, 2010–2016: Implications for Future Health Reforms. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2018;62(6):667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz K, Schwartz T. Uninsured young adults: A profile and overview of coverage options. Washington, DC2008. [Google Scholar]
- 17.Park MJ, Paul Mulye T, Adams SH, et al. The Health Status of Young Adults in the United States. Journal of Adolescent Health. 2006;39(3):305–317. [DOI] [PubMed] [Google Scholar]
- 18.Mulye TP, Park MJ, Nelson CD, et al. Trends in Adolescent and Young Adult Health in the United States. Journal of Adolescent Health. 2009;45(1):8–24. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 20.Carethers JM. Clinical and Genetic Factors to Inform Reducing Colorectal Cancer Disparitites in African Americans. Front Oncol. 2018;8:531–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guda K, Veigl ML, Varadan V, et al. Novel recurrently mutated genes in African American colon cancers. Proc Natl Acad Sci U S A. 2015;112(4):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathot L, Kundu S, Ljungström V, et al. Somatic Ephrin Receptor Mutations Are Associated with Metastasis in Primary Colorectal Cancer. Cancer Research. 2017;77(7):1730. [DOI] [PubMed] [Google Scholar]
- 23.Carethers JM, Murali B, Yang B, et al. Influence of race on microsatellite instability and CD8+ T cell infiltration in colon cancer. PLoS One. 2014;9(6):e100461–e100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Kweon S-S, Tanikawa C, et al. Large-Scale Genome-Wide Association Study of East Asians Identifies Loci Associated With Risk for Colorectal Cancer. Gastroenterology. 2019;156(5):1455–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Chen VW, Martin J, et al. Subsite-Specific Colorectal Cancer Incidence Rates and Stage Distributions among Asians and Pacific Islanders in the United States, 1995 to 1999. Cancer Epidemiology Biomarkers & Prevention. 2004;13(7):1215. [PubMed] [Google Scholar]
- 26.Maus MKH, Hanna DL, Stephens CL, et al. Distinct gene expression profiles of proximal and distal colorectal cancer: implications for cytotoxic and targeted therapy. Pharmacogenomics J. 2015;15(4):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinicrope FA, Mahoney MR, Yoon HH, et al. Analysis of Molecular Markers by Anatomic Tumor Site in Stage III Colon Carcinomas from Adjuvant Chemotherapy Trial NCCTG N0147 (Alliance). Clin Cancer Res. 2015;21(23):5294–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Understanding Racial and Ethnic Differences in Health in Late Life: A Research Agenda. Washington, DC2004. [PubMed] [Google Scholar]
- 29.Lee S, Martinez G, Ma GX, et al. Barriers to health care access in 13 Asian American communities. American journal of health behavior. 2010;34(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz JN. Patient Preferences and Health Disparities. Jama. 2001;286(12):1506–1509. [DOI] [PubMed] [Google Scholar]
- 31.Fiscella K, Franks P, Gold MR, et al. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. Jama. 2000;283(19):2579–2584. [DOI] [PubMed] [Google Scholar]
- 32.Ashton CM, Haidet P, Paterniti DA, et al. Racial and ethnic disparities in the use of health services: bias, preferences, or poor communication? Journal of general internal medicine. 2003;18(2):146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manuel JI. Racial/Ethnic and Gender Disparities in Health Care Use and Access. Health Serv Res. 2018;53(3):1407–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ananthakrishnan AN, Schellhase KG, Sparapani RA, et al. Disparities in Colon Cancer Screening in the Medicare Population. JAMA Internal Medicine. 2007;167(3):258–264. [DOI] [PubMed] [Google Scholar]
- 35.Kandula NR, Wen M, Jacobs EA, et al. Low rates of colorectal, cervical, and breast cancer screening in Asian Americans compared with non-Hispanic whites. Cancer. 2006;107(1):184–192. [DOI] [PubMed] [Google Scholar]
- 36.Jones RM, Devers KJ, Kuzel AJ, et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. American journal of preventive medicine. 2010;38(5):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sentell T, Braun KL, Davis J, et al. Colorectal cancer screening: low health literacy and limited English proficiency among Asians and Whites in California. Journal of health communication. 2013;18(sup1):242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narcisse M-R, Felix H, Long CR, et al. Frequency and predictors of health services use by Native Hawaiians and Pacific Islanders: evidence from the U.S. National Health Interview Survey. BMC Health Services Research. 2018;18(1):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starr RR, Taflinger FV, Teel CM. Insights in Public Health: Public Health Perspectives on Colorectal Cancer Screening. Hawai’i Journal of Medicine & Public Health. 2014;73(7):223. [PMC free article] [PubMed] [Google Scholar]
- 40.Domingo J-LB, Chen JJ, Braun KL. Colorectal Cancer Screening Compliance among Asian and Pacific Islander Americans. Journal of Immigrant and Minority Health. 2018;20(3):584–593. [DOI] [PubMed] [Google Scholar]
- 41.Moy KL, Sallis JF, David KJ. Health indicators of Native Hawaiian and Pacific Islanders in the United States. J Community Health. 2010;35(1):81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrone S Understanding barriers to health care: a review of disparities in health care services among indigenous populations. International Journal of Circumpolar Health. 2007;66(3):188–198. [DOI] [PubMed] [Google Scholar]
- 43.Vega WA, Rodriguez MA, Gruskin E. Health disparities in the Latino population. Epidemiologic reviews. 2009;31:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filippi MK, Braiuca S, Cully L, et al. American Indian perceptions of colorectal cancer screening: viewpoints from adults under age 50. Journal of Cancer Education. 2013;28(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson CS, Oman M, Patel AM, et al. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. Journal of gastrointestinal oncology. 2016;7(Suppl 1):S32–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friese B, Grube JW, Seninger S, et al. Drinking behavior and sources of alcohol: differences between Native American and White youths. J Stud Alcohol Drugs. 2011;72(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Wilson SE, Stewart DB, et al. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer epidemiology. 2011;35(5):417–422. [DOI] [PubMed] [Google Scholar]
- 48.El-Haddad B, Dong F, Kallail KJ, et al. Association of marital status and colorectal cancer screening participation in the USA. Colorectal Disease. 2015;17(5):O108–O114. [DOI] [PubMed] [Google Scholar]
- 49.Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. The American journal of gastroenterology. 1999;94(10):3039. [DOI] [PubMed] [Google Scholar]
- 50.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(2):104–117. [DOI] [PubMed] [Google Scholar]
- 51.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: a cancer journal for clinicians. 2018;68(4):250–281. [DOI] [PubMed] [Google Scholar]


