Abstract
Objectives:
Oncology clinical trials use a variety of clinical endpoints. Patients’ understanding of the differences between clinical endpoints is important because misperceptions of treatment efficacy may affect treatment decisions. The objective of this literature review is to find and synthesize available empirical publications assessing patients’ understanding of common oncology clinical endpoints.
Methods:
We conducted a literature search of 5 databases and 3 conferences, limiting the search to articles and abstracts published in English through September 2018. We reviewed the titles and abstracts for inclusion, then reviewed full texts to determine if they reported empirical research studies focused on (1) clinical endpoints, (2) oncology, and (3) patient understanding. The original search identified 497 publications, of which 13 met the inclusion criteria.
Results:
Available literature yields little information on this topic. The few publications that do exist suggest that healthcare professionals and cancer patients generally do not discuss clinical endpoint concepts and that patients can be confused about the purpose of a treatment based on misperceptions about endpoints.
Conclusions:
Research is needed on how to discuss oncology clinical endpoints with patients.
Practice Implications:
Patient-friendly definitions of clinical endpoints may help healthcare providers communicate important information about treatments to patients.
Keywords: Progression-free survival, patients, regulatory science, clinical endpoints, patient understanding
1. Introduction
The past decade has seen a dramatic rise in the number of oncology drugs brought to market. In 2018, 29% (17/59) of the novel drugs approved by the Food and Drug Administration (FDA) were oncology drugs—more than any other therapeutic area [1]. Clinical trials of cancer drugs use a variety of clinical endpoints to assess efficacy. Commonly used clinical endpoints include overall survival (OS), disease-free survival (DFS), objective response rate (ORR), and progression-free survival (PFS) [2]. While OS is the most reliable direct measure of effectiveness in oncology drug trials [3], its use can be complicated by cross-over (when subsequent cancer therapy potentially confounds survival analysis) and other feasibility issues (such as requiring longer follow-ups compared to other clinical endpoints). Most drug approvals from 2008 through 2014 were approved on the basis of improvements in tumor measures such as ORR or PFS [4, 5].
Informed decision-making necessitates clear communication of potential risks and benefits of a treatment. Efficacy endpoint terms are complex, and patients’ understanding of terms such as “progression-free survival” and “overall response rate” may be misaligned with the technical clinical definition. Patients’ understanding of the differences between various clinical endpoints is noteworthy because patients’ expectations about whether a treatment can help them live longer can affect treatment decisions [6, 7]. For patients to make informed decisions about their treatment, a clear understanding of the treatment’s benefits, harms, and uncertainties is critical. Without this understanding, patients may opt for a treatment that offers only marginal efficacy improvement at the expense of functional impairment due to side effects of the treatment. Further, without this understanding, patients may have unrealistic expectations about the potential clinical benefit of their treatment.
In recent years, various organizations have produced information about clinical endpoints for patients (e.g., https://www.cancer.gov/publications/dictionaries/cancer-terms). At the same time, for many patients in the United States, a prominent source of information about clinical endpoints is direct-to-consumer (DTC) ads for prescription oncology drugs. With a recent increase in the availability of new cancer-related drugs, substantial growth in national television ads for new cancer medications is likely [8]. In fact, at least 4 oncology prescription drugs were advertised via DTC television ads in the United States in 2018 [9]. Information conveyed in oncology ads is often complicated, both in terms of the indication and the clinical endpoints. See Figure 1 for example endpoints and associated promotional claims.
Figure 1.
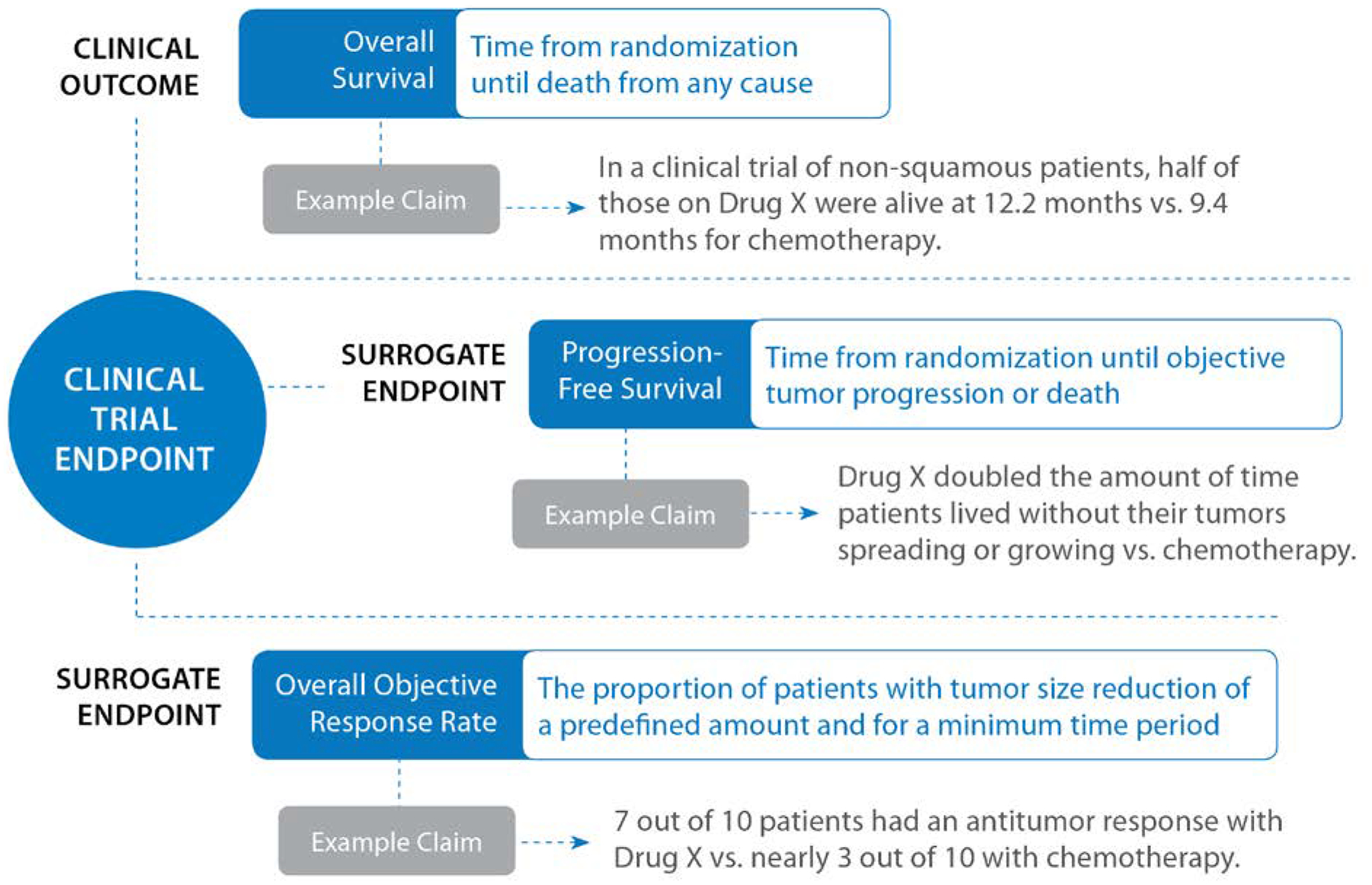
Examples of Clinical Endpoints and Associated Promotional Claims Used in Oncology
The goal of this literature review was to examine existing research on consumers’ and patients’ understanding of common oncology clinical endpoints, including OS, ORR, and PFS. In examining the existing research, we sought to learn what oncology patients understand about different clinical endpoints when evaluating treatments. Our consideration of related topics also guided this review, including ways in which knowledge or expectations surrounding the goals of treatment may or may not influence decision-making. With this literature review, we summarize available research relevant to an overarching research question and describe a path forward for future research.
The objective of this literature review was to find and synthesize available empirical publications assessing consumers’ and patients’ understanding of oncology clinical endpoints. The main goal was to address the following research question:
To what extent do consumers (patients) understand common oncology clinical endpoints (e.g., overall survival, response rate, progression-free survival)?
2. Methods
We sought published scholarship any time through September 2018 as a source of information about consumers’ and patients’ understanding of oncology clinical endpoints. We limited our search to English-language journals in a series of prominent databases (PubMed, Business Source Corporate, PsycINFO, Biomedical Reference Collection, Science Direct). To explore recent grey literature, e.g. abstracts and other reports aside from journal articles, we also searched conference abstracts from the American Society of Clinical Oncology annual meeting for the years 2017 and 2018 as well as the session and paper titles from the 2017 San Antonio Breast Cancer Research symposium—the largest conference on breast cancer—for any relevant publications. We considered publications focusing on adult populations only. We generated a pool of publications using unique search terms for our searches of databases (Table 1) and conference proceedings. To ensure the inclusion of critical literature related to the research question, two authors also conducted a hand search to supplement the database search. Three authors (JD, KFP, VB) started by searching the reference lists of relevant review articles and other key articles that were either identified by the search results or known to the researchers through previous work on oncology prescription drug promotion. The original search yielded 510 records, and a hand search found an additional 7 records (Figure 2). After removing duplicates, we were left with 497 records. To verify consistency in coding, 10% of the publications (n=50) were dual reviewed by two authors (JD, KFP) to remove any publications that were clearly not relevant. Any disagreements about inclusion were resolved through discussion with the first author (VB). The authors had consistent inclusion decisions, so the remaining publications were divided amongst two authors (JD, KFP) to flag for full text consideration. Eighteen publications were flagged for full-text review. Two authors (JD, VB) dual reviewed the 18 publications for inclusion and excluded publications that did not assess the outcome of interest (patients’ perceptions of clinical endpoints) or the study’s research question and publications that were not empirical research studies. Any disagreements were resolved through discussion with the team. Following final full-text review, our search yielded a total of 13 publications.
Table 1.
Key Search Terms
|
Endpoint Terms “overall survival” OR “progression-free survival” OR “event-free survival” OR “disease-free survival” OR (“response rate*” AND trial* AND endpoint*) OR “tumor response” OR “overall response rate*” OR “objective response rate*” OR “duration of response” OR “time to progression” OR “time to treatment failure” OR “surrogate outcome*” OR “surrogate endpoint*” OR “secondary endpoint*” OR “clinical endpoint*” OR “clinical trial endpoint*” OR “clinical trial outcome*” (in the abstract or title) AND Oncology Terms oncology OR oncological OR hematological OR cancer* (in the abstract or title or keyword) AND Patient Understanding Terms “patient* knowledge” OR “patient* understand*” OR “patient* perception*” OR “patient* comprehen*” OR “patient* view*” OR “patient* misunderstand*” OR “patient* misunderstood” OR “patient* perceive*” OR “consumer* knowledge” OR “consumer* understand*” OR “consumer* perception*” OR “consumer* comprehen*” OR “consumer* view*” OR “consumer* misunderstand*” OR “consumer* misunderstood” OR “consumer* perceive*” OR “lay knowledge” OR “lay understand*” OR “lay perception*” OR “lay comprehen*” OR “lay view*” OR “lay misunderstand*” OR “lay misunderstood” OR “lay perceive*” OR “public* knowledge” OR “public* understand*” OR “public* perception*” OR “public* comprehen*” OR “public* view*” OR “public* misunderstand*” OR “public* misunderstood” OR “public perceive*” (in the abstract or title) OR ((patient* OR consumer* OR lay OR public) AND (misunderstand* OR misunderstood* OR understand* OR knowledge* OR perception* OR perceive* OR comprehen* OR view*)) (in the title) |
Figure 2.
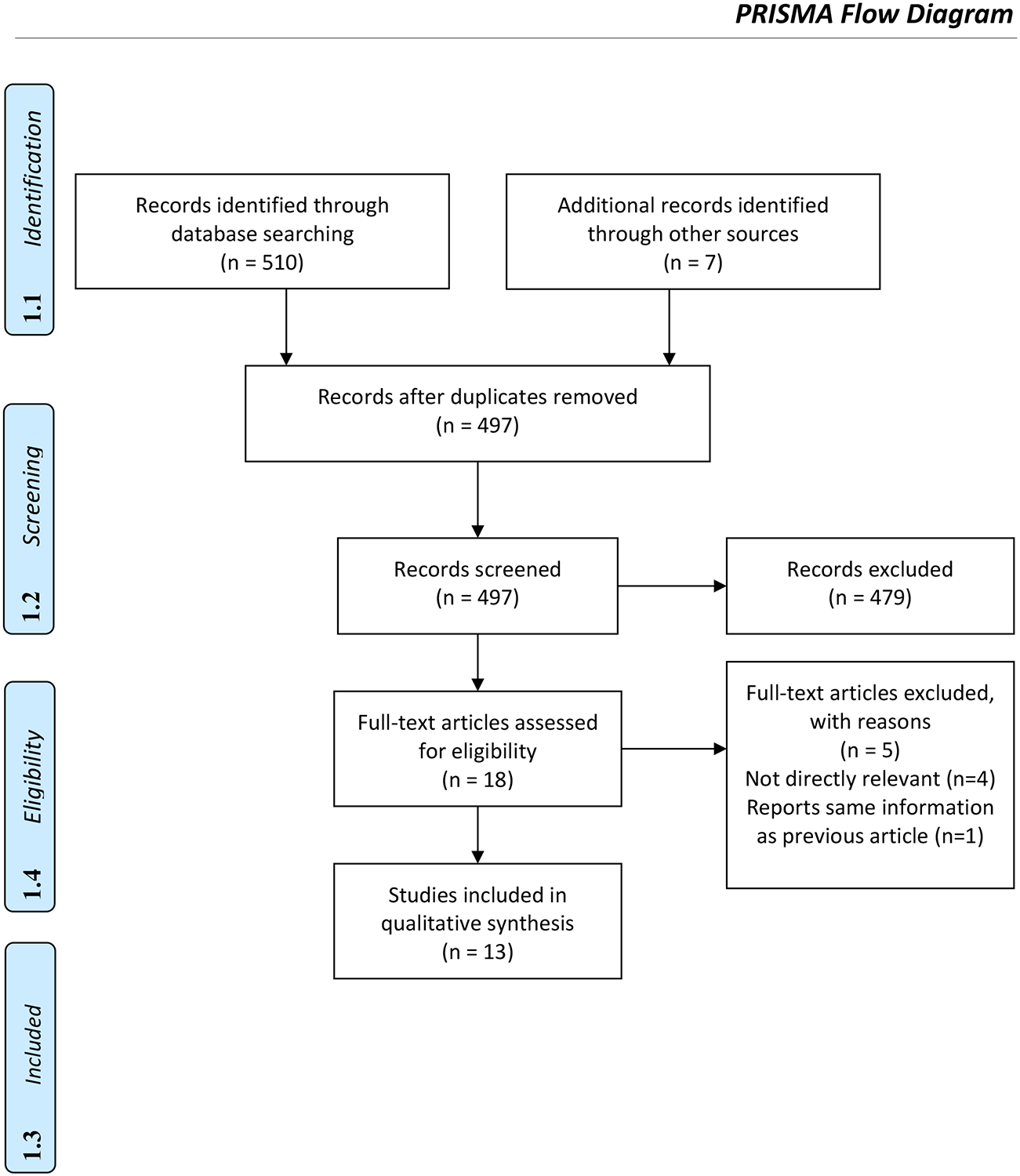
Summary of the Article Search and Selection
3. Results: Summary of Available Research
3.1. Study characteristics
The 13 publications provide a rich diversity in aims, methods, sample sizes, and country of origin (see Table 2) [10–22]. Seven publications used quantitative methods [10, 11, 15, 17, 19, 21, 22], 4 used mixed-methods [12, 13, 16, 20], 1 was a literature review [14] and 1 was a qualitative study [18]. Sample sizes ranged from 20 to 560. Eight publications were peer-reviewed articles [10–17] and 5 were published conference abstracts [18–22]. Three publications came from the VALue to patients of PROgression Free Survival (AVALPROFS) study, which we considered to be the most relevant study to our topic [12, 13, 18]. Two publications [12, 18] focused on patients’ awareness of clinical endpoint concepts, eight publications [10–12, 14, 17, 18, 21, 22] focused on patients’ understanding of clinical endpoint concepts or treament intent, seven of the available publications included data related to patterns in patients’ treatment preferences and tradeoffs based on clinical endpoint information [10, 12, 13, 15, 16, 19, 20].
Table 2.
Study Characteristics and Major Findings
| Article author, year | Study design and data collection mode | Population and sample size and country | Clinical Endpoint | Key measures | Major findings |
|---|---|---|---|---|---|
| Bridges et al. (2012) [10] | Quantitative (cross-sectional online survey) Conjoint analysis methodology to explore trade-off preferences |
Advanced non-small cell lung cancer patients (N = 89) UK |
PFS | Dependent variables: Treatment preferences Benefits that patients judged sufficient to compensate for the risks associated with therapy for advanced NSCLC NCSLC patients’ stated treatment preferences |
Patients’ understanding of clinical endpoint concepts and treatment intent Patients indicated in pretest interviews that they understood the meaning of PFS as time to disease progression. However, they were unaware of the difference between PFS and OS. Patients’ perceptions of clinical endpoint importance and perceived tradeoffs Patients ranked the relative importance of PFS (4, 5, and 7 months) along with a variety of disease symptoms of increasing severity. Having an improvement in disease symptoms with 7 months PFS was most important, followed by 5 months, and 4 months. As severity of treatment risks increased, patients’ preferences for these treatments diminished. |
| Catt et al. (2015) [18] [conference abstract] |
Qualitative (semi-structured individual interviews) | Patients with metastatic cancer (N = 11) UK |
PFS | Dependent variables: Patients’ familiarity with the phrase “progression free survival” Patients’ beliefs about the aims and benefits of new treatment for advanced cancer. |
Patients’ awareness of clinical endpoint concepts
Only one patient (9%) recalled the phrase PFS used in clinical consultations and four (36%) had no idea what this term meant. Patients’ understanding of clinical endpoint concepts and treatment intent Four patients (36%) incorrectly believed the therapeutic aim of further treatment was to extend survival. 8 (73%) believed the aim of the treatment was to slow the cancer. Four patients (36%) who had not yet begun treatment believed the benefits of their new treatment included extending life. |
| Craft et al. (2005) [11] | Quantitative (longitudinal in-person survey) | Patients with advanced cancer receiving palliative therapies (N = 181) Australia |
N/A | Dependent variables: Knowledge of treatment intent (treatment wasn’t curative, treatment was intended to improve QOL or control illness) Independent variables: Age, marital status, education, country of birth, place of residence, occupation, cancer type, type of therapy, course of illness, site of disease, time to death, psychosocial classification |
Patients’ understanding of clinical endpoint concepts and treatment intent Less than half (47.4%) of participants correctly understood that their treatment was not curative. Responses to this question were measured at week 1 and week 12. The number of patients that reported that their treatment was curative at week 1 was higher than the number at week 12. Nearly half of those undergoing chemotherapy thought the treatment goal was curative versus 28% receiving supportive care. Non-ambulatory patients and patients closer to death were more likely than others to understand the goal of treatment was not curative. |
| Fallowfield et al. (2017) [12] | Mixed-method (cross-sectional survey and in-person or telephone interviews) | Patients diagnosed with metastatic cancer, with a predicted life expectancy of at least 6 months, who were about to commence treatment with prescription drugs that demonstrated only progression-free survival (PFS) or modest overall survival (OS) benefits at the time the study commenced (N = 90) Oncologists who prescribed drugs that demonstrated only PFS or modest OS benefits at the time the study commenced (N = 32) UK |
PFS | Dependent variables: Patients’ understanding and expectations of the phrase PFS Patients’ understanding of treatment aims The benefits patients expected from treatment Patients’ answers to time-trade-off questions |
Patients’ awareness of clinical endpoint concepts During conversations with the patient about the cancer treatment, 99% of oncologists reported discussing the aims of treatment, but only 28% (n = 25) of oncologists reported explicitly using the terms “progression-free survival” or “progression-free interval.” When asked, only 4 of 90 patients recalled its use. Patients’ understanding of clinical endpoint concepts and treatment intent Understanding of the term “PFS” was mixed. 57% (N = 51) of patients reported they had no idea or were unclear about what PFS meant. 32% (N = 29) thought it was about controlling cancer (which was somewhat in line with PFS definition), and 11% (N = 10) thought it was about extending life. The majority (92%) (N = 83) believed that an aim of their treatment was to slow or stop the cancer. 50% (N = 45) also believed that this meant living longer. 39% (N = 35) said living longer was the most important aim of treatment, and 62% (N = 56) thought that their understanding of treatment aims would be achieved. When asked about benefits they expected from treatment, 86% (N = 77) believed it would keep the cancer under control, 54% (N = 49) believed it would make them feel better, and 39% (N = 35) believed it would help them to live longer. Patients’ perceptions of clinical endpoint importance and perceived tradeoffs In a time-trade-off analysis, patients were significantly less likely to feel that the benefit of controlling the cancer was worthwhile as the severity of the side effects increased. |
| González et al. (2016) [19] [conference abstract] |
Quantitative (cross-sectional online survey) Discrete choice experiment (DCE) |
Patients with a self-reported physician diagnosis of renal cell carcinoma (N = 201) US |
PFS OS |
Dependent variables: Patients’ perceptions of and preference weights for PFS and OS efficacy measures. |
Patients’ perceptions of clinical endpoint importance and perceived tradeoffs
Perceptions of a treatment’s PFS was not correlated with perceptions of long-term and uncertain efficacy outcomes (i.e., survival). When selecting a preferred treatment, the most important attribute to patients was long-term survival, followed by PFS, treatment cost, adverse events, and route and frequency of administration. Patients tended to focus on improved chances of long-term survival and largely discounted PFS as a measure of treatment efficacy. Among patients with metastatic disease, a 2.5-month increase in PFS was rated as important as a 10% increase in survival. |
| Jenkins et al. (2018) [13] | Mixed-method (cross-sectional survey and in-person or telephone interviews) Secondary data analysis |
Patients diagnosed with metastatic cancer, with a predicted life expectancy of at least 6 months, who were about to commence treatment with prescription drugs that demonstrated only PFS or modest OS benefits at the time the study commenced (N = 90) UK |
PFS | Dependent variables: (measured at baseline and 6 weeks) Patients’ perceptions of the trade-off between PFS and quality of life Independent variables: Type of cancer patient |
Patients’ perceptions of clinical endpoint importance and perceived tradeoffs
As the severity of the possible side effects increased, patients were less likely to indicate that the benefit of controlling the cancer (PFS) would be worthwhile (95% of patients indicated controlling cancer was worth it if they experienced Grade I side effects vs. 44% for Grade III side effects). This pattern was found at baseline and at 6 weeks on treatment, and the same pattern was found for patients with lung, melanoma, and breast cancer. As side effect severity increased, patients wanted the treatment to control the cancer for longer to make the treatment worthwhile. |
| Johnson et al. (2015) [14] | Literature review | N/A | N/A | Definitions of curative |
Patients’ understanding of clinical endpoint concepts and treatment intent There were a variety of ways in which cancer “cure” was described and differences across physicians, academics and patients were noted. It was much more common for documents from healthcare professionals and academics to use language related to disease progression and survival whereas patients tended to describe “cure” in terms of successful eradication of cancerous cells. |
| O’Donnell et al. (2018) [20] [conference abstract] |
Mixed-method (Structured discrete choice trade-off exercise and in-person interviews) | Ambulatory clinic patients with advanced metastatic cancer (lung, colorectal, or ovarian) who had completed at least 3 months of chemotherapy (N = 20) Canada |
PFS OS |
Dependent variables: Discrete choice trade-off exercise between treatment options with the same OS but different PFS, treatment time, and toxicity levels. |
Patients’ perceptions of clinical endpoint importance and perceived tradeoffs
Most participants (85%) (N = 17) chose treatment with shorter treatment time and lower toxicity levels even when PFS was shorter. Two patients (10%) chose treatment with higher toxicity but longer PFS, and one patient (5%) always chose the most aggressive treatment regardless of PFS and toxicity. |
| Postmus et al. (2018) [15] | Quantitative (cross-sectional online survey) Decision-analysis methodology |
Multiple myeloma patients (N = 560) UK |
PFS | Dependent variables: Patients’ preference weights for PFS and side effects (ranging from mild to life-threatening) of two hypothetical treatments Patients’ perceptions of the trade-off between PFS and treatment side effects Independent variables: Age, time of diagnosis, employment status, number of previous treatments, number of dependents, current side effects or other health problems, or regular contact with support group |
Patients’ perceptions of clinical endpoint importance and perceived tradeoffs
The average preference weight given to PFS was higher than the average weight given to severe or life-threatening treatment toxicity or mild or moderate chronic toxicity. The majority of participants (58%) considered increasing the probability of being progression-free for 1 year or longer from 50% to 90% to be more important than simultaneously decreasing the probability of experiencing severe or life-threatening toxicity from 80% to 20% and the probability of experiencing mild or moderate chronic toxicity from 85% to 45%. The amount of weight given to PFS was not associated with age, time of diagnosis, race, employment status, number of previous treatments, number of dependents, current side effects or other health problems, or regular contact with support group. |
| Protiere et al. (2017) [16] | Mixed-method (Q-sort methodology with quantitative and qualitative components) |
N = 146; Cancer patients in remission at 18 months of diagnosis (n = 52) General population (n = 46) Oncologists (n = 27) Healthcare decision-makers (n = 19) Pharmaceutical industry representatives (n = 2) France |
PFS OS |
Dependent variables: Respondent perceptions of trade-offs between quality of life and length of life for a hypothetical drug for advanced cancer Independent variables: Type of population (health decision-makers, oncologists, cancer patients, general population) |
Patients’ perceptions of clinical endpoint importance and perceived tradeoffs
13 patients, 25 general population participants, 18 oncologists, and 16 healthcare decision-makers endorsed the view that quality of life is more important than length of life and that an effective treatment would allow the patient to live a life comparable to their life before the disease. 2 patients and 1 general population participant and 4 oncologists endorsed the viewpoint that a gain in OS or decreasing the risk of disease progression is preferred even if it implies a decrease in quality of life and negative impacts on family, social, and professional life. Healthcare decision makers did not endorse this viewpoint. |
| Sheik-Yousouf et al. (2010) [21] [conference abstract] |
Quantitative (cross-sectional survey) | Patients with metastatic breast cancer (N = 52) and oncologists (N = 28) Canada |
PFS, OS, ORR | Dependent variables: Respondents’ perceptions of clinically important endpoints in the treatment of metastatic breast cancer Independent variables: Type of population (oncologist vs. patient) |
Patients’ understanding of clinical endpoint concepts and treatment intent Study examined the importance of clinical endpoints/outcomes (OS, PFS, ORR, and QoL) among physicians and patients. The abstract discussed PFS only as a treatment goal from physicians. For patients, the treatment goals were OS, ORR, and QoL. 88% of patients believed the primary goal of their treatment was to prolong life; 63% also believed slowing tumor growth was a goal of treatment. 50% of patients indicated that improving symptoms and pain (i.e., QoL) were important treatment goals. 54% of patients and 52% of oncologists said OS was the most important endpoint considered in choosing a specific therapy. 48% of oncologists thought PFS was the most important endpoint and 17% of patients thought shrinking tumor size was the most important endpoint. Oncologists considered much smaller absolute improvements in OS and PFS (2–6 months) as significant enough to adopt therapies, while almost half (46%) of patients thought that a treatment would need to extend their life by at least 12 months to make it worthwhile. |
| Shimer et al. (2018) [22] | Quantitative (cross-sectional survey) | Patients admitted with acute leukemia (N = 90) and their treating hematologists US |
N/A | Dependent variables: Patients’ perceptions of treatment goals, chance of cure, expected survival Independent variables: Population (physicians and patients) Relapse (nonrelapsed, relapsed) |
Patients’ understanding of clinical endpoint concepts and treatment intent Most patients (82.2%) and physicians (78.9%) shared a goal of cure. Compared with physician estimates, significantly more non-relapsed (58% vs. 5%) and relapsed (39% vs. 0%) patients reported they had a greater than 80% chance of cure. |
| Temel et al. (2011) [17] | Quantitative (experimental randomized controlled trial, longitudinal) | Ambulatory patients newly diagnosed with metastatic non-small cell lung cancer (N = 151) US |
N/A | Dependent variables: (Measured at 12, 18, and 24 weeks) Effect of early palliative care on longitudinal perceptions of illness understanding Accuracy of patients’ perceptions of prognosis and therapy goals Independent variable: Type of care (standard vs. early palliative) |
Patients’ understanding of clinical endpoint concepts and treatment intent Despite having terminal cancer, at baseline 31% (n = 45) reported that their cancer was curable. 33% of participants did not think their cancer was curable, but they did think that the goal of their therapy was to get rid of all their cancer. 97% (n = 138) indicated that a goal of treatment is to help them live longer and 69% (n = 84) reported that a goal of treatment was to get rid of all their cancer. There was a significant (p = .02) positive effect of early palliative care on illness understanding by patients over time: 82.5% of patients assigned to early palliative care either remained or became accurate in the perception that the cancer was not curable compared with 59.6% of patients receiving standard care. Type of care did not significantly affect perceptions of the goals of treatment at any time point; the majority of patients continued to report that an aim of therapy was to get rid of all the cancer. Time of assessment (baseline vs. 12, 18, and 24 weeks) did not significantly affect perceptions that the goal of the treatment was to live longer. |
Note. ORR = objective response rate; OS = overall survival; PFS = progression-free survival; QoL = quality of life
3.2. Patients’ awareness of clinical endpoint concepts
How often do healthcare professionals explicitly discuss clinical endpoint information with patients? In our review, relevant research has yet to produce a robust body of evidence on this question. Most publications on endpoints did not explicitly present evidence on patient-physician conversation or patient recollection of such exchanges. Only two of the 13 publications (15%) reported on whether clinical endpoint information was discussed by physicians and remembered by patients [12, 18]. Both focused on PFS. Available evidence from those publications that did present conversation data suggests that physician-patient interaction typically does not involve direct reference to clinical endpoint terms or phrases. Clinical endpoint language appears to not be used during conversations between patients and their healthcare professionals. Fallowfield and colleagues [12] queried 32 UK oncologists and 90 of their patients who were being treated for advanced disease. They found that only a minority of oncologists (28%) reported using phrases like “progression-free survival” or “progression-free interval” when talking with their cancer patients, and fewer than 5% of patients remembered oncologists referencing such phrases. Using semi-structured interviews with 11 patients with metastatic cancer, Catt and colleagues [18] found that only one patient (9%) recalled the phrase PFS being used in clinical consultations and four (36%) had no idea what this term meant. One stated, “sounds positive, hopeful to me as it’s got the word survival in it.”
3.3. Patients’ understanding of clinical endpoint concepts and treatment intent
Eight of the publications (62%) examined patients’ understanding of clinical endpoints or treatment aims or goals [10–12, 14, 17, 18, 21, 22] (see Table 2). Of those, two publications focused on understanding of PFS [10, 12], and six focused on perceptions or discussions of treatment intent [11, 14, 17, 18, 21, 22]. In the Fallowfield et al. [12] study, 57% (51/90) of patients reported they had no idea what PFS meant or were unclear about what it meant, 32% thought it was about controlling cancer, and 11% thought PFS involved extending life. In other words, a minority of patients claimed to know the definition of PFS, meaning an even smaller proportion of patients likely actually knew the definition. When asked about the aims of their treatment, a vast majority (92%) of patients in the Fallowfield et al. [12] study correctly believed that an aim of treatment was to slow or stop their cancer, but 50% also believed that slowing or stopping their cancer meant living longer. Similarly, Bridges et al. [10] noted that although pretest interviews suggested that patients understood the meaning of PFS as time to disease progression, patients tended to not see a distinction between the concepts of PFS and OS.
Six (46%) publications assessed patients’ understanding of their treatment intent – that is, what was the goal of treatment [11, 17, 18, 21, 22] or examined patient and provider definitions of what it means to cure cancer [14]. Three studies examined whether patients diagnosed with advanced cancer understood the intent of their treatment, that is, their treatment was not curative [11, 17, 22]. Two of those publications [11,17] found that a sizeable minority of patients with incurable cancers thought that their treatment was intended to cure their cancer and one publication found that patients were more likely than physicians to report a greater than 80% change of cure [22]. In the aforementioned Catt et al. [18] study, 73% (8/11) of respondents believed that the aim of treatment was to slow their cancer, but 36% of patients (4/11) incorrectly believed the therapeutic aim of further treatment was to extend survival.
Although these findings are not directly related to specific clinical endpoints, they highlight the potential for inaccurate understanding of a treatment’s intent. In addition, the literature review by Johnson et al. [14] suggests that patients and physicians have different definitions on what it means to “cure” cancer which may contribute to why findings for physicians and patients can appear misaligned.
For example, a longitudinal study of 181 cancer patients with advanced disease [11] found that at baseline 46% (77/163) correctly understood that their treatment was not curative, 29% thought that the aim of their treatment was curative and 22% did not know whether the aim was curative. The misperception of drug treatment as curative diminished over time. Patients undergoing chemotherapy were also more likely to believe a treatment was curative than those receiving supportive care.
A 2011 study by Temel and colleagues [17], found that, at baseline, 32% (46/145) of patients diagnosed with terminal cancer reported that their cancer was curable (reflecting an inaccurate view of their prognosis); and 69% (86/124) reported that the goal of their treatment was to get rid of all of their cancer (reflecting inaccurate expectations regarding their cancer therapy). The authors also found that 33% of participants had conflicting illness perceptions wherein they believed that their cancer was incurable while simultaneously reporting that the goal of their therapy was to eliminate their cancer.
Finally, Sheik-Yousouf et al [21] examined perceptions and perceived importance of treatment goals among physicians and patients. For patients, the treatment goals were OS, ORR, and quality of life (QoL). The authors found that 88% of patients indicated the primary goal of their treatment was to prolong life. In this study, 54% of patients believed that prolonging survival was the most important endpoint in accepting metastatic breast cancer therapy with a much smaller percentage (17%) thinking that shrinking tumor size was the most important.
3.4. Patients’ perceptions of clinical endpoint importance and perceived tradeoffs
A majority of the articles we studied focused on patient understanding and preferences regarding treatment in light of endpoint perceptions. Seven publications (54%) explored patients’ perceptions of clinical endpoint importance and perceived tradeoffs. Three of these looked at PFS and OS [16, 19, 20] and the remaining focused on PFS [10, 12, 13, 15]. Although preferences are individual, these publications show patterns in patients’ preferences for clinical endpoint information. When asked in the context of an array of drug considerations, patients do sometimes report that factors such as PFS evidence are important. Postmus and colleagues [15], for example, reported that patients tended to say PFS likelihood is more important than toxicity when considering treatment options. This pattern also appears to be generalizable across demographic groups: patient-allotted weight for PFS as a factor to consider was not associated with age, time of diagnosis, race, employment status, number of previous treatments, number of dependents, current side effects or other health problems, or regular contact with a support group.
O’Donnell et al. [20] explicitly stated to patients that a certain treatment option had the same OS time and found that patients favored less toxicity over lengthened PFS—the opposite from what Posthmus and colleagues found. Jenkins and colleagues [13] provided relevant evidence in this regard: they found the patient value of PFS outcomes to be at least partially a function of the severity of the presented side effects. Fallowfield et al. [12] similarly found patients’ perceptions of the value of controlling cancer to be a function of side effect severity. Another study found that patients with renal cell carcinoma tended to focus on improved chances of long-term survival and largely discounted PFS as a measure of treatment efficacy [19].
4. Discussion and Conclusion
4.1. Discussion
Our search for potentially relevant literature revealed a lack of published empirical evidence specifically relevant to the question of how well consumers and patients understand the clinical endpoints commonly used in oncology. Not only did our search yield only a small set of directly pertinent publications, but our assessment of those publications suggested inconclusive answers to some questions about what patients are aware of and understand about clinical endpoints. Many relevant studies of patient preferences, for example, have not included specific measures of clinical endpoint concept awareness and comprehension. Our search of the available literature also suggests researchers know little about how (or whether) patients understand the clinical meaning of endpoints. Based on the two publications we identified that answered this question most directly, the evidence suggests potential for confusion or misunderstanding and warrants future research.
Comprehension of medical concepts such as clinical endpoints reflects a nested set of conditions, each necessary for us to be able to label a person as having a substantive grasp of a technical idea. These conditions include patients’ awareness, understanding, and preferences for using clinical endpoint information in weighing treatment options. For example, patients need first to be aware of a concept before being able to define that concept. Even if a person is aware of a concept, they may not be able to explain a concept in their own language or using language that they commonly use in talking with a healthcare professional. In the case of clinical endpoints related to evidence for oncology prescription drug efficacy and outcomes, these 13 publications do not demonstrate widespread awareness of clinical endpoint phrases nor do they demonstrate extensive ability to explain or define clinical endpoint concepts in a way that is fully understood by patients. Importantly, though, that evidence rests on only two publications in which researchers have directly assessed knowledge. Empirical evidence is most abundant on the last dimension, patient preferences, although reported preferences likely are affected by awareness and understanding of endpoint concepts, so they should be assessed in that light.
In the few available publications of clinical endpoint perceptions among patients, we found evidence that clinical endpoints are not be especially present or prominent in the minds of patients currently. Many cancer patients report they have little or no awareness of clinical endpoint concepts in general, despite clinical endpoint data being used in oncology prescription drug promotion. The available literature also suggests cancer patients in recent years have not heard about the clinical endpoints regularly in their discussions with healthcare professionals (although only a small minority of publications present empirical evidence on such conversations) and general population patients are unlikely to have heard such concepts any more regularly. Even if healthcare professionals sometimes do mention such concepts, cancer patients are not often processing those references sufficiently to affect awareness or understanding of endpoint concepts. We need further research to assess the prevalence and potential of patient-physician conversations about clinical endpoints.
The fact that we do not yet have much data on previous patient exposure to clinical endpoint information also should constrain our interpretation of data we do have on patient choices and preferences when they are presented with information. We do not know whether some available data on patients’ perceptions of oncology drugs reflect extensive misunderstanding of clinical endpoint concepts, per se, or whether people tend to guess in the face of presented choices. Protiere and colleagues [16], for example, reported on patients’ preferences for treatment considerations (such as quality of life or length of life) and yet did not directly report evidence of patient comprehension of drug efficacy concepts such as clinical endpoints beyond noting that pilot testing with patients suggested that survey questions were well understood. Thus, patient preference evidence to date may be constrained by patients’ misperceptions of treatment efficacy (and also by a lack of exposure to extensive information about clinical endpoints). We do not have large-scale studies of clinical endpoint preferences to date, meaning available samples may not have been sufficiently large to allow extensive subgroup analysis. Moreover, without evidence of substantive understanding of clinical endpoint concepts, it is unclear whether expressed preferences for PFS over toxicity reflect what would be the case if more patients accurately comprehended clinical endpoint concepts or if patients had an opportunity to learn about clinical endpoints.
One theme that emerged from the available literature is the extent to which many patients incorrectly viewed non-curative oncology prescription drugs as a tool to cure a person of cancer rather than as a tool to achieve other cancer treatment goals. Challenges in the current levels of patient understanding of these concepts could be due to (1) conversations with healthcare professionals in which an absence of disease progression may be described (or implied) as a curative treatment [14], (2) exposure to suboptimal explanations of clinical endpoint data (in any of a variety of sources), (3) patients’ optimistic bias regarding treatment possibilities that might affect how they understand clinical endpoint concepts, or (4) patients’ active avoidance of treatment information [23]. In other words, we do not know if people lack exposure to information (although we have some initial evidence that is the case) or if they tend to interpret information they have encountered in unrealistic ways (or both). Inaccurate viewing of the goals of therapy might reflect a widespread tendency to view all drug treatment associated with cancer as a tool to cure or defeat the disease altogether rather than for other potential purposes. Even if patients hold accurate views about the medical goals of a treatment option, they nonetheless may be overly optimistic about the possibility of their own personal benefit from that treatment [12, 22]. In other words, they might understand that the formal goal of a treatment is to slow down progression of cancer, but they also might privately think they will benefit by lengthened survival. Such perceptions could affect stated treatment preferences.
4.1.1. Avenues for Future Research
As we note in our description above, the available literature on patient awareness or comprehension of oncology clinical endpoint concepts has been limited not only in terms of the volume of available publications but also in terms of the data reported. Beyond those general limitations, we also note some methodological concerns and avenues for future research.
4.1.1.1. Expanding the types of clinical endpoint concepts studied
Even though we did find several useful papers on patients’ perceptions of clinical endpoint data or clinical endpoint considerations, it is also noteworthy that we do not have equal amounts of evidence for all specific types of clinical endpoints. We primarily found evidence regarding patients’ perceptions related to PFS and OS, with nearly all publications focusing on PFS specifically (e.g., Fallowfield et al. [12]). Because this is a new area of inquiry, the existing publications have focused on the clinical endpoint terms themselves. Future research should focus on whether patients are familiar with and understand clinical endpoint concepts explained in plain language (e.g., living longer versus “overall survival”).
4.1.1.2. Expanding sample sizes and populations studied
Much of the limited evidence on patients’ perceptions of clinical endpoints comes from small samples. This issue reflects the small samples that sometimes are used for clinical research with cancer patients (which has been a primary source for the available literature to date). Although even small samples can provide useful data, such relatively small samples can limit the possibility of subgroup analysis or understanding of the variables that predict key outcomes. That pattern suggests a need for future work to explore individual-level differences. For instance, factors such as education, numeracy, and health literacy might interact with understanding and decision-making. If we are to understand how patients develop understanding of endpoint information as they progress from undiagnosed to diagnosed, it could be useful to have data on the general population’s perceptions as well. Given the complexity surrounding these nuanced medical terms, those with lower motivation or ability to process these concepts may be more vulnerable to misunderstanding, suggesting the utility of future research with a range of patients from various backgrounds.
4.1.1.3. Other unaddressed research questions
Comprehension of clinical endpoint concepts is multifaceted. Not only can we distinguish between simple concept awareness and one’s ability to explain a concept to a family member or a friend, but we also could potentially assess the extent to which patients understand why clinical endpoints are used as reference points in studies. Do patients understand not only that the various endpoints are different from one another but also that the extent of evidence available in support of each type of estimate might vary (e.g., we might only have PFS data in some cases)? Patient literacy regarding drug efficacy data is a part of a larger concern regarding health literacy that researchers have raised in recent years (e.g., Berkman, Davis, & McCormack [24]) and remains a relevant focus for future inquiry.
Multimodal research that includes qualitative (e.g., focus groups) and quantitative (e.g., surveys or experimental studies) research methods would help address these questions. Measures of patient awareness and perception likely should balance the need to offer concept definitions against the value of soliciting information from patients without simultaneously teaching them about specific concepts. Because we have at least some evidence that many patients have never heard specific clinical endpoint phrases, researchers may benefit from giving respondents a chance to demonstrate previous exposure to the concepts before asking them about their understanding.
Although we have some evidence cancer patients do not generally discuss specific endpoint concepts with healthcare professionals, we need more research on patient-physician conversations about clinical endpoints. We also lack information on patient exposure to endpoint concepts in other aspects of their lives outside of the examination room. One source of relevant interaction and information is discussion with caregivers, so it could be worthwhile to understand whether someone is a caregiver to someone else with cancer and the extent to which caregivers tend to be aware of and to understand endpoint concepts. Insofar as caregivers play an important role in decision-making, it could be worthwhile to study caregivers’ understanding of endpoint concepts. We also know patients get at least some medical information from mass media and online sources [25], suggesting the utility of future work to understand patient engagement with misinformation in electronic media.
4.1.2. Limitations
Some limitations to our literature review should be noted. First, we chose to include published abstracts from a few recent, representative conferences to ensure we included current research. However, this approach may have missed relevant research presented at other conferences. Also, published abstracts provide less information than full articles, making it difficult to evaluate the research reported. Finally, because of the heterogeneity of the identified publications, we did not conduct a quality assessment as part of our literature review; therefore, we cannot formally evaluate the strength of the evidence.
4.2. Conclusion
Providing clear and accurate information respects patients’ autonomy to make decisions based on what is known about the true benefits and risks of a treatment option [26]. It can also contribute to improved patient experiences and their resultant health outcomes [27]. With the rise in oncology drugs being brought to market, an increase in exposure to news stories and DTC advertising for oncology drugs among general populations is likely to ensue. This presents a potential challenge for information accuracy. Currently, approval for many advertised treatments has relied on endpoints related to tumor measurement that can be complicated. We need research on how to introduce clinical endpoint concepts to consumers and patients and to understand their comprehension of these clinical endpoints. The little evidence we do have suggests that cancer patients face challenges in gaining information about clinical endpoints. For example, we have reports that suggest healthcare professionals and cancer patients are not generally discussing clinical endpoints specifically, although we do not have data on how healthcare professionals may convey the clinical concepts. We also have evidence of the potential for patient confusion or misunderstanding of treatments approved on the basis of various clinical endpoints—patients sometimes appear to hold inaccurate perceptions about drug treatment benefits—which also warrants future research. For patients to be able to make choices that are consistent with their needs and preferences, they need information about the expected benefits and risks of each treatment option.
4.3. Practice Implications
As noted by Kim and colleagues [28], patient-friendly definitions of clinical endpoints may help healthcare providers communicate important information about treatments to patients. Care should be taken not to burden patients with too much information, but rather to clarify what is currently known about treatment options and their potential benefits and risks in a patient-friendly manner. This patient-centered approach could help patients make more informed treatment decisions. Our literature review reveals several areas for future research needed to achieve this goal.
Funding statement:
This work was funded by a contract from the US Food and Drug Administration.
Footnotes
Declarations of interest statement: None.
Patient Identifying Information
We confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the article.
References
- [1].Food and Drug Administration.. Novel drug approvals for 2018. FDA Web site. https://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm592464.htmPublished2018. Accessed October 4, 2019.
- [2].Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–77. doi: 10.1200/JCO.2015.61.6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Food and Drug Administration. Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics. FDA Web site. http://www.fda.gov/downloads/Drugs/Guidances/ucm071590.pdf. Published 2007. Accessed October 4, 2019.
- [4].Beaver JA, Howie LJ, Pelosof L, et al. A 25-year experience of us food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncology. 2018;4:849–56. doi: 10.1001/jamaoncol.2017.5618 [DOI] [PubMed] [Google Scholar]
- [5].Kim C, Prasad V. Strength of validation for surrogate end points used in the US Food and Drug Administration’s approval of oncology drugs. Mayo Clin Proc. 2016;91:713–25. doi: 10.1016/j.mayocp.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marta GN, Del Nero LG, Marta GN, et al. Treatment priorities in oncology: do we want to live longer or better? Clinics. 2014;69:509–14. doi: 10.6061/clinics/2014(08)02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Matsuyama R, Reddy S, Smith TJ. Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol. 2006;24:3490–6. doi: 10.1200/JCO.2005.03.6236 [DOI] [PubMed] [Google Scholar]
- [8].McCaffrey K. Oncology Marketing Goes Prime Time: Pharma Brands Move to DTC Advertising to Capture Market Share. New York, NY: MM&M/Haymarket Media, Inc.; 2017. http://media.mmm-online.com/documents/306/mm_m_oncology_marketing_ebook_76427.pdf. Accessed October 4, 2019. [Google Scholar]
- [9].iSpot.tv. Rx: Cancer TV commercials. iSpot.tv Web site. https://www.ispot.tv/browse/7k.bv/pharmaceutical-and-medical/rx-cancer. Published 2018. Accessed October 4, 2019.
- [10].Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77:224–31. doi: 10.1016/j.lungcan.2012.01.016 [DOI] [PubMed] [Google Scholar]
- [11].Craft PS, Burns CM, Smith WT, Broom DH. Knowledge of treatment intent among patients with advanced cancer: a longitudinal study. Eur J Cancer Care. 2005;14:417–25. doi: 10.1111/j.1365-2354.2005.00601.x [DOI] [PubMed] [Google Scholar]
- [12].Fallowfield LJ, Catt SL, May SF, et al. Therapeutic aims of drugs offering only progression-free survival are misunderstood by patients, and oncologists may be overly optimistic about likely benefits. Support Care Cancer. 2017;25:237–44. doi: 10.1007/s00520-016-3408-7 [DOI] [PubMed] [Google Scholar]
- [13].Jenkins V, Farewell V, May S, et al. Do drugs offering only PFS maintain quality of life sufficiently from a patient’s perspective? Results from AVALPROFS (Assessing the ‘VALue’ to patients of PROgression Free Survival) study. Support Care Cancer. 2018;26:3941–9. doi: 10.1007/s00520-018-4273-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Johnson P, Greiner W, Al-Dakkak I, Wagner S. Which metrics are appropriate to describe the value of new cancer therapies? Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/865101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Postmus D, Richard S, Bere N, et al. Individual trade-offs between possible benefits and risks of cancer treatments: results from a stated preference study with patients with multiple myeloma. Oncologist. 2018;23:44–51. doi: 10.1634/theoncologist.2017-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Protiere C, Baker R, Genre D, Goncalves A, Viens P. Marketing authorization procedures for advanced cancer drugs: exploring the views of patients, oncologists, healthcare decision makers, and citizens in France. Med Decis Making. 2017;37:555–66. doi: [DOI] [PubMed] [Google Scholar]
- [17].Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29:2319–26. doi: 10.1200/jco.2010.32.4459 [DOI] [PubMed] [Google Scholar]
- [18].Catt S, Jenkins V, Matthews L, McKenzie M, Garth J, Fallowfield L. The AVALPROFS study: assessing the VALue of PROgression Free Survival. Psychooncology. 2015;24:10. [Google Scholar]
- [19].González JM, Doan J, Gebben DJ, Fishman M. Patient preferences for metastatic renal cell carcinoma treatments: a discrete-choice experiment. BJU Int. 2016;118:22–3. doi: 10.1111/bju.13694 [DOI] [PubMed] [Google Scholar]
- [20].O’Donnell J, Robinson AG, Booth CM, Koven R, Eisenhauer EA, Brundage MD. Imaging progression-free survival: how much does it matter to patients? Paper presented at: ASCO Quality Care Symposium; September 28, 2018; Phoenix, AZ https://meetinglibrary.asco.org/record/166475/abstract. Accessed October 4, 2019. [Google Scholar]
- [21].Sheik-Yousouf A, Gandhi S, Dukhovny S, Verma S. A comparison of physician and patient perceptions of clinically important endpoints in the treatment of metastatic breast cancer (MBC). Eur J Cancer Suppl. 2010;8:77. doi: 10.1016/s1359-6349(10)70094-x [DOI] [Google Scholar]
- [22].Shimer S, Canavan M, Capasso R, Gore S, Tannenbaum S, Babar T. Prognostic understanding in acute leukemia: patient-physician differences. J Clin Oncol. 2018;36. doi: 10.1200/JCO.2018.36.15_suppl.e22124 [DOI] [Google Scholar]
- [23].McCloud RF, Jung M, Gray SW, Viswanath K. Class, race and ethnicity and information avoidance among cancer survivors. Br J Cancer. 2013;108:1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berkman ND, Davis TD, McCormack L. Health literacy: what is it? J Health Commun. 2010;15:9–19. doi: 10.1080/10810730.2010.499985 [DOI] [PubMed] [Google Scholar]
- [25].Boudewyns V, Southwell BG, Betts KR, Gupta CS, Paquin RS, O’Donoghue AC, Vazquez N. Awareness of misinformation in health-related advertising: a narrative review of the literature In Southwell BG, Thorson EA, Sheble L (Eds.) Misinformation and mass audiences. Austin, TX: University of Texas Press, 2018, pp. 35–50. [Google Scholar]
- [26].Epstein R, Street RL. Patient-centered communication in cancer care: promoting healing and reducing suffering. NIH Report No. 07–6225. Bethesda, MD: National Cancer Institute, U.S. Department of Health and Human Services, National Institutes of Health; 2007. [Google Scholar]
- [27].Mollica MA, Lines LM, Halpern MT, et al. Patient experiences of cancer care: scoping review, future directions, and introduction of a new data resource: Surveillance Epidemiology and End Results-Consumer Assessment of Healthcare Providers and Systems (SEER-CAHPS). Patient Exp J. 2017;4:103–21. [Google Scholar]
- [28].Kim J, Gao J, Amiri-Kordestani L, Beaver JA, Kluetz P. Patient-friendly language to facilitate treatment choice for patients with cancer. Oncologist. 2019;24:1011–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


