Abstract
The pubertal period is a time of rapid increase in the incidence of anxiety disorders, and thus, pubertal hormones may play a role in the precipitation of anxious psychopathology. DHEA, a steroid hormone that surges in adolescence, has been previously linked to anxiety, although the direction of this effect has been mixed. Using a cross-sectional design in a sample of 286 adolescent girls, the present study examined associations between salivary DHEA concentrations and self-report and interview-based measures of anxiety while controlling for pubertal status, menarche status, assessment time of day, and other hormones including testosterone, estradiol, and progesterone. Increased salivary DHEA concentrations were associated with more self-reported anxiety symptoms, increased anxiety symptom counts based on clinical interview, and increased probability of an anxiety disorder. Out of all anxiety symptom domains examined, generalized anxiety disorder symptoms were the best predictor of salivary DHEA concentrations after controlling for pubertal development. Collectively, our findings suggest relevance for DHEA in the development of anxiety in the pubertal period, as well as a robust relationship between DHEA and emerging symptoms of pathological worry during adolescence. The present study underscores the importance of examining associations between DHEA concentrations and anxiety in longitudinal designs.
Keywords: adolescence, anxiety, DHEA, generalized anxiety disorder, hormones, puberty
Introduction
Anxiety disorders are the most common form of psychopathology and often begin early in development (Beesdo, Knappe, & Pine, 2009; Beesdo, 2010). Anxiety symptoms generally increase during adolescence and the average age of onset for any anxiety disorder is approximately 11 (Kessler et al., 2005). Thus, adolescence may be an important developmental period in which youth are specifically at risk for increases in anxiety (Fuhrmann, Knoll, & Blakemore, 2015). Considering the tremendous negative impact of anxiety across the lifespan, there is great interest in identifying contributing factors to the developmental increases in anxiety observed during adolescence.
Previous research has highlighted a role of early pubertal maturation, specifically, in conferring risk for anxiety. Across multiple studies, early pubertal maturation has been linked to increased social anxiety symptoms (Blumenthal et al., 2009; Blumenthal et al., 2011; Deardorff et al., 2007), increased symptoms of state and trait anxiety (Zehr et al., 2007), greater prevalence of lifetime anxiety (Graber et al., 2004), and increased anxiety symptoms and probability of panic attacks (Hayward et al., 1997). In many studies, these effects were stronger in girls than boys (Blumenthal et al., 2011; Reardon et al., 2009), but more recent studies have found no sex differences in this effect (Hamlat et al., 2018; Ullsperger & Nikolas, 2017).
Advanced pubertal status independent of early pubertal timing has also been linked to anxiety. In a study by Hayward and colleagues (1992), advanced pubertal status as measured by Tanner stages was associated with higher likelihood of history of panic attacks. In another study, increased pubertal developmental was associated with increased anxiety symptoms in boys, even when controlling for age (Susman et al., 1991). Similarly, Huerta and Brizuela-Gamino found that pubertal status correlated positively with trait anxiety (2002). Taken together, these reports point to a role for pubertal changes in the increase in anxiety symptoms during adolescence.
The onset of puberty is characterized by marked increases in activity of the hypothalamic-pituitiary-gonadal (HPG) and hypothalamic-pituitary-adrenal (HPA) axes (Buck Louis et al., 2008). Increases in activity of the HPA axis, commonly referred to as adrenarche, typically begin between the ages of 6 and 8 in both sexes (Buck Louis et al., 2008). In this process, the central nervous system (CNS) signals the hypothalamus to release adrenocorticotropin-releasing hormone (CRH), which stimulates the pituitary gland to release adrenocortical tropic hormone (ACTH), which activates the adrenal cortex to release androstenedione and dehydroepiandrosterone (DHEA). Subsequently, DHEA aids in the development of pubic hair, armpit hair, and skin changes (Buck Louis et al., 2008).
In adolescence, differential exposure to sex steroid hormones activate neural circuitry previously organized perinatally, bringing about cognitive and behavioral changes (Pheonix, Goy, & Young, 1967). Further, more recent research has suggested that steroid hormones can also have organizational effects on neural circuitry underlying behavior into puberty and adolescence, and that these organizational effects can be activated later in life by future hormonal exposure (Sisk & Zehr, 2005; Romeo, 2003; Schulz et al., 2009; Schulz & Sisk, 2016). Thus, previously reported associations between puberty and elevations in anxiety could potentially reflect the impact of specific hormones that surge during adrenarche.
Previous literature examining anxiety and hormonal influence have suggested links between DHEA concentrations and anxiety, although the direction of these effects have been mixed (Jezova & Hlavacova, 2008). First, some studies have reported anxiolytic effects of DHEA. Aside from their function in the development of secondary sex characteristics, DHEA and its sulphate counterpart, DHEA-S, are secreted in response to stressors (Lennartsson et al., 2012; Izawa et al., 2008), and have been reported to antagonize the neurotoxic effects of cortisol (Buoso et al., 2011; Kamin & Kertes, 2017). Thus, DHEA is theorized to be protective against the detrimental effects of elevated cortisol on psychological well-being (Kamin & Kertes, 2017). Along these lines, administration of exogenous DHEA has been shown to reduce self-reported anxiety in a sample of male and female schizophrenic patients (Strous et al., 2003), and to reduce anxiety-like behavior in rats (Maayan et al., 2006). Additionally, lower DHEA in response to an acute social stressor was found to be associated with increased negative mood during and after the stressor in a sample of undergraduate men (Izawa et al., 2008).
Conversely, studies have also suggested links between greater DHEA concentrations and anxiety. One study found that male and female children who exhibited greater internalizing problems in elementary school went on to have a greater DHEA response to an anxiety induction in adolescence (Han et al., 2015). In another study on male and female adolescents, while DHEA was not directly associated with anxiety, larger DHEA concentrations were indirectly associated with social anxiety symptoms mediated by pituitary gland volume (Murray et al., 2016). Additionally, a study in adult males found increased DHEA concentrations in participants with panic disorder as compared to healthy controls (Brambilla et al., 2005). Moreover, studies have also encountered positive associations between anxiety and DHEA-S, a metabolite of DHEA. Greater morning serum DHEA-S concentrations were shown to be associated with greater self-reported anxiety in medication-free male and female patients experiencing a major depressive episode (Hsiao, 2006). Another study in a sample of women with polycystic ovarian syndrome found greater concentrations of DHEA-S in patients with major depressive disorder and generalized anxiety disorder as compared to controls with no depression or anxiety disorders (Annagür et al., 2013). Finally, a recent review summarizing studies on endocrine function and anxiety development in adolescence has posited that androgens, including DHEA and testosterone, may disrupt top-down control of the amygdala by the orbitofrontal cortex (OFC) in adolescence, contributing to greater amygdala reactivity, and that this increase in amygdala reactivity contributes to the development of anxiety (Spielberg et al., 2019). As DHEA increases earlier in development than testosterone, DHEA may contribute to beginning the process of OFC-amygdala decoupling as early as adrenarche, which may explain why the peak onset of anxiety disorders in adolescence (i.e., 11 years old; Kessler et al., 2005) occurs prior to the peak effects of gonadarche. Taken together, current findings on the direction of associations between DHEA concentrations and anxiety are mixed, leaving a considerable gap in our knowledge of the relationship between DHEA and anxiety, especially during adolescence.
The present study sought to examine associations between salivary DHEA concentrations and anxiety symptoms and diagnoses during the pubertal period. Our primary goal was to determine whether salivary DHEA concentrations were correlated with anxiety symptom severity and anxiety disorder status. We pursued this aim using both self-report and interviewer-based measures to examine the robustness of potential associations. Additionally, we aimed to examine DHEA–anxiety associations across different anxiety symptom domains. Importantly, we aimed to investigate these associations while also accounting for other indicators of pubertal development such as age and self-reported pubertal development, as well as other sex hormones, testosterone, estradiol, and progesterone. To this end, the present study used a cross-sectional design to examine associations between salivary DHEA concentrations and self-reported and interview-based anxiety measures in a sample of 286 female youth between the ages of 8 and 14. While the literature presents mixed results, previous studies examining DHEA-anxiety associations in child/adolescent populations have suggested that greater concentrations of androgens like DHEA are associated with greater anxiety (Han et al., 2015; Murray et al., 2016; Spielberg et al., 2019). Thus, we hypothesized that increased salivary DHEA concentrations would be associated with greater scores on anxiety self-report measures and greater probability of having an anxiety disorder. Further, we aimed to examine which specific anxiety symptom domains might best account for associations with DHEA.
Method
Participants
317 female girls and adolescents from the community surrounding Stony Brook University participated for monetary compensation. Participants were recruited using a commercial mailing list of families that have an 8- to 14-year-old female living at home. Letters were sent to the families describing the study prior to an initial screening call. Families were screened according to the following criteria: the child must live with at least one biological parent, the child and caretaker must speak English, and the child must not have a significant developmental or medical disability. 23 participants were excluded from the final sample for insufficient hormone data and an additional 8 participants were excluded for incomplete self-report data. Thus, the final sample consisted of 286 subjects. The sample had a mean age of 12.57 years (SD = 1.72) and was composed of multiple ethnicities, including 87.8% Caucasian, 7.5% Black, and 4.7% Latino. Informed consent was obtained prior to participation and the research protocol was approved by the Institutional Review Board at Stony Brook University.
Self-Report Measures
Pubertal Development Scale (PDS): The PDS is a self- and parent-report measure of physical development for youth under the age of 16 (Petersen et al., 1988). The female version of the PDS was used, which ask respondents to report on their level of development on indices such as body hair, skin change, breast development, and growth spurt. Responses are coded on a four-point scale ranging from “no development” to “completed development”. Ratings are averaged to create an overall score of pubertal development. The scale has been shown to have good reliability and validity and is correlated with measures of pubertal development derived from physical examination (Petersen et al., 1988). Validation studies of the PDS find that more than 85% agreement within one Tanner stage was obtained for most assessed measures (e.g., female breast and pubic hair; Schmitz et al., 2004). Cronbach’s alpha for the PDS in the present sample was .83. In addition to the total score, scores on item 5 of the PDS were used as a dichotomous measure of menarche status (i.e., whether participants have experienced their first occurrence of menstruation). In the present sample, 55.3% (n = 157) endorsed reaching menarche on item 5 of the PDS whereas 44.7% (n = 127) did not.
Screen for Child Anxiety Related Disorders (SCARED): The SCARED is a 41-item parent- and self-report inventory that screens for symptoms of anxiety in children. The SCARED is comprised of five subscales: panic/somatic, generalized anxiety, separation anxiety, social phobia, and school avoidance. The SCARED has been shown to be reliable and valid (Birmaher et al., 2009). Cronbach’s alpha for the SCARED in the present sample was .91.
Diagnostic Interview
Kiddie Schedule for Affective Disorders and Schizophrenia - Present and Lifetime Version for DSM-IV (K-SADS-PL): The K-SADS is a semi-structured interview for DSM-IV which assesses for current and past episodes of psychopathology in children and adolescents. The K-SADS has been found to generate reliable and valid child psychiatric diagnoses (Kaufman et al., 1997). Prevalence of cases per anxiety disorder in the present sample are depicted in Table 1. Cohen’s κ was computed to examine inter-rater reliability for anxiety diagnoses based on 10% of the total sample. Inter-rater agreement across anxiety diagnoses ranged between .73 – .91.
Table 1.
K-SADS anxiety diagnoses by disorder
| Threshold Cases (n) | Subthreshold Cases (n) | |
|---|---|---|
| Anxiety Disorders | ||
| Agoraphobia | 1 | 2 |
| Anxiety Disorder NOS | 0 | 1 |
| Generalized Anxiety Disorder | 17 | 21 |
| Obsessive-Compulsive Disorder | 8 | 8 |
| Panic Disorder | 4 | 3 |
| Separation Anxiety Disorder | 4 | 16 |
| Social Anxiety Disorder | 13 | 14 |
| Specific Phobia | 27 | 39 |
| No Anxiety Disorder | 168 | - |
Procedure
Participants attended one laboratory visit. All participants first provided written informed consent, completed self-report questionnaires including the SCARED and PDS, and provided salivary samples. Next, adolescents completed a battery of EEG tasks (not reported here) while their parent completed the K-SADS with a clinical psychology graduate student interviewer. Following completion of the EEG tasks, the adolescent participant completed the K-SADS with the same interviewer. The results of other tasks administered during the same experimental session are presented elsewhere (Levinson, Speed, & Hajcak, 2018; Jackson, Nelson, Meyer, & Hajcak, 2017; Luking, Nelson, Infantolino, Sauder, & Hajcak, 2017; Nelson & Hajcak, 2017; Speed, Nelson, Auerbach, Klein, & Hajcak, 2016; Ferri, Bress, Eaton, & Proudfit, 2014; Gorday & Meyer, 2018).
Hormone Assay
To examine salivary concentrations of estradiol, progesterone, DHEA, and testosterone, salivary samples were collected using SalivaBio passive drool saliva collection aids and SalivaBio 2 mL cryovials (Salimetrics, State College, PA). 20 minutes prior to providing the salivary sample, participants were asked to rinse their mouths with water to prevent the inclusion of food particles in the sample. When providing the sample, participants were instructed to drool into the collection tube until a sample volume between 1 and 1.5 mL was reached. Samples were immediately frozen and stored at −20°C. Samples were assayed at the Salimetrics’ SalivaLab (Carlsbad, CA) using Salimetrics Salivary Assay Kits without modifications to the manufacturer’s protocol. The minimum detection limits for DHEA, estradiol, progesterone, and testosterone were 5 pg/mL (range from 10.2 – 1000 pg/mL) 0.1 pg/mL (range from 1 – 32 pg/mL), 5 pg/mL (range from 10 – 2430 pg/mL), and 0.1 pg/mL (range from 6.1 – 600 pg/mL), respectively.
The mean intra-assay coefficient of variation for DHEA was 5.6% and the mean inter-assay coefficient of variation was 8.2%. The mean intra-assay coefficient of variation for estradiol was 7.1%, and the mean inter-assay coefficient of variation was 7.5%. The mean intra-assay coefficient of variation for progesterone was 6.2%, and the average inter-assay coefficient of variation was 7.6%. The mean intra-assay coefficient of variation for testosterone was 4.6%, and the average inter-assay coefficient of variation was 9.8%.
Nominal levels of cross-reactivity were detected for the estradiol samples in regard to both estriol and estrone, and no significant levels of cross-reactivity occurred regarding antibodies and progesterone, DHEA, or testosterone. There is minimal cross-reactivity for the progesterone assay to corticosterone and no detected cross-reactivity to estradiol, testosterone, or DHEA. There is minimal cross-reactivity for the testosterone assay to progesterone or estradiol and no detected cross-reactivity to corticosterone or DHEA. Finally, there is minimal cross-reactivity for the DHEA assay to DHEA-S and androstenedione, and no detected cross-reactivity to estradiol, progesterone, or testosterone.
Data Analysis
Primary analyses focused on associations between continuous measures of anxiety, puberty, and salivary hormone concentrations. For these analyses, child total and subscale scores on the Screen for Child Anxiety Related Disorders (SCARED) were utilized as a measure of continuous anxiety symptoms. Also, child scores on the Pubertal Development Scale (PDS) were utilized as a measure of pubertal development and scores on item 5 of the PDS were used as a distinct dichotomous measure of menarche status. Menarche status was controlled for in the below analyses because salivary concentrations of sex hormones are known to vary as a function of the menstrual cycle. Associations between salivary hormone concentrations and time of day of the testing session were also examined to account for diurnal rhythms in hormone secretion. Finally, a linear regression was conducted to examine associations between DHEA and continuous anxiety symptoms while controlling for testosterone, PDS scores, and time of day.
Further, we also sought to examine whether hormones were associated with continuous counts of anxiety symptoms endorsed during administration of the K-SADS. Symptom counts were computed by summing the number of current symptoms endorsed for each anxiety disorder (i.e., generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, agoraphobia, separation anxiety disorder, and specific phobia). Next, correlations were conducted to examine associations between hormone concentrations, PDS scores, menarche status, race, time of day, and anxiety symptom counts, and a linear regression was conducted to examine associations between DHEA and anxiety symptom counts while controlling for testosterone and PDS scores.
Moreover, to assess which anxiety symptom domains might best account for variance in salivary DHEA concentrations, we conducted two stepwise regressions. In the first, PDS scores and all SCARED subscale scores (i.e., the panic, GAD, separation anxiety, social anxiety, and school avoidance subscales) were entered as independent variables predicting salivary DHEA concentrations. In the second stepwise regression, PDS scores and symptom counts for each individual anxiety disorder (i.e., generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, agoraphobia, separation anxiety disorder, and specific phobia) were entered as independent variables predicting salivary DHEA concentrations.
Next, in exploratory analyses, we also sought to examine DHEA as a function of categorical diagnoses of anxiety disorders. Because there were relatively few cases of each disorder, we combined threshold and subthreshold diagnoses of anxiety disorders, and further examined the distinction between phobia-only and other anxiety disorders (i.e., generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, agoraphobia, separation anxiety disorder, and anxiety disorder not otherwise specified). Next, correlations were conducted to examine associations between hormone concentrations, PDS scores, menarche status, time of day, and anxiety disorder status, and a logistic regression was conducted to examine associations between DHEA and anxiety disorder status while controlling for PDS scores.
Results
Associations between hormones, PDS scores and continuous self-reported anxiety symptoms
First, we examined associations between salivary hormone concentrations, time of day, menarche status, race, PDS scores, and total SCARED scores. PDS scores were positively associated with menarche status (r(284)= .86, p < .001), DHEA (r(286)= .40, p < .001), testosterone (r(286)= .37, p < .001), estradiol (r(286)= .23, p < .001), progesterone (r(285)= .28, p < .001), and SCARED total scores (r(286)= .23, p < .001). PDS scores were negatively associated with time of day of the testing session (r(286)= −.14, p = .02). Increased total SCARED scores were associated with positive menarche status (r(286)= .18, p < .01), higher DHEA (r(286)= .25, p < .001) and testosterone (r(286)= .14, p < .05) concentrations but were unrelated to estradiol (r(286)= .08, p = .21) and progesterone (r(285)= .06, p = .33) concentrations. Later time of day was associated with lower concentrations of progesterone (r(284)= −.16, p < .01). However, time of day was unrelated with salivary DHEA concentrations (r(285)= −.07, p = .22), testosterone concentrations (r(285)= −.04, p = .50), and estradiol concentrations (r(285)= .01, p = .89). Finally, we found no differences in PDS scores or salivary concentrations of DHEA, estradiol, progesterone, and testosterone due to race (all p’s > .05). We also found no differences in scores on the SCARED due to race, except for a difference in scores on the SCARED separation anxiety subscale (F(2, 275) = 7.42, p < .01). Post-hoc t-tests revealed that the scores on the separation anxiety subscale were higher for Black (M = 5.90, SD = 3.15) participants as compared to White (M = 3.65, SD = 2.74; t(263) = −3.58, p < .001) and Hispanic (M = 2.77, SD = 2.28; t(32) = 3.12, p < .01) participants. However, scores on this subscale did not differ between White and Hispanic participants (t(255) = 1.14, p = .23).
Higher salivary DHEA concentrations were associated with increased scores on the panic, generalized anxiety, and social anxiety SCARED subscales (all ps < .001). Higher salivary testosterone concentrations were associated with increased scores on the generalized anxiety and social anxiety SCARED subscales (all ps < .01). Correlations between DHEA, testosterone, PDS scores, and SCARED subscale scores are depicted in Table 2.
Table 2.
Correlations between DHEA, PDS, and combined SCARED subscale scores (N = 286)
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | DHEA | - | ||||||||
| 2. | Testosterone | .70** | - | |||||||
| 3. | PDS Score | .40** | .37** | - | ||||||
| 4. | SCARED Panic | .21** | .09 | .23** | - | |||||
| 5. | SCARED GAD | .28** | .21** | .32** | .66** | - | ||||
| 6. | SCARED Separation Anxiety | −.01 | −.03 | −.18** | .37** | .36** | - | |||
| 7. | SCARED Social Anxiety | .24** | .16** | .22** | .41** | .51** | .23** | - | ||
| 8. | SCARED School Avoidance | .10 | .04 | .14** | .54** | .56** | .37** | .34** | - | |
| 9. | SCARED Total Score | .25** | .14* | .23** | .83** | .87** | .59** | .70** | .69** | - |
Note.
p < .05
p < .01.
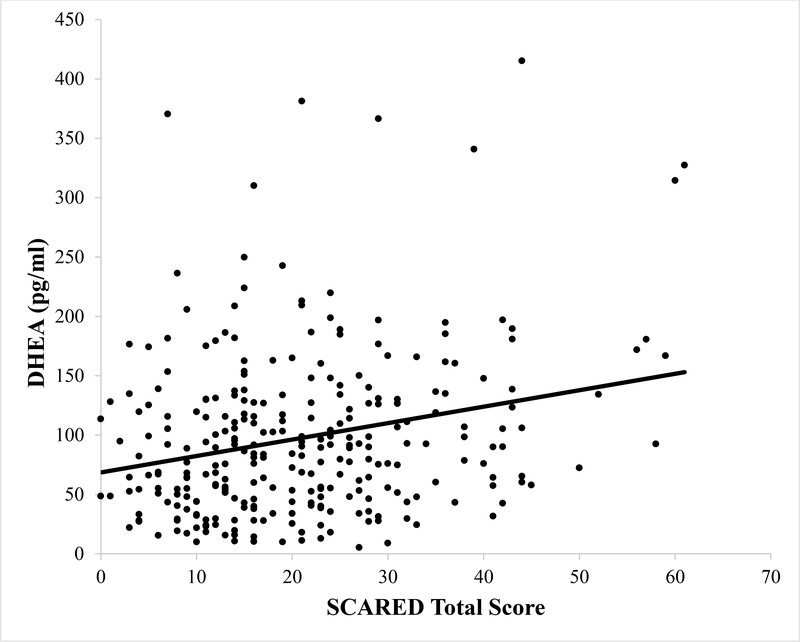
Next, a linear regression was conducted predicting SCARED total scores with salivary DHEA concentrations, testosterone concentrations, and PDS score entered as predictors. A significant regression equation was found, F(3, 285) = 8.69, p < .001, with an R2 of .09. Higher DHEA (β = .24, t(285) = 2.94, p = .01) and increased PDS scores (β = .16, t(285) = 2.62, p < .01) independently predicted greater SCARED scores. However, testosterone concentrations did not significantly predict SCARED scores (β = −.09, t(285) = −1.09, p = .28). Thus, greater DHEA was associated with increased anxiety symptoms, even when controlling for salivary testosterone concentrations and pubertal development (i.e., PDS scores).1
Associations between hormones, PDS scores, and continuous anxiety symptom counts
Increased anxiety symptom counts derived from a clinical interview (i.e., the K-SADS) were associated with increased salivary DHEA concentrations (r(281)= .21, p < .001) and marginally associated with increased testosterone concentrations (r(281)= .11, p = .06). However, anxiety symptom counts were not associated with estradiol concentrations (r(281)= .03, p = .65), progesterone concentrations (r(281)= .10, p = .11), PDS scores (r(281)= .09, p = .15), menarche status (r(280)= .07, p = .22), or time of day of the testing session (r(281)= .02, p = .78). There were also no differences in anxiety symptom counts or scores due to race (r(271) = −.02, p = .70).
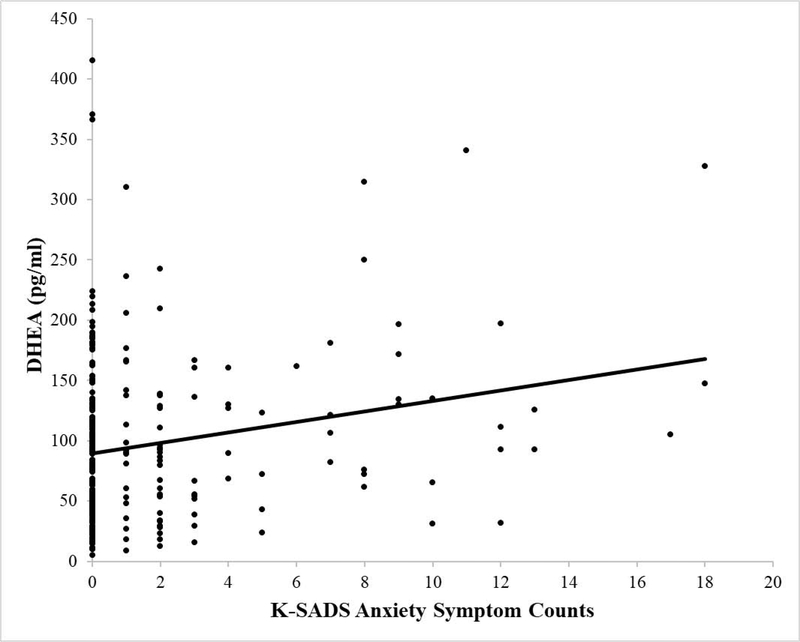
Next, a linear regression predicting anxiety symptom counts with salivary DHEA concentrations, salivary testosterone concentrations, and PDS score entered as predictors revealed a significant regression equation, F(2, 280) = 4.44, p < .01, with an R2 of .05. Greater DHEA significantly predicted increased anxiety symptom counts (β = .25, t(280) = 2.99, p < .01), while testosterone concentrations (β = −.07, t(280) = −.82, p = .41) and PDS scores (β = .005, t(280) = .07, p = .94) were unrelated to anxiety symptom counts. Thus, greater DHEA was associated with increased anxiety symptom counts, even when controlling for testosterone concentrations and pubertal development (i.e., PDS scores).2
Stepwise regressions predicting salivary DHEA concentrations
Next, we conducted two stepwise regressions with DHEA as the dependent variable to examine which anxiety symptom domains might best predict concentrations of DHEA in adolescence. In the first regression, PDS scores and all SCARED subscale scores (i.e., the panic, GAD, separation anxiety, social anxiety, and school avoidance subscales) were entered as predictor variables. In the first step (F(1, 285) = 55.33, p < .001), PDS score was the best single predictor of salivary DHEA concentrations (β = 6.87, t(285) = 7.44, p < .001), accounting for 16% of the variance in salivary DHEA concentrations. In the second step (F(2, 285) = 32.56, p < .001), PDS score (β = 5.99, t(285) = 6.22, p < .001) and the GAD subscale (β = 2.68, t(285) = 2.89, p < .01) were significant independent predictors of salivary DHEA concentrations, with the addition of the GAD subscale accounting for an additional 2% of the variance in salivary DHEA concentrations. All other predictor variables (i.e., the panic, separation anxiety, social anxiety, and school avoidance subscales) failed to account for a significant increment in salivary DHEA concentrations at the .05 level. Thus, the stepwise regression analysis suggested that PDS score and the GAD subscale were the best independent predictors of salivary DHEA concentrations — and together accounted for 18% of the variance in salivary DHEA concentrations.
We then repeated these analyses using KSADS-based symptom counts for each individual anxiety disorder (i.e., generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, agoraphobia, separation anxiety disorder, and specific phobia) in a second stepwise regression predicting salivary DHEA concentrations. In the first step (F(1, 280) = 58.64, p < .001), PDS score was the best single predictor of salivary DHEA concentrations (β = 6.96, t(280) = 7.66, p < .001), accounting for 17% of the variance in salivary DHEA concentrations. In the second step (F(2, 280) = 34.11, p < .001), PDS score (β = 6.73, t(280) = 7.47, p < .001) and the GAD symptom counts (β = 7.72, t(280) = 2.84, p < .01) were significant independent predictors of salivary DHEA concentrations, with the addition of the GAD symptom counts accounting for an additional 2% of the variance in salivary DHEA concentrations. All other predictor variables (i.e., social anxiety disorder, obsessive-compulsive disorder, panic disorder, agoraphobia, separation anxiety disorder, and specific phobia symptom counts) failed to account for a significant increment in salivary DHEA concentrations at the .05 level. Thus, the stepwise regression analysis suggested that PDS score and GAD symptom counts were the best independent predictors of salivary DHEA concentrations —and together accounted for 19% of the variance in salivary DHEA concentrations.
Finally, given that race was associated with scores on the SCARED separation anxiety subscale, race was entered as a covariate in the above analyses involving the SCARED. Results from the multiple regression predicting the SCARED total scores were not changed. Specifically, the regression model was significant (p < .05), and DHEA concentrations and PDS scores were significant predictors of SCARED total scores (p’s < .05), whereas testosterone concentrations and race were not significant predictors of SCARED total scores (p’s > .05). Additionally, results from the stepwise regression predicting DHEA concentrations were not changed. In the first step, PDS score was the best single predictor of DHEA concentrations, accounting for 17% of the variance in DHEA concentrations. In the second step, PDS score and the GAD subscale were significant independent predictors of DHEA concentrations, with the addition of the GAD subscale accounting for an additional 2% of the variance in DHEA concentrations. All other predictor variables (i.e., the panic, separation anxiety, social anxiety, and school avoidance subscales, and race) failed to account for a significant increment in DHEA concentrations at the .05 level.
Associations between hormones, PDS scores, and categorical anxiety diagnoses
First, we explored whether hormones and PDS scores were correlated with anxiety disorder status (any anxiety disorder vs. no anxiety disorders). Anxiety disorder status did not relate to hormones, PDS scores, menarche status, or time of day of the testing session (all p’s > .10). Next, we removed individuals whose only anxiety disorder was a specific phobia from the anxiety disorder group and placed them in the “no anxiety disorder” group, and again explored whether hormones and PDS scores correlated with anxiety disorder status. Specific phobia was chosen to be removed from the anxiety disorder group in light of the above findings that the GAD anxiety domain was the best predictor of salivary DHEA concentrations. As such, we posited that individuals with specific phobia may have fears that are too circumscribed in nature, and thus, that specific phobia may be unrelated with DHEA. Anxiety disorder status was significantly correlated with DHEA (r(285)= .16, p < .01) such that those with a greater DHEA level were more likely to have an anxiety disorder.3 However, anxiety disorder status was not associated with testosterone, estradiol, progesterone, PDS scores, menarche status, or time of day (all ps > .10).
Finally, a logistic regression was conducted examining associations between anxiety disorder status and salivary DHEA concentrations while controlling for PDS scores. As shown in Table 3, greater DHEA was significantly associated with the presence of an anxiety disorder (odds ratio = 1.01, 95% CI = 1.00–1.01, p < 0.05). However, anxiety disorder status was not associated with PDS score (odds ratio = .95, 95% CI = .66–1.35, p = 0.76). Thus, participants with an anxiety disorder had greater salivary concentrations of DHEA as compared to participants without an anxiety disorder and this association was not driven by pubertal development.4,5
Table 3:
Logistic regression predicting anxiety disorder status
| Variables | R2 | X2 | Adjusted Odds Ratio | 95% CI |
|---|---|---|---|---|
| .034 | 6.854* | |||
| DHEA | 1.005 | 1.00–1.01* | ||
| PDS Score | .945 | .66–1.35 | ||
Note. Logistic regression was used for the dichotomous dependent variable anxiety disorder status (0=absent, 1=present). The chi-square statistic is reported for the overall omnibus test for the logistic regression. Nagelkerke R2 is reported for the logistic regression.
p<0.05.
Finally, 157 participants, or 55.3% of the sample, reported having reached menarche based on item 5 of the PDS; these participants were more advanced in age (t = −14.02, p < .001), had higher PDS scores (t = −28.22, p < .001), had greater salivary concentrations of DHEA (t = −7.56, p < .001), testosterone (t = −5.78, p < .001), estradiol (t = −3.50, p < .001), and progesterone (t = −4.85, p < .001), and had higher SCARED total scores (t = −3.08, p = .002). Menarche status was highly correlated with overall PDS scores (r(283)= .86, p < .001). Thus, to determine if pubertal status interacted with DHEA to predict anxiety, we examined interactions between pubertal development (i.e., PDS scores) and salivary DHEA concentrations in additional analyses. When controlling for PDS and including a PDS × DHEA interaction term, the interaction term was non-significant in predicting SCARED total scores, K-SADS anxiety symptom counts, and categorical anxiety disorder status (all p’s > .10); however, DHEA was a significant predictor of SCARED total scores, K-SADS anxiety symptom counts, and categorical anxiety disorder status (all p’s < .05). Thus, the association between DHEA levels and anxiety was not moderated by pubertal development.
Discussion
The present study examined associations between salivary hormone concentrations and anxiety symptoms and diagnoses during the pubertal period in a relatively large sample of girls. Results in the full sample indicated that greater DHEA was associated with increased self-reported anxiety symptoms and increased anxiety symptoms on the K-SADS clinical interview—results that remained even when controlling for salivary testosterone concentrations, pubertal status, menarche status, and time of day. With regard to SCARED subscales, salivary DHEA concentrations were positively associated with scores on the panic, GAD, and social anxiety subscales. These findings are in line with previous studies that have shown positive associations between DHEA and panic disorder (Brambilla et al., 2005), GAD (Annagür et al., 2013), and social anxiety symptoms (Murray et al., 2016).
Additionally, when pubertal development scores and anxiety symptom domains from the SCARED and K-SADS symptom counts were entered into stepwise regressions predicting salivary DHEA concentrations, the GAD symptom domain was the best predictor of salivary DHEA concentrations in both of the stepwise regression models, and was the second-best predictor of DHEA after pubertal development scores. Again, this finding aligns with previous work that has found greater DHEA-S concentrations in those with GAD as opposed to healthy controls (Annagür et al., 2013). Furthermore, these findings provide some specificity as to which anxiety symptoms and phenotypes might be most associated with DHEA.
Moreover, while the odds ratio was modest, suggesting an increased risk of .5%, greater salivary DHEA concentrations were associated with increased probability of having an anxiety disorder based on the K-SADS interview in the full sample. Notably, while associations were not apparent between K-SADS diagnoses and salivary DHEA concentrations with specific phobia cases included, this association became apparent upon the exclusion of cases with specific phobia as the only anxiety disorder. Thus, it may be that DHEA concentrations are less related to specific phobias, which are circumscribed in nature. This is bolstered by the present findings that the GAD symptom domain is best associated with DHEA, as GAD is characterized by prevalent worry in multiple life domains. Altogether, these findings align with those of previous studies revealing greater concentrations of DHEA in individuals with anxiety diagnoses (Annagür et al., 2013), and positive associations between DHEA concentrations and anxiety severity (Hsiao, 2006; Murray et al., 2016).
While later time of day of the testing session was associated with lower levels of progesterone, time of day was not associated with salivary concentrations of DHEA, testosterone, or estradiol, which are also known to demonstrate diurnal fluctuations. While it is unclear why this was the case, we also observed that time of day was negatively associated with PDS scores, suggesting that participants with more advanced pubertal development participated earlier in the day as compared to participants with less advanced pubertal development. As such, effects of age or pubertal development may have partially confounded the association between time of day and hormone levels.
The present results provide an improved understanding of the mixed findings that have been reported previously with respect to associations between DHEA concentrations and anxiety among adolescents. As mentioned previously, late childhood and early adolescence is marked by adrenarche, which consists in marked increases in activity of the HPA axis, resulting in a surge of DHEA concentrations (Forbes & Dahl, 2009; Romeo, 2003). Considering that greater DHEA concentrations were found to be associated with greater anxiety in the present study, it may be the case that individual differences in the amount of DHEA secreted during adrenarche may play a role in precipitating anxious symptoms and psychopathology. This is in line with previous studies suggesting that androgens, such as testosterone and DHEA, may precipitate anxiety by disrupting top-down control of the orbitofrontal cortex on amygdala reactivity to threat (Spielberg et al., 2019). The present findings also align with previous studies that have suggested that DHEA is positively associated, whether directly or indirectly, with anxiety in adolescent girls and boys (Han et al., 2015; Murray et al., 2016; Spielberg et al., 2019), and diverge from findings in previous studies conducted in clinical and adult samples of both sexes, which have shown anxiolytic effects of DHEA (Strous et al., 2003; Izawa et al., 2008). Importantly, the present study leveraged a large sample of 286 adolescent girls and found positive associations between DHEA and anxiety across three separate indices: self-reported symptom scores, and interview-based symptom counts, and interview-based categorical diagnoses. Thus, the present results provide robust evidence of links between DHEA and anxiety in adolescent girls and suggests a need for further research in the mechanisms underlying these associations.
The present findings also align with previous work suggesting that DHEA is an allosteric antagonist of the gamma-aminobutyric acidA (GABAA) receptor (Gartside et al., 2010; Krug et al., 2008; Park-Chung et al., 1999). As such, it is possible that DHEA may reduce neuronal inhibition via GABA in the amygdala and exhibit anxiogenic effects (Sanders & Shekhar, 1995; Nuss, 2014; Majewska, 1992). On the other hand, allopregnanolone, a metabolite of progesterone, is a known positive allosteric modulator of the GABAA receptor and a potent anxiolytic (Schule et al., 2014). As such, the lack of associations between salivary progesterone concentrations and anxiety in the present study does not align with previous findings on the impact of allopregnanolone on anxiety, but may be due to the age distribution of the present sample, as 44.7% of the sample had not yet reached menarche and showed substantially lower levels of progesterone.
There are multiple limitations that warrant consideration. First, as a limitation of the present study, we did not examine longitudinal associations between anxiety and DHEA over adolescence. Thus, as a future direction, studies should elucidate the associations between DHEA and anxiety with longitudinal designs to develop a better understanding of how these variables interact over time. Second, the present study examined associations between DHEA and anxiety solely in adolescent girls. Thus, the present findings may not generalize to adolescent boys, and future studies should examine this issue in large samples comprised of both sexes. Third, while the present study controlled for menarche status, the present study did not account for menstrual cycle phases in participants who had reached menarche, which could have influenced concentrations of sex hormones. Future studies exploring associations between anxiety and sex hormones in girls should account for menstrual cycle phases according to published guidelines (Allen et al., 2016; Schmalenberger & Eisenlohr-Moul, 2019). Fourth, the present study did not assess DHEA-S concentrations, and thus, was not able to examine unique associations of DHEA and DHEA-S with measures of anxiety. Future studies should examine and compare associations between both forms of DHEA and anxiety. Fifth, while the present study controlled for time of day of the testing session statistically, it is most ideal to control for time of day of hormone sampling during data collection. Additionally, salivary concentrations of DHEA were measured at a single time interval only for each participant. Future studies should control for time of day during data collection (i.e., by scheduling all hormone sampling to take place in the afternoon) to control for diurnal rhythms in hormone secretion and should also employ repeated measures of salivary hormone concentrations, as salivary hormone concentrations may be influenced by other internal and environmental factors, such as pulsatile release and perceived stress. Finally, given the relatively small number of cases reaching clinical threshold for each specific anxiety disorder in the present study, we were not able to examine specificity in associations between DHEA and anxiety on a diagnostic level. Thus, future studies should aim to explore specificity in clinical populations.
In conclusion, the present study examined associations between salivary DHEA concentrations and anxiety symptoms and diagnoses during the pubertal period while controlling for salivary testosterone concentrations and pubertal development and found that greater salivary DHEA concentrations were associated with increased self-reported anxiety symptoms, increased anxiety symptom counts based on the K-SADS interview, and increased probability of an anxiety disorder. Further, we found that out of all anxiety symptom domains examined (i.e., generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, panic disorder, agoraphobia, separation anxiety disorder, and specific phobia), the generalized anxiety disorder symptom domain was the best predictor of salivary DHEA concentrations, even after controlling for pubertal development and menarche status. Taken together, our findings suggest relevance for DHEA in the development of anxiety in the pubertal period as well as a robust relationship between DHEA and emerging symptoms of pathological worry during adolescence. The present findings underscore the importance of examining whether increased DHEA during and following adrenarche prospectively predicts increases in anxiety and worry in longitudinal designs.
Figure 1.
Scatter plot depicting the association between DHEA concentrations in picograms per milliliter (pg/ml) and self-reported total anxiety symptoms on the SCARED questionnaire. The line represents the line of best fit.
Figure 2.
Scatter plot depicting the association between DHEA concentrations in picograms per milliliter (pg/ml) and total interview-based anxiety symptom counts based on the K-SADS interview. The line represents the line of best fit.
Highlights.
Greater DHEA was associated with increased self-reported anxiety symptoms
Greater DHEA was linked to increased probability of current anxiety diagnoses
DHEA was associated with anxiety when controlling for gonadal hormones and puberty
Among all anxiety disorders, GAD symptoms were most robustly correlated with DHEA
Acknowledgments
This work was supported by the following grants: NIMH T32 MH093311 to Elizabeth Mulligan and NIMH R01 MH97767 to Greg Hajcak.
Footnotes
Results of this linear regression were consistent when menarche status was entered as a predictor instead of PDS total scores. A significant regression equation was found, F(3, 283) = 7.29, p < .001, with an R2 of .07. Higher DHEA (β = .26, t(285) = 3.12, p < .01) was associated with greater SCARED scores. However, testosterone concentrations (β = −.07, t(285) = −.90, p = .37) and menarche status (β = .10, t(285) = 1.53, p = .13) were not independently associated with SCARED scores. Thus, greater DHEA was associated with increased anxiety symptoms even when controlling for testosterone concentrations and menarche status.
Results of this linear regression were consistent when menarche status was entered as a predictor instead of PDS total scores. A significant regression equation was found, F(3, 279) = 4.40, p < .01, with an R2 of .05. Higher DHEA (β = .26, t(285) = 3.03, p < .01) was associated with greater anxiety symptom counts. However, testosterone concentrations (β = −.07, t(285) = −.83, p = .41) and menarche status (β = −.01, t(285) = −.22, p = .83) were not independently associated with anxiety symptom counts. Thus, greater DHEA was associated with increased anxiety symptom counts even when controlling for testosterone concentrations and menarche status.
When individuals with a sole anxiety disorder of specific phobia were removed from both the “anxiety” and “no anxiety” groups, the association between anxiety disorder status and DHEA remained significant (r(254)= .15, p < .05).
Results of this logistic regression were consistent when menarche status was entered as a predictor instead of PDS scores. Greater DHEA was significantly associated with the presence of an anxiety disorder (odds ratio = 1.01, 95% CI = 1.00–1.01, p < 0.05). However, anxiety disorder status was not associated with menarche status (odds ratio = .94, 95% CI = .43–2.00, p = 0.84).
When participant age was substituted for PDS scores in the regressions predicting SCARED total scores, anxiety disorder status, and anxiety symptom counts, DHEA remained a significant predictor (all ps < .05) of SCARED scores, anxiety disorder status, and anxiety symptom counts, while age was not significantly associated with SCARED scores, anxiety disorder status, or anxiety symptom counts (all ps > .10).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL, McKee SA, & Allen SS (2016). Determining menstrual phase in human biobehavioral research: A review with recommendations. Experimental and clinical psychopharmacology, 24(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annagür BB, Tazegül A, Uguz F, Kerimoglu ÖS, Tekinarslan E, & Celik Ç (2013). Biological correlates of major depression and generalized anxiety disorder in women with polycystic ovary syndrome. Journal of psychosomatic research, 74(3), 244–247. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, & Pine DS (2009). Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics, 32(3), 483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Pine DS, Lieb R, & Wittchen HU (2010). Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Archives of general psychiatry, 67(1), 47–57. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child & Adolescent Psychiatry, 38(10), 1230–1236. [DOI] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Babson KA, Gahr JL, Trainor CD, & Frala JL (2011). Elevated social anxiety among early maturing girls. Developmental Psychology, 47(4), 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Trainor CD, Babson KA, & Bunaciu L (2009). Interactive roles of pubertal timing and peer relations in predicting social anxiety symptoms among youth. Journal of Adolescent Health, 44(4), 401–403. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Mellado C, Alciati A, Pisu MG, Purdy RH, Zanone S, … & Biggio G (2005). Plasma concentrations of anxiolytic neuroactive steroids in men with panic disorder. Psychiatry research, 135(3), 185–190. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Gray LE, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, … & Bourguignon JP (2008). Environmental factors and puberty timing: expert panel research needs. Pediatrics, 121(Supplement 3), S192–S207. [DOI] [PubMed] [Google Scholar]
- Buoso E, Lanni C, Molteni E, Rousset F, Corsini E, & Racchi M (2011). Opposing effects of cortisol and dehydroepiandrosterone on the expression of the receptor for activated C kinase 1: implications in immunosenescence. Experimental gerontology, 46(11), 877–883. [DOI] [PubMed] [Google Scholar]
- Deardorff J, Hayward C, Wilson KA, Bryson S, Hammer LD, & Agras S (2007). Puberty and gender interact to predict social anxiety symptoms in early adolescence. Journal of Adolescent Health, 41(1), 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J, Bress JN, Eaton NR, & Proudfit GH (2014). The impact of puberty and social anxiety on amygdala activation to faces in adolescence. Developmental Neuroscience, 36(3–4), 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2010). Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain and cognition, 72(1), 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, & Blakemore SJ (2015). Adolescence as a sensitive period of brain development. Trends in cognitive sciences, 19(10), 558–566. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Griffith NC, Kaura V, & Ingram CD (2010). The neurosteroid dehydroepiandrosterone (DHEA) and its metabolites alter 5-HT neuronal activity via modulation of GABAA receptors. Journal of Psychopharmacology, 24(11), 1717–1724. [DOI] [PubMed] [Google Scholar]
- Gorday JY, & Meyer A (2018). Linking puberty and error- monitoring: Relationships between self- reported pubertal stages, pubertal hormones, and the error- related negativity in a large sample of children and adolescents. Developmental psychobiology. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, & Lewinsohn PM (2004). Is pubertal timing associated with psychopathology in young adulthood?. Journal of the American Academy of Child & Adolescent Psychiatry, 43(6), 718–726. [DOI] [PubMed] [Google Scholar]
- Hamlat EJ, Snyder HR, Young JF, & Hankin BL (2019). Pubertal timing as a transdiagnostic risk for psychopathology in youth. Clinical Psychological Science, 7(3), 411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Miller JG, Cole PM, Zahn- Waxler C, & Hastings PD (2015). Adolescents’ internalizing and externalizing problems predict their affect- specific HPA and HPG axes reactivity. Developmental psychobiology, 57(6), 769–785. [DOI] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Wilson DM, & Hammer LD (1997). Psychiatric risk associated with early puberty in adolescent girls. Journal of the American Academy of Child & Adolescent Psychiatry. [PubMed] [Google Scholar]
- Hsiao CC (2006). Positive correlation between anxiety severity and plasma levels of dehydroepiandrosterone sulfate in medication- free patients experiencing a major episode of depression. Psychiatry and clinical neurosciences, 60(6), 746–750. [DOI] [PubMed] [Google Scholar]
- Huerta R, & Brizuela-Gamiño OL (2002). Interaction of pubertal status, mood and self-esteem in adolescent girls. The Journal of reproductive medicine, 47(3), 217–225. [PubMed] [Google Scholar]
- Izawa S, Sugaya N, Shirotsuki K, Yamada KC, Ogawa N, Ouchi Y, … & Nomura S (2008). Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biological Psychology, 79(3), 294–298. [DOI] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, Meyer A, & Hajcak G (2017). Pubertal development and anxiety risk independently relate to startle habituation during fear conditioning in 8–14 year- old females. Developmental psychobiology, 59(4), 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova D, & Hlavacova N (2008). Endocrine factors in stress and psychiatric disorders: focus on anxiety and salivary steroids. Annals of the New York Academy of Sciences, 1148(1), 495–503. [DOI] [PubMed] [Google Scholar]
- Kamin HS, & Kertes DA (2017). Cortisol and DHEA in development and psychopathology. Hormones and behavior, 89, 69–85. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Krug AW, Ziegler CG, Bornstein SR (2008) DHEA and DHEA-S, and their Functions in the Brain and Adrenal Medulla In: Ritsner MS, Weizman A (eds) Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders. Springer, Dordrecht [Google Scholar]
- Lennartsson AK, Kushnir MM, Bergquist J, & Jonsdottir IH (2012). DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biological psychology, 90(2), 143–149. [DOI] [PubMed] [Google Scholar]
- Levinson AR, Speed BC, & Hajcak G (2018). Neural response to pleasant pictures moderates prospective relationship between stress and depressive symptoms in adolescent girls. Journal of Clinical Child & Adolescent Psychology, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Nelson BD, Infantolino ZP, Sauder CL, & Hajcak G (2017). Internal consistency of functional magnetic resonance imaging and electroencephalography measures of reward in late childhood and early adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(3), 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan R, Touati-Werner D, Ram E, Strous R, Keren O, & Weizman A (2006). The protective effect of frontal cortex dehydroepiandrosterone in anxiety and depressive models in mice. Pharmacology Biochemistry and Behavior, 85(2), 415–421. [DOI] [PubMed] [Google Scholar]
- Majewska MD (1992). Neurosteroids: endogenous bimodal modulators of the GABAA receptor mechanism of action and physiological significance. Progress in neurobiology, 38(4), 379–394. [DOI] [PubMed] [Google Scholar]
- Murray CR, Simmons JG, Allen NB, Byrne ML, Mundy LK, Seal ML, … & Whittle S (2016). Associations between dehydroepiandrosterone (DHEA) levels, pituitary volume, and social anxiety in children. Psychoneuroendocrinology, 64, 31–39. [DOI] [PubMed] [Google Scholar]
- Nelson BD, & Hajcak G (2017). Anxiety and depression symptom dimensions demonstrate unique associations with the startle reflex in anticipation of unpredictable threat in 8 to 14 year-old girls. Journal of Abnormal Child Psychology, 45(2), 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss P (2015). Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatric disease and treatment, 11, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, & Farb DH (1999). Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain research, 830(1), 72–87. [DOI] [PubMed] [Google Scholar]
- Patton GC, Hibbert ME, Carlin J, Shao Q, Rosier M, Caust J, & Bowes G (1996). Menarche and the onset of depression and anxiety in Victoria, Australia. Journal of Epidemiology & Community Health, 50(6), 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, & Young WC (1967). Sexual behavior: General aspects. Neuroendocrinology, 2, 163–196. [Google Scholar]
- Reardon LE, Leen-Feldner EW, & Hayward C (2009). A critical review of the empirical literature on the relation between anxiety and puberty. Clinical psychology review, 29(1), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD (2003). Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. Journal of neuroendocrinology, 15(12), 1185–1192. [DOI] [PubMed] [Google Scholar]
- Sanders SK, & Shekhar A (1995). Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacology Biochemistry and Behavior, 52(4), 701–706. [DOI] [PubMed] [Google Scholar]
- Schmalenberger KM, & Eisenlohr-Moul TA (2019). Studying the menstrual cycle as an independent variable: Practical recommendations and tools for getting started. 10.31219/osf.io/94jua [DOI]
- Schmitz KE, Hovell MF, Nichols JF, Irvin VL, Keating K, Simon GM, … & Jones KL (2004). A validation study of early adolescents’ pubertal self-assessments. The Journal of Early Adolescence, 24(4), 357–384. [Google Scholar]
- Schüle C, Nothdurfter C, & Rupprecht R (2014). The role of allopregnanolone in depression and anxiety. Progress in neurobiology, 113, 79–87. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, & Sisk CL (2009). Back to the future: the organizational–activational hypothesis adapted to puberty and adolescence. Hormones and behavior, 55(5), 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, & Sisk CL (2016). The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience & Biobehavioral Reviews, 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, & Zehr JL (2005). Pubertal hormones organize the adolescent brain and behavior. Frontiers in neuroendocrinology, 26(3–4), 163–174. [DOI] [PubMed] [Google Scholar]
- Speed BC, Nelson BD, Auerbach RP, Klein DN, & Hajcak G (2016). Depression Risk and Electrocortical Reactivity during Self-Referential Emotional Processing in 8 to 14 Year-Old Girls. Journal of Abnormal Psychology, 125(5), 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Schwarz JM, & Matyi MA (2019). Anxiety in Transition: Neuroendocrine Mechanisms Supporting the Development of Anxiety Pathology in Adolescence and Young Adulthood. Frontiers in neuroendocrinology, 100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, & Weizman A (2003). Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Archives of general psychiatry, 60(2), 133–141. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, & Chrousos GP (1991). Negative affect and hormone levels in young adolescents: Concurrent and predictive perspectives. Journal of Youth and Adolescence, 20(2), 167–190. [DOI] [PubMed] [Google Scholar]
- Ullsperger JM, & Nikolas MA (2017). A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk?. Psychological bulletin, 143(9), 903. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Culbert KM, Sisk CL, & Klump KL (2007). An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Hormones and behavior, 52(4), 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]