Abstract
Background
The concurrent use of cigarettes with other tobacco products, such as smokeless tobacco (SLT), is increasingly common. Extant work with cigarette smokers who also use SLT is based heavily on retrospective reports and between-group comparisons. The purpose of this study was to assess prospectively the patterns of dual users’ product use and nicotine exposure on days when cigarettes were smoked exclusively (single use) versus concurrently with SLT (dual use).
Design
Forty-six dual cigarette-SLT users recorded their product use in real time via ecological momentary assessment for a 2-week longitudinal design. They responded to questions about situational factors (eg, location, mood) using this same diary, and collected saliva samples each night for later cotinine measurement. At the end of this 2-week period, users reported on their reasons for and beliefs about SLT use.
Results
Cotinine levels were significantly higher on dual versus single use days (mean±SEM=374.48±41.08 ng/mL vs 300.17±28.13 ng/mL, respectively; p<0.01), and the number of cigarettes logged was higher on dual versus single use days (11.13±0.98 vs 9.13±1.11, respectively; p<0.01). Product use was distinguished by situational factors, with the strongest predictor being location of use. Moreover, the most common reason for initiating (56.52%) and continuing (67.39%) SLT use was to circumvent indoor smoking restrictions.
Conclusions
Results support the idea of product supplementation rather than replacement among this convenience sample of dual users. For smokers whose primary motivation for SLT use involves situations where they would otherwise be tobacco free, the potential benefits of clean indoor air laws may be diminished.
INTRODUCTION
The tobacco use landscape in the USA has been shifting over recent decades, with cigarette smoking now at an all-time low (~14%)1 and use of other tobacco products either increasing or remaining stable.2,3 Also more popular than in previous years is the concurrent use of cigarettes with other tobacco products, including loose or pouched smokeless tobacco (SLT).4,5 Indeed, nearly 40% of current cigarette smokers have reported use of at least one other tobacco product,6 with nearly 8% reporting that the other tobacco product is a form of SLT.5 The use of SLT alone and in combination with cigarettes is most prevalent among young Caucasian males,7–10 and this disparity is most pronounced among those who reside in rural regions (eg, West Virginia, Kentucky, Mississippi).11,12
Cigarette smokers’ increasing use of other tobacco products like SLT is not surprising given the myriad of challenges to smoking they have faced over these same decades, and their exposure to tobacco industry marketing that encourages the use of cigarette alternatives. The number of states with comprehensive indoor (ie, workplace, restaurants) smoke-free laws increased from 0 to 27 in the period of 2000–2015.13 Today, smoking bans also exist for some outdoor spaces (eg, college campuses, playgrounds),14,15 multiunit housing facilities,16 and personal vehicles with children (for review, see Hyland et al14). In anticipation of this changing tide, both cigarette and SLT manufacturers began the development and/or marketing of SLT products for use in smoking-restricted locations.17,18 Advertisements for SLT products such as those pouched and spitless in nature informed smokers that they could be used ‘anywhere’ and ‘anytime’.19 Many smokers now report use of SLT in places where they cannot smoke.9,20,21 Another tactic employed by tobacco manufacturers was to tout SLT as a harm reduction product.18,22 Emphasis was put on snus, a moist snuff product with relatively low levels of tobacco-specific carcinogens.23 Interestingly, whereas a minority of smokers (eg, ~11%–24%) believe that SLT products are less harmful than are cigarettes,24,25 a notable portion of smokers have confirmed their use of SLT as a method for quitting cigarettes.26,27
Importantly, much of what is known about dual use of cigarettes and SLT is based on retrospective reports and between-group comparisons. In one such study, 28 cigarette smokers who used SLT daily reported a lower number of cigarettes per day (CPD; n = 13) than smokers who used SLT non-daily or never (n=20). Other work suggests no differences in the average number of CPD between smokers with varying levels of SLT use (daily, non-daily, not at all) (n = 17–19; n = 17–18) (ref 29 30, respectively). For this latter work, cotinine levels were higher for daily cigarette smokers who used SLT daily versus non-daily or never, but were comparable between those who used SLT non-daily versus never.30 Of course, different groups of cigarette smokers may use SLT for different reasons (eg, circumvent smoking restrictions; quit cigarettes), and consequently have different patterns of use and nicotine exposure.
The present study was designed to prospectively assess patterns of dual cigarette-SLT use via ecological momentary assessment (EMA) methods. Primary aims were to compare smokers’ cigarette use and nicotine exposure (salivary cotinine levels) on days when SLT was also used (dual use days) versus on days when SLT was not used (single use days). Secondary aims were to evaluate whether contextual factors (eg, smoking-restricted vs non-restricted locations) differentiated type of product used (cigarettes vs SLT), and to describe the reasons for and beliefs about SLT use among a sample of dual users who were not currently interested in quitting smoking.
METHODS
Inclusion/exclusion criteria
Dual cigarette-SLT users were recruited from March 2015 to May 2017 in various counties throughout West Virginia (eg, Monongalia, Marion, McDowell, Raleigh) via fliers, online postings and word of mouth. Inclusion criteria included being 18–60 years of age; smoking ≥5 CPD for ≥1 year; and using SLT ≥2 times per day for ≥4 days per week for ≥6 months. These cut-offs for CPD and SLT were chosen to ensure sufficient power to detect differences between single versus dual use days for primary outcomes. To verify tobacco use status, participants also were required to provide an exhaled air carbon monoxide (CO) level of ≥7 ppm (CoVita; Haddonfield, NJ) and a urinary cotinine >3 (NicAlert; Nymox Pharmaceutical; Hasbrouck Heights, NJ). Exclusion criteria included diagnosis of schizophrenia or bipolar disorder; current pregnancy (verified by urinalysis) or breast feeding; use of marijuana >5 days in the past month; any other illicit drug use in the past 3 months; use of alcohol >15 days in the past month; regular use of other tobacco products; or active engagement in tobacco cessation. A power analysis indicated that ~35 participants were needed to detect small-medium differences between single versus dual use days for primary outcomes (logged CPD and cotinine levels), with a desired power of 0.80 and a type I error rate of 0.05.
Procedures
Using a longitudinal study design, dual cigarette-SLT users provided responses to assessments via EMA device every day for 2 consecutive weeks. They also visited the laboratory on four occasions for screening and training (day 1), compliance checks (days 3, 9 and 15) and/or completion of questionnaires (day 15). Participants were paid $50 on day 3, $100 on day 9 and $150 on day 15 for a total of $300 for study completion.
Baseline (day 1)
Following informed consent, participants completed questionnaires that assessed demographics, medical and drug use history, as well as dependence on cigarettes (Fagerström Test for Cigarette Dependence; FTCD)31 and SLT (Severson Smokeless Tobacco Dependency Scale).32 FTCD scores range from 0 to 10: 0–2 (very low dependence), 3—4 (low dependence), 5 (medium dependence), 6–7 (high dependence) and 8–10 (very high dependence). Severson Smokeless Tobacco Dependency Scale scores range from 0 to 19, with higher scores indicating greater levels of dependence. Participants also provided urine and breath samples for verification of tobacco use status, and females were tested for pregnancy. Those deemed eligible were then trained on all study procedures outlined below, and left the laboratory with relevant supplies and instruction materials.
Assessment period (days 1–15)
For 14 consecutive days, participants used their own brand of cigarette and SLT products ad libitum. They also engaged daily with an EMA monitoring device by logging all cigarettes and/or SLT uses immediately before the product was used. For a randomly selected portion of these logged products, participants were further prompted to complete questions that addressed mood, withdrawal symptoms and situational factors. Items related to mood (eg, sad, happy, enthusiastic, bored, calm/relaxed) and withdrawal (eg, craving, difficulty concentrating, irritable) were measured using a visual analogue scale that ranged from 0 (not at all) to 100 (extremely). Situational factors were measured via multiple choice or yes/no items. Situational factors included location (eg, home, other’s home, vehicle, workplace, outside, bar, restaurant, other), cigarette availability (ie, easily, with difficulty, no), smoking norms (ie, allowed, discouraged, forbidden), social context (eg, with others, others smoking, and so on) and activities (eg, working, inactive/leisure, eating/drinking, between activities, other). The number of prompts randomly selected for these additional questions was based on participants’ self-reported number of CPD at baseline in order to standardise the number of prompts across participants. Participants also answered these same questions in response to random prompts that occurred independent of product use. At the end of the day, participants again completed questionnaires (eg, withdrawal symptoms, mood), as well as tallied and logged any products used but not recorded in real time during the day. Similar sampling procedures have been outlined in extensive detail elsewhere.33,34 Participants also were required to collect the filters from all cigarettes smoked for storage in containers prelabelled for each day of the week, as well as to collect a saliva sample each night using kits provided to them.
Study visits (days 3, 9, 15)
At each visit, participants returned their spent cigarette filters, saliva samples and EMA device. The number of cigarette filters returned was counted and compared with the number of cigarettes logged via monitoring device. The devices were checked to evaluate compliance, with a minimum threshold of 80% responses to random prompts.34,35 If compliance was <80%, participants were counselled by staff and provided with additional device training.
During the final study visit (day 15), participants were asked to choose their reason(s) for initiating use of SLT and again for their current use of SLT from the following options: (1) to improve health; (2) to assist with quitting smoking; (3) to use in places where I can’t smoke; and (4) other. When ‘other’ was chosen, participants were asked to describe their other reason(s). In addition, participants were asked to report their beliefs about SLT21: (1) snuff/dip/chew products are (less/more/same/don’t know) harmful than cigarettes; (2) snus products are (less/more/same/don’t know) harmful than cigarettes; (3) snuff/dip/chew products (help/do not help/don’t know) smokers quit cigarettes; (4) snus products (help/do not help/don’t know) smokers quit cigarettes; (5) I use smokeless tobacco when I cannot smoke: (true/false/not sure).
Materials
EMA device
The mobile devices used were BLU Dash 5.0 (BLU Products, Doral, FL) with an Android operating system. Each device was preloaded with software customised specifically for this study (https://www.utas.edu.au/health/research/groups/school-of-medicine/behavioural-and-situational-research-group-bsrg/hbart). Participants were not able to access features of the device other than the software used for data collection.
Saliva samples
SalivaBio Oral Swabs (Salimetrics, State College, PA) were used for the collection of passive drool samples. Abstinence from food and drink was required for 1 hour prior to sample collection. During collection, participants were instructed to rinse their mouth with water, wait for 10 min and then place the cotton swab in their mouth for 2 min. The swab was then placed into a plastic vial and stored in the participants’ freezer until their next scheduled in-person visit. These instructions were also provided via the monitoring device, which allowed for recording the completion of this task. Once returned to the lab, saliva samples were stored at −80°C until assayed. Cotinine levels were determined by liquid chromatography with tandem mass spectrometry using extraction and processing methods described by Cappendijk and colleagues.36 The limit of quantification was 1 ng/mL.
Data analysis
Primary outcomes
EMA records were used to categorise study days as ‘dual use’ (days when both cigarettes and SET were logged) or ‘single use’ (days when only cigarettes were logged, there was only 1 day in which only SLT was logged). CPD and cotinine levels were averaged across days for each participant and compared between dual and single use days via dependent samples t-tests (all p<0.05).
Secondary outcomes
A secondary aim was to determine whether cigarettes and SLT were being used under different circumstances (eg, location, mood). A subset of participants provided a large enough sample (n=13 for 733 assessments combined) to calculate the area under the curve for the receiver operating characteristic curve (ALTC-ROC). An ALTC-ROC value was generated for each participant, then weighted by the inverse of the SE and averaged across participants to create one ALTC-ROC value. AUC-ROC values range from 0.5 (no discrimination between products) to 1.0 (complete discrimination) and describe the probability of accurately identifying which product was used based on the situational factor. These values were calculated for the following items: location (home; work; bar/restaurant/other; vehicle; or outside); smoking restrictions (forbidden; discouraged; or allowed); craving; and affect. Affect was derived from a factor analysis of 14 mood items followed by a Varimax rotation. Data were determined to be suitable for factor analysis based on a significant Bartlett test (X2(91) = 4521.3, p<0.001), and an overall Kaiser-Meyer-Olkin value of 0.91. All factor loadings were 0.4 and larger (range = 0.4–0.9). Affect consisted of seven of the 14 items: ‘irritable’, ‘angry/frustrated’, ‘calm/relaxed’, ‘happy’, ‘miserable’, ‘bored’ and ‘enthusiastic’. Following calculation of ALTC/ROC values, weighted t-tests were performed to determine whether the obtained values were significantly larger than 0.5 (ie, chance). Analyses were performed using R statistical software (http://www.r-project.org/) in the ROCR, pROC, psych, weights and GRArotation libraries and outcomes were considered statistically significant when p<0.05. Another secondary aim was to describe the reasons for and beliefs about SLT use among our sample. For these questions, the proportion of participants who endorsed each response option is provided.
RESULTS
Missing data
Excluded from all analyses were individuals who failed to respond to prompts or log their product use (n=7), or to return saliva samples (n = 3) entirely. For the remaining completers, data were missing on 3.4% of study days for logs of product use and on 2.8% of study days for cotinine levels. For situational and mood items, an inconsistency between logging and the program algorithm resulted in missing data for a notable number of participants (n=33). The remaining 13 participants provided a total of 733 combined assessments for cigarette and SLT logs.
Participants
Of 69 participants who consented to participate, 56 (81.2%) were enrolled and 46 (68.7%) completed the study. Seventy per cent of non-completers failed to respond to random prompts or to log their used products via EMA device. Non-completers did not differ from completers on any baseline demographic characteristic. Table 1 outlines these characteristics for those who completed the study.
Table 1.
Participants’ baseline characteristics
| M (SD) or % | |
|---|---|
| Male | 93.5% |
| Non-Hispanic, Caucasian | 97.8% |
| Age (years) | 30.39 (8.99) |
| Cigarettes per day | 19.50 (8.63) |
| Years smoking | 10.74 (6.47) |
| Expired air CO (ppm) | 25.52 (14.63) |
| FTCD score* | 6.02 (2.50) |
| SLT products | |
| Snuff/dip/chew | 80.4% |
| Snus | 6.5% |
| Multiple | 13.0% |
| Wintergreen/mint | 65.2% |
| SLT uses/day | 4.27 (2.21) |
| SLT days/week | 5.74 (1.54) |
| Years SLT use | 9.32 (7.94) |
| SSTDS score† | 8.30 (3.75) |
Primary outcomes
EMA-based logs of SLT use
SLT use was logged on 7 days/week for 35.6%, 5–6 days/week for 53.3% and 3–4 days/week for 11.1% of participants. Daily SLT users logged an average of 3.38 (SD = 1.61) dips/day, while non-daily users reported using an average of 2.42 (SD = 1.44) dips/day. Of the 25 participants who reported daily use at baseline, 72.0% logged SLT on 13–14 study days and 28.0% logged SLT on 10–12 study days. The mode was 2 dips/day for daily users and 1 dip/day for non-daily users. One-third of participants logged an average of 2 or fewer dips/day, 51.1% logged 2–4 dips/day and 15.6% logged 4 or more dips/day. The mean dips/week was 17.22 (SD = 11.03).
Dual versus single use days
Both cigarettes and SLT products (ie, dual use) were logged on 85.0% of study days. As for single use, only cigarettes were logged on 13.7% of study days and only SLT was logged on 0.2% of study days. Logged cigarettes differed significantly between single (M = 9.13, SEM = 1.11) and dual use days (M = 11.13, SEM=0.98), t(25)= −3.25, p = 0.00 (see figure 1). Cotinine levels also differed significantly between these days: 300.17ng/mL (SEM=28.13) for single use versus 374.49 ng/mL (SEM=41.09) for dual use, t(23)= −2.95, p = 0.01 (see figure 2). These same analyses were run after removal of outliers (eg, participants with only one single use day). For this analysis, logged cigarettes did not differ significantly between single (M = 11.26, SEM = 1.27) and dual use days (M = 12.49, SEM = 1.31), t(16) = −1.69, p = 0.11. Cotinine levels remained significantly different between days: 322.75 ng/mL (SEM=31.36) for single use versus 401.53 ng/mL (SEM=52.08) for dual use, t(16) = −2.43, p=0.03.
Figure 1.
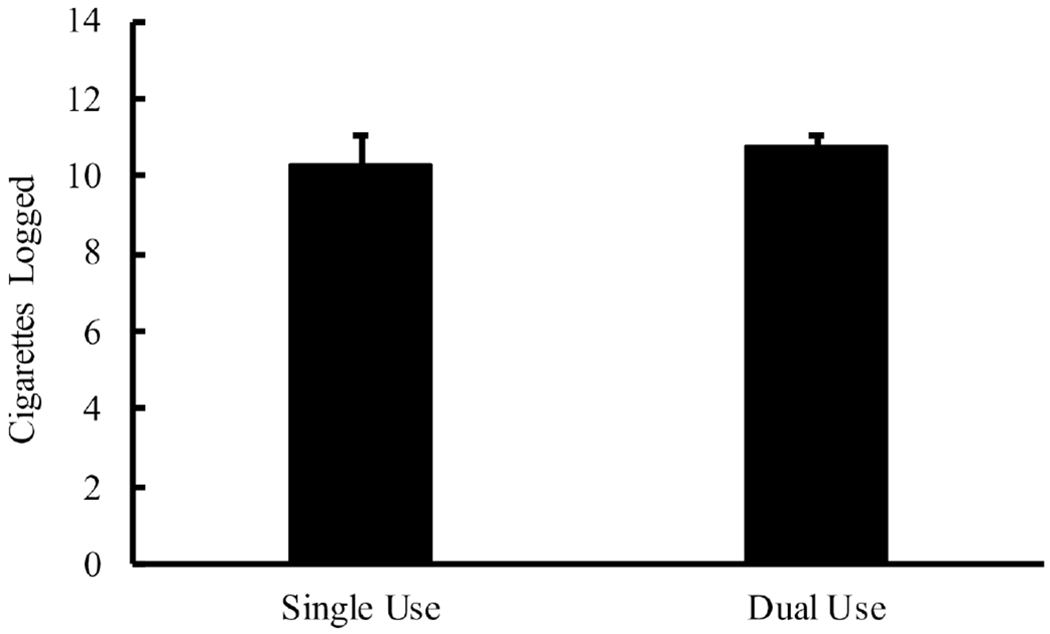
Mean (SEM) number of cigarettes logged between single use (cigarette only) and dual use (cigarette+smokeless tobacco (SLT)) days, which were significantly different at p<0.05.
Figure 2.
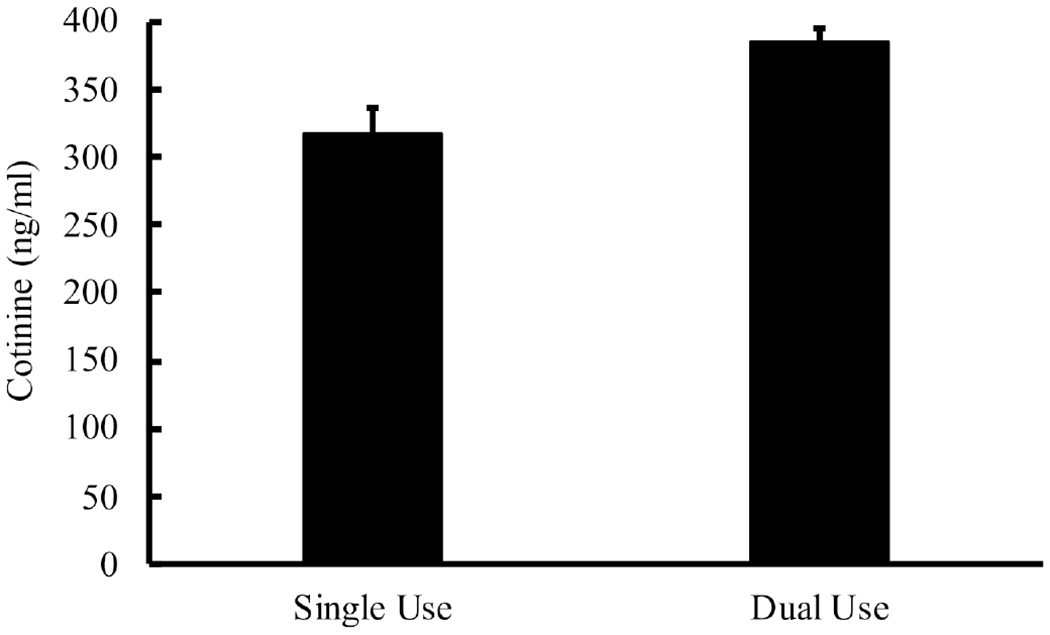
Mean (SEM) cotinine levels between single use (cigarette only) and dual use (cigarette+smokeless tobacco (SLT)) days, which were significantly different at p<0.05.
Secondary outcomes
Stimulus control of product use
Figure 3 shows AUC-ROC values for situational factors, all of which were statistically significant (all p’s<0.01). Cigarette use was logged most often at home (53.8%), outside (18.1%), in a vehicle (11.0%) and at work (7.7%). SLT use was logged most often at home (59.2%), at work (11.5%), outside (10.6%) and in a vehicle (9.9%). Cigarette use was logged 92.0% of the time in smoking-permitted locations and 8.0% of the time in smoking-discouraged/forbidden locations, respectively. SLT use was logged 82.4% vs 17.6% of the time in these respective locations.
Figure 3.
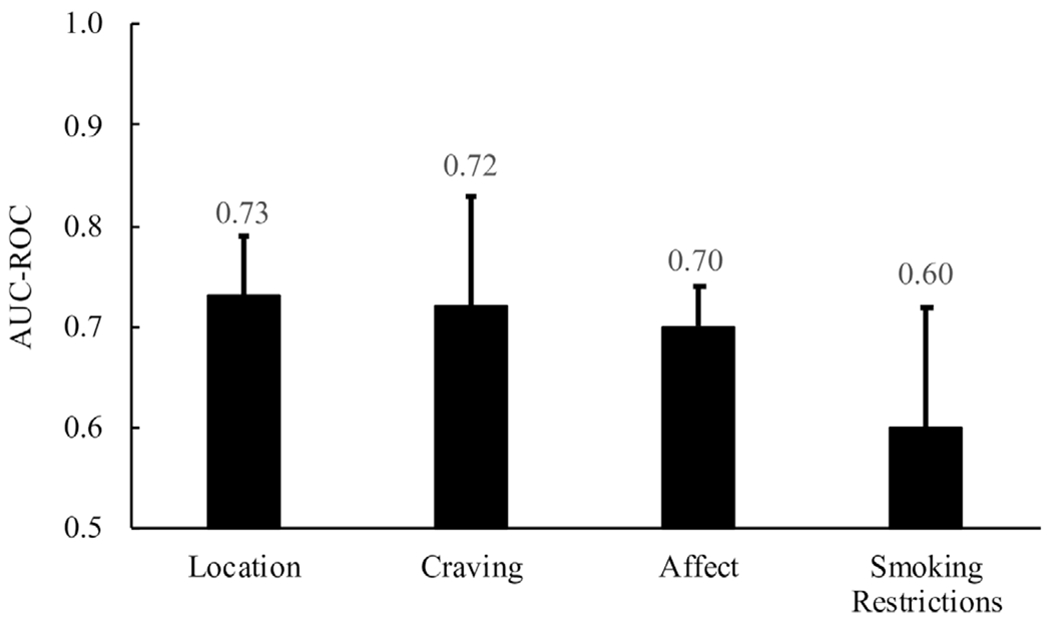
AUC-ROC (SEM) values across situational domains(all p’s<0.01). AUC-ROC, area under the curve for the receiver operating characteristic curve.
SLT reasons/beliefs
Nearly all participants (97.8%) reported that they use SLT on at least some occasions when they cannot smoke cigarettes. Using SLT for this same purpose was the most commonly reported reason for initiating SLT use (56.5%), as well as for continuing to use SLT today (67.4%). Other reasons reported were to aide smoking cessation (10.9% initiation; 10.9% continuation), to improve health (4.4% initiation; 0.0% continuation), other (eg, 13.0% for both initiation and continuation) and more than one of these reasons (15.2% initiation; 8.8% continuation). For the option of ‘other’, reasons given mentioned social use, convenience and relaxation.
When asked whether traditional SLT use is as harmful as cigarette smoking, 63.0% reported ‘same’, 17.4% reported ‘less’, 13.0% reported ‘more’ and 6.5% reported ‘don’t know’. When this same question was asked for the moist product snus, 45.7% reported ‘same’, 19.6% reported ‘less’, 10.9% reported ‘more’ and 23.9% reported ‘don’t know’. Participants also were asked whether they believe that SLT helps with quitting cigarettes, with answers being ‘does help’ (39.1% for traditional; 23.9% for snus), ‘does not help’ (47.8% for traditional; 50.0% for snus) and ‘don’t know’ (13.0% for traditional; 26.1% for snus).
DISCUSSION
This study is the first to use a prospective longitudinal design to characterise patterns of product use and nicotine exposure among a convenience sample of dual cigarette-SLT users. For this sample, the average number of cigarettes smoked per day was higher on days when cigarettes were used concurrently with SLT than versus exclusively. Consequently, cotinine levels were higher on days when SLT was also used. Moreover, the type of product used was associated with situational factors, with the strongest situational predictor being location of use. Together, results do not support the idea of product replacement for this sample of dual users.
The dual users sampled here reported their use of SLT primarily for circumvention of indoor smoking restrictions (similar to McClave-Regan and Berkowitz).21 Indeed, those enrolled were not actively trying to quit cigarettes, and very few reported that they initiated and/or continued SLT use as a cessation or harm reduction method. Additionally, about half reported that they do not believe that traditional SLT or snus assists with quitting (consistent with McClave-Regan and Berkowitz).21 For smokers whose sole motivation for SLT use involves situations where they would otherwise be tobacco free, the potential benefits of clean indoor air laws may be diminished. Relative to exclusive cigarette or SLT use, dual use has been associated with more negative outcomes: increased risk of nicotine dependence,5,30 decreased likelihood of successful quit attempts26,30 and higher rates of serious medical conditions.37,38 Even for smokers who use SLT specifically for harm reduction or cessation, the benefits remain unknown. Some work shows that use of SLT as a cessation aid is more likely among dual cigarette-SLT users than cigarette-only smokers.39 There also exists little evidence that, in the USA, dual cigarette-SLT users switch to exclusive SLT use.40,41 In extant randomised controlled trials, poor long-term smoking cessation rates have been observed for snus relative to controls ,42,43 Importantly, smokers may find SLT products less appealing than cigarettes because of unacceptable sensory characteristics, minimal withdrawal relief and/or poor nicotine delivery capabilities.44–46
Notably, the large majority of these dual users stated that both traditional SLT and snus are as or more harmful than are cigarettes. This finding is consistent with other work with either cigarette smokers or SLT users.21,25,47,48 Arguments have been made for the evaluation of dual use patterns within the context of SLT harm perceptions.25,48,49 That is, perhaps smokers would be more likely to replace rather than supplement cigarettes with SLT if they were told, and believed, that doing so would significantly reduce their risks of tobacco-related disease.49 Still others suggest that such a promotion of SLT products would fail to result in health benefits at the population level.50
Given the observational nature of our study, we are unable to answer many of these questions that surround the dual use debate. Another study limitation includes the generalisability of results given our convenience sample. Those enrolled were overwhelmingly Caucasian males, and recruited from relatively rural geographic locations; however, this demographic reflects what has been reported repeatedly in the literature.7–10 Results also may not generalise to those with a different pattern of dual use (eg, daily SLT users who are non-daily smokers). For the assessment of SLT consumption, measures are crude relative to those for cigarettes. The size/weight and duration of a single bout of SLT use (eg, one ‘pinch’ of chew) were not considered, factors which likely affect nicotine exposure.
Additional work is needed to replicate these findings, and should consider examining patterns of use as a function of SLT use reasons and beliefs. That is, dual use patterns may differ by participants’ motivation for using SLT, as well as their beliefs about such products in terms of harm reduction. Characterising such differences in the patterns of dual use is important for understanding user toxicant exposure and subsequent health risks. Indeed, regulators would benefit from a better understanding of the context of SLT use among smokers as they make decisions about how these products are marketed. These same ideas might be applied to smokers’ use of other products, such as electronic cigarettes, which are used at a much higher rate than SLT products.5,6 Major LJS cigarette manufacturers are now entering the electronic cigarette market,51 as they have done in the past for the SLT market. They also may be employing the same marketing strategy as that used for SLT, with some advertisements positioning electronic cigarettes as an alternative product for use in smoking-restricted situations.52
What this paper adds.
Previous work about dual use of cigarettes and smokeless tobacco (SLT) is primarily based on retrospective reports and between-group comparisons and little is known about patterns of use in this population.
This is the first study to assess prospectively the patterns of product use and nicotine exposure for dual users on days when cigarettes were smoked exclusively (single use) versus concurrently with SLT (dual use).
Relative to single use days, dual use days revealed a larger number of cigarettes smoked and higher levels of cotinine.
The patterns of dual use among these samples do not support the idea of product replacement.
Acknowledgments
Funding Financial support was provided by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the US Food and Drug Administration (R03DA037583; PI MDB, consultant SGF). Additional support was provided by the National Institute of General Medical Sciences (T32GM081741; NJF and JEOH), and the US Centers for Disease Control and Prevention (U481006466AR; PI GD, Co-I MDB) to the West Virginia Prevention Research Center.
Footnotes
NOTICE WARNING CONCERNING COPYRIGHT RESTRICTIONS
The copyright law of the United States [Title 17, United States Code] governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the reproduction is not to be used for any purpose other than private study, scholarship, or research. If a user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use,” that use may be liable for copyright infringement. This institution reserves the right to refuse to accept a copying order if, in its judgement, fulfillment of the order would involve violation of copyright law. No further reproduction and distribution of this copy is permitted by transmission or any other means.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH, the FDA or the CDC.
Competing interests SGF has consulted for various pharmaceutical companies on matters relating to smoking cessation. All other authors declare no conflicts of interest.
Patient consent for publication Not reguired.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable reguest.
REFERENCES
- 1.Wang TW, Asman K, Gentzke AS, et al. Tobacco Product Use Among Adults - United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67:1225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delnevo CD, Wackowski OA, Giovenco DP, et al. Examining market trends in the United States smokeless tobacco use: 2005-2011. Tob Control 2014;23:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipari RN, Van Horn SL. Trends in smokeless tobacco use and initiation: 2002 to 2014 The CBHSQ Report; 2017. [PubMed] [Google Scholar]
- 4.Lee YO, Hebert CJ, Nonnemaker JM, et al. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med 2014;62:14–19. [DOI] [PubMed] [Google Scholar]
- 5.Sung H-Y, Wang Y, Yao T, et al. Polytobacco use and nicotine dependence symptoms among US adults, 2012–2014. Nic Tob Res 2018;20:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-Product use by adults and youths in the United States in 2013 and 2014. N Engl J Med 2017;376:342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasky TM, Hinton A, Doogan NJ, et al. Characteristics of the tobacco user adult cohort in urban and rural Ohio, tobacco reg sci 2018;4:614–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaffee BW, Cheng J. Cigarette and smokeless tobacco perception differences of rural male youth. Tob Regul Sci 2018;4:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y-C, Rostron BL, Day HR, et al. Patterns of use of smokeless tobacco in US adults, 2013–2014.Ami Public Health 2017;107:1508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts ME, Doogan NJ, Stanton CA, et al. Rural versus urban use of traditional and emerging tobacco products in the United States, 2013–2014. Am J Public Health 2017;107:1554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SS H, Homa DM, Wang T, et al. State-Specific patterns of cigarette smoking, smokeless tobacco use, and e-cigarette use among adults - United States, 2016. Prev Chronic Dis 2019; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen K, Marshall L, Hu S, et al. State-specific prevalence of current cigarette smoking and smokeless tobacco use among adults aged ≥18 years - United States, 2011-2013. MMWR Morb Mortal Wkly Rep 2015;64:532–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Tynan MA, Holmes CB, Promoff G, et al. State and local comprehensive smoke-free laws for Worksites, restaurants, and bars - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:623–6. [DOI] [PubMed] [Google Scholar]
- 14.Hyland A, Barnoya J, Corral JE. Smoke-Free air policies: past, present and future. Tob Control 2012;21:154–61. [DOI] [PubMed] [Google Scholar]
- 15.Wang TW, Tynan MA, Hallett C, et al. Smoke-Free and tobacco-free policies in colleges and universities — United States and territories, 2017. MMWR Morb Mortal Wkly Rep 2018;67:686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder K, Vick JH, King BA. Smoke-Free multiunit housing: a review of the scientific literature. Tob Control 2016;25:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter CM, Connolly GN, Ayo-Yusuf OA, et al. Developing smokeless tobacco products for smokers: an examination of tobacco industry documents. Tob Control 2009;18:54–9. [DOI] [PubMed] [Google Scholar]
- 18.Hendlin YH, Elias J, Ling PM. The Pharmaceuticalization of the tobacco industry. Ann Intern Med 2017;167:278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timberlake DS, Pechmann C, Tran SY, et al. A content analysis of camel Snus advertisements in print media. Nicotine Tob Res 2011;13:431–9. [DOI] [PubMed] [Google Scholar]
- 20.Campbell BK, Le T, Gubner NR, et al. Health risk perceptions and reasons for use of tobacco products among clients in addictions treatment. Addict Behav 2019;91:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClave-Regan AK, Berkowitz J. Smokers who are also using smokeless tobacco products in the US: a national assessment of characteristics, behaviours and beliefs of ‘dual users’. Tob Control 2011;20:239–42. [DOI] [PubMed] [Google Scholar]
- 22.Mejia AB, Ling PM. Tobacco industry consumer research on smokeless tobacco users and product development. Am J Public Health 2010;100:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepanov I, Jensen J, Hatsukami D, et al. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob Res 2008;10:1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feirman SP, Donaldson EA, Parascandola M, et al. Monitoring harm perceptions of smokeless tobacco products among U.S. adults: health information national trends survey 2012, 2014, 2015. Addict Behav 2018;77:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wackowski OA, Ray AE, Stapleton JL. Smokers’ perceptions of risks and harm from snus relative to cigarettes: a latent profile analysis study. Addict Behav 2019;91:171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messer K, Vijayaraghavan M, White MM, et al. Cigarette smoking cessation attempts among current US smokers who also use smokeless tobacco. Addict Behav 2015;51:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodu B, Phillips CV. Switching to smokeless tobacco as a smoking cessation method: evidence from the 2000 National health interview survey. Harm Reduct J 2008;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodu B, Cole P. Smokeless tobacco use among men in the United States, 2000 and 2005. J Oral Pathol Med 2009;38:545–50. [DOI] [PubMed] [Google Scholar]
- 29.Tomar SL. Snuff use and smoking in U.S. men: implications for harm reduction. Am J Prev Med 2002;23:143–9. [DOI] [PubMed] [Google Scholar]
- 30.Tomar SL, Alpert HR, Connolly GN. Patterns of dual use of cigarettes and smokeless tobacco among US males: findings from national surveys. Tob Control 2010;19:104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Addiction 1991;86:1119–27. [DOI] [PubMed] [Google Scholar]
- 32.Severson HH, Akers L, Andrews JA. Development of a smokeless tobacco dependence scale. Paper presented at the world conference on tobacco or health Helsinki, Finland; 2003. [Google Scholar]
- 33.Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol 2006;74:1153–61. [DOI] [PubMed] [Google Scholar]
- 34.Shiffman S, Dunbar MS, Li X, et al. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One 2014;9:e89911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiffman S, Dunbar MS, Ferguson SG. Stimulus control in intermittent and daily smokers. Psychol Addict Behav 2015;29:847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappendijk SLT, Pirvan DF, Miller GL, et al. In vivo nicotine exposure in the zebra finch: a promising innovative animal model to use in neurodegenerative disorders related research. Pharmacol Biochem Behav 2010;96:152–9. [DOI] [PubMed] [Google Scholar]
- 37.Chao A, Thun MJ, Henley SJ, et al. Cigarette smoking, use of other tobacco products and stomach cancer mortality in US adults: the cancer prevention study II. Int J Cancer 2002;101:380–9. [DOI] [PubMed] [Google Scholar]
- 38.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. The Lancet 2006;368:647–58. [DOI] [PubMed] [Google Scholar]
- 39.Kalkhoran S, Grana RA, Neilands TB, et al. Dual use of smokeless tobacco or e-cigarettes with cigarettes and cessation. Am J Health Behav 2015;39:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macy JT, Li J, Xun P, Li P, Xun CC, et al. Dual trajectories of cigarette smoking and smokeless tobacco use from adolescence to midlife among males in a midwestern US community sample. NICTOB 2016;18:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam J, Day HR, Rostron BL, et al. A systematic review of transitions between cigarette and smokeless tobacco product use in the United States. BMC Public Health 2015;15:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagerstrom K, Rutqvist LE, Hughes JR. Snus as a smoking cessation aid: a randomized placebo-controlled trial. Nicotine Tob Res 2012;14:306–12. [DOI] [PubMed] [Google Scholar]
- 43.Hatsukami DK, Severson H, Anderson A, et al. Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching. Tob Control 2016;25:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blank MD, Eissenberg T. Commentary on Brose et al. . (2015): Protecting individual and public health by regulating electronic cigarette nicotine delivery. Addiction 2015;110:1169–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meier E, Burris JL, Wahlquist A, et al. Perceptions of snus among US adult smokers given free product. Nicotine Tob Res 2017;20:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wackowski OA, Lewis MJ, Delnevo CD. Interviews with smokers about smokeless tobacco products, risk messages and news articles: Table 1. Tob Control 2016;25:671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi K, Bestrashniy J, Forster J. Trends in awareness, use of, and beliefs about electronic cigarette and Snus among a longitudinal cohort of US Midwest young adults. Nic Tob Res 2018;20:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control 2017;26:e49–58. [DOI] [PubMed] [Google Scholar]
- 49.Kozlowski LT, Sweanor DT. Young or adult users of multiple tobacco/nicotine products urgently need to be informed of meaningful differences in product risks. Addict Behav 2018;76:376–81. [DOI] [PubMed] [Google Scholar]
- 50.Mejia AB, Ling PM, Glantz SA. Quantifying the effects of promoting smokeless tobacco as a harm reduction strategy in the USA. Tob Control 2010;19:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levy DT, Sweanor D, Sanchez-Romero LM, et al. Altria-Juul Labs deal: why did it occur and what does it mean for the US nicotine delivery product market. Tob Control 2019:tobaccocontrol-2019-055081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy DT, Chaloupka F, Lindblom EN, et al. The US cigarette industry: an economic and marketing perspective. tob regul sci 2019;5:156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


