Abstract
Due to improvements in the number of cancer survivors and survival time, there is a growing interest in healthy behaviors, such as physical activity (PA), and their potential impact on cancer- and non-cancer-related morbidity in individuals with cancer. Commissioned by the Spanish Society of Medical Oncology (SEOM), in this review, we sought to distill the most recent evidence on this topic, focusing on the mechanisms that underpin the effects of PA on cancer, the role of PA in cancer prevention and in the prognosis of cancer and practical recommendations for clinicians regarding PA counseling. Despite the available information, the introduction of exercise programs into the global management of cancer patients remains a challenge with several areas of uncertainty. Among others, the most effective behavioral interventions to achieve long-term changes in a patient’s lifestyle and the optimal intensity and duration of PA should be defined with more precision in future studies.
Keywords: Oncology, Cancer, Exercise, Physical activity, Exercise-oncology
Introduction
Regular and adequate physical activity (PA) is associated with key benefits to human health, such as improvements in weight control, muscular and cardiorespiratory fitness, bone and functional health and a reduced risk of falls and several noncommunicable diseases, including diabetes, cardiovascular disease, depression and some cancers [1].
Due to improvements in the management of cancer, the number of cancer survivors and survival time are increasing. Consequently, interest in healthy behaviors, such as PA, and their potential impact on cancer- and non-cancer-related morbidity in these individuals has rapidly increased [2].
Commissioned by the Spanish Society of Medical Oncology (SEOM), in this review, we sought to distill the most recent evidence on this topic, focusing on the mechanisms that underpin the effects of PA on cancer, describing the role of PA in cancer prevention and prognosis, and providing practical recommendations to clinicians on managing PA counseling.
Biological mechanisms underpinning the potential anticancer effects of exercise
Of note, PA is any bodily movement produced by skeletal muscles that requires energy expenditure, whereas exercise is a subset of PA that is planned, structured and repetitive and that has a final or an intermediate objective of improving or maintaining physical fitness. The epidemiological evidence regarding the risk of some cancers mainly refers to regular PA (usually self-reported, i.e., through questionnaires). Thus, regular exercise or “exercise training” is a proxy, but not a perfect surrogate, for PA and is thought to induce more profound molecular adaptations than PA.
There is growing evidence from preclinical research that regular exercise can influence cancer development or the rate of tumor growth once malignancy has initiated. For instance, a recent meta-analysis of 28 preclinical studies in breast tumors (n = 2085 animals) found large favorable effects for exercise training on proliferation and apoptosis [3]. Exercise is also emerging as a potential coadjuvant treatment; when combined with cyclophosphamide, exercise delays murine breast tumor growth versus chemotherapy alone [4], and similar findings have been reported for exercise combined with anthracyclines [5–7]. There is, however, heterogeneity among studies in the tumor models used, ranging from tumor transplant (where there is also substantial variability, e.g., syngeneic versus xenograft models) to carcinogen-induced or genetically engineered mouse models [8]. The type of exercise training to which mice are typically subjected to before/upon tumor inoculation also varies between studies (forced treadmill, forced swimming, voluntary wheel running). The duration of exercise in the trials typically consists of several weeks (~ 4 to 10 weeks), which can be translated to human “years.”
Exercise can have an impact on tumor development, growth or dissemination through several mechanisms. First, exercise might help to prevent cancer by reducing the circulating levels of several mediators, such as insulin growth factor-1 (IGF-1) [9–14], a mitogen that triggers cell proliferation [15]. Exercise can also reduce the levels of hyperphosphorylated retinoblastoma protein (Rb) in a chemically induced rat model of mammary carcinogenesis [16, 17], increase ß-catenin phosphorylation in colon polyps [18, 19], and reduce the levels of micro-RNA 21 [20].
Exercise can upregulate tumor suppressors, such as the tumor suppressor programmed cell death protein 4 in a murine model of estrogen receptor-positive breast cancer (BC) [21]. In addition, exercise-induced catecholamines might reduce BC development through activation of the Hippo tumor suppressor pathway [22] and exercise-induced increased p53 activation, leading to tumor prevention, as shown in mouse models of skin [13, 23] and lung [24] cancer.
Exercise training can stimulate apoptosis, as shown in xenograft models of lung adenocarcinoma [24] and human pancreatic and prostate cancers [25, 26] and in murine models of skin tumorigenesis [12] and mammary carcinoma [4, 16, 27]. Exercise also exerts proapoptotic effects on cultured prostate cancer cells [28], estrogen receptor-positive BC cells [29, 30] and lymph node metastases of prostate tumor cells [25]. Exercise additionally reduces the levels of the antiapoptotic protein B cell lymphoma 2 [16, 31] and stimulates the proapoptotic proteins Bax and Bak [4, 16, 24] and the protein kinase AMPK [32, 33].
Hypoxia and poor blood supply promote an aggressive cancer phenotype and contribute to ineffective systemic therapy [34]. In this respect, exercise may promote a shift toward a more “normalized” tumor microenvironment by improving intratumoral perfusion/vascularization, at least in orthotopic murine models of human BC [4, 35] and prostate cancer [36–38] and in xenografts of different tumors (melanoma, pancreas) [39].
Exercise might also attenuate the development of metastases. Mouse exercise training can decrease catenin while increasing E-cadherin inside tumors [19, 40]. Importantly, cadherins act as glue between epithelial cells, and their loss can favor malignancy by allowing the disaggregation of cells, which can then invade locally or metastasize [40]. Moderate-intensity mouse swimming can suppress liver cancer metastases via boosting the activity of dopamine receptor 2 [40]. Exercise may also modulate blood–brain barrier integrity by maintaining the expression levels of occludin or claudin-5 proteins [41], thereby preventing metastatic progression to the brain [42]. On the other hand, inflammatory cells within the tumor microenvironment supply bioactive molecules that sustain cancer hallmarks [43–45]. In this context, mouse exercise training decreases macrophage infiltration in allogeneic lymphoid tumors [46], Ehrlich tumor cells [47] and colon polyps [18].
One major potential “anticancer” effect of exercise lies in an enhancement of immune function [43, 48]. At moderate intensities, exercise can stimulate the innate immune system, especially natural killer (NK) cells [49, 50]. A 6-week mouse wheel running program had preventive effects against the development of several types of tumors (melanoma, liver and lung cancers), and the effect was mediated by improved NK cell infiltration into the tumors, which in turn was mediated by the enhanced tumor expression of ligands for several NK cell-activating receptors [51]. A previous study showed that exercise training increased the cytolytic capacity of resident peritoneal macrophages against mastocytoma cells [52]. Mouse exercise training could also polarize the immunological response toward an efficient “antitumor” macrophage profile 1, which is linked to the production of T-helper 1 cytokines [52–56]. Short-term (6-day) moderate exercise before the injection of melanoma cells into mice decreased their metastatic spread, which was partially mediated by increased antitumor macrophage cytotoxicity [57]. Preliminary data from mice [53, 58–60] and cancer patients suggest that exercise training may help to reduce the immunosuppressive effects of T regulatory lymphocytes [61]. Finally, regular exercise can increase alveolar macrophage antitumor cytotoxicity in vitro, which would mediate a protective effect against mouse lung metastases [62, 63].
Importantly, skeletal muscle, especially during contractions, releases molecules collectively known as “myokines” into the bloodstream, which act systemically to induce a myriad of health-promoting effects, such as decreased inflammation and reduced insulin resistance [64]. Some myokines might also induce direct anticancer effects (via the stimulation of apoptosis in tumor cells), such as oncostatin M in hormone-sensitive BC [30] or secreted protein acidic and rich in cysteine (SPARC, also known as osteonectin) in colon cancer [65]. The aforementioned exercise-induced infiltration of NK cells into tumors seems to be mediated by the release of interleukin 6 by muscle into the bloodstream [49, 51, 66].
Physical activity and cancer prevention
According to the World Health Organization (WHO), up to 31% of the adult population worldwide and 35% in Europe are physically inactive [67]. PA is difficult to measure for the following reasons: (1) there are at least four domains: occupational, household, transportation and leisure time; (2) PA questionnaires on past and current activity are subject to recall bias; and (3) objective methods (pedometers or accelerometers) can only be used in prospective studies for short time periods, and they may not always represent overall PA. Fortunately, smartphones and other devices now allow PA monitoring and will hopefully provide more accurate measures in the future.
Body mass is related to PA and cancer risk, acting as a confounder. However, the prevention of adiposity may mediate the relationship between PA and cancer, and controlling for adiposity could lead to underestimating the real effect of PA [68].
Of note, 1 metabolic equivalent (MET) is the rate of energy expenditure while resting or 3.5 ml O2/kg body weight/min on average. Moderate PA (e.g., brisk walking) usually requires an energy expenditure of 3–6 MET, whereas vigorous PA (e.g., jogging) requires an energy expenditure above 6 MET. The WHO recommends that adults engage in ≥ 150 min/week of moderate PA or ≥ 75 min/week of vigorous PA or a combination thereof. If a person does an ~ 3 MET activity (e.g., brisk walking on a level surface) for 1 h, he or she has done 3 MET-hours of PA. If this person does this same PA on every day of the week, he/she has done 21 (= 3 × 7) MET-hours/week. If a person does an ~ 8 MET activity (e.g., jogging) for 1 h on each day of the week, he/she has done 56 (= 8 × 7) MET-hours/week.
The World Cancer Research Fund and the American Institute for Cancer Research periodically publish the conclusions of a panel reviewing evidence linking food, nutrition and PA with cancer risk [69]. The evidence is classified as follows: (1) convincing: available results support a causal relationship; (2) probable: evidence supports a probable causal relationship; and (3) limited: results are not considered sufficient to rate the relationship as convincing or probable. In the last category, a distinction is made between limited-suggestive evidence when an effect is reported but there were methodological problems and limited-not conclusive evidence when there were insufficient data and/or the results were too heterogeneous. The panel concluded that regular, sustained PA protects against several types of cancer independent of body fat [69]. This evidence comes from high-income countries and is mainly based on leisure-time PA. The three tumors with the most solid results are colon, postmenopausal BC and endometrial.
Colorectal cancer
The evidence for colon cancer is judged as “convincing,” with an overall risk reduction of approximately 20% in the most physically active group compared with the less active group [70]. The effect is weaker or absent for rectal cancer. However, a pooled analysis of 12 prospective cohort studies with information on leisure-time PA at baseline compared the group at the 90th percentile of PA with the group under the 10th percentile and showed a reduced incidence of both colon (13% reduction) and rectal cancer (12%) after controlling for body mass index (BMI) [71]. Regarding the amount of PA required to obtain maximum benefit, a cohort of more than 40,000 men in the USA (The Health Professionals Follow-up Study) showed that aerobic PA seems to be more beneficial and that overall PA is more relevant than the intensity of PA [72]. Finally, while a benefit was observed in men meeting current guidelines (17% risk reduction), the maximum benefit (32% risk reduction) was observed for PA ≥ 30 metabolic equivalents (MET)-hours/week, which is equivalent to 10 h or more of walking/week [72]. A meta-analysis evaluating the dose–response shape of PA for different endpoints, including colon cancer, showed that major gains occurred at lower levels of activity (up to 50 MET-hours/week), while a decrease in risk was minimal at levels higher than 50–65 MET-hours/week [73].
Breast cancer
The evidence for postmenopausal BC is judged as “probable” [74]. Most studies show a protective effect with a 13% decreased risk in high versus low PA groups [74]. For recreational PA, a nonlinear dose–response was observed with a greater decrease in the risk for PA activity at > 20 MET-hours/week [74]. The pooling analysis with information on baseline leisure PA showed a reduction of 7% in the incidence of BC between the 90th and the 10th percentiles of PA [71]. Evidence for PA in premenopausal BC was rated as “limited-suggestive” for total PA and as “probable” for vigorous-intensity PA [74]. In Canada, a cohort study with 39,000 women reported a clear downward trend of BC incidence based on the number of MET-hours/week, which was mainly due to the risk reduction observed for premenopausal tumors [75]. Finally, a case–control study in Spain showed a reduced risk of 5% per 6 MET-hours/week [76]. The protection was particularly important for nulliparous women (12% risk reduction per 6 MET-hours/week) [76].
Endometrial cancer
The evidence for endometrial cancer was rated as “probable,” and the results showed a lower risk of endometrial cancer with higher levels of PA [77]. A meta-analysis reported a 20% risk reduction in high versus low PA groups [78]. This inverse association was only observed in overweight/obese women [78]. The pooled analysis of PA at baseline in 12 cohorts showed a risk reduction of 21% between the two extreme deciles of PA before taking BMI into account, while adjusting for BMI reduced the benefit to a nonsignificant risk reduction of 2% [71]. In the stratified analyses, PA was only associated with endometrial cancer in women with a BMI equal to or greater than 25 [71].
Lung cancer
A recent report classifies the evidence for lung cancer as “limited-suggestive” [69]. Leisure-time PA was considered in a systematic review, showing a clear inverse association with all histological lung cancer subtypes but only among former or current smokers [79]. The pooled analysis of cohort studies on leisure-time PA reported a 27% reduction in lung cancer incidence in the highest decile of PA compared with the lowest [71]. Again, the effect was only observed among smokers [71].
Liver cancer
The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) report classifies the evidence for liver cancer as “limited-suggestive” [80]. In the joint analysis of liver cancer incidence in 12 cohorts according to recreational PA at baseline, the highest decile had a hazard ratio (HR) of 0.73 before taking BMI into account and decreased to a nonsignificant HR of 0.81 when BMI was included as a confounder [71].
Esophageal cancer
There is limited but suggestive evidence of a protective effect of PA against esophageal adenocarcinoma and squamous cell carcinomas [81]. A meta-analysis found a risk ratio (RR) of 0.79 for esophageal adenocarcinoma and a nonsignificant RR of 0.94 for esophageal squamous cell carcinoma [82]. The pooled analyses of 1,44 million individuals from 12 cohorts showed an approximately 40% risk reduction in esophageal adenocarcinomas and a 24% risk reduction in esophageal squamous tumors for participants in the 90th percentile of leisure-time PA at baseline compared with the lowest PA group [71].
Stomach cancer
The recent WCRF/AICR update still considers limited-not conclusive evidence available for stomach cancer [83]. A previous meta-analysis on gastric cancer estimated an RR of 0.82 for high versus low PA [82]. The pooled analysis of leisure-time PA at baseline in the 12 cohorts showed a 22% risk reduction for gastric cardia tumors when BMI was not taken into account, but stratification by BMI showed that the protective effect was only observed among overweight/obese people [71].
Prostate cancer
There is a limited-not conclusive evidence of a link between PA and prostate cancer [84]. A systematic review showed substantial heterogeneity among 85 studies: 22 reported a statistically significant risk reduction, 25 reported a nonsignificant risk reduction, 31 did not find any association, and eight found an adverse effect of PA [85]. A higher incidence of prostate cancer (4% increase) was observed among the 10% more physically active participants in the pooled analysis of 12 cohort studies compared with those with a lower decile of activity. The authors hypothesized that this result could be due to a higher probability of prostate cancer screening in physically active men [71].
Ovarian cancer
The evidence for ovarian cancer was considered limited-not conclusive [86]. While most case–control studies found significant risk reductions among very active women, most cohort studies failed to show a clear effect [87]. The pooling analysis of 1.44 million participants in 12 prospective cohorts in the USA and Europe did not find a protective effect of high leisure-time PA for this tumor [71]. The Nurses’ Health Study, a prospective cohort with updated information on leisure-time PA, revealed an increased risk for both low and high levels of premenopausal PA, while no association was observed in postmenopausal women [88].
Pancreatic cancer
The WCRF/AICR report considers limited-not conclusive evidence for pancreatic cancer [89]. A meta-analysis yielded a statistically significant RR of 0.89 for high versus low PA [90]. Stronger effects were observed in case–control studies and for younger populations [90]. The pooling analysis of 12 cohort studies showed a non-statistically significant reduction of 5% in the most active group at baseline, but this effect was no longer observed when BMI was considered [71]. The EPIC-Norfolk cohort communicated a decreased risk in the highest category of total PA among participants younger than 60 years independent of BMI, while no effect was observed in older people [91].
Kidney cancer
The WCRF/AICR panel considers limited-not conclusive evidence for kidney cancer [92]; however, a meta-analysis in 2013 estimated a 12% risk reduction in the high PA group that was stronger when combining only high-quality studies [93]. The pooled analysis of the 12 cohorts showed a risk reduction of 16% independent of BMI among the most active group [71].
Bladder cancer
The evidence for bladder cancer is judged as limited-not conclusive [94]. A meta-analysis showed an RR of 0.85 for high versus low PA [95]. Moreover, the joint analysis of 12 prospective cohorts found a significantly reduced risk of bladder tumors in participants for the highest decile of leisure-time PA at baseline (HR = 0.88) [71].
Other tumors
A systematic review and meta-analysis on PA and hematologic cancers showed a reduced risk for non-Hodgkin lymphoma and nonsignificant results for multiple myeloma and leukemias [96]. The pooled analysis of 12 cohorts found a protective effect of PA against myeloid leukemia, myeloma and head–neck carcinomas [71]. Interestingly, malignant melanomas were more frequent in participants at the highest decile of leisure-time PA, a finding attributed to greater sun exposure due to outdoor activity and an increased risk of sunburn [71].
Summary and future directions
PA clearly reduces the risk of colon, BC and endometrial cancer. Furthermore, recent epidemiological studies suggest a protective effect for most cancer sites.
There is no conclusive evidence regarding the amount of PA needed to significantly reduce cancer risk, although it is likely tumor dependent.
New devices that routinely collect information on PA may help to increase the accuracy of PA measures and reduce information bias.
Effect of physical activity on the prognosis of cancer
Several reviews and meta-analyses of observational studies have suggested the benefit of PA on cancer outcomes. Most of the studies included breast cancer (Tables 1, 2) and colon cancer survivors (Table 3). A few studies have been conducted on patients with other types of neoplasms, such as prostate (Table 4), esophageal, lung and kidney cancer (Table 5). In these studies, PA is reported as lifetime PA in the latest years before or after diagnosis. The outcomes reported are usually overall survival, cancer-related survival, cancer recurrence and quality of life (QoL).
Table 1.
Meta-analysis of observational and interventional studies on the impact of exercise on breast cancer outcome
| References | Population | PA | Outcome | Results |
|---|---|---|---|---|
| Lahart et al. [97] |
123,574 BC survivors 1994–2014 Most studies observational |
Pre-diagnosis | All-cause mortality | HR 0.82 (95% CI 0.75–0.96) |
| BC mortality | HR 0.73 (95% CI 0.54–0.98) | |||
| BC events | HR 0.72 (95% CI 0.56–0.91) | |||
| After diagnosis | All-cause mortality | HR 0.52 (95% CI 0.43–0.64) | ||
| BC mortality | HR 0.59 (95% CI 0.45–0.78) | |||
| BC events | HR 0.79 (95% CI 0.63–0.98) | |||
| Lahart et al. [98] |
5761 BC survivors from 63 randomized trials PA intervention |
After diagnosis | All-cause mortality | No data |
| BC recurrence | No data | |||
| HRQoL, emotional function, perceived physical function, anxiety, and cardiorespiratory fitness | Small to moderate improvement |
BC, breast cancer; HRQoL, health-related quality of life; PA, physical activity
Table 2.
Summary of prospective observational studies on physical activity and prognosis in breast cancer patients
| Study/references | Population | LTPA | Outcome | Results |
|---|---|---|---|---|
|
Holmes et al. [99] Nurses’ Health Study |
2987 Nurses with stage I-III BC, 1984–1998 |
After diagnosis ≥ 9 MET-h/week |
BC-specific mortality | HR 0.50 (95% CI 0.34–0.74) |
|
Irwin et al. [100] HEAL Study |
933 Women with BC 1995–1998 |
Pre-diagnosis ≥ 9 MET-h/week |
Overall survival | HR 0.69 (95% CI 0.45–1.06) |
|
After diagnosis ≥ 9 MET-h/week |
Overall survival | HR 0.33 (95% CI 0.15–0.73) | ||
|
Bao et al. [101] Shanghai BCSS |
518 Women with TNBC |
After diagnosis ≥ 7.6 MET-h/week or ≥ 2.5 MET-h/week |
BC-specific mortality | HR 0.58 (95% CI 0.39–0.86) |
| BC recurrence | HR 0.67 (95% CI 0.46–0.96) | |||
|
Schmidt et al. [102] Germany |
3393 Women with early BC 50–74 year |
Pre-diagnosis ≥ 42 MET-h/week |
All-cause mortality | HR 0.66 (95% CI 0.47–0.92) |
| BC mortality | HR 0.80 (95% CI 0.53–1.21) | |||
| Cancer recurrence | HR 0.65 (95% CI 0.44–0.97) | |||
|
Holick et al. [103] Florida-Boston |
4482 Invasive BC 1998–2001 |
After diagnosis ≥ 21 MET-h/week |
BC mortality | HR 0.51 (95% CI 0.29–0.89) |
| All-cause mortality | HR 0.44 (95% CI 0.32–0.60) | |||
|
Ammitzboll et al. [104] Danish Diet, Cancer and Health Cohort |
959 BC survivors |
After diagnosis ≥ 8 MET-h/week |
All-cause mortality | HR 0.67 (95% CI 0.47–0.99) |
|
Friedenreich et al. [105] Canadian |
1233 BC survivors 1995–1997 |
Pre-diagnosis 46.9 MET-h/w |
BC mortality | HR 0.56 (95% CI 0.38–0.82) |
| BC recurrence | HR 0.66 (95% CI 0.48–0.91) | |||
|
Sternfeld et al. [106] LACE Study |
Multivariable 1970 BC survivors |
PA 6 months prior to diagnosis | BC mortality | No association confirmed |
| BC recurrence | No association confirmed | |||
| All-cause mortality | HR 0.66 (95% CI 0.42–1.03) | |||
|
Irwin et al. [107] Women’s Health Initiative |
4643 BC (in situ + invasive) |
Prior to diagnosis ≥ 9 MET-h/week |
All-cause mortality | HR 0.61 (95% CI 0.44–0.87) |
|
After diagnosis ≥ 9 MET-h/week |
BC mortality | HR 0.61 (95% CI 0.43–0.99) | ||
| All-cause mortality | HR 0.54 (95% CI 0.38–0.79) | |||
|
Bertram et al. [108] WHEL Study |
2361 Women with stage I-III BC | Baseline active | All-cause mortality | HR 0.47 (95% CI 0.26–0.84) |
| BC events | No effect | |||
| Adherence to activity guidelines after 1 year post-diagnosis | All-cause mortality | HR 0.65 (95% CI 0.47–0.91) | ||
| BC events | No effect | |||
|
Bradshaw et al. [109] Long Island BC Study |
1033 BC (in situ + invasive) 1995–1996 |
After diagnosis ≥ 9 MET-h/week |
All-cause mortality | HR 0.33 (95% CI 0.22–0.48) |
| BC mortality | HR 0.27 (95% CI 0.15–0.46) |
BC, breast cancer; LTPA, leisure-time physical activity; MET-h/week, metabolic equivalent task hours per week; TNBC: triple-negative breast cancer
BC events: BC progression, new primary BC, recurrence of BC
Table 3.
Summary of observational studies on physical activity and prognosis in colorectal cancer patients
| Study | Population | LTPA | Outcome | Results |
|---|---|---|---|---|
| Walter et al. [110] | 3121 CRC patients |
Latest LTPA ≥ 56 MET-h/week |
Overall mortality CRC mortality |
HR 0.75 (95% CI 0.61–0.91) HR 0.81 (95% CI 0.64–1.02) |
|
Arem et al. [111] AARP Diet and Health Study |
3797 CRC patients 1759 CRC patients |
Pre-diagnosis LTPA > 7 MET-h/week Post-diagnosis LTPA > 7 MET-h/week |
Overall mortality Overall mortality |
HR 0.80 (95% CI 0.68–0.95) HR 0.69 (95% CI 0.49–0.98) |
|
Meyerhardt et al. [112] CALGB 89803 |
832 Patients with stage III CRC |
Post-diagnosis LTPA > 18 MET-h/week |
Disease-free survival | HR 0.51 (95% CI 0.26–0.97) |
|
van Blarigan et al. [113] CALGB 89803 |
992 Patients with stage III colon cancer |
Post-diagnosis LTPA ≥ 8.75 MET-h/week |
Overall survival | HR 0.64 (95% CI 0.45–0.92) |
|
Meyerhardt et al. [112] Nurses’ Health Study |
57 Women with stage I-III CRC |
Post-diagnosis LTPA > 18 MET-h/week |
CRC mortality Overall mortality |
HR 0.39 (95% CI 0.18–0.82) HR 0.43 (95% CI 0.25–0.74) |
| Campbell et al. [114] | 2293 Patients with stage I-III CRC |
Pre-diagnosis LTPA ≥ 8.75 MET-h/week Post-diagnosis LTPA ≥ 8.75 MET-h/week |
All-cause mortality All-cause mortality |
RR 0.72 (95% CI 0.58–0.89) RR 0.58 (95% CI 0.47–0.71) |
CRC, colorectal cancer; LTPA, leisure-time physical activity; MET-h/week, metabolic equivalent task hours per week
Table 4.
Summary of observational studies on physical activity and prognosis in prostate cancer patients
| Study | Population | LTPA | Outcome | Results |
|---|---|---|---|---|
| Richman et al. [115] |
N = 1455 Non-metastatic PC |
Walk briskly ≥ 3 h/week |
Rate of progression | HR 0.43 (95% CI 0.21–0.91) |
| Friedenreich et al. [116] |
N = 830 Stage II–IV PC 1997–2000 |
Post-diagnosis total activity > 119 MET-hours/week | All-cause mortality | HR 0.58 (95% CI 0.42–0.79) |
| Pre- and post-diagnosis activity | PC mortality | HR 0.56 (95% CI 0.35–0.90) | ||
| > 18 MET-hours/week | All-cause mortality | HR 0.66 (95% CI 0.49–0.88) | ||
|
Kenfield et al. [117] Health Professional Follow-up Study |
N = 2705 Non-metastatic PC 1990–2008 |
Post-diagnosis walking ≥ 90 min per week | All-cause mortality | HR 0.54 (95% CI 0.41–0.71) |
| Post-diagnosis walking ≥ 3 h per week or vigorous activity | All-cause mortality | HR 0.51 (95% CI 0.36–0.72) |
LTPA, leisure-time physical activity; MET-h/week, metabolic equivalent task hours per week; PC, prostate cancer
Table 5.
Prospective observational studies on physical activity and prognosis in other cancers
| Study | Population | LTPA | Outcome | Results |
|---|---|---|---|---|
|
Liss et al. [118] Texas and San Diego |
222,163 Kidney cancer survivors 1998–2004 | Any PA | Kidney cancer-specific mortality | HR 0.50 (95% CI 0.27–0.93) |
|
Sloan et al. [119] Rochester, US |
1466 Lung cancer survivors 1997–2009 | Physically active |
Recurrence rate Overall survival |
81% versus 82% (P = 0.62) 8.4 year versus 4.4 year (P < 0.0001) |
|
Wang et al. [120] Chinese |
303 Early esophageal cancer survivors |
After surgery > 9 MET-h/week |
All-cause mortality Risk of recurrence |
HR 0.67 (95% CI 0.48–0.92) HR 0.31 (95% CI 0.22–0.43) |
HR, hazard ratio; LTPA, leisure-time physical activity; MET, metabolic equivalent; PA, physical activity
Epidemiologic and observational studies show a decrease in the risk of cancer recurrence and all-cause mortality in patients who practice regular PA [121–123]. A systematic review of studies published through June 2013 concluded that PA performed before or after cancer diagnosis is associated with a reduced mortality risk among BC and colorectal cancer survivors [124]. Mortality in adult survivors of childhood cancer was inferior in those patients who practiced vigorous exercise after diagnosis in a large multicentric observational study [125]. In 2015, Lahart et al. [97] published a meta-analysis of 22 studies analyzing the impact of PA on BC outcomes. A literature search was performed using PubMed, EMBASE and CENTRAL databases from 1995 to October 2014. In 40% of the observational studies, the risk of relapse and death in BC survivors decreased in most physically active women. Most studies included an analysis of leisure-time PA and only a few of interventional PA programs. The majority of the studies did not perform a multivariable analysis to exclude the effect of known confounding factors, and less than half included clinical prognostic factors, such as stage, nodal status, age or type of treatment. These studies found a positive impact on all-cause and BC mortality in patients who practiced moderate or intense lifetime PA before the diagnosis of BC and in recent years before diagnosis. However, the authors recommend interpreting these results with caution due to the large heterogeneity of the studies. A post-diagnosis activity of at least 10 MET-hours/week was associated with a decrease in all-cause, and BC mortality and was not influenced by the heterogeneity; however, not all the studies could corroborate a decrease in recurrence risk. The “After Breast Cancer Pooling Project” included more than 13,000 women from four prospective cohorts of BC survivors in the USA and Shanghai and analyzed the association between PA at 18–48 months after diagnosis and risk of all-cause and BC-specific mortality and BC recurrence [126]. BC mortality was reduced in patients who achieved 18.7 or more MET-hours/week, and no association was found between PA and BC recurrence [126]. A comprehensive review of sixty-three interventional studies on women after BC adjuvant therapy concluded that PA interventions might have certain beneficial effects on QoL, cardiorespiratory fitness and psychological and social functions, but conclusions about BC recurrence, BC mortality and all-cause mortality could not be made [98].
Several prospective observational studies and meta-analyses in patients with colorectal cancer have suggested the benefit of PA before and after diagnosis in terms of improvements on all-cause and cancer-specific mortality after controlling for other confounding factors, such as BMI, sex, number of positive lymph nodes, age, baseline performance status (PS), adjuvant chemotherapy regimen or recurrence-free survival period [110, 111, 127, 128]. Similarly, three observational prospective studies in prostate cancer found a strong inverse relationship between exercise and the risk of cancer progression regardless of other known prognostic factors [115–117]. A Chinese study in patients who underwent esophagectomy for esophageal cancer supported the benefit of PA (> 9 MET-hours/week) on recurrence risk and all-cause mortality [120]. Data from a prospective observational study in kidney cancer survivors investigating PA and diet changes suggested a decrease in the recurrence rate in patients who did any PA compared with those that were totally inactive [118]. The only study in lung cancer survivors showed better overall survival in patients who met > 9 MET-hours/week, but no difference in the recurrence rate was observed [119].
In conclusion, the real impact of PA on the risk of relapse and cancer mortality is not well-defined. PA may contribute to reduced cancer-related mortality and all-cause mortality in cancer survivors by modifying fat accumulation and improving cardiovascular and skeletal muscle function [129]. Numerous prospective observational studies consistently showed the benefit of PA on cancer outcomes; however, most of these studies were based on measures from self-reported questionnaires, including heterogeneous populations, and only a few performed a multivariable analysis to exclude the contribution of other confounding factors. Interventional studies with reliable and objective measures of PA in homogeneous populations are needed to confirm the data from observational studies and to evaluate the real effect of exercise on cancer prognosis.
Exercise-oncology: a pragmatic point of view for clinicians
Exercise-oncology is a new field of cancer care with the goal of the appropriate and rationale introduction of exercise programs into the overall management of cancer patients to take advantage of the numerous benefits associated with PA. Several major comprehensive cancer centers have created exercise-oncology units to implement these programs in a timely and organized manner. A collaborative work among rehab specialists, physiotherapists and exercise physiologists, as well as oncologists and radio-oncology specialists, is developed in these units.
Exercise has demonstrated numerous benefits on the QoL of patients with cancer throughout the history of the disease, ameliorating the negative impact of cancer on physical and psychological health and having a positive impact on patient survival [130–133].
Despite these benefits, many questions about PA/exercise in cancer patients remain, as it is particularly challenging to elucidate how much exercise is needed to achieve patient improvements and how the exercise should be recommended and monitored by clinicians.
General PA/exercise recommendations for cancer patients
In 2010, the first exercise guidelines were published by a roundtable of the American College of Sports Medicine (ACSM) based on general WHO PA guidelines to the general population. These guidelines consist of a minimum exercise recommendation: 150 min of moderate-intensity exercise in 3–5 days combining 2 days of resistance exercise and 3 days of aerobic exercise or 70 min of high-intensity exercise combining 1 day of resistance exercise and 2 days of aerobic exercise [133].
Exercise in the cancer treatment continuum
First, it is important to highlight that exercise is feasible, effective and safe in patients with cancer throughout the course of the disease. However, there are specific recommendations for the different moments of the disease and its therapies.
Presurgical exercise
It has been shown that presurgical high-intensity interval training in cancer patients is feasible and effective in improving cardiorespiratory fitness, which is typically measured as peak oxygen uptake (VO2peak); this training makes sense when patients need to achieve a specific VO2peak to undergo surgery, as noted for patients with lung cancer [134]. The intervention was based on high-intensity aerobic exercise (cycling) from 50 to 100% of VO2peak for 30 min, 5 days per week.
In another study in patients with BC, a presurgical intervention consisting of 180 min of moderate aerobic exercise and 40 min of strength training per week was associated with physiological changes and alterations in gene expression in tumor tissue (notably, downregulation of pathways related to cell cycle, RNA transport and DNA replication) [135].
Exercise during chemotherapy
Several studies using PA concomitantly with neoadjuvant and adjuvant chemotherapy have been performed with different approaches, demonstrating safety, effectiveness and fitness improvements [136].
Exercise programs concomitant with neoadjuvant chemotherapy are usually focused on improving the VO2peak level or maintaining it at the baseline range after cancer treatment. Interventions are based on at least 3 days per week with different durations (from 4 to 12 weeks) in 30- to 60-min session with variable intensities, which range from 55 to 60% of VO2peak at the start to 70–100% of VO2peak at the end [137].
Exercise interventions concomitant with adjuvant therapy must take into account a safe starting time to be sure that surgical wounds are completely scarred. Different reviews have shown that exercise improves fitness capacity [138] and might reduce some cancer-related side effects, such as fatigue [136]. However, interventions in these studies were heterogeneous and did not often describe the intensity or type of exercise used. A recent meta-analysis suggested that a workload of 600 MET (intensity-minutes) was associated with a clinically significant improvement in fitness capacity, suggesting that a 10-week program of 90 min/week of supervised training at 70% of VO2peak may be sufficient [139–141]. Another meta-analysis found that cancer survivors who completed 15 MET-h/week presented a 27% lower risk of cancer mortality with respect to controls, and this effect was greater in patients who were sedentary at pre-diagnosis (35% lower risk) [142].
Despite the aggressiveness of cancer therapies, medium to high-intensity exercise and different types of exercise interventions are well-tolerated by most patients. Both previous reviews mentioned above focused on exercise intervention during neoadjuvant and adjuvant treatments, including high-intensity intervention [137, 143].
In addition, resistance training has been shown to be safe and effective in preventing lean body mass loss and reducing body fat mass during neoadjuvant and adjuvant treatments [144].
Exercise in cancer survivors
It is well-known that cancer survivors obtain an improvement in QoL, body composition and physical fitness with exercise [130, 131, 144–146]. Again, the challenge in this population is to determine how much exercise is needed to achieve the maximum benefits. Related to exercise intensity, Gil-Rey et al. [147] showed that cancer survivors have an important reduction in their fitness capacity after cancer therapy and therefore suggest a reduction in exercise intensity at the beginning of training (i.e., 41–64% of VO2max). However, high-intensity training is feasible, safe and effective for cancer patients, and a shorter time of training is likely sufficient to obtain benefits, which should be taken into account in the implementation of exercise strategies [148].
More research is needed to determine the dose–response relationship between exercise and physical improvements given that some data from past clinical studies have suggested that an exercise intensity higher than that included in the general WHO recommendations might be needed to improve patients’ health status [147].
Exercise in patients with advanced and metastatic disease
Previous studies and reviews have shown that exercise is a safe and effective tool to improve fitness and functional capacity, strength, QoL and fatigue. Fitness and functional capacity were assessed by the VO2peak and 6-min walking tests, showing significantly better results compared with the control group. In these studies, the aerobic exercise intensity ranged from 55 to 75% of VO2peak [15, 16, 19, 29]. Muscle strength was assessed with the one-repetition maximum (1RM) or estimated 1RM test (lower and upper limbs), and exercise intensity in these studies ranged from 40 to 80% of 1RM. Program durations ranged from 5 to 12 weeks [22, 25]. With respect to body composition, significant changes were observed in lean mass, but no changes in fat mass, body mass or BMI were observed in previous studies. The low intensity of the exercise intervention (from 55 to 70% of VO2peak) might be a reason for these inconsistent results [15, 16, 33].
Exercise supervision
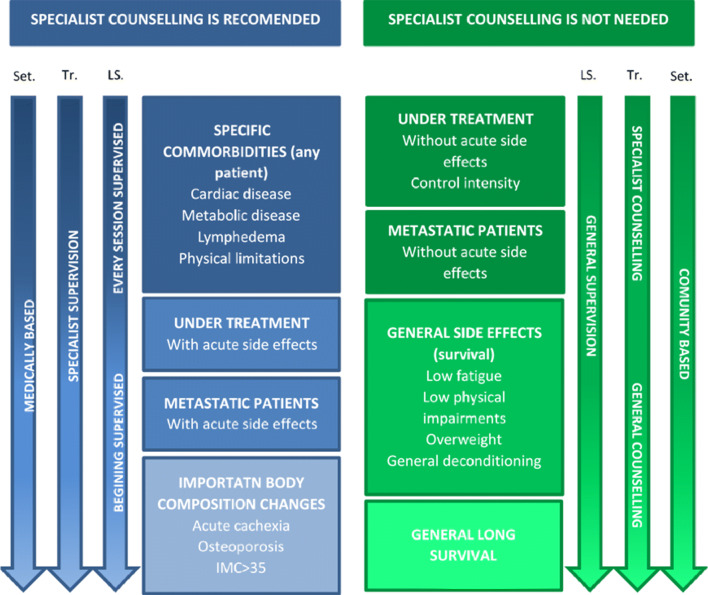
Although the benefits of exercise are well-established, the exercise dose–response and the best type of exercise in terms of duration and intensity remain unclear, making it difficult to establish how to provide specific recommendations to each individual patient and how to supervise the patient’s exercise by clinicians. With these caveats in mind, it might be wise to differentiate between patients who clearly need specialist counseling (as those under active treatment or metastatic patients, and all patients with side effects who limit them physically) and patients who do not (survival with limited side effects) (Fig. 1). An exercise-oncology specialist is an exercise professional with a previous background that includes a general qualification in exercise and health with specific knowledge in oncology items. Related to the oncology items, general knowledge about cancer biology, biomarkers and treatments and their side effects should be used to adapt to and individualize exercise to patients’ needs.
Fig. 1.
Distinguishing between patients who need specialist counseling and those who do not.
Adaptation to the triage model for population-based screening of cancer survivors for weight management and physical activity interventions. Modified from National Academies of Sciences, Engineering, and Medicine 2018 [149]. Set. = setting; Tr. = training of professional; LS. = level of supervision. Specialist refers to clinicians, physical therapists, occupational therapists, dieticians, and clinical exercise physiologists
Challenges for patients: general supervision
For those patients who do not present the need for specialist counseling, the control of patients by informed clinicians could be sufficient to achieve reasonable results. In this respect, there are some specific guidelines that could be followed by patients and supervised by a nonspecialist with the help of different tests, devices or scales. For example, following WHO/ACSM guidelines or achieving more than 10,000 steps per day [150] are reasonable goals for cancer survivors (Table 6).
Table 6.
General challenges for patients without specialist counseling needs based on existing guidelines
| Recommendation | Challenge | Intensity |
|---|---|---|
| WHO/ACSM guidelines |
150 min per week 30 min/3 times “aerobic” exercise 30 min/2 times strength exercises |
Moderate |
|
75 min per week 25 min/2 times aerobic exercise 25 min/1 time strength exercises |
High intensity | |
| Survival recommendations [99, 107, 147, 151] | 9 MET corresponding to 180 min of walking | 5 km/h |
| Review psychological benefits [152] | 12 MET; 90–120 min | Moderate intensity |
| Minimum step recommendations [150] | < 5000 steps/day | “Sedentary lifestyle index” |
| 5000–7499 steps/day | It is typical of daily activity excluding sports/exercise and might be considered “low active” | |
| 7500–9999 | “Somewhat active” | |
| ≥ 10,000 steps/day | “Active” | |
| Individuals who take > 12,500 steps/day | “Highly active” |
MET, metabolic equivalent
New technologies are improving methods to supervise the quality and quantity of exercise [153]. While behavioral interventions using text messages (with or without educational material and internet support) have produced limited effects on exercise adherence, mobile applications have been shown to be an effective and useful tool for both patients and providers to establish a healthy lifestyle. To achieve significant changes, it has been observed that these apps should include self-monitoring merged with other motivational techniques (goal setting, feedback on performance, review of goals, prompts, planning or barrier identifications, among others), allowing better supervision and control for patients and trainers [153].
Challenges for clinicians: learning exercise techniques
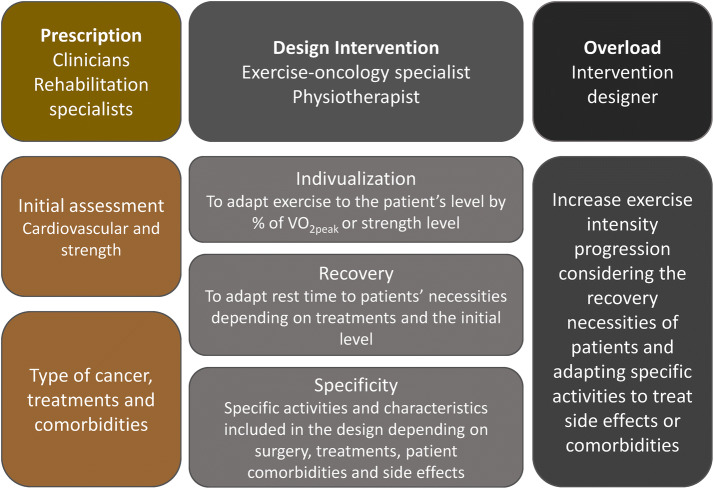
At present, exercise provides empowerment among health care providers, presenting a new challenge for them. One of the most important issues to address is who might prescribe and control exercise. It is possible that multidisciplinary committees, including oncologists, rehab departments and exercise physiologists, should be created to provide patients with the best counseling and training physicians to help individuals not requiring special help (Fig. 2).
Fig. 2.
Specialists and activities that should be developed by each specialist to achieve an adequate exercise intervention for oncologic patients while taking into account training principles adapted to exercise-oncology
However, while this scenario seems far away, other achievable proposals are feasible. The education of clinicians taking care of cancer patients and survivors about exercise techniques and control is a crucial point. Education and training should ideally start during university studies, although few institutions worldwide provide exercise theory and training to their future professionals. This education would have an impact not only in cancer patients but also on many other common pathologies such as cardiovascular, metabolic, joint and other diseases [154]. In accordance, patients’ associations playing a crucial role at present in providing exercise training and assistance until exercise will be included in the usual care.
Future lines of research
Despite the increasing number of studies addressing the benefits of exercise for cancer patients and survivors in the last 5 years, further research remains essential to clarify many unanswered questions. The establishment of new, more concrete guidelines for exercise in cancer patients is necessary not only for the exercise-oncology specialist but also for oncologists and other clinicians who take care of cancer patients.
There is an urgent need to further clarify the biological mechanism that makes exercise an effective method of intervention to decrease cancer incidence and mortality and to improve overall health in cancer patients. Studies of the modification of biological biomarkers before and after exercise are crucial for understanding the underlying mechanisms through which exercise can exert its influence on cancer biology. Several preclinical studies (discussed above) and clinical studies of small sample sizes have provided preliminary evidence on the relevance of the immune system, cytokines and insulin-related pathways [155]; however, because the evidence is preliminary, larger and statistically powerful studies are required.
In addition, new studies aimed at identifying the optimal intensity and duration of PA are needed. The characteristics of cancer survivors differ from those of the healthy population to whom the recommendations of the different health organizations are directed.
The best method of introducing exercise into the lifestyle of patients is also a matter to be addressed. The most effective behavioral interventions to achieve long-term changes in a patient’s lifestyle must be defined, bearing in mind that cancer diagnosis and treatment are “learning moments” in which patients are willing to change their daily activities to improve their health. The feasibility of using new technologies, such as mobile health applications and wrist and watch bands, as well as interventions based on social networks should be investigated to favor adherence and motivation to these programs of adapted PA.
In addition, future research on intervention in metastatic cancer stages should be performed due to the lack of knowledge in this area and the potential interest in improving the tolerance and effectiveness of treatments and the QoL of patients, many of whom can live today for many years after relapse due to the effective and sustained disease palliation that can be achieved with modern systemic treatments.
Conclusions
Regular PA is associated with major benefits to human health, including a reduced risk of some cancers.
The mechanisms through which exercise exerts its antitumor activity are still poorly understood but might be related to a direct effect on tumor cells (inhibition of tumor cell proliferation, induction of apoptosis, upregulation of tumor suppressor genes, anti-inflammatory effects) or to an enhancement of immune function.
There is convincing evidence that regular PA reduces the risk of colorectal cancer, while the reduction in postmenopausal BC and endometrial cancer risk is judged as probable. The effect of PA on the risk of other tumors is less evident but still possible.
Several epidemiological studies have suggested an association of regular PA with reduced cancer-related and all-cause mortality in some tumor types, particularly BC and colorectal cancer. The minimum amount of PA needed to achieve such a benefit is still unknown, although the US recommendations suggest that a minimum 10 MET-hours/week (equivalent to ≥ 150 min of moderate-intensity PA) is needed.
Exercise-oncology is a field of cancer care in which the goal is the introduction of exercise programs into the overall management of cancer patients. The first exercise guidelines for cancer patients were published in 2010 by the ACSM. These guidelines, which are mainly based on general WHO guidelines to the general population, consider that regular PA in cancer patients is safe and exerts positive effects in patients at multiple levels, particularly QoL. Exercise programs in cancer patients are feasible along the course of the disease, including the presurgical period, during adjuvant antitumor medical treatment (including chemotherapy) and in cancer survivors; a summary of these recommendations is shown in Table 7. However, the experience with regular exercise in metastatic cancer patients is limited.
Table 7.
| Cancer treatment moment | Type of exercise | Description | Intensity | Duration (start with…) |
Examples |
|---|---|---|---|---|---|
| Pre-surgery without other treatment | Endurance | High-intensity interval training | 60–90% of VO2peak [157, 158] | 20–35 min |
Walking Running Spinning |
| Strength | Global strength exercises |
Exercises with patient’s own body weight 0–40% of 1RM |
2 sets of 10 repetitions | Global strength circuits | |
| Stretching | All body | Passive or active stretches | 30 s/exercise |
Yoga Stretching classes |
|
| Surgery/mammary reconstructions | Endurance | Light intensity, avoid pain | Depending on patient’ mobility [143] | 20–40 min |
Walking biking Avoid swimming |
| Strength | Rehabilitation recommendations | Rehabilitation recommendations | Rehabilitation recommendations |
Upper-limb surgery: light arm mobility and postural exercises Abdominal surgery: hypopressive abdominal exercises, and postural exercises—including isometric exercises |
|
| Stretching | Rehabilitation recommendations | Rehabilitation recommendations | Rehabilitation recommendations | Passive stretching without pain, focusing on affected area, when specialist allows it | |
| Under chemo/radiotherapy | Endurance | Adapted intervention to patient needs | From 41 to 64% (moderate) to 80–90% of VO2peak (high intensity, if the patient was previously active) | 3 days per week/20–35 min |
Walking Dance Bike Spinning Running Avoid swimming |
| Strength | Global strength to prevent sarcopenia or cachexia | From light movements to 40–60% of 1RM at first [159]. Depending on the patient’s comorbidities [143] | 2 days per week, 2 sets of 10 repetitions |
Yoga Pilates Elastic bands Global strength circuits Machines |
|
| Stretching | Stretch gently all body. Special care with radiation areas. Avoid pain | Passive stretches | 30 s/exercise |
Yoga Stretching classes |
|
| Under hormone therapy | Endurance | Moderate to high intensity depending on the patient’s previous situation. Rest for 48 h after high-intensity training | From 41–64% (moderate) to 80–90% of VO2peak (high intensity) if the patient was previously active | 3 days per week/30–40 min |
Walking Dance Bike Spinning Running Swimming |
| Strength | Moderate to high intensity depending on the patient’s situation. Rest for 48 h after high-intensity training | From light movements to 40–60% of 1RM [159] at first. Depending on the patient’s comorbidities [143] | 2 days per week, 2 sets of 10 repetitions |
Yoga Pilates Elastic bands Global strength circuits Machines |
|
| Stretch | All body | Passive or active stretches | 30 s/exercise |
Yoga Stretching classes |
|
| Survivors | Endurance | Moderate to high intensity depending on previous patients’ situation | From 41 to 64% (moderate) to 80–100% of VO2peak (intense) if the patient was initially active | 3 days per week/30–40 min |
Walking Dance Bike Spinning Running Swimming |
| Strength | Moderate to high intensity depending on the patient’s previous situation. Special care for patients with functional limitations. Avoid pain. | From light movements to 40–60% of 1RM [159] at first. Depending on the patient comorbidities [143] | 2 days per week, 2 sets of 10 repetitions |
Yoga Pilates Elastic bands Global strength circuits Machines |
|
| Stretch | All body | Passive or active stretches | 30 s/exercise |
Yoga Stretching classes |
RM, repetition maximum; VO2peak, peak oxygen uptake
The introduction of exercise programs into the global management of cancer patients remains a challenge due to conceptual and logistic issues. The most effective behavioral interventions to achieve long-term changes in a patient’s lifestyle must be defined. New technologies, such as mobile health applications and wrist and watch bands (the so-called “mHealth”), can be of great help to monitor the compliance to these programs. The optimal intensity and duration of PA should be defined with more precision in future studies. Regarding logistics, the intervention of both exercise-oncology specialists and trained clinicians is probably necessary at different time points to provide the best care. Several major comprehensive cancer centers have created exercise-oncology units to implement these programs in a timely and organized manner, and these models could serve as a reference for other institutions.
Acknowledgements
We would like to thank to Fernando Rico-Villademoros, MD (COCIENTE S.L., Madrid, Spain) for his editorial assistance; his participation was funded by the Spanish Society of Medical Oncology (SEOM).
Author’s contribution
All authors were involved in the conception of this work, drafting and/or revising the manuscript, and approved the final version.
Funding
This positioning statement was funded by the Spanish Society of Medical Oncology (SEOM).
Compliance with ethical standards
Conflict of interest
MP declares that she have no conflict of interest; SC declares that she have no conflict of interest; JA received institutional research grants from Merck Serono and Novartis; CE received speaker’s honoraria from AstraZeneca, Roche, Novartis and Pfizer; MAS-P received consulting/advisory fees from AstraZeneca, Pfizer and Amgen, and speakers’ honoraria from Amgen, Roche and Pfizer; AL declares that he have no conflict of interest; MM has received research grants from Roche and Novartis, consulting/advisory fees from AstraZeneca, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA Taiho Oncology and Pfizer, and speakers’ honoraria from AstraZeneca, Amgen, Roche/Genentech, Novartis and Pfizer.
Ethical approval
The manuscript does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent/ethical approval is not required for this type of project.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. Pollán and S. Casla-Barrio have equally contributed to this work.
Contributor Information
A. Lucia, Email: alejandro.lucia@universidadeuropea.es
M. Martín, Email: mmartin@geicam.org
References
- 1.World Health Organization . Physical activity. Geneva: WHO; 2018. [Google Scholar]
- 2.National Cancer Institute. Cancer survivors and physical activity. 2018. https://progressreport.cancer.gov/after/physical_activity. Accessed 5 June 2019.
- 3.Figueira A, Cortinhas A, Soares J, Leitão J, Ferreira R, Duarte J. Efficacy of exercise on breast cancer outcomes: a systematic review and meta-analysis of preclinical data. Int J Sports Med. 2018;39:327–342. doi: 10.1055/s-0044-101149. [DOI] [PubMed] [Google Scholar]
- 4.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, Palmer G, Jones LW, Dewhirst MW. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015;107:djv040. doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones LW, Eves N, Courneya K, Chiu B, Baracos V, Hanson J, Johnson L, Mackey J. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clin Cancer Res. 2005;11:6695–6698. doi: 10.1158/1078-0432.CCR-05-0844. [DOI] [PubMed] [Google Scholar]
- 6.Jones LW, Fels DR, West M, Allen JD, Broadwater G, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RC, Povsic TJ, Peppercorn J, Marcom PK, Blackwell KL, Kimmick G, Turkington TG, Dewhirst MW. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res. 2013;6:925–937. doi: 10.1158/1940-6207.CAPR-12-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturgeon K, Schadler K, Muthukumaran G, Ding D, Bajulaiye A, Thomas NJ, Ferrari V, Ryeom S, Libonati JR. Concomitant low-dose doxorubicin treatment and exercise. Am J Physiol Regul Integr Comp Physiol. 2014;307:R685–R692. doi: 10.1152/ajpregu.00082.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Casado A, Martín-Ruiz A, Pérez LM, Provencio M, Fiuza-Luces C, Lucia A. Exercise and the hallmarks of cancer. Trends Cancer. 2017;3:423–441. doi: 10.1016/j.trecan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Colbert LH, Mai V, Tooze J, Perkins S, Berrigan D, Hursting S. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis. 2006;27:2103–2107. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- 10.Leung P-S, Aronson WJ, Ngo TH, Golding LA, Barnard RJ. Exercise alters the IGF axis in vivo and increases p53 protein in prostate tumor cells in vitro. J Appl Physiol. 2004;96:450–454. doi: 10.1152/japplphysiol.00871.2003. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang P, Jiang Y, Doan HM, Xie L, Vasquez D, Welti R, Su X, Lu N, Herndon B, Yang SS, Jeannotte R, Wang W. Weight loss via exercise with controlled dietary intake may affect phospholipid profile for cancer prevention in murine skin tissues. Cancer Prev Res. 2010;3:466–477. doi: 10.1158/1940-6207.CAPR-09-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie L, Jiang Y, Ouyang P, Chen J, Doan H, Herndon B, Sylvester JE, Zhang K, Molteni A, Reichle M, Zhang R, Haub MD, Baybutt RC, Wang W. Effects of dietary calorie restriction or exercise on the PI3K and ras signaling pathways in the skin of mice. J Biol Chem. 2007;282:28025–28035. doi: 10.1074/jbc.M604857200. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, King B, Ewert E, Su X, Mardiyati N, Zhao Z, Wang W. Exercise activates p53 and negatively regulates IGF-1 pathway in epidermis within a skin cancer model. PLoS ONE. 2016;11:e0160939. doi: 10.1371/journal.pone.0160939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Z, Jiang W, Zacher JH, Neil ES, McGinley JN, Thompson HJ. Effects of energy restriction and wheel running on mammary carcinogenesis and host systemic factors in a rat model. Cancer Prev Res. 2012;5:414–422. doi: 10.1158/1940-6207.CAPR-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Zhu Z, Thompson HJ. Effects of physical activity and restricted energy intake on chemically induced mammary carcinogenesis. Cancer Prev Res. 2009;2:338–344. doi: 10.1158/1940-6207.CAPR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z, Jiang W, Sells JL, Neil ES, McGinley JN, Thompson HJ. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol Biomark Prev. 2008;17:1920–1929. doi: 10.1158/1055-9965.EPI-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baltgalvis KA, Berger FG, Peña MMO, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol. 2008;104:1137–1143. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- 19.Ju J, Nolan B, Cheh M, Bose M, Lin Y, Wagner GC, Yang CS. Voluntary exercise inhibits intestinal tumorigenesis in Apc Min/+ mice and azoxymethane/dextran sulfate sodium-treated mice. BMC Cancer. 2008;8:316. doi: 10.1186/1471-2407-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horak M, Zlamal F, Iliev R, Kucera J, Cacek J, Svobodova L, Hlavonova Z, Kalina T, Slaby O, Bienertova-Vasku J. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PLoS ONE. 2018;13:e0191060. doi: 10.1371/journal.pone.0191060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khori V, Amani Shalamzari S, Isanejad A, Alizadeh Ali M, Alizadeh S, Khodayari S, Khodayari H, Shahbazi S, Zahedi A, Sohanaki H, Khaniki M, Mahdian R, Saffari M, Fayad R. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: possible underlying pathway of miR-21. Eur J Pharmacol. 2015;765:179–187. doi: 10.1016/j.ejphar.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF, Pedersen BK, Hojman P. Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 2017;77:4894–4904. doi: 10.1158/0008-5472.CAN-16-3125. [DOI] [PubMed] [Google Scholar]
- 23.Lu YP, Lou YR, Nolan B, Peng QY, Xie JG, Wagner GC, Conney AH. Stimulatory effect of voluntary exercise or fat removal (partial lipectomy) on apoptosis in the skin of UVB light-irradiated mice. Proc Natl Acad Sci USA. 2006;103:16301–16306. doi: 10.1073/pnas.0607789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins KA, Park D, Lee GY, Curran WJ, Deng X. Exercise-induced lung cancer regression: mechanistic findings from a mouse model. Cancer. 2014;120:3302–3310. doi: 10.1002/cncr.28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Cui XX, Huang MT, Liu Y, Shih WJ, Lin Y, Lu YP, Wagner GC, Conney AH. Inhibitory effect of voluntary running wheel exercise on the growth of human pancreatic Panc-1 and prostate PC-3 xenograft tumors in immunodeficient mice. Oncol Rep. 2008;19:1583–1588. [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng X, Cui X-X, Huang M-T, Liu Y, Wagner GC, Lin Y, Shih WJ, Lee M-J, Yang CS, Conney AH. Inhibition of progression of androgen-dependent prostate LNCaP tumors to androgen independence in SCID mice by oral caffeine and voluntary exercise. Nutr Cancer. 2012;64:1029–1037. doi: 10.1080/01635581.2012.716899. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Z, Jiang W, McGinley JN, Thompson HJ. Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. J Appl Physiol. 2009;106:911–918. doi: 10.1152/japplphysiol.91201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soliman S, Aronson WJ, Barnard RJ. Analyzing serum-stimulated prostate cancer cell lines after low-fat, high-fiber diet and exercise intervention. Evid Based Complement Altern Med. 2011;2011:529053. doi: 10.1093/ecam/nep031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnard RJ, Hong Gonzalez J, Liva ME, Ngo TH. Effects of a low-fat, high-fiber diet and exercise program on breast cancer risk factors in vivo and tumor cell growth and apoptosis in vitro. Nutr Cancer. 2006;55:28–34. doi: 10.1207/s15327914nc5501_4. [DOI] [PubMed] [Google Scholar]
- 30.Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metabol. 2011;301:E504–E510. doi: 10.1152/ajpendo.00520.2010. [DOI] [PubMed] [Google Scholar]
- 31.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piguet A-C, Saran U, Simillion C, Keller I, Terracciano L, Reeves HL, Dufour J-F. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J Hepatol. 2015;62:1296–1303. doi: 10.1016/j.jhep.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Sanchis-Gomar F. Sestrins: novel antioxidant and AMPK-modulating functions regulated by exercise? J Cell Physiol. 2013;228:1647–1650. doi: 10.1002/jcp.24338. [DOI] [PubMed] [Google Scholar]
- 34.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 35.Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, Potter MQ, Jung Moon E, Schroeder T, Herndon JE, Dewhirst MW. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 2010;108:343–348. doi: 10.1152/japplphysiol.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, Dewhirst MW, Dyck JRB, Nagendran J, Flores CT, Betof AS, Nelson ER, Pollak M, Dash RC, Young ME, Freedland SJ. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. 2012;113:263–272. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough DJ, Nguyen LMD, Siemann DW, Behnke BJ. Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. J Appl Physiol. 2013;115:1846–1854. doi: 10.1152/japplphysiol.00949.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 2014;106:dju036. doi: 10.1093/jnci/dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, Till JE, Sturgeon K, Zaslavsky A, Chen CS, Ryeom S. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7:65429–65440. doi: 10.18632/oncotarget.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang QB, Zhang BH, Zhang KZ, Meng XT, Jia QA, Zhang QB, Bu Y, Zhu XD, Ma DN, Ye BG, Zhang N, Ren ZG, Sun HC, Tang ZY. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene. 2016;35:4122–4131. doi: 10.1038/onc.2015.484. [DOI] [PubMed] [Google Scholar]
- 41.Wolff G, Davidson SJ, Wrobel JK, Toborek M. Exercise maintains blood–brain barrier integrity during early stages of brain metastasis formation. Biochem Biophys Res Commun. 2015;463:811–817. doi: 10.1016/j.bbrc.2015.04.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia W, Lu R, Martin TA, Jiang WG. The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (review) Mol Med Rep. 2014;9:779–785. doi: 10.3892/mmr.2013.1875. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17:620–632. doi: 10.1038/nrc.2017.78. [DOI] [PubMed] [Google Scholar]
- 45.Wiggins JM, Opoku-Acheampong AB, Baumfalk DR, Siemann DW, Behnke BJ. Exercise and the tumor microenvironment. Exerc Sport Sci Rev. 2018;46:56–64. doi: 10.1249/JES.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 46.Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol. 2004;96:2249–2256. doi: 10.1152/japplphysiol.01210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida PWM, Gomes-Filho A, Ferreira AJ, Rodrigues CEM, Dias-Peixoto MF, Russo RC, Teixeira MM, Cassali GD, Ferreira E, Santos IC, Garcia AMC, Silami-Garcia E, Wisløff U, Pussieldi GA. Swim training suppresses tumor growth in mice. J Appl Physiol. 2009;107:261–265. doi: 10.1152/japplphysiol.00249.2009. [DOI] [PubMed] [Google Scholar]
- 48.Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 49.Idorn M, Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med. 2016;22:565–577. doi: 10.1016/j.molmed.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Kruijsen-Jaarsma M, Revesz D, Bierings MB, Buffart LM, Takken T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. 2013;19:120–143. [PubMed] [Google Scholar]
- 51.Pedersen L, Idorn M, Olofsson Gitte H, Lauenborg B, Nookaew I, Hansen Rasmus H, Johannesen Helle H, Becker Jürgen C, Pedersen Katrine S, Dethlefsen C, Nielsen J, Gehl J, Pedersen Bente K, Thor Straten P, Hojman P. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metabol. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Lu Q, Ceddia MA, Price EA, Ye SM, Woods JA. Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. Am J Physiol Regul Integr Comp Physiol. 1999;276:R482–R489. doi: 10.1152/ajpregu.1999.276.2.R482. [DOI] [PubMed] [Google Scholar]
- 53.Abdalla DR, Murta EFC, Michelin MA. The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7,12-dimethylbenzanthracene. Eur J Cancer Prev. 2013;22:251–258. doi: 10.1097/CEJ.0b013e3283592cbb. [DOI] [PubMed] [Google Scholar]
- 54.Abdalla DR, Aleixo AAR, Murta EFC, Michelin MA. Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncol Lett. 2014;7:886–890. doi: 10.3892/ol.2013.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kizaki T, Takemasa T, Sakurai T, Izawa T, Hanawa T, Kamiya S, Haga S, Imaizumi K, Ohno H. Adaptation of macrophages to exercise training improves innate immunity. Biochem Biophys Res Commun. 2008;372:152–156. doi: 10.1016/j.bbrc.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Koelwyn GJ, Wennerberg E, Demaria S, Jones LW. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology (Williston Park) 2015;29(908–20):22. [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy EA, Davis JM, Brown AS, Carmichael MD, Mayer EP, Ghaffar A. Effects of moderate exercise and oat β-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J Appl Physiol. 2004;97:955–959. doi: 10.1152/japplphysiol.00252.2004. [DOI] [PubMed] [Google Scholar]
- 58.Goh J, Tsai J, Bammler TK, Farin FM, Endicott E, Ladiges WC. Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS ONE. 2013;8:e80123. doi: 10.1371/journal.pone.0080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McClellan JL, Steiner JL, Day SD, Enos RT, Davis MJ, Singh UP, Murphy EA. Exercise effects on polyp burden and immune markers in the ApcMin/+ mouse model of intestinal tumorigenesis. Int J Oncol. 2014;45:861–868. doi: 10.3892/ijo.2014.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, Ma Y. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22:643–652. doi: 10.1111/j.1600-0838.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 61.Hampras SS, Nesline M, Wallace PK, Odunsi K, Furlani N, Davis W, Moysich KB. Predictors of immunosuppressive regulatory T lymphocytes in healthy women. J Cancer Epidemiol. 2012;2012:191090. doi: 10.1155/2012/191090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis JM, Kohut ML, Jackson DA, Colbert LH, Mayer EP, Ghaffar A. Exercise effects on lung tumor metastases and in vitro alveolar macrophage antitumor cytotoxicity. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1454–R1459. doi: 10.1152/ajpregu.1998.274.5.R1454. [DOI] [PubMed] [Google Scholar]
- 63.Frellstedt L, Waldschmidt I, Gosset P, Desmet C, Pirottin D, Bureau F, Farnir F, Franck T, Dupuis-Tricaud M-C, Lekeux P, Art T. Training modifies innate immune responses in blood monocytes and in pulmonary alveolar macrophages. Am J Respir Cell Mol Biol. 2014;51:135–142. doi: 10.1165/rcmb.2013-0341OC. [DOI] [PubMed] [Google Scholar]
- 64.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28:330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 65.Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, Koyama R, Wada S, Higashi A, Kokura S, Ichikawa H, Yoshikawa T. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2012;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 66.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases—myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization . Assessing national capacity for the prevention and control of noncommunicable diseases. Geneva: WHO; 2012. [Google Scholar]
- 68.Leitzmann M, Powers H, Anderson AS, Scoccianti C, Berrino F, Boutron-Ruault M-C, Cecchini M, Espina C, Key TJ, Norat T, Wiseman M, Romieu I. European code against cancer 4th edition: physical activity and cancer. Cancer Epidemiol. 2015;39:S46–S55. doi: 10.1016/j.canep.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 69.World Cancer Research Fund/American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 70.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and colorectal cancer. 2017. http://wcrf.org/colorectal-cancer-2017. Accessed 5 June 2019.
- 71.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami H-O, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw K-T, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keum N, Bao Y, Smith-Warner SA, Orav J, Wu K, Fuchs CS, Giovannucci EL. Association of physical activity by type and intensity with digestive system cancer risk. JAMA Oncol. 2016;2:1146–1153. doi: 10.1001/jamaoncol.2016.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJL, Forouzanfar MH. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of breast cancer. 2010. http://www.aicr.org/continuous-update-project/reports/Breast-Cancer-2010-Report.pdf. Accessed 10 April 2017.
- 75.Catsburg C, Kirsh VA, Soskolne CL, Kreiger N, Bruce E, Ho T, Leatherdale ST, Rohan TE. Associations between anthropometric characteristics, physical activity, and breast cancer risk in a Canadian cohort. Breast Cancer Res Treat. 2014;145:545–552. doi: 10.1007/s10549-014-2973-z. [DOI] [PubMed] [Google Scholar]
- 76.Lope V, Martín M, Castelló A, Casla S, Ruiz A, Baena-Cañada JM, Casas AM, Calvo L, Bermejo B, Muñoz M, Ramos M, de Juan-Ferré A, Jara C, Antón A, Jimeno MÁ, Lluch A, Antolín S, García-Sáenz JÁ, Estévez P, Arriola-Arellano E, Gavilá J, Pérez-Gómez B, Carrasco E, Pollán M. Physical activity and breast cancer risk by pathological subtype. Gynecol Oncol. 2017;144:577–585. doi: 10.1016/j.ygyno.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 77.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of endometrial cancer. 2013. http://www.wcrf.org/sites/default/files/Endometrial-Cancer-2013-Report. Accessed 5 June 2019.
- 78.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30:397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 79.Brenner DR, Yannitsos DH, Farris MS, Johansson M, Friedenreich CM. Leisure-time physical activity and lung cancer risk: a systematic review and meta-analysis. Lung Cancer. 2016;95:17–27. doi: 10.1016/j.lungcan.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 80.World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project report: diet, nutrition, physical activity and liver cancer. 2015. http://www.wcrf.org/sites/default/files/Liver-Cancer-2015-Report.pdf. Accessed 5 June 2019.
- 81.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and oesophageal cancer. 2016. http://www.wcrf.org/sites/default/files/Oesophageal-Cancer-2016-Report.pdf. Accessed 5 June 2019.
- 82.Behrens G, Jochem C, Keimling M, Ricci C, Schmid D, Leitzmann MF. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol. 2014;29:151–170. doi: 10.1007/s10654-014-9895-2. [DOI] [PubMed] [Google Scholar]
- 83.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and stomach cancer. 2016. http://www.wcrf.org/sites/default/files/Stomach-Cancer-2016-Report.pdf. Accessed 5 June 2019.
- 84.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity, and prostate cancer. 2014. http://www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf. Accessed 5 June 2019.
- 85.Shephard RJ. Physical activity and prostate cancer: an updated review. Sports Med. 2017;47:1055–1073. doi: 10.1007/s40279-016-0648-0. [DOI] [PubMed] [Google Scholar]
- 86.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of ovarian cancer. 2014. http://www.dietancancerreport.org/cup/cupresources.php. Accessed 5 June 2019.
- 87.Cannioto RA, Moysich KB. Epithelial ovarian cancer and recreational physical activity: a review of the epidemiological literature and implications for exercise prescription. Gynecol Oncol. 2015;137:559–573. doi: 10.1016/j.ygyno.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang T, Eliassen AH, Hankinson SE, Okereke OI, Kubzansky LD, Wang M, Poole EM, Chavarro JE, Tworoger SS. A prospective study of leisure-time physical activity and risk of incident epithelial ovarian cancer: impact by menopausal status. Int J Cancer. 2016;138:843–852. doi: 10.1002/ijc.29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.World Cancer Research Fund/American Institute for Cancer Research. Continous Update Project Expert Report 2018. Food, nutrition, physical activity, and the prevention of pancreatic cancer. Available at http://dietandcancerreport.org. Accesed 4 June 2019.
- 90.Farris MS, Mosli MH, McFadden AA, Friedenreich CM, Brenner DR. The association between leisure time physical activity and pancreatic cancer risk in adults: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2015;24:1462–1473. doi: 10.1158/1055-9965.EPI-15-0301. [DOI] [PubMed] [Google Scholar]
- 91.Noor NM, Banim PJR, Luben RN, Khaw K-T, Hart AR. Investigating physical activity in the etiology of pancreatic cancer. Pancreas. 2016;45:388–393. doi: 10.1097/MPA.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and kidney cancer. 2015. www.wcrf/kidney-cancer-2015. Accessed 5 June 2019.
- 93.Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer. 2013;108:798–811. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and bladder cancer. 2015. http://www.wcrf.org/sites/default/files/Bladder-Cancer-2015-Report.pdf. Accessed 5 June 2019.
- 95.Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. The association between physical activity and bladder cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1862–1870. doi: 10.1038/bjc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jochem C, Leitzmann MF, Keimling M, Schmid D, Behrens G. Physical activity in relation to risk of hematologic cancers: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2014;23:833–846. doi: 10.1158/1055-9965.EPI-13-0699. [DOI] [PubMed] [Google Scholar]
- 97.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54:635–654. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 98.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018;1:CD011292. doi: 10.1002/14651858.CD011292.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 100.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao P-P, Zhao G-M, Shu X-O, Peng P, Cai H, Lu W, Zheng Y. Modifiable lifestyle factors and triple-negative breast cancer survival. Epidemiology. 2015;26:909–916. doi: 10.1097/EDE.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt ME, Chang-Claude J, Vrieling A, Seibold P, Heinz J, Obi N, Flesch-Janys D, Steindorf K. Association of pre-diagnosis physical activity with recurrence and mortality among women with breast cancer. Int J Cancer. 2013;133:1431–1440. doi: 10.1002/ijc.28130. [DOI] [PubMed] [Google Scholar]
- 103.Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomark Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 104.Ammitzbøll G, Søgaard K, Karlsen RV, Tjønneland A, Johansen C, Frederiksen K, Bidstrup P. Physical activity and survival in breast cancer. Eur J Cancer. 2016;66:67–74. doi: 10.1016/j.ejca.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 105.Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124:1954–1962. doi: 10.1002/ijc.24155. [DOI] [PubMed] [Google Scholar]
- 106.Sternfeld B, Weltzien E, Quesenberry CP, Castillo AL, Kwan M, Slattery ML, Caan BJ. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomark Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, Wactawski-Wende J, Craft L, Lane D, Martin LW, Chlebowski R. Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer Prev Res. 2011;4:522–529. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bertram LAC, Stefanick ML, Saquib N, Natarajan L, Patterson RE, Bardwell W, Flatt SW, Newman VA, Rock CL, Thomson CA, Pierce JP. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011;22:427–435. doi: 10.1007/s10552-010-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bradshaw PT, Ibrahim JG, Khankari N, Cleveland RJ, Abrahamson PE, Stevens J, Satia JA, Teitelbaum SL, Neugut AI, Gammon MD. Post-diagnosis physical activity and survival after breast cancer diagnosis: the Long Island Breast Cancer Study. Breast Cancer Res Treat. 2014;145:735–742. doi: 10.1007/s10549-014-2966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walter V, Jansen L, Knebel P, Chang-Claude J, Hoffmeister M, Brenner H. Physical activity and survival of colorectal cancer patients: population-based study from Germany. Int J Cancer. 2017;140:1985–1997. doi: 10.1002/ijc.30619. [DOI] [PubMed] [Google Scholar]
- 111.Arem H, Pfeiffer RM, Engels EA, Alfano CM, Hollenbeck A, Park Y, Matthews CE. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the National Institutes of Health—AARP diet and health study. J Clin Oncol. 2015;33:180–188. doi: 10.1200/JCO.2014.58.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 113.van Blarigan EL, Fuchs CS, Niedzwiecki D, Zhang S, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, Atienza D, Messino M, Kindler H, Venook A, Ogino S, Giovannucci EL, Ng K, Meyerhardt JA. Association of survival with adherence to the american cancer society nutrition and physical activity guidelines for cancer survivors after colon cancer diagnosis. JAMA Oncol. 2018;4:783–790. doi: 10.1001/jamaoncol.2018.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 115.Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70:576–585. doi: 10.1016/j.eururo.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 117.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liss M, Natarajan L, Hasan A, Noguchi JL, White M, Parsons JK. Physical activity decreases kidney cancer mortality. Curr Urol. 2017;10:193–198. doi: 10.1159/000447180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sloan JA, Cheville AL, Liu H, Novotny PJ, Wampfler JA, Garces YI, Clark MM, Yang P. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health Qual Outcomes. 2016;14:66. doi: 10.1186/s12955-016-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang L, Wang C, Wang J, Huang X, Cheng Y. Longitudinal, observational study on associations between postoperative nutritional vitamin D supplementation and clinical outcomes in esophageal cancer patients undergoing esophagectomy. Sci Rep. 2016;6:1572–1581. doi: 10.1038/srep38962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gunnell AS, Joyce S, Tomlin S, Taaffe DR, Cormie P, Newton RU, Joseph D, Spry N, Einarsdóttir K, Galvão DA. Physical activity and survival among long-term cancer survivor and non-cancer cohorts. Front Public Health. 2017;5:19. doi: 10.3389/fpubh.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, Deng Y, Chen Q, Wei S, Nie S, Liu L. The dose–response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50:339–345. doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 124.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 125.Scott JM, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC, Gibson T, Armenian SH, Nilsen TS, Oeffinger KC, Ness KK, Adams SC, Robison LL, Armstrong GT, Jones LW. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol. 2018;4:1352–1358. doi: 10.1001/jamaoncol.2018.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beasley JM, Kwan ML, Chen WY, Weltzien EK, Kroenke CH, Lu W, Nechuta SJ, Cadmus-Bertram L, Patterson RE, Sternfeld B, Shu X-O, Pierce JP, Caan BJ. Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat. 2012;131:637–643. doi: 10.1007/s10549-011-1770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeon J, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, Wigler DS, Atienza D, Messino M, Kindler H, Venook A, Fuchs CS, Meyerhardt JA. Impact of physical activity after cancer diagnosis on survival in patients with recurrent colon cancer: findings from CALGB 89803/alliance. Clin Colorectal Cancer. 2013;12:233–238. doi: 10.1016/j.clcc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 129.Haykowsky MJ, Scott JM, Hudson K, Denduluri N. Lifestyle interventions to improve cardiorespiratory fitness and reduce breast cancer recurrence. Am Soc Clin Oncol Educ Book. 2017;37:57–64. doi: 10.1200/EDBK_175349. [DOI] [PubMed] [Google Scholar]
- 130.Casla S, Hojman P, Márquez-Rodas I, López-Tarruella S, Jerez Y, Barakat R, Martín M. Running away from side effects: physical exercise as a complementary intervention for breast cancer patients. Clin Transl Oncol. 2015;17:180–196. doi: 10.1007/s12094-014-1184-8. [DOI] [PubMed] [Google Scholar]
- 131.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.cd007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.cd008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, GalvÃO DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, Von Gruenigen VE, Schwartz AL. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 134.Jones LW, Eves ND, Peddle CJ, Courneya KS, Haykowsky M, Kumar V, Winton TW, Reiman T. Effects of presurgical exercise training on systemic inflammatory markers among patients with malignant lung lesions. Appl Physiol Nutr Metabol. 2009;34:197–202. doi: 10.1139/H08-104. [DOI] [PubMed] [Google Scholar]
- 135.Ligibel JA, Irwin M, Dillon D, Barry W, Giobbie-Hurder A, Frank E, Winer EP, McTiernan A, Cornwell M, Pun M, Brown M, Jeselsohn R. Impact of pre-operative exercise on breast cancer gene expresision. In: Proceedings of the thirty-ninth annual CTRC–AACR San Antonio breast cancer symposium. San Antonio: American Association for Cancer Research; 2017. p. 77.
- 136.Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001. doi: 10.1002/14651858.CD005001.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loughney L, West MA, Kemp GJ, Grocott MPW, Jack S. Exercise intervention in people with cancer undergoing neoadjuvant cancer treatment and surgery: a systematic review. Eur J Surg Oncol. 2016;42:28–38. doi: 10.1016/j.ejso.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 138.Loughney L, West MA, Kemp GJ, Grocott MPW, Jack S. Exercise intervention in people with cancer undergoing adjuvant cancer treatment following surgery: a systematic review. Eur J Surg Oncol. 2015;41:1590–1602. doi: 10.1016/j.ejso.2015.08.153. [DOI] [PubMed] [Google Scholar]
- 139.Beaudry RI, Liang Y, Boyton ST, Tucker WJ, Brothers RM, Daniel KM, Rao R, Haykowsky MJ. Meta-analysis of exercise training on vascular endothelial function in cancer survivors. Integr Cancer Ther. 2018;17:192–199. doi: 10.1177/1534735418756193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 141.Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Taylor SJC, Rosario DJ, Thaha MA, Bourke L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9:CD010192. doi: 10.1002/14651858.CD010192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jones LW, Liu Q, Armstrong GT, Ness KK, Yasui Y, Devine K, Tonorezos E, Soares-Miranda L, Sklar CA, Douglas PS, Robison LL, Oeffinger KC. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32:3643–3650. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015;6:115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Padilha CS, Marinello PC, Galvão DA, Newton RU, Borges FH, Frajacomo F, Deminice R. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv. 2017;11:339–349. doi: 10.1007/s11764-016-0592-x. [DOI] [PubMed] [Google Scholar]
- 145.Jones LW, Courneya KS, Vallance JKH, Ladha AB, Mant MJ, Belch AR, Stewart DA, Reiman T. Association between exercise and quality of life in multiple myeloma cancer survivors. Support Care Cancer. 2004;12:780–788. doi: 10.1007/s00520-004-0668-4. [DOI] [PubMed] [Google Scholar]
- 146.Mills RC. Breast cancer survivors, common markers of inflammation, and exercise: a narrative review. Breast Cancer Basic Clin Res. 2017;11:117822341774397. doi: 10.1177/1178223417743976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gil-Rey E, Quevedo-Jerez K, Maldonado-Martin S, Herrero-Román F. Exercise intensity guidelines for cancer survivors: a comparison with reference values. Int J Sports Med. 2014;35:e1–e9. doi: 10.1055/s-0034-1389972. [DOI] [PubMed] [Google Scholar]
- 148.Toohey K, Pumpa K, McKune A, Cooke J, Semple S. High-intensity exercise interventions in cancer survivors: a systematic review exploring the impact on health outcomes. J Cancer Res Clin Oncol. 2018;144:1–12. doi: 10.1007/s00432-017-2552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.National Academies of Sciences EM, Health and Medicine Division. Board on health care services, national cancer policy forum. Incorporating weight management and physical activity throughout the cancer care continuum. In: Proceedings of a workshop. Washington, DC: National Academies Press; 2018. [PubMed]
- 150.Tudor-Locke C, Bassett DR. How many steps/day are enough? Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 151.Chen X, Lu W, Zheng W, Gu K, Matthews CE, Chen Z, Zheng Y, Shu XO. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res. 2011;4:1409–1418. doi: 10.1158/1940-6207.CAPR-10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carayol M, Bernard P, Boiché J, Riou F, Mercier B, Cousson-Gélie F, Romain AJ, Delpierre C, Ninot G. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol. 2013;24:291–300. doi: 10.1093/annonc/mds342. [DOI] [PubMed] [Google Scholar]
- 153.Valle C. Enhancing benefits of exercise for the survivor using emerging technology. In: ASCO Annual Meeting. Chicago; 201.
- 154.Joyner MJ, Sanchis-Gomar F, Lucia A. Exercise medicine education should be expanded. Br J Sports Med. 2017;51:625–626. doi: 10.1136/bjsports-2016-096620. [DOI] [PubMed] [Google Scholar]
- 155.McDermott LA, Murphy MH, McNeilly AM, Rankin JP, Gracey JH. Biological markers as an outcome measure of exercise in cancer rehabilitation: a systematic review. J Cancer Res Ther. 2018;14:267–277. doi: 10.4103/0973-1482.191036. [DOI] [PubMed] [Google Scholar]
- 156.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 158.Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76:248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Travier N, Velthuis MJ, Steins Bisschop CN, van den Buijs B, Monninkhof EM, Backx F, et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med. 2015;13:121. doi: 10.1186/s12916-015-0362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]