Abstract
The oxytocin (OXT) and dopamine systems synergistically facilitate striatal reactivity. Abnormal striatal activation has repeatedly been observed in patients with bipolar disorder (BD); however, such abnormality remains unclear in BD II. Here we aimed to investigate whether the corticostriatal connectivity was altered and the possible relationships among corticostriatal connectivity, OXT, and dopamine systems in BD II. Twenty-five BD II patients, as defined by the DSM-V, and 29 healthy controls (HC) were enrolled in this study. Plasma OXT was measured and striatal dopamine transporter (DAT) availability was assessed using [99mTc]TRODAT-1 single-photon emission computed tomography (SPECT). Brain network functional connectivity (FC) was measured during the resting-state using functional magnetic resonance imaging, and the dorsal caudate (DC) was selected as the seed region. The results showed that the OXT level was significantly lower in the BD II patients, while the striatal DAT availability was not significantly different between the BD II and HC groups. The BD II patients exhibited significantly lower FC between the DC and the executive control network (dorsolateral prefrontal, anterior cingulate cortex, and posterior parietal cortex) as compared with the HC. Only observed in HC, the DC-posterior parietal cortex FC was negatively correlated with the OXT level and striatal DAT availability. Our findings in the HC support a model in which the OXT and dopamine systems act in tandem to regulate corticostriatal circuitry, while the synergistic interaction was perturbed in BD II. Taken together, these results implied a maladaptive neuroplasticity in BD II.
Subject terms: Bipolar disorder, Molecular neuroscience
Introduction
The oxytocin (OXT) and dopamine systems interact and synergistically facilitate striatal reactivity for proper social behaviors1. Both social behaviors and the corticostriatal circuitry are mediated by OXT2,3 and dopamine4. Oxytocinergic neurons are projected from the hypothalamus to both the ventral tegmental area and substantia nigra1, while dopaminergic neurons are projected from the ventral tegmental area and substantia nigra to the ventral and dorsal areas of the striatum5. The dorsal striatum is involved in the reward system, reactive to social stimuli, as social behaviors may be differentiated by the corticostriatal functional connectivity (FC) strength in animals6 or by the caudate FC patterns in humans7.
The caudate is one of the most important neural substrates of the corticostriatal circuitry and is associated with cognition8. The projections from the prefrontal (associative circuit) and anterior cingulate (limbic circuit) cortex terminate in the caudate, then the caudate projects to other parts of the basal ganglia and back to the cortex via the thalamus8,9. Such corticostriatal circuitry is essential in motivating behaviors, and its dysfunction is well characterized in neuropsychiatric disorders10,11, including bipolar disorder (BD)10.
Previous neuroimaging studies have unraveled neuroplastic changes in the corticostriatal circuitry of patients with BD in terms of structural volume10, intrinsic FC12, and activity that may influence emotional processing13. Furthermore, altered FC between the caudate and default mode network (DMN)14 and between the caudate and executive control network (ECN)15,16 has been observed in BD patients as compared with controls. The ECN involves the dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex (ACC), and posterior parietal cortex (PPC)17. Different activation patterns in these networks may contribute to mood dysregulation18 and may distinguish BD from unipolar depression15,19. However, these patterns remain elusive in patients with BD II, because previous studies did not include BD II patients or distinguish them from BD I patients, even though BD II patients are as disabled as BD I patients20,21. In the current study, we aimed to investigate whether the caudate-seeded connectivity was altered in the DMN and ECN in BD II.
The dopamine hypothesis is a key theory in BD22, and BD is associated with abnormal dopamine transporter (DAT) levels23 and genetic factors of DAT24. As striatal dopamine deficits predict reductions in striatal FC in major depression25, we aimed to explore the relationships between possible FC alterations and striatal DAT in BD II. In addition, we have previously reported that BD II patients exhibited significant dysregulation in the plasma OXT level26. As the corticostriatal circuitry is mediated by OXT2,3 and dopamine4, we also aimed to explore the association between corticostriatal FC and OXT in BD II.
Methods
Subjects
All patients were recruited from the psychiatric outpatient department at National Cheng Kung University Hospital, while all healthy controls (HC) were recruited from the community through advertisement. All participants, aged between 18 and 70 years, were recruited and evaluated by an attending psychiatrist using the Chinese version of the Mini International Neuropsychiatry Interview27, the 17-item Hamilton Depression Rating Scale (HDRS) and the 11-item Young Mania Rating Scale (YMRS).
All patients were diagnosed by a psychiatrist to determine eligibility according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). The exclusion criteria for all the participants were as follows: (1) major mental illnesses (other than BD II for the BD II patients); (2) a history of head trauma, organic mental disease, or other neurological disorders; (3) inflammatory diseases, serious surgical conditions, or severe physical illnesses, such as acute coronary syndrome, kidney dialysis, or transplant; and (4) plans for pregnancy, breastfeeding, or a positive pregnancy test.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of National Cheng Kung University Hospital. All participants provided their written informed consent.
Experimental design
After enrollment into this study, the administration of medications, such as mood stabilizers (i.e., valproic acid or lithium), was recorded and adjusted according to the clinical manifestation and patients’ tolerance. There was no main regimen adjustment during the study.
Levels of plasma oxytocin
Blood samples for the OXT assay were collected in 5-mL EDTA tubes and stored at 4 °C in a fridge. Plasma was isolated by centrifugation at 1800×g for 15 min at 4 °C and immediately stored at ‒80 °C. OXT immunoreactivity levels were quantified in duplicate using a commercial OXT ELISA kit (Enzo Life Sciences, NY, USA, formerly Assays Designs, MI, USA). The detection range was from 12.35 to 1000 pg/ml, while the sensitivity (i.e., the minimum detectable dose of OXT) of our assay was <4.92 pg/ml. There was no extraction. The intra-assay precision and inter-assay precision of the assay were <10 and 12%, respectively (coefficient of variance (c.v.) (%) = SD/mean × 100; intra-assay: c.v. <10%; inter-assay: c.v. <12%).
[99mTc]TRODAT-1 single-photon emission computed tomography (SPECT)
The imaging procedure was identical to that used in our previous study. For detailed information regarding the selective labeling of DAT and obtaining/reconstructing/co-registering SPECT images, please refer to our published papers28–30. The MRI images were used as a reference, and so the slice thickness of the co-registered images was the thickness of the T2-weighted MRI images (3.3 mm). Regions of interest were the striatum (basal ganglia, caudate nucleus, and putamen) and occipital cortex, and the ratio of the radioactivity [(St – Oc)/Oc ratio] was then derived by dividing the difference between the average activity in the striatum (St) and the average activity in the occipital cortex (Oc) by the average activity in the occipital cortex (Oc)31.
Image acquisition
Resting-state functional MRI images were acquired using a 3.0 Tesla MRI scanner (MR750, GE Medical Systems, Milwaukee, WI, USA) with an 8-channel head coil in the Mind Research and Imaging Center of National Cheng Kung University. High-resolution T1-weighted 3-dimensional structural images ([TR]/[TE] = 7.7 ms/2.9 ms, flip angle = 12°, field-of-view = 224 mm2, in-plane matrix size = 256 × 256, slice thickness = 1 mm, and slices = 166) and T2*-weighted echoplanar imaging sequences ([TR]/[TE] = 2000 ms/33 ms, flip angle = 90°, field-of-view = 240 mm2, in-plane matrix size = 64 × 64, slice thickness = 3 mm, and slices = 40) were conducted to obtain high-resolution anatomical T1 images and functional MRI images. The first five functional scans of each resting-state functional MRI series were discarded for signal saturation and magnetic field stabilization. The participants remained awake during the scan (eyes closed, head still but relaxed, and without thinking about anything in particular). Head cushions and earplugs were provided to reduce head motion and noise, respectively.
Image preprocessing
Preprocessing was performed using the DPARSF toolbox (State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, China) with Statistical Parametrical Mapping 12 (SPM 12, Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm) in MATLAB 2016a (MathWorks Inc., Natick, MA USA). All functional images were subjected to slice timing, realignment for head-motion correction, and co-registration against each individual’s anatomical image, as well as normalization against the International Consortium for Brain Mapping (ICBM) space template of East Asian brains. Subjects with head motion of any volume >2 mm or 2° were excluded from further processing. The images were re-sampled to an isotropic 2 mm3 voxel size during the normalization step and then spatially smoothed using a 3D Gaussian kernel of 6 mm full-width at half-maximum. Linear trends were then removed from the resulting time series, and the time series was temporally band-pass filtered (0.01–0.1 Hz) in order to extract the low-frequency oscillations associated with spontaneous neuronal activity.
Definition of caudate seed and caudate-seeded functional connectivity maps
The left and right dorsal caudate (DC) seeds (3 mm radius), centered at MNI coordinates (±13, 15, and 9), were identified based on the published literature32. The mean time-series activity in the seed region of each subject was extracted. The DC-seeded FC maps were then generated. Each individual-level FC map obtained was then converted into a z-map using Fisher’s r-to-z transformation for second-level group analyses.
Additional test to examine specificity
One potential concern was whether the results were generic across the whole basal ganglia or specific to the DC. To further address this concern, we performed identical functional analyses using seeds at the inferior ventral striatum, ventral rostral putamen, and dorsal caudal putamen33. If similar differences were observed with other seeds in the basal ganglia, then the differences in the DC FC were not specific. Conversely, if comparable connectivities between groups were observed within other seeds in the basal ganglia, then the results were strengthened, because they were specific to the DC.
Statistical analyses
SPSS Statistics 20.0 (SPSS Inc., Chicago, IL) was used for the rest of the analyses. Results were considered significant at p < 0.05 (two-tailed). A two-sample t-test (or a Mann–Whitney U test, if the sample was not distributed in a Gaussian manner) was conducted to examine between-group (BD II patients vs. HC) differences in demographic characteristics, HDRS score, YMRS score, plasma level of OXT, and striatal DAT availability.
Image analysis
A two-sample independent t-test was employed to analyze the FC maps using SPM 12 (Wellcome Trust Centre for Neuroimaging, London, https://www.fil.ion.ucl.ac.uk/spm/). Statistical maps were computed to identify changes in the DC-seeded FC for between-group comparisons. Significance was thresholded at the voxel-level family-wise error rate (FWE)-corrected p = 0.05 for whole-brain multiple comparisons.
Two two-sample t-tests were performed to determine the correlations between the DC-seeded FC and the OXT level or striatal DAT availability, respectively. We entered the demeaned (in SPM) values as a regressor to identify brain regions with either positive or negative correlations with the DC-seeded FC in the BD II patients or HC. Significance was thresholded at the uncorrected voxel-level p = 0.001, followed by the FWE-corrected cluster-level at p = 0.05.
To display 3D imaging, we used MRIcroGL for 3D rendering (Department of Psychology, University of South Carolina http://www.mccauslandcenter.sc.edu/mricrogl). To further show the regression results in scatterplots, we extracted the DC-seeded FC values in the PPC (peak MNI coordinates [–28, –48, 56] and [–24, –66, 58], radius = 6 mm). The corresponding correlation coefficients (r) and p values were analyzed using SPSS Statistics 20.0.
Results
Twenty-five BD II patients and 29 HC were enrolled in this study. The BD II patients received mood stabilizer treatment, including valproic acid (n = 10, 40.0%), valproic acid plus antipsychotics (n = 10, 40.0%), and only antipsychotics (n = 3, 12.0%).
There were no significant differences between the BD II patients and HC in the demographic data (Table 1). The BD II patients in this study scored 5.29 ± 5.24 on the HDRS and 1.30 ± 2.03 on the YMRS, and 16 of them were euthymic (scores of <7 on the HDRS and YMRS; Table 1). The BD II patients, in comparison to the HC, demonstrated a higher YMRS score and a lower OXT level, but there was no significant difference in striatal DAT availability (Table 1).
Table 1.
Demographic data and baseline information.
| BD II patients (n = 25) | Controls (n = 29) | p value | |
|---|---|---|---|
| Age, years | 36.08 ± 11.64 | 32.83 ± 10.96 | 0.400 |
| Gender, female (%) | 17 (68%) | 17 (59%) | 0.576 |
| Body mass indexa | 27.52 ± 5.72 | 25.78 ± 5.85 | 0.281 |
| Education, yearsb | 14.20 ± 3.14 | 15.36 ± 2.02 | 0.273 |
| HDRS scorec | 5.29 ± 5.24 | 2.92 ± 1.41 | 0.300 |
| YMRS scored | 1.30 ± 2.03 | 0.00 ± 0.00 | <0.001* |
| Oxytocin (pg/mL)e | 167.27 ± 130.21 | 248.45 ± 52.99 | 0.012* |
| DAT availabilityf | 1.47 ± 0.22 | 1.43 ± 0.26 | 0.591 |
The data are presented as the means ± SD.
*p < 0.05.
aOne bipolar disorder (BD) II patient did not have body weight and body height measurements and was excluded from this calculation.
bFour controls did not answer the years of education question and were excluded from this calculation.
cOne BD II patient and 4 controls did not complete the 17-item Hamilton Depression Rating Scale (HDRS) and were excluded from this calculation.
dTwo BD II patient did not complete the 11-item Young Mania Rating Scale (YMRS) and were excluded from this calculation.
eFour BD II patients and eight controls did not undergo plasma oxytocin level measurement and were excluded from this calculation.
fTwo BD II patients did not undergo TRODAT and were excluded from this dopamine transporter (DAT) availability calculation.
The BD II patients exhibited significantly decreased FC between the left DC and the dlPFC, ACC, orbitofrontal cortex, temporal, supplementary motor area, pons, and cerebellum as compared with the HC (Fig. 1 and Table 2). Because the right DC-seeded FC analyses yielded similar results (i.e., DC-ECN hypo-connectivity, see Supplementary Table S1), we presented only the left DC-seeded FC maps in the following correlational analyses. In contrast, there were no significant group differences using seeds at the inferior ventral striatum, ventral rostral putamen, and dorsal caudal putamen as independent coordinates to validate our findings. These supplementary analyses supported the specificity of the DC-ECN circuitry in the BD II patients.
Fig. 1. Regions showed significant functional connectivity with the left dorsal caudate in between-group comparisons comparing bipolar II disorder patients < healthy controls.
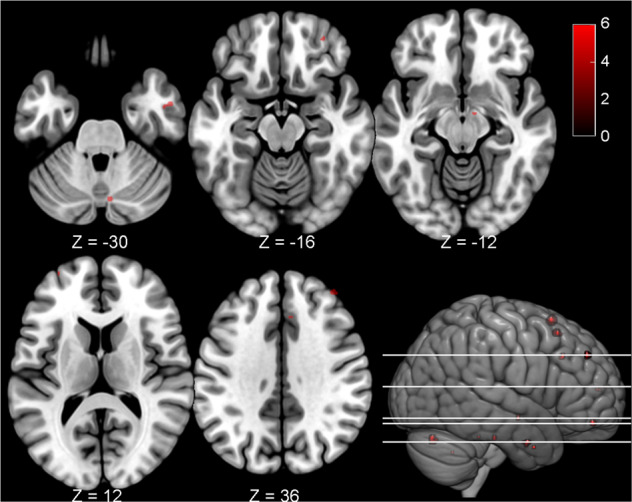
The bipolar II disorder patients exhibited impaired functional connectivity between the left dorsal caudate and the pons, temporal, orbitofrontal, dorsolateral prefrontal, anterior cingulate cortex, supplementary motor area, and cerebellum as compared with the controls. Each region’s coordinates are listed in Table 2. Results were thresholded at the FWE-corrected voxel level p = 0.05. The color bar denotes the t-scores. Figures are displayed according to neurological convention (left = left).
Table 2.
Peak MNI coordinates for the regions exhibiting significant resting-state functional connectivity with the left dorsal caudate for between-group differences comparing BD II patients < healthy controls.
| Region | Lateral | Cluster | BA | t score | Peak coordinate | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Dorsolateral prefrontal cortex | R | 7 | 9 | 6.40 | 40 | 46 | 36 |
| Dorsolateral prefrontal cortex | L | 2 | 10 | 5.61 | –32 | 58 | 12 |
| Supplementary motor area | R | 20 | 6 | 6.26 | 6 | 18 | 64 |
| Supplementary motor area | R | 11 | 6 | 6.08 | 10 | 20 | 54 |
| Middle temporal gyrus | R | 9 | 21 | 6.05 | 52 | –2 | –30 |
| Anterior cingulate cortex | R | 4 | 32 | 5.87 | 6 | 26 | 34 |
| Pons | R | 3 | — | 5.79 | 10 | –8 | –12 |
| Pons | R | 2 | — | 5.75 | 8 | –26 | –28 |
| Superior frontal gyrus | L | 2 | 10 | 5.68 | –20 | 52 | 10 |
| Temporal pole | R | 2 | 38 | 5.67 | 36 | 4 | –34 |
| Orbitofrontal cortex | R | 5 | 11 | 5.66 | 32 | 48 | –16 |
| Cerebellum (Crus I) | R | 12 | — | 6.05 | 8 | –74 | –28 |
| Cerebellum (Hemisphere III) | L | 1 | — | 5.54 | –14 | –38 | –26 |
| Cerebellum (Anterior lobe) | L | 1 | — | 5.52 | –20 | –58 | –38 |
Peak coordinates refer to the Montreal Neurological Institute (MNI) space. Significance was thresholded at the FWE-corrected voxel level p = 0.05.
No region was found in the contrast of bipolar disorder (BD) II patients > healthy controls.
BA Brodmann area.
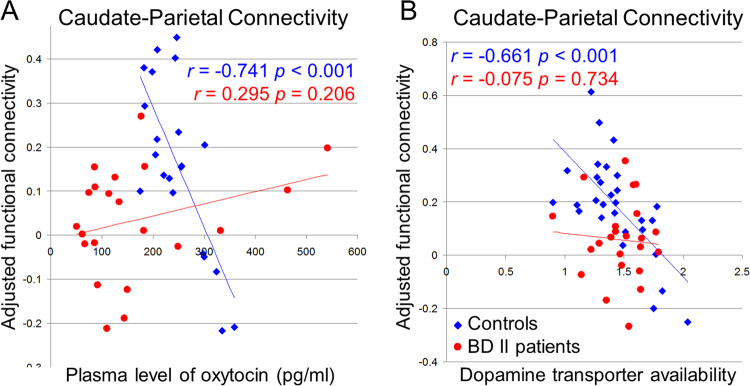
Among the HC, the DC-dlPFC and DC-PPC FC were negatively correlated with the plasma OXT level, while the DC-PPC FC was negatively correlated with the striatal DAT availability (Fig. 2 and Table 3). No correlation was found in the BD II patients.
Fig. 2. The caudate functional connectivity covaries with the oxytocin and dopamine transporter availabilities.
The oxytocin and dopamine transporter (DAT) availabilities were correlated negatively with the dorsal caudate-posterior parietal cortex functional connectivity only in the healthy controls (blue diamond) and not in the bipolar II disorder (BD II) patients (red dot). The scatterplots disclose the relationships between the dorsal caudate-seeded functional connectivity around the peak voxel (see Table 3) and a oxytocin and b dopamine transporter (DAT) availability. The corresponding correlation coefficients (r) and p values are provided. Significance was thresholded at the uncorrected voxel level p = 0.001, followed by the FWE-corrected cluster level p = 0.05.
Table 3.
Functional connectivity of the left dorsal caudate co-varying with oxytocin and left striatal dopamine transporter levels in healthy controls.
| Peak coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Direction | Region | Lateral | Cluster | BA | t score | x | y | z |
| Oxytocin levela | ||||||||
| Negative | Posterior parietal cortex | L | 6206 | 7 | 5.04 | −28 | −48 | 56 |
| Dorsolateral prefrontal cortex | L | 141 | 10 | 3.95 | −34 | 58 | 12 | |
| Lingual gyrus | L | 348 | 18 | 4.42 | −14 | −72 | −4 | |
| Lingual gyrus | R | 298 | 19 | 4.06 | 16 | −62 | 0 | |
| Superior occipital gyrus | R | 161 | 19 | 3.96 | 18 | −82 | 32 | |
| Positive | Cerebellum (Crus II) | L | 167 | – | 5.10 | −32 | −82 | −42 |
| Striatal DAT availabilityb | ||||||||
| Negative | Posterior parietal cortex | L | 395 | 7 | 4.46 | −24 | −66 | 58 |
| Positive | Cerebellum (Crus II) | L | 763 | – | 5.77 | −14 | −88 | −38 |
| Cerebellum (Crus II) | R | 180 | – | 4.55 | 50 | −60 | −50 | |
Peak coordinates refer to the Montreal Neurological Institute (MNI) space. Significance was thresholded at the uncorrected voxel level p = 0.001, followed by the FWE-corrected cluster level p = 0.05.
No correlation was found in the bipolar disorder (BD) II patients.
BA Brodmann area.
aFour BD II patients and eight controls did not have the plasma oxytocin level recorded and were excluded from this analysis.
bTwo BD II patients did not undergo TRODAT and were excluded from this dopamine transporter (DAT) availability calculation.
Discussion
Our results in the HC support a model in which the OXT and dopamine systems act in tandem to regulate the DC-ECN circuitry. However, such synergistic interaction was perturbed in the BD II patients, who had a lower OXT level and a lower DC-ECN hypo-connectivity as compared with the HC. Taken together, these results imply a maladaptive neuroplasticity in BD II. Also, our findings of alterations in FC between the DC and the orbitofrontal and anterior cingulate cortex were in line with the results of previous activity studies of BD13,34.
In the BD II patients, we found a dysfunctional DC-ECN connectivity (Fig. 1) and disrupted correlations of the DC-ECN connectivity with OXT and striatal DAT, implicating a perturbed reward system in BD II. Our reasoning was supported by the low OXT level in the BD II patients (Table 1). As OXT increases the FC between the corticostriatal circuitry3 and facilitates the DC-dlPFC loops for stable social bond formation2, the low OXT level in the BD II patients may be the reason for the dysfunctional corticostriatal circuitry, and may further result in poor social cognitive function35. Consistent with this observation, impaired ECN has also been found in premenstrual dysphoric disorder36 and repetitive negative thinking37, which are both related to altered reward systems (reinforced reward sensitivity38 and enhanced regret for no reward39, respectively).
In the HC, there were significant correlations between the DC-PPC FC and OXT level, as well as striatal DAT, suggesting oxytocinergic modulation of dopaminergic reward systems. Such OXT-driven dopaminergic modulation may have influenced social behaviors through control of motor activity in prior animal studies1,40. Consistent with previous findings, our research further demonstrated that the DC-PPC connectivity may be a fundamental neural signature in the corticostriatal circuitry. The DC-PPC connectivity has been reported in meta-analytic FC and diffusion tensor imaging studies41, and co-activation of DC-PPC is associated with decision-making42–44. Moreover, intranasal OXT may decrease dopamine release in the PPC and enhance attractiveness in a positron emission tomography (PET) study45. Given that the higher the OXT or DAT, the lower the DC-PPC connectivity (Fig. 2a, b), and the fact that the PPC is involved in spatial explorations46,47, our data provide a possible underlying mechanism of oxytocinergic modulation of dopaminergic reward systems dampening non-social exploration in the HC.
Our data showed hypo-connectivity between the DC and the cerebellum (Crus I) in the BD II patients (Table 1). In contrast, the DC-cerebellum (Crus II) FC was positively correlated with OXT and striatal DAT in the HC (Table 2). Both Crus I and Crus II may be involved in cognitive processing as they were more connected to the cortical ECN (dlPFC and PPC) during pain48. Furthermore, Crus I and Crus II are connected to the thalamus48, and altered cerebello-thalamo-cortical networks were found to be associated with psychosis49, and may be a heritable trait in schizophrenia50. Collectively, the DC-Crus II FC may interact with OXT and striatal DAT in HC, while the disrupted cerebello-DC-cortical circuitry may underlie the neuropathology of BD II.
Limitations
Our study had limitations in the relatively small sample size. As a cross-sectional study, understanding consequential or causal roles among OXT, striatal DAT, and corticostriatal connectivity was difficult to affirm. OXT immunoreactivity levels cannot be used to accurately infer true values of OXT, and therefore cannot be compared between studies51–53. Nevertheless, it is not our purpose in the current imaging study to measure absolute values of OXT in the BD II patients; rather, we used these assays for the comparison of relative levels of peripheral OXT between groups54, as well as investigating the relationship between OXT levels and functional connectivity. Another concern was that the plasma OXT level may not predict the central concentration51; however, other imaging studies found that the plasma OXT level was positively correlated with left caudate activation, and may augment the reward systems55. We did not find alterations in the DMN FC and striatal DAT availability; nevertheless, stability of the DMN may reflect a state of remission in BD56. In the same way, the lack of between-group differences in striatal DAT availability may have also resulted from the fact that most of the BD II patients in this study were in a euthymic state (Table 1), and therefore had no impaired striatal activity.
Conclusion
To the best of our knowledge, this was the first study to provide important and novel insights into the unexplored corticostriatal circuitry in BD II patients. The results extend existing knowledge from animal studies and offer some useful implications for neurobiological research going forward. The DC-PPC connectivity is a critical circuitry in the oxytocinergic modulation of dopaminergic reward systems, and dysfunctional DC-ECN circuitry implicates a maladaptive neuroplasticity in BD II patients, specifically. Other regions, such as cerebellum, are easily overlooked, but may also have important regulatory roles in the corticostriatal circuitry in BD II.
Supplementary information
Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 107-2314-B-006-082, MOST 107-2320-B-006-016, MOST 107-2320-B-006-071, MOST 108-2320-B-006-047-MY3, and MOST 108-2321-B-006-026-MY2) and National Cheng Kung University Hospital (NCKUH-10703005). The authors thank all the participants in this study and extend particular appreciation to En-Ju Lin and Chien Ting Lin from National Cheng Kung University, and Professor Yuan-Hwa Chou from Taipei Veterans General Hospital, for their technical and experimental assistance. We thank the Mind Research and Imaging Center (MRIC) at National Cheng Kung University for consultation and instrument availability. The MRIC is supported by the Ministry of Science and Technology.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-00972-6).
References
- 1.Charlet A, Grinevich V. Oxytocin mobilizes midbrain dopamine toward sociality. Neuron. 2017;95:235–237. doi: 10.1016/j.neuron.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Tops M, Koole SL, H IJ, Buisman-Pijlman FT. Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacol. Biochem. Behav. 2014;119:39–48. doi: 10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Bethlehem RAI, et al. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Transl. Psychiatry. 2017;7:e1099. doi: 10.1038/tp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atzil S, et al. Dopamine in the medial amygdala network mediates human bonding. Proc. Natl Acad. Sci. USA. 2017;114:2361–2366. doi: 10.1073/pnas.1612233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amadei EA, et al. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature. 2017;546:297–301. doi: 10.1038/nature22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham E, et al. The human coparental bond implicates distinct corticostriatal pathways: longitudinal impact on family formation and child well-being. Neuropsychopharmacology. 2017;42:2301–2313. doi: 10.1038/npp.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haber SN. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Marchand WR, Yurgelun-Todd D. Striatal structure and function in mood disorders: a comprehensive review. Bipolar Disord. 2010;12:764–785. doi: 10.1111/j.1399-5618.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd GM. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 2013;14:278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoddard J, et al. Aberrant intrinsic functional connectivity within and between corticostriatal and temporal-parietal networks in adults and youth with bipolar disorder. Psychol. Med. 2016;46:1509–1522. doi: 10.1017/S0033291716000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, et al. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord. 2012;14:432–441. doi: 10.1111/j.1399-5618.2012.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Y, et al. Aberrant resting-state functional connectivity in the default mode network in pediatric bipolar disorder patients with and without psychotic symptoms. Neurosci. Bull. 2019;35:581–590. doi: 10.1007/s12264-018-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Z, et al. Altered resting-state cerebral blood flow and functional connectivity of striatum in bipolar disorder and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;90:177–185. doi: 10.1016/j.pnpbp.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Thomas SA, et al. Preliminary analysis of resting state functional connectivity in young adults with subtypes of bipolar disorder. J. Affect. Disord. 2019;246:716–726. doi: 10.1016/j.jad.2018.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niendam TA, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst M, et al. Pubertal maturation and sex effects on the default-mode network connectivity implicated in mood dysregulation. Transl. Psychiatry. 2019;9:103. doi: 10.1038/s41398-019-0433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han KM, De Berardis D, Fornaro M, Kim YK. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;91:20–27. doi: 10.1016/j.pnpbp.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Rosa AR, et al. Functional impairment and disability across mood states in bipolar disorder. Value Health. 2010;13:984–988. doi: 10.1111/j.1524-4733.2010.00768.x. [DOI] [PubMed] [Google Scholar]
- 21.Yin L, et al. Inflammation and decreased functional connectivity in a widely-distributed network in depression: centralized effects in the ventral medial prefrontal cortex. Brain. Behav. Immun. 2019;80:657–666. doi: 10.1016/j.bbi.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashok AH, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol. Psychiatry. 2017;22:666–679. doi: 10.1038/mp.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang CC, et al. Dopamine transporter gene may be associated with bipolar disorder and its personality traits. Eur. Arch. Psychiatry Clin. Neurosci. 2015;265:281–290. doi: 10.1007/s00406-014-0570-0. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton JP, et al. Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: a concurrent (11)C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl. Psychiatry. 2018;8:264. doi: 10.1038/s41398-018-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lien YJ, et al. Plasma oxytocin levels in major depressive and bipolar II disorders. Psychiatry Res. 2017;258:402–406. doi: 10.1016/j.psychres.2017.08.080. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:34–57. [PubMed] [Google Scholar]
- 28.Lee LT, et al. Lower availability of striatal dopamine transporter in generalized anxiety disorder: a preliminary two-ligand SPECT study. Int. Clin. Psychopharmacol. 2015;30:175–178. doi: 10.1097/YIC.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 29.Chang WH, et al. Unaltered dopamine transporter availability in drug-naive patients with schizophrenia after 6 months of antipsychotics treatment: a naturalistic study. J. Clin. Psychopharmacol. 2017;37:21–26. doi: 10.1097/JCP.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 30.Tai YC, et al. Availability of striatal dopamine transporter in healthy individuals with and without a family history of ADHD. J. Atten. Disord. 2019;23:665–670. doi: 10.1177/1087054716654570. [DOI] [PubMed] [Google Scholar]
- 31.Hwang WJ, Yao WJ, Wey SP, Ting G. Reproducibility of 99mTc-TRODAT-1 SPECT measurement of dopamine transporters in Parkinson’s disease. J. Nucl. Med. 2004;45:207–213. [PubMed] [Google Scholar]
- 32.Di Martino A, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 33.Felger JC, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killgore WD, Gruber SA, Yurgelun-Todd DA. Abnormal corticostriatal activity during fear perception in bipolar disorder. Neuroreport. 2008;19:1523–1527. doi: 10.1097/WNR.0b013e328310af58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaskinn A, et al. Impairment in emotion perception from body movements in individuals with bipolar I and bipolar II disorder is associated with functional capacity. Int. J. Bipolar Disord. 2017;5:13. doi: 10.1186/s40345-017-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen N, et al. Resting-state functional connectivity in women with PMDD. Transl. Psychiatry. 2019;9:339. doi: 10.1038/s41398-019-0670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lydon-Staley DM, et al. Repetitive negative thinking in daily life and functional connectivity among default mode, fronto-parietal, and salience networks. Transl. Psychiatry. 2019;9:234. doi: 10.1038/s41398-019-0560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko CH, et al. Gonadotrophic hormone and reinforcement sensitivity systems in women with premenstrual dysphoric disorder. Psychiatry Clin. Neurosci. 2014;68:785–794. doi: 10.1111/pcn.12189. [DOI] [PubMed] [Google Scholar]
- 39.Allaert J, De Raedt R, Vanderhasselt M-A. When choosing means losing: Regret enhances repetitive negative thinking in high brooders. J. Exp. Soc. Psychol. 2019;85:103850. [Google Scholar]
- 40.Patel JC, Rossignol E, Rice ME, Machold RP. Opposing regulation of dopaminergic activity and exploratory motor behavior by forebrain and brainstem cholinergic circuits. Nat. Commun. 2012;3:1172. doi: 10.1038/ncomms2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson JL, et al. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 43.Verney SP, Brown GG, Frank L, Paulus MP. Error-rate-related caudate and parietal cortex activation during decision making. Neuroreport. 2003;14:923–928. doi: 10.1097/01.wnr.0000072842.93264.b6. [DOI] [PubMed] [Google Scholar]
- 44.Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol. Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Striepens N, et al. Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology. 2014;39:74–87. doi: 10.1016/j.psyneuen.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Denny-Brown D, Chambers RA. The parietal lobe and behavior. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1958;36:35–117. [PubMed] [Google Scholar]
- 47.Save E, Buhot MC, Foreman N, Thinus-Blanc C. Exploratory activity and response to a spatial change in rats with hippocampal or posterior parietal cortical lesions. Behav. Brain Res. 1992;47:113–127. doi: 10.1016/s0166-4328(05)80118-4. [DOI] [PubMed] [Google Scholar]
- 48.Diano M, et al. Cerebellar clustering and functional connectivity during pain processing. Cerebellum. 2016;15:343–356. doi: 10.1007/s12311-015-0706-4. [DOI] [PubMed] [Google Scholar]
- 49.Bernard JA, Orr JM, Mittal VA. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. 2017;14:622–628. doi: 10.1016/j.nicl.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao H, Ingvar M, Hultman CM, Cannon T. Evidence for cerebello-thalamo-cortical hyperconnectivity as a heritable trait for schizophrenia. Transl. Psychiatry. 2019;9:192. doi: 10.1038/s41398-019-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 52.Leng, G. & Sabatier, N. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J. Neuroendocrinol.28, 12413 (2016). [DOI] [PMC free article] [PubMed]
- 53.Robinson KJ, Hazon N, Lonergan M, Pomeroy PP. Validation of an enzyme-linked immunoassay (ELISA) for plasma oxytocin in a novel mammal species reveals potential errors induced by sampling procedure. J. Neurosci. Methods. 2014;226:73–79. doi: 10.1016/j.jneumeth.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 54.Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017;13:700–709. doi: 10.1038/nrendo.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rilling JK, et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Syan SK, et al. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J. Psychiatry Neurosci. 2018;43:298–316. doi: 10.1503/jpn.170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.