Abstract
INTRODUCTION:
Endoscopic submucosal dissection (ESD) could become a standard treatment for early stage esophageal neoplasia. Recurrence sometimes develops close to a previous ESD scar. These lesions are predictably difficult to treat with ESD because of severe fibrosis. We evaluated the clinical outcomes of ESD for esophageal neoplasia located close to a previous ESD scar.
METHODS:
This was a retrospective observational study in a single institution. A total of 549 consecutive patients with 927 esophageal lesions were treated with ESD. The primary outcomes were resectability and adverse events of esophageal neoplasia located close to previous ESD scars (ESD scar group) than in primary esophageal ESD (primary group). Furthermore, predictive factors of perforation were examined.
RESULTS:
A total of 545 primary and 29 ESD scars in consecutive patients were evaluated. En bloc and complete (R0) resection rates in the ESD scar group were lower than those in the primary group (79.3% vs 98.3%, P < 0.01 and 75.9% vs 93.4%, P < 0.01). Perforations occurred more frequently in the ESD scar group (10.3% vs 2.0%, P = 0.03). The ESD scar group was a predictive factor for perforation (odds ratio = 10.37, 95% confidence interval: 2.15–49.94, P = 0.004). There were similar results for inverse probability of treatment weighting methods (odds ratio = 6.78, 95% confidence interval: 1.40–32.98, P = 0.018).
DISCUSSION:
ESD for esophageal neoplasia located close to a previous ESD scar was difficult to completely resect and increased the likelihood of perforation but could be a treatment option.
INTRODUCTION
Esophageal cancer has a poor prognosis because of its progression speed and difficulty of detection in the early stage (1,2). Recently, new optical imaging techniques, especially narrow-band imaging endoscopy, have improved detection of esophageal neoplasia in the early stage (3,4). After endoscopic resection, there has also been improved survival (5–7). Endoscopic submucosal dissection (ESD) has been accepted in eastern countries with higher en bloc and complete resection rates for superficial esophageal neoplasia compared with those of endoscopic mucosal resection (EMR), regardless of the tumor's size or of presence of ulceration (8). For these reasons, ESD could become the standard treatment of early stage esophageal neoplasia (9–11).
Nevertheless, residual mucosa after esophageal ESD retains a high potential for the development of metachronous neoplasia. Most patients with squamous cell carcinoma (SCC) have multiple Lugol-voiding lesions that are known to be precancerous lesions secondary to alcohol consumption (12). In these patients, Lugol-voiding lesions are sometimes found on the cut margin of primary ESD. In these reasons, recurrent neoplasia sometimes develops close to a previous ESD scar. Additional esophagectomy and chemoradiotherapy for esophageal cancer have high treatment-related mortality and morbidity associated with low quality of life (QoL) (13,14). However, such lesions are predictably difficult to treat with ESD because of severe submucosal fibrosis caused by primary ESD that can cause increase adverse events and longer procedure times (15). Favorable outcomes with acceptable adverse events were reported in local recurrence of gastric cancers after ESD (16–18). Patients who had residual or local recurrent lesions had frequent perforations and required longer procedure times than those with primary lesions even after colonic EMR (19–22). Esophageal ESD is more difficult to perform than stomach because of the narrow lumen and thin wall.
Because there have been no reports of repeated ESD for esophageal neoplasia located close to a previous ESD scar, this study aimed to evaluate the outcomes of repeated ESD for esophageal neoplasia located close to a previous ESD scar.
METHODS
Patients
This was a retrospective observational study that was conducted at a single referral hospital in Japan. A total of 549 consecutive patients with 927 superficial esophageal neoplasias including patients with intraepithelial neoplasia who were treated with ESD between May 2004 and March 2016 (Figure 1) were enrolled (23). The initial or largest lesion was the target in patients with multiple lesions. The exclusion criterion was missing data in patients. We divided the enrolled patients into 2 groups: the patients who were treated for esophageal neoplasia located close to a previous ESD scar (ESD scar group) and those who were treated for primary esophageal neoplasia that was not close to an ESD scar (primary group). The ESD scar group was defined as having lesions localized at treated scars of previous ESD and at least one part of lesions endoscopically.
Figure 1.
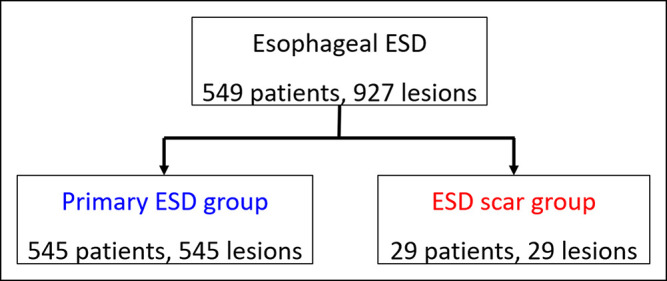
Diagram of the study design. ESD, endoscopic submucosal dissection.
Written informed consent was obtained from each patient. The ethics committee of the Osaka City University Graduate School of Medicine approved the study's protocol (number 3987). The study's information was available to the public on the internet, and the participants were offered the opportunity to opt out.
Outcomes
The primary outcomes of this study were clinical outcomes including en bloc resection rate, complete (R0) resection rate, and adverse events of the ESD scar group. These patients were compared with the primary group. Secondary outcomes were predictive factors of esophageal perforation.
Endoscopic submucosal dissection procedure
A complete description of the ESD procedure has been previously reported (10,24–26). We used a single-channel endoscope (GIF-Q260J; Olympus Medical Co. Ltd, Tokyo, Japan) and an electrosurgical generator (ICC 200 or VIO300D; ERBE Elektromedizin GmbH, Tübingen, Germany). The primary electrosurgical knives were a bipolar needle knife (B knife; Xemex Co., Tokyo, Japan) and a monopolar needle knife (Flush knife, DK2618JN; Fujifilm Medical, Tokyo, Japan). We sometimes used a hook knife (KD-620; Olympus Medical Co. Ltd) if severe fibrosis was observed. Marking dots were placed outside the margin of the lesion using iodine staining for SCC or indigo carmine and using narrow-band imaging magnification endoscopy in adenocarcinoma. In the ESD scar group, submucosal injection and mucosal incision were started from the part without scar-induced fibrosis, the well-lifted part of the oral side. Submucosal dissection also started from the nonfibrotic area; then, the fibrotic area was dissected at last. The circumference of esophagus was measured as the proportion of the esophageal circumference based on its division into 12 equal parts when the esophageal lumen was spread to its maximum width using full insufflation (e.g., 9/12 and 11/12) (24). The measured values were represented as percentages.
Definitions
An en bloc resection was defined as a resection in one piece that included all markings. A complete (R0) resection was defined as an en bloc resection with histologically cancer-free margins (6,10). Perforation was defined as a visible hole in the esophageal wall, exposing the mediastinal space during endoscopic procedure. Delayed perforation was defined as perforation detected after an ESD procedure but not during it. Delayed bleeding was defined as bleeding with hematemesis or melena that required endoscopic reintervention or transfusion after an ESD procedure. Esophageal stricture was defined when a standard upper gastrointestinal endoscope with a 9.2-mm diameter (GIF-Q260; Olympus Medical Co. Ltd) could not be passed through the treatment site. The endoscopist was considered to be an expert if they had an experience performing >30 esophageal ESDs and was considered a trainee if they had performed ≤30 esophageal ESDs.
Pathological examination
After fixation in formalin, the resected specimens were cut into 2-mm slices. Histological type, depth of invasion, lateral and vertical margins, and lymphovascular invasion were evaluated in each slice.
Statistical analyses
The data were expressed as means and standard deviations for continuous variables, and comparisons were performed using Student t-test. The data were presented as numbers for categorical variables, and comparisons were performed using the χ2 test or Fisher exact test when necessary. Crude logistic regression analysis was used to evaluate the simultaneous effects of 18 variables (Table 4) on perforation, and the risk factors for perforation were estimated by calculating the odds ratios (ORs) and the 95% confidence intervals (CIs). Generalized estimating equations were used to analyze the repeated measures data (27). Multivariate logistic analysis was used for variables with P value < 0.05. We used 17 variables that may influence perforation to generate propensity scores ranging from 0 to 1 using logistic regression. The validity of the model was assessed by estimating the area under the receiver operating characteristics (ROC) curve using C-statistics. The reliability of the model was evaluated using the Hosmer–Lemeshow test for goodness-of-fit. We also used the inverse probability of treatment weighting (IPTW) method based on propensity scoring, which is an approach used to adjust for confounding factors between binary groups without reducing the sample size (28,29). We adjusted for confounding factors using the estimated propensity scores to assign weights to the data. Analyses involving IPTW linear regressions for perforation were performed. The statistical analyses were performed using IBM SPSS software, version 23.0 for Windows (IBM Corporation, Armonk, NY). All statistical tests were two-sided, and a value of P < 0.05 was considered statistically significant.
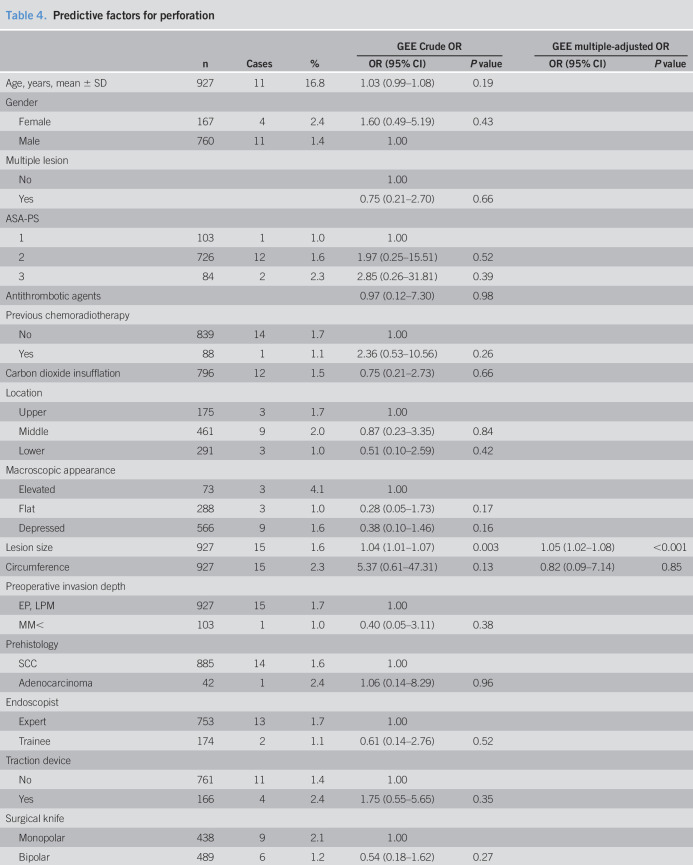
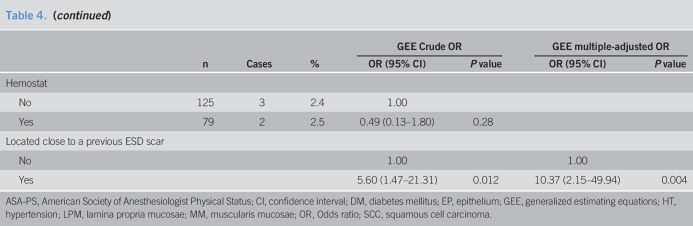
Table 4.
Predictive factors for perforation
RESULTS
Clinicopathological characteristics of the study subjects and each lesion
A total of 549 patients with 927 lesions were treated with ESD in our institution (Figure 1). Of these, 29 patients were in the ESD scar group and 545 were in the primary group.
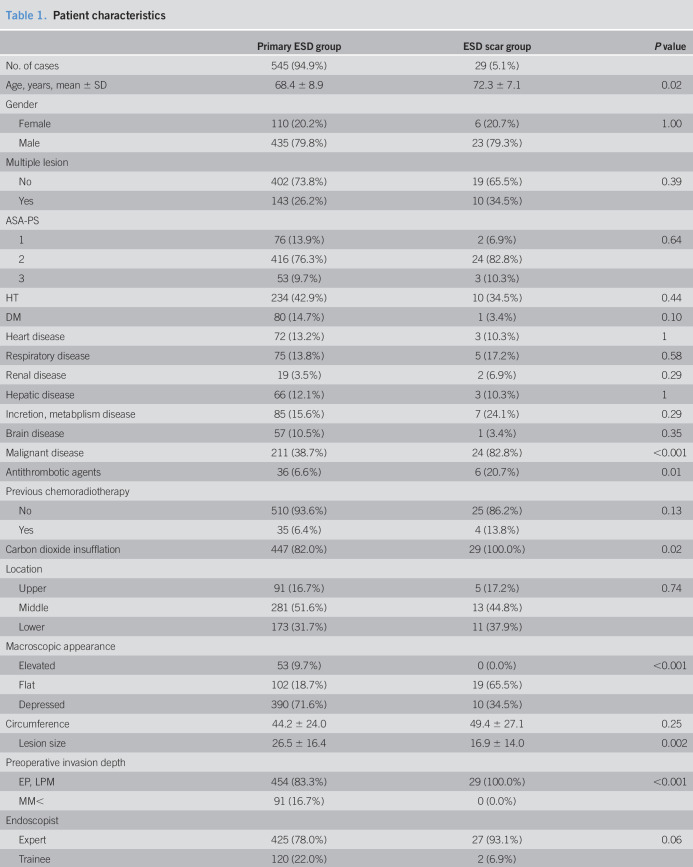
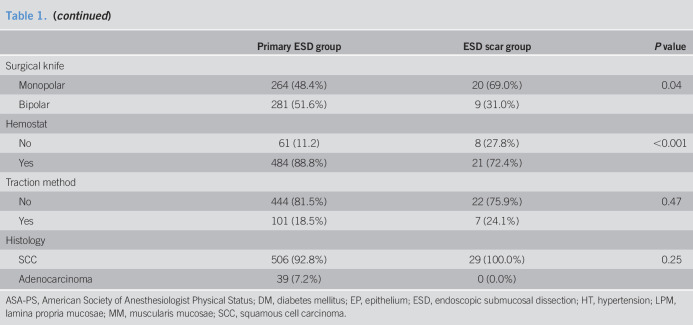
The clinicopathological characteristics of the study subjects are shown in Table 1. Compared with patients of the primary group, patients in the ESD scar group were significantly older, had a higher incidence of malignant disease, used antithrombotic agents more significantly, had a higher incidence of flat lesion, had smaller-size lesions, and were diagnosed in shallower depths. Monopolar knife and carbon dioxide insufflation were used more frequently, whereas hemostat was used lower in the ESD scar group than in the primary group.
Table 1.
Patient characteristics
Clinical outcomes
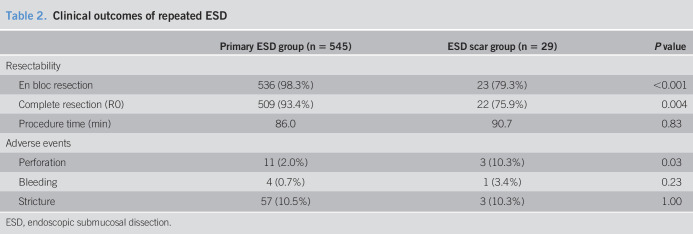
Clinical outcomes and adverse events are shown in Table 2. Depth of invasion of all lesions in the ESD scar group were limited to the lamina propria mucosa without lymphovascular invasion. En bloc and complete resection rates in the ESD scar group (79.3% and 75.9%) were significantly lower than those in the primary group (98.3% and 93.4%). There was a higher incidence of perforation in the ESD scar group (10.3%) than in the primary group (2.0%, P = 0.03). However, these could be treated with endoscopic closure and conservative management (Table 3 and Figure 2). There were no significant differences in delayed bleeding, esophageal stricture, and procedure time between the groups. We examined clinical outcomes associated with positional relationship between lesions and scars 15 lesions which were mostly located on previous scars or 14 lesions adjacent to scars. En bloc and complete resection rates of the lesions which were mostly located on scars (60.0% and 60.0%) were significantly lower than those of the lesions adjacent to scars (100.0% and 92.9%, P < 0.01 and P < 0.01). The lesions which were mostly located on scars had frequent perforation (13.3%) than did the lesions adjacent to scars (7.1%, P = 0.03). Third recurrent lesions located close to second ESD scar were observed 10.3% (3/29) during a median follow-up period of 52 (range 18–98) months. One lesion was a suspected local recurrence in a patient who had positive lateral margins at the second ESD. The others were suspected metachronous recurrences near the second ESD scar, whose margins were negative in the second ESD. All these were treated with repeated ESD.
Table 2.
Clinical outcomes of repeated ESD
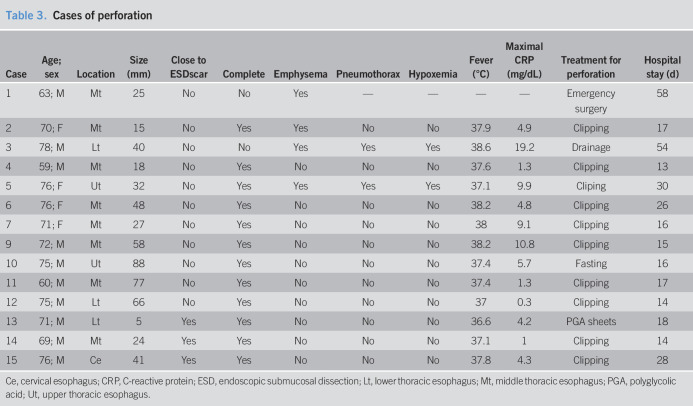
Table 3.
Cases of perforation
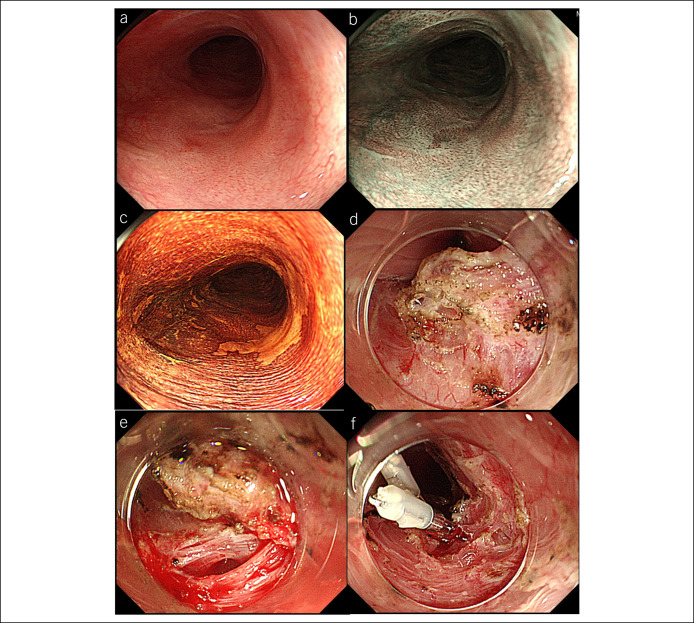
Figure 2.
A patient of ESD for esophageal cancer located close to previous ESD scar. (a–c) Esophageal cancer located close to previous ESD scar using white light imaging, narrow-band imaging, and Lugol staining. (d) Submucosal fibrosis was observed during ESD. (e, f) Perforation was closed using endoclips. ESD, Endoscopic submucosal dissection.
Predictive factors for esophageal perforation
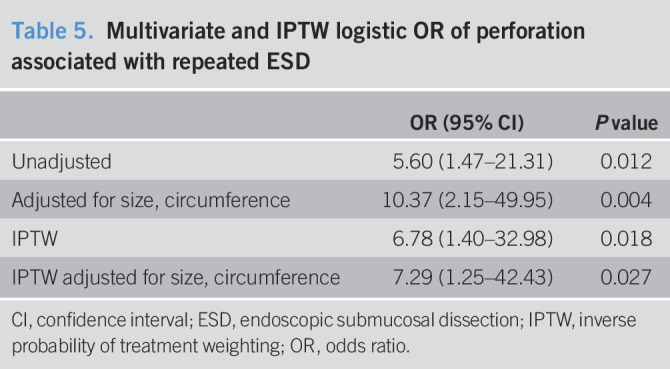
Esophageal perforation occurred in 15 patients (2.7%) including one delayed perforation (No. 10, Table 3). We evaluated the predictive factors for esophageal perforation in patients who were treated with ESD for esophageal neoplasia (Table 4). Larger lesion size (OR = 1.04, 95% CI: 1.01–1.07, P = 0.003) and ESD scar group (OR = 5.60, 95% CI: 1.47–21.3, P = 0.012) compared with the primary group were predictive factors of perforation using crude logistic regression analysis. Larger lesion size (OR = 1.05, 95% CI: 1.02–1.08, P < 0.001) and lesions located close to a previous esophageal ESD scar were independent predictive factors for perforation after adjustment for lesion size and circumference using multivariate logistic regression analysis (OR = 10.37, 95% CI: 2.15–49.94, P = 0.004, Table 5).
Table 5.
Multivariate and IPTW logistic OR of perforation associated with repeated ESD
Evaluation using the inverse probability of treatment weighting method
The propensity score model was well-calibrated (Hosmer–Lemeshow test: P = 0.99) and showed good discrimination between the groups (C-statistic = 0.94). After adjusting the model for differences in relation to the baseline risk factors using the IPTW method with the generalized estimating equations, we determined that esophageal perforation increased in association with lesion located close to a previous esophageal ESD scar (OR = 6.78, 95% CI: 1.40–32.98, P = 0.018) (Table 5). After adjustment for lesion size and circumference using multivariate logistic regression analysis, a similar result was observed (OR = 7.29, 95% CI: 1.25–42.43, P = 0.027).
DISCUSSION
The findings of this study showed that repeated ESD for esophageal neoplasia located close to a previous ESD scar had lower en bloc and complete resection rates with higher incidence of perforation compared with conventional esophageal ESD for primary lesions. Repeated ESD was an independent risk factor for esophageal perforation using multivariate regression analysis after adjusting for differences in the baseline risk factors using the IPTW method with propensity score. Most lesions adjacent to the scar were completely resected, whereas the lesions which were mostly located on scars were difficult to resect with frequent perforation because of widespread severe fibrosis. However, perforations could be closed endoscopically.
Large lesion size was a reported risk factor for positive resection margins after ESD for superficial esophageal cancer, which could cause local recurrence (30). In esophageal ESD, because esophageal stricture may occur because of resection with wide circumference, minimal margin resection is important to avoid esophageal stricture (24,25,31). In patients treated with repeated ESD, because our study included few patients treated with positive resection margin of primary ESD, patients with residual cancer may be few. Multiple Lugol-voiding lesions (which are well known precancerous lesions) make it difficult to determine cut margins or widths of resection for colesions. Such colesions may sometimes develop into secondary esophageal neoplasia. Even if no colesions were observed during primary ESD, secondary lesions sometimes arise close to primary ESD scar because the residual mucosa after esophageal ESD retains a high potential for the development of metachronous neoplasia in the esophagus because of alcohol consumption (12). Therefore, we sometimes encountered esophageal neoplasia located close to primary ESD scar.
Those lesions located close to ESD scar make it difficult to perform ESD because of severe fibrosis due to primary ESD. Favorable en bloc and R0 resection rates with acceptable perforation rates were reported for second ESD for local recurrence or residual gastric cancer after primary ESD and which were similar with the results of primary ESD (16–18). By contrast, patients who had residual or local recurrent lesions even after EMR in the colon had frequent perforations and required longer treatment time than those who underwent ESD for primary colorectal lesions (19–22). Similar results were observed in our study, possibly associated with thinner esophageal submucosal and muscle layer than those in the stomach. Lower resection rates were observed in this study compared with those of studies of the colorectum because it is known that more severe submucosal fibrosis was observed after ESD compared with after EMR due to differences of resection depth and the burning effect of electrocautery (16,18,22). There were some lesions after chemoradiotherapy that also affected submucosal fibrosis in our study. The procedure time was similar between the groups, possibly affected by the difference of endoscopists and lesion size between the 2 groups.
It was reported that mucosal deficiency larger than 75% of the circumference of the esophagus was a risk factor for intraoperative perforation (32). Because we needed to predict perforation before ESD, our study used only preoperative circumference, possibly resulting in the finding of absence of risk by multivariate regression model in this larger volume study. A previous study found that most perforations occurred in the ESD by nonexperienced endoscopists (11). However, experts tended to have perforation rates comparable with those of trainees in this study because experienced endoscopists tended to treat repeated ESD for the lesions located close to previous ESD scars.
The lesions located close to previous ESD scar tended to be smaller, flatter, more superficial, and in older patients than primary lesions. Nevertheless, lower en bloc and complete resection rates with higher incidence of perforation and similar procedure time were observed in the ESD scar group. Suitable surveillance every 6 months may make it possible to detect early stages, resulting in limited lamina propria mucosa depth in histological assessment. It is of interest to note that these patients had more malignant disease and had used antithrombotic agents. Although they may depend on older age than primary lesions, associations between these factors and metachronous esophageal neoplasia would be hopefully elucidated in the future. Because of the difficulty of ESD, experienced endoscopists tended to perform repeated ESD with an increasing use of carbon dioxide. The monopolar surgical knife was selected because it cuts more sharply than does the bipolar knife. The hook knife was sometimes used to avoid perforation. Less use of hemostats may have led to less intraoperative bleeding in the ESD scar group with fibrotic tissue. In the context of such selection bias, the outcomes of repeated ESD were worse than those of primary ESD.
Some other methods were considered for the esophageal neoplasia located close to previous ESD scars. Esophagectomy can achieve R0 resection; however, it carries higher mortality and morbidity associated with worse QoL than do endoscopic procedures (13,14). Although chemoradiotherapy may cure the lesions, no response and local recurrence would be predicted with several adverse events and worse QoL. A case series found that argon plasma coagulation was useful in esophageal neoplasia located close to previous ESD scars with few adverse events (33). However, the absence of pathological assessment after argon plasma coagulation may lead to local and distant recurrence, whereas ESD can enable an accurate pathological assessment. Because it is sometimes difficult to diagnose invasion depth due to fibrosis of scar in lesions located close to a previous ESD scar, repeated ESD may be beneficial in pathological assessment, even if there is lower resectability with higher perforation (16). All perforations were managed with a conservative treatment, and it was possible to control third lesions located close to second ESD scars using repeated ESD and endoscopic follow-up, even if there were not resected en bloc. Using carbon dioxide insufflation and several closing techniques of the perforation site reduced worsening after perforation (34–36).
Our study has some limitations. First, because this was a single tertiary center study with a small sample size, generalization may not be possible. Most patients who underwent repeated ESD were performed by experienced endoscopists because of their case difficulty. The small sample size may have affected the reliability with wide 95% CIs. Further multicenter large-scale study should be conducted in the future. Second, selection bias can persist in retrospective studies because the relationship between perforation and repeated ESD may have been affected by confounding factors, including age, gender, lesion size, and clinical characteristics. The IPTW method was used to evaluate these causal effects that were independent of the confounding effects without reducing the sample size (37). Third, most lesions were SCC in this study because most esophageal cancer is SCC in Japan as in eastern Asian countries as well. Nevertheless, the results of this study would be helpful for esophageal adenocarcinoma that is predominant in western countries because similar situations would be experienced.
In conclusion, in repeated ESD for esophageal neoplasia located close to a previous ESD scar, it was difficult to achieve complete resection and there was greater likelihood of perforation than with primary esophageal ESD. Nevertheless, ESD could be an option to treat esophageal neoplasia located close to a previous ESD scar with acceptable adverse event rates.
CONFLICTS OF INTEREST
Guarantor of the article: Yasuaki Nagami, MD, PhD.
Specific author contributions: None to report.
Financial support: None to report.
Potential competing interests: None to report.
ACKNOWLEDGMENT
We would like to thank Editage (www.editage.jp) for English language editing.
Study Highlights.
WHAT IS KNOWN
✓ ESD is useful for esophageal neoplasia.
✓ Recurrence sometimes develops close to a previous ESD scar.
✓ No information for the treatment of such lesions.
WHAT IS NEW HERE
✓ ESD for lesions located close to a previous ESD scar was difficult with a lower resection rate and more frequent perforation.
✓ All perforations could be treated with endoscopic closure and conservative management.
✓ Repeated ESD could resect re-recurrence in the same location.
TRANSLATIONAL IMPACT
✓ Repeated ESD could be an option to treat esophageal neoplasia located close to a previous ESD scar with acceptable adverse event rates.
REFERENCES
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71–96. [DOI] [PubMed] [Google Scholar]
- 3.Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: A multicenter randomized controlled trial. J Clin Oncol 2010;28:1566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagami Y, Tominaga K, Machida H, et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: A prospective comparative study using propensity score matching. Am J Gastroenterol 2014;109:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara R, Tanaka H, Iishi H, et al. Long-term outcome of esophageal mucosal squamous cell carcinoma without lymphovascular involvement after endoscopic resection. Cancer 2008;112:2166–72. [DOI] [PubMed] [Google Scholar]
- 6.Nagami Y, Ominami M, Shiba M, et al. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig Liver Dis 2017;49:427–33. [DOI] [PubMed] [Google Scholar]
- 7.Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860–6. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066–72. [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: A meta-analysis. Dig Dis Sci 2014;59:1862–9. [DOI] [PubMed] [Google Scholar]
- 10.Nagami Y, Machida H, Shiba M, et al. Clinical efficacy of endoscopic submucosal dissection for adenocarcinomas of the esophagogastric junction. Endosc Int Open 2014;2:E15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: A multicenter retrospective cohort study. Endoscopy 2015;47:775–83. [DOI] [PubMed] [Google Scholar]
- 12.Katada C, Yokoyama T, Yano T, et al. Alcohol consumption and multiple dysplastic lesions increase risk of squamous cell carcinoma in the esophagus, head, and neck. Gastroenterology 2016;151:860–9.e7. [DOI] [PubMed] [Google Scholar]
- 13.Min YW, Lee H, Song BG, et al. Comparison of endoscopic submucosal dissection and surgery for superficial esophageal squamous cell carcinoma: A propensity score-matched analysis. Gastrointest Endosc 2018;88:624–33. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Ding H, Chen T, et al. Outcomes of endoscopic submucosal dissection vs esophagectomy for T1 esophageal squamous cell carcinoma in a real-world cohort. Clin Gastroenterol Hepatol 2019;17:73–81.e3. [DOI] [PubMed] [Google Scholar]
- 15.Huh CW, Lee HH, Kim BW, et al. Predictive factors of submucosal fibrosis before endoscopic submucosal dissection for superficial squamous esophageal neoplasia. Clin Transl Gastroenterol 2018;9:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higashimaya M, Oka S, Tanaka S, et al. Endoscopic submucosal dissection for residual early gastric cancer after endoscopic submucosal dissection. Gastrointest Endosc 2013;77:298–302. [DOI] [PubMed] [Google Scholar]
- 17.Hoteya S, Iizuka T, Kikuchi D, et al. Secondary endoscopic submucosal dissection for residual or recurrent tumors after gastric endoscopic submucosal dissection. Gastric Cancer 2014;17:697–702. [DOI] [PubMed] [Google Scholar]
- 18.Sekiguchi M, Suzuki H, Oda I, et al. Favorable long-term outcomes of endoscopic submucosal dissection for locally recurrent early gastric cancer after endoscopic resection. Endoscopy 2013;45:708–13. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi R, Hirasawa K, Ikeda R, et al. The feasibility of colorectal endoscopic submucosal dissection for the treatment of residual or recurrent tumor localized in therapeutic scar tissue. Endosc Int Open 2017;5:E1242–E1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroki Y, Hoteya S, Mitani T, et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol 2010;25:1747–53. [DOI] [PubMed] [Google Scholar]
- 21.Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015;16:14–21. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto T, Saito Y, Matsuda T, et al. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc 2011;25:255–60. [DOI] [PubMed] [Google Scholar]
- 23.Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th edition: Part I. Esophagus 2017;14:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagami Y, Ominami M, Shiba M, et al. Prediction of esophageal stricture in patients given locoregional triamcinolone injections immediately after endoscopic submucosal dissection. Dig Endosc 2018;30:198–205. [DOI] [PubMed] [Google Scholar]
- 25.Nagami Y, Shiba M, Ominami M, et al. Single locoregional triamcinolone injection immediately after esophageal endoscopic submucosal dissection prevents stricture formation. Clin Transl Gastroenterol 2017;8:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ominami M, Nagami Y, Shiba M, et al. Comparison of propofol with midazolam in endoscopic submucosal dissection for esophageal squamous cell carcinoma: A randomized controlled trial. J Gastroenterol 2018;53:397–406. [DOI] [PubMed] [Google Scholar]
- 27.Smith PJ, Hadgu A. Sensitivity and specificity for correlated observations. Stat Med 1992;11:1503–9. [DOI] [PubMed] [Google Scholar]
- 28.Fukunaga S, Nagami Y, Shiba M, et al. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc 2017;85:143–52. [DOI] [PubMed] [Google Scholar]
- 29.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Stat Med 2004;23:2937–60. [DOI] [PubMed] [Google Scholar]
- 30.Wen J, Linghu E, Yang Y, et al. Relevant risk factors and prognostic impact of positive resection margins after endoscopic submucosal dissection of superficial esophageal squamous cell neoplasia. Surg Endosc 2014;28:1653–9. [DOI] [PubMed] [Google Scholar]
- 31.Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009;41:661–5. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi M, Yano T, Kato T, et al. Risk factors for intraoperative perforation during endoscopic submucosal dissection of superficial esophageal squamous cell carcinoma. World J Gastroenterol 2017;23:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tahara K, Tanabe S, Ishido K, et al. Argon plasma coagulation for superficial esophageal squamous-cell carcinoma in high-risk patients. World J Gastroenterol 2012;18:5412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ominami M, Nagami Y, Tanaka C, et al. Endoscopic technique for closure of a large gastric tube perforation by using endoclips with line-assisted complete closure. Endoscopy 2019;51:E49–E50. [DOI] [PubMed] [Google Scholar]
- 35.Nagami Y, Shiba M, Ominami M, et al. A novel endoscopic technique for closure of a large esophageal perforation using the clip-and-snare method with the prelooping technique. Endoscopy 2016;48(Suppl 1):E250–1. [DOI] [PubMed] [Google Scholar]
- 36.Nagami Y, Shiba M, Arakawa T. Use of PGA sheets in the endoscopic closure of a perforation after endoscopic submucosal dissection for gastric-tube cancer. Am J Gastroenterol 2016;111:768. [DOI] [PubMed] [Google Scholar]
- 37.Gotoda T, Hatta W. Are randomized control studies needed to evaluate the efficacy of treatment techniques that are clearly minimally invasive and already widely used? Gastrointest Endosc 2017;85:153–4. [DOI] [PubMed] [Google Scholar]