Abstract
Neurocritical care is an approach of comprehensive care through multidisciplinary coordination and implementation of neuroprotective strategies to reduce the risk of neurologic injury among critically ill patients. Premature infants are at a special risk of sustaining brain injury and having adverse neurodevelopmental outcome. The pathogenesis of “encephalopathy of prematurity” is tightly linked to hemodynamic instability during postnatal transition, immaturity of the cerebral vascular bed and nervous system, and the commonly encountered inflammation in an intensive care setting. Clinical assessment aided by renewed monitoring techniques, together with therapies supported by best available evidence may provide opportunities to salvage these vulnerable brains. Indeed, to promote optimal brain development and to ensure neurodevelopmental intact survival is of imperial priority in the modern care of preterm infants.
Keywords: Neurocritical care, Premature infant, Brain injury, Near-infrared spectroscopy, Neuroprotection, Neurodevelopmental disability
Disease burden of preterm birth
Preterm birth, defined as born before 37 completed weeks of gestation, is a disease of worldwide epidemic. Recent estimates show that approximately 10.6% of all live births globally are preterm, which brings about a global incidence of preterm birth of nearly 15 million per year [1,2]. Preterm birth is the leading cause of neonatal death (i.e. during the first four weeks of life or days 0–27) and death in children under age 5 [3]. Preterm birth is further classified as moderate to late preterm (32–37 weeks), very preterm (28–32 weeks), and extremely preterm birth (less than 28 weeks) with the majority of preterm deliveries in the developed countries occur in the late preterm period. With fast advances in maternal-fetal medicine and neonatal intensive care, survival of extremely preterm infants, or even of infants born near the limit of viability, have increased significantly over the past decades. However, complications arisen from premature birth remain significant in the survivors, and the incidence and severity of the complications are positively correlated to the degree of immaturity. Most important complications of prematurity include long-term neurodevelopmental problems, such as cerebral palsy (CP), motor and cognitive impairment, visual and auditory deficits, and behavioral problems [4]. These not only cause an emotional burden for families but also an economic burden on society. The societal cost of prematurity in the United States-accounting for medical costs, educational costs, and lost productivity-has been estimated to be at least 26.2 billion dollars each year [5]. As such, the focus of care in premature infants has shifted to not just survival but intact neurodevelopmental outcome as well as reasonable quality of life for the increasing number of NICU survivors and their families.
In recent years, there are around 200,000 infants born in Taiwan annually, and the preterm birth rate is estimated to be 8–10% [6,7]. Of these preterm births, very low birth weight (VLBW) infants, defined as those with a body weight of 1500 g at birth, represent 0.8% of live births yet account for over 50% of perinatal deaths in Taiwan [6]. It has been shown that the high infant mortality rate, as compared to our neighbor Japan, is attribute to our high preterm birth rate. Under our National Health policy, Ministry of Health and Welfare Central Health Insurance Agency of Taiwan government reported that the average cost for each preterm infant under 3 years of age ranged from 80,000 to 100,000 NT each year (presented by Taiwanese Department of Health in the 27th Annual Meeting of Taiwan Society of Neonatology 3-12-2017.) It is obvious that the disease burden comes not only from keeping the preterm infants alive, but also from remedying of the debilitating associated morbidities in the survivors.
Concept of neurocritical care
The concept of neurocritical care was initiated in adult intensive care for patients with severe neurological and neurosurgical conditions. By implementing multidisciplinary care and adherence to evidence based guidelines, patients who received neurocritical care have shown improved survival and shortened hospital stay in adults, and better survival and improved functional outcomes in children with traumatic brain injury [[8], [9], [10], [11]]. Conventionally, the goal of intensive care is to stabilize patient's cardiorespiratory system for adequate delivery of sufficient oxygen to the tissue to meet metabolic demands. Neurocritical care provides an interface between the brain and other organ systems for comprehensive medical and specialized neurological support by integrating and balancing the management of both the brain and the body [12].
The first Neonatal Neurocritical Care Service was established in 2008 at the University of California San Francisco (UCSF). The highlight of this program emphasized the importance of early collaboration of neonatologists and pediatric neurologists in the care of newborn infants at risk for neurologic compromise. To achieve standardized management and to improve neurodevelopmental outcomes, crucial elements of the program also involve education to nurses and other medical providers of brain-focused care, application of new therapies and diagnostic tools, and establishing pathways or care bundles to enable consistency of care and reduce error [13]. The NICU (neonatal intensive care unit) experience of adapting therapeutic hypothermia (TH) as a treatment modality for term and near term infants with perinatal asphyxia, and the compiling evidence of its beneficial treatment effects in decreasing mortality and neurodevelopmental deficits in the survivors [14] has accelerated the development of NeuroNICUs across the United States and beyond. The other major group of NICU admissions at risk of brain injury are neonates born extremely preterm.
For preterm infants, neurocritical care should be considered in a significantly different prospect than that for other population. First of all, preterm brain injury occurs at various stages of early and ongoing brain development, therefore the impact of disturbed normal maturation of the brain should be taken into account. Secondly, other than exposure to the unprotected ex-utero environment, in a way for preterm infants neurocritical care seems to emphasize in the prevention of specific insults that may lead to brain injuries to occur as compared to remedying neurological consequences of acute insults that had already happened, such as hypoxic-ischemic encephalopathy following acute myocardial infarction or birth asphyxia.
Brain injury in the premature infants
Studies of brain injury in the premature infants started in the 1970s. Based on both animal experiments and clinical correlates between imaging and functional outcomes, we have since gained much better insights in the pathophysiology of premature brain injury [15]. The most important acquired brain lesions of premature neonates are periventricular-intraventricular hemorrhages (PIVH) and (diffuse) white matter injury (WMI). Diagnosis of PIVH has been relied on bedside cranial ultrasound studies. Papile et al. first classified the widely used severity of PIVH into four grades with Grade III and IV denoting severe IVH with ventricular enlargement and parenchymal involvement [16]. Volpe later modified the grading system based on the presence and amount of blood in the germinal matrix and lateral ventricle into: Grade I germinal matrix hemorrhage (GMH) with no or minimal IVH (<10% of the ventricular area), Grade II IVH 10–50% of ventricular area, and Grade III IVH >50% of ventricular area, often with distension of lateral ventricle. Volpe added a specific notion of presence (and the location and extend) of periventricular echodensity to signify periventricular hemorrhagic infarction (PVHI) and involvement of white matter injury in the severe form of PIVH, which accounts for most of the morbidity attributable to IVH per se [17,18]. This type of lesion occurs in only 4–5% of all very low birth weight (VLBW, birth weight of <1500 g) preterm infants, but the incidence increases significantly to 45% in those born with extremely low birth weight (ELBW, birth weight of <1000 g) [[19], [20], [21]], and up to 75% of the involved infants develop mild to severe PIVH-related sequelae in later life [22,23].
PIVH develops from the fragile vascular network of GM where neurons and glial cells arise. In human, GM starts to involute around 28-week gestation and often disappears at term. Predisposing factors of PIVH include a GM with an immature vasculature, a pressure passive cerebral circulation, and hemodynamic perturbations in sick premature infants [24]. In >90% of the cases, PIVH occurs during the first 3 postnatal days when the immature cardiorespiratory system is challenged to make the hemodynamic adaptation from fetal-to-neonatal life that comes too early [18].
Another form of brain injury in the premature infants is WMI, with periventricular leukomalacia (PVL) being the fundamental and most well-known feature. PVL is defined as focal periventricular necrosis associated with more diffuse reactive gliosis and microglial activation in the surrounding cerebral white matter [17,18]. Banker and Larroche first described the lesion in detail and related it to cardiorespiratory events and cerebral ischemia causing injuries in the watershed periventricular white matter [25]. The neuropathology of dWMI was later elaborated by Volpe el al. and termed as “encephalopathy of prematurity,” which consist of primary destructive disease (acute cell death) and secondary maturational and trophic disturbances causing failure of pre-OL differentiation and hypomyelination [26]. It is generally accepted that the main initiating pathogenetic mechanisms of PVL are ischemia and inflammation, and further dWMI arise from myelination failure and disturbed connectivity of CNS pathways [17]. Inflammation leads to activation of microglia, free radical attack, and excitotoxicity [27]. For instance, bacterial sepsis activates toll-like receptors that are present on the surface of microglia in white matter. The activation of microglia leads to a release of free radicals as well as pro-inflammatory cytokines, and injury to the developing pre-oligodendrocytes, axons, and neurons in the white matter [27]. The activation of pro-inflammatory cytokines suppresses the synthesis of growth factors, such as insulin-like growth factor (IGF)-1, that are important for brain growth and differentiation [28]. Evidence to link brain injury caused by PIVH-hemorrhagic infarction and dWMI with poor neurodevelopmental outcomes have been supported by neuroimaging and cohort follow up reports worldwide. Involved neurodevelopmental domains include motor function, cognition, behavior, hearing, and vision, and the impact could last into adulthood for the survivors [29].
Neuroprotective maneuvers for the premature neonates
In this review, we will discuss the mainstay of neuroprotective interventions in today's standard of care for preterm infants, and some strategies that have gained ample attention but are still pending to win general consensus. The backbone of the discussion is summarized in Table 1. Since researches in neuroprotection for preterm infants are on-going and with rapid progression, the discussion here although aiming at as up-to-date as possible, could not cover all the work that has been done so far. It should better serve as a scheme to lay out how neurocritical care of premature infants can be structured.
Table 1.
Neuroprotective strategies for preterm neonates.
| Prenatal |
| Prevent premature birth Antenatal corticosteroids Antenatal magnesium sulfate (MgSO4) |
| Intrapartum |
| Delayed cord clamping (DCC) |
| Postnatal |
| A. Maintain stable cerebral blood flow (CBF) |
| Hemodynamic monitoring to ensure systemic perfusion |
| NIRS for regional cerebral tissue oxygenation (rScO2) |
| Transcutaneous CO2 monitor |
| B. EEG monitoring and seizure control |
| EEG, amplitude-integrated EEG (aEEG) antiepileptic medications |
| C. Neuroimaging |
| Cranial ultrasound (CUS) and MRI |
| E. General Care |
| Optimal nutrition |
| Inflammatory cytokines |
| F. Pharmacological prevention |
| Erythropoietin (EPO) |
| G. Pain control and sedation |
| H. Developmental care |
Abbreviation: NIRS: Near infrared spectroscopy.
Prenatal management
Prevention of premature birth is the single most effective way to eliminate related sequelae. If preterm birth is inevitable, antenatal corticosteroids effectively enhance fetal lung maturity and reduce postnatal hypoxia and hypercarbia events that may lead to PIVH. Guidelines of antenatal corticosteroids during imminent preterm delivery was initially established in 1995 [30], and later studies have confirmed use of antenatal corticosteroids compared to non-use is associated with a lower rate of PIVH as well as decreased short- and long-term neurodevelopmental impairment among VLBW and even ELBW infants born at 23–25 weeks gestation [31,32].
Magnesium sulfate has long been used in pregnant women for tocolysis, or for eclampsia seizure prevention. Following observational reports in 1990s and later randomized control trials on the neuroprotective effects of MgSO4, recent meta-analysis confirmed an association between prenatal exposure to magnesium sulfate and less frequent subsequent neurologic morbidities in that magnesium sulfate given to women with imminent preterm delivery reduces cerebral palsy at 2 years of age by about 30% and the number needed to treat is 63 (95% confidence interval 43 to 87) [33].
Delivery room management
Upon delivery, clamping of the umbilical cord physiologically terminates placental-fetal circulation. From fetal point of view, occlusion of the umbilical vein decreases right atrial preload by 40–50% whereas occlusion of the umbilical artery results in an immediate increase in LV afterload. Both the decrease in preload and the increase in afterload negatively affect cardiac contractility and output. In preterm infants with limited myocardial reserve, this drastic hemodynamic change has a negative impact on postnatal circulatory adaptation and causes low cardiac output (shock), fluctuations in cerebral blood flow, which might further results in cerebral ischemia or hemorrhage. Delayed cord clamping (DCC) usually allows 30–60 s’ of the placental transfusion to replenish neonatal preload before cord clamping, if performed following lung aeration to drop pulmonary pressure, may help establishing a postnatal serial circulation and achieve a smoother transition [34]. Although delayed “physiological” clamping after lung aeration showed better perfusion and less bradycardia in animal models [35], results from recent enthusiastic clinical trials on if DCC aids in decrease of IVH or improves long-term neurological outcomes in preterm infants are still inconclusive [36,37].
NICU neurocritical care
Maintain stable cerebral blood flow and oxygen delivery based on hemodynamic monitoring
Implementation of neurocritical care in NICU can be divided into 4 domains: assessment, monitoring, protection, and development. Since neurological and other physical examination in the sick tiny premature infants are often limited and variable depends on their degree of maturity, monitor by electronic devices resumes as a crucial approach of assessing patients’ disease progress and response to treatment.
As discussed earlier, maintenance of circulatory homeostasis during immediate postnatal transition is a real challenge in the premature infants. Successful transition from a parallel fetal circulation into a serial neonatal circulation relies on mature lungs to open alveoli and achieve optimal oxygenation, so to mitigate pulmonary pressure and reduce right to left shunting via foramen ovale and ductus arteriosus. Hemodynamic instability and unstable cerebral blood flow (CBF) can play a key role in prematurity-related brain injuries through causing weakening and rupture of the GM vessels (PIVH), or triggering repeated ischemia-reperfusion oxidative stress, leading to WMI [24,38]. Nevertheless, in preterm infants within the first 48 h after birth, there is no positive association with blood pressure and systemic blood flow [39], and no consensus on how to define adequate blood pressure, especially in the ELBW infants. How to establish clear and firm hemodynamic boundaries, as a part of neuroprotective strategy, is still a clinical challenge that we are facing.
Although there are various modalities for bedside hemodynamic monitoring, from conventional heart rate, blood pressure, O2 saturation measurement to Doppler ultrasound assessment of cardiac output and regional organ blood flow, none of them has taken into account of the distributive factors of cerebral circulation, specifically mediators of local vascular beds, and the operation of autoregulation. For sick preterm infants, cerebrovascular autoregulation, the mechanisms that control blood flow to the brain, is easily perturbed. Once the limits of cerebral autoregulation are reached, cerebral perfusion pressures will vary directly with arterial blood pressure, and such pressure-passive perfusion has been identified as a critical mechanism for PIVH [18].
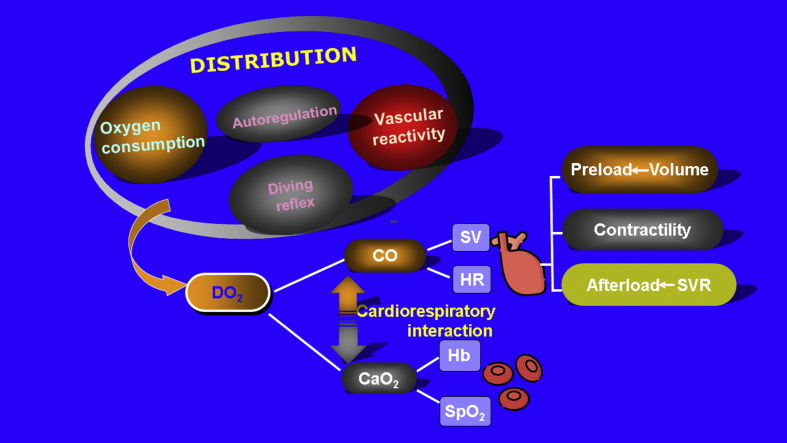
Unfortunately, the lower limits of autoregulation in neonates are not precisely known. A comprehensive graphic illustration of how tissue oxygen delivery is determined by macro- and microcirculation is illustrated in Fig. 1. It is crucial to recollect the pathophysiological relevance of factors contribute to circulatory compromise since the merit of neurocritical care relies on correct interpretation of monitored data to direct appropriate patient-specific interventions. Fig. 1.
Fig. 1.
Determinants of cerebral blood flow and oxygen delivery.
Abbreviations used: CO: cardiac output; CaO2: arterial oxygen content; DO2: oxygen delivery; Hb: hemoglobin; SpO2: Oxygen saturation; SVR: systemic vascular resistance.
Near infrared spectroscopy (NIRS)
Near infrared spectroscopy (NIRS)-derived monitoring of regional tissue oxygen saturation provides a continuous, non-invasive measurement of end-organ perfusion and oxygen metabolism. Cerebral NIRS (cNIRS) has been increasing used in the very preterm infant. Regional cerebral oxygen saturation (rScO2) represents oxygen supply to the brain, while cerebral fractional tissue oxygen extraction, which is the ratio between rScO2 and systemic arterial oxygen saturation, reflects cerebral oxygen utilization. NIRS then can be applied to assess cerebral autoregulation, blood volume, flow, and balance between oxygen supply and demand [40]. Recent European multicenter randomized controlled trial (the SafeboosC study) demonstrated that using a dedicated treatment guideline in combination with cerebral NIRS monitoring was able to reduce the hypoxic burden in extremely preterm neonates in the first days after birth [41]. Further follow-up study from this SafeboosC cohort showed that the early burden of hypoxia was associated with the occurrence of severe PIVH [42]. A phase III SafeboosC trial of infants less than 28 weeks GA has been initiated in 2018 with the objectives to investigate if NIRS in combination with an evidence-based treatment guideline, compared with treatment as usual, during the first 72 h of life will result in a reduction in death or survival with severe brain injury at 36 weeks postmenstrual age. Hopefully through this study interventions guided by cNIRS monitoring as a neuroprotective maneuver in preterm infants could be consolidated.
For the sake of reiterating pathophysiology of cerebral oxygenation derangement and how clinical intervention could eliminate subsequent injuries, treatment guidelines based on cNIRS in the SafeboosC trial is summarized in Table 2 [43].
Table 2.
Pathophysiologically Oriented Treatment Guideline for off range rScO2.
| rScO2 low (<55%) | rScO2 high (>85%) |
A. Cardiovascular
|
A. Respiratory
|
Abbreviations: rScO2: regional cerebral oxygen saturation; CBF: cerebral blood flow; CO: cardiac output; PDA: patent ductus arteriosus; MAP: mean airway pressure; MV: minute ventilation.
∗1. This treatment guideline is targeted at premature infants, specifically those ELBW infants and within 72 h of life.
∗2. Modified from recommendations of SafeBoosC phase II randomised clinical trial.
Non-invasive CO2 monitor
Inadvertent hypercarbia or hypocarbia is common in NICU ventilated patients. Alterations in PaCO2 can significantly affect cerebral hemodynamics in that hypocarbia and hypercarbia decreases and increases CBF, respectively [44,45]. Both extremes and fluctuations of PaCO2 are associated with severe intraventricular hemorrhage, whereas hypocarbia, cerebral vasoconstriction and ischemia can lead to PVL. Using end-tidal CO2 monitor and NIRS, Dix et al. revealed that CO2 fluctuations are associated with changes in cerebral oxygenation and electrical activity in the first 72 h of life in preterm infants [46]. Therefore, to avoid significant hypocarbia and hypercarbia, and to prevent related brain injury, it might be prudent to incorporate continuous non-invasive CO2 monitor into standard of care in the ventilated ELBW infants [47].
EEG monitoring and seizure control
Electroencephalography (EEG) is a common method of monitoring brain function. Due to technical difficulties in obtaining a conventional EEG in the tiny premature infants from lack of spaces for leads placement and disturbed signal quality in a NICU setting, amplitude-integrated EEG (aEEG) has been adopted as an alternative for continuous brain function monitoring [48]. Developed in the 1960s by Prior and colleagues, aEEG in its simplest form is a processed single-channel EEG that is filtered and time-compressed [49]. Today a 2-channel or 4-channel (C3–P3, C4–P4) aEEG is more commonly used. For preterm infants, interpretation of aEEG had been mostly based on Hellstrom-Westas’ classification using background analysis and sleep wake cycling (SWC) [50]. However, researchers later discovered that in very preterm infants aEEG patterns change not only with increasing gestational age, and also as chronological and postmenstrual age (PMA) advances [[51], [52], [53]]. So far in the neurocritical care of preterm infants, aEEG has been used in the assessment of cortical functional maturity, for seizure detection and monitoring, and in the prediction of long-term neurological outcome, particularly in extremely preterm infants [54]. Diagnostic algorithms have been proposed based on EEG readings by ICNs with brain-focused care, and there're also clinical evidence to support that aEEG or EEG recorded within early postnatal (<7) days correlates with later neurodevelopmental outcome [55].
The use of anti-epileptic medication in preterm infants so far are still based on experience from adult or pediatric patients, and there's a lack of clear understanding of what the impact of immaturity on the neurophysiology and pharmacokinetics of seizure and its treatment might have. It is suggested that standardized seizure guideline agreed upon by the neurocritical care team should be established in advance to specify which drugs shoul be used, in what order, and the dosages recommended.
Neuroimaging
Neuroimaging of preterm infants has become part of routine clinical care, with cranial ultrasonography (cUS) and MRI being the two commonly used modalities. Clinical management that would be taken based on neuroimage findings include drainage of post-hemorrhagic hydrocephalus, early developmental intervention, prognostic prediction and parental consultation. The widely used Papile's grading of PIVH was based on cUS findings. Today, cUS is still considered effective for the diagnosis of a large GMH-IVH, but less so for detection of WMI [56]. On the other hand, MRI may show lesions which are not always easy to interpret and may be cause for concern for parents [57]. It is suggested that using an MRI scoring system for the scan taken at term equivalent age (TEA) may help to assess scans in a systematic way and to predict neurodevelopmental outcome [58].
There are many caveats in incorporating cUS and MRI as part of the neurocritical care for the very and extremely preterm infants. In this imperative review by de Vries et al. [59] the authors came to the following conclusions: For prediction of CP, sequential cUS during admission, at discharge and at TEA by an experienced professional should be able to recognize most of the lesions which will result in moderate to severe CP, GMFCS level III–V. For better understanding of more subtle disabilities, MRI is helpful, especially when not only using conventional imaging but also using advanced MR techniques. MRI at discharge may be too early to reliably assess myelination of the posterior limb of the internal capsule (PLIC), which is one of the most important additional findings and predictors of CP obtained with conventional MRI. Performing a TEA MRI has the advantage of assessing subtle white matter lesions, cerebellar lesions and myelination of the PLIC [59].
General care
Meticulous NICU care provide opportunities to protect the vulnerable preterm brain not only by attempting to reduce the incidence of severe IVH but also by reducing other morbidities such as growth restriction and nosocomial infection, all of which likely would impact brain development and neurodevelopmental outcome.
Faltering growth is common in preterm infants. Nutritional supply interrupted by preterm birth often causes suboptimal energy provision and deficiency in macro- and micronutrients, which subsequently jeopardizes normal brain development. Early aggressive nutritional support has been proven safe and is advocated in the standard of care for even the tiniest ELBW infants [60]. Evidence of early nutrition affecting morbidity and neurodevelopmental outcome has been clearly provided by Ehrenkranz et al. From the classic study they demonstrated that in ELBW infants faster weight gain in the first weeks after birth is associated with improved neurodevelopmental outcome in a dose dependent manner. When the rate of weight gain velocity increased from 12.0 to 21.0 g·kg−1·day−1, the incidence of CP, low Bayley II Mental and Psychomotor Developmental Indices, abnormal neurological examination and neurodevelopmental impairment each decreased by at least 50% [61].
Standardized neonatal intensive care by itself may also help achieving better neurodevelopmental outcome in the preterm infants. For instance, complying with a central line care bundle may decrease line-associated infection and late onset sepsis. And as previously discussed that inflammation and cytokine surge induced by bacterial infection can cause WMI, could be so avoided [62]. Another example is the now well-known association between necrotizing enterocolitis (NEC) and later neurodevelopmental disorder in ELBW infants, which was first described by Salhab et al., in 2004 [63].
Pharmacological prevention
Erythropoietin (EPO)
Anemia of prematurity (AOP) is a premature birth-related complication arisen mainly from shifting of erythropoietin (EPO) production from low hypoxemia-sensitive liver in fetal stage to higher efficient renal production when close to term gestation [64], and iatrogenic effects such as phlebotomy blood loss in the NICU. Treatment of AOP with recombinant human erythropoietin was first reported by Halperin et al., in 1990 [65]. At the same time, there was also evolution in understanding of erythropoietin's role in neuroprotection. EPO and its receptor are upregulated by exposure to hypoxia and proinflammatory cytokines after brain injury. Binding of EPO and its receptors results in neurotrophic effects of neurogenesis, oligodendrogenesis, and angiogenesis, thus aids in recovery of locally injured neuronal cells. It also has neuroprotective effects from setting off glial cells' release of mediators causing neuro-inflammation and cell death [66]. Following results of a meta-analysis showing preterm infants who received EPO had better neurodevelopmental outcome than those who received placebo [67], a phase 3 clinical trial (Preterm Erythropoietin Neuroprotection Trial, PENUT), was conducted to assess the safety and efficacy of early high-dose erythropoietin for neuroprotection in extremely preterm infants. Results of this multi-centered trial involving 731 ELBW infants were recently published which showed no significant difference between the EPO group and the placebo group in the incidence of death or severe neurodevelopmental impairment at 2 years of age. There were also no differences between the groups in the rates of any major complications of prematurity such as ROP or intracranial hemorrhage [68].
Pain control and sedation
It was once questioned if neonates were able to perceive pain due to the immaturity of the sensory nervous system. Strong evidence of pain sensation in preterm infants were later provided by neuroanatomical and behavioral studies [69]. Preterm newborns in the NICU experience frequent and repetitive painful stimuli from procedures such as heel pricking, venous puncture or endotracheal intubation. There are increasing concerns of what could be the consequences of early life pain and how is it going to affect the infants as they grow? A growing body of evidence reports long-term consequences of early life pain in humans show the association between early life pain and long-term neurosensory, cognitive, and psycho-behavioral disorders [70,71]. However, without methods to subjectively measure the degree of pain, and with current uncertainty of the effect of analgesics and sedatives exposure in the developing nervous system, assessment of pain and how to best alleviate it remains an important issue to be addressed in the critical care of preterm neonates.
Developmental care
Initiated in 1986, Newborn Individualized Developmental Care and Assessment Program (NIDCAP) is an individualized care giving principle to reduce stress and promote physiologic stability for better neurological outcome of sick neonates [72]. While the evidence base for NIDCAP specifically in improving neurodevelopmental outcome is limited [73], it introduced the concept of physical care that addresses the environmental impact on the developing brain and considering patients’ progress through developmental stages. Nowadays, a team consisted of parents, developmental specialists, occupational therapists, physical therapist along with neonatologists and neurologists are all crucial elements of a preterm infants care, either during the hospitalization or at the time of discharge.
Conclusion and prospective
Walking through the path of establishing a comprehensive brain-focused intensive care, we have gained insights and humble success in salvaging the vulnerable brains of those extremely premature infants. However, injury to and subnormal development of the periventricular white matter is still common. Future direction of neuroprotection in the premature infants are aiming at both pharmacological prevention, specifically using agents with anti-inflammatory (e.g. melatonin) or neurotrophic properties (e.g. IGF-1), and repair the injured brain with progenitor cells such as mesenchymal stem cells. Both interventions are now under substantial investigation and are showing promising potential to be able to close the gap between survival and intact neurological, mental and behavioral outcome in the survivors of premature infants.
We are hoping that neurologically intact survival can be achieved in all babies that are born preterm, even in those most vulnerable ones with ELBW.
Conflicts of interest
The author declares no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Chawanpaiboon S., Vogel J.P., Moller A.B., Lumbiganon P., Petzold M., Hogan D. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 19 February 2018. Preterm birth.https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth [Google Scholar]
- 3.Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron I.S., Rey-Casserly C. Extremely preterm birth outcome: a review of four decades of cognitive research. Neuropsychol Rev. 2010;20:430–452. doi: 10.1007/s11065-010-9132-z. [DOI] [PubMed] [Google Scholar]
- 5.Patel R.M., Kandefer S., Walsh M.C., Bell E.F., Carlo W.A., Laptook A.R. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Y.Y., Wang S.H., Chou H.C., Chen C.Y., Hsieh W.S., Tsao P.N. Morbidity and mortality of very low birth weight infants in Taiwan-Changes in 15 years: a population based study. J Formos Med Assoc. 2016;115:1039–1045. doi: 10.1016/j.jfma.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Su B.H., Hsieh W.S., Hsu C.H., Chang J.H., Lien R., Lin C.H. Neonatal outcomes of extremely preterm infants from taiwan: comparison with Canada, Japan, and the USA. Pediatr Neonatol. 2015;56:46–52. doi: 10.1016/j.pedneo.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Suarez J.I. Outcome in neurocritical care: advances in monitoring and treatment and effect of a specialized neurocritical care team. Crit Care Med. 2006;34(9 Suppl):S232–S238. doi: 10.1097/01.CCM.0000231881.29040.25. [DOI] [PubMed] [Google Scholar]
- 9.Kramer A.H., Zygun D.A. Neurocritical care: why does it make a difference? Curr Opin Crit Care. 2014;20:174–181. doi: 10.1097/MCC.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 10.Vavilala M.S., Kernic M.A., Wang J., Kannan N., Mink R.B., Wainwright M.S. Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med. 2014;42:2258–2266. doi: 10.1097/CCM.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pineda J.A., Leonard J.R., Mazotas I.G., Noetzel M., Limbrick D.D., Keller M.S. Effect of implementation of a paediatric neurocritical care programme on outcomes after severe traumatic brain injury: a retrospective cohort study. Lancet Neurol. 2013;12:45–52. doi: 10.1016/S1474-4422(12)70269-7. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda Y. Neurocritical care update. J Intensive Care. 2016;4:36. doi: 10.1186/s40560-016-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonifacio S.L., Van Meurs K. Neonatal neurocritical care: providing brain-focused care for all at risk neonates. Semin Pediatr Neurol. 2019;32:100774. doi: 10.1016/j.spen.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs S.E., Berg M., Hunt R., Tarnow-Mordi W.O., Inder T.E., Davis P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013:CD003311. doi: 10.1002/14651858.CD003311. [DOI] [PubMed] [Google Scholar]
- 15.Salmaso N., Jablonska B., Scafidi J., Vaccarino F.M., Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papile L.A., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 17.Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inder T.E., Perlman J.M., Volpe J.J. Chapter 24 - preterm intraventricular hemorrhage/posthemorrhagic hydrocephalus. In: Volpe J.J., Inder T.E., Darras B.T., de Vries L.S., du Plessis A.J., Neil J.J., editors. Volpe's neurology of the newborn. 6th ed. Elsevier; 2018. pp. 637–698. e21. [Google Scholar]
- 19.Jain N.J., Kruse L.K., Demissie K., Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neona. 2009;22:491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 20.Stoll B.J., Hansen N.I., Bell E.F., Shankaran S., Laptook A.R., Walsh M.C. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukerji A., Shah V., Shah P.S. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. 2015;136:1132–1143. doi: 10.1542/peds.2015-0944. [DOI] [PubMed] [Google Scholar]
- 22.Sherlock R.L., Anderson P.J., Doyle L.W., Victorian Infant Collaborative Study G. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81:909–916. doi: 10.1016/j.earlhumdev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Luu T.M., Ment L.R., Schneider K.C., Katz K.H., Allan W.C., Vohr B.R. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perlman J.M. The relationship between systemic hemodynamic perturbations and periventricular-intraventricular hemorrhage--a historical perspective. Semin Pediatr Neurol. 2009;16:191–199. doi: 10.1016/j.spen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Banker B.Q., Larroche J.C. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 26.Volpe J.J. Confusions in nomenclature: "periventricular leukomalacia" and "white matter injury"-identical, distinct, or overlapping? Pediatr Neurol. 2017;73:3–6. doi: 10.1016/j.pediatrneurol.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Volpe J.J., Kinney H.C., Jensen F.E., Rosenberg P.A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011;29:423–440. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen-Pupp I., Hellström-Westas L., Cilio C.M., Andersson S., Fellman V., Ley D. Inflammation at birth and the insulin-like growth factor system in very preterm infants. Acta Paediatr. 2007;96:830–836. doi: 10.1111/j.1651-2227.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- 29.Linsell L., Malouf R., Morris J., Kurinczuk J.J., Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Dev Med Child Neurol. 2016;58:554–569. doi: 10.1111/dmcn.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. J Am Med Assoc. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 31.Roberts D., Brown J., Medley N., Dalziel S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:Cd004454. doi: 10.1002/14651858.CD004454.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlo W.A., McDonald S.A., Fanaroff A.A., Vohr B.R., Stoll B.J., Ehrenkranz R.A. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks' gestation. J Am Med Assoc. 2011;306:2348–2358. doi: 10.1001/jama.2011.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle L.W., Crowther C.A., Middleton P., Marret S., Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009:Cd004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Tarnow-Mordi W., Morris J., Kirby A., Robledo K., Askie L., Brown R. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017;377:2445–2455. doi: 10.1056/NEJMoa1711281. [DOI] [PubMed] [Google Scholar]
- 35.Bhatt S., Alison B.J., Wallace E.M., Crossley K.J., Gill A.W., Kluckow M. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013;591:2113–2126. doi: 10.1113/jphysiol.2012.250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fogarty M., Osborn D.A., Askie L., Seidler A.L., Hunter K., Lui K. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:1–18. doi: 10.1016/j.ajog.2017.10.231. [DOI] [PubMed] [Google Scholar]
- 37.Rabe H., Gyte G.M., Diaz-Rossello J.L., Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2019;9:Cd003248. doi: 10.1002/14651858.CD003248.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesoulis Z.A., Mathur A.M. Cerebral autoregulation, brain injury, and the transitioning premature infant. Front Pediatr. 2017;5:64. doi: 10.3389/fped.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groves A.M., Kuschel C.A., Knight D.B., Skinner J.R. Relationship between blood pressure and blood flow in newborn preterm infants. Arch Dis Child Fetal Neonatal Ed. 2008;93:F29–F32. doi: 10.1136/adc.2006.109520. [DOI] [PubMed] [Google Scholar]
- 40.Wolf M., Greisen G. Advances in near-infrared spectroscopy to study the brain of the preterm and term neonate. Clin Perinatol. 2009;36(4):807–834. doi: 10.1016/j.clp.2009.07.007. vi. [DOI] [PubMed] [Google Scholar]
- 41.Hyttel-Sorensen S., Pellicer A., Alderliesten T., Austin T., van Bel F., Benders M. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ. 2015;350:g7635. doi: 10.1136/bmj.g7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plomgaard A.M., Alderliesten T., Austin T., van Bel F., Benders M., Claris O. Early biomarkers of brain injury and cerebral hypo- and hyperoxia in the SafeBoosC II trial. PloS One. 2017;12 doi: 10.1371/journal.pone.0173440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellicer A., Greisen G., Benders M., Claris O., Dempsey E., Fumagalli M. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology. 2013;104:171–178. doi: 10.1159/000351346. [DOI] [PubMed] [Google Scholar]
- 44.Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev. 2005;81:423–428. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Noori S., Anderson M., Soleymani S., Seri I. Effect of carbon dioxide on cerebral blood flow velocity in preterm infants during postnatal transition. Acta Paediatr. 2014;103:e334–e339. doi: 10.1111/apa.12646. [DOI] [PubMed] [Google Scholar]
- 46.Dix L.M.L., Weeke L.C., de Vries L.S., Groenendaal F., Baerts W., van Bel F. Carbon dioxide fluctuations are associated with changes in cerebral oxygenation and electrical activity in infants born preterm. J Pediatr. 2017;187:66–72.e1. doi: 10.1016/j.jpeds.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 47.Hochwald O., Borenstein-Levin L., Dinur G., Jubran H., Ben-David S., Kugelman A. Continuous noninvasive carbon dioxide monitoring in neonates: from theory to standard of care. Pediatrics. 2019;144:e20183640. doi: 10.1542/peds.2018-3640. [DOI] [PubMed] [Google Scholar]
- 48.Hellstrom-Westas L., Rosen I. Electroencephalography and brain damage in preterm infants. Early Hum Dev. 2005;81:255–261. doi: 10.1016/j.earlhumdev.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Prior P.F., Maynard D.E., Sheaff P.C., Simpson B.R., Strunin L., Weaver E.J. Monitoring cerebral function: clinical experience with new device for continuous recording of electrical activity of brain. Br Med J. 1971;2:736–738. doi: 10.1136/bmj.2.5764.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellström-Westas L., Rosén I., de Vries L.S., Greisen G. Amplitude-integrated EEG classification and interpretation in preterm and term infants. NeoReviews. 2006;7:e76. [Google Scholar]
- 51.Burdjalov V.F., Baumgart S., Spitzer A.R. Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates. Pediatrics. 2003;112:855–861. doi: 10.1542/peds.112.4.855. [DOI] [PubMed] [Google Scholar]
- 52.Soubasi V., Mitsakis K., Nakas C.T., Petridou S., Sarafidis K., Griva M. The influence of extrauterine life on the aEEG maturation in normal preterm infants. Early Hum Dev. 2009;85:761–765. doi: 10.1016/j.earlhumdev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson N.J., Oberdorfer L., Koolen N., O'Toole J.M., Werther T., Klebermass-Schrehof K. Functional maturation in preterm infants measured by serial recording of cortical activity. Sci Rep. 2017;7:12969. doi: 10.1038/s41598-017-13537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ł Karpiński, Mazela J. Amplitude-integrated electroencephalography use in preterm infants: current knowledge and applications. NeoReviews. 2015;16:e526. [Google Scholar]
- 55.Fogtmann E.P., Plomgaard A.M., Greisen G., Gluud C. Prognostic accuracy of electroencephalograms in preterm infants: a systematic review. Pediatrics. 2017;139:e20161951. doi: 10.1542/peds.2016-1951. [DOI] [PubMed] [Google Scholar]
- 56.van Haastert I.C., Groenendaal F., Uiterwaal C.S., Ju Termote, van der Heide-Jalving M., Eijsermans M.J. Decreasing incidence and severity of cerebral palsy in prematurely born children. J Pediatr. 2011;159:86–91.e1. doi: 10.1016/j.jpeds.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 57.Pearce R., Baardsnes J. Term MRI for small preterm babies: do parents really want to know and why has nobody asked them? Acta Paediatr. 2012;101:1013–1015. doi: 10.1111/j.1651-2227.2012.02767.x. [DOI] [PubMed] [Google Scholar]
- 58.Kidokoro H., Neil J.J., Inder T.E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34:2208–2214. doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Vries L.S., Benders M.J., Groenendaal F. Imaging the premature brain: ultrasound or MRI? Neuroradiology. 2013;55(Suppl 2):13–22. doi: 10.1007/s00234-013-1233-y. [DOI] [PubMed] [Google Scholar]
- 60.Mihatsch W.A., Braegger C., Bronsky J., Cai W., Campoy C., Carnielli V. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin Nutr. 2018;37:2303–2305. doi: 10.1016/j.clnu.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 61.Ehrenkranz R.A., Dusick A.M., Vohr B.R., Wright L.L., Wrage L.A., Poole W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 62.Morris M., Cleary J.P., Soliman A. Small baby unit improves quality and outcomes in extremely low birth weight infants. Pediatrics. 2015;136:e1007–e1015. doi: 10.1542/peds.2014-3918. [DOI] [PubMed] [Google Scholar]
- 63.Salhab W.A., Perlman J.M., Silver L., Sue Broyles R. Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants <1000 g. J Perinatol. 2004;24:534–540. doi: 10.1038/sj.jp.7211165. [DOI] [PubMed] [Google Scholar]
- 64.Ohls R.K. 116 - developmental erythropoiesis. In: Polin R.A., Abman S.H., Rowitch D.H., Benitz W.E., Fox W.W., editors. Fetal and neonatal physiology. 5th ed. Elsevier; 2017. pp. 1112–1134. e4. [Google Scholar]
- 65.Halperin D.S., Wacker P., Lacourt G., Felix M., Babel J.F., Aapro M. Effects of recombinant human erythropoietin in infants with the anemia of prematurity: a pilot study. J Pediatr. 1990;116:779–786. doi: 10.1016/s0022-3476(05)82671-x. [DOI] [PubMed] [Google Scholar]
- 66.Rangarajan V., Juul S.E. Erythropoietin: emerging role of erythropoietin in neonatal neuroprotection. Pediatr Neurol. 2014;51:481–488. doi: 10.1016/j.pediatrneurol.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer H.S., Reibel N.J., Buhrer C., Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a meta-analysis. Pediatrics. 2017;139:e20164317. doi: 10.1542/peds.2016-4317. [DOI] [PubMed] [Google Scholar]
- 68.Juul S.E., Comstock B.A., Wadhawan R., Mayock D.E., Courtney S.E., Robinson T. A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med. 2020;382:233–243. doi: 10.1056/NEJMoa1907423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anand K.J. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- 70.Ranger M., Grunau R.E. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 2014;4:57–67. doi: 10.2217/pmt.13.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams M.D., Lascelles B.D.X. Early neonatal pain-A review of clinical and experimental implications on painful conditions later in life. Front Pediatr. 2020;8:30. doi: 10.3389/fped.2020.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Als H., Lawhon G., Brown E., Gibes R., Duffy F.H., McAnulty G. Individualized behavioral and environmental care for the very low birth weight preterm infant at high risk for bronchopulmonary dysplasia: neonatal intensive care unit and developmental outcome. Pediatrics. 1986;78:1123–1132. [PubMed] [Google Scholar]
- 73.Ohlsson A., Jacobs S.E. NIDCAP: a systematic review and meta-analyses of randomized controlled trials. Pediatrics. 2013;131:e881–e893. doi: 10.1542/peds.2012-2121. [DOI] [PubMed] [Google Scholar]