Abstract
Febrile infection-related epilepsy syndrome (FIRES) is an intractable neurological disease characterized by an unexplained refractory status epilepticus triggered by febrile infection. A Consensus definition of FIRES was proposed in 2018, and its clinical features and prognosis are gradually being clarified. However, the development of effective treatments has been hindered as the etiology of this rare disease is as yet unelucidated. The basic approach to the management of FIRES, like other forms of epilepsy, is based on the control of seizures, however seizures are extremely intractable and require intravenous administration of large doses of anticonvulsants, mainly barbiturates. This treatment strategy produces various complications including respiratory depression and drug hypersensitivity syndrome, which make it more difficult to control seizures. Consequently, it is crucial to predict these events and to formulate a planned treatment strategy. As well, it is important to grow out of conventional treatment strategies that rely on only anticonvulsants, and alternative therapies are gradually being developed. One such example is the adoption of a ketogenic diet which may lead to reduced convulsions as well as improve intellectual prognosis. Further, overproduction of inflammatory cytokines in the central nervous system has been shown to be strongly related to the pathology of FIRES which has led to attempts at immunomodulation therapy including anti-cytokine therapy.
Keywords: Febrile infection-related epilepsy syndrome (FIRES), New-onset refractory status epilepticus (NORSE), Burst-suppression coma, Ketogenic diet, Anti-cytokine therapy
Febrile infection-related epilepsy syndrome (FIRES) is an intractable neurological disease characterized by an unexplained refractory status epilepticus following febrile infection [1,2]. In adults, the term “new-onset refractory status epilepticus (NORSE) syndrome” is often used and both conditions have been considered to be the same disease concept in principle [3]. A consensus definition for FIRES and NORSE was proposed in 2018 [4,5]. In this proposal, NORSE was defined as a clinical presentation, not a specific diagnosis, in a patient without active epilepsy or other preexisting relevant neurological disorder, with new onset of refractory status epilepticus without a clear acute or active structural, toxic or metabolic cause, while FIRES was categorized as a subcategory of NORSE, applicable for all ages, that requires a prior febrile infection starting between 2 weeks and 24 h prior to onset of refractory status epilepticus, with or without fever at onset of status epilepticus. Since these definitions were created with the intention of early diagnosis and treatment of NORSE and FIRES, a more specific diagnosis may be achieved following a detailed etiological investigation [4]. For example, under the current definitions, the exclusion of anti-NMDA receptor encephalitis is deemed unnecessary and a diagnosis of NORSE and FIRES can be made even if the anti-NMDA receptor antibody is positive. Conversely, tests for autoantibodies including anti-NMDA receptor antibodies have been reported to be negative in pediatric FIRES [6], suggesting a gap between the consensus definition and the previously reported concept of FIRES. An understanding is shared among clinicians that there is a highly uniform group that fulfills NORSE/FIRES criteria with no identifiable causes, which is called ‘cryptogenic’ NORSE/FIRES [7]. This review mainly discusses this cryptogenic NORSE and FIRES.
Clinical features of FIRES
Clinical symptoms
In FIRES, fever is observed in many cases prior to the appearance of neurological symptoms, and seizures develop after an incubation period of about 3–7 days [8]. Seizures gradually increase after about a week, leading to intractable cluster seizures. These seizures are characterized by many focal seizures around the face [9,10]. Because seizures at the nadir are markedly refractory to anticonvulsants, it is necessary to administer large amounts of anticonvulsants intravenously. As a result, intensive care such as artificial ventilation is required, and various therapeutic problems will be encountered as described below [11]. Furthermore, since markedly intractable seizures persist for a long time, it is extremely difficult to withdraw the drug. The frequency of seizures gradually decreases and treatments can be tapered, but in many cases epileptic seizures last a lifetime, leaving neurological sequelae such as intellectual disability [1,12,13]. Thus, an important feature of FIRES is that it is an intractable chronic neurological disease of acute onset.
Laboratory findings
Various theories, including the role of inflammatory mechanisms, have been speculated as the cause of FIRES [8,14]. An inflammatory cause is supported by the frequent observation of transient increases in cerebrospinal fluid pleocytosis. Additionally, abnormally high levels of pro-inflammatory cytokines and chemokines are found in the cerebrospinal fluid in the early stage of onset, presumably due to strong activation of the immune system in the central nervous system [15,16]. Such marked pro-inflammatory cytokine changes may be seen in infectious encephalitis but not in many autoimmune neurological disorders. However, the link between FIRES and any particular pathogen has not been proven. As mentioned above, anti-neural antibodies are negative for cryptogenic FIRES. FIRES is not caused by SCN1A, POLG, PCDH19 mutations or rare copy number variations [17], while IL1RN gene polymorphism was associated with FIRES [18]. Non-specific hippocampal abnormalities may be detected by neuroimaging including brain MRI [19]. In severe cases, abnormal signals are also detected in the thalamus, basal ganglia, and cerebral cortex. Lesions within the claustrum may be observed in adult patients with refractory status epilepticus following febrile illness and is considered to be a concept that overlaps with FIRES [[20], [21], [22]].
Neurocritical care of FIRES
General management
It is essential to understand the natural course of FIRES in the practical management. In FIRES, prolonged status epilepticus is rather infrequent, and thus seizures are not often accompanied by sudden deterioration of general condition. Characteristically, the post onset progression is relatively slow and the occurrence of respiratory and circulatory insufficiency in the early stages of disease is rare. Rather, the problem may be due to cardio-respiratory suppression consequent to long-term administration of barbiturates. Based on this context of prolonged intravenous administration, paralytic ileus often renders intestinal nutrition difficult. In rare instances, systemic inflammatory response syndrome with cytokine storm may occur [23]. Hemophagocytic lymphohistiocytosis is another complication of FIRES and the co-occurrence of these rare disorders may be attributed to a common immune dysregulation [24].
Neurocritical care and examination
Another characteristic of FIRES is that cerebral edema is rarely observed [19]; thus, careful management of intracranial pressure is not necessary in most cases. In the acute phase of FIRES, electroencephalography (EEG) findings best reflect disease status; hence, continuous EEG monitoring is essential [25]. Burst-suppression by intravenous barbiturate has been regarded as a standard care for initial treatment, and sometimes complete suppression may be required. Even if clinical seizures are not observed, subclinical seizures often appear on the EEG periodically, and continuous EEG monitoring is essential for assessing the therapeutic effect. In severe cases, periodic discharges may persist during intermittent seizures, and if these findings are observed, seizures often recur when the anticonvulsant is reduced [22]. Thus, in FIRES, the fundamental approach lies in the adjustment of anticonvulsants based on EEG findings.
Targeted temperature management
There is no definitive consensus on the effectiveness of targeted temperature management, including cerebral hypothermia, in the management of FIRES. Rather than cerebral protection, the goal of hypothermia treatment in FIRES is to control seizures, and in this regard the treatment strategy differs from other acute neurological disorders. Since there is exacerbation of seizures during fever conditions, controlling body temperature (brain temperature) may be beneficial for the suppression of convulsions. Additionally, cerebral hypothermia may mediate a reduction in the strong inflammatory response often observed in FIRES. To date, there have been only a few cases of cerebral hypothermia for FIRES [26], and there is no fixed protocol; hence, further study is needed.
Problems in treating FIRES
Two challenging issues are faced in the treatment of FIRES, the exceptionally refractory nature of the seizures and the necessity of prolonged management in intensive care unit. In order to confront these issues, prior comprehension of treatment-related problems and the formulation of a long-term strategy based on such understanding are necessary.
Anticonvulsant use
Due to the extreme intractability of the seizures in FIRES, barbiturates or benzodiazepines are used in almost all cases [10]. Barbiturates are used particularly for cases where benzodiazepines such as midazolam have been ineffective in the suppression of seizures and high doses (up to 20 mg/kg/hr for thiopental) are may be required. There have also been reports of the need for the use of inhaled anesthetics due to uncontrolled seizures even with maximal doses of barbiturates [27]. The cardio-respiratory suppression is inevitable in these cases, and thus the administration of artificial ventilation by the use of vasopressors and intravenous hyperalimentation are necessary. There has been debate on such extensive treatment for the purpose of complete suppression of seizures in FIRES, and the question of how to set therapeutic goals must be solved in the future. Along with the issue of dosage, the duration of treatment is another clinically important issue concerning the administration of barbiturates. In addition to the harmful effects of barbiturates, a poor prognosis has been reported as a consequence of the use of large doses of barbiturates for long periods in the management of FIRES [1,28]. While there is controversy regarding this report, efforts should be made to minimize the administration period of barbiturates.
Method and timing of withdrawal from intravenous barbiturates
Typically, seizures can often be managed after the induction of burst-suppression coma for status epilepticus along with the combined use of other intravenous anticonvulsants. However, seizures often recur after tapering barbiturate and drug dosages must be increased. Therefore, replacement therapy for intravenous barbiturate is important during the recovery period, and several methods have been proposed. The ketogenic diet described below is a particularly effective treatment for FIRES and lidocaine, phosphenytoin, levetiracetam, etc. are effective as intravenous drugs [29,30]. Oral antiepileptic drugs such as topiramate and potassium bromide may also work [29]. However, since the effectiveness of drugs varies from case to case, effective combinations of drugs must be determined through trial and error.
Adverse effects of anticonvulsants
In FIRES, long-term administration of large doses of anticonvulsants often causes side effects. Hepatic dysfunction is often observed in FIRES, but most of which present after drug treatment has started and are presumed to be drug-induced hepatic disorders. Venous thrombosis may occur as iatrogenic complication of prolonged catheterization. In addition, arrhythmia was frequently found during the acute stage, possibly due to multiple medications or neurogenic arrhythmia [11]. The most important of these adverse effects is drug hypersensitivity syndrome. This is mainly caused by anticonvulsants, particularly intravenous barbiturate. The majority of these symptoms present as drug exanthemas and often resolve following withdrawal of the causative drug. However, in many cases, the simultaneous administration of a large number of anticonvulsants at this point makes it extremely difficult to identify the causative agent. Moreover, it is not uncommon for drug eruptions to reappear even after changing anticonvulsants, which greatly restricts the use of anticonvulsants. However, a reduction in the anticonvulsant dosage worsens seizures. Moreover, hypersensitivity syndrome is often accompanied by fever. There is a report on a FIRES case with a progression from drug hypersensitivity syndrome to multiple organ failure such as shock and renal failure, leading rapid deterioration of the general condition [23]. Thus, drug hypersensitivity syndrome is an important factor in determining the prognosis of FIRES, its occurrence must be considered while treatment is being implemented. Up to present time, there is no known method for predicting or preventing the occurrence of drug hypersensitivity syndrome.
Emerging treatments
Existing treatments for FIRES are still inadequate and there are many associated problems, including adverse reactions [Fig. 1]. This suggests that therapeutic strategies that rely on antiepileptic drugs have their limitations, and there is an urgent need to develop therapeutics based on different methodologies. Recent research has provided new solutions, some of which have already been applied in the clinical practice [Table 1].
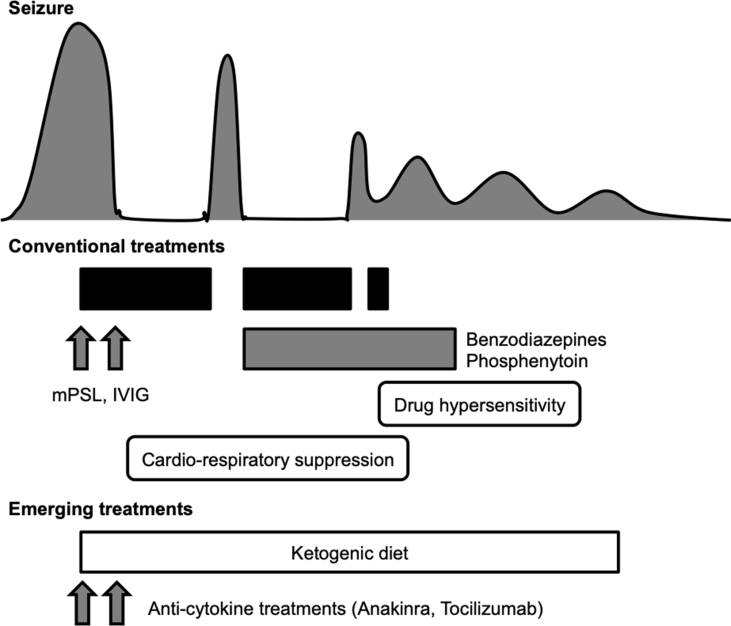
Fig. 1.
Clinical and therapeutic course of cryptogenic FIRES. In the typical cases of cryptogenic FIRES, patients reaches a nadir at about one week after the onset, when they require high-dose intravenous barbiturate as well as other intravenous anticonvulsants and immunomodulators. Such extensive treatments frequently cause many adverse events including cardio-respiratory suppression and drug hypersensitity syndrome. Emerging treatments including ketogenic diet and anti-cytokine agents are promising options to minimize therapeutic complications. Abbreviations used: IVIG: intravenous immune; mPSL: methylprednisolone.
Table 1.
Emerging treatments for febrile infection-related epilepsy syndrome.
| Treatment | Mechanism of action | Efficacy on FIRES | Reference |
|---|---|---|---|
| Ketogenic diet | Unknown | Suppress refractory status epilepticus Improve cognitive outcome |
31, 32, 33 |
| Pulsed methylprednisolone and intravenous immunoglobulin | Reduce production of pro-inflammatory cytokines? | Suppress refractory status epilepticus | 34 |
| Anakinra (a human IL-1β receptor antagonist) | Blocking of IL-1β | Suppress refractory status epilepticus | 36, 37, 38 |
| Tocilizumab (a humanized anti-human IL-6 receptor monoclonal antibody) | Blocking of IL-6 | Suppress refractory status epilepticus | 39 |
Abbreviations: IL-1β: interleukin 1β; IL-6: interleukin-6.
Ketogenic diet
The report on the effectiveness of the ketogenic diet in the management of FIRES has generated significant interest on its usefulness in FIRES [31]. While ketogenic diet therapy has been used for epilepsy for a long time, its effectiveness has recently been rediscovered as it may prove effective for intractable epilepsy. Its high effectiveness for FIRES in particular has attracted attention. It has been reported that ketogenic diet therapy not only reduced seizures in FIRES [32] but also improved intellectual prognosis [33]. The introduction of the ketogenic diet during the acute phase of FIRES remains challenging due to the difficulty of oral intake. Carbohydrate restriction alone may protect against seizure activity because some cases show dramatic seizure reduction soon after glucose was removed from intravenous fluids. However, there are concerns about the safety of maintenance of a state of starvation in severe cases and the effect on long-term prognosis is unknown. Additionally, as the ketogenic diet has proved ineffective in a few cases, further investigation on its indication/contraindication and standard regimen in the treatment of FIRES is needed.
Immunomodulatory treatment
As the etiology of FIRES is still unknown, its treatments that have been reported so far have mainly been symptomatic. Based on the hypothesis that neuroinflammation is associated with FIRES, various immunomodulatory therapies including the use of corticosteroids have been attempted. Although many reports do not provide evidence supportive of any short-term effect on seizures, there have been reports of the efficacy of pulsed methylprednisolone and intravenous immunoglobulin (IVIg) in febrile refractory status epilepticus, including FIRES [34]. While the mechanism is unknown, it is possible that the suppression of excessive cytokine production in the central nervous system modifies the pathology.
Anti-cytokine therapy
In addition to the use of nonspecific immunomodulatory therapies such as steroids and IVIg for the treatment of FIRES, close attention is being paid to new modalities involving the targeting of inflammatory cytokines. Animal studies have shown that inflammatory cytokines such as interkleukin-1β (IL-1β), IL-6, and TNFα are closely related to convulsions [35], and attempts have been made to suppress convulsions by inhibiting related pathways. One turning point was the discovery of elevated levels of cytokines in the cerebrospinal fluid in FIRES [15], and this finding has accelerated the clinical trial of anti-cytokine therapy. Anakinra, a human IL-1β receptor antagonist (IL1RA), has been reported to suppress seizures in FIRES [36,37]. Mechanistically, FIRES is associated with reduced expression of intracellular IL1RA isoforms and a functional deficiency in IL1RA inhibitory activity [38]. Furthermore, tocilizumab, a humanized anti-human IL-6 receptor monoclonal antibody, also suppressed status epilepticus in NORSE [39]. IL-6 is one of the molecules that are significantly increased in the cerebrospinal fluid in FIRES, and the success of these treatments has simultaneously proved that inflammatory cytokines actually promote the pathology of FIRES.
Conclusion
With the rapid progress in the treatment of epilepsy, FIRES/NORSE remains a difficult disease to treat and the prognosis is extremely poor. Basic and clinical studies on this disease have not sufficiently advanced, and one of the causes is that it is an extremely rare disease. To overcome this problem, attempts are being made to identify clinical features and biomarkers through multicenter research. Emerging anti-cytokine therapies are highly encouraging treatments and we hope that further research will overcome this disastrous disease.
Conflict of interest
Authors declare no conflict of interest to disclose.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Kramer U., Chi C.S., Lin K.L., Specchio N., Sahin M., Olson H. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. 2011;52:1956–1965. doi: 10.1111/j.1528-1167.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- 2.van Baalen A., Vezzani A., Hausler M., Kluger G. Febrile infection-related epilepsy syndrome: clinical review and hypotheses of epileptogenesis. Neuropediatrics. 2017;48:5–18. doi: 10.1055/s-0036-1597271. [DOI] [PubMed] [Google Scholar]
- 3.Gaspard N., Foreman B.P., Alvarez V., Cabrera Kang C., Probasco J.C., Jongeling A.C. Critical Care EEGMRC. New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. 2015;85:1604–1613. doi: 10.1212/WNL.0000000000001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch L.J., Gaspard N., van Baalen A., Nabbout R., Demeret S., Loddenkemper T. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59:739–744. doi: 10.1111/epi.14016. [DOI] [PubMed] [Google Scholar]
- 5.Gaspard N., Hirsch L.J., Sculier C., Loddenkemper T., van Baalen A., Lancrenon J. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): state of the art and perspectives. Epilepsia. 2018;59:745–752. doi: 10.1111/epi.14022. [DOI] [PubMed] [Google Scholar]
- 6.van Baalen A., Hausler M., Plecko-Startinig B., Strautmanis J., Vlaho S., Gebhardt B. Febrile infection-related epilepsy syndrome without detectable autoantibodies and response to immunotherapy: a case series and discussion of epileptogenesis in FIRES. Neuropediatrics. 2012;43:209–216. doi: 10.1055/s-0032-1323848. [DOI] [PubMed] [Google Scholar]
- 7.Iizuka T., Kanazawa N., Kaneko J., Tominaga N., Nonoda Y., Hara A. Cryptogenic NORSE: its distinctive clinical features and response to immunotherapy. Neurol Neuroimmunol Neuroinflamm. 2017;4:e396. doi: 10.1212/NXI.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Baalen A., Hausler M., Boor R., Rohr A., Sperner J., Kurlemann G. Febrile infection-related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia. 2010;51:1323–1328. doi: 10.1111/j.1528-1167.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakuma H. Acute encephalitis with refractory, repetitive partial seizures. Brain Dev. 2009;31:510–514. doi: 10.1016/j.braindev.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Sakuma H., Awaya Y., Shiomi M., Yamanouchi H., Takahashi Y., Saito Y. Acute encephalitis with refractory, repetitive partial seizures (AERRPS): a peculiar form of childhood encephalitis. Acta Neurol Scand. 2010;121:251–256. doi: 10.1111/j.1600-0404.2009.01198.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee H.F., Chi C.S. Febrile infection-related epilepsy syndrome (FIRES): therapeutic complications, long-term neurological and neuroimaging follow-up. Seizure. 2018;56:53–59. doi: 10.1016/j.seizure.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Howell K.B., Katanyuwong K., Mackay M.T., Bailey C.A., Scheffer I.E., Freeman J.L. Long-term follow-up of febrile infection-related epilepsy syndrome. Epilepsia. 2012;53:101–110. doi: 10.1111/j.1528-1167.2011.03350.x. [DOI] [PubMed] [Google Scholar]
- 13.Lam S.K., Lu W.Y., Weng W.C., Fan P.C., Lee W.T. The short-term and long-term outcome of febrile infection-related epilepsy syndrome in children. Epilepsy Behav. 2019;95:117–123. doi: 10.1016/j.yebeh.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Nabbout R., Vezzani A., Dulac O., Chiron C. Acute encephalopathy with inflammation-mediated status epilepticus. Lancet Neurol. 2011;10:99–108. doi: 10.1016/S1474-4422(10)70214-3. [DOI] [PubMed] [Google Scholar]
- 15.Sakuma H., Tanuma N., Kuki I., Takahashi Y., Shiomi M., Hayashi M. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J Neurol Neurosurg Psychiatry. 2015;86:820–822. doi: 10.1136/jnnp-2014-309388. [DOI] [PubMed] [Google Scholar]
- 16.Kothur K., Bandodkar S., Wienholt L., Chu S., Pope A., Gill D. Etiology is the key determinant of neuroinflammation in epilepsy: elevation of cerebrospinal fluid cytokines and chemokines in febrile infection-related epilepsy syndrome and febrile status epilepticus. Epilepsia. 2019;60:1678–1688. doi: 10.1111/epi.16275. [DOI] [PubMed] [Google Scholar]
- 17.Appenzeller S., Helbig I., Stephani U., Hausler M., Kluger G., Bungeroth M. Febrile infection-related epilepsy syndrome (FIRES) is not caused by SCN1A, POLG, PCDH19 mutations or rare copy number variations. Dev Med Child Neurol. 2012;54:1144–1148. doi: 10.1111/j.1469-8749.2012.04435.x. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh M., Kobayashi K., Ohmori I., Tanaka Y., Tanaka K., Inoue T. Cytokine-related and sodium channel polymorphism as candidate predisposing factors for childhood encephalopathy FIRES/AERRPS. J Neurol Sci. 2016;368:272–276. doi: 10.1016/j.jns.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 19.Culleton S., Talenti G., Kaliakatsos M., Pujar S., D'Arco F. The spectrum of neuroimaging findings in febrile infection-related epilepsy syndrome (FIRES): a literature review. Epilepsia. 2019;60:585–592. doi: 10.1111/epi.14684. [DOI] [PubMed] [Google Scholar]
- 20.Meletti S., Slonkova J., Mareckova I., Monti G., Specchio N., Hon P. Claustrum damage and refractory status epilepticus following febrile illness. Neurology. 2015;85:1224–1232. doi: 10.1212/WNL.0000000000001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meletti S., Giovannini G., d'Orsi G., Toran L., Monti G., Guha R. New-onset refractory status epilepticus with claustrum damage: definition of the clinical and neuroimaging features. Front Neurol. 2017;8:111. doi: 10.3389/fneur.2017.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saito Y., Maegaki Y., Okamoto R., Ogura K., Togawa M., Nanba Y. Acute encephalitis with refractory, repetitive partial seizures: case reports of this unusual post-encephalitic epilepsy. Brain Dev. 2007;29:147–156. doi: 10.1016/j.braindev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa C., Natsume J., Yamamoto H., Ishihara N., Tashiro A., Kidokoro H. Autopsy findings of a patient with acute encephalitis and refractory, repetitive partial seizures. Seizure. 2016;35:80–82. doi: 10.1016/j.seizure.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Farias-Moeller R., LaFrance-Corey R., Bartolini L., Wells E.M., Baker M., Doslea A. Fueling the FIRES: hemophagocytic lymphohistiocytosis in febrile infection-related epilepsy syndrome. Epilepsia. 2018;59:1753–1763. doi: 10.1111/epi.14524. [DOI] [PubMed] [Google Scholar]
- 25.Okumura A., Komatsu M., Abe S., Kitamura T., Matsui K., Ikeno M. Amplitude-integrated electroencephalography in patients with acute encephalopathy with refractory, repetitive partial seizures. Brain Dev. 2011;33:77–82. doi: 10.1016/j.braindev.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Lin J.J., Lin K.L., Hsia S.H., Wang H.S., Group C.S. Therapeutic hypothermia for febrile infection-related epilepsy syndrome in two patients. Pediatr Neurol. 2012;47:448–450. doi: 10.1016/j.pediatrneurol.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Miras Veiga A., Moreno D.C., Menendez A.I., Siscart I.M., Fernandez M.D., Sanchez E.G. Effectiveness of electroconvulsive therapy for refractory status epilepticus in febrile infection-related epilepsy syndrome. Neuropediatrics. 2017;48:45–48. doi: 10.1055/s-0036-1584939. [DOI] [PubMed] [Google Scholar]
- 28.Kramer U., Chi C.S., Lin K.L., Specchio N., Sahin M., Olson H. Febrile infection-related epilepsy syndrome (FIRES): does duration of anesthesia affect outcome? Epilepsia. 2011;52(Suppl. 8):28–30. doi: 10.1111/j.1528-1167.2011.03230.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin J.J., Lin K.L., Wang H.S., Hsia S.H., Wu C.T. Effect of topiramate, in combination with lidocaine, and phenobarbital, in acute encephalitis with refractory repetitive partial seizures. Brain Dev. 2009;31:605–611. doi: 10.1016/j.braindev.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Ueda R., Saito Y., Ohno K., Maruta K., Matsunami K., Saiki Y. Effect of levetiracetam in acute encephalitis with refractory, repetitive partial seizures during acute and chronic phase. Brain Dev. 2015;37:471–477. doi: 10.1016/j.braindev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Nabbout R., Mazzuca M., Hubert P., Peudennier S., Allaire C., Flurin V. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 32.Peng P., Peng J., Yin F., Deng X., Chen C., He F. Ketogenic diet as a treatment for super-refractory status epilepticus in febrile infection-related epilepsy syndrome. Front Neurol. 2019;10:423. doi: 10.3389/fneur.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh R.K., Joshi S.M., Potter D.M., Leber S.M., Carlson M.D., Shellhaas R.A. Cognitive outcomes in febrile infection-related epilepsy syndrome treated with the ketogenic diet. Pediatrics. 2014;134:e1431–e1435. doi: 10.1542/peds.2013-3106. [DOI] [PubMed] [Google Scholar]
- 34.Lin J.J., Wang Y., Lan S.Y., Chan O.W., Hsia S.H., Chou M.L. Combination of intravenous immunoglobulin and steroid pulse therapy improves outcomes of febrile refractory status epilepticus. Epilepsy Res. 2018;142:100–105. doi: 10.1016/j.eplepsyres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Vezzani A., French J., Bartfai T., Baram T.Z. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenney-Jung D.L., Vezzani A., Kahoud R.J., LaFrance-Corey R.G., Ho M.L., Muskardin T.W. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80:939–945. doi: 10.1002/ana.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dilena R., Mauri E., Aronica E., Bernasconi P., Bana C., Cappelletti C. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection-related epilepsy syndrome. Epilepsia Open. 2019;4:344–350. doi: 10.1002/epi4.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarkson B.D.S., LaFrance-Corey R.G., Kahoud R.J., Farias-Moeller R., Payne E.T., Howe C.L. Functional deficiency in endogenous interleukin-1 receptor antagonist in patients with febrile infection-related epilepsy syndrome. Ann Neurol. 2019;85:526–537. doi: 10.1002/ana.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jun J.S., Lee S.T., Kim R., Chu K., Lee S.K. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol. 2018;84:940–945. doi: 10.1002/ana.25374. [DOI] [PubMed] [Google Scholar]