Abstract
Perinatal intraventricular hemorrhage (IVH) with or without development of posthemorrhagic hydrocephalus (PHH) in premature neonates may lead to severe neurological disability. Although the percentage of preterm infants developing IVH has been greatly reduced in the last three decades, increased survival of these very immature infants has meant that large IVH with subsequent PHH is still a serious unsolved problem. Early cerebrospinal fluid diversion as a temporizing measure or a permanent shunt is the treatment of choice. This review summarizes the surgical modalities, techniques, and their complications in the management of IVH and PHH in premature infants. Though there is no level-one evidence to support the superiority of any of the currently available managements in the initial treatment of PHH over others, this review aims to provide pediatric neurosurgeons a comprehensive understanding of the pros and cons of various surgical treatment modalities, focusing on the temporizing measures before the infants is heavy enough to undergo ventriculoperitoneal shunt insertion. Based on the patient's condition, the facility and man power of the institution with minimal complication rate, the pediatric neurosurgeons may choose the best initial approach for the management of IVH and PHH in premature infants.
Keywords: Case management, Posthemorrhagic hydrocephalus, Premature infant, Preterm infant, Intraventricular hemorrhage, Ventriculoperitoneal shunt
The basic anatomic structure of the germinal matrix (GM) of the premature infants may predispose them to intraventricular hemorrhage (IVH). The veins in this region make a 180° turn at the caudate nuclei and drain via internal cerebral veins. This anatomical arrangement may predispose to turbulence in blood flow and promotes platelet aggregation and vascular instability. In infants of less than 28 weeks gestation, the hemorrhage tends to occur in the head of the caudate nucleus. In full-tem infants, the choroid plexus is more likely to be the location of hemorrhage [1,2]. In premature infants, the periventricular capillaries appear as immature vascular rete, and the germinal matrix has both a gelatinous consistency and a high fibrinolytic activity. These vascular natures also play an important role in the development of IVH, which leads to a disruption in the cerebrospinal fluid (CSF) and ventricular dilatation. It is well known that 40–50% of hydrocephalus cases in premature infants, occur following GM hemorrhage (GMH). Such hemorrhages are reported to arise commonly among infants with <1500 g birth weight and <32 weeks old. Of the premature infants with IVH, 20%–50% will go on to develop ventriculomegaly, either transient or progressive. Although survival for extremely low gestational age newborns has improved in the past three decades, the continued improvements in neonatal intensive care, the survival of infants including those that develop post-hemorrhagic hydrocephalus (PHH) continues to be a challenge to the pediatric neurosurgeons [3]. This review will discuss the surgical management of IVH and PHH in the preterm infants and aims to provide neurosurgeons with the evidence needed to choose the best initial approach for PHH treatment, yet with minimal complication rate.
Intraventricular hemorrhage in premature infants
Clinical features
The premature IVH can present as a catastrophic event, saltatory, or as a clinical silent phenomenon. It usually occurs within 48 h of birth and 50% will occur in the first 24 h. A later onset is not uncommon, especially following a secondary hypoxic insult such as pneumothorax.
Catastrophic deterioration evolves in minutes to hours. Aggressive neurosurgical intervention is rarely considered as many of these infants do not survive this event. The saltatory or subacute presentation is seen principally in an infant with a smaller hemorrhage that evolves over hour to days. There may be a subtle change in the decreased alertness and activity, hypotonia, abnormally tight popliteal angle, abnormal eye movements, and respiratory difficulties. Many IVHs are clinically silent and is one diagnosed by cranial ultrasonography. An unexplained decline in the hematocrit, decreased tone and activity may suggest that an IVH has occurred [4].
Management of IVH
The incidence of IVH in premature infants who weigh less than 1500 g decreased from 35%–70% to 15%–20% in the past three decades [5,6]. Less than half of these children will develop transient or progressive ventriculomegaly. Considerable ventriculomegaly can occur before any increase in intracranial pressure (ICP) or head circumference is noted. It seems reasonable to treat IVH aggressively; however, too-aggressive therapy may change a small lesion to a large one. We suggest early consultation with pediatric neurosurgeon as early as possible when premature IVH with more than a subependymal hemorrhage or mild progression of ventricular dilatation is diagnosed.
Pharmacological treatment
In the acute hemorrhage maintaining cerebral perfusion and ICP may be undertaken medically by decreasing the PaCO2, and perhaps using some medicine. However, a large multicenter randomized trial showed that reduction of CSF production by acetazolamide and furosemide had a worse outcome in the treated arm [3].
Drainage, irrigation, and fibrinolytic therapy (DRIFT)
Based on the hypothesis that reducing pressure, free iron, and proinflammatory and profibrotic cytokines may reduce death and severe disability, prevention of PHH in newborn infants by DRIFT has been conducted in UK since 2003. A total of 77 premature babies with IVH were studied. Though DRIFT improves cognitive function in the 52 out of the 66 surviving babies after 10-year follow-up, secondary IVH is a major complication that up to 35% of the infants who received DRIFT had secondary IVH compared with 8% of the standard group. In addition, DRIFT did not reduce shunt surgery or death when tested in a multicenter, randomized trial in UK. DRIFT is still not a standard treatment for the prematurity with IVH [3,[7], [8], [9]].
Neuroendoscopic lavage
A study demonstrated that neuroendoscopic lavage in 19 neonates with IVH had less shunt, fewer infections, and multiloculated hydrocephalus than the 10 neonates treated with various conventional modalities. A multicenter, prospective study to verify the outcome is warranted [10].
Management of post-hemorrhagic hydrocephalus (PHH)
Transient or progressive ventricular dilatation is seen in 20%–50% patients with premature IVH. Early ventriculoperitoneal (VP) shunt insertion is associated with a high failure rate and many complications in premature infants with PHH; hence, temporizing measures are always instituted until the infant is mature enough at age and/or weight. Many different approaches have been taken to achieve temporary ventricular decompression in infants with PHH [11]. Therapeutic serial lumbar and ventricular tapping remain temporizing options in the treatment of PHH used by 10% and 30% of the neonatal units.
Serial lumbar punctures
Lumbar puncture (LP) is only useful when the ventricles are in communication with the lumbar subarachnoid space [3]. Daily LPs can be used if it is necessary to stabilize the head circumference, and usually up to 10 ml of CSF is removed per LP [4]. It is often difficult to remove sufficient quantities of CSF in these infants, and, again, the child is exposed to contamination each time. Spinal osteomyelitis has been associated with repeated LPs [12]. It has been suggested that daily LPs need to occur for at least 7 days to be effective whereas the repeated procedures can be traumatic for the premature infants and, therefore, not always the preferred treatment. Randomized controlled trials have failed to demonstrate a significant effect of serial LPs on the rates of morbidity, mortality or conversion to permanent VP shunt in the treatment of PHH [[13], [14], [15], [16]]. Lumbar tappings are also associated with increased risk of CSF infection [17]. Hence, early repeated LPs cannot be recommended as the mainstay treatment for neonates at risk of, or actually developing PHH and spinal taps are increasingly considered a temporizing measure in the management of PPH only for an initial period. If frequent multiple LPs are needed other measures can be used as described below [18].
Serial ventricular punctures
Repeated ventricular taps incur a new injury to the frontal lobe with each pass of a needle (“puncture porencephaly”). Each access exposes the child to the risk of infection. Ventricular taps have fallen out of favor due to the risk of porencephalic cyst formation, infection, and loculated hydrocephalus [4,19]. In general, ventricular puncture should be reserved for treating infants in extremis.
Temporary devices
Most of the temporary devices which include external ventricular drainage (EVD), ventriculosubgaleal shunt (VSGS), and ventricular access device (VAD) can be performed at the bedside with or without anesthesia. However, with the advancement of neonatal neurocritical care and anesthesiology the role of beside surgery of PHH became less significant considering the higher infection rate in the neonatal unit than in the operation theater. The three temporary measures involve ventriculostomy. During cannulation of the frontal horn, Kocher's point is landmarked as a point of entry through the frontal bone for an intraventricular catheter to drain CSF. In adult or children, it is located 2.5–3 cm lateral to the midline (at approximately the mid-pupillary line) and approximately 1 cm anterior to the coronal suture. In premature infants, because of small head size, the Kocher's point is located 1.5–2 cm lateral to the midline and approximately 0.5–1 cm anterior to the coronal suture. The distance and depth can be measured accurately by preoperative bedside ultrasound. In addition, since the fontanel is widely open in premature infants with PHH, it is suggested to draw an imaginary line between the bilateral edges of the fontanel as the future coronal suture, then the Kocher's point can be properly located [Fig. 1]. Mostly, the non-dominant, right frontal horn is preferred unless the right side is not suitable to use due to any reason.
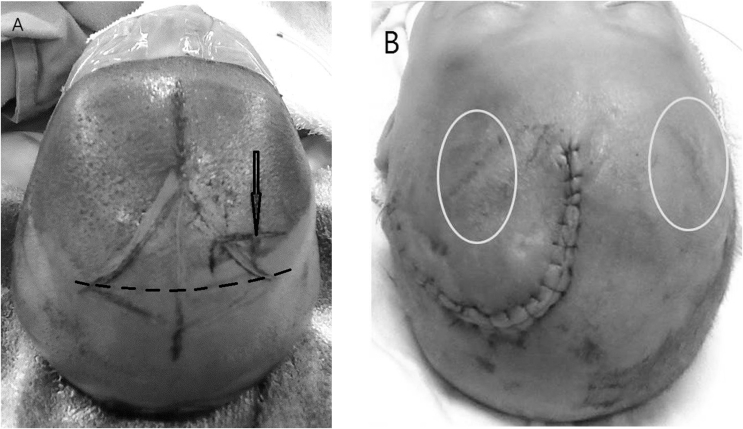
Fig. 1.
(A) The Kocher's point for a proper frontal entry of the ventricular catheter is sometimes hard to locate due to the widely open fontanel in premature infant and the inexperienced neurosurgeons. In premature infants with PHH, it is suggested to draw an imaginary line between the bilateral edges of the fontanel as the future location of the coronal suture (dotted line), then the Kocher's point can be properly located (bold arrow). The Kocher's point is located 1.5–2 cm lateral to the midline and approximately 0.5–1 cm anterior to the coronal suture. (B) This patient was referred to the author from other institution due to repeated malfunction of the ventricular reservoirs and recurrent hydrocephalus. The old incisions for bilateral ventricular reservoir placement were located too anteriorly to the Kocher's point (circles). The baby just underwent a burr-hole typed ventriculoperitoneal shunt insertion after the body weight was good enough.
External ventricular drainage (EVD)
The chances of infection and the long duration required for EVD make it an unattractive option.
The EVD procedures were performed with a ventricular catheter connected to a closed drainage plastic container via subcutaneous tunneling. EVD has the physiological advantage of continuous clearance of bloody CSF [20,21]. Therefore, EVD reduces the spikes of intracranial hypertension which can occur with intermittent tapping. It also gives an opportunity to titrate the amount of CSF drained in order to prevent problems of underdrainage. On the contrary, the continuous drain may induce overdrainage and results in trapped and loculated ventricle. Considering the small amount of CSF, 10 mL/kg/day, that needs to be drained in premature infants with PHH [21], the advantage of continuous drainage of EVD becomes less significant. The disadvantages of EVD include infection, need for repeated CSF studies, and repeated rotation of the EVD site, obstruction and dislodgement of the catheter, and CSF leakage from the exit site of EVD. Though a long-segment of EVD can be performed to reduce infection and to reduce rotation of the EVD site, EVD is not a favored way to manage PHH in pediatric neurosurgery [11,[22], [23], [24]].
Ventriculosubgaleal shunt (VSGS)
Since the first VSGS was performed in 1896 by von Mikulicz, VSGS has been used in chronic postoperative CSF fistulas, tumor, recurrent subdural hematoma, acute head trauma, and repeated VP shunt infections [[25], [26], [27], [28], [29], [30], [31], [32]].
A VSGS consists of a shunt tube with one end in the lateral ventricle while the other end is inserted into the subgaleal space of the scalp. This will allow for the membranes of the scalp to absorb the excess CSF [Fig. 2]. VSGS is preferred by many institutions because it is a simple and rapid method, precludes the need for repeated aspiration without causing electrolyte and nutritional losses. VSGS is associated with lower infection rates than EVD due to the closed system of CSF drainage and lack of external tubes.
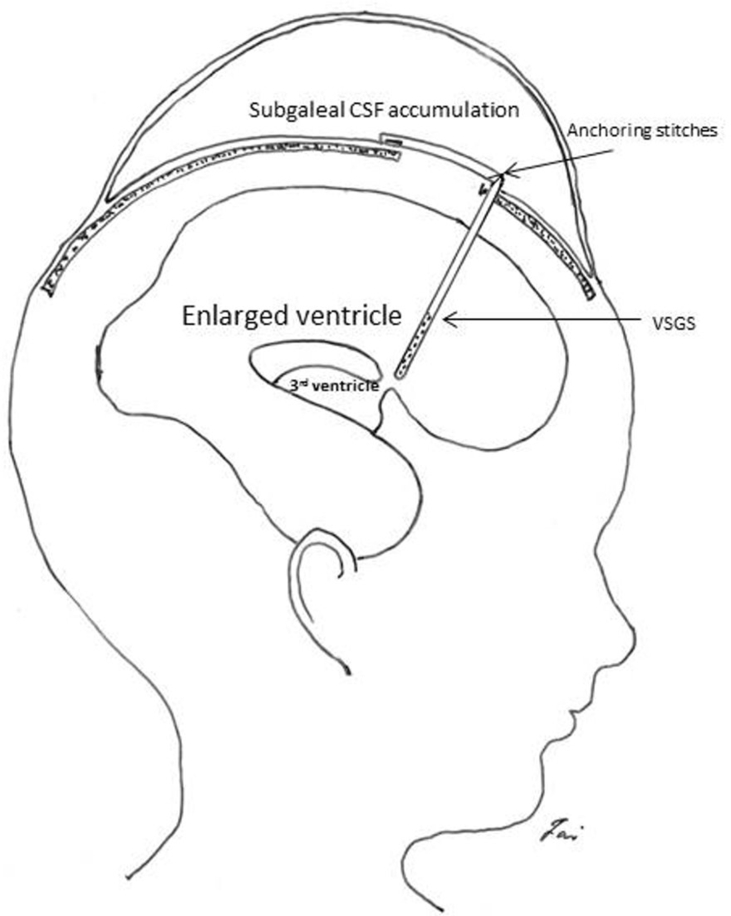
Fig. 2.
The cartoon shows the ventriculosubgaleal shunt. The ventricular catheter can be connected to a subgaleal catheter with a right-angle connector or a low-pressure valve (not shown). A one-way mechanism is needed by using a subgaleal catheter with slits or a low-pressure valve.
Surgical techniques of VSGS
It is important to identify the coronal sutures and locate the Kocher's point for the entrance of ventricular catheter [Fig. 1]. Other important factor includes not grasping the extremely thin skin of the premature infants during the whole procedure [Fig. 3]. In the supine position, the first step is placement of a ventricular catheter into the frontal horn. Next, the ventricular catheter is connected to either a reservoir or via a right angled connector to a short piece of close-end tubing with slits on it in order to establish one-way flow from the ventricle into the subgaleal pocket. If a reservoir is not used, the tubing is secured to the periosteum with suture to prevent catheter migration into or out of the lateral ventricle. To prevent this, the use of a reservoir is suggested. A subgaleal pocket is formed with blunt dissection with finger sweep or blunt tipped Metz scissors with curved ends. In the latter case, care must be given to dissecting the subgaleal space and not a more superficial or deeper layer. Since larger subgaleal pockets can prolong the longevity of the VSGS, always try to dissect out laterally toward each ear, posteriorly over the occiput, not onto the forehead taking care when crossing the midline and avoid button holing the skin. The subgaleal catheter is situated to drain in the direction of the pocket. The wound is closed in two layers.
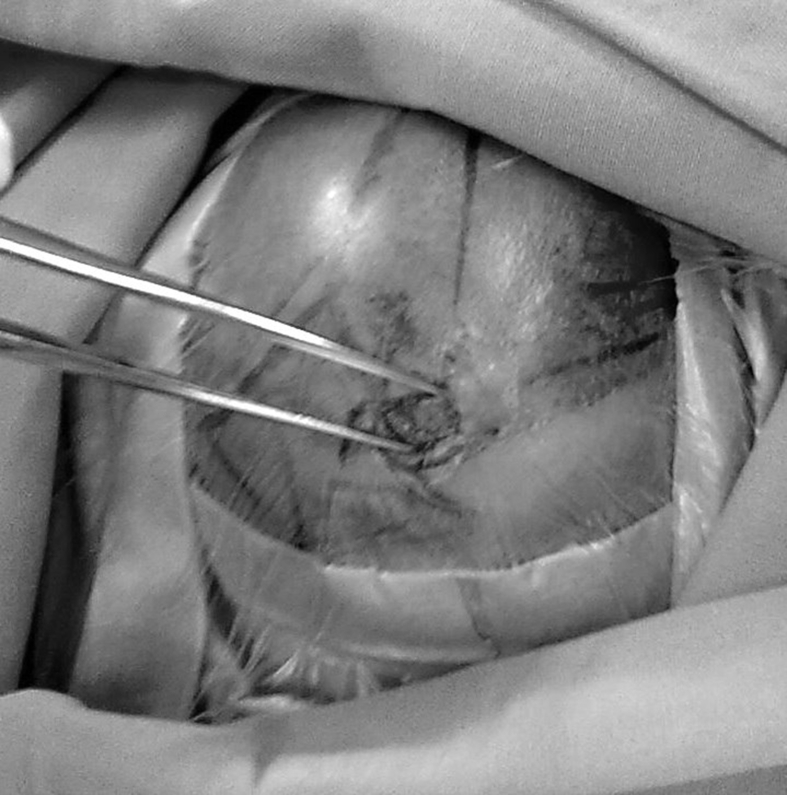
Fig. 3.
A non-grasping technique is used during the whole shunting procedure in premature infants to prevent skin injury. The two arms of a forceps can be used as retractor to separate the incision and prevent injury to the thin skin.
Longevity (survival duration) and evaluation of the effectiveness of VSGS
The average longevity of the primary VSGS was 35.1 and 37.4 days, respectively in two studies. Some of the infants may need two VSG shunts [19,33]. The survival duration of the VSGS is variable depending on several factors that include the absorptive capacity of the subgaleal space and the creation of an ample space during dissection. However, in some cases the ventricles might re-enlarge due to the large collection of CSF exceeding the absorptive capacity of the subgaleal space. This is an indication for a shunt conversion [34].
The effectiveness of VSGS is judged by reduction of head circumference, softening of the anterior fontanel, and reduction of ventricular size as seen on cranial ultrasound or other neuroimaging studies. In cases of VSGS failure, either the previously soft, fluctuant subgaleal pocket became tense with fluid, or the subgaleal space became obliterated without the presence of subgaleal fluid. Cranial imaging confirm ventricular enlargement with either scenario.
Complications of VSGS
The drawback of VSGS is the cosmetically unappealing swelling caused by the CSF collection in the subgaleal space [22]. Most of the parents accept the transient cosmetic problem if they are made aware of the significant scalp swelling. The complications of VSGS include wound leakage before or when sutures were removed, kinking and blockage of the catheter, migration of the catheter from the ventricle or its slippage into the lateral ventricle, VSGS-related death due to intraoperative rapid decompression of a poorly myelinated neonatal brain under high pressure, resulting in numerous intracerebral hemorrhages [19]. In a study of Willis et al., a unacceptably high infection rate of VSGS was noted that even made VSGS no longer performed at their institution. The infection includes infection of VSGS per se, and 3/4 of the infections occurred after the VSGS were converted to a VP shunt [22]. They proposed the cause of infection as below: the formation of pseudomeningocele in the subgaleal space causes further thinning of the extremely thin skin of the premature infants promoting colonization by skin flora. They suggested CSF sampling before conversion VSGS to a permanent shunt and changes in the proximal hardware (ventricular catheter and the valve) of VSGS at the time of insertion of the VP shunt, as the VSG shunts have been in situ for a prolonged period in contact with potentially colonized CSF, may decrease the infection rates [22]. In most of the VSGS complications, the shunts needed to be removed. Another rare, potential complication of VSGS is the secondary subgaleal encephaloceles that resulted in seizure, meningitis, abscess formation, and infarction of herniated brain parenchyma [35] [Fig. 4]. The other unique complications of VSGS include the requirement of CSF taps shortly before a permanent VP shunt is placed when CSF absorption from the subgaleal pocket is no longer adequate to control the hydrocephalus and severe skull deformity [36]. It should be noted that infants should have their head turned every couple of hours in order to avoid skull deformation, which can be severe.
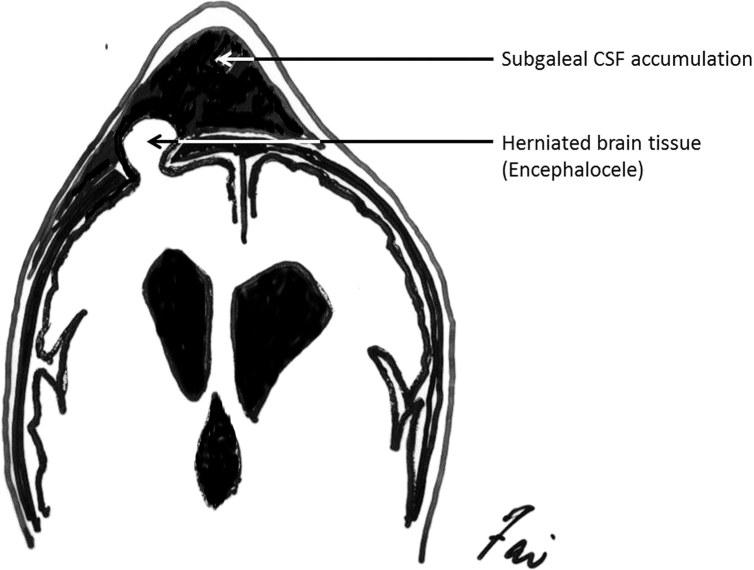
Fig. 4.
A rare but unique complication of VSGS, the secondary subgaleal encephaloceles that has to be recognized, especially when the patient has unusual presentation of seizure, meningitis, abscess formation, and infarction of herniated brain parenchyma.
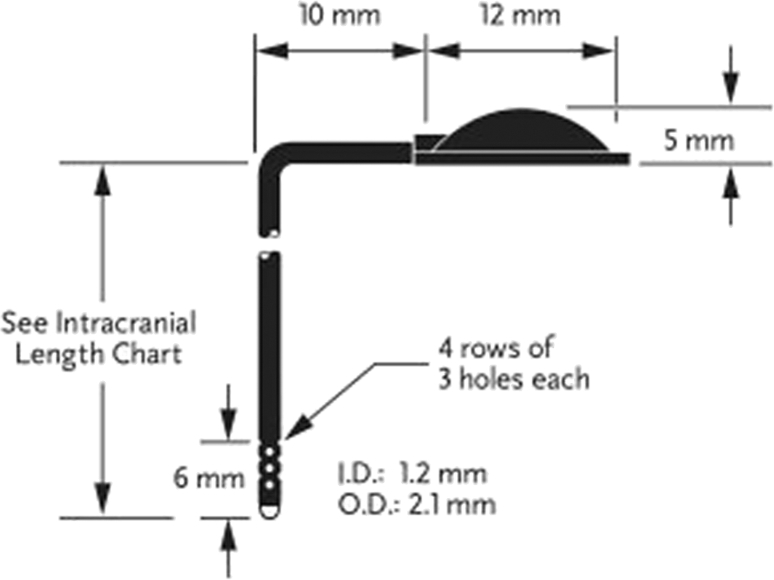
Ventricular access device (VAD), subcutaneous ventricular reservoir, neonatal reservoir, Ommaya reservoir [Fig. 5]
Fig. 5.
A ventricular catheter with integrated reservoir (CSF ventricular reservoir, Medtronic Inc.) from one of the many manufactures. The intracranial length of 3.5 to 4 cm is most commonly used in premature infants.
One of the most acceptable forms of temporized drainage for the prematurity is the implantation of VADs, which located in subgaleal space are repeatedly tapped for decompression of intracranial hypertension and antibiotic and fibrinolytic agents are administered via the reservoir if needed [37]. It also allows easy conversion to a permanent shunt as the suitable proximal hardware is already in place [22]. The drawback of VAD is the need for repeated punctures of the reservoir with an additional risk of infection though it is much less than that of EVD. Other drawbacks include labor-intensive, wound problems, occlusion, not providing constant decompression of the ventricles, and the loss of protein- and electrolyte-laden CSF [[37], [38], [39]]. In the author's institution, a tertiary medical center, VAD is routinely used as the first choice in PHH with minimal complications.
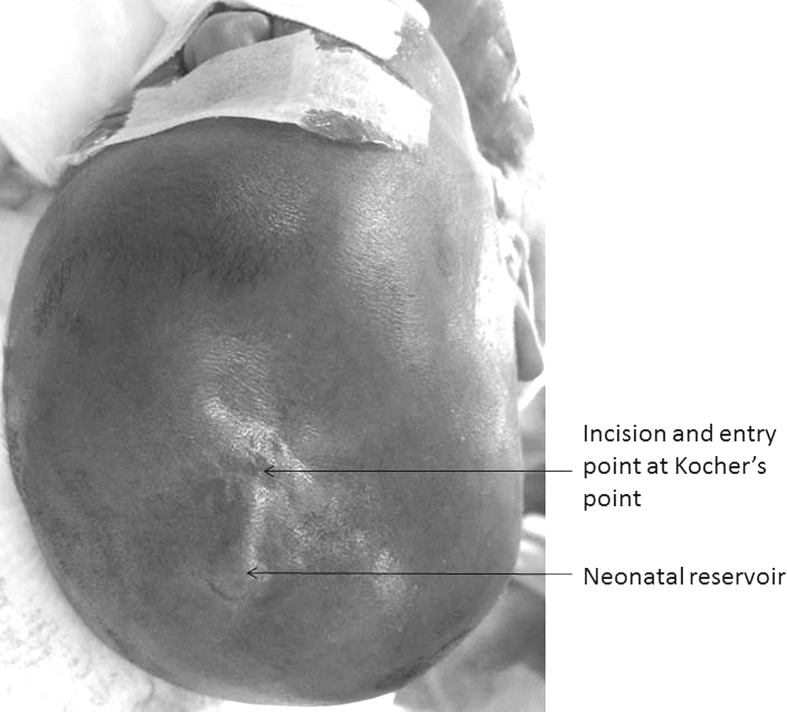
Surgical techniques of neonatal reservoir [Fig. 6]
Fig. 6.
The photo shows the proper setting of the ventricular access device (VAD). The patient is going to receive the conversion of a temporizing VAD to a ventriculoperitoneal shunt.
The right frontal and parietal regions are shaved and prepped. The skin just anterior to the coronal suture and over the parietal bone is infiltrated with normal saline. The frontal part is for the entrance of ventricular catheter and the parietal part is for the placement of the subcutaneous reservoir. A linear or semi-lunar incision is made anterior to the Kocher's entry point, so that the ventricular catheter later placed does not erode through the incision line. The coronal suture is confirmed again then the dura over the Kocher's point is coagulated with a bipolar unit and incised. The subgaleal space over the parietal bone is dissected bluntly, then the reservoir dome is placed underneath the skin. The ventricular catheter is inserted into the right lateral ventricle, the incision is then closed in two layers. A 27-gauge needle is used to confirm the function of the reservoir by aspiration of the CSF smoothly.
Treatment protocol of VAD
The reservoir is tapped through the scalp on a regular basis to remove CSF and maintain a stable clinical condition which includes normal increase of head circumference, soft fontanel, and sonogram. In the supine position, a scalp needle of 25-gauge or a smaller one is used to tap the reservoir. The frequency and amount of CSF aspiration is tailored for each infant and is determined by the opening and closing pressures, respectively. When the infant lies in supine position, the water-level of the scalp needle at the forehead level is about 3 cm of intraventricular pressure. Since the normal intraventricular pressure of the premature infant is subatmospheric, we aspirate the CSF of an amount till the closing pressure is just below the level of the forehead. While we like to keep the opening pressure lower than 8 cm, which is the upper limit of the normal ICP of full-term infants. If the opening pressure is higher than 8 cm, the frequency of tapping is increased, for example from once daily to twice daily. It is better to keep the ventricle system mild dilated than collapsed and anterior fontanel soft than marked depressed. Sonograms can be used to confirm the ventricular size. If the frequency of tapping can be tapered according to the lowering of opening pressure, decreasing amount of CSF in each tap, and the symptomatic hydrocephalus dissipates, the reservoir may be no longer needed. In this situation, we do not routinely remove the VAD or we will wait for at least four years if the parents insist to have it removed. If it becomes evident that permanent CSF diversion is necessary, the VAD will be converted to VP shunt when the baby's weight or age is good enough.
Comparison between the temporary measures (Table 1)
Table 1.
Comparison between different temporary measures.
| EVD | VSGS | VAD | |
|---|---|---|---|
| Close system | – | + | + |
| Continuous drainage | + | ± or + | – |
| Repeated aspiration | – | – | + |
| Labor intense | + | + | ++ |
| Risk of over-drainage or under-drainage | + | + | – |
| Electrolyte and nutritional loss | + | – | + |
| Risk of infection | ++ | + | + |
| CSF studies before conversion to a permanent shunt | ++ | ++ | + |
| Changes of the hardware when converting to a permanent shunt | +++ | +++ | ± |
| Survival duration | Short | Average 30 days | Long |
| Unique complications and drawbacks | – | Catheter migration, unappealing scalp swelling, skull deformity, secondary subgaleal encephalocele | – |
Abbreviations: EVD: external ventricular drainage; VSGS: ventriculosubgaleal shunt; VAD: ventricular access device.
The rates of VP shunt requirement and device infection were similar between patients treated with VAD versus the VSGS [40]. The VSGS requires less labor-intensive management by ventricular tapping; the VSGS patients also attained higher weights and more optimal surgical candidacy at the time of VP shunt insertion. However, some complications are unique to VSGS, it is thus important for the treating physicians to be aware of these rare, potential complications while using VSGS in the management of PHH. Finally, the potential differences in long-term developmental and neurological outcomes between VSGS and VAD warrant further study [40].
Permanent surgical management
VP shunt
Early VP shunt insertion is associated with a high failure rate and many complications; hence, temporizing measures are always instituted until the infant is mature (age and/or weight) enough [18]. VP shunting is indicated when the ventricles continue to enlarge at a body weight exceeds 2 kg and CSF protein levels are below 1–1.5 g/L, then VP shunt insertion is appropriate [3]. Though these minimal requirements are subjective, they indicate fewer complications [22,41].
Endoscopic third ventriculostomy (ETV)
ETV offers an alternative to a shunt in selected patients with obstructive hydrocephalus; however, ETV alone has low success rate for premature infants with PHH because the age and cause of hydrocephalus of these infants are unfavorable factors of a successful ETV [42]. Combined ETV and choroid plexus cauterization may be effective on the treatment of a selected subsets of premature infants with PHH though further study is warranted [43].
Discussion
Significant progress has been made to reduce neonatal mortality, and neurological complication of prematurity. Although it is likely that fewer premature infants will suffer IVH in the future, the treatment, reduction of perioperative complications and surgical outcome of IVH in premature infants remain challenging to pediatric neurosurgeons. This review aims to provide pediatric neurosurgeons a comprehensive understanding of various surgical treatment modalities, focusing on the temporizing measures before the infants is heavy and old enough to undergo permanent surgical management. The pediatric neurosurgeons may choose the best initial approach for the management of IVH and PHH in premature infants based on the facility and man power of the institution and the evidence of previous studies. A successful transition from the temporizing measures to permanent shunt insertion or resolution of hydrocephalus with minimal perioperative complication is the key to a good surgical outcome of these premature infants with IVH and PHH.
Conflict of interest
Authors have declared that no competing interests exist.
Acknowledgment
The writing of this article was partly supported by grants from the Ministry of Science and Technology, Taiwan (MOST108-2314-B-002-086 to Dr. MF Kuo).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Hambleton G., Wigglesworth J.S. Origin of intraventricular haemorrhage in the preterm infant. Arch Dis Child. 1976;51:651–659. doi: 10.1136/adc.51.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hambleton G., Wigglesworth J.S. Pathogenesis of intraventricular hemorrhage. J Pediatr. 1979;94:159–161. doi: 10.1016/s0022-3476(79)80388-1. [DOI] [PubMed] [Google Scholar]
- 3.Whitelaw A., Aquilina K. Management of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2012;97:F229–F233. doi: 10.1136/adc.2010.190173. [DOI] [PubMed] [Google Scholar]
- 4.Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 2012;9:242–258. doi: 10.3171/2011.12.PEDS11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papile L.A., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gram. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 6.du Plessis A.J. The role of systemic hemodynamic disturbances in prematurity-related brain injury. J Child Neurol. 2009;24:1127–1140. doi: 10.1177/0883073809339361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luyt K., Jary S., Lea C., Young G.J., Odd D., Miller H. Ten-year follow-up of a randomised trial of drainage, irrigation and fibrinolytic therapy (DRIFT) in infants with post-haemorrhagic ventricular dilatation. Health Technol Assess. 2019;23:1–116. doi: 10.3310/hta23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitelaw A., Evans D., Carter M., Thoresen M., Wroblewska J., Mandera M. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007;119:e1071–e1078. doi: 10.1542/peds.2006-2841. [DOI] [PubMed] [Google Scholar]
- 9.Whitelaw A., Pople I., Cherian S., Evans D., Thoresen M. Phase 1 trial of prevention of hydrocephalus after intraventricular hemorrhage in newborn infants by drainage, irrigation, and fibrinolytic therapy. Pediatrics. 2003;111:759–765. doi: 10.1542/peds.111.4.759. [DOI] [PubMed] [Google Scholar]
- 10.Schulz M., Bührer C., Pohl-Schickinger A., Haberl H., Thomale U.W. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J Neurosurg Pediatr. 2014;13:626–635. doi: 10.3171/2014.2.PEDS13397. Erratum in J Neurosurg Pediatr. 2014;13:706. [DOI] [PubMed] [Google Scholar]
- 11.Gurtner P., Bass T., Gudeman S., Penix J., Philput C., Schinco F. Surgical management of posthemorrhagic hydrocephalus in 22 low-birth-weight infants. Childs Nerv Syst. 1992;8:198–202. doi: 10.1007/BF00262844. [DOI] [PubMed] [Google Scholar]
- 12.Bergman I., Wald E., Meyer J., Painter M. Epidural abscess and vertebral osteomyelitis following serial lumbar punctures. Pediatrics. 1983;72:476–480. [PubMed] [Google Scholar]
- 13.Anwar M., Kadam S., Hiatt I.M., Hegyi T. Serial lumbar punctures in prevention of post-hemorrhagic hydrocephalus in preterm infants. J Pediatr. 1985;107:446–450. doi: 10.1016/s0022-3476(85)80532-1. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani J.F., Pasternak J.F., Mathew O.P. Failure of daily lumbar punctures to prevent the development of hydrocephalus following intraventricular hemorrhage. J Pediatr. 1980;97:278–281. doi: 10.1016/s0022-3476(80)80495-1. [DOI] [PubMed] [Google Scholar]
- 15.Johnson A., Wincott E., Grant A., Elbourne D. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation: results at 30 months. Arch Dis Child. 1994;71:F147. doi: 10.1136/fn.71.2.f147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dykes F.D., Dunbar B., Lazarra A., Ahmann P.A. Posthemorrhagic hydrocephalus in high-risk preterm infants: natural history, management, and long-term outcome. J Pediatr. 1989;114:611–618. doi: 10.1016/s0022-3476(89)80707-3. [DOI] [PubMed] [Google Scholar]
- 17.Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database Syst Rev. 2001:CD000216. doi: 10.1002/14651858.CD000216. [DOI] [PubMed] [Google Scholar]
- 18.Zaben M., Finnigan A., Bhatti M.I., Leach P. The initial neurosurgical interventions for the treatment of posthaemorrhagic hydrocephalus in preterm infants: a focused review. Br J Neurosurg. 2016;30:7–10. doi: 10.3109/02688697.2015.1096911. [DOI] [PubMed] [Google Scholar]
- 19.Fulmer B.B., Grabb P.A., Oakes W.J., Mapstone T.B. Neonatal ventriculosubgaleal shunts. Neurosurgery. 2000;47:80–83. doi: 10.1097/00006123-200007000-00018. discussion 83–4. [DOI] [PubMed] [Google Scholar]
- 20.Beni-Adani L., Ben-Sira L., Constantini S. Surgical treatment of post hemorrhagic hydrocephalus (PHH) In: Berger I., Schimmel M.S., editors. Hot topics in neonatal neurology. Nova Science Publishers Inc.; 2008. pp. 171–210. [Google Scholar]
- 21.Bassan H., Eshel R., Golan I., Kohelet D., Ben-Sira L., Mandel D. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with posthemorrhagic hydrocephalus. Eur J Paediatr Neurol. 2012;16:662–670. doi: 10.1016/j.ejpn.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Willis B.K., Kumar C.R., Wylen E.L., Nanda A. Ventriculosubgaleal shunts for posthemorrhagic hydrocephalus in premature infants. Pediatr Neurosurg. 2005;41:178–185. doi: 10.1159/000086558. [DOI] [PubMed] [Google Scholar]
- 23.Berger A., Weninger M., Reinprecht A., Haschke N., Kohlhauser C., Pollak A. Long-term experience with subcutaneously tunneled external ventricular drainage in preterm infants. Childs Nerv Syst. 2000;16:103–110. doi: 10.1007/s003810050022. [DOI] [PubMed] [Google Scholar]
- 24.Hudgins R.J., Boydston W.R., Gilreath C.L. Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device. Pediatr Neurosurg. 1998;29:309–313. doi: 10.1159/000028744. [DOI] [PubMed] [Google Scholar]
- 25.Köksal V., Öktem S. Ventriculosubgaleal shunt procedure and its long-term outcomes in premature infants with post-hemorrhagic hydrocephalus. Childs Nerv Syst. 2010;26:1505–1515. doi: 10.1007/s00381-010-1118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dardenne G. Le traitment par le drainage ventriculaire souscutane des fistules de liquide cephalo-rachidien postoperatoires rebelles. Acta Neurol Belg. 1964;64:1202–1211. [PubMed] [Google Scholar]
- 27.Ferreira N.P., Correa J. Derivacao liquorica ventriculo-subcutanea na terapeutica cirurgica das fistulas liquoricas pos-operatorias rebeldes. Neurobiologica. 1972;35:97–104. [Google Scholar]
- 28.Perret G.E., Graf C.J. Subgaleal shunt for temporary ventricle decompression and subdural drainage. J Neurosurg. 1977;47:590–594. doi: 10.3171/jns.1977.47.4.0590. [DOI] [PubMed] [Google Scholar]
- 29.Savitz M.H., Katz S.S. Ventriculoperitoneal shunting for acute head trauma. Crit Care Med. 1983;11:290–293. doi: 10.1097/00003246-198304000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Van Calenbergh F., Goffin J., Casaer P., Plets C. Use of a ventriculosubgaleal shunt in the management of hydrocephalus in children with posterior fossa tumors. Childs Nerv Syst. 1996;12:34–37. doi: 10.1007/BF00573852. [DOI] [PubMed] [Google Scholar]
- 31.Savitz M.H., Malis L.I. Subgaleal shunting: a 20-year experience. Neurosurg Focus. 2000;9:1–5. doi: 10.3171/foc.2000.9.6.11. [DOI] [PubMed] [Google Scholar]
- 32.Steinbok P., Cochrane D. Ventriculosubgaleal shunt in the management of recurrent ventriculoperitoneal shunt infection. Childs Nerv Syst. 1994;10:536–539. doi: 10.1007/BF00335079. [DOI] [PubMed] [Google Scholar]
- 33.Tubbs R.S., Smyth M.D., Wellons J.C., III, Blount J.P., Grabb P.A., Oakes W.J. Life expectancy of ventriculosubgaleal shunt revisions. Pediatr Neurosurg. 2003;38:244–246. doi: 10.1159/000069827. [DOI] [PubMed] [Google Scholar]
- 34.Drapkin A., Levine M.E., Yang W.C. Ventriculo-subgaleal shunt: evaluation by computed tomography. Acta Neurochir. 1980;55:107–115. doi: 10.1007/BF01808925. [DOI] [PubMed] [Google Scholar]
- 35.Seeburg D., Ahn E., Huisman T. Secondary pediatric encephalocele after ventriculosubgaleal shunting for posthemorrhagic hydrocephalus. Neuropediatrics. 2014;45:252–255. doi: 10.1055/s-0033-1363298. [DOI] [PubMed] [Google Scholar]
- 36.Lam Herman P., Heilman Carl B. Ventricular access device versus ventriculosubgaleal shunt in post hemorrhagic hydrocephalus associated with prematurity. J Matern Fetal Neonatal Med. 2009;22:1097–1101. doi: 10.3109/14767050903029576. [DOI] [PubMed] [Google Scholar]
- 37.McComb J.G., Ramos A.D., Platzker A.C., Henderson D.J., Segall H.D. Management of hydrocephalus secondary to intraventricular hemorrhage in the preterm infant with a subcutaneous ventricular catheter reservoir. Neurosurgery. 1983;13:295–300. doi: 10.1227/00006123-198309000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Marlin A.E. Protection of the cortical mantle in premature infants with posthemorrhagic hydrocephalus. Neurosurgery. 1980;7:464–468. doi: 10.1227/00006123-198011000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Levy M.L., Masri L.S., McComb J.G. Outcome for preterm infants with germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery. 1997;41:1111–1118. doi: 10.1097/00006123-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Wang J.Y., Amin A.G., Jallo G.I., Ahn E.S. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr. 2014;14:447–454. doi: 10.3171/2014.7.PEDS13552. [DOI] [PubMed] [Google Scholar]
- 41.Eid S., Iwanaga J., Oskouian R.J., Loukas M., Jerry Oakes W., Tubbs R.S. Ventriculosubgaleal shunting - a comprehensive review and over two-decade surgical experience. Childs Nerv Syst. 2018;34:1639–1642. doi: 10.1007/s00381-018-3887-6. [DOI] [PubMed] [Google Scholar]
- 42.Kulkarni A.V., Drake J.M., Mallucci C.L., Sgouros S., Roth J., Constantini S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 2009;155:254–259. doi: 10.1016/j.jpeds.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 43.Warf B.C., Campbell J.W., Riddle E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst. 2011;27:1063–1071. doi: 10.1007/s00381-011-1475-0. [DOI] [PubMed] [Google Scholar]