Abstract
In an influential theoretical model, human sensorimotor control is achieved by a Bayesian decision process, which combines noisy sensory information and learned prior knowledge. A ubiquitous signature of prior knowledge and Bayesian integration in human perception and motor behavior is the frequently observed bias toward an average stimulus magnitude (i.e., a central-tendency bias, range effect, regression-to-the-mean effect). However, in the domain of eye movements, there is a recent controversy about the fundamental existence of a range effect in the saccadic system. Here we argue that the problem of the existence of a range effect is linked to the availability of prior knowledge for saccade control. We present results from two prosaccade experiments that both employ an informative prior structure (i.e., a nonuniform Gaussian distribution of saccade target distances). Our results demonstrate the validity of Bayesian integration in saccade control, which generates a range effect in saccades. According to Bayesian integration principles, the saccadic range effect depends on the availability of prior knowledge and varies in size as a function of the reliability of the prior and the sensory likelihood.
Keywords: saccades, saccadic accuracy, range effect, Bayesian sensorimotor integration, central-tendency bias
Introduction
Uncertainty about parameters of the outside world, such as the distance, size, color, or the speed of an object, poses a fundamental problem for human behavior, which is based on reliable interaction with the environment (Wolpert & Landy, 2012; Faisal et al., 2008). During the last two decades, it has become evident that the brain readily combines learned prior knowledge about the statistics of environmental properties Θ and currently available sensory information S (likelihood) in order to obtain parameter estimates with lower uncertainty than either the prior knowledge or the sensory likelihood alone (Körding & Wolpert, 2004; Vilares et al., 2012). According to the Bayesian brain hypothesis (Knill & Pouget, 2004; Petzschner et al., 2015), the estimation of environmental parameters can be mathematically formulated as the product of the prior belief distribution Q(Θ) on the parameter Θ and the likelihood distribution L(S|Θ):
| (1) |
Under the assumption of Gaussian distributions, the mean μΘ|S of the posterior distribution is the weighted average of the prior mean μΘ and the likelihood mean μS|Θ, where the weights are given by the relative uncertainties (i.e., variances) of the distributions:
| (2) |
Central-tendency bias as a consequence of Bayesian decisions in perception and action
It follows from Equation 2 that the optimal Bayesian estimate of an environmental parameter Θ is biased toward a central magnitude represented by the mean of the prior. The size of such a prior-evoked central-tendency bias is determined by the reliabilities of both the prior and the likelihood information. In the case of low prior uncertainty of the true value of Θ and/or unreliable sensory information, the prior belief generates a regression-to-the-mean effect on true values of Θ that differ from μΘ, that is, from a person's central a priori expectation (Figure 1A). On the other hand, a weak central-tendency bias results from weak influence of the prior on the posterior estimate, that is, when the sensory likelihood is clearly more reliable than the prior information. In recent years, the Bayesian framework has emerged as a universal account for the influence of prior knowledge in human sensorimotor behavior. Prior-evoked central-tendency effects, i.e., the characteristic overestimation of small stimulus magnitudes and underestimation of large stimulus magnitudes within the range of possible true values of Θ, have been reported across sensory modalities, for different environmental parameters, and across different motor tasks (Körding & Wolpert, 2004; Vilares et al., 2012; Jazayeri & Shadlen, 2010; Sciutti et al., 2014; Sato & Kording, 2014; Olkkonen et al., 2014; Kok et al., 2013; Grau-Moya et al., 2012; Wiener et al., 2016; Trommershäuser et al., 2003; Petzschner & Glasauer, 2011).
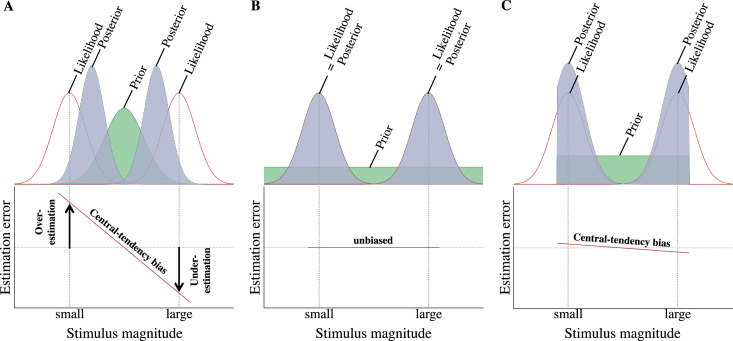
Figure 1.
(A) An informative central prior (green area) leads to a bias of the sensory measurements (likelihoods, red curves) toward the peak of the prior. The posterior estimate (gray area) has higher precision than the prior or the likelihood but is systematically shifted toward the center of the range of stimulus magnitudes, which generates the characteristic central-tendency bias. As an observed behavioral consequence, the central-tendency bias is expressed by a negative trend of estimation errors measured as a function of stimulus magnitudes, which turns the overestimation of small stimulus magnitudes into an underestimation of large stimulus magnitudes. (B) An uninformative (flat) prior (green area) does not generate a central-tendency bias. The posterior estimates (gray areas) have the same precision as the sensory judgments (likelihoods, red curves) and are not shifted from the likelihoods. As a consequence, the observed average measurement error is unbiased with no systematic over- or underestimation of stimulus magnitudes within the test range. (C) A weakly informative uniform prior with truncated support, which assigns equal probabilities to the stimulus magnitudes within a certain range, leads to the prediction of an attenuated central-tendency bias. The posterior estimates (gray areas) have higher precision than the likelihoods (red curves) and are truncated at the boundaries of the prior.
Evidence against a central-tendency bias in saccades
Saccadic eye movements are the most frequent single movements humans produce during their lifetime, and precise oculomotor control is the foundation of the saccade-fixation strategy of human vision (Land, 1999). Goal-directed saccades toward targets at distances of 10 degrees or more are typically characterized by a systematic undershoot of approximately 10%. It is largely undisputed that this robust property of the saccadic system represents a deliberate oculomotor control strategy aiming at minimizing saccadic costs (flight time or energy expenditure) and avoiding corrective secondary saccades for which the eye must reverse the direction of the initial saccade. There is, however, a long-standing debate about the existence of a regression-to-the mean effect in saccadic eye movements. Two recent studies claim to provide evidence against a central-tendency bias in the saccadic system (Gillen et al., 2013; Nuthmann et al., 2016). Given the important implications of a possible absence of one of the most fundamental sensorimotor functions in the saccadic system, this claim should be considered carefully.
The debate about the presence of a central-tendency bias in saccades dates back to the initial report of the so-called range effect within a simple saccade-targeting experiment published by Kapoula (1985) (see also Kapoula & Robinson, 1986). In this study, subjects made goal-directed saccades from a fixation stimulus toward a small square-shaped target stimulus that appeared randomly across trials at one out of five different distances ranging from 2.7○ to 9.5○ in one block of the experiment and from 7○ to 21.9○ in a different block. The main finding was that subjects systematically overshot the targets at the near end of the range of target distances and undershot the targets at the far end. Since the two test ranges overlapped, it was found that subjects either under- or overshot the overlapping target distances depending on whether the distances where framed within the proximal or the distal range. Kapoula's work is widely cited and has become a standard reference for a similar effect in eye movements during reading (McConkie et al., 1988; Rayner, 1998).
The reliability of Kapoulas's (1985) results has been questioned for reasons such as a small number of participants (four and two participants for testing the proximal and distal saccade-amplitude ranges) together with a marginal effect size, however, most importantly, because of several failed attempts to replicate the results (Vitu, 1991; Gillen et al., 2013; Nuthmann et al., 2016; Gillen & Heath, 2014; Heath et al., 2015). Recently, Nuthmann et al. (2016) reported a series of three prosaccade experiments beginning with an as-close-as-possible replication of Kapoulas's original experiment, plus an extended experiment with increased statistical power and an additional range of very short saccade amplitudes, and finally a third experiment including memory-guided saccades. In all three experiments, saccadic landing positions did not express a systematic overshoot of short distances and an undershoot of far distances in any of the experiments of the study. Furthermore, Gillen et al. (2013) concluded that there is no range effect in stimulus-driven saccades from a very similar experiment. Subjects in their study made saccades toward targets within ranges between either 3○ and 13○ or 10.5○ and 20.5○ of eccentricity. In this study, target-presentation times of 50 ms were used in order to decrease the frequency of secondary saccades. Gillen et al. (2013) found no evidence of a saccadic range effect, but they did replicate the finding of a general saccadic undershoot across target distances. The same results were found in two further studies (Gillen & Heath, 2014; Heath et al., 2015), where no range effect was found for prosaccades, and in a study by Vitu (1991), who tested the range effect in saccadic eye movements using isolated words at different distances as saccade targets and found, again, no evidence for a range effect.
Is there really no range effect in saccades?
So is there really no central-tendency bias in saccades as in almost all other human motor systems and perceptual judgments? We think this conclusion cannot be made from the abovementioned studies. Our main argument is that previous studies on the saccadic range effect employ uniform priors and so hardly meet the conditions for a manifestation of a central-tendency bias according to Bayesian theory (Figure 1a). In the following section, we show how uniform priors with limited or unlimited support represent a limiting case in the Bayesian sensorimotor model, which lead to the prediction of strongly reduced or absent central-tendency effects.
What follows from a uniform prior according to Bayesian theory
A common characteristic of previous studies on the central-tendency bias in saccades is that all target positions during the experiments were tested equally frequently, that is, the probability that a target appeared at a specific location in these experiments was uniformly distributed. As the prior represents the subjects’ learned expectation on the probability of target locations, it is straightforward to assume that subjects employed a uniform prior distribution when estimating target locations for saccade planning. We will consider the consequences of two alternative uniform priors that may result from these experimental designs: first, a uniform prior with wide (i.e., effectively unlimited) support (Figure 1B) and, second, a uniform prior with compact support in the limits of the test range used in the experiments (Figure 1C). When the prior is effectively uninformative in the sense that a person assigns a priori equal probabilities over a wide range of possible target locations (flat prior with unlimited support), the posterior estimate would not be biased toward a central location regardless of the actual precision of the sensory likelihood (Figure 1B). Thus, an uninformative flat prior represents a special case for which the Bayesian model predicts the absence of a central-tendency bias.
However, since the range of target locations is in fact limited, one could argue that instead of leading to an uninformative flat prior, these experimental settings might lead to a prior probability distribution that uniformly supports positions within the range but is zero outside of the range used in the experiment. Thus, we also consider the consequences of a weakly informative prior with uniform compact support in the limits of the test range (Figure 1C). Interestingly, Bayesian integration of a uniform compact prior and Gaussian likelihood densities leads to truncated Gaussian posterior densities near the boundaries of the test range and generates an attenuated central-tendency bias. However, in comparison to a Gaussian prior (Figure 1A), a weakly informative uniform prior with limited support generates a substantially smaller central-tendency bias. This theoretical consideration of the consequences of a uniform compact prior is completed by the following two points: First, even small overestimations of the true limits of the compact support would transform a uniform compact prior (Figure 1C) into an essentially uninformative flat prior (Figure 1B). Second, most of the previous studies on the saccadic range effect additionally employ clearly identifiable target stimuli (low sensory uncertainty), which further decreases the influence of prior knowledge in the computation of the posterior estimate according to Bayesian theory. Taken together, the employment of uniform prior probability distributions over target locations and clearly identifiable target stimuli strongly operates against the manifestation of a saccadic range effect. This consequence of Bayesian integration theory could therefore well explain the mixed findings in the literature on the saccadic range effect with a number of studies that did not find a saccadic range effect and others reporting a very small effect.
There is a study by Heath et al. (2015) that provides an exception to the use of uniformly distributed target-location frequencies. This study tested prosaccades for three different target distances (10.5, 15.5, and 20.5 degrees) while in some conditions of the experiment, either the closest or the farthest target was present five times more often than the other two locations. Heath at al. (2015) did not find evidence for a shift of landing positions in prosaccades toward the target location with the higher a priori probability. However, it seems at least questionable whether the use of only three and highly distinct target positions, each 5 degrees apart, would actually lead to the formation of a common, continuous prior distribution.
However, the effect of using prior knowledge and sensory information according to Bayesian integration principles for saccade control and the existence of a prior-evoked range effect in the saccadic system can be tested using either a more informative prior (see Experiment 1) or by manipulating the uncertainty of the prior and the likelihood within a full-factorial experimental design (see Experiment 2).
Central-tendency bias and general oculomotor undershoot strategy must not be mutually exclusive
Before we present our experiments and hypotheses, we wish to emphasize that we do not consider the saccadic range effect and a general saccadic undershoot strategy as mutually exclusive saccadic biases. In fact, we believe that noisy sensory input and prior knowledge can generate a central-tendency bias in the sensory localization of saccade targets such that positions of near targets are systematically overestimated and positions of far targets are systematically underestimated. However, this does not necessarily affect a general oculomotor strategy to generate hypometric saccades toward these biased target-position estimates in order to overall reduce saccadic flight time or energy expenditure. From this consideration, it follows that the term range effect should not be used as a synonym for over- and undershoots as the landing positions of generally hypometric saccades toward a near target need not necessarily overshoot the target even if its position was perceptually overestimated in the first place. The critical test of a central-tendency bias in saccades is the prediction of a relative difference of landing positions for targets at identical absolute positions that are framed as either relatively near or relatively far depending on the range of target eccentricities in the experiment. For example, within a range of proximal target eccentricities from 2° to 12○, a target at 10○ should elicit a larger undershoot than the identical target within a set of distal targets ranging from 8○ to 18○, if the position of these targets is underestimated in the proximal condition and overestimated in the distal condition depending on the availability of priors that favors distances shorter than 10○(proximal range) or longer (distal range) than 10○.
Aims of the present study
The purpose of the present study is to examine the consistency of saccade control under the influence of informative prior knowledge with Bayesian integration theory. Experiment 1 was designed as a close replication of the experiments reported by Kapoula (1985), Nuthmann et al. (2016), and Gillen et al. (2013) with the exception that we include an informative Gaussian prior. In Experiment 1, subjects made saccades from a fixation stimulus toward a target stimulus (a small square) appearing at variable eccentricities within two partially overlapping sets of eccentricity ranges (2○–12○ and 8.5○–18.5○). Furthermore, target presentation times were either 50 ms as in Gillen et al. (2013) or 500 ms, that is, longer than a typical saccadic reaction time, as in Kapoula (1985) and Nuthmann et al. (2016). Most important, the probability of target locations within the test ranges was not uniformly distributed but randomly drawn from a centered Gaussian probability distribution leading to a higher probability of target locations near the center of the test range and a decreasing probability of target locations near the boundaries of the test ranges. We predict that the underlying informative prior structure leads to the development of a central-tendency bias in saccadic eye movements. In more detail, we expect that subjects systematically overestimate the position of targets at the near end of both test ranges and systematically underestimate the distance of targets at their far ends, leading to corresponding modulations of saccadic responses and, most important, to the prediction of different saccadic landing positions at overlapping target distances. Overlapping targets in Experiment 1 (between 8.5○and 12○) should elicit stronger undershoot in the proximal condition of the experiment (as we predict target-position estimates to be biased toward the center of the proximal range at 7○) than in the distal condition (as the target-position estimates should be biased toward the center of the distal range at 13.5○).
However, the Bayesian saccade planning model outlined in this work does not only predict different saccadic responses on identical targets in different eccentricity ranges but also predicts that saccadic behavior within the same range of target distances can be modulated by varying the uncertainty of the prior and the sensory likelihood. Experiment 2 thus extends and completes the first experiment by manipulating both the reliability of the prior (weak vs. informative) and the reliability of the sensory likelihood (narrow vs. wide). In this experiment, we tested saccades within one range of target distances from 4○ to 20○. In order to manipulate the reliability of the sensory likelihood, the targets of this experiment consisted of a cloud of points, which were randomly scattered according to a two-dimensional Gaussian (centered at the true target position) with either a low standard deviation (i.e., narrow likelihood condition) or a high standard deviation (i.e., wide likelihood condition). Furthermore, the probability distribution of the varying target positions corresponded to either a broad Gaussian distribution (i.e., weak prior) or a narrow Gaussian (i.e., strong prior) for either half of the participants. Following Equation 2, we expected that saccadic errors toward the same saccade target distances within the same range of target eccentricities are modulated by both the reliability of the prior and the likelihood. More specifically, we expected that the combination of a weak prior and precise sensory information (narrow likelihood) generates the smallest centering bias, and vice versa, we expected that an informative prior together with increased sensory uncertainty (wide likelihood) leads to the strongest bias.
Method
Experiment 1
Participants
Ten students (nine female, ages between 19 and 36 years, mean = 22.6 years) of the University of Potsdam participated in Experiment 1. Subjects received study credit or a total of 14€ for their participation in two sessions. All participants had normal or corrected-to-normal vision.
Experimental setup
Testing took place in a dimly lit room of the Engbert Lab at the University of Potsdam. Subjects sat 70 cm in front of a computer screen with their heads stabilized on a chinrest. The Mitsubishi Diamond Pro 2070 SB monitor (Mitsubishi Electric Corporation, Tokyo, Japan) had a physical size of 40 × 30 cm, a resolution of 1,280 × 1,024, and a refresh rate of 85 Hz. Eye movements were recorded with an EyeLink 1000 desktop-mount system (SR Research, Ottawa, Ontario, Canada) with a sampling rate of 1 kHz. The experiment was programmed using the Psychophysics Toolbox (Brainard & Vision, 1997; Pelli, 1997; Kleiner et al., 2007) and the Eyelink Toolbox (Cornelissen et al., 2002) extensions for (MATLAB, 2015) and was executed on a Windows 7 machine.
Eye-tracking procedure
Eye movements were recorded binocular. The eye tracker was calibrated with a standard 9-point calibration and validation procedure before the beginning of each block and routinely after every 15th trial. At the beginning of each trial, a fixation check was performed. If it failed, a drift correction was performed. An early recalibration was performed if the fixation check still failed after three drift corrections.
Statistical analyses
Statistical analyses (of both experiments) were performed within the R language for statistical computing (R Core Team, 2016). Linear mixed-effect models were performed with the lme4 package (Bates et al., 2015); figures were computed with the ggplot2 package (Wickham, 2009).
Stimuli and experimental procedure
Each session included 10 training trials at the beginning and 260 test trials. Stimuli were presented in white against a gray background. Each trial began with the presentation of a white fixation cross (extending 0.5○ × 0.5○) either on the left or the right side of the monitor. A fixation check within an invisible squared box centered on the fixation cross with a side length of 1○ ensured an appropriate gaze position before the presentation of the target. A successful fixation check triggered the onset of a variable foreperiod interval between 250 and 550 ms, which was randomly drawn for each trial from a uniform distribution. After the foreperiod, the fixation cross disappeared and the target stimulus was simultaneously flipped to the screen without any time delay.
The target stimulus was a small white square with a side length of 0.5○. The target appeared at variable distances that were randomly drawn from two different (truncated) Gaussian prior distributions: The proximal prior, , was sampled from a normal distribution with a mean target distance of 7○ and a standard deviation of 2.6○, truncated at 2○and 12○. Thus, when the sampled value fell outside the truncated range, another sample was drawn. Targets in the distal prior condition, , were drawn from a normal distribution with a mean distance of 13.5○ and a standard deviation of 2.6○, truncated at 8.5○and 18.5○.
For half of the trials, the target was presented for 50 ms, after which the screen was blanked until the eyes moved at least half of the distance toward the target (see below). For the other half of trials, the target remained visible for 500 ms or until the initial saccade was made toward the target. The 50-ms versus 500-ms target presentation times appeared equally often but randomly distributed across the trials within a session.
After the eyes of the subject crossed an invisible boundary halfway between the location of the fixation cross and the target (typically during the initial saccade), three or five small dots appeared within the target square for the secondary task to match the experimental procedure with Kapoula's (1985) initial report of the range effect. The boundary procedure was used to ensure that the dots had no influence on the programming of the initial saccade toward the target. Subjects responded to the number of dots with a key press (“left arrow” = three dots, “right arrow” = five dots). If no response was obtained within 2 s after the onset of the dots, the trial was terminated. Three or five dots appeared equally often across the trials, but the order was randomized.
Because the implicit learning of the two priors of target distances through the experiment is prone to transfer-of-learning effects across the prior conditions, we made an attempt to largely separate the learning conditions for the two priors. For each participant, the two prior conditions were tested in sessions on different days while the order of testing the proximal and distal priors was counterbalanced across participants. Furthermore, in the proximal prior condition, the fixation cross appeared at the left side of the screen and the target was presented to the right. This was reversed in the distal prior condition, that is, the fixation cross appeared at the right side of the screen and the targets to its left.1
Data preprocessing
Separately for each trial of the experiment, the series of recorded eye positions was translated into eye velocities and classified as saccades if a minimum of six successive eye positions exceeded a velocity threshold, defined as four times the standard deviation above the median eye velocity of the trial (Engbert & Mergenthaler, 2006). Additionally, saccades had to be detected from both eyes with a substantial temporal overlap. Trials with missing samples of eye positions (e.g., because of eye blinks) were removed from all later analyses. Saccades falling below an amplitude of one degree were declined as microsaccades. Initial saccades were identified as the first saccadic event detected 80 ms or later after target onset. Saccades that appeared earlier than 80 ms after target onset were classified as anticipatory saccades. Trials with anticipatory saccades were discarded. Of the trials, 23.8% were discarded due to either missing eye-position recordings or the detection of anticipatory saccades. For each valid trial, the realized target distance was recalculated based on the actual horizontal fixation position (instead of the position of the fixation cross) in an 80-ms window after target onset. Saccades’ landing positions were identified as the average horizontal fixation position of both eyes within an 80-ms window beginning 25 ms after the identified offset of the saccade. The time window was chosen as to exclude the frequently present overshoot of the eye-position data at the end of saccades (see Figure 2).
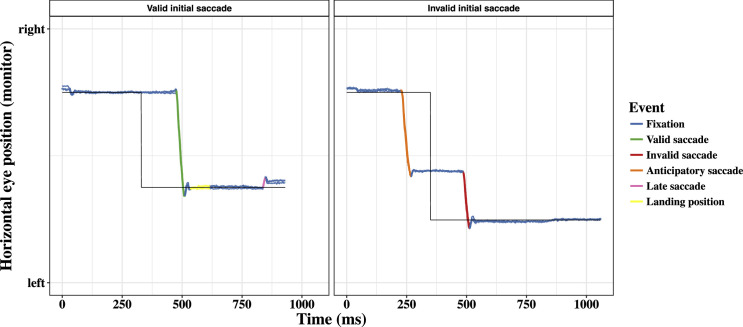
Figure 2.
Horizontal viewing positions of the left and the right eye on the screen in a valid (left panel) and invalid (right panel) trial. Fixations (blue) and saccades are highlighted in different color. The black line represents the position of the fixation/target stimulus on the screen. The target appeared to the left of the fixation stimulus in both trials. The left panel shows a valid first saccade (green), the time window that was employed to determine the landing position of the initial saccade (yellow), and a late saccades (pink). The right panel shows an early “anticipatory” saccade before target onset. The first saccade after target onset was classified as invalid.
Experiment 2
We report only those aspects of the experimental setup and procedures of Experiment 2 that differed from Experiment 1.
Participants, apparatus, and procedures
Subjects were four students of the University of Potsdam (all female, ages 21, 23, 24, and 26 years) who participated in exchange of study credit or a total of 21€ for their participation in three sessions. All participants had normal or corrected-to-normal vision. Subjects sat 60 cm in front of the computer screen (Mitsubishi Diamond Pro 2070 SB) in a dimly lit room with their heads stabilized on a chinrest. Movements of the right eye were recorded. The eye tracker was calibrated with a standard 9-point calibration and validation procedure before the beginning of each block and after every 12th trial.
Stimuli and experimental procedure
Stimuli were presented in white against a black background. Each trial began with the presentation of a white fixation circle with a diameter of 0.8○ on the left side of the monitor. The target appeared after a constant foreperiod of 750 ms.
To ensure that fixation was maintained at the starting position, we performed two fixation checks: one before and one after the foreperiod. The second fixation check triggered the simultaneous removal of the fixation stimulus and the presentation of the target stimulus.
The target stimulus consisted of a cloud of dots consisting of 29 white pixels that were drawn randomly from a two-dimensional Gaussian distribution centered at distances of 4○, 6○, 8○, 10○, 12○, 14○, 16○, 18○, or 20○ from the starting fixation position. The standard deviation of the underlying Gaussian was either 0.1○ (narrow likelihood) or 2.5○ (wide likelihood). The frequencies of the targets to appear at one of the nine positions across the test range were nonuniformly distributed and differed between the two prior conditions (weak vs. informative) according to Table 1.
Table 1.
Frequency table of realized target distances across 410 trials of an experimental session separated by prior condition.
| Target distances | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prior | 4○ | 6○ | 8○ | 10○ | 12○ | 14○ | 16○ | 18○ | 20○ |
| Strong | 20 (4.8%) | 34 (8.3%) | 52 (12.7%) | 64 (15.6%) | 70 (17.1%) | 64 (15.6%) | 52 (12.7%) | 34 (8.3%) | 20 (4.8%) |
| Weak | 42 (10.2%) | 44 (10.7%) | 46 (11.2%) | 48 (11.7%) | 50 (12.2%) | 48 (11.7%) | 46 (11.2%) | 44 (10.7%) | 42 (10.2%) |
The target remained visible for 500 ms or until the initial saccade was made toward the target. After the subjects’ eyes crossed an invisible boundary halfway between the location of the fixation circle and the target (typically during the initial saccade), an additional green or red pixel was displayed at the center of the target. Subjects responded to the color of the central dot with a key press (green = 1, red = 3). If no response was obtained within 2 s after the onset of the dot, the trial was terminated. Green and red dots appeared equally often and in randomized order.
Each subject was tested in three different sessions, all conducted on the same day with a minimum of a 1-hr break between sessions. Each session consisted of 410 trials. The first session was particularly designed to learn the prior. In this session, only the precise target (drawn from a Gaussian with a standard deviation of 0.1○) was employed. In the next two sessions, wide and narrow likelihood trials were equally often presented in a randomized order.
Results
Experiment 1
Central-tendency bias (range effect)
Saccades’ landing-position errors were calculated by subtracting target positions from landing positions. Thus, positive saccadic errors represent saccadic overshoots of the target and negative values represent undershoots. Both the distance of the target before the initial saccade and the saccadic error were measured in degrees of visual angle. In order to test whether there is a central-tendency bias in saccadic eye movements under experimental conditions with an informative prior, we first performed linear regressions of landing-site errors on target distance separately for prior condition and participant. The slopes quantify the modulation of saccadic errors as a function of target distance within the test ranges. Since the Bayesian saccade planning model predicts that target positions at the near ends of the test ranges are overestimated (i.e., favoring positive landing-site errors) and targets positions at the far ends are systematically underestimated (i.e., favoring negative landing-site errors), negative regressions slopes were expected. Figure 3 shows the landing-site errors of all valid saccades for the 10 participants of the experiment as a function of the distance of the target separated by the proximal (red) and distal (blue) prior conditions. Solid regression lines represent significant slopes (all ps < 0.5). Note that we pooled the data from the 50-ms and 500-ms presentation-time conditions as it turned out that presentation time had no significant effect on the spatial accuracy of the saccades in our experiment (see the results of the mixed-effect model below).
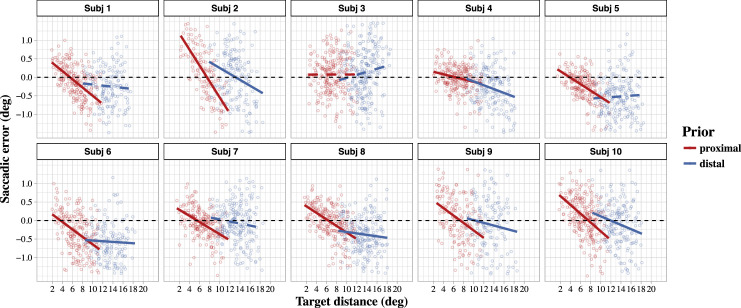
Figure 3.
Landing-site errors as a function of target distance separated by prior conditions and subjects (red = proximal prior over target distances ranging from 2○ to 12○, blue = distal prior over target distances from 8.5○ to 18.5○). Solid lines represent significant regression slopes.
In the proximal prior condition spanning target distances from 2○ to 12○, all but one subject showed the expected negative linear trend in saccadic errors with increasing target distance. Moreover, the predicted overestimation of near target positions and underestimation of far target positions in the proximal range is reflected by a systematic overshoot of the near targets and undershoot of far targets in all subjects that exhibited the negative linear trend. For the distal prior condition (targets between 8.5○ and 18.5○), the result is not as ideal-typical as the results of the proximal prior condition. Six out of 10 participants show the expected negative trends, with three participants further showing an overshoot of the near targets within the distal range. Four subjects (1,3,5, and 7) show no systematic modulation of saccadic errors by target distance within the distal range.
As a second step in our analysis of the central-tendency bias, we focused on the group level and conducted (generalized) linear mixed-effects models for both the proximal and the distal prior conditions based on the pooled data of the 10 participants. Saccadic landing-position errors were predicted as a linear function of the target distance. The independent variable target distance was centered separately within each prior condition so that the model intercepts represent the optimal target distance (i.e., the distance with no systematic landing-site error) relative to the center of the distal and proximal ranges. The model included the intercept, the target distance, the presentation time (50 vs. 500 ms), and the interaction as fixed factors and subject as a crossed random factor. Estimates with t values larger than 2 were considered significant at the 5% level (Kliegl et al., 2013). Table 2 shows the fixed-effect results of the linear mixed model.
Table 2.
Linear mixed model statistics for landing-site errors separated by prior conditions.
| Proximal range | Distal range | ||||
|---|---|---|---|---|---|
| Variables | Estimate | t value | Estimate | t value | |
| Intercept | −0.06506 | −1.207 | −0.300080 | −3.515 | |
| Distance | −0.10439 | −5.289 | −0.057054 | −3.107 | |
| Time | −0.05224 | −1.533 | 0.003722 | 0.063 | |
| Distance:Time | −0.01901 | −1.217 | −0.015258 | −0.772 | |
For the proximal range, we found a significant negative relation between the target distance and the saccadic error. None of the other effects, including the intercept, were significant. These results suggest that targets below 7○were systematically overshot and targets above 7○were undershot. Moreover, as the intercept did not deviate from the center of the proximal range, we conclude that the transition of saccadic overshoot responses to saccadic undershoots appears at the center of the range of target distances in this experimental condition. Thus, the results for the proximal conditions demonstrate a clear range effect in saccades and agree almost ideal-typical with the predictions of the Bayesian saccade-planning model. Target presentation time did not modulate saccadic accuracy, suggesting that the precision of the sensory localization of the position of the target did not differ between the 50-ms and the 500-ms presentation condition. The mixed model analysis for the distal condition revealed a significant negative trend in saccadic errors as a function of target distance plus a significantly negative intercept. No other effects were significant. These results confirm that saccadic errors within the distal range depend on the distance of target within the range such that increasing target distances elicit increasing undershoots of target positions. However, as reflected in the negative intercept of the model and visualized in Figure 4, saccadic responses toward targets below 13○(i.e., the center of the distal range) lack a systematic overshoot component. However, as already pointed out, the absence of an overshoot component could be due to a general oculomotor undershoot strategy, especially to distant target stimuli. Thus, the key test for the presence of a range effect in the saccadic responses in this experiment is the comparison of saccadic errors in overlapping saccade-target distances of the proximal and the distal ranges. Figure 4 suggests that the landing-position errors of saccades toward the same absolute target distances are substantially different between the distal and proximal conditions and were systematically biased toward the centers of the respective range. In order to test this difference, we ran a separate linear regression analysis on saccadic errors within the overlapping range of target distances (i.e., from 8.5○ to 12○) employing target distance and range (proximal vs. distal) as predictors. Results are reported in Table 3. We found again a highly significant negative linear trend in saccadic error by target distance (i.e., increasing undershoots with increasing target distance). Most important, we also found a highly significant effect of the range of target distances such that targets from 8.5○ to 12○ in the distal condition elicited larger undershoot errors than the corresponding targets in the proximal condition. This range-contingent difference of saccadic landing positions on identical targets can not be explained by a general undershoot tendency of the saccadic system but matches our key prediction for a saccadic range effect as a result of biased target-position estimates based on the availability of informative prior knowledge.
Figure 4.
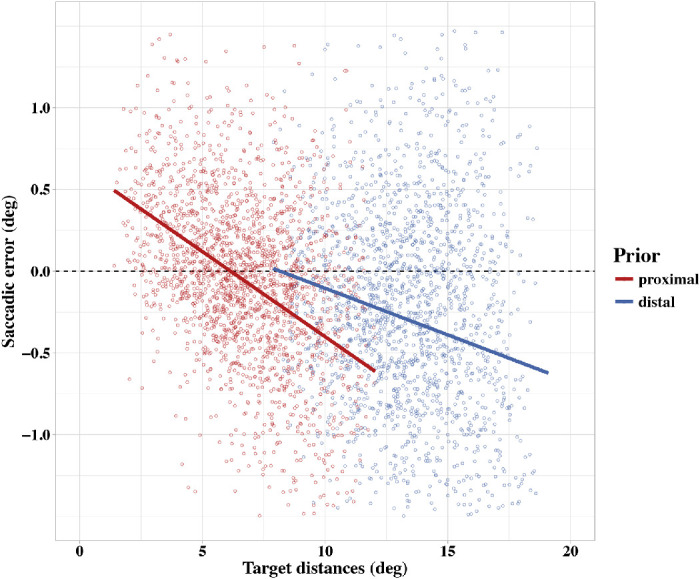
Landing-site errors as a function of target distance separated by prior conditions (red = proximal prior, blue = distal prior). Regression lines are based on the fixed-effect estimates of the linear mixed model.
Table 3.
Linear regression statistics for saccadic errors in overlapping target distances (from 8.5○ to 12○) with predictors target distance and range (proximal/baseline vs. distal).
| Variables | Estimate | SE | t value |
|---|---|---|---|
| Intercept | −0.374*** | 0.0300 | −12.46 |
| Distance | −0.109*** | 0.0273 | −4.00 |
| Range (distal) | 0.238*** | 0.0428 | 5.56 |
| Distance:Range | 0.030 | 0.0382 | 0.78 |
* p < .05; **p < .01; ***p < .001.
Experiment 2
The purpose of Experiment 2 was to find out whether the central-tendency bias in saccades can be modulated by the reliability of the prior information and the quality of the likelihood as predicted by the Bayesian framework within a constant range of target eccentricities. We performed a linear mixed model analysis predicting landing-position errors as a function of target distance (centered for the statistical analysis within the test range from 4○–20○), reliability of the prior (weak vs. informative), reliability of the likelihood (narrow vs. wide), and all possible interactions. Subjects were included as random intercepts. Since we expected the smallest range effect for the combination of weak prior information and precise sensory measurements (i.e., narrow likelihood), the levels of the factor prior were represented numerically as weak = 0 and informative = 1, and the levels of the factor likelihood as narrow = 0 and wide = 1, so that the fixed-effects estimates of the linear mixed model could be interpreted relative to the baseline condition “weak prior and narrow likelihood.” Estimates with t values larger than 2 were considered significant at the 5% level. Table 4 shows the coefficients of the fixed effects of our analysis.
Table 4.
Fixed-effects statistics for landing-site errors as a function of target distance, prior (weak vs. informative), and likelihood (wide vs. narrow). Intercept and target-distance parameters represent the statistical estimates for the baseline condition “weak prior/narrow likelihood.”
| Variables | Estimate | t value |
|---|---|---|
| Intercept | −0.378953 | −6.635 |
| Target distance | −0.030631 | −4.031 |
| Prior | 0.105725 | 1.308 |
| Likelihood | −0.858606 | −16.095 |
| Target distance:Prior | −0.028139 | −2.375 |
| Target distance:Fac.Likelihood | −0.110098 | −10.333 |
| Prior:Likelihood | −0.035268 | −0.468 |
| Target distance:Prior:Likelihood | −0.045889 | −2.772 |
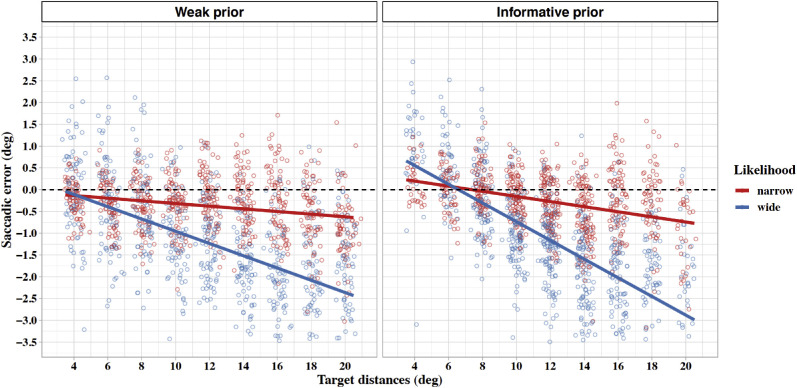
First, with respect to saccades based on weak prior knowledge and good sensory quality (narrow likelihood), we found both a significantly negative intercept and a negative slope, which reveals a slight but highly significant constant undershoot tendency together with an increase of the saccadic error with increasing target distance. These results are in good agreement with the results reported by Gillen et al. (2013). Additionally, we found a significant main effect of the likelihood manipulation with noisy targets, leading to a slightly stronger general undershoot. However, most important, as expected from the Bayesian saccade-planning hypothesis, the critical interactions of target distance and prior, and of target distance and likelihood, were both significant. The interactions are shown in Figure 5. These results reveal that saccadic errors toward targets in the same eccentricity range are modulated by both the reliability of the prior and the reliability of the likelihood. In more detail, increasing reliability of the prior (from weak to informative) led to a steeper negative slope, which is compatible with the predicted increase of the central-tendency bias in target-position estimates with increased reliability of the prior. In contrast, the manipulation of the sensory likelihood shows that in this case, a steeper slope is related to decreasing reliability of sensory information (from narrow to wide likelihood), also as predicted by Bayesian model. Finally, we also found a significant three-way interaction of the fixed-effects distance, prior, and likelihood, which shows that the combination of an informative prior together with noisy sensory data (wide likelihood) leads to the strongest range effect. The results demonstrate that the error of saccades toward targets with the same absolute eccentricity and within the same range depends on the reliability of the prior and the likelihood in a way that is fully compatible with the Bayesian prediction. Furthermore, we found again an additional general undershooting bias. Interestingly, due to this additional effect, saccades undershoot also targets at the near boundary of the test range under the weak prior condition. However, with informative prior knowledge, we observed a systematic overshoot of the two nearest target distances within the range as revealed by the intercepts of both the narrow and wide likelihood conditions under the influence of an informative prior. As can be seen in Figure 5, these intercepts intersect the dashed line, which represents the transition from over- to undershoots, next to the two most proximal target eccentricities.
Figure 5.
Landing-site errors as a function of target distance separated by the reliability of the prior (left panel = weak prior, right panel = informative prior) and by the reliability of the likelihood (red = narrow likelihood, blue = wide likelihood). Regression lines are based on parameter estimates from the linear mixed-effect model.
Discussion
In this study, we examined the use of Bayesian integration of prior knowledge and sensory information in the control of goal-directed saccadic eye movements. Our findings lend strong support to the assumption that learned prior knowledge is used for saccade planning according to Bayesian integration principles and that the saccadic range effect, i.e., a systematic saccadic bias toward the center of a certain range of target positions, is linked to the availability of prior knowledge.
Our hypotheses were derived from Bayesian integration of prior knowledge and incoming sensory measurements of environmental parameters, which represents a key principle in human sensorimotor control (Faisal et al., 2008; Körding & Wolpert, 2004). Thus, a key aspect of our experiments is that target locations within the test ranges vary according to a Gaussian probability distribution so that targets in the middle of the test ranges have a higher probability than targets near the boundaries of the test ranges. It has been demonstrated in a number of other studies that subjects implicitly learn such a prior probability structure during the experiment and use their prior knowledge to improve motor actions based on uncertain sensory information (Vilares et al., 2012; Jazayeri & Shadlen, 2010; Rhodes & Woodgate, 2015).
In Experiment 1, we tested saccades within two overlapping ranges of target distances, a proximal range between 2○ and 12○ (average target distance of 7○) and a distal range between 8.5○ to 18.5○ (average target distance 13○). We found that targets appearing at identical positions within both ranges elicited different saccadic landing-position errors. Moreover, the differences in the saccadic errors between the proximal and the distal range agreed well with the prediction of the Bayesian model as we found that saccadic landing positions at distal targets in the proximal range largely undershoot their counterparts in the proximal range, suggesting that targets at the far end of the proximal range are biased toward the center of the proximal range while identical targets in the distal range are biased toward the center of the distal range. This finding demonstrates that saccadic responses are not fully determined by the absolute spatial position of the target but are systematically biased according to the range of other targets in the experiment, hence showing the existence of a saccadic range effect.
Additionally, in the proximal range, we observed a systematic overshoot of all target distances below the center of the range and a systematic undershoot of targets appearing further away, which demonstrates the existence of a saccadic range effect that is almost perfectly in line with the qualitative predictions of Bayesian sensorimotor integration based on the availability of informative prior knowledge. Notably, as revealed by separate analysis for each individual, this result was consistently obtained across the 10 participants with only one subject who did not express a systematic landing-site bias. In the distal range, only a minimal overshoot tendency at the group level was observed for the nearest target distances within the range. However, we still observed a strong effect of target distances within the distal range such that the observed saccadic undershoots systematically increased from the nearest targets in this range, which elicited no undershoot, to a strong undershoot of the most distal targets in the distal range. The lack of a significant overshoot component in the distal range can be explained by a general saccadic undershoot strategy that was found in many previous studies and that is particularly characteristic for more distal saccades (Becker & Fuchs, 1969; Weber & Daroff, 1971; Deubel et al., 1986; Abrams et al., 1989; Tian et al., 2013). A common explanation of this robust property of the saccadic system is to minimize saccadic flight time or energy expenditure. Interestingly, the debate about systematic saccadic errors is sometimes reduced to arguing for either a range effect or a general undershoot tendency. However, we think it is very likely that different biases such as the range effect (Kapoula, 1985) and an additional undershooting strategy (Becker & Fuchs, 1969; Frost & Pöppel, 1976), originating at different levels of the saccade generation process, can be very well in place at the same time.
In Experiment 2, we further manipulated the reliability of the prior and the likelihood and found that saccadic errors are modulated as predicted by the Bayesian assumption that prior knowledge and sensory likelihood are combined based on weights representing their relative reliability. In particular, we found the weakest systematic saccadic bias under the condition of weak prior information and high quality of the sensory measurements (narrow likelihood) and the strongest bias under the condition of informative prior and noisy likelihood, which is in full agreement with the Bayesian predictions. This result is in general agreement with a large number of findings in other sensorimotor domains, which have led to the widely held assumption that the integration of prior knowledge and sensory information represents a key principle of human sensorimotor control (Faisal et al., 2008). Against the backdrop of such a general sensorimotor integration framework, it becomes clear that the long-standing debate about the existence of a saccadic range effect is inherently a debate about whether the integration of prior knowledge and sensory measurements also applies to the saccadic system or whether the generation of saccades represents an important exception of this fundamental principle. Our results, which clearly contradict the latter, lend further support to the generalizability of the Bayesian sensorimotor framework as an universal account for human sensorimotor control.
Several further implications of our findings stand out: It is obvious from the modulations of saccadic errors both between ranges of stimulus magnitudes (Experiment 1) and within the same range (Experiment 2) that subjects learn the statistical properties of the task (i.e., the prior) and that this knowledge has a systematic influence on the accuracy of saccadic eye movements. Thus, our results clearly contradict the claim that “stimulus-driven saccades operate independent of the relational properties associated with the target eccentricities within a given block of trials” (Gillen et al., 2013, p. 173). This claim was based on results from experiments in which all target locations in a test range were equally likely (uniform prior; see also Nuthmann et al. 2016; Vitu, 1991). Against the backdrop of our findings and in the light of Bayesian decision theory, an opposite conclusion would be more appropriate: The fact that saccadic errors in the previous studies did not express a significant difference between target locations actually reflects that the relational properties associated with the target eccentricities were learned (i.e., equal probabilities) and appropriately employed by the subjects. As we have pointed out before (see Figure 1), the consequence of a uniform prior plus clearly visible targets (low sensory uncertainty) is the absence or near absence of a range effect. This direct consequence of a Bayesian target-localization process during saccade control is particularly instructive, since the debate about the existence of a saccadic range effect is based on mixed findings from experiments with uniform priors. Our results provide important insights into the perceptual processes that contribute to the generation of goal-directed saccades, and the idea of Bayesian saccade planning allows a reevaluation of previous studies on the saccadic range effect.
Finally, we would like to link our results to saccade control during reading, since eye movements during reading represent an important example of goal-directed saccades within an ecologically valid task. The systematic overshoot of near word centers and the systematic undershoot of distant word centers is one of the most robust oculomotor effects in reading (McConkie et al., 1988; Krügel & Engbert, 2010) and is represented in most of the computational reading models (Engbert et al., 2005; Reichle et al., 2003; McDonald et al., 2005; Reilly & Radach, 2006). Based on eye-movement data from continuous reading, Engbert and Krügel (2010) demonstrated that the systematic landing position error in eye movements during reading is compatible with the framework of Bayesian saccade planning (see also Krügel & Engbert, 2014). However, such an account is called into question by the fundamental claim that there is no range effect in the saccadic system. Thus, our results, which show a range effect that depends on the presence of an informative prior, has important implications for our understanding of eye-movement control during reading. Furthermore, the principles of Bayesian decision theory provide a plausible and unified framework to explain why the range effect in saccades during reading is about five times larger than in simple stimulus-elicited saccades such as in our experiments or by Kapoula (1985). Given the exceptionally high amount of practice of skilled readers, it is plausible to assume an informative prior of saccade target distances in reading. Additionally, it is more difficult to localize word centers during reading than to localize a single target in a simple prosaccade experiment. Thus, it is plausible to assume that during reading, eye movements are based on informative prior knowledge and noisy sensory measurements of target positions (wide likelihood), which leads to a strong influence of the prior and consequently to a large range effect as we have demonstrated in Experiment 2. Based on such reasoning, we implemented a computational model of Bayesian saccade planning during reading based on sensory information about word boundaries that reproduce the complex pattern of experimental within-word landing positions in reading very well both for average landing positions as well as for saccades’ landing site variability (Krügel & Engbert, 2014). The model assumes, for example, that the sensory localization of the boundaries of N+2 words during reading is less reliable than for N+1 words, providing a plausible explanation for the modulation of the range effect in skipped words during reading.
Acknowledgments
The authors thank Matthew Heath, Western University, London, Ontario, for his valuable comments on a earlier version of the manuscript. They acknowledge financial support by Deutsche Forschungsgemeinschaft (Grants EN 471/15-1 and SFB 1294, project B03, project no. 318763901).
Commercial relationships: none.
Corresponding author: André Krügel.
Email: kruegel@uni-potsdam.de.
Address: Experimental and Biological Psychology, Department of Psychology, University of Potsdam, Potsdam, Germany.
Footnotes
Note that the accuracy of left versus right directed saccades was explicitly tested both in Nuthmann et al. (2016) and in Gillen et al. (2015) with the consistent finding that the spatial accuracy of left versus right directed saccades is comparable without significant differences. Kapoula (1985) also pooled left and right saccades for their analyses, although it was not reported whether left versus right saccades were explicitly tested for differences.
References
- Abrams R. A., Meyer D. E., & Kornblum S. (1989). Speed and accuracy of saccadic eye movements: Characteristics of impulse variability in the oculomotor system. Journal of Experimental Psychology: Human Perception and Performance, 15, 529. [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., & Walker S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Becker W., & Fuchs A. (1969). Further properties of the human saccadic system: Eye movements and correction saccades with and without visual fixation points. Vision Research, 9, 1247–1258. [DOI] [PubMed] [Google Scholar]
- Brainard D. H., & Vision S. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Cornelissen F. W., Peters E. M., & Palmer J. (2002). The eyelink toolbox: Eye tracking with MATLAB and the psychophysics toolbox. Behavior Research Methods, 34, 613–617. [DOI] [PubMed] [Google Scholar]
- Deubel H., Wolf W., & Hauske G. (1986). Adaptive gain control of saccadic eye movements. Human Neurobiology, 5, 245–253. [PubMed] [Google Scholar]
- Engbert R., & Krügel A. (2010). Readers use Bayesian estimation for eye movement control. Psychological Science, 21, 366–371. [DOI] [PubMed] [Google Scholar]
- Engbert R., & Mergenthaler K. (2006). Microsaccades are triggered by low retinal image slip. Proceedings of the National Academy of Sciences, 103, 7192–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R., Nuthmann A., Richter E. M., & Kliegl R. (2005). SWIFT: A dynamical model of saccade generation during reading. Psychological Review, 112, 777. [DOI] [PubMed] [Google Scholar]
- Faisal A. A., Selen L. P., & Wolpert D. M. (2008). Noise in the nervous system. Nature Reviews Neuroscience, 9, 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost D., & Pöppel E. (1976). Different programming modes of human saccadic eye movements as a function of stimulus eccentricity: Indications of a functional subdivision of the visual field. Biological Cybernetics, 23, 39–48. [DOI] [PubMed] [Google Scholar]
- Gillen C., & Heath M. (2014). Perceptual averaging governs antisaccade endpoint bias. Experimental Brain Research, 232, 3201–3210. [DOI] [PubMed] [Google Scholar]
- Gillen C., Weiler J., & Heath M. (2013). Stimulus-driven saccades are characterized by an invariant undershooting bias: No evidence for a range effect. Experimental Brain Research, 230, 165. [DOI] [PubMed] [Google Scholar]
- Grau-Moya J., Ortega P. A., & Braun D. A. (2012). Risk-sensitivity in Bayesian sensorimotor integration. PLoS Computational Biology, 8, e1002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath M., Gillen C., & Weiler J. (2015). The antisaccade task: Vector inversion contributes to a statistical summary representation of target eccentricities. Journal of Vision, 15(4), 4, doi: 10.1167/15.4.4. [DOI] [PubMed] [Google Scholar]
- Jazayeri M., & Shadlen M. N. (2010). Temporal context calibrates interval timing. Nature Neuroscience, 13, 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoula Z. (1985). Evidence for a range effect in the saccadic system. Vision Research, 25, 1155–1157. [DOI] [PubMed] [Google Scholar]
- Kapoula Z., & Robinson D. (1986). Saccadic undershoot is not inevitable: Saccades can be accurate. Vision Research, 26, 735–743. [DOI] [PubMed] [Google Scholar]
- Kleiner M., Brainard D., Pelli D., Ingling A., Murray R., & Broussard C. (2007). What's new in psychtoolbox-3. Perception, 36, 1. [Google Scholar]
- Kliegl R., Hohenstein S., Yan M., & McDonald S. A. (2013). How preview space/time translates into preview cost/benefit for fixation durations during reading. The Quarterly Journal of Experimental Psychology, 66, 581–600. [DOI] [PubMed] [Google Scholar]
- Knill D. C., & Pouget A. (2004). The Bayesian brain: The role of uncertainty in neural coding and computation. Trends in Neurosciences, 27, 712–719. [DOI] [PubMed] [Google Scholar]
- Kok P., Brouwer G. J., van Gerven M. A., & de Lange F. P. (2013). Prior expectations bias sensory representations in visual cortex. Journal of Neuroscience, 33, 16275–16284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körding K. P., & Wolpert D. M. (2004). Bayesian integration in sensorimotor learning. Nature, 427, 244–247. [DOI] [PubMed] [Google Scholar]
- Krügel A., & Engbert R. (2010). On the launch-site effect for skipped words during reading. Vision Research, 50, 1532–1539. [DOI] [PubMed] [Google Scholar]
- Krügel A., & Engbert R. (2014). A model of saccadic landing positions in reading under the influence of sensory noise. Visual Cognition, 22, 334–353. [Google Scholar]
- Land M. F. (1999). Motion and vision: why animals move their eyes. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 185, 341–352. [DOI] [PubMed] [Google Scholar]
- MATLAB. (2015). MATLAB r2015a [Computer software manual]. (2015). Natick, MA: MathWorks. [Google Scholar]
- McConkie G. W., Kerr P. W., Reddix M. D., & Zola D. (1988). Eye movement control during reading: I. The location of initial eye fixations on words. Vision Research, 28, 1107–1118. [DOI] [PubMed] [Google Scholar]
- McDonald S. A., Carpenter R., & Shillcock R. C. (2005). An anatomically constrained, stochastic model of eye movement control in reading. Psychological Review, 112, 814. [DOI] [PubMed] [Google Scholar]
- Nuthmann A., Vitu F., Engbert R., & Kliegl R. (2016). No evidence for a saccadic range effect for visually guided and memory-guided saccades in simple saccade-targeting tasks. PLoS One, 11, e0162449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen M., McCarthy P. F., & Allred S. R. (2014). The central tendency bias in color perception: Effects of internal and external noise. Journal of Vision, 14(11), 5, doi: 10.1167/14.11.5. [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The videotoolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [PubMed] [Google Scholar]
- Petzschner F. H., & Glasauer S. (2011). Iterative bayesian estimation as an explanation for range and regression effects: A study on human path integration. Journal of Neuroscience, 31, 17220–17229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzschner F. H., Glasauer S., & Stephan K. E. (2015). A Bayesian perspective on magnitude estimation. Trends in Cognitive Sciences, 19, 285–293. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2016). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rayner K. (1998). Eye movements in reading and information processing: 20 years of research. Psychological Bulletin, 124, 372. [DOI] [PubMed] [Google Scholar]
- Reichle E. D., Rayner K., & Pollatsek A. (2003). The E-Z Reader model of eye-movement control in reading: Comparisons to other models. Behavioral and Brain Sciences, 26, 445–476. [DOI] [PubMed] [Google Scholar]
- Reilly R. G., & Radach R. (2006). Some empirical tests of an interactive activation model of eye movement control in reading. Cognitive Systems Research, 7, 34–55. [Google Scholar]
- Rhodes D., & Woodgate P. J. (2015). Guidance of movements by prior experience: A Bayesian account of reach performance. Journal of Neuroscience, 35, 439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., & Kording K. P. (2014). How much to trust the senses: Likelihood learning. Journal of Vision, 14(13), 13, doi: 10.1167/14.13.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciutti A., Burr D., Saracco A., Sandini G., & Gori M. (2014). Development of context dependency in human space perception. Experimental Brain Research, 232, 3965. [DOI] [PubMed] [Google Scholar]
- Tian J., Ying H. S., & Zee D. S. (2013). Revisiting corrective saccades: Role of visual feedback. Vision Research, 89, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommershäuser J., Maloney L. T., & Landy M. S. (2003). Statistical decision theory and the selection of rapid, goal-directed movements. Journal of the Optical Society of America A, 20, 1419–1433. [DOI] [PubMed] [Google Scholar]
- Vilares I., Howard J. D., Fernandes H. L., Gottfried J. A., & Kording K. P. (2012). Differential representations of prior and likelihood uncertainty in the human brain. Current Biology, 22, 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitu F. (1991). Against the existence of a range effect during reading. Vision Research, 31, 2009–2015. [DOI] [PubMed] [Google Scholar]
- Weber R. B., & Daroff R. B. (1971). The metrics of horizontal saccadic eye movements in normal humans. Vision Research, 11, 921–IN2. [DOI] [PubMed] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant graphics for data analysis. New York, NY: Springer-Verlag. [Google Scholar]
- Wiener M., Michaelis K., & Thompson J. C. (2016). Functional correlates of likelihood and prior representations in a virtual distance task. Human Brain Mapping, 37, 3172–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D. M., & Landy M. S. (2012). Motor control is decision-making. Current Opinion in Neurobiology, 22, 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]