Abstract
Systematic shortening or lengthening of target objects during saccades modifies saccade amplitudes and perceived size of the objects. These two events are concomitant when size change during the saccade occurs asymmetrically, thereby shifting the center of mass of the object. In the present study, we asked whether or not the two are necessarily linked. We tested human participants in symmetrical systematic shortening and lengthening of a vertical bar during a horizontal saccade, aiming to not modify the saccade amplitude. Before and after a phase of trans-saccadic changes of the target bar, participants manually indicated the sizes of various vertically oriented bars by open-loop grip aperture. We evaluated the effect of trans-saccadic changes of bar length on manual perceptual reports and whether this change depended on saccade amplitude. As expected, we did not induce any change in horizontal or vertical components of saccade amplitude, but we found a significant difference in perceived size after the lengthening experiment compared to after the shortening experiment. Moreover, after the lengthening experiment, perceived size differed significantly from pre-lengthening baseline. These findings suggest that a change of size perception can be induced trans-saccadically, and its mechanism does not depend on saccadic amplitude change.
Keywords: perception, eye movement, vision
Introduction
Saccadic eye movements provide a fundamental possibility to explore our visual environment. These movements are highly accurate despite variations in muscle conditions such as fatigue or aging. The accuracy is maintained by means of a motor learning mechanism called saccadic adaptation. It can occur naturally (i.e., when extraocular muscles are weakened) or it can be induced experimentally, such as by the use of a double-step paradigm (McLaughlin, 1967). For this latter situation, it is well established that the saccades to small targets adapt their amplitude when the target is horizontally shifted during the saccade to another position with respect to the original one. A large number of studies used many variations of the double-step paradigm to demonstrate the conditions under which saccade amplitude would adapt to changes in the post-saccadic target shift, either along or orthogonal to the main direction of the saccade (Deubel, 1987; Ethier, Zee, & Shadmehr, 2008; Hopp & Fuchs, 2006; Kojima, Iwamoto, & Yoshida, 2005; McLaughlin, 1967; Miller, Anstis, & Templeton, 1981; Rahmouni & Madelain, 2019; Watanabe, Ogino, Nakamura, & Koizuka, 2003). In all of these studies, the target of saccade was represented by a small point that shifted on-axis or cross-axis with respect to the main direction of the saccade. A particular manipulation of saccadic adaptation was done using spatially extended targets in reading studies (Lavergne, Vergilino-Perez, Collins, & Doré-Mazars, 2010; McConkie, Kerr, Reddix, Zola, & Jacobs, 1989). In this context, it was highlighted that the target size was a crucial parameter to execute within-object saccades and that successful saccadic adaptation was obtained by a systematic change in object size (Lavergne et al., 2010). Our previous work investigated the saccadic adaptation process toward spatially extended targets that systematically changed the horizontal size during saccade execution (Bosco, Lappe, & Fattori, 2015). Specifically, we observed that saccade amplitudes were modified according to the direction of the size change (shortening or lengthening). In this particular study, the target was a bar that changed horizontal size leading to a displacement of the center of mass in one direction or another. The center of mass represented the reference position within the target area where saccades consistently landed (Bosco et al., 2015; He & Kowler, 1991; Kowler & Blaser, 1995; McGowan, Kowler, Sharma, & Chubb, 1998; Melcher & Kowler, 1999).
Interestingly, the manipulation performed in Bosco et al. (2015) also influenced the visual perception of size of the target object. The modification of size perception followed the direction of saccadic amplitude adaptation: If saccade amplitudes became smaller, the perceived size estimates became smaller; if saccade amplitudes became larger, the perceived size estimates also became larger (Bosco et al., 2015). The observed modification of size perception was in line with several studies that demonstrated an interaction between the motor adaptation with a distortion of visual localization of the target executed by hand pointing or by perceptual reports (Awater, Burr, Lappe, Morrone, & Goldberg, 2005; Bahcall & Kowler, 1999; Bruno & Morrone, 2007; Collins, Doré-Mazars, & Lappe, 2007; Garaas & Pomplun, 2011; Gremmler, Bosco, Fattori, & Lappe, 2014; Zimmermann & Lappe, 2010). Several more recent studies have indicated that adaptation of visual features can be found without saccadic adaptation (Herwig & Schneider, 2014; Herwig, Weiß, & Schneider, 2015; Herwig, Weiß, & Schneider, 2018; Köller, Poth, & Herwig, 2020; Paeye, Collins, Cavanagh, & Herwig, 2018; Valsecchi & Gegenfurtner, 2016; Valsecchi, Cassanello, Herwig, Rolfs, & Gegenfurtner, 2020). Specifically, features for which this phenomenon occurs are spatial frequency (Herwig & Schneider, 2014; Herwig et al., 2018), shape (Herwig et al., 2015; Köller et al., 2020; Paeye et al., 2018) and size (Bosco et al., 2015; Valsecchi & Gegenfurtner, 2016; Valsecchi et al., 2020). Particular focus has to be given to these two latter studies that tested paradigms able to demonstrate a size recalibration independent of saccade adaptation. However, in these studies visual size adjustment or visual size comparison tasks were used instead of an independent manual report of perceived size, as in Bosco et al. (2015).
To investigate whether or not trans-saccadic size perception changes can occur in the absence of saccadic adaptation, in the present work we changed the orientation of the visual target from horizontal to vertical and tested the modification of the perception of the vertical size of the target. In a paradigm similar to that used by Bosco et al. (2015), we presented different sizes before and after a phase of systematic trans-saccadic size changes. During this phase, the bars were vertically increased or reduced in length in a symmetrical way so as to keep the center of mass stable. We hypothesized that, if the horizontal saccade amplitude remained stable after the adaptation phase and a modification of size perception occurred, then this could not be related to the mechanism of saccadic adaptation but required a distinct mechanism of trans-saccadic size adaptation.
Materials and methods
Participants
A total of 15 right-handed participants (six females and nine males; ages 19–34 years) with normal or corrected-to-normal vision took part in the study. Ten participants completed the shortening and lengthening main experiments. Each participant completed these two main experiments in separate sessions at least 2 days apart. For the shortening main experiment, the data of one of the 10 participants had to be excluded because of problems with the eye tracking during the size-change phase.
The remaining five participants took part in the replication experiment. Three of those participants executed the shortening replication experiment and three participants the lengthening replication experiment; hence, one participant executed both replication experiments. The replication experiment consisted of repeating the manual shortening and lengthening experiments performed in Bosco et al. (2015).
No participant had a history of musculoskeletal or neurological disorders. All participants were naïve to the experimental purpose of the study and gave informed consent to participate in the experiments. All procedures were approved by the Ethical Committee of the Medical Faculty of the Eberhard Karls University of Tübingen under the reference number 138/2017B02. All procedures were in accordance with the tenets of the Declaration of Helsinki.
Apparatus and setup
In the shortening and lengthening experiments, participants were seated in front of a monitor (ViewPixx /3D; ViewPixx Technologies, Saint-Bruno, QC, Canada) which displayed target stimuli within a visible display of 52 × 29.5 cm. To stabilize head position, the participants placed their head on a chin rest located 73.5 cm from the screen, which resulted in a visual field of 32.28 × 21.86°. The display had a resolution of 1920 × 1080 pixels and a frame rate of 100 Hz. For stimulus presentation, we used MATLAB (MathWorks, Natick, MA) with the Psychophysics Toolbox extension (Brainard, 1997). The stimuli were blue, green, and red dots and 10 differently sized red vertical bars (vertical sizes: 2.11°, 2.36°, 2.61°, 2.87°, 3.12°, 3.38°, 3.63°, 3.89°, 4.13°, 4.39°). Eye movements were monitored by the EyeLink 1000 system (SR Research, Mississauga, ON, Canada), which sampled gaze positions with a frequency of 1000 Hz. Viewing was binocular, but the dominant eye was recorded. Hand position was measured by an ART motion capture system (frequency of acquisition, 60 Hz; Advanced Realtime Tracking, Weilheim, Germany), which follows the trajectory of the hand in three dimensions by recording infrared light reflection on passive markers placed on the index finger and thumb. All markers were held in place on the participant's skin with small pieces of adhesive tape that allowed freedom of movement of the hand and fingers.
During the shortening and lengthening experiments, participants were asked to use a head rest with a single resting point for the chin in order to maintain a stable head position through the entire session. The participants were informed not to move their head from the beginning of the experiment. The calibration was performed with EyeLink software at the beginning of each recording session using a nine-point calibration grid that allowed precise measurements of horizontal and vertical eye position. All sessions were conducted in a room with dim background lighting. During the recording, only the participant and the experimenter were allowed in the room in order to maintain a calm environment for the recording session. For the replication experiment, the same setup of shortening and lengthening experiments was used.
Behavioral tasks
In the shortening and lengthening experiments, participants were tested in three successive phases: pre-size-change estimation phase, shortening or lengthening size-change phase, and post-size-change estimation phase. The pre-size-change and post-size-change estimation phases consisted of 30 trials in which participants were required to manually indicate the vertical size of a vertical red bar presented at 13.32° on the right of the initial fixation target. The sequence of events of the pre-size-change manual estimation phase is illustrated in Figure 1A. The pre-size-change manual estimation phase consisted of two randomized conditions distributed in 30 trials in which the fixation target was either blue or green for the no-saccade and saccade conditions, respectively. If the blue fixation target appeared on the screen, subjects were required to fixate it. After 1 second, a vertical red bar was presented for 1 second. Thereafter, an acoustic signal informed the participant to manually indicate the perceived size of the bar by extending the thumb and the index finger while maintaining fixation on the blue fixation target. If the fixation target was green, subjects looked at it and after 1 second a red bar was presented on the screen. Participants held their gaze on the green fixation target for 1 second more and then an acoustic signal notified them to perform a saccade toward the bar and to manually indicate the perceived size of the bar. All participants indicated the size of the bars at the same hand position to avoid any effect of distance on the estimation of size. The distance between the subject's eyes and the screen was kept constant to obtain a consistent vergence angle.
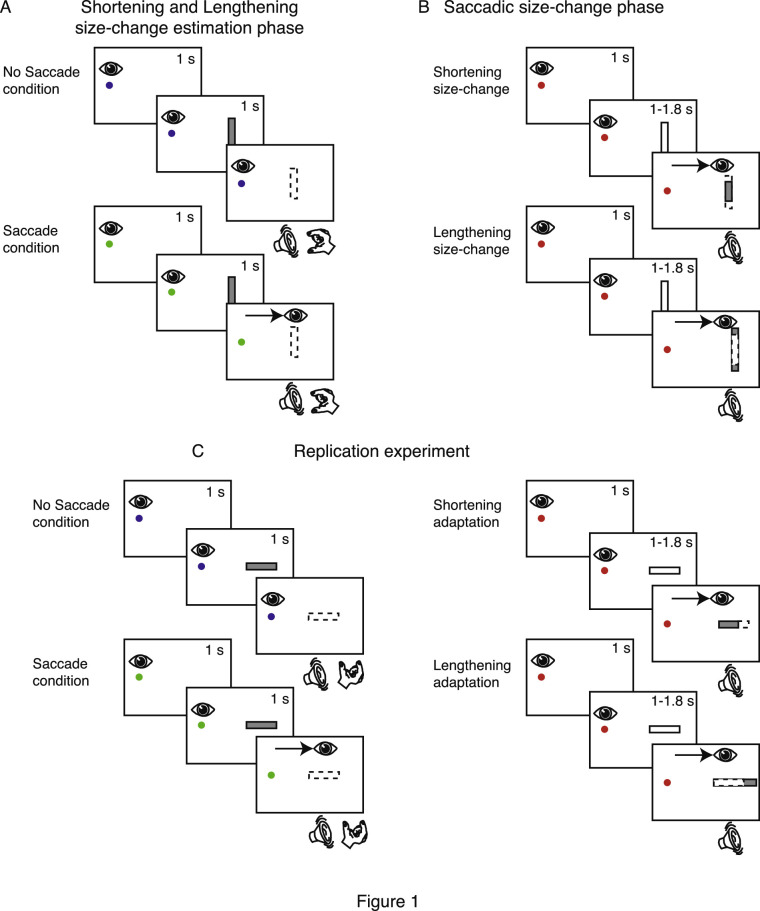
Figure 1.
Task design. (A) Shortening and lengthening estimation phase: (Top row) No-saccade condition trial. Subjects were instructed to fixate at the blue fixation target shown as a small circle. After 1 second, a bar was flashed (gray rectangle) for 1 second; following an acoustic signal, subjects had to indicate (while maintaining fixation) the perceived size of the bar by the grip aperture. (Bottom row) Saccade condition trial. Subjects fixated at the green fixation target. After 1 second, a bar was projected for 1 second. An acoustic signal was then activated, prompting the subject to perform a saccade toward the bar and thereafter indicate the perceived size of the bar. As soon as the start of the saccade was detected, the bar disappeared from the screen. (B) Saccadic size-change phase: (Top row) Shortening size-change phase. At the beginning of the trial, the red fixation target was presented, and the subject's gaze was directed toward it. After 1 second, a red bar appeared, but the subject had to continue to fixate at the fixation target. After a randomized time (1–1.8 second), an acoustic signal informed the subject to execute a saccade toward the bar. As soon as the saccade was detected, the bar was symmetrically decreased in size by 30% of its length. (Bottom row) Lengthening saccadic size-change phase, which was structured in the same way as the shortening saccadic size-change phase, with the difference that the bar was symmetrically increased in size by 30% during saccade execution. (C) Replication experiment: (Left) The no-saccade condition and saccade condition were the same as for the shortening and lengthening size-change estimation phases in A, but the bars presented were horizontally oriented. (Right) The shortening and lengthening adaptation phases, for which the task sequence was the same as in B, but saccades were performed to horizontal bars that decreased and increased, respectively, but not symmetrically (Bosco et al., 2015).
The trans-saccadic size-change phase could be shortening or lengthening, depending on the type of experiment. We used a double-step protocol with delayed saccades in which we manipulated the size of the target bars while the saccade was in flight. The trial sequence is shown in Figure 1B. For the first 20 trials (pre-size-change trials), one of 10 vertical bars was presented 13.32° to the right of the fixation target. Bar presentation occurred 1 second after the appearance of the fixation target. Participants were required to maintain their fixation on the initial fixation point for 1 to 1.8 seconds until an acoustic signal informed them to perform a saccade to the bar. For the remaining 360 trials (size-change trials), the sequence of events was the same, but the bar was symmetrically shortened (Figure 1B, top, shortening experiment) or lengthened (Figure 1B, bottom, lengthening experiment) by 30% of its original length during saccade execution. Saccade onset was detected when the eye movement exceeded a distance of 2° from the fixation target. As soon as the saccade onset was detected, the bar was decreased or increased to a new size. After the size-change trials, we again tested the participants in the post size-change manual estimation phase (Figure 1A). This phase was structured identically to the pre-size-change manual estimation phase with randomization of the no-saccade and saccade conditions. During both estimation phases, participants had no visual feedback for their hands because they maintained their gaze on the stimuli projected on the screen and their hands were outside the field of view.
Replication experiment
We further conducted a replication experiment illustrated in Figure 1C that replicated the experiment reported in Bosco et al. (2015). We performed this experiment to verify that the results obtained in the present study were not due to a difference in the experimental equipment from the previous study. In this experiment, participants had to indicate manually the perceived size of horizontal bars presented on the screen to the right of the fixation point. Participants sat in front of the ViewPixx /3D monitor at a distance of 73.5 cm with a resulting visual field of 32.28 × 21.86°. The stimuli and structure of the replication experiment were identical to those of the main experiments, except for the orientation of the presented bars, which was horizontal. This configuration allowed the change in size to occur “on axis” with respect to the main direction of the saccade. In this configuration, we expected an adaptation of the horizontal amplitude of the saccade along with a change in perceived size of the bar as reported in Bosco et al. (2015). In one session, participants executed the shortening adaptation; in the other, the lengthening adaptation. During the pre-estimation and post-estimation phases, an acoustic signal informed the participant to manually indicate the perceived size of the horizontal bars. Blue and green colors of stimuli indicated the no-saccade and saccade conditions, respectively. The number of trials was the same as in the main experiments. In all experiments, the sequence of bar sizes and the conditions were randomly created by MATLAB code. No indication about eye landing position on the bar was provided. All participants received the same instructions.
Data analysis
We wrote custom software in MATLAB to compute the distance between index and thumb markers during the manual estimation phases. Grip aperture was calculated considering trial intervals in which velocities of the index and thumb markers remained at <5 mm/second. Grip aperture was defined as the median distance within this interval (Bosco et al., 2015). Saccadic amplitude was calculated by determining gaze position directly before saccade onset (i.e., at the time when the target was presented) and after the saccade reached its end position. The change in saccade amplitude after the size-change phase was computed as the difference of amplitudes between the last 20 trials of the late-size-change phase and the first 20 pre-size-change trials, averaged across sizes and participants. We compared saccadic amplitude changes between the pre-size-change and late-size-change trials by a two-tailed t-test within the shortening and lengthening experiments. Following the hypothesis that no saccadic parameters change after adaptation, we also assessed the change in saccade angle. We calculated the angle between the horizontal line connecting the fixation point and the center of the bar and the line connecting the fixation point and the saccade endpoints. We then compared the saccade angles between pre-size-change and late-size-change trials by a two-tailed t-test within the shortening and lengthening experiment. We extracted the averaged time for the start and end of saccades of the size-change phase in the shortening and lengthening experiments. Then, we plotted them relative to the target size-change presentation. We calculated the amount of grip aperture change after the saccadic size-change phase as the difference between post-estimation and pre-estimation trials and compared them using two-tailed t-test analyses. Two-tailed t-test analyses were also used to evaluate significant differences between the grip aperture change that occurred in the shortening and lengthening experiments. We used an ANOVA repeated-measures analysis to evaluate the effect of phase (factor 1) and size (factor 2) on grip apertures and their interaction after the saccadic size-change phase.
In the replication experiment, for each participant we compared pre-adaptation trials and late-adaptation trials in the saccadic adaptation phase and pre- and post-grip aperture measures in the estimation phases by a bootstrapping method (10,000 iterations with replacement). Synthetic standard errors were created by drawing N averaged grip apertures from the N repetitions of pre-adaptation and post-adaptation trials recorded during the estimation phases and N averaged saccadic amplitudes during the saccadic adaptation phase. Ten thousand iterations were performed, and confidence intervals were estimated as the range that delimited 95% of the computed standard errors.
For all statistical analyses, the significant criterion was set to p < 0.05.
Results
In the two main experiments, we evaluated shortening and lengthening size-change paradigms in which spatially extended objects oriented perpendicular to the main direction of the saccadic movement were reduced and increased in size, respectively. Given the orientation of the saccadic target and the fact that size change occurred symmetrically, we did not expect any modification of the horizontal and vertical saccadic amplitude. In this way, we aimed to evaluate whether we could induce a modification of perceived size without a change in the amplitude of the saccade.
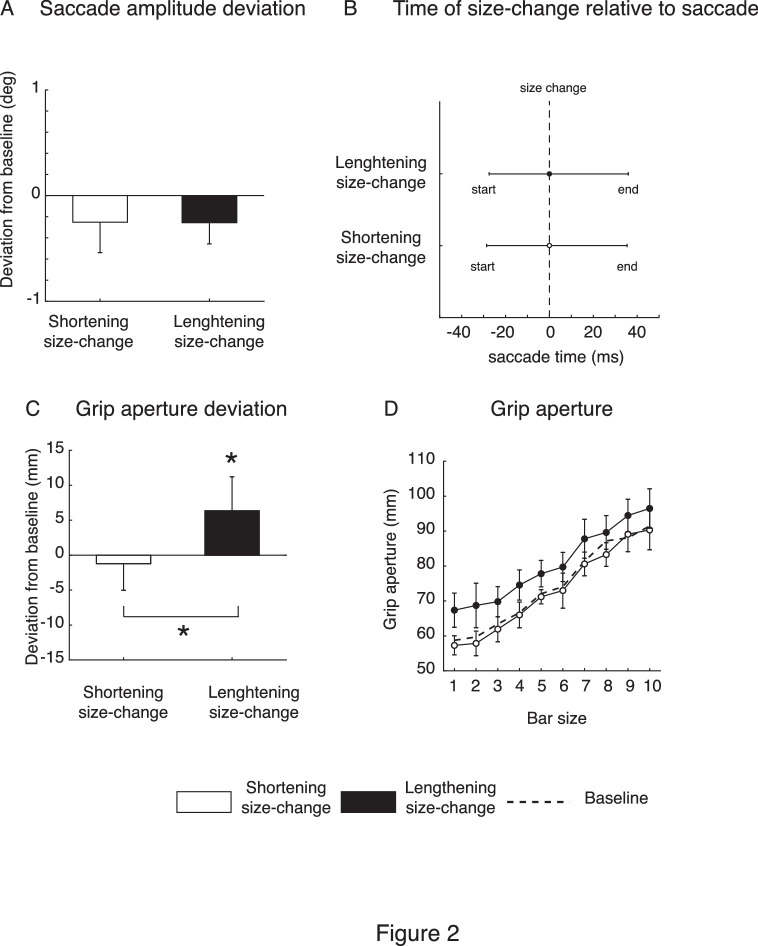
Before analyzing the changes in perceived size we therefore checked whether saccade amplitudes changed during the experiment. We first checked that the start positions at the beginning of the size-change phase and those at the end of the size-change phase did not differ. This ensured that any possible amplitude change could be ascribed to landing point modifications rather than a shift of the participant's fixation position inside the tracker window in the direction of the future position of the saccade target. There was no significant difference between the mean fixation position in the first and the last 20 trials among all participants in both experiments (t-test, p > 0.05). We then calculated the difference in saccade amplitudes between the pre- and late-size-change trials. Figure 2A shows the saccade amplitude change for the shortening (white) and lengthening (black) experiments as the difference between the horizontal average saccade amplitudes in the pre-size-change trials and the average saccade amplitudes in the late-size-change trials. As expected, there was no significant modification of saccade amplitude in either experiment with mean values of –0.25 ± 0.28° in the shortening experiment and –0.26 ± 0.2° in the lengthening experiment (t-tests not significant; p > 0.05 for both shortening and lengthening size-change trials). Also, saccade amplitude changes were not different between shortening and lengthening conditions (t-test, p > 0.05). Similarly to saccade amplitude, no significant modification of saccade angle between pre-size-change and late-size-change trials in shortening and lengthening experiments was found (mean ± SD: pre-size-change trial = –0.02 ± 0.0058; shortening size-change trial = –0.01 ± 0.0079; lengthening size-change trial = –0.02 ± 0.0075; t-test, p > 0.05).
Figure 2.
(A) Mean deviation of saccade amplitude from baseline for the shortening size-change trials (white column) and the lengthening size-change trials (black column), averaged across participants and sizes. (B) Time of target size change presentation relative to the start and end of the saccade for the shortening (white dot) and lengthening (black dot) experiments. (C) Mean deviation of grip aperture from baseline for the shortening size-change trials (white column) and the lengthening size-change trials (black column) averaged across participants and sizes. (D) Distribution of mean post grip apertures for the shortening and lengthening size-change trials (white and black dots, respectively). Dotted line indicates the baseline (pre-grip apertures) averaged across shortening and lengthening sessions. Error bars indicate SE; *p < 0.05.
We next checked that the presentation times of the size changes occurred similarly during the saccade in both conditions. To evaluate that there were no differences in the size-change presentation during the size-change trials in the shortening and lengthening experiments, we calculated the start and the end times of the saccades relative to the target size-change times (averaged time reported in Figure 2B). In both shortening and lengthening experiments, the size-change presentation always occurred between the start and the end of the saccade. Specifically, in the shortening experiment, the size-change presentation occurred on average 28.64 ms after the start of the saccade and 35.43 ms before the end of the saccade. In the lengthening experiment, the size-change presentation occurred on average 27.5 ms after the start of the saccade and 35.99 ms before the end of the saccade.
We then returned to our main hypothesis regarding adaptation of size perception in the absence of saccadic adaptation. Before and after the saccadic size-change phase, we asked participants to indicate the perceived vertical size of the presented bar via the aperture of the thumb and the index fingers (grip aperture). We did so in two conditions: (1) participants had to keep fixation (no-saccade condition) and (2) participants made saccades to the peripheral bar (saccade condition). A two-tailed t-test showed no significant differences in the grip apertures between the two conditions in either the pre- or post-estimation phases of the shortening and lengthening experiments, respectively (shortening experiment: no saccade vs. saccade pre-estimation phase p > 0.05 and no saccade vs. saccade post-estimation phase p > 0.05; lengthening experiment: no saccade vs. saccade pre-estimation phase p > 0.05 and no saccade vs. saccade post-estimation phase p > 0.05). Thus, we analyzed the averaged manual perceived sizes by collapsing the saccade and no-saccade conditions. Figures 2C and 2D show the perceived sizes calculated as grip apertures. As we did not find a significant difference between the pre-estimation phases in the shortening and lengthening experiments, we averaged the grip apertures across the two pre-estimation phases in Figure 2D (t-test, p > 0.05), creating a common baseline. To evaluate the effect of phase and size on the perceived sizes of each experiment, we performed repeated-measures ANOVAs, with two levels for factor 1 (phase) and 10 levels for factor 2 (size). The shortening experiment (white) shows only a significant main effect of size, where F(9, 72) = 22.1868 and p < 0.05. No significant main effects of phase and no interaction were found; for phase, F(1, 8) = 0.09 and p = 0.77, and, for interaction, F(9, 72) = 0.48 and p = 0.87. The lengthening experiment (black) showed significant main effects for phase and size: for phase, F(1, 9) = 10.83 and p < 0.05, and, for size, F(9, 81) = 41.46 and p < 0.05. However, there was no significant interaction: F(9, 81) = 1.04 and p = 0.41. The direct comparison of the shortening and lengthening post-estimation phases by t-test analysis revealed a significant difference (t-test, p < 0.05), with the shortening post-estimation phase being significantly smaller than the lengthening post-estimation phase (Figure 2B). In addition, the lengthening experiment showed a significant increase of perceived size with respect to the baseline.
Overall, the lack of saccade amplitude modification was expected, as no changes of the spatial characteristics of the center of mass of the stimuli were generated during the saccadic size-change phase and, consequently, modification of the perceived size was not dependent on the saccade parameters.
Replication experiment
The combined observation of the saccadic amplitude and the perceived size modifications showed a behavior that was not consistent with the saccadic amplitude parameter. To test whether this inconsistency might have been caused by particular features in the setup, we performed a replication experiment identical to the task used in Bosco et al. (2015). In this experiment, the stimuli were a fixation point presented on the left and a horizontal bar presented on the right of the screen. In the pre- and post-estimation phases, participants were asked to estimate the size of the bar presented by grip aperture; in the adaptation phase, they were required to perform a saccade toward the bar. During the saccade, the bar was shortened or lengthened by removing or adding a segment on the right edge of the bar; thus, bar size was not changed symmetrically but only on the right side. This manipulation allowed us to confirm the results of the previous paper and the effectiveness of our experimental setup.
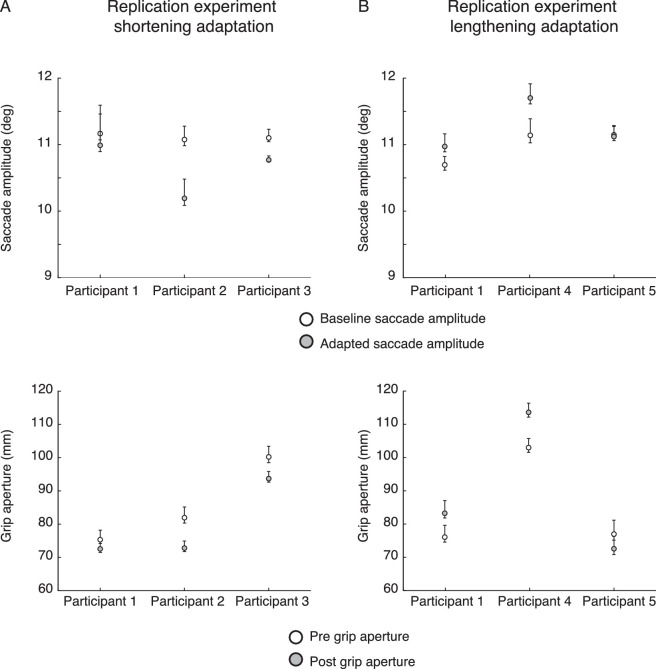
Figure 3A (top) shows individual horizontal saccadic amplitudes relative to baseline trials (white circle) and late adaptation trials (grey circles) with the corresponding bootstrap-estimated CIs (10,000 iterations) measured in the shortening adaptation. Participants 2 and 3 significantly reduced the horizontal saccadic amplitude (CIs not overlapped). Consistent with the saccadic parameter, the same participants showed significant reduction of the grip aperture after the saccadic adaptation phase (Figure 3A, bottom). Participant 1 showed a consistent but not significant effect on saccadic amplitude and grip aperture.
Figure 3.
(A) Shortening adaptation: (Top) Averaged saccade amplitudes at baseline (white dots) and for the adaptation trials (grey dots), with lines corresponding to bootstrap-estimated 95% CIs. (Bottom) Averaged grip apertures during pre-estimation phase (white dots) and post-estimation phase (grey dots), with lines corresponding to bootstrap-estimated 95% CIs. (B) Lengthening adaptation: (Top) Averaged saccade amplitudes at baseline (white dots) and for the adaptation trials (grey dots), with lines corresponding to bootstrap-estimated 95% CIs. (Bottom) Averaged grip apertures during pre-estimation phase (white dots) and post-estimation phase (grey dots), with lines corresponding to bootstrap-estimated 95% CIs.
In the lengthening adaptation, participants 1 and 4 consistently and significantly increased the saccadic amplitude and grip aperture as shown in Figure 3B (CIs not overlapped). Participant 5 had no effect in either the saccadic amplitude or the grip aperture (CIs overlapped). In line with the results of Bosco et al. (2015), the change in horizontal saccadic amplitude in the direction of size change coincided with a systematic change in perceived object size. This confirms that the modification of saccadic amplitude is consistent with the direction of the stimulus size change only when this change is “on axis” with the main direction of the saccade and the shift of the center of mass of stimulus occurs. However, in light of the data shown in the main experiments of the present work, the concomitant and consistent changes in perceived object size represent an effect whose mechanisms are not dependent on the saccadic motor parameter changes.
Discussion
In this study, we collected data in naïve participants in two experiments consisting of manual reporting of the size of objects presented before and after a shortening and lengthening trans-saccadic size-change paradigm. For each experiment, participants were tested on separate days to induce changes in size perception not dependent on the modification of saccade amplitude normally induced by a classical saccadic adaptation procedure. To obtain this, participants performed hundreds of saccades toward a vertical spatially extended target that decreased or increased its vertical size symmetrically according to the type of experiment. Under these experimental conditions, we hypothesized that saccadic amplitude would not be modified and that the changes in size perception may occur in a way that is not dependent on saccadic motor properties.
Consistent with this expectation, we did not find any significant modification of saccade amplitude and saccade angles that could be concomitant with the direction of the size change of the target. Other authors investigated the saccadic adaptation process to targets shifting not only on axis with respect to the main saccade direction but also cross axis (Chen-Harris, Joiner, Ethier, Zee, & Shadmehr, 2008; Deubel, 1987; Ethier et al., 2008; Rahmouni & Madelain, 2019). In all cases, they found consistent changes in saccadic amplitude according to the direction of target shift, confirming that “the saccadic system's flexibility in response to change in environmental contingencies is certainly a general learning phenomenon” (Rahmouni & Madelain, 2019). However, in all of these studies, the targets were represented by small and localized points that shifted in position with respect to the initial step. Under the present experimental conditions, a reduction and growth of the target area occurred symmetrically, maintaining a constant center of mass to which the saccades could be directed (Bosco et al., 2015; He & Kowler, 1991; Kowler & Blaser, 1995; McGowan et al., 1998; Melcher & Kowler, 1999). Thus, it is expected that there should be no horizontal or vertical modification of the saccade amplitude.
Despite no change in saccade amplitude, our data further show that the perceptual reports of object size differed significantly after the lengthening compared to the shortening experiment. After the lengthening experiment, the same bar appeared longer than after the shortening experiment, consistent with the different directions of the trans-saccadic size change in these experiments. Moreover, in the lengthening experiment, the perceived size of the bars also changed with respect to the baseline size percept before the lengthening phase. Also, the analysis showed that the size perception changed significantly and consistently with the direction of the size change.
We did not obtain an opposite significant difference from baseline in the shortening experiment. This lack of significant modification of perceived size in the shortening experiment suggests that the same procedure may not be valid for both directions. This is reminiscent of several peculiarities of saccadic adaptation that also suggest different mechanisms for lengthening and shortening (Panouillères, Weiss, Urquizar, Salemme, Munoz, & Pélisson, 2009; Pélisson, Alahyane, Panouillères, & Tilikete, 2010; Schnier & Lappe, 2011).
Many studies demonstrated that saccadic adaptation includes a mechanism that calibrates visual space perception by observing and correcting mismatches between the peripheral view of a target and the central view of the same target after a saccade toward it (Awater et al., 2005; Bahcall & Kowler, 1999; Bruno & Morrone, 2007; Collins et al., 2007; Garaas & Pomplun, 2011; Gremmler et al., 2014; Zimmermann & Lappe, 2010; Zimmermann & Lappe, 2016). As mentioned earlier, similar to changing position of the target in the traditional saccadic adaptation paradigm, other features such as spatial frequency (Herwig & Schneider, 2014), shape (Herwig et al., 2015; Köller et al., 2020; Paeye et al., 2018), and size (Bosco et al., 2015; Valsecchi & Gegenfurtner, 2016; Valsecchi et al., 2020) also appear adaptive across saccades. However, similar effects on shape and size perception have also been observed when objects were successively presented in peripheral and foveal view while observers maintained fixation. Valsecchi and Gegenfurtner (2016) demonstrated that changes in size comparison between foveal and peripheral views of an object can occur in the absence of eye movement if an object changes size while it is shifted from a peripheral to a central view by saccade-like image motion. Although this, like our present results, suggests a mechanism of perceptual adaptation that is not linked to saccadic adaptation, in their case of saccade-like image motion, Paeye et al. (2018) similarly demonstrated that a systematic change of stimulus shape with and without saccade execution leads to a change in shape perception when comparing foveal and peripheral views. They proposed that a generative associative process, independent of saccade execution, contributes to the perception of shape across peripheral and foveal viewpoints. Recently, Valsecchi et al. (2020) showed that saccadic adaptation and size recalibration share the same temporal development. However, because size recalibration of the presented visual objects generalized to the opposite hemifield but saccadic adaptation did not, they concluded that distinct learning mechanisms were involved. This is in line with the present results that directly demonstrate that size recalibration is independent from the saccade amplitude adaptation. Our present results, as well as those of Valsecchi and Gegenfurtner (2016), may be consistent with such an explanation, but in our experimental condition the recalibration of size affected independent manual estimates of perceived peripheral size rather than visual comparison between learned objects.
Conclusions
Our results suggest that a manipulation of the target size that, in general, did not change the spatial property relevant for the saccadic system influences the interaction between saccadic motor process and object size perception. Here, we propose that the modification of size perception relies on a mechanism that dynamically recalibrates the size of the stimuli on the basis of prediction about what will appear on the fovea in a subsequent action and not on the basis of modified saccadic motor parameters.
Acknowledgments
This work has received funding from the European Union's Horizon 2020 research and innovation programme (under Marie Skłodowska-Curie Grant agreement no. 734227–Platypus) and from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), PRIN 2017–Prot. 2017KZNZLN.
Commercial relationships: none.
References
- Awater H., Burr D., Lappe M., Morrone M. C., & Goldberg M. E. (2005). Effect of saccadic adaptation on localization of visual targets. Journal of Neurophysiology, 93(6), 3605–3614. [DOI] [PubMed] [Google Scholar]
- Bahcall D. O., & Kowler E. (1999). Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature, 400(6747), 864–866. [DOI] [PubMed] [Google Scholar]
- Bosco A., Lappe M., & Fattori P. (2015). Adaptation of saccades and perceived size after trans-saccadic changes of object size. The Journal of Neuroscience, 35(43), 14448–14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Bruno A., & Morrone M. C. (2007). Influence of saccadic adaptation on spatial localization: Comparison of verbal and pointing reports. Journal of Vision, 7(5):16, 1–13, 10.1167/7.5.16. [DOI] [PubMed] [Google Scholar]
- Chen-Harris H., Joiner W. M., Ethier V., Zee D. S., & Shadmehr R. (2008). Adaptive control of saccades via internal feedback. The Journal of Neuroscience, 28(11), 2804–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Doré-Mazars K., & Lappe M. (2007). Motor space structures perceptual space: Evidence from human saccadic adaptation. Brain Research, 1172(1), 32–39. [DOI] [PubMed] [Google Scholar]
- Deubel H. (1987). Adaptivity of gain and direction in oblique saccades. In O'Regan J. K., Levy-Schoen A. (Eds.), Eye movements from physiology to cognition (pp. 181–190). Amsterdam, The Netherlands: Elsevier Science Publishers. [Google Scholar]
- Ethier V., Zee D. S., & Shadmehr R. (2008). Changes in control of saccades during gain adaptation. The Journal of Neuroscience, 28(51), 13929–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaas T. W., & Pomplun M. (2011). Distorted object perception following whole-field adaptation of saccadic eye movements. Journal of Vision, 11(1):2, 1–11, 10.1167/11.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremmler S., Bosco A., Fattori P., & Lappe M. (2014). Saccadic adaptation shapes visual space in macaques. Journal of Neurophysiology, 111(9), 1846–1851. [DOI] [PubMed] [Google Scholar]
- He P., & Kowler E. (1991). Saccadic localization of eccentric forms. Journal of the Optical Society of America A, 8, 440–449. [DOI] [PubMed] [Google Scholar]
- Herwig A., & Schneider W. X. (2014). Predicting object features across saccades: Evidence from object recognition and visual search. Journal of Experimental Psychology: General, 143(5), 1903–1922. [DOI] [PubMed] [Google Scholar]
- Herwig A., Weiß K., & Schneider W. X. (2015). When circles become triangular: How transsaccadic predictions shape the perception of shape. Annals of the New York Academy of Sciences, 1339(1), 97–105. [DOI] [PubMed] [Google Scholar]
- Herwig A., Weiß K., & Schneider W. X. (2018). Feature prediction across eye movements is location specific and based on retinotopic coordinates. Journal of Vision, 18(8)13, 1–13, 10.1167/18.8.13. [DOI] [PubMed] [Google Scholar]
- Hopp J. J., & Fuchs A. F. (2006). Amplitude adaptation occurs where a saccade is represented as a vector and not as its components. Vision Research, 46(19), 3121–3128. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Iwamoto Y., & Yoshida K. (2005). Effect of saccadic amplitude adaptation on subsequent adaptation of saccades in different directions. Neuroscience Research, 53(4), 404–412. [DOI] [PubMed] [Google Scholar]
- Köller C. P., Poth C. H., & Herwig A. (2020). Object discrepancy modulates feature prediction across eye movements. Psychological Research, 84(1), 231–244. [DOI] [PubMed] [Google Scholar]
- Kowler E., & Blaser E. (1995). The accuracy and precision of saccades to small and large targets. Vision Research, 35(12), 1741–1754. [DOI] [PubMed] [Google Scholar]
- Lavergne L., Vergilino-Perez D., Collins T., & Doré-Mazars K. (2010). Adaptation of within-object saccades can be induced by changing stimulus size. Experimental Brain Research, 203, 773–780. [DOI] [PubMed] [Google Scholar]
- McConkie G., Kerr P. W., Reddix M., Zola D., & Jacobs A. M. (1989). Eye movement control during reading: II. Frequency of refixating a word. Perception & Psychophysics, 46, 245–253. [DOI] [PubMed] [Google Scholar]
- McGowan J. W., Kowler E., Sharma A., & Chubb C. (1998). Saccadic localization of random dot targets. Vision Research, 38(6), 895–909. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. C. (1967). Parametric adjustment in saccadic eye movements. Perception & Psychophysics, 2(8), 359–362. [Google Scholar]
- Melcher D., & Kowler E. (1999). Shapes, surfaces and saccades. Vision Research, 39(17), 2929–2946. [DOI] [PubMed] [Google Scholar]
- Miller J. M., Anstis T., & Templeton W. B. (1981). Saccadic plasticity: parametric adaptive control by retinal feedback. Journal of Experimental Psychology: Human Perception and Performance, 7(2), 356–366. [DOI] [PubMed] [Google Scholar]
- Paeye C., Collins T., Cavanagh P., & Herwig A. (2018). Calibration of peripheral perception of shape with and without saccadic eye movements. Attention, Perception, & Psychophysics, 80(3), 723–737. [DOI] [PubMed] [Google Scholar]
- Panouillères M., Weiss T., Urquizar C., Salemme R., Munoz D. P., & Pélisson D. (2009). Behavioral evidence of separate adaptation mechanisms controlling saccade amplitude lengthening and shortening. Journal of Neurophysiology, 101(3), 1550–1559. [DOI] [PubMed] [Google Scholar]
- Pélisson D., Alahyane N., Panouillères M., & Tilikete C. (2010). Sensorimotor adaptation of saccadic eye movements. Neuroscience & Biobehavioral Reviews, 34(8), 1103–1120. [DOI] [PubMed] [Google Scholar]
- Rahmouni S., & Madelain L. (2019). Inter-individual variability and consistency of saccade adaptation in oblique saccades: Amplitude increase and decrease in the horizontal or vertical saccade component. Vision Research, 160, 82–98. [DOI] [PubMed] [Google Scholar]
- Schnier F., & Lappe M. (2011). Differences in intersaccadic adaptation transfer between inward and outward adaptation. Journal of Neurophysiology, 106(3), 1399–1410. [DOI] [PubMed] [Google Scholar]
- Valsecchi M., Cassanello C., Herwig A., Rolfs M., & Gegenfurtner K. R. (2020). A comparison of the temporal and spatial properties of trans-saccadic perceptual recalibration and saccadic adaptation. Journal of Vision, 20(4):2, 1–15, 10.1167/jov.20.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi M., & Gegenfurtner K. R. (2016). Dynamic re-calibration of perceived size in fovea and periphery through predictable size changes. Current Biology, 26(1), 59–63. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Ogino S., Nakamura T., & Koizuka I. (2003). Saccadic adaptation in the horizontal and vertical directions in normal subjects. Auris Nasus Larynx, 30, 41–45. [DOI] [PubMed] [Google Scholar]
- Zimmermann E., & Lappe M. (2010). Motor signals in visual localization. Journal of Vision, 10(6):2, 1–11, 10.1167/10.6.2. [DOI] [PubMed] [Google Scholar]
- Zimmermann E., & Lappe M. (2016). Visual space constructed by saccade motor maps. Frontiers in Human Neuroscience, 10, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]