Abstract
Binocular rivalry has become an important index of visual performance, both to measure ocular dominance or its plasticity, and to index bistable perception. We investigated its interindividual variability across 50 normal adults and found that the duration of dominance phases in rivalry is linked with the duration of dominance phases in another bistable phenomenon (structure from motion). Surprisingly, it also correlates with the strength of center–surround interactions (indexed by the tilt illusion), suggesting a common mechanism supporting both competitive interactions: center–surround and rivalry.
In a subset of 34 participants, we further investigated the variability of short-term ocular dominance plasticity, measured with binocular rivalry before and after 2 hours of monocular deprivation. We found that ocular dominance shifts in favor of the deprived eye and that a large portion of ocular dominance variability after deprivation can be predicted from the dynamics of binocular rivalry before deprivation. The single best predictor is the proportion of mixed percepts (phases without dominance of either eye) before deprivation, which is positively related to ocular dominance unbalance after deprivation. Another predictor is the duration of dominance phases, which interacts with mixed percepts to explain nearly 50% of variance in ocular dominance unbalance after deprivation. A similar predictive power is achieved by substituting binocular rivalry dominance phase durations with tilt illusion magnitude, or structure from motion phase durations. Thus, we speculate that ocular dominance plasticity is modulated by two types of signals, estimated from psychophysical performance before deprivation, namely, interocular inhibition (promoting binocular fusion, hence mixed percepts) and inhibition for perceptual competition (promoting longer dominance phases and stronger center–surround interactions).
Keywords: homeostatic plasticity, psychophysics, binocular vision, competitive interactions
Introduction
Binocular rivalry is a classic example of bistable perception, where visual awareness oscillates between two percepts (i.e., clockwise and counter-clockwise oriented gratings) despite constant and incoherent stimulation of the two eyes (e.g., clockwise to the left eye and counter-clockwise to the right eye). Much research has been dedicated to identifying the mechanisms leading to the perceptual switches. A major role is typically assigned to reciprocal inhibitory interactions across neuronal populations encoding each eye's percept, at multiple stages of visual processing (Blake, 1989; Laing & Chow, 2002; Li, Rankin, Rinzel, Carrasco, & Heeger, 2017; Logothetis, Leopold, & Sheinberg, 1996; Noest, van Ee, Nijs, & van Wezel, 2007; Said & Heeger, 2013; Seely & Chow, 2011; Tong, Meng, & Blake, 2006; van Loon et al., 2013; Xu et al., 2016)—and similar models apply to other bistable/rivalrous phenomena, such as structure from motion rivalry (Brascamp, Becker, & Hambrick, 2018; van Loon et al., 2013), but see (Gallagher & Arnold, 2014; Sandberg et al., 2016).
In formal rivalry models, mutual inhibition simultaneously influences two distinct properties of rivalry. On the one hand, interocular inhibition is critical for instantiating the competition between eyes for visual awareness (Klink, Brascamp, Blake, & van Wezel, 2010; Said, Egan, Minshew, Behrmann, & Heeger, 2013), and decreased inhibition implies increased periods of nonrivalrous mixed perception, that is, periods where neither eye dominates perception but the two images mix into a fused percept (Robertson, Ratai, & Kanwisher, 2016). In contrast, increased inhibition is related to longer durations of dominance phases, i.e. periods during which the image in either eye completely dominates perception. This finding is supported by magnetic resonance spectroscopy (MRS) evidence in humans, indicating that higher levels of the inhibitory neurotransmitter gamma-amino butyric acid (GABA) in the occipital cortex is related to longer dominance phases (Pitchaimuthu et al., 2017; van Loon et al., 2013; but see Gallagher & Arnold, 2014; Sandberg et al., 2016). A recent study (Mentch, Spiegel, Ricciardi, & Robertson, 2019) specifically supported the hypothesis that two distinct forms of GABAergic inhibition may be differently involved in regulating these two rivalry properties: mixed percepts and dominance phase durations.
Intracortical GABAergic inhibition is not only instrumental in modulating interocular suppression or summation, but it also affects selectivity in many other dimensions (Cook, Hammett, & Larsson, 2016; Frangou et al., 2019; van Loon et al., 2013). In particular, one fundamental perceptual mechanism relying on inhibition is surround suppression, whereby visual processing of one stimulus is affected—through competitive interactions—by the stimuli in the surrounding visual field. Surrounding inhibition may modify the quality of perception, for example, by inducing a shift of perceived orientation of a target grating, which is tilted away from the orientation of the surrounding grating, the so-called tilt illusion (Clifford, 2014). Theoretical models of the tilt illusion suggest a neural mechanism (or mechanisms) that involves inhibition in V1 (Blakemore, Carpenter, & Georgeson, 1970; Blakemore, Muncey, & Ridley, 1973; Clifford, Wenderoth, & Spehar, 2000; Schwartz, Sejnowski, & Dayan, 2009; Series, Lorenceau, & Fregnac, 2003; Solomon & Morgan, 2006). Furthermore, this phenomenon has been linked to GABAergic inhibition through MRS in humans (Cook et al., 2016; Pitchaimuthu et al., 2017; Schallmo, Sponheim, & Olman, 2015; Seymour, Stein, Clifford, & Sterzer, 2018; Song, Sandberg, Andersen, Blicher, & Rees, 2017).
Recent evidence indicates that inhibitory GABAergic signaling also plays a critical role in setting the level of neuroplasticity in the visual cortex, both during development and in the adult brain. Neuroplasticity refers to the capability of neural networks to change and adapt to the external environment (Berardi, Pizzorusso, & Maffei, 2000; Scheyltjens & Arckens, 2016). This property is maximal during early childhood, when sensory systems gradually organize and fine tune in response to environmental inputs (Berardi et al., 2000; Hubel & Wiesel, 1970; Hubel, Wiesel, & LeVay, 1977; Wiesel & Hubel, 1963). Abnormal visual experience in this early critical period can produce long-lasting structural and functional changes. For example, monocular deprivation during the critical period leads to long-lasting suppression of the deprived eye, a medical condition called amblyopia (Berardi et al., 2000; Levi, McKee, & Movshon, 2011; Maurer, Lewis, & Mondloch, 2005; Ostrovsky, Andalman, & Sinha, 2006). These forms of neuroplasticity decrease with age, in parallel with the development of intracortical inhibitory signaling, particularly GABAergic (Bono & Clopath, 2019; Spolidoro, Sale, Berardi, & Maffei, 2009). Consequently, when deprivation occurs in the adult individual, long-lasting effects are not usually seen.
However, mounting evidence suggests that the adult sensory cortex does retain a degree of plasticity (Baseler et al., 2002; Wandell & Smirnakis, 2009). Functional changes have been observed with perceptual learning (Dosher & Lu, 2017; Fiorentini & Berardi, 1980; Karni & Sagi, 1991, 1993; Watanabe & Sasaki, 2015), and short-term visual deprivation (Binda & Lunghi, 2017; Kwon, Legge, Fang, Cheong, & He, 2009; Lunghi, Berchicci, Morrone, & Di Russo, 2015; Lunghi, Burr, & Morrone, 2011; Lunghi, Burr, & Morrone, 2013; Zhang, Bao, Kwon, He, & Engel, 2009; Zhou, Clavagnier, & Hess, 2013; Zhou, Reynaud, & Hess, 2014). Studies on adult human plasticity have used a variety of visual manipulations; Zhang et al., (2009) showed that a few hours deprivation of one cardinal orientation leads to enhanced sensitivity to the deprived orientation—the opposite effect observed during development when the deprived orientation response is suppressed (Blakemore & Campbell, 1969). Similarly, 2 hours of monocular contrast deprivation is followed by a transient boost of the deprived eye (Binda & Lunghi, 2017; Lunghi, Berchicci, et al., 2015; Lunghi et al., 2011; Lunghi et al., 2013; Lunghi, Emir, Morrone, & Bridge, 2015; Zhou et al., 2013; Zhou et al., 2014)—opposite to the amblyopia induced by monocular deprivation during the critical period. Importantly, in amblyopic patients, the transient boost is accompanied by an improvement in visual acuity that persists for months after patching (Lunghi, Sframeli, et al., 2019), suggesting that this form of homeostatic plasticity can be conducive to other more permanent system rearrangements.
Despite the well-established replicability of the effect (Binda et al., 2018; Binda & Lunghi, 2017; Lunghi, Berchicci, et al., 2015; Lunghi et al., 2011; Lunghi et al., 2013; Lunghi, Emir, et al., 2015; Lunghi, Sframeli, et al., 2019; Zhou et al., 2013; Zhou et al., 2014; Zhou, Reynaud, Kim, Mullen, & Hess, 2017), the response to short-term deprivation presents a high level of interindividual variability, possibly reflecting an individual susceptibility to neuronal plasticity. Interestingly, this variability has been related to cortical inhibitory mechanisms, given the strong correlation between the transient boost of the deprived eye and the decrease in GABA concentration in occipital cortex measured by MRS (Lunghi, Emir, et al., 2015).
Given this link between homeostatic plasticity and GABA, we investigate here whether the interindividual variability on short-term plasticity can be explained, at least partially, by other perceptual tasks strongly mediated by intracortical inhibitory circuitry. We measured interindividual variability in a relatively large group of participants, ocular dominance short-term plasticity, binocular rivalry, structure from motion rivalry, and tilt illusion induced by surround context.
Methods
Human subjects
Experimental procedures were approved by the regional ethics committee (Comitato Etico Pediatrico Regionale—Azienda Ospedaliero-Universitaria Meyer—Firenze; protocol “Plasticita’ del Sistema visivo”) and are in line with the declaration of Helsinki. Written informed consent was obtained from each participant, which included consent to process and preserve the data and publish them in anonymous form.
We recruited 54 volunteers (40 females, 14 males; mean age, 26.5 ± 3.5 years) who were selected to have (i) normal or corrected-to-normal vision and normal color vision, (ii) no known history of amblyopia, eye surgery, or other active eye disease (such as keratoconus), (iii) typical ocular dominance balance measured with binocular rivalry (before deprivation). We included all participants with a ratio of mean phase durations of the dominant over the nondominant eye smaller than 0.2 log units.
Experimental design and procedure
Each participant reported to our laboratory at 9.00 a.m. (after breakfast) and performed three psychophysical tasks: tilt illusion, structure from motion, and binocular rivalry. Owing to a technical failure, data from four participants in the structure from motion and tilt illusion tasks are not available. Immediately after these baseline measures, a subset of 34 participants agreed to undergo a procedure of short-term monocular deprivation described elsewhere in this article.
Stimuli and apparatus
Tilt illusion
The tilt illusion stimulus was generated with PsychoPhysics Toolbox routines for MATLAB and presented at a distance of 57 cm on a Sony Trinitron Multiscan E230 linearized monitor (1024 × 768 pixels, 100 Hz) by a Macbook Pro (OSX El Capitan, 10.11.6). The stimulus comprised a centrally presented target grating (diameter: 1.2° of visual angle, or deg) of variable orientation, surrounded by an annular grating (diameter 2.88˚) with the same spatial frequency (2 cpd) and contrast (21% Michelson). The target grating and the surround annulus differed in orientation by either –15° or +15° and could be presented around ±45˚. To minimize the impact of vertical/horizontal edges on orientation judgments, the stimulus was shown at the center of a mid-level grey disk (diameter 21.3˚), overlaid with a small white fixation point (diameter 0.15˚).
The target and surround were presented simultaneously within a temporal Gaussian window of 100 ms full width at half maximum. Three seconds after the stimulus offset, observers performed a two-alternative forced choice task, reporting the orientation of the target (more vertical or horizontal, ignoring the surround). Participants completed 120 trials, divided into four blocks. For the first 12 trials, target orientation was chosen between 33˚ and 57˚ (where smaller values indicate more vertical stimuli). For the rest of the trials, the target orientation was determined by fitting two independent Gaussian functions (one per surround orientation) and homing in on the median of each curve using two independent adaptive procedures.
The median of the Gaussian fit to the complete dataset (60 trials per surround orientation) estimated the point of subjective equality to 45 deg, that is, the target orientation that is reported as closer to vertical on 50% of trials. The distance between the point of subjective equality to 45 deg for the two surround orientations was taken as the magnitude index of the tilt illusion. Positive values indicate a repulsive effect of the surround orientation over the perceived target orientation.
Structure from motion
The apparatus for generating the structure from motion stimulus was the same as for the tilt illusion stimulus. The structure from motion stimulus was made of 300 dots (diameter 0.3˚), randomly placed within a rectangular aperture (8˚ wide and 14˚ tall) shown at screen center and overlaid with a red 0.15˚ fixation mark. One-half of the dots were black (0.08 cd/m2) and moved rightwards; the rest were white (68 cd/m2) and moved leftwards, both with a linear velocity profile that followed a cosine function, peaking at 3.9°/s at screen center.
The resulting stimulus could be perceived as a cylinder rotating about its vertical axis at 60°/s (taking 6 seconds to complete a revolution), with bistable direction: clockwise (with white dots forming the front surface, and black dots forming the rear one) and counter-clockwise (with black dots forming the front surface). A third possibility was a mixed percept, where neither clockwise nor counter-clockwise coherent percept dominated perception exclusively. The stimulus was played for 10 trials of 59s each (and 1-second intertrial intervals) and observers continuously reported their perception by pressing one of the three keys, corresponding with clockwise, counter-clockwise, and mixed percepts.
Binocular rivalry
Stimuli for binocular rivalry were two luminance contrast modulated Gaussian-vignetted sinusoidal gratings (orientation: ±45°, σ: 2°, spatial frequency: 2 cpd, contrast: 50%), presented dichoptically on a uniform equiluminant background, with a binocular central black fixation point and a squared black frame to facilitate fusion. Visual stimuli were generated by the ViSaGe (CRS, Cambridge Research Systems, Rochester, UK) housed in a PC (Dell) controlled by MATLAB programs and displayed on a linearized monitor (Barco CDCT 6551, 800 × 600 pixels, 140 Hz). Dichoptic presentation was achieved through CRS ferromagnetic shutter goggles (Cambridge Research Systems) that alternated the two gratings at each frame. Observers had their head stabilized with a chin rest and viewed the display at a distance of 57 cm.
Binocular rivalry was measured in two short sessions (each comprising two runs of 3 minutes each), immediately before and after the short-term monocular deprivation. During each trial, participants continuously reported their perception (clockwise, or +45° oriented grating, counter-clockwise, or –45° oriented grating, and mixed) by pressing one of three keys. Participants were instructed to classify the stimuli as mixed when none of the two gratings appeared to clearly dominate perception. Each participant was given ample opportunity to practice the task (associating the appropriate response key to each monocularly presented stimulus, and familiarizing with the binocular rivalry phenomenon) before starting the experiment.
Monocular deprivation
Monocular deprivation was achieved by patching the dominant eye, defined as the eye with longer mean phase duration in binocular rivalry, for 2 hours. Dominance was also confirmed using the Porta test (Lunghi, Berchicci, et al., 2015).
As in previous studies (Binda & Lunghi, 2017; Lunghi et al., 2011; Lunghi et al., 2013), the eye patch was made of a translucent plastic material that allowed light to reach the retina (attenuation 15%), but no pattern information, as assessed by the Fourier transform of a natural world image seen through the eye patch. During the 2 hours of monocular deprivation, participants were free to read, work at the computer, or walk around the laboratory (but not to eat or sleep).
To quantify the plasticity effect elicited by monocular deprivation, we computed an ocular dominance index, calculated as in Equation 1, and examined it before versus after deprivation
| (1) |
where p is the ratio between the time spent seeing with either the deprived (d) or nondeprived (nd) eye over the total dominance time (i.e., mixed excluded) and OD is ocular dominance. In previous work, the plasticity effect was evaluated by comparing the dominance of the deprived eye after deprivation versus the same value before deprivation (Lunghi, Sframeli, et al., 2019). Here we chose to assess it using only data acquired after deprivation to have an index that is statistically independent from measurements of the predeprivation sessions. This difference is important, given that we aimed to correlate the plasticity effect with the predeprivation dynamic parameters, like mixed percept and phase duration. Given that we selected participants with balanced eyes before deprivation, the ocular dominance index of Equation 1 is very similar to that used in previous research (Lunghi, Daniele, et al., 2019).
Reliability analysis
To assess the reliability and consistency of our dataset, we used a split-half approach (Allen, 2017). For the tilt illusion, we randomly assigned trials to two subsets and correlated them across participants. For binocular rivalry, we correlated the parameters estimated in the first one-half of the data acquisition with that of the second half. Similarly, for the structure from motion test, we split the ten 60-second-long trials into two sets (the first five in one set, the rest in the other) and correlated them across participants. These correlation coefficients are reported in Table 1, with their associated p values and lgBF values, i.e. the base-10 logarithm of the JZS Bayes Factor. For this and the other analyses in the paper, lgBF quantifies the evidence for or against the null hypothesis, with |lgBF| > 0.5 indicating substantial evidence for (negative lgBF) or against (positive lgBF) the null hypothesis. In addition, we used the Spearman-Brown prediction formula (Spearman, 1910, 1987) to transform these coefficients into reliability estimates, reported in the last column of the Table 1.
Table 1.
Split-half Reliability Analysis.
| Parameter | Rho | p value | lgBF | Reliability |
|---|---|---|---|---|
| BR mpd before | 0.85 | <0.001 | 13.39 | 0.92 |
| BR % mixed before | 0.59 | <0.001 | 3.84 | 0.75 |
| BR mpd after | 0.81 | <0.001 | 6.3 | 0.89 |
| BR % mixed after | 0.72 | <0.001 | 3.98 | 0.83 |
| SFM mpd | 0.80 | <0.001 | 9.41 | 0.89 |
| Tilt illusion size | 0.64 | <0.001 | 4.54 | 0.78 |
Results
We measured interindividual variability across 50 healthy adult observers, in three tasks: tilt illusion, structure from motion, and binocular rivalry. Our primary aim was to establish whether performance in any of these tasks could predict the homeostatic plasticity induced by monocular deprivation. In addition, we investigated the associations between the three tasks, measured before the monocular deprivation procedure.
Figures 1A and 1B show example results for the tilt illusion task: psychometric curves obtained for one participant with a continuously changing target and surround stimuli oriented at ±15° from the target. The perceived orientation of the target was repulsed away from the surround orientation. The repulsive illusory effect was present in all tested participants. The mean orientation difference, 14.7 ± 5.53° (mean ± standard error across participants), was highly significant, t(49) = –18.92, p < 0.001, lgBF = 21.0. This finding was not associated with any change of steepness of the psychometric functions, t(49) = 0.67, p = 0.508, lgBF = –0.7. A split-half reliability test (see Methods and Table 1) showed that the internal consistency of the tilt illusion magnitude is 0.78, moderately high.
Figure 1.
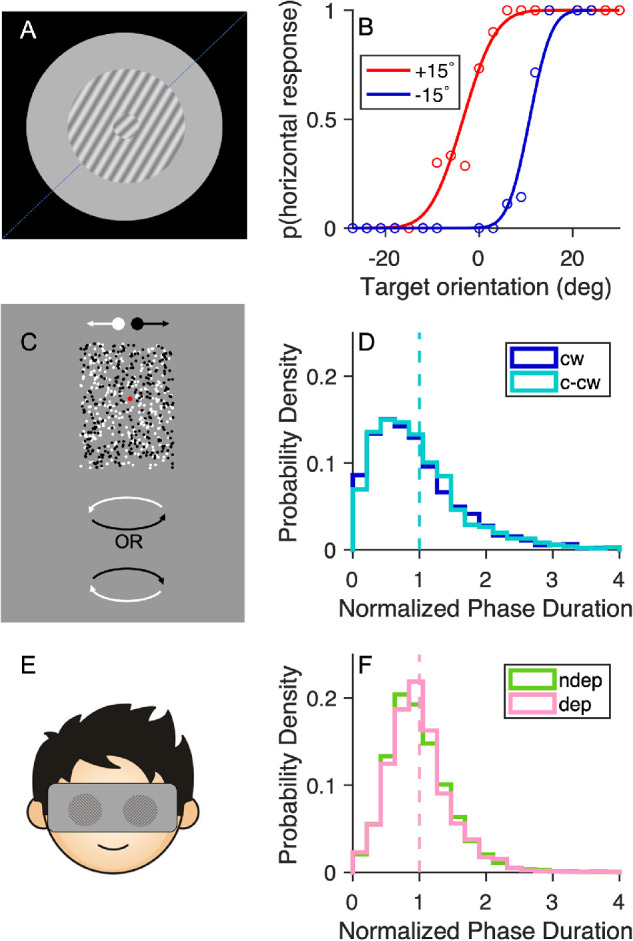
The three psychophysical tasks. (A, B) Tilt illusion. (A) Stimulus arrangement, showing an example case where the target is oriented at 45° (dashed line) but appears as tilted clockwise owing to the surround (tilted counter-clockwise from 45°). (B) Example of psychometric curves for trials with surround tilted ±15° from the target; the separation of the curves (measured by the difference in their points of subjective equality [PSEs] to 45°) estimates the magnitude of the tilt illusion. (C, D) Structure from motion. (C) Two clouds of white and black dots moving rightwards or leftwards, respectively, could be perceived as a cylinder rotating about its vertical axis, with bistable direction: clockwise or counter-clockwise. (D) Probability density function of the normalized phase durations for each percept. The dashed line shows the mean of the distributions, which by definition is equal to 1. (E, F) Binocular rivalry. (E) Stimulus display, made of two orthogonal Gabors presented dichoptically. (F) Probability density function of the normalized phase durations for each eye (deprived and nondeprived). Same format as in (D).
Figures 1C and 1D show results for the structure from motion task. For each participant, we normalized phase durations for the two main percepts (clockwise or counter-clockwise rotation) to their mean, then computed a probability density function, which was finally averaged across participants. The mean phase durations were 10.6 ± 1.15 seconds (mean ± standard error of the mean), for the clockwise percept, and 10.6 ± 1.12 seconds for the counter-clockwise percept. Mixed precepts amounted to a small proportion of the total viewing time 10.81 ± 1.09%. The split-half analysis revealed that mean phase durations had a reliability of 0.89, moderately high.
Finally, Figures 1E and 1F show the results for the binocular rivalry task. For each observer, we normalized the phase duration for the deprived (pink) and nondeprived eye (green) to their mean; the probability density functions were then averaged across participants, yielding very similar curves for the two eyes. The average phase durations were 4.43 ± 0.23 seconds (mean ± standard error of the mean) for the dominant eye and of 4.2 ± 0.22 seconds for the nondominant one. The proportion of viewing time during which each eye dominated percept was 0.47 ± 0.05 for the dominant eye, and 0.43 ± 0.05 for the nondominant, corresponding with a mean ocular dominance index of 0.52 ± 0.05. The mixed percepts dominated for a small proportion of the total viewing time (9.97 ± 0.78%). We used the split-half analysis based on the temporal order of the participant's reports to compute the reliability of both these parameters, separately for sessions before and after deprivation. In all cases, reliability indices are 0.75 or greater (see Table 1).
We found systematic associations between performance in the three tasks (all performed before deprivation).
Figure 2 explores the two main parameters describing binocular rivalry: mean phase durations (averaged for the two eyes) and proportion of mixed percepts, which are themselves not correlated: r(34) = –0.21, p = 0.23, lgBF = –0.6. We found a tight correlation between binocular rivalry mean phase durations and the magnitude of the tilt illusion, r(50) = 0.49, p <0.001, lgBF = 1.9, across participants, those with slower binocular rivalry dynamics are those showing stronger tilt illusion effects (Figure 2A). The correlation remains significant, r(49) = 0.41, p = 0.003, lgBF = 0.89, after excluding the outlier in the top right of Figure 2A.
Figure 2.
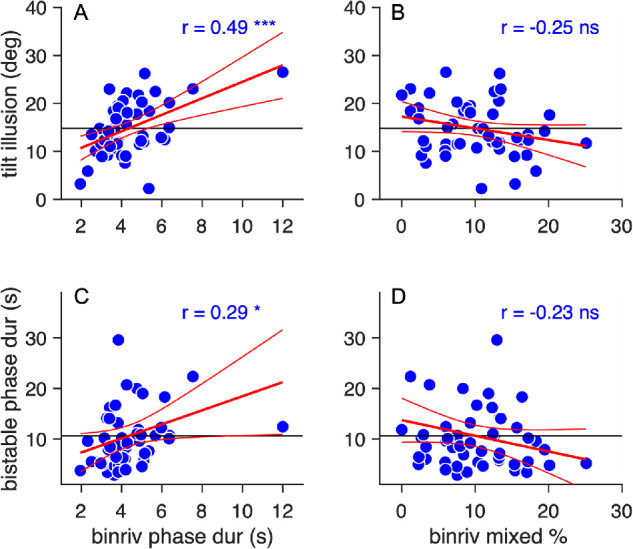
Associations between task performances. Associations between predeprivation performance in binocular rivalry, measured as mean phase duration (A and C) or proportion of mixed percepts (B and D), and magnitude of the tilt illusion (A and B) or mean phase durations in the structure from motion (C and D). Each symbol is one participant; the continuous horizontal line shows the mean of the values on the ordinate; the red lines show the best fit linear function with CIs. Text insets give the Pearson's correlation with associated sample size and p value.
We also found a mild positive correlation between mean phase durations in binocular rivalry and structure from motion, r(50) = 0.29, p <0.05, lgBF = –0.1, implying a portion of shared variance in the rate of perceptual alternation in the two bistable phenomena (Figure 2C).
The other main parameter of binocular rivalry, the percentage of mixed percept, was not significantly correlated with either tilt illusion magnitude, r(50) = –0.25, p = 0.07, lgBF = –0.3, Figure 2B,or structure from motion mean phase durations, r(50) = –0.23, p = 0.22, lgBF = –0.6, Figure 2D. Also, the magnitude of the tilt illusion was not significantly correlated with the structure from motion mean phase durations, r(50) = 0.21, p = 0.138, lgBF = –0.48; not shown.
Having established the pattern of correlations at baseline, we investigated the ability of these measures to predict the effect of monocular deprivation on the dynamics of binocular rivalry.
In line with previous studies, we found that monocular deprivation affected ocular dominance, shifting it in favor of the deprived eye, t(33) = –9.07, p < 0.001, Figure 3B. This was primarily due to an increase in phase durations for the deprived eye, t(33) = –7.095, p < 0.001, and also to a smaller and nonsignificant decrease for the nondeprived eye, t(33) = 1.792, p = 0.082, resulting in a significant Time × Eye interaction in a two-way ANOVA for repeated measures, F(1,33) = 56.35235, p < 0.001, Figure 3A. Figure 3C and 3D show that deprivation affected the distribution of phase durations for each eye, normalized to its predeprivation mean. The distribution of phase durations for the deprived eye became broader and shifted toward the longer durations (the mean moving from 4.43 ± 0.23 seconds to 5.67 ± 0.25 seconds), whereas the distribution of phase durations for the nondeprived eye was only marginally shifted in the opposite direction (mean before, 4.20 ± 0.22 seconds; mean after, 3.77 ± 0.15 seconds). Despite these changes, distributions maintained their typical gamma-like characteristics.
Figure 3.
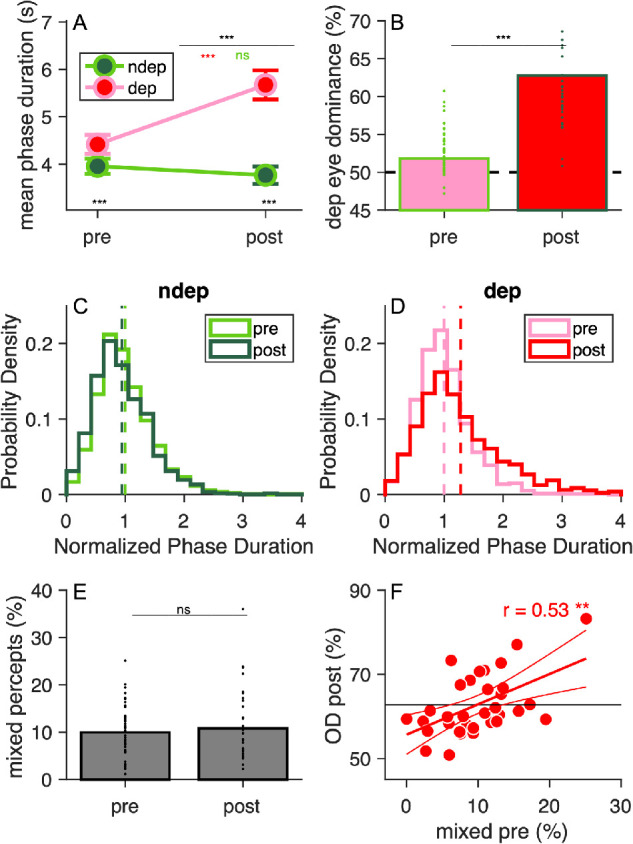
Effects of monocular deprivation. (A) Mean phase durations for each eye (deprived and nondeprived), before and after deprivation. Error bars are standard errors across participants; the top black stars indicate the significance of the Time × Eye interaction; red and green stars give the significance of the change across deprivation for the deprived and nondeprived eye respectively; black stars near the x-axis give the significance of the difference across eyes, before and after deprivation. (B) Dominance of the deprived eye (Equation 1), measured before and after deprivation. Each dot is one participant; bars give the mean across participants and black stars show the significance of the paired t test. (C, D) Probability density functions of the mean phase durations for each eye (nondeprived and deprived, respectively), normalized to the respective pre-deprivation mean. Dashed lines show the means, which by definition are equal to 1 for before deprivation, but shift right or left for the after deprivation, reflecting the plasticity effect. (E) Percentage of mixed percepts, before and after deprivation. Each dot is one participant; bars give the mean across participants and text shows the significance of the paired t test. (F) Association between postdeprivation ocular dominance (OD post) and the percentage of mixed percepts before deprivation. Each symbol is one participant; the continuous horizontal line shows the mean OD post; the red line shows the best fit linear function with CI s. Text insets give the Pearson's correlation with associated sample size and p-value. ns, nonsignificant. *p < 0.05; **p < 0.01; ***p < 0.001 .
Finally, Figure 3E plots the proportion of mixed percepts before and after deprivation, which were unaffected, t(33) = 0.97, p = 0.33, lgBF = –0.5. Note that this measurement was tightly correlated before/after deprivation, r(34) = 0.66, p < 0.001, lgBF = 3.1, suggesting that the failure to measure a change cannot be due to its unreliability (see also the split-half reliability reported in Table 1).
As shown in Figure 3B, ocular dominance shows considerable interindividual variability. However, this is unrelated before/after deprivation, r(34) = 0.13, p = 0.45, lgBF = –0.8: our participants were selected to have balanced eyes, and a slight ocular preference before deprivation is not predictive of a larger (or smaller) ocular dominance imbalance after deprivation.
Although the baseline ocular dominance is not predictive of the plasticity effect, we found that the prominence of mixed percepts does predict the effect of monocular deprivation. Specifically, we found a positive correlation between the percentage of mixed percepts before deprivation and the ocular imbalance after deprivation, r(34) = 0.53, p < 0.01, lgBF = 1.3. This robust association implies that participants who experienced more mixed percepts before deprivation tend to show a stronger dominance of the deprived eye, after deprivation (Figure 3F).
Ocular dominance after deprivation was not correlated with the other parameters describing perceptual performance before deprivation (Figures 4A–4C): the mean duration of binocular rivalry phases, averaged for the two eyes, r(34) = –0.06, p = 0.750, lgBF = –0.85, the magnitude of the tilt illusion, r(30) = –0.09, p = 0.619, lgBF = –0.80, or the mean duration of structure from motion phases, r(30) = –0.15, p = 0.418, lgBF = –0.71. However, entering any of these parameters in a multiple regression model significantly increased its ability to capture interindividual variability in ocular dominance after deprivation compared with a single-regressor model including only the prominence of mixed percepts (Equation 3). The single-regressor model explains 27.6% of postdeprivation ocular dominance variance, R2 = 0.276, adjusted R2 = 0.254, F (32,1) = 12.22, p < 0.001.
| (3) |
Figure 4.
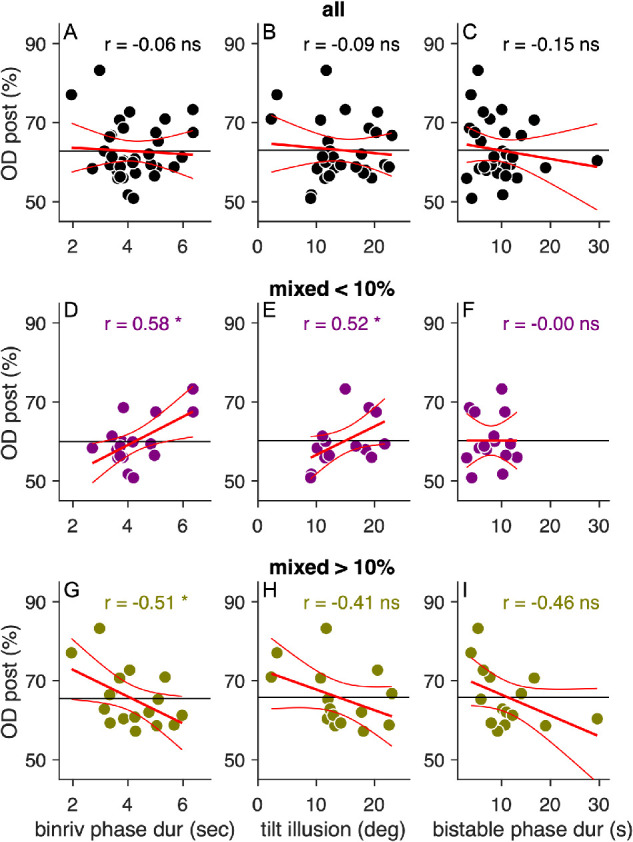
Associations between postdeprivation ocular dominance and predeprivation psychophysical performance. (A–C) Association between postdeprivation ocular dominance and the three main parameters of predeprivation performance, besides mixed percepts in binocular rivalry (shown in Figure 3F): the mean duration of binocular rivalry phases (A), magnitude of the tilt illusion (B), and mean duration of structure from motion phases (C). None of these parameters is directly related to the plasticity effect. However, all three interact significantly with binocular rivalry mixed percepts in explaining postdeprivation ocular dominance, as shown by the multiple regression analysis. This is visualized in D through I, which display the same correlations as in A through C, measured in two subsamples of participants: those with mixed percepts lower (D–F) or higher (G–I) than the median value (10%). Same conventions as in Figure 3F.
A more complex model (Equation 4) considering both mixed percepts and the mean duration of binocular rivalry phases (averaged for the two eyes), both measured before deprivation, allows for explaining 43.8% of variability in postdeprivation ocular dominance, R2 = 0.43, adjusted R2 = 0.38, F(30,2) = 7.788, p = 0.001.
| (4) |
The multiple regression analysis reveals a significant interaction term (β3Mixed*PhaseDur = –0.581, confidence interval [CI] = [–0.989 to –0.173]), implying that the effect of mean phase duration varies depending on the percentage of mixed percepts. We explored this with Figures 4D and 4G, by plotting the postdeprivation ocular imbalance against the mean duration of binocular rivalry phases, separately for participants with higher or lower prominence of mixed percepts before deprivation (median split). Longer phase durations predict a reduced effect of deprivation, but only for individuals experiencing a relatively high percentage of mixed percepts (Figure 4G), not for the other half of the sample (Figure 4D).
A similar picture emerges when entering the model with the magnitude of the tilt illusion (Figures 4B, 4E, and 4H) and the mean duration of structure from motion phases (Figures 4C, 4F, and 4I). Both parameters are correlated with binocular rivalry mean phase durations (see Figure 2); consequently, they both play a similar role in the multiple regression model.
The model including magnitude of the tilt illusion, Equation 5, R2 = 0.391, adjusted R2 = 0.33, F(30,2) = 5.560, p = 0.004, reveals a significant interaction between tilt illusion magnitude and the percentage of mixed percepts in pre-deprivation binocular rivalry, β3Mixed*TiltIll = –0.11, CI = [–0.21 to –0.01].
| (5) |
Similarly, the model including the mean duration of structure from motion phases (Equation 6), R2 = 0.41, adjusted R2 = 0.35, F(30,2) = 6.032, p = 0.003, reveals a significant interaction between structure from motion phase durations and the percentage of mixed percepts in pre-deprivation binocular rivalry, β3Mixed*SFM = –0.144, CI = [–0.280 to –0.008].
| (6) |
Equations 4 to 6 have similar adjusted R2, and they all represent better models than the single-predictor model (Equation 3). We also tested a more complex model including all these parameters and their interaction, but this did not further increase the adjusted R2, R2 = 0.499, adjusted R2 = 0.339, F(30,2) = 2.619, p = 0.037.
A similar pattern of results was obtained when measuring plasticity as the change of ocular dominance before versus after deprivation (instead of ocular dominance after deprivation). In particular, the interaction indices for all three models described in Equations 4 to 6 are significant, β3Mixed*PhaseDur = -51.992, CI = [–91.215 to –12.769], p = 0.003; β3Mixed*TiltIll = –12.237, CI = [–21.026 to –3.448], p = 0.003; β3Mixed*SFM = –13.624, CI = [–25.931 to –1.317], p = 0.004.
Discussion
The main aim of our research was to test the possibility of predicting the strength of homeostatic sensory plasticity, induced through short-term monocular deprivation, based on performance in standard, well-known psychophysical tests: binocular rivalry, structure from motion, and tilt illusion. As a preliminary step toward this goal, we also investigated interindividual variability in each task and checked for potential associations between tasks at baseline (before the monocular deprivation procedure).
We found a surprising and strong association between the dynamics of binocular rivalry and the magnitude of the tilt illusion. These tasks differ on a number of levels (one requires continuous reporting of percept, the other relies on a standard two-alternative forced choice task; one is strictly foveal, the other measures the influence of a parafoveal region) and their association has never been hypothesized. However, inhibitory mechanisms are assumed to underlay both phenomena. The tilt illusion may be mediated by inhibitory and disinhibitory interactions between spatial frequency and orientation-selective mechanisms at multiple levels of the cortical processing hierarchy. Theoretical models of the tilt illusion suggest a neural mechanism (or mechanisms) that involves inhibition in V1 (Blakemore et al., 1970; Blakemore et al., 1973; Clifford et al., 2000; Schwartz et al., 2009; Series et al., 2003; Solomon & Morgan, 2006). In line with this, recent MRS studies in humans have linked occipital GABA levels with the magnitude of tilt illusion (Cook et al., 2016; Seymour et al., 2018; Song et al., 2017). Combining MRS studies on tilt illusion and binocular rivalry, showing that occipital GABA is positively correlated with tilt illusion magnitude (Song et al., 2017) and positively correlated with the duration of mean phases of binocular rivalry (van Loon et al., 2013), one would predict a positive correlation between tilt illusion magnitude and binocular rivalry phase durations. This pattern of results is precisely that we report.
We also found that the dynamics of the two bistable phenomena (duration of perceptual phases in binocular rivalry and structure from motion) were mildly correlated—participants with longer phase durations in binocular rivalry, tended to experience longer phases in structure from motion. There is considerable evidence that different bistable phenomena share at least partially some underlying mechanisms (Leopold & Logothetis, 1999), generally involving reciprocal inhibition between competing neural representations (Blake, Brascamp, & Heeger, 2014; Blake & Wilson, 2011; Brascamp, Klink, & Levelt, 2015). However, previous studies find weak (nonsignificant) correlations between binocular rivalry and bistable structure from motion dynamics (Brascamp et al., 2018; Cao, Wang, Sun, Engel, & He, 2018), attributing this lack of correlation to the possibility that the two sets of stimuli may be processed by very different brain mechanisms. Here we did observe a correlation between these two widely different stimuli; however, the correlation is weak and hence not inconsistent with previous reports. Moreover, one factor potentially playing an important role in explaining this correlation is motor noise, shared between the two forms of bistability (both reported by continuous key presses) (Gallagher & Arnold, 2014), but see also (Brascamp et al., 2018). Lacking a rivalry replay control condition to isolate the impact of motor noise on rivalry reports, we cannot exclude that this phenomenon contributes to the correlations we observed.
Although the main parameter used to describe the dynamics of binocular rivalry is the duration of perceptual phases (or the rate of perceptual switches), a second parameter is gaining interest in the literature: the proportion of mixed percepts, where images in the two eyes, rather than competing, fuse in a mixed percept. A recent meta-analysis (Brascamp et al., 2018) reported a lack of consensus on the way mixed percepts are quantified and analyzed, as well as on the importance that is attached to parameter. In many binocular rivalry experiments, participants are not given the option to report mixed percepts—they can only report which eye is relatively dominant (Brascamp et al., 2018; Pitchaimuthu et al., 2017). In contrast, several recent studies have specifically examined individual differences in the predominance of mixed perception, either focusing on different mixed percepts subtypes (Sheynin, Proulx, & Hess, 2019) or, for example, relating it to autistic spectrum disorders and the alterations of cortical inhibition that may accompany them (Robertson, Kravitz, Freyberg, Baron-Cohen, & Baker, 2013; Said et al., 2013). Mentch et al. (2019) went further, suggesting that the proportion of mixed percepts and the duration of exclusive-dominance phases are independently regulated by two distinct inhibitory pathways. Specifically, they show how the duration of mixed percepts and that of exclusive dominance during binocular rivalry are modulated differently by GABA-A (less mixed, no change in percepts durations) and GABA-B (mainly more percepts durations, and slightly less mixed) agonists. In line with this finding, we find that binocular rivalry mean phase durations and mixed percepts are not significantly correlated, despite a nonsignificant negative trend that may be a byproduct of mixed percepts usually occurring at the transition between phases, implying that participants with shorter phases will tend to have more mixed percepts. We speculate that mixed percepts may indicate interocular summation, the logic opposite of interocular inhibition, which would be consistent with Mentch et al.'s (2019) observation that pharmacologically increasing GABA leads to a decrease of mixed percepts, but only if the manipulation occurs at the GABA-A receptor. This implies that mixed percepts are a stimulus-specific index of bistability—explaining its lack of correlation with any parameter of structure from motion and tilt illusion. It may also explain its importance in predicting the change of binocular rivalry with monocular deprivation, which is thought to modulate the balance between eyes by interfering with interocular inhibition, and has been correlated with MRS estimates of occipital GABA levels (Lunghi, Emir, et al., 2015). Our results regarding mixed percepts are in contrast with those of Sheyin et al. (2019): we found no change in mixed percepts while they found an increase both in proportion and phase duration after deprivation. This difference could be due to different methodologic procedures used; in fact, Sheyin et al. (2019) instructed their participants to differentiate between different kinds of mixed percepts (piecemeal vs superimposition), whereas we decided to avoid this as a possible confusion for our participant.
We measured the effects of monocular deprivation on binocular rivalry on a sample of 34 participants, a larger sample than those used in previous studies from our laboratory (Binda & Lunghi, 2017; Lunghi et al., 2011; Lunghi et al., 2013) and others’ (Zhou et al., 2013). Besides confirming the robustness and replicability of the monocular deprivation effect, these figures give us a chance to leverage the substantial interindividual variability of the effect to assess the predictive power of several perceptual indices measured before monocular deprivation. The proportion of mixed percepts was found to be stable before and after monocular deprivation, and to have the greatest predictive power on the monocular deprivation effect. However, including binocular rivalry mean phase durations as a second predictor allowed for the substantially larger portion (almost 50%) of variance in the monocular deprivation effect. Similar, although lower, predictive power was achieved by substituting binocular rivalry mean phase durations with structure from motion phase durations or tilt illusion magnitude (as may be expected, given the positive correlation among these three variables, discussed elsewhere in this article). In all models, a key component was the interaction between mixed percepts and phase durations (or tilt illusion magnitude), which may be taken to suggest that at least two different mechanisms may influence the effect of monocular deprivation, possibly related to two (or more) distinct inhibitory mechanisms acting at the level of the visual cortex.
In fact, anatomophysiologic evidence indicates that the population of GABAergic interneurons in V1 is extremely heterogeneous (Trachtenberg, 2015), comprising at least two very distinct classes of cells (Maffei, Lambo, & Turrigiano, 2010; Priya et al., 2019; Scheyltjens & Arckens, 2016). Parvalbumin cells (Scholl, Pattadkal, Dilly, Priebe, & Zemelman, 2015) mediate local inhibitory interactions and could mediate the reciprocal inhibition between neighboring ocular dominance cells (Hensch & Quinlan, 2018; Saiepour et al., 2015; Trachtenberg, 2015). Somatostatin expressing inhibitory neurons, in contrast, act at a more global level, mediating long-range interactions across remote visual field regions and have been specifically associated with center–surround interactions (Adesnik, Bruns, Taniguchi, Huang, & Scanziani, 2012; Vangeneugden et al., 2019; Yazdani, Serrano-Pedraza, Whittaker, Trevelyan, & Read, 2015). Somatostatin expressing inhibitory neurons have been suggested to play a role in short-term plasticity, proposing that their modulation during physical exercise (Lunghi, Sframeli, et al., 2019) could be responsible for the enhanced plasticity reported in Lunghi & Sale (2015). Interestingly, our findings show that variability in short-term plasticity is explained by at least two independent factors related, possibly, to GABAergic inhibition. This finding may suggest an influence of parvalbumin cells inhibitory mechanisms that modulates interocular inhibition revealed by mixed percepts measures and of long-range mechanisms through somatostatin expressing inhibitory neurons that modulate center–surround inhibition (Clifford, 2014) and dynamics of bistability. The interaction between the two factors observed in our results is reminiscent of a normalization model (Reynolds & Heeger, 2009), whereby relatively low local inhibition (high prevalence of mixed percepts) is permissive for long-range inhibition to drive the plasticity effect: slow bistable dynamics or strong center–surround interactions predict smaller plasticity, but high local inhibition (low prevalence of mixed percepts) obscure the effects of the other inhibitory input. We acknowledge that this model is entirely speculative and, although indirectly supported by evidence of multiple sources, requires further research.
In conclusion, we find that a significant portion of interindividual variability in short-term plasticity may be predicted based on performance on standard psychophysical tasks. However, the key predictive parameters are derived from binocular rivalry measurements. This could be a simple consequence of rivalry being our probe for short-term plasticity, or it could be indicative of binocular rivalry being highly informative of the functional properties of early visual cortex.
Acknowledgments
This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 801715-PUPILTRAITS), by European programme Flag-ERA JTC 2019 (grant DOMINO) and by the Italian Ministry of University Research under the PRIN2017 programme (grants MISMATCH and 2017SBCPZY). The authors would like to thank Claudia Lunghi for her help in the implementation of the study.
Commercial relationships: none.
Corresponding author: Paola Binda.
Email: paola.binda@unipi.it.
Address: Department of Translational Research on New Technologies in Medicine and Surgery, University of Pisa. Via San Zeno 31, 56123 Pisa (PI).
References
- Adesnik H., Bruns W., Taniguchi H., Huang Z. J., & Scanziani M. (2012). A neural circuit for spatial summation in visual cortex. Nature , 490(7419), 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. (2017). Reliability, split-half. The SAGE Encyclopedia of Communication Research Methods. Thousand Oaks, CA: Sage. [Google Scholar]
- Baseler H. A., Brewer A. A., Sharpe L. T., Morland A. B., Jagle H., & Wandell B. A. (2002). Reorganization of human cortical maps caused by inherited photoreceptor abnormalities. Nature. Neuroscience , 5(4), 364–370. [DOI] [PubMed] [Google Scholar]
- Berardi N., Pizzorusso T., & Maffei L. (2000). Critical periods during sensory development. Current Opinion in Neurobiology , 10(1), 138–145. [DOI] [PubMed] [Google Scholar]
- Binda P., Kurzawski J. W., Lunghi C., Biagi L., Tosetti M., & Morrone M. C. (2018). Response to short-term deprivation of the human adult visual cortex measured with 7T BOLD. Elife , 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda P., & Lunghi C. (2017). Short-term monocular deprivation enhances physiological pupillary oscillations. Neural Plasticity, 2017, 6724631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R. (1989). A neural theory of binocular rivalry. Psychological Review , 96(1), 145–167. [DOI] [PubMed] [Google Scholar]
- Blake R., Brascamp J., & Heeger D. J. (2014). Can binocular rivalry reveal neural correlates of consciousness? Philosophical Transactions of the Royal Society of London. Series B, Biological Science , 369(1641), 20130211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R., & Wilson H. (2011). Binocular vision. Vision Research , 51(7), 754–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., & Campbell F. W. (1969). On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. Journal of Physiology , 203(1), 237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Carpenter R. H., & Georgeson M. A. (1970). Lateral inhibition between orientation detectors in the human visual system. Nature , 228(5266), 37–39. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Muncey J. P., & Ridley R. M. (1973). Stimulus specificity in the human visual system. Vision Research , 13(10), 1915–1931. [DOI] [PubMed] [Google Scholar]
- Bono J., & Clopath C. (2019). Synaptic plasticity onto inhibitory neurons as a mechanism for ocular dominance plasticity. PLoS Computational Biology , 15(3), e1006834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brascamp J. W., Becker M. W., & Hambrick D. Z. (2018). Revisiting individual differences in the time course of binocular rivalry. Journal of Vision , 18(7), 3. [DOI] [PubMed] [Google Scholar]
- Brascamp J. W., Klink P. C., & Levelt W. J. (2015). The 'laws' of binocular rivalry: 50 years of Levelt's propositions. Vision Research , 109(Pt A), 20–37. [DOI] [PubMed] [Google Scholar]
- Cao T., Wang L., Sun Z., Engel S. A., & He S. (2018). The independent and shared mechanisms of intrinsic brain dynamics: Insights from bistable perception. Frontiers in Psychology, 9, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford C. W. (2014). The tilt illusion: Phenomenology and functional implications. Vision Research, 104, 3–11. [DOI] [PubMed] [Google Scholar]
- Clifford C. W., Wenderoth P., & Spehar B. (2000). A functional angle on some after-effects in cortical vision. Proceedings. Biological Sciences , 267(1454), 1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook E., Hammett S. T., & Larsson J. (2016). GABA predicts visual intelligence. Neuroscience Letters, 632, 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher B., & Lu Z. L. (2017). Visual perceptual learning and models. Annual Review of Vision Science, 3, 343–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A., & Berardi N. (1980). Perceptual learning specific for orientation and spatial frequency. Nature , 287(5777), 43–44. [DOI] [PubMed] [Google Scholar]
- Frangou P., Emir U. E., Karlaftis V. M., Nettekoven C., Hinson E. L., Larcombe S., et al. (2019). Learning to optimize perceptual decisions through suppressive interactions in the human brain. Nature Communications , 10(1), 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. M., & Arnold D. H. (2014). Interpreting the temporal dynamics of perceptual rivalries. Perception , 43(11), 1239–1248. [DOI] [PubMed] [Google Scholar]
- Hensch T. K., & Quinlan E. M. (2018). Critical periods in amblyopia. Visual Neuroscience, 35, E014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., & Wiesel T. N. (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology , 206(2), 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., & LeVay S. (1977). Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences , 278(961), 377–409. [DOI] [PubMed] [Google Scholar]
- Karni A., & Sagi D. (1991). Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity. Proceedings of the National Academy of Science of the United States of America , 88(11), 4966–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A., & Sagi D. (1993). The time course of learning a visual skill. Nature , 365(6443), 250–252. [DOI] [PubMed] [Google Scholar]
- Klink P. C., Brascamp J. W., Blake R., & van Wezel R. J. (2010). Experience-driven plasticity in binocular vision. Current Biology , 20(16), 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M., Legge G. E., Fang F., Cheong A. M., & He S. (2009). Adaptive changes in visual cortex following prolonged contrast reduction. Journal of Vision , 9(2), 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing C. R., & Chow C. C. (2002). A spiking neuron model for binocular rivalry. Journal of Computational Neuroscience , 12(1), 39–53. [DOI] [PubMed] [Google Scholar]
- Leopold D. A., & Logothetis N. K. (1999). Multistable phenomena: Changing views in perception. Trends in Cognitive Science , 3(7), 254–264. [DOI] [PubMed] [Google Scholar]
- Levi D. M., McKee S. P., & Movshon J. A. (2011). Visual deficits in anisometropia. Vision Research , 51(1), 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Rankin J., Rinzel J., Carrasco M., & Heeger D. J. (2017). Attention model of binocular rivalry. Proceedings of the National Academy of Science of the United States of America , 114(30), E6192–E6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N. K., Leopold D. A., & Sheinberg D. L. (1996). What is rivalling during binocular rivalry? Nature , 380(6575), 621–624. [DOI] [PubMed] [Google Scholar]
- Lunghi C., Berchicci M., Morrone M. C., & Di Russo F. (2015). Short-term monocular deprivation alters early components of visual evoked potentials. Journal of Physiology , 593(19), 4361–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., Burr D. C., & Morrone C. (2011). Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Current Biology , 21(14), R538–539. [DOI] [PubMed] [Google Scholar]
- Lunghi C., Burr D. C., & Morrone M. C. (2013). Long-term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. Journal of Vision , 13(6). [DOI] [PubMed] [Google Scholar]
- Lunghi C., Daniele G., Binda P., Dardano A., Ceccarini G., Santini F., et al. (2019). Altered visual plasticity in morbidly obese subjects. iScience, 22, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., Emir U. E., Morrone M. C., & Bridge H. (2015). Short-term monocular deprivation alters GABA in the adult human visual cortex. Current Biology, 25(11), 1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., & Sale A. (2015). A cycling lane for brain rewiring. Current Biology, 25(23), R1122–R1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., Sframeli A. T., Lepri A., Lepri M., Lisi D., Sale A., et al. (2019). A new counterintuitive training for adult amblyopia. Annals of Clinical and Translational Neurology, 6(2), 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A., Lambo M. E., & Turrigiano G. G. (2010). Critical period for inhibitory plasticity in rodent binocular V1. Journal of Neuroscience, 30(9), 3304–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer D., Lewis T. L., & Mondloch C. J. (2005). Missing sights: Consequences for visual cognitive development. Trends in Cognitive Science, 9(3), 144–151. [DOI] [PubMed] [Google Scholar]
- Mentch J., Spiegel A., Ricciardi C., & Robertson C. E. (2019). GABAergic inhibition gates perceptual awareness during binocular rivalry. Journal of Neuroscience, 39(42), 8398–8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noest A. J., van Ee R., Nijs M. M., & van Wezel R. J. (2007). Percept-choice sequences driven by interrupted ambiguous stimuli: A low-level neural model. Journal of Vision, 7(8), 10. [DOI] [PubMed] [Google Scholar]
- Ostrovsky Y., Andalman A., & Sinha P. (2006). Vision following extended congenital blindness. Psychological Science, 17(12), 1009–1014. [DOI] [PubMed] [Google Scholar]
- Pitchaimuthu K., Wu Q. Z., Carter O., Nguyen B. N., Ahn S., Egan G. F., et al. (2017). Occipital GABA levels in older adults and their relationship to visual perceptual suppression. Scientific Reports, 7(1), 14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya R., Rakela B., Kaneko M., Spatazza J., Larimer P., Hoseini M. S., et al. (2019). Vesicular GABA transporter is necessary for transplant-induced critical period plasticity in mouse visual cortex. Journal of Neuroscience , 39(14), 2635–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. H., & Heeger D. J. (2009). The normalization model of attention. Neuron , 61(2), 168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. E., Kravitz D. J., Freyberg J., Baron-Cohen S., & Baker C. I. (2013). Slower rate of binocular rivalry in autism. Journal of Neuroscience , 33(43), 16983–16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. E., Ratai E. M., & Kanwisher N. (2016). Reduced GABAergic action in the autistic brain. Current Biology , 26(1), 80–85. [DOI] [PubMed] [Google Scholar]
- Said C. P., Egan R. D., Minshew N. J., Behrmann M., & Heeger D. J. (2013). Normal binocular rivalry in autism: Implications for the excitation/inhibition imbalance hypothesis. Vision Research, 77, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said C. P., & Heeger D. J. (2013). A model of binocular rivalry and cross-orientation suppression. PLoS Computational Biology , 9(3), e1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiepour M. H., Rajendran R., Omrani A., Ma W. P., Tao H. W., Heimel J. A., et al. (2015). Ocular dominance plasticity disrupts binocular inhibition-excitation matching in visual cortex. Current Biology , 25(6), 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg K., Blicher J. U., Del Pin S. H., Andersen L. M., Rees G., & Kanai R. (2016). Improved estimates for the role of grey matter volume and GABA in bistable perception. Cortex, 83, 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallmo M. P., Sponheim S. R., & Olman C. A. (2015). Reduced contextual effects on visual contrast perception in schizophrenia and bipolar affective disorder. Psychological Medicine , 45(16), 3527–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyltjens I., & Arckens L. (2016). The current status of somatostatin-interneurons in inhibitory control of brain function and plasticity. Neural Plasticity, 2016, 8723623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B., Pattadkal J. J., Dilly G. A., Priebe N. J., & Zemelman B. V. (2015). Local integration accounts for weak selectivity of mouse neocortical parvalbumin interneurons. Neuron , 87(2), 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Sejnowski T. J., & Dayan P. (2009). Perceptual organization in the tilt illusion. Journal of Vision , 9(4), 19 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J., & Chow C. C. (2011). Role of mutual inhibition in binocular rivalry. Journal of Neurophysiology , 106(5), 2136–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Series P., Lorenceau J., & Fregnac Y. (2003). The “silent” surround of V1 receptive fields: Theory and experiments. Journal of Physiology Paris , 97(4–6), 453–474. [DOI] [PubMed] [Google Scholar]
- Seymour K. J., Stein T., Clifford C. W. G., & Sterzer P. (2018). Cortical suppression in human primary visual cortex predicts individual differences in illusory tilt perception. Journal of Vision , 18(11), 3. [DOI] [PubMed] [Google Scholar]
- Sheynin Y., Proulx S., & Hess R. F. (2019). Temporary monocular occlusion facilitates binocular fusion during rivalry. Journal of Vision , 19(5), 23. [DOI] [PubMed] [Google Scholar]
- Solomon J. A., & Morgan M. J. (2006). Stochastic re-calibration: Contextual effects on perceived tilt. Proceedings. Biological Sciences , 273(1601), 2681–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Sandberg K., Andersen L. M., Blicher J. U., & Rees G. (2017). Human occipital and parietal GABA selectively influence visual perception of orientation and size. Journal of Neuroscience , 37(37), 8929–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. (1910). Correlation calculated from faulty data. British Journal of Psychology, 1904–1920. [Google Scholar]
- Spearman C. (1987). The proof and measurement of association between two things. By C. Spearman, 1904. American Journal of Psychology , 100(3–4), 441–471. [PubMed] [Google Scholar]
- Spolidoro M., Sale A., Berardi N., & Maffei L. (2009). Plasticity in the adult brain: Lessons from the visual system. Experimental Brain Research , 192(3), 335–341. [DOI] [PubMed] [Google Scholar]
- Tong F., Meng M., & Blake R. (2006). Neural bases of binocular rivalry. Trends in Cognitive Science , 10(11), 502–511. [DOI] [PubMed] [Google Scholar]
- Trachtenberg J. T. (2015). Parvalbumin interneurons: All forest, no trees. Neuron , 87(2), 247–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. M., Knapen T., Scholte H. S., St John-Saaltink E., Donner T. H., & Lamme V. A. (2013). GABA shapes the dynamics of bistable perception. Current Biology , 23(9), 823–827. [DOI] [PubMed] [Google Scholar]
- Vangeneugden J., van Beest E. H., Cohen M. X., Lorteije J. A. M., Mukherjee S., Kirchberger L., et al. (2019). Activity in lateral visual areas contributes to surround suppression in awake mouse V1. Current Biology , 29(24), 4268–4275.e4267. [DOI] [PubMed] [Google Scholar]
- Wandell B. A., & Smirnakis S. M. (2009). Plasticity and stability of visual field maps in adult primary visual cortex. Nature Reviews. Neuroscience , 10(12), 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., & Sasaki Y. (2015). Perceptual learning: Toward a comprehensive theory. Annual Review of Psychology, 66, 197–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., & Hubel D. H. (1963). Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology, 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- Xu H., Han C., Chen M., Li P., Zhu S., Fang Y., et al. (2016). Rivalry-like neural activity in primary visual cortex in anesthetized monkeys. Journal of Neuroscience , 36(11), 3231–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani P., Serrano-Pedraza I., Whittaker R. G., Trevelyan A., & Read J. C. (2015). Two common psychophysical measures of surround suppression reflect independent neuronal mechanisms. Journal of Vision , 15(13), 21. [DOI] [PubMed] [Google Scholar]
- Zhang P., Bao M., Kwon M., He S., & Engel S. A. (2009). Effects of orientation-specific visual deprivation induced with altered reality. Current Biology , 19(22), 1956–1960. [DOI] [PubMed] [Google Scholar]
- Zhou J., Clavagnier S., & Hess R. F. (2013). Short-term monocular deprivation strengthens the patched eye's contribution to binocular combination. Journal of Vision , 13(5), 12. [DOI] [PubMed] [Google Scholar]
- Zhou J., Reynaud A., & Hess R. F. (2014). Real-time modulation of perceptual eye dominance in humans. Proceedings. Biological Sciences , 281(1795) 20141717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Reynaud A., Kim Y. J., Mullen K. T., & Hess R. F. (2017). Chromatic and achromatic monocular deprivation produce separable changes of eye dominance in adults. Proceedings. Biological Sciences , 284(1867) 20171669. [DOI] [PMC free article] [PubMed] [Google Scholar]


