Highlights
-
•
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary liver carcinoma.
-
•
The indications for live transplantation for cHCC-CCA remain controversial.
-
•
In this case series, patients who met the Milan criteria on the pathological examination had good prognosis.
-
•
By itself, cHCC-CCA was not a contraindication for liver transplantation.
Abbreviations: AFP, alpha-fetoprotein; CCA, cholangiocarcinoma; cHCC-CCA, combined hepatocellular-cholangiocarcinoma; CT, computed tomography; FDG-PET, F-18 fluorodeoxyglucose positron emission tomography; DCP, des-gamma carboxyprothrombin; HCC, hepatocellular carcinoma; LDLT, living donor liver transplantation; LT, liver transplantation; MELD, model for end-stage liver disease; MRI, magnetic resonance image; SUV, standardized uptake value
Keywords: Combined hepatocellular-cholangiocarcinoma, Hepatocellular carcinoma, Liver transplantation
Abstract
Introduction
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary liver carcinoma whose components include both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA). Indications for liver transplantation for cHCC-CCA remain controversial.
Case presentations
Four patients underwent living donor liver transplantation (LDLT) for cHCC-CCA. All patients had multiple tumor nodules preoperatively diagnosed as HCC. Postoperative pathological examinations revealed that one of the tumors was cHCC-CCA. Cases 1 and 2 underwent LDLT for cirrhosis with HCC tumors that met Milan criteria. Case 3 underwent LDLT for recurrent HCC tumors with nonalcoholic steatohepatitis. Although the preoperative examinations showed that the patient met the Kyoto, but not Milan criteria, postoperative pathological examinations revealed that the patient did meet Milan criteria. The three patients who met Milan criteria on postoperative pathological examination had no recurrences after LDLT. Case 4 had multiple tumors with alcoholic liver cirrhosis. Although the preoperative examinations showed that the patient did not meet Milan criteria—but did meet Kyoto criteria—, on postoperative pathological examinations, the patient met neither Millan nor Kyoto criteria. He died of tumor recurrence 15 months after LDLT.
Discussion and conclusions
Our experiences suggested that patients who meet Millan or Kyoto criteria experienced acceptable outcomes of LDLT for cHCC-CCA. By itself, cHCC-CCA is not contraindication for liver transplantation.
1. Introduction
Primary liver carcinoma, including hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (CCA), is the second leading cause of cancer-related deaths worldwide [1]. Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary liver carcinoma with both HCC and CCA components in one tumor nodule [[2], [3], [4]].
Some reports have shown worse prognosis for cHCC-CCA than that for HCC in liver transplantation (LT) [[5], [6], [7], [8]]. However, because of the rarity of cHCC-CCA, few reports have described the outcomes of LT for cHCC-CCA. Therefore, the indications of LT for cHCC-CCA remain controversial. The present study examined four cases at our institute. The research work has been reported in line with the process criteria [9].
2. Case presentations
2.1. Patients
All patients who underwent LT between January 2005 and December 2018 at our institute were enrolled. The study protocol was approved by the Ethics Committee of our institute and performed in accordance with the 1964 Declaration of Helsinki and its later amendments. A total of 835 patients underwent LT, four of whom underwent living donor LT (LDLT) for cHCC-CCA. All four patients had multiple tumor nodules and were diagnosed with HCC preoperatively. One of the multiple tumors turned out to be cHCC-CCA by postoperative pathological examination. The pathological diagnoses were made in accordance with the 2019 World Health Organization (WHO) classification based on hematoxylin and eosin staining [4]. The clinical features of the four patients are summarized in Table 1.
Table 1.
Clinicopathological features of the four cases with cHCC-CCA.
| Case | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 65 | 62 | 67 | 45 |
| Sex | M | M | F | M |
| Background liver disease | HCV-LC | HBV-LC | NASH | Alcoholic LC |
| Child-Pugh | C(11) | C(11) | B(8) | C(10) |
| CEA [ng/mL] | 2.4 | 3.1 | 5 | 11 |
| CA19-9 [U/mL] | 40 | 33.1 | 34.8 | <0.6 |
| AFP [ng/mL] | 85.8 | 6.7 | 2075 | 642.3 |
| DCP [mAU/mL] | 84 | 59 | 380 | 18 |
| Milan criteria | Within | Within | (Beyond→) Within* | Beyond |
| Kyoto criteria | Within | Within | Within | (Within→) Beyond** |
| Tumor number (total) | 3 | 2 | 3 | >10 |
| Tumor number (cHCC-CCA) | 1 | 1 | 1 | 1 |
| Tumor size(cHCC-CCA) [cm] | 1.4 | 1.1 | 2.1 | 3.6 |
| Tumor size(maximum) [cm] | 2.9 | 1.1 | 3 | 3.6 |
| Recurrence | – | – | – | Multiple liver cancer |
| Recurrence[month] | – | – | – | 9 |
| Outcome | Alive | Alive | Alive | Death |
| Survival time[month] | 65 | 66 | 11 | 15 |
| Cause of death | – | – | – | Liver cancer |
*Case 3 did not meet the Milan criteria preoperatively. However, she met the criteria on the postoperative examination. ** Case 4 met Kyoto criteria preoperatively, but did not meet Kyoto criteria on the postoperative examination. M: male, F: female, HCV: hepatitis C virus, HBV: hepatitis B virus, NASH: non-alcoholic steatohepatitis, LC: liver cirrhosis.
2.2. Case 1: a 65-year-old man
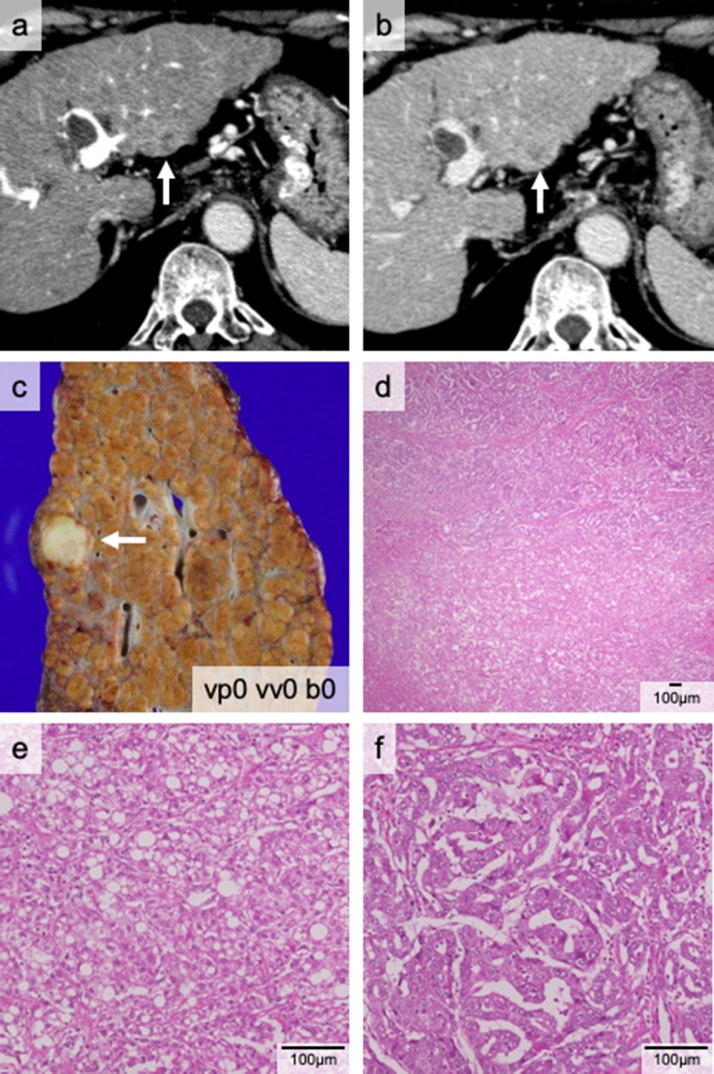
The patient was referred to our institute for hepatitis C virus-associated cirrhosis with multiple liver tumors in segments 4 and 8. He previously underwent a partial liver resection for HCC. Computed tomography (CT) showed two tumors (maximum size 21 mm) (Fig. 1a and b). F-18 fluorodeoxyglucose positron emission tomography (FDG-PET) / CT failed to detect a high standardized uptake value (SUV) tumor. His des-gamma carboxyprothrombin (DCP) and α-fetoprotein (AFP) levels were 84 mAU/mL and 85.8 ng/mL, respectively. His Child-Pugh (C-P) score was grade C (11 points) and his Model for End-stage Liver Disease (MELD) score was 12 points. Since he met the Milan criteria [10], LDLT with the right lobe graft was performed. Postoperative pathological examination revealed three tumors (Fig. 1c): one of which was diagnosed as cHCC-CCA (Fig. 1d–f). The other two tumors were HCC. He lived without tumor recurrence for over five years.
Fig. 1.
The cHCC-CCA nodule in Case 1. The computed tomography (CT) scan shows a tumor in segment 2, which is enhanced in the portal phase (a) and washed out in the late phase (b). This tumor macroscopically appeared solid white (c). Pathological findings indicating that the nodule consists of two components (d): CCA (e) and HCC (f).
2.3. Case 2: a 62-year-old man
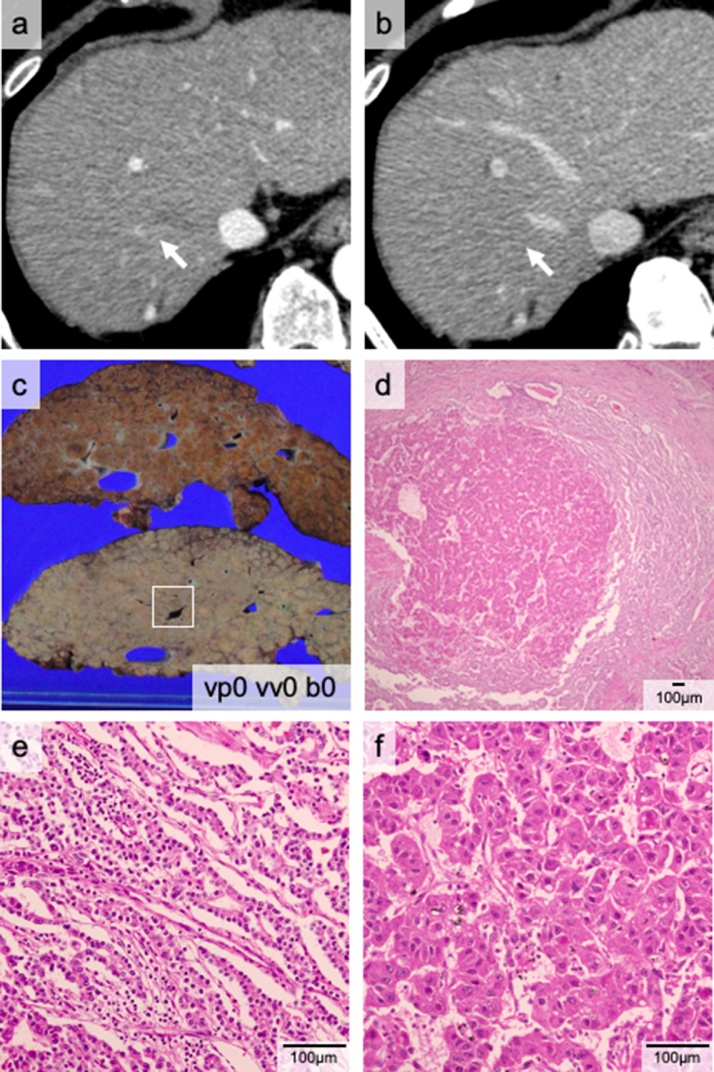
The patient was referred to our institute for hepatitis B virus-associated cirrhosis with multiple HCCs. He had a history of distal gastrectomy for a gastric ulcer at 20 years of age. He had been treated with entecavir hydrate. The patient developed recurrent encephalopathy and esophageal varices hemorrhage in the year before the LT. His C-P score was grade C (11 points) and his MELD score was 16 points. Preoperative examination revealed three liver tumors (Fig. 2a and b) that were 20 mm or less in diameter, thereby meeting Milan criteria. Contrast-enhanced magnetic resonance image (MRI) revealed tumors with arterial phase hyper-enhancement and a washed-out appearance, typical of HCC. The preoperative examination also detected ascending colon cancer. Ileocecal resection was performed first. We determined that his cancer was early-stage, and LDLT was performed one month later. Pathological examination showed two viable liver tumors (Fig. 2c): one cHCC-CCA (Fig. 2d–f) and one HCC. He experienced no recurrences for over five years.
Fig. 2.
The cHCC-CCA nodule of Case 2. The computed tomography (CT) scan shows a small enhanced nodule (white arrow) in the portal phase (a). The nodule is not visible in the late phase (b). cHCC-CCA (white square) (c). Microscopically, the tumor consists of two components: CCA (e) and HCC (f).
2.4. Case 3: a 67-year-old woman
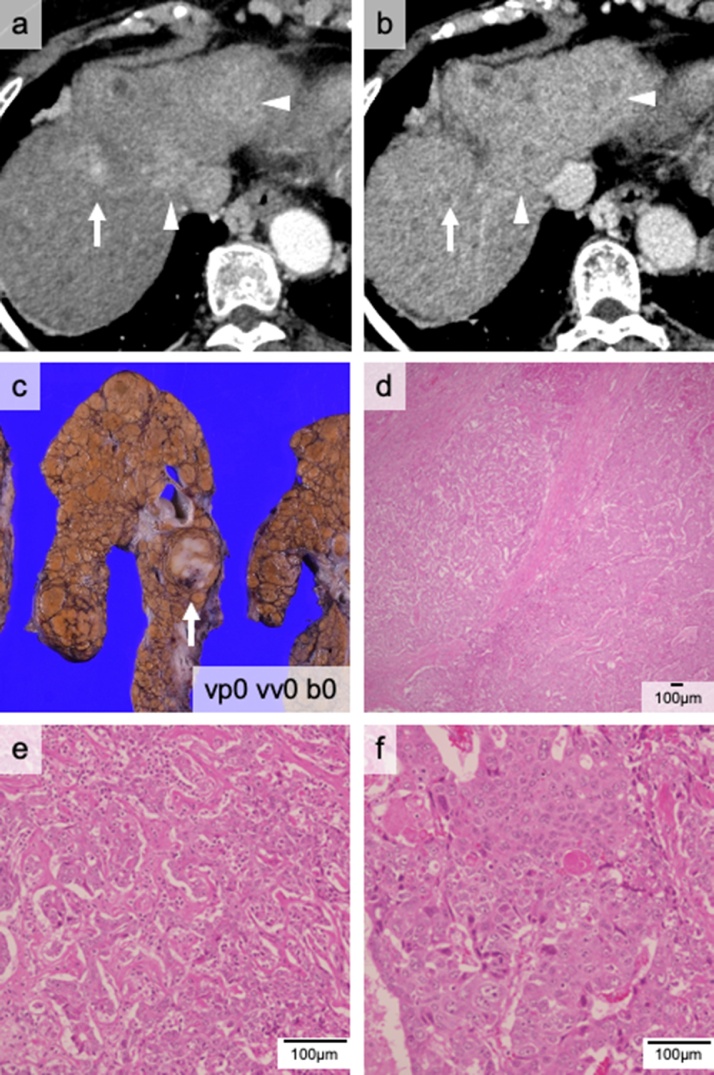
The patient had been followed for non-alcoholic steatohepatitis. She underwent a partial hepatectomy for an HCC at 59 years of age and had twice undergone radiofrequency ablation for HCC recurrence. She was referred to our institute for multiple liver tumors with worsening liver function. Preoperative CT examination suggested five tumors with a maximum diameter of 29 mm, thus not meeting Milan criteria (Fig. 3a and b). However, her DCP level was 380 mAU/mL, meeting Kyoto criteria (ten or fewer tumors, maximum tumor size of 5 cm or less, and DCP level of 400 mAU/mL or less), as it is an extended criterion for LDLT [11]. FDG-PET / CT didn’t detect a high SUV tumor, and contrast-enhanced MRI results were typical for HCC. Her C-P score was grade B (8 points). We performed a LDLT using a right liver graft. The postoperative pathological examination revealed three viable tumors with a maximum diameter of 30 mm. Therefore, she retrospectively met Milan criteria. One tumor was cHCC-CCA (21 mm in diameter); the other two were HCC (Fig. 3c–f). DCP and AFP levels, which are tumor markers, decreased to within the normal range postoperatively. She lived without tumor recurrence for approximately one year after LDLT.
Fig. 3.
The cHCC-CCA nodule of Case 3. The computed tomography (CT) scan indicates three tumors in portal (a) and late (b) phases. The tumor—indicated by the white arrow—is the cHCC-CCA nodule; the other tumors (arrowheads) are HCC nodules (c). Pathological findings indicate that the nodule consists of two components (d): CCA (e) and HCC (f).
2.5. Case 4: a 45-year-old man
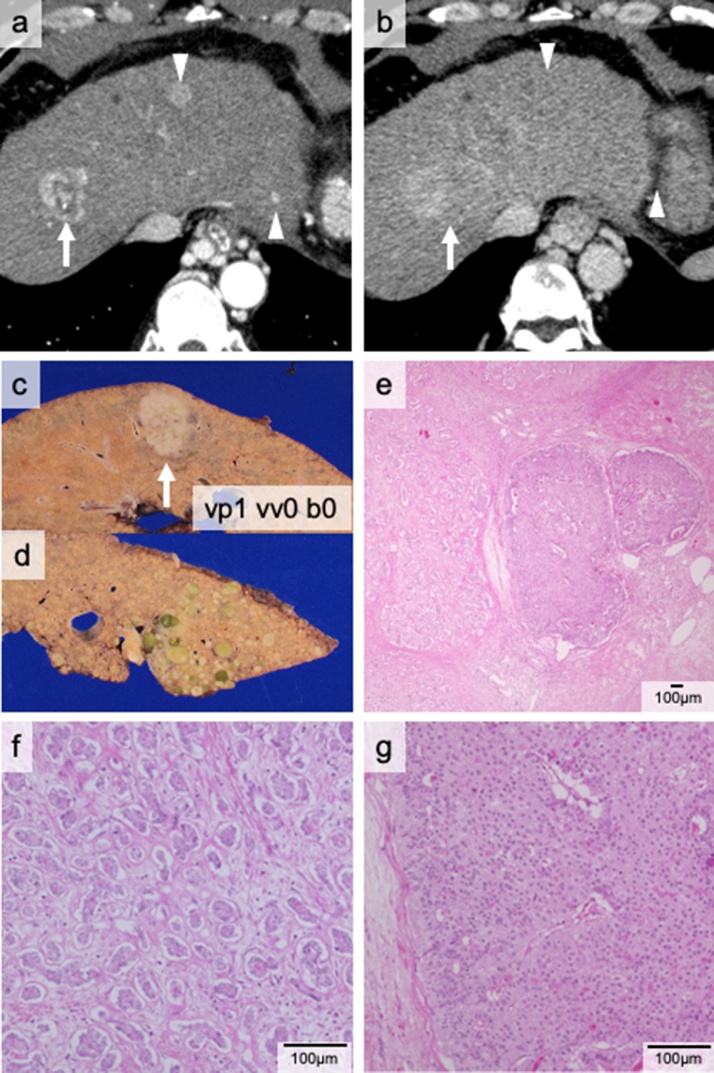
The patient was referred to our institute for alcoholic cirrhosis with multiple liver tumors. He underwent laparoscopic partial liver resection for HCC five years previously and also received transcatheter arterial chemoembolization for HCC recurrence. The preoperative CT examination revealed five classical HCC tumors (Fig. 4a and b). His C-P score was grade C (10 points) and his MELD score was 20 points. The patient’s AFP level was 642.3 ng/mL and his DCP level (18 mAU/mL) was within normal limits. His condition met Kyoto criteria and LDLT was performed using a right liver graft. Pathological examination revealed more than ten viable tumors in the recipient's liver. The largest tumor was diagnosed as cHCC-CCA (36 mm in diameter) (Fig. 4c and e–g); the other tumors were HCCs (Fig. 4d). Therefore, the pathological examination revealed retrospectively that his condition met neither Kyoto nor Milan criteria. The postoperative AFP level temporarily dropped to within the normal range; however, nine months after LDLT, his AFP and DCP levels were elevated (18.8 ng/mL and 80 mAU/mL, respectively), and CT examination revealed multiple liver tumors with hypervascularities, suggesting multiple HCC recurrence. He died of liver failure and HCC recurrence 15 months after the LDLT.
Fig. 4.
The cHCC-CCA nodule of Case 4. The computed tomography (CT) scan indicates three tumors in the portal (a) and late (b) phases. The arrow indicates the cHCC-CCA nodule while the arrowheads show HCC nodules. Macroscopically, the cHCC-CCA nodule—indicated by the arrow—is white (c), while the multiple HCC nodules are green (d). The microscopic findings show that the nodule consists of two components (e): CCA (f) and HCC (g), surrounded by a CCA component.
3. Discussion
The preoperative diagnosis of cHCC-CCA is difficult because it has few specific imaging characteristics [12]. The strategy for preoperative diagnosis of cHCC-CCA has been studied before [[13], [14], [15]], however, in the present study—in spite of the multimodal preoperative examination (PET/CT, MRI, or tumor markers, etc.)—all four patients received LDLT for preoperative diagnoses of multiple HCC tumors. Postoperative pathological examinations incidentally revealed that one of the multiple tumors was cHCC-CCA and that the remaining tumors were HCC. In this study, the cHCC-CCA characteristics were not captured, even after checking the images retrospectively. If these patients had been diagnosed with cHCC-CCA preoperatively would they have demonstrated indications for LT?
Some studies reported poor outcomes of LT for cHCC-CCA. Analyses based on large-scale data, including the Surveillance Epidemiology and End Results (SEER) and United Network for Organ Sharing (UNOS) databases suggested worse outcomes of LT for cHCC-CCA than for HCC. The analyses also indicated that the therapeutic effects of LT for cHCC-CCA were limited [[6], [7], [8]]. However, several reports showed that small cHCC-CCA tumors (2 cm or less), were associated with better prognosis [16,17]. Therefore, no conclusions have been reached regarding the LT indications for cHCC-CCA.
In general, patients with cHCC-CCA have background liver diseases [18,19]. Thus, LT for cHCC-CCA theoretically reduces the recurrence rate as well as LT does for HCC. All patients in this case series had serious liver dysfunction. Although the patient with tumors that met neither Millan nor Kyoto criteria relapsed immediately after LDLT, the three patients who met Milan criteria had no recurrence after LDLT. These findings suggested that cHCC-CCA was not an absolute contraindication for LT.
4. Conclusions
In this series, the patients with cHCC-CCA who met Milan criteria on postoperative pathological examination had no recurrence after LDLT. Thus, cHCC-CCA itself is not a contraindication for liver transplantation in patients with cirrhosis.
Declaration of Competing Interest
None.
Funding
This work was partially supported by the Grants-in-Aid for Scientific Research (19K09145).
Ethical approval
The study protocol was approved by the Ethics Committee of the Graduate School of Medicine, Kyoto University (R1473-2, and R1737), and performed in accordance with the 1964 Helsinki declaration and its later amendments.
Consent
The patients provided consent for publication.
Author contribution
TI was involved in the data collection, data analysis, and manuscript writing. TI was involved in data collection and analysis and is responsible for the manuscript. SS was involved in pathological analysis. SO, KF, TI, SY, SS, and KH was involved in date collection. KT and SU participated in critically revising the manuscript. All authors have read and approved the final version of the manuscript.
Registration of research studies
-
1
Name of the registry: Research Registry.
-
2
Unique identifying number or registration ID: researchregistry5626.
-
3
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browse-the-registry#home/.
Guarantor
The guarantor of this study is TI.
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
Takashi Ito: Data curation, Investigation, Writing - original draft. Takamichi Ishii: Conceptualization, Methodology, Writing - review & editing. Shinji Sumiyoshi: Data curation, Visualization. Satoshi Ogiso: Investigation. Ken Fukumitsu: Investigation. Takashi Ito: Investigation. Shintaro Yagi: Investigation. Satoru Seo: Investigation. Koichiro Hata: Investigation. Kojiro Taura: Methodology. Shinji Uemoto: Supervision.
Acknowledgement
The authors would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Llovet J.M., Zucman-Rossi J., Pikarsky E., Sangro B., Schwartz M., Sherman M. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 2.Allen R.A., Lisa J.R. Combined liver cell and bile duct carcinoma. Am. J. Pathol. 1949;25(4):647–655. [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman Z.D., Ishak K.G., Langloss J.M., Sesterhenn I.A., Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55(1):124–135. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Sempoux C., Kakar S., Kondo F., Schirmacher P. Combined hepatocellular-cholangiocarcinoma and undifferentiated primary liver carcinoma. In: Arends M.J., Fukuyama M., Klimstra D.S., editors. WHO Classification of Tumours: Digestive System Tumours, 5th. IARC; Lyon: 2019. p. 260. [Google Scholar]
- 5.Chang C.C., Chen Y.J., Huang T.H., Chen C.H., Kuo F.Y., Eng H.L. Living donor liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma: experience of a single center. Ann. Transplant. 2017;22:115–120. doi: 10.12659/aot.900779. [DOI] [PubMed] [Google Scholar]
- 6.Groeschl R.T., Turaga K.K., Gamblin T.C. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. J. Surg. Oncol. 2013;107(6):608–612. doi: 10.1002/jso.23289. [DOI] [PubMed] [Google Scholar]
- 7.Garancini M., Goffredo P., Pagni F., Romano F., Roman S., Sosa J.A. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20(8):952–959. doi: 10.1002/lt.23897. [DOI] [PubMed] [Google Scholar]
- 8.Vilchez V., Shah M.B., Daily M.F., Pena L., Tzeng C.W., Davenport D. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford) 2016;18(1):29–34. doi: 10.1016/j.hpb.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., SCARE Group The PROCESS 2018 statement: updating consensus Preferred Reporting of CasE Series in Surgery (PROCESS) guidelines. Int. J. Surg. 2018;60:279–282. doi: 10.1016/j.ijsu.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Ito T., Takada Y., Ueda M., Haga H., Maetani Y., Oike F. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13(12):1637–1644. doi: 10.1002/lt.21281. [DOI] [PubMed] [Google Scholar]
- 12.Maximin S., Ganeshan D.M., Shanbhogue A.K., Dighe M.K., Yeh M.M., Kolokythas O. Current update on combined hepatocellular-cholangiocarcinoma. Eur. J. Radiol. Open. 2014;1:40–48. doi: 10.1016/j.ejro.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panjala C., Senecal D.L., Bridges M.D., Kim G.P., Nakhleh R.E., Nguyen J.H. The diagnostic conundrum and liver transplantation outcome for combined hepatocellular-cholangiocarcinoma. Am. J. Transplant. 2010;10(5):1263–1267. doi: 10.1111/j.1600-6143.2010.03062.x. [DOI] [PubMed] [Google Scholar]
- 14.Potretzke T.A., Tan B.R., Doyle M.B., Brunt E.M., Heiken J.P., Fowler K.J. Imaging features of biphenotypic primary liver carcinoma (Hepatocholangiocarcinoma) and the potential to mimic hepatocellular carcinoma: LI-RADS analysis of CT and MRI features in 61 cases. AJR Am. J. Roentgenol. 2016;207(1):25–31. doi: 10.2214/AJR.15.14997. [DOI] [PubMed] [Google Scholar]
- 15.Fowler K.J., Sheybani A., Parker R.A., Doherty S., Brunt E.M., Chapman W.C. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am. J. Roentgenol. 2013;201(2):332–339. doi: 10.2214/AJR.12.9488. [DOI] [PubMed] [Google Scholar]
- 16.Jung D.H., Hwang S., Song G.W., Ahn C.S., Moon D.B., Kim K.H. Longterm prognosis of combined hepatocellular carcinoma-cholangiocarcinoma following liver transplantation and resection. Liver Transpl. 2017;23(3):330–341. doi: 10.1002/lt.24711. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R., Togashi J., Akamatsu N., Sakamoto Y., Kokudo N. Impact of incidental/misdiagnosed intrahepatic cholangiocarcinoma and combined hepatocellular cholangiocarcinoma on the outcomes of liver transplantation: an institutional case series and literature review. Surg. Today. 2017;47(8):908–917. doi: 10.1007/s00595-017-1472-3. [DOI] [PubMed] [Google Scholar]
- 18.Yin X., Zhang B.H., Qiu S.J., Ren Z.G., Zhou J., Chen X.H. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann. Surg. Oncol. 2012;19(9):2869–2876. doi: 10.1245/s10434-012-2328-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.H., Hsieh S.Y., Chang C.J., Lin Y.J. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J. Gastroenterol. Hepatol. 2013;28(1):122–127. doi: 10.1111/j.1440-1746.2012.07289.x. [DOI] [PubMed] [Google Scholar]