Abstract
Neuronal intranuclear inclusion disease (NIID) is a rare, neurodegenerative disorder characterized by the presence of eosinophilic hyaline intranuclear inclusions, which are ubiquitin-positive and p62-positive, in neuronal and somatic cells; this can be observed on skin biopsy. Although patients with NIID present with a variety of symptoms that often make the diagnosis difficult, characteristic high-signal intensity of the corticomedullary junction on diffusion-weighted imaging (DWI) often provides a clue to the diagnosis of NIID. We present a case of NIID in a 57-year-old woman who only had recurrent vomiting for four years, which is uncommon as the presenting symptom; moreover, DWI showed no apparent abnormality until a slightly abnormal intensity lesion appeared at the right frontal corticomedullary junction seven years after the first episode of recurrent vomiting. Skin biopsies revealed multiple p62-positive nuclear inclusions, and genetic test showed GGC repeat expansion in NOTCH2NLC; this may form the genetic basis for NIID. Retrospectively, we found that abnormal cerebellar signals besides the vermis in the fluid attenuation inversion recovery (FLAIR) images were detected early-on in the disease. Periodic vomiting may be the only symptom of NIID in the early stages of the disease, and cerebellar abnormalities in FLAIR may serve as an important finding in the diagnosis of NIID, even in the absence of characteristic clinical symptoms or abnormal DWI signals at the cerebral corticomedullary junction.
Keywords: Antibody, Immune disorder, Immune response, Immunoglobulin, Immunology, Inflammation, Nervous system, Neurology, Neuroscience, Neuronal intranuclear inclusion disease, Recurrent vomiting, Diffusion-weighted imaging, Cerebellum, Leukoencephalopathy
Antibody; Immune disorder; Immune response; Immunoglobulin; Immunology; Inflammation; Nervous system; Neurology; Neuroscience; Neuronal intranuclear inclusion disease; Recurrent vomiting; Diffusion-weighted imaging; Cerebellum; Leukoencephalopathy
1. Introduction
Neuronal intranuclear inclusion disease (NIID) is a slowly progressive neurodegenerative disorder characterized by the presence of eosinophilic hyaline intranuclear inclusions, which are also ubiquitin-positive and p62-positive, in neuronal and somatic cells [1]. In 2019, the causative gene of NIID was found to be a GGC repeat expansion in the 5′ region of the NOTCH2NLC gene, which could be read by long-read sequencing, and has been used in the diagnosis of NIID [2]. NIID patients present with a wide variety of clinical manifestations, which account for the difficulty in diagnosing NIID [1]. In recent years, confirming intranuclear inclusions by skin biopsies, along with observing high-signal intensity of the corticomedullary junction on diffusion-weighted imaging (DWI), have emerged as useful diagnostic tools for NIID [3, 4]. This DWI abnormality, which is highly specific and sensitive to NIID, has been reported to become stronger or weaker during the disease course [5, 6]. Here, we report a woman with NIID who had recurrent vomiting, a rare symptom of NIID. This was her predominant symptom, and her DWI showed no apparent abnormality at the corticomedullary junction for the first seven years.
2. Case report
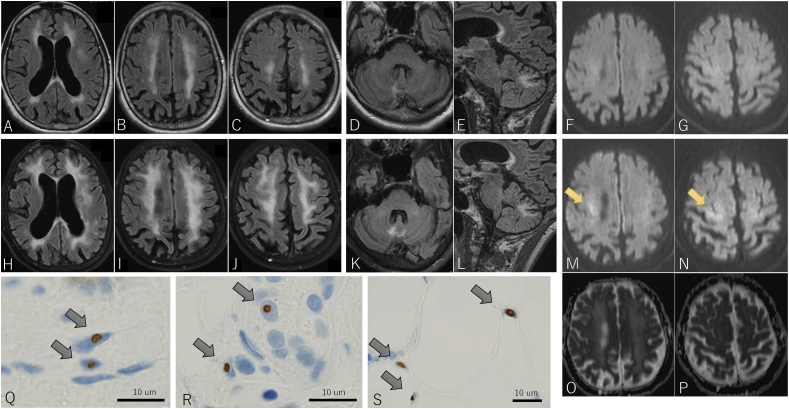
A 57-year-old woman was referred to our department for consultation because of a brain abnormality on magnetic resonance imaging (MRI). She was healthy, except for recurrent vomiting, which would occur when she was busy and last for approximately two weeks. This pattern had been repeating since she was 53 years old. Eleven days before consultation, she experienced sudden nausea and vomiting. She continued to vomit and, due to dehydration, was admitted to our hospital nine days before consultation. Her nausea was unresponsive to antiemetics, and she repeatedly vomited several times an hour, even when there was nothing left to throw up. Enhanced computed tomography (CT) scan was performed, but it did not reveal any abnormalities in the gastrointestinal tract. Brain MRI taken to investigate the cause of her vomiting demonstrated cerebral atrophy and leukoencephalopathy (Figure 1A–C). Fluid attenuation inversion recovery (FLAIR) images demonstrated high-signal intensity in the right cerebellar hemisphere, beside the vermis, in addition to cerebral leukoencephalopathy (Figure 1D, E). There were no abnormal findings on DWI (Figure 1F, G). Her older sister had a similar medical history; she vomited repeatedly in her 40s, was diagnosed with leukoencephalopathy at age 51, became bedridden at age 52, lost her hearing and sight at age 55, and died of aspiration pneumonia at age 57. No autopsy was performed, and a diagnosis of leukoencephalopathy was made. Our patient had no children, and her parents who were deceased did not show similar symptoms.
Figure 1.
Brain MRI findings at the age of 57 (A–G) and 60 (H–P), and skin biopsy findings (Q–S). Fluid-attenuated inversion recovery (FLAIR) image shows cerebellar atrophy and a high intensity lesion in the cerebral white matter (A–C). FLAIR image shows high intensity lesion in the right cerebellar hemisphere near the vermis (D, E). Diffusion-weighted imaging (DWI) shows no obvious abnormality (F, G). FLAIR images show progressive cerebral atrophy, an enlarged high intensity lesion in the cerebral white matter (H–J), and an enlarged high intensity lesion in the cerebral hemisphere (K, L). DWI shows a small high intensity lesion in the corticomedullary junction in the right prefrontal cortex (M, N, arrows) with slightly high signal on apparent diffusion coefficient (ADC) maps (O, P). P62-positive nuclear inclusion bodies are found in fibroblasts (Q), sweat gland cells (R), and adipocytes (S).
Our current patient was emaciated, but she was alert and her Mini-Mental State Examination (MMSE) and Addenbrooke's Cognitive Examination Revised (ACE-R) were within normal limits (30/30 and 98/100, respectively). Tendon reflexes in the lower extremities were decreased. She could walk smoothly, but she had position sense disturbance apparent in both toes and could not stand on one foot. Nerve conduction study revealed mild motor and sensory conduction delay. Considering the possibility of mitochondrial disease from her physical and examination findings, she was treated with oral ubidecarenone for outpatient follow-up.
At the age of 58, she had urinary retention; however, her neurological and brain MRI findings were largely unchanged. At age 60, she was brought to our hospital again with severe vomiting. She had emaciation, fever of 38.6 °C, and sinus tachycardia of 115 bpm. She had difficulty standing, even with a broad base, due to the weakness of proximal lower extremities and worsening of position sense disturbance. There was no miosis, dysarthria, or limb ataxia. Her cognitive function had deteriorated; MMSE was 28/30 and ACE-R was 79/100. FLAIR image demonstrated progression of atrophy and of leukoencephalopathy (Figure 1H–J) and expansion of the abnormal intensity lesion in her cerebellum (Figure 1K, L). On DWI, small high intensity lesions appeared at the right frontal corticomedullary junction (Figure 1M, N, arrow), with slightly high signal on apparent diffusion coefficient (ADC) maps (Figure 1O, P), which suggested NIID. Skin biopsies revealed multiple p62-positive nuclear inclusions in fibroblasts, sweat gland cells, and adipocytes (Figure 1Q–S, arrows). Blood genetic test revealed GGC repeat expansion in NOTCH2NLC, and the diagnosis of NIID was made.
2.1. Consent
We obtained written informed consent from the patient.
3. Discussion
The important clinical issue in this case was that the patient presented with recurrent vomiting as her predominant symptom, which is rare in NIID. Moreover, she did not demonstrate typical DWI findings for the first seven years. It was suggested that these clinical features may have prolonged the diagnosis.
The majority of NIID cases present with a combination of several symptoms, including progressive dementia in sporadic cases (94.7%) and muscle weakness in familial cases (94.7%). Only approximately 15–30% of NIID cases present with vomiting [1], and there are few reports of NIID cases in which recurrent vomiting presents as one of the main symptoms [7, 8]. On initial admission, except for weak ataxia that she did not notice, our patient presented with only recurrent vomiting, and such a case of NIID has not been reported previously. Periodic vomiting in NIID is considered to be an autonomic symptom [7, 8]. In the present case, since there was no gastrointestinal abnormality on the CT scan, since fatigue was a trigger, and since urinary retention appeared later, the possibility of autonomic symptoms was considered. As physicians, we must become more aware that NIID patients may present with recurrent vomiting as their cardinal symptom.
Our case did not demonstrate characteristic, abnormal DWI findings. Seven years after presenting with periodic vomiting, only small high intensity lesions were found. Abnormal DWI in the cerebral corticomedullary junction has proved to be highly sensitive for detecting NIID (100% of sporadic cases and 81% of familial cases) [1]. However, some cases where abnormal findings of DWI are not apparent, some of which are due to signal changes during the disease course, have been reported [5, 6, 9]. Therefore, it should be considered that NIID patients may not produce apparent DWI abnormalities, depending on the timing of imaging. It was reported that cerebellar abnormalities, including high-signal intensity on FLAIR in the cerebellar hemisphere besides the vermis, are specific findings in NIID [10]. This cerebellar abnormality was confirmed in our case even when there were no DWI abnormalities, which indicates that abnormal cerebellar findings on FLAIR may be effective for diagnosing NIID, regardless of the absence of DWI abnormalities. However, it has been reported that high-signal intensity on FLAIR in the cerebellar hemisphere, besides the vermis, can also be observed in patients with fragile X–associated tremor/ataxia syndrome (FXTAS) [11]. Moreover, NIID and FXTAS have similar clinical presentation, family history, and pathologic changes showing intranuclear eosinophilic inclusions [12]. However, there are some differences between these two diseases, such as the localization of neuronal loss, the presence of oligodendrocyte inclusions, and the findings of skin biopsy specimens [13]. In order to distinguish NIID from FXTAS, it is important to perform a detailed histopathological study including skin biopsy and genetic testing.
Family history of our case was a limitation. Her older sister's medical history suggested a possibility of familial NIID; however, we could not confirm it because the older sister had not undergone an autopsy. Because familial NIID may show characteristics different from those of sporadic NIID [1], our case may have presented with symptoms and DWI findings that are uncommon in sporadic NIID.
4. Conclusion
In summary, we encountered a patient with NIID with recurrent vomiting as the predominant symptom without apparent DWI abnormalities for the first seven years. Even if the diagnosis of NIID is difficult based on clinical symptoms and DWI findings, cerebellar abnormality on FLAIR may help in the diagnosis of NIID.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Dr. Shinji Kagami for the skin biopsy.
References
- 1.Sone J., Mori K., Inagaki T., Katsumata R., Takagi S., Yokoi S. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. 2016;139:3170–3186. doi: 10.1093/brain/aww249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sone J., Mitsuhashi S., Fujita A., Mizuguchi T., Hamanaka K., Mori K. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat. Genet. 2019;51:1215–1221. doi: 10.1038/s41588-019-0459-y. [DOI] [PubMed] [Google Scholar]
- 3.Sone J., Tanaka F., Koike H., Inukai A., Katsuno M., Yoshida M. Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology. 2011;76:1372–1376. doi: 10.1212/WNL.0b013e3182166e13. [DOI] [PubMed] [Google Scholar]
- 4.Sone J., Kitagawa N., Sugawara E., Iguchi M., Nakamura R., Koike H. Neuronal intranuclear inclusion disease cases with leukoencephalopathy diagnosed via skin biopsy. J. Neurol. Neurosurg. Psychiatry. 2014;85:354–356. doi: 10.1136/jnnp-2013-306084. [DOI] [PubMed] [Google Scholar]
- 5.Kawarabayashi T., Nakamura T., Seino Y., Hirohata M., Mori F., Wakabayashi K. Disappearance of MRI imaging signals in a patient with neuronal intranuclear inclusion disease. J. Neurol. Sci. 2018;388:1–3. doi: 10.1016/j.jns.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Chen L., Wu L., Li S., Huang Q., Xiong J., Hong D. A long time radiological follow-up of neuronal intranuclear inclusion disease: two case reports. Medicine (Baltim.) 2018;97 doi: 10.1097/MD.0000000000013544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sone J., Hishikawa N., Koike H., Hattori N., Hirayama M., Nagamatsu M. Neuronal intranuclear hyaline inclusion disease showing motor-sensory and autonomic neuropathy. Neurology. 2005;65:1538–1543. doi: 10.1212/01.wnl.0000184490.22527.90. [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Liu X., Du Y., Lin Y., Li C., Liu C. A case of recurrent vomiting: extending the spectrum of neuronal intranuclear inclusion disease. Neurol. Sci. 2019;40:2661–2664. doi: 10.1007/s10072-019-03986-1. [DOI] [PubMed] [Google Scholar]
- 9.Okubo M., Doi H., Fukai R., Fujita A., Mitsuhashi S., Hashiguchi S. GGC repeat expansion of NOTCH2NLC in adult patients with leukoencephalopathy. Ann. Neurol. 2019;86:962–968. doi: 10.1002/ana.25586. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama A., Sato N., Kimura Y., Maekawa T., Enokizono M., Saito Y. MR imaging features of the cerebellum in adult-onset neuronal intranuclear inclusion disease: 8 cases. Am. J. Neuroradiol. 2017;38:2100–2104. doi: 10.3174/ajnr.A5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilha I.G., Nunes R.H., Scortegagna F.A., Pedroso J.L., Marussi V.H., Rodrigues M.R. MR imaging features of adult-onset neuronal intranuclear inclusion disease may be indistinguishable from fragile X-associated tremor/ataxia syndrome. AJNR Am. J. Neuroradiol. 2018;39:E100–101. doi: 10.3174/ajnr.A5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelpi E., Botta-Orfila T., Bodi L., Marti S., Kovacs G., Grau-Rivera O. Neuronal intranuclear (hyaline) inclusion disease and fragile X-associated tremor/ataxia syndrome: a morphological and molecular dilemma. Brain. 2017;140:e51. doi: 10.1093/brain/awx156. [DOI] [PubMed] [Google Scholar]
- 13.Sone J., Nakamura T., Koike H., Katuno M., Tanaka F., Iwasaki Y. Reply: neuronal intranuclear (hyaline) inclusion disease and fragile X-associated tremor/ataxia syndrome: a morphological and molecular dilemma. Brain. 2017;140:e52. doi: 10.1093/brain/awx158. [DOI] [PubMed] [Google Scholar]