Summary
IL-7 receptor signaling is essential for the generation and maintenance of conventional T cells. Immunosuppressive Foxp3+ Treg cells, however, express uniquely low amounts of the IL-7-proprietary IL-7Rα so that they are impaired in IL-7 signaling. Because Treg cells depend on IL-2, the loss of IL-7Rα has been considered irrelevant for Treg cells. In contrast, here, we report that IL-7Rα downregulation is necessary to maximize IL-2R signaling. Although IL-7Rα overexpression promoted IL-7 signaling, unexpectedly, IL-2 signaling was suppressed in the same cells. Mechanistically, we found that γc, which is a receptor subunit shared by IL-7R and IL-2R, directly binds and pre-associates with IL-7Rα, thus limiting its availability for IL-2R binding. Consequently, overexpression of signaling-deficient, tailless IL-7Rα proteins inhibited IL-2R signaling, demonstrating that IL-7Rα sequesters γc and suppresses IL-2R signaling by extracellular interactions. Collectively, these results reveal a previously unappreciated regulatory mechanism of IL-2 receptor signaling that is governed by IL-7Rα abundance.
Subject Areas: Molecular Biology, Immunology
Graphical Abstract

Highlights
-
•
The availability of γc is limited, so that IL-7Rα and IL-2Rβ compete for γc to signal
-
•
IL-7Rα has high affinity for γc, and it outcompetes IL-2Rβ for binding to γc
-
•
Foxp3+ Treg cells express low amounts of IL-7Rα, which frees γc for IL-2Rβ binding
-
•
Forced IL-7Rα expression sequesters γc and impairs IL-2R signaling in Treg cells
Molecular Biology; Immunology
Introduction
Interleukin-7 (IL-7) signaling is critical for T cell development and homeostasis, as demonstrated by impaired thymopoiesis and survival defects in T cells of IL-7- or IL-7R-deficient mice (Von Freeden-Jeffry et al., 1995, Peschon et al., 1994; Hong et al., 2014; Fry and Mackall, 2001). IL-7 signaling is transduced through the heterodimeric IL-7 receptor, which is composed of a common γ-chain (γc) that is shared with IL-2 and other γc family cytokines (Waickman et al., 2015; Rochman et al., 2009) and the IL-7-proprietary IL-7Rα-chain (IL-7Rα) (Park et al., 2000). Signaling by IL-7 is triggered by ligand-induced dimerization of IL-7Rα and γc, which results in conformational changes of the receptor subunits and the transactivation of the receptor-associated kinases JAK1 and JAK3 (Wang et al., 2009). This event is followed by tyrosine phosphorylation of the IL-7Rα intracellular domain and the recruitment and activation of downstream signaling molecules, such as STAT5a/b and the PI-3 kinase (Waickman et al., 2015; Rochman et al., 2009). IL-7 signaling in T cells is important because it upregulates the expression of anti-apoptotic genes, such as Bcl2 and Mcl1, and promotes the expression of trophic proteins such as glucose transporter-1 (Surh and Sprent, 2008; Yu et al., 2003; Rathmell et al., 2001). Excessive or prolonged IL-7 signaling, on the other hand, can result in cytotoxicity (Kimura et al., 2013) or lymphomas (Mertsching et al., 1995), so that the timing and magnitude of IL-7 signaling are tightly controlled during T cell development and differentiation.
A major mechanism by which IL-7 signaling is fine-tuned in T cells is based on the highly dynamic expression of IL-7Rα. During T cell development in the thymus, the earliest T cell precursors express large amounts of IL-7Rα proteins, which is required for the cells to transition from the double-negative (DN) stage into immature CD4, CD8 double-positive (DP) cells (Hong et al., 2012; Akashi et al., 1997, Von Freeden-Jeffry et al., 1997). In DP thymocytes, however, IL-7Rα expression ceases and is only re-induced upon TCR-signaling-mediated positive selection (Yu et al., 2006). Moreover, assessments of IL-7Rα regulation in mature T cells revealed that TCR activation and signaling by IL-7 and other γc family cytokines can downregulate IL-7Rα expression (Park et al., 2004; Xue et al., 2002), whereas, TGF-β, TNF-α, and glucocorticoids can induce IL-7Rα gene expression (Ouyang et al., 2013; Johnson and Jameson, 2012; Park et al., 2004; Lee et al., 2005). Mechanistically, several nuclear factors that control IL-7Rα expression in T cells have been identified: the transcription factors GABP, Runx1, Foxo1, and glucocorticoid receptors upregulate IL-7Rα gene expression (Egawa et al., 2007; Xue et al., 2004; Lee et al., 2005; Kerdiles et al., 2009), whereas the transcriptional repressors Foxp1 and Gfi1 suppress IL-7Rα expression (Feng et al., 2011; Ligons et al., 2012; Park et al., 2004). Thus, IL-7Rα expression is determined by a complex regulatory network that adjusts the amount of IL-7Rα protein in accordance with its IL-7 requirement in a cell- and stage-specific manner.
Although all conventional T cells express high levels of IL-7Rα, Foxp3+ CD25+ regulatory T cells (Tregs) are distinct, as they express uniquely low levels of cell surface IL-7Rα (Seddiki et al., 2006; Liu et al., 2006; Cozzo et al., 2003). In fact, the loss of IL-7Rα is such a distinguishing trait of Foxp3+ Treg cells that it is now routinely used to identify Treg cells among human peripheral blood lymphocytes with high accuracy and reproducibility (Banham, 2006). However, the molecular mechanism that suppresses IL-7Rα expression on Treg cells and the biological significance of IL-7Rα downregulation remain unclear. Initially, IL-7Rα expression was thought to be suppressed on Foxp3+ Treg cells as a consequence of strong TCR/CD28 stimulation that is necessary for thymic Treg cell generation (Tai et al., 2005; Cozzo et al., 2003). Alternatively, it was proposed that Foxp3 proteins could directly inhibit Il7r promoter activity, as indicated by Foxp3-ChIP assay results (Liu et al., 2006). However, whether IL-7Rα downregulation is an epiphenomenon of Foxp3+ Treg cell differentiation or whether the suppression of IL-7Rα expression in Foxp3+ Treg cells has a functional role in Treg cell biology has not been determined.
Here, we addressed this question by generating and analyzing Foxp3+ Treg cells that express high levels of IL-7Rα. We came to the surprising conclusion that the downregulation of IL-7Rα expression is not only associated with Foxp3+ Treg cell differentiation but also with maximizing IL-2 receptor signaling in Treg cells. Because Foxp3+ Treg cells primarily utilize IL-2, and not IL-7, for their generation and survival (Fontenot et al., 2005), some studies considered IL-7 signaling to be superfluous and not a factor that interfered with Treg cell function (Peffault De Latour et al., 2006). In contrast to this supposition, our results showed that forced IL-7Rα expression strongly interfered with IL-2 receptor signaling in Treg cells, and that it significantly blunted downstream STAT5 phosphorylation. Notably, the diminished IL-2 responsiveness induced by increased abundance of IL-7Rα was not due to a decrease in IL-2 receptor expression. Instead, we propose that unliganded IL-7Rα and γc proteins pre-associate through their extracellular domains to sequester γc from other cytokine proprietary receptors, such as IL-2Rβ. In support of this hypothesis, we found that recombinant IL-7Rα ectodomain proteins directly bind to surface γc even in the absence of IL-7 and that a signaling-incompetent tailless IL-7Rα construct was able to impair IL-2 receptor signaling in Treg cells. Collectively, these findings highlight a novel regulatory mechanism of cytokine receptor cross talk that utilizes IL-7Rα expression as a means to control γc cytokine responsiveness.
Results
γc Binds IL-7Rα Independently of IL-7
To assess whether γc availability limits the cytokine responsiveness of CD4 T cells, we quantified the number of cell surface γc, IL-7Rα, and IL-2Rβ cytokine receptors using fluorescence-conjugated antibodies and fluorescent beads (Figure 1A) (Gonnord et al., 2018). Saturating amounts of phycoerythrin (PE)-conjugated anti-γc, anti-IL-7Rα, and anti-IL-2Rβ monoclonal antibodies were used to stain freshly isolated LN CD4 T cells, and then the mean fluorescence intensity (MFI) of each stained sample was assessed to calculate the number of receptors based on standard curves. Standard curves were generated by flow cytometric data from the analysis of latex beads coated with a predetermined number of PE molecules and by plotting the number of PE molecules versus the MFI of the individual beads (Figure S1) (Gonnord et al., 2018).
Figure 1.
Pre-assembly of γc Cytokine Receptor Complexes on Mature T Cells
(A) Surface IL-7Rα proteins outnumber γc proteins on naive CD4+ T cells. Surface γc, IL-7Rα, and IL-2Rβ proteins were quantified on naive (CD44lo) Foxp3– CD4+ T cells from Foxp3-EGFP reporter mice. Saturating concentrations of PE-conjugated antibodies and a standard curve generated using Quantum R-PE MESF beads were used to determine the number of receptor number per cell. Bar graphs show summary of three independent experiments with three mice and mean and SEM are shown.
(B) SPR analysis of IL-7Rα binding to γc. Binding sensorgrams (black lines) of IL-7Rα over immobilized γc are displayed and globally fit to a single step kinetic model (red lines) to determine the kon and koff rate constants.
(C) Binding sensorgrams of IL-2Rβ over an immobilized γc sensor chip. The inset shows a dose-response curve plotting the maximal responses (Rmax, depicted by the dashed boxes) for each IL-2Rβ concentration. The plot of Rmax values versus IL-2Rβ concentrations was non-linearly fit to a single-site binding affinity model to determine a Kd value.
See also Figure S1.
Using this assay, we found that the number of cytokine receptor molecules substantially differed among each sample and that IL-7Rα was abundant (~4,300 molecules/cell), but IL-2Rβ was only minimally expressed (~75 molecules/cell) on naive CD4 T cells (Figure 1A). The γc receptor itself, on the other hand, was present at ~2,300 molecules per cell, a quantity significantly less than that of IL-7-proprietary IL-7Rα but vastly more than that of IL-2Rβ (Figure 1A). A corollary of this observation is that γc availability would differ depending on the cytokine; therefore, γc is not a limiting factor for IL-2Rβ signaling but is insufficient for maximal ligand binding and signaling of IL-7Rα. Altogether, these results demonstrate a molecular stoichiometry among γc family cytokine receptors in which γc availability is not static but varies depending on the cytokine of interest.
An underlying assumption in determining γc availability is based on γc being freely available and not pre-associated with other molecules that could impede γc recruitment to proprietary receptors. However, there is an increasing body of evidence indicating that this is not the case. Structural studies together with protein binding assays have demonstrated cytokine-free binding of γc to proprietary cytokine receptors (Gonnord et al., 2018, Mcelroy et al., 2012). In fact, we previously demonstrated that IL-7Rα can directly bind and sequester γc proteins, making them unavailable to other proprietary receptors (Hong et al., 2014; Park et al., 2016). The pre-association of γc with its proprietary receptors raises an interesting quandary: low-abundance receptors, such as IL-2Rβ, should be disadvantaged compared with abundantly expressed receptors, such as IL-7Rα, when competing for γc. Therefore, we thought it important to understand γc availability in the context of cytokine receptor pre-association, which alters the accessibility of γc to cytokine proprietary receptors.
To directly visualize the propensity of γc to associate with IL-2Rβ and IL-7Rα, we measured the binding affinities of the extracellular domains (ECDs) of these receptors using surface plasmon resonance (SPR). The IL-7Rα ECD binds the immobilized γc ECD with a kon (on-rate) of 5.93 ± 0.28 x 103 M−1 s−1 and a koff (off-rate) of 2.21 ± 0.18 x 10−4 s−1, yielding a Kd of 37.3 ± 0.4 nM (Figure 1B). The recombinant IL-2Rβ ECD binds immobilized γc ECD with dramatically weaker binding affinity of 577 ± 40 μM (Figure 1C). Altogether, IL-7Rα ECD binds γc more than 15,000-fold stronger than the IL-2Rβ ECD. Thus, the vastly higher affinity of IL-7Rα for γc, combined with its dramatically greater abundance than IL-2Rβ on CD4 T cells, suggests that IL-7Rα outcompetes IL-2Rβ in accessing γc. In support of this idea, IL-2 receptor signaling in naive CD4 T cells was highly inefficient, and these cells did not respond to IL-2 or to IL-15 (Cho et al., 2010).
Foxp3 Suppresses IL-7Rα Transcription and Expression in Foxp3+ Treg Cells
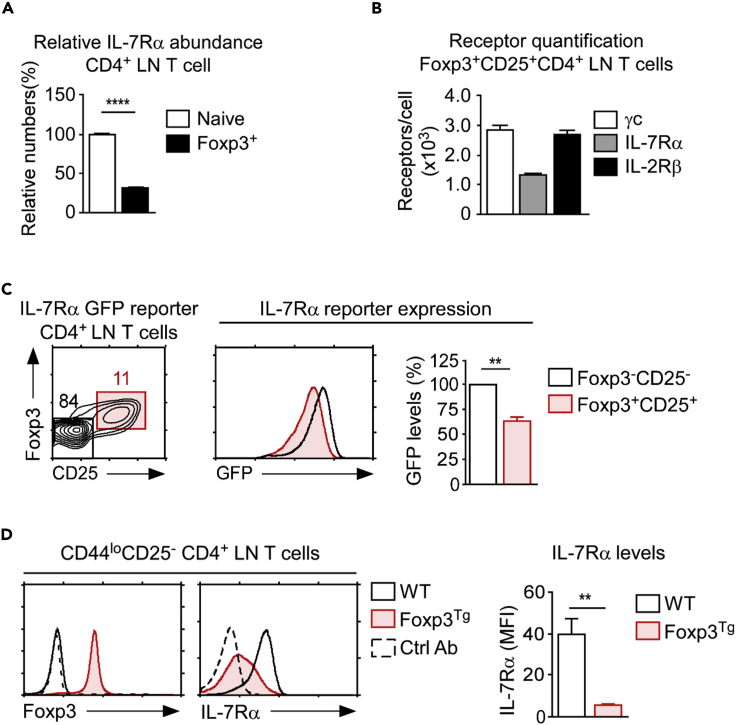
Because the survival of conventional CD4 T cells depends on IL-7 and not on IL-2 (Fry and Mackall, 2001; Surh and Sprent, 2008), a lack of IL-2 signaling can be expected to minimally affect the homeostasis of peripheral CD4 T cells. In contrast to conventional CD4 T cells, however, Foxp3+ T regulatory CD4 T cells (Tregs) require IL-2 for their development and survival (Fontenot et al., 2005). This selective IL-2 requirement raises the question of how Foxp3+ Treg cells deal with γc sequestration by IL-7Rα, which impedes IL-2 signaling. Here, we considered that Foxp3+ Treg cells are uniquely low in IL-7Rα expression compared with conventional CD4 T cells (Figure 2A) (Seddiki et al., 2006; Liu et al., 2006; Cozzo et al., 2003). We further hypothesized that this loss in IL-7Rα numbers would lead to the release of pre-associated γc and increase its availability for IL-2 receptor signaling. Indeed, results from cytokine receptor quantification supported this idea, as we found that the number of IL-7Rα molecules on Foxp3+ Treg cells was reduced to such a low level that they were outnumbered by γc proteins (Figure 2B). On the other hand, IL-2Rβ expression was increased to such level that it was greater than that of IL-7Rα (Figure 2B). As a corollary, a substantial fraction of γc molecules would be freely available to IL-2Rβ for IL-2 binding and signaling. We propose that the downregulation of IL-7Rα is a newly identified mechanism by which pre-associated γc is freed up for use in IL-2 signaling of Foxp3+ Treg cells.
Figure 2.
Foxp3 Suppresses IL-7Rα Transcription and Cell Surface Expression
(A) Relative expression of surface IL-7Rα on naive CD4+ LN T cells and Foxp3+ CD4+ Treg cells in Foxp3-EGFP reporter mice. Bar graphs show summary of three independent experiments with three mice with mean and SEM shown. Unpaired two-tailed Student's t test, where ∗∗∗∗p < 0.0001.
(B) γc, IL-7Rα, and IL-2Rβ protein expression was quantified on Foxp3+ CD4+ Treg cells as identified as EGFP+ CD4+ LN T cells from Foxp3-EGFP reporter mice. Saturating concentration of PE-conjugated antibodies to each cytokine receptor and a standard curve generated by Quantum R-PE MESF beads were used to determine the receptor number per cell. Bar graphs show summary of three independent experiments with three mice and mean with SEM shown.
(C) Reduced IL-7Rα-reporter activity in Foxp3+ Treg cells. IL-7Rα-transcription was assessed in Foxp3+CD25+ Treg cells from IL-7Rα-GFP mice (Ligons et al., 2012) (left). GFP levels were assessed in CD4+ T cells surface stained for CD25 and stained for intranuclear Foxp3 (right). Results are representative of two independent experiments utilizing four mice with mean and SEM shown. Unpaired two-tailed Student's t test, where ∗∗p < 0.01.
(D) Enforced Foxp3 expression downregulates IL-7Rα expression. Surface IL-7Rα expression was assessed on naive (CD44loCD25–) CD4+ LN T cells from the WT and Foxp3 transgenic mice (Tai et al., 2013) (left). IL-7Rα levels (MFI, mean ± SEM) were determined from two independent experiments with a total of four mice (right). Unpaired two-tailed Student's t test, where ∗ ∗∗p < 0.01.
See also Figures S2 and S3.
Next, we wished to ascertain whether the downregulation of IL-7Rα is indeed an active mechanism operating in Foxp3+ Treg cells and not simply a bystander effect caused by high-affinity TCR signaling that is typically associated with Treg cell differentiation (Jordan et al., 2001; Lee et al., 2012). Multiple mechanisms regulate the expression of IL-7Rα on T cells. They include internalization/degradation of the receptor, inhibition of protein translation, and the regulation of gene transcription. To determine whether IL-7Rα expression is transcriptionally suppressed in Foxp3+CD25+ Tregs, we utilized IL-7Rα-GFP gene reporter mice to address this question (Ligons et al., 2012). Because the GFP abundance was significantly lower in Foxp3+CD25+ Treg cells than it was in Foxp3−CD25- naive CD4+ T cells (Figure 2C), the results suggested that IL-7Rα expression was downregulated by transcriptional mechanisms in Foxp3+CD25+ Treg cells.
Foxp3 is a potent transcriptional repressor (Fontenot et al., 2003; Hori et al., 2003; Arvey et al., 2014), and we also aimed to demonstrate that Foxp3 can directly suppress IL-7Rα transcription. To this end, we employed a Foxp3 transgenic mouse model (Foxp3Tg), in which Foxp3 is driven by the human CD2 promoter to be overexpressed in all T-lineage cells (Tai et al., 2013). In these mice, Foxp3 proteins are forced to be expressed even in naive CD44loCD25- CD4 T cells, whereas no Foxp3 protein was expressed in the corresponding WT naive CD4 T population (Figure 2D, left). Notably, forced expression of Foxp3 was sufficient to reduce surface IL-7Rα expression on naive CD44loCD25- CD4 T cells (Figure 2D, right) but did not affect IL-2Rβ expression (Figure S2), indicating that the Foxp3 protein is directly responsible for the loss of IL-7Rα expression on Treg cells. In agreement with these findings, results from computational analyses of the Il7r enhancer region revealed a highly evolutionarily conserved forkhead transcription factor-binding domain, further bolstering a direct suppressive role for Foxp3 in Il7r gene transcription (Figure S3). Collectively, these results indicate that IL-7Rα expression is suppressed in Foxp3+ Treg cells as a direct consequence of Foxp3 and that the loss of IL-7Rα is an intrinsic feature of Foxp3+ Treg cells that prevents γc from pre-associating with IL-7Rα and promotes IL-2 receptor signaling.
Forced IL-7Rα Expression on Mature Foxp3+ CD25+ Treg Cells
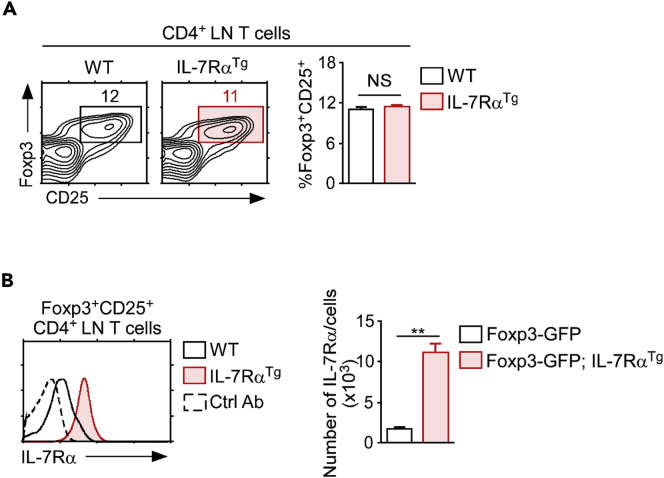
To test whether such a decrease in IL-7Rα abundance is necessary for efficient IL-2R signaling in Foxp3+ Treg cells, we next sought to determine whether the failure to downregulate IL-7Rα on Foxp3+ Treg cells impairs IL-2R signaling and its downstream effects. To this end, we utilized IL-7RαTg mice where a murine IL-7Rα cDNA is expressed under the control of the human CD2 promoter/enhancer such that all T lineage cells, including Foxp3+ Treg cells, overexpress IL-7Rα (Yu et al., 2004). The transgenic expression of IL-7Rα did not show any detrimental effects on Foxp3+CD25+ Treg cell differentiation or homeostasis because the frequency of Treg cells among CD4 LN T cells remained unaltered (Figure 3A) and it did not affect their survival (Figure S4A). However, IL-7RαTg Foxp3+ Treg cells were indeed distinct from WT Foxp3+ Treg cells owing to the dramatically increased abundance of IL-7Rα on IL-7RαTg Foxp3+CD25+ mature Treg cells (Figure 3B). In fact, it is likely that the increased amount of IL-7Rα and IL-7 signaling compensates for any potential loss in IL-2R signaling. Notably, we did not find any discernable changes among other γc family cytokine receptors on IL-7RαTg T cells, including the expression of IL-2Rα and IL-2Rβ (Figure S4B). The results from an RT-PCR array analysis of purified WT and IL-7RαTg Treg cells also failed to show any significant changes in the mRNA expression of IL-2R-associated signaling molecules, such as JAK1, JAK3, STAT5a, and STAT5b (Figure S5). Thus, any potential changes in IL-2R signaling in IL-7RαTg Treg cells can be directly and specifically attributed to the increased expression of IL-7Rα.
Figure 3.
Forced IL-7Rα Expression on Foxp3+ Treg Cells
(A) Foxp3+ CD4+ Tregs from WT and IL-7RαTg mice. Percentage of Foxp3+CD25+ Treg cells was assessed among the CD4+ LN T cells from the indicated mice (left). Bar graph shows a summary of two independent experiments each with four mice (right) and mean with SEM shown. Unpaired two-tailed Student's t test, where NS, not significant.
(B) Surface IL-7Rα expression was assessed on the Foxp3+CD25+ cells from WT and IL-7RαTg LN CD4+ T cells (left). Numbers of IL-7Rα molecules per cell on Foxp3+CD25+ Treg cells was assessed among the CD4+ LN T cells from the indicated mice (right). Bar graph shows summary of three independent experiments each with three mice (right) and mean with SEM shown.
Unpaired two-tailed Student's t test, where ∗∗p < 0.01. See also Figures S4 and S5.
Increased IL-7Rα Expression Impedes IL-2 Receptor Signaling in Foxp3+ Treg Cells
To directly assess cytokine receptor signaling in Foxp3+ Treg cells, we developed an intracellular staining protocol that permitted the simultaneous detection of both nuclear Foxp3 proteins and the phosphorylated form of STAT5 (pSTAT5)—the canonical downstream target of both IL-2 and IL-7 signaling (Transparent Methods). Here, we found that Foxp3+ Treg cells robustly induced STAT5 phosphorylation upon IL-2 stimulation but minimally induced pSTAT5 expression upon IL-7 signaling in vitro (Figure 4A). These results were expected, and they agreed with results showing diminished IL-7Rα expression but abundant IL-2Rβ expression in Treg cells (Figure 2B) (Seddiki et al., 2006; Liu et al., 2006; Cozzo et al., 2003). In contrast, naive CD4 T cells failed to respond to IL-2 stimulation, but these cells were highly sensitive to IL-7 stimulation (Figure 4A).
Figure 4.
IL-7Rα Expression Interferes with IL-2 Signaling in Foxp3+ Treg Cells
(A) Cytokine signaling in Foxp3+ Treg cells. IL-2- and IL-7-induced STAT5 phosphorylation was determined in Foxp3+CD25+ and Foxp3−CD25− CD4+ T cells (left, middle). Phospho-STAT5 (pSTAT5) induction was determined as fold increase over medium (mean ± SEM) from four independent experiments (right). Unpaired two-tailed Student's t test, where ∗∗∗p < 0.001.
(B) IL-7 signaling in IL-7RαTg Foxp3+ Treg cells. WT and IL-7RαTg LN cells were stimulated with IL-7 (0.1 ng/mL), and pSTAT5 contents were assessed in Foxp3+CD25+CD4+ T cells. Results are representative of three independent experiments.
(C) IL-7Rα expression dampens IL-2 signaling in Foxp3+ regulatory T cells. WT or IL-7RαTg LN cells were stimulated with increasing amounts of IL-2, and pSTAT5 contents were assessed in Foxp3+CD25+ Treg cells. pSTAT5 induction was determined as the fold increase over medium (mean ± SEM). Graph shows the results based on two WT and two IL-7RαTg mice and is representative of two independent experiments with three WT and three IL-7RαTg mice in total. Significance was determined by two-way ANOVA, where ∗∗p < 0.01.
(D) IL-7Rα expression inhibits Foxp3+ Treg cell differentiation in vitro. Naive CD4+ T cells from WT or IL-7RαTg mice were stimulated with plate-bound anti-CD3/CD28 antibodies, TGF-β (5 ng/mL), and increasing amounts of recombinant IL-2. The number of Foxp3+ CD4+ T cells was determined 4 days after cell differentiation. Graph shows the results based on two WT and two IL-7RαTg mice and is representative of two independent experiments with four WT and four IL-7RαTg mice in total, showing mean with SEM. Significance was determined by two-way ANOVA, where ∗∗∗p < 0.001.
(E) IL-7Rα expression suppresses IL-2-induced CD25 expression on in vitro differentiated Foxp3+ Treg cells. Naive CD4+ T cells from WT or IL-7RαTg mice were stimulated with plate-bound anti-CD3/CD28 antibodies, TGF-β (5 ng/mL) and IL-2 (10 ng/mL). CD25 expression was determined on Foxp3+ CD4+ T cells after 4 days of differentiation. Results show summary of two independent experiments with three WT and three IL-7RαTg mice, and mean with SEM. Unpaired two-tailed Student's t test, where ∗∗∗p < 0.001.
(F) Adoptive cell transfer of WT and IL-7RαTg Foxp3+ Treg cells. Contour plots show the relative frequencies of CD45.2+ IL-7RαTg Foxp3+ Treg cells and congenic CD45.1+ WT Foxp3+ Treg cells before (left) and after (right) adoptive transfer into IL-7-deficient RagKO host mice. Graph shows the ratio of WT versus IL-7RαTg Foxp3+ Treg cells based on two independent experiments with a total of four host mice, showing mean with SEM.
Unpaired two-tailed Student's t test, where ∗p < 0.05
In the IL-7RαTg Foxp3+ Treg cells, we found that cytokine receptor signaling was substantially altered compared with that of the WT Foxp3+ Treg cells. Forced IL-7Rα expression dramatically increased IL-7 signaling (Figure 4B), but simultaneously, IL-2 receptor signaling was significantly impaired (Figure 4C). Indeed, IL-7RαTg Foxp3+ Treg cells displayed consistently decreased levels of pSTAT5 compared with those in the WT Treg cells upon IL-2 signaling. Additionally, IL-2-induced in vitro differentiation of naive CD4+ T cells into Foxp3+ Treg cells was dramatically impaired when IL-7Rα was overexpressed (Figure 4D), and the increased abundance of IL-7Rα also interfered with IL-2-mediated induction of CD25 (Figure 4E). Collectively, these results suggest a negative regulatory effect of IL-7Rα on IL-2 signaling in Foxp3+ Treg cells.
To further demonstrate the biological significance of IL-7Rα downregulation in maximizing IL-2 signaling, we aimed to assess whether the failure to suppress IL-7Rα expression would impair the IL-2-dependent survival of Foxp3+ Treg cells. To this end, we mixed WT Foxp3+ Treg cells with IL-7RαTg Foxp3+ Treg cells and adoptively transferred them into IL-7-deficient lymphopenic hosts (Rag2KOIL-7KO mice) to make them entirely dependent on IL-2 for their survival (Schmaler et al., 2015; Simonetta et al., 2014; Kim et al., 2012). If IL-7Rα interferes with IL-2 signaling, we expected to observe a preferential loss of IL-7RαTg Foxp3+ Treg cells because of insufficient IL-2 signaling in these cells. We found this was precisely the case (Figure 4F). In this experiment, we isolated CD4 LN T cells from CD45.2+ IL-7RαTg Foxp3-GFP reporter mice and mixed them at a 1:1 ratio with CD4 LN T cells that were purified from congenic CD45.1+ WT Foxp3-GFP reporter mice. In the donor T cell inoculum, we confirmed that CD45.2+ and CD45.1+ Treg cells were represented at a 1:1 ratio when gated on Foxp3-GFP reporter expressing CD4 T cells (Figure 4F). Two weeks after transfer into Rag2KOIL-7KO host mice, donor T cells were recovered from host LNs and assessed for the frequency of CD45.2 Treg cells as compared with the frequency of CD45.1-origin Treg cells. Strikingly, we observed a substantial decrease in Foxp3+ Treg cells that were forced to express IL-7Rα (CD45.2+ IL-7RαTg Foxp3-GFP) when placed under strict IL-2-dependent survival conditions. Collectively, these results agree with and illustrate a detrimental role of IL-7Rα in IL-2 receptor signaling in vivo.
The IL-7Rα Extracellular Domain Is Sufficient to Desensitize IL-2 Receptors in Foxp3+ Tregs
As the molecular basis of IL-7Rα-mediated suppression of IL-2R signaling, we proposed that IL-7Rα would sequester γc molecules whose availability for dimerization with proprietary cytokine receptors is limited. A prediction of this γc sequestration model is that the extracellular domain of IL-7Rα would be sufficient to desensitize IL-2R signaling. Alternatively, we also considered a model in which IL-7Rα competes with IL-2Rβ for downstream intracellular signaling components, such as JAK1 or STAT5, and thus constrains IL-2R signaling.
To differentiate between these two possible models, we generated mice expressing “tailless” IL-7Rα311Tg proteins. The IL-7Rα311Tg construct encodes for the N-terminal 311 amino acids of IL-7Rα, which comprise the entire ectodomain, the transmembrane domain, and 71 amino acids of the cytosolic tail. Specifically, it lacks the C-terminal 128 amino acids, which contains all four tyrosine residues required for intracellular signal transduction (Figure S6A). Expression of the IL-7Rα311 transgene was driven by the human CD2-promoter/enhancer regulatory elements and thus was first expressed at the DN stage of thymocyte development (Figure S6B). To confirm that the IL-7Rα311 construct indeed lacked any signaling ability, we bred IL-7Rα311Tg mice with IL-7RαKO mice to generate IL-7Rα311Tg/KO mice. The number of LN T cells in the IL-7Rα311Tg/KO mice was significantly diminished and was comparable with the number observed in IL-7RαKO mice (Figure 5A). The few LN T cells that we recovered failed to induce STAT5 phosphorylation upon IL-7 stimulation (Figure 5B). These results demonstrate that IL-7Rα311Tg is truly signaling incompetent and cannot restore IL-7 receptor signaling in IL-7RαKO T cells.
Figure 5.
The IL-7Rα Extracellular Domain Suffices to Dampen IL-2 Signaling in Foxp3+ Treg Cells
(A) Number of LN T cells in IL-7Rα311Tg/KO mice. Bar graph shows the total LN T cell numbers (mean ± SEM) from 2 WT, 5 IL-7RαKO, and 8 IL-7Rα311Tg/KO mice. Unpaired two-tailed Student's t test, where ∗∗∗p < 0.001.
(B) Truncated IL-7Rα311 protein fails to transduce IL-7 signaling. IL-7Rα311Tg/KO mice express only a truncated form of IL-7Rα protein, which lacks the 148 distal amino acids of the intracellular membrane domain (total 195 amino acids). WT or IL-7Rα311Tg/KO LN cells were stimulated with IL-7 (0.1 ng/mL) and assessed for pSTAT5 contents. Results are representative of three independent experiments.
(C) IL-7Rα311 overexpression does not affect the IL-7 signaling of Foxp3+CD25+ Treg cells. Surface IL-7Rα expression (left) and IL-7-induced STAT5 phosphorylation (right) were assessed in Foxp3+CD25+ Treg cells from WT and IL-7Rα311Tg mice. Results are representative of three independent experiments.
(D) Truncated IL-7Rα311 suppresses IL-2 signaling in trans in Foxp3+ Treg cells. WT or IL-7Rα311Tg LN cells were stimulated with increasing concentrations of IL-2. pSTAT5 induction was determined in Foxp3+CD25+ CD4+ T cells. Results are shown as the fold increase over medium (mean ± SEM), and they are based on three independent experiments each with two mice.
Statistical significance was determined by two-way ANOVA, where ∗∗∗p < 0.001. See also Figure S6.
To determine what effect this truncated IL-7Rα311 construct had on IL-2R responsiveness in Foxp3+ Treg cells, we analyzed IL-7Rα311Tg mouse on WT background. In these mice, Treg cells express the truncated IL-7Rα311 construct (Figure 5C), without affecting the expression of the IL-2 receptor subunits, including the IL-2Rα and IL-2Rβ (Figure S6C). However, when stimulated with IL-2, the IL-7Rα311Tg Foxp3+ Treg cells still displayed a profound reduction in IL-2R responsiveness, which was comparable with that observed in the Treg cells with full-length IL-7RαTg proteins (Figure 5D). These results demonstrate that the extracellular domain of IL-7Rα is sufficient to inhibit IL-2 receptor responsiveness in Foxp3+ Treg cells, presumably through competition for the limited amount of γc. Furthermore, these data strongly suggest that the transcriptional inhibition of IL-7Rα expression by Foxp3 is necessary to reduce the competition for γc binding, thereby maximizing IL-2 receptor responsiveness.
IL-7Rα Promotes Th17 Cell Differentiation in the Absence of IL-7
While IL-2R signaling promotes the generation and maintenance of Foxp3+ T regulatory cells, the differentiation of pro-inflammatory Th17 CD4+ T cells can be antagonized by IL-2-induced STAT5 (Laurence et al., 2007). Indeed, when naive CD4+ T cells are skewed under Th17 differentiating conditions, IL-17 production can be significantly inhibited by IL-2 stimulation (Figure 6A) (Laurence et al., 2007). Because our in vitro assays demonstrated that IL-7Rα suppresses IL-2R signaling, we hypothesized that the absence of IL-7Rα would increase IL-2 signaling and thus conversely suppress Th17 cell generation. To test this, we utilized a conditional IL-7Rα−cKO mouse in which IL-7Rα was deleted at the DP stage of thymic development (Mccaughtry et al., 2012). In these mice, mature CD4SP thymocytes do not express IL-7Rα (Figure S7), but the lack of IL-7Rα expression does not affect CD4SP T cell development (Mccaughtry et al., 2012). Thus, we FACS-sorted naive CD4SP thymocytes from WT and IL-7Rα-cKO mice and differentiated them under Th17-skewing conditions for 5 days. In support of our hypothesis, we observed a significant reduction in the efficiency of Th17 differentiation in CD4SP thymocytes lacking IL-7Rα expression compared with the efficiency in their IL-7Rα-sufficient WT counterparts (Figure 6B).
Figure 6.
IL-7Rα Expression Promotes Th17 Cell Differentiation
(A) IL-2 signaling inhibits IL-17 production by CD4 T cells activated under Th17-skewing conditions. Naive CD4 T cells enriched from WT mice were stimulated with 1 μg/mL plate-bound anti-CD3/CD28 for 5 days in the presence of 5 ng/mL TGF-β, 30 ng/mL IL-6, 10 μg/mL anti-IL-4, and 10 μg/mL anti-IFN-γ and the indicated concentration of IL-2. Levels of IL-17A and IFN-γ production were assessed by flow cytometry following re-stimulation with PMA/ionomycin in the presence of brefeldin A. Results are the summary of three independent experiments, showing mean with SEM.
(B) Loss of IL-7Rα expression reduces IL-17 production in CD4SP thymocytes. TCRβhiCD4SP thymocytes from WT and IL-7Rα-cKO mice were sorted and stimulated with 1 μg/mL plate-bound anti-CD3/CD28 for 5 days in the presence of 5 ng/mL TGF-β, 30 ng/mL IL-6, 10 μg/mL anti-IL-4, and 10 μg/mL anti-IFN-γ. IL-17A and IFN-γ production was assessed by flow cytometry following re-stimulation with PMA/ionomycin in the presence of brefeldin A. Results are based on three independent experiments with a total of three WT and three IL-7Rα-cKO mice, showing mean with SEM. Unpaired two-tailed Student's t test, where ∗∗p < 0.01.
(C) IL-7Rα overexpression increases IL-17 production in CD4+ LN T cells. Naive CD4+ LN T cells were enriched by magnetic selection and stimulated with 1 μg/mL plate-bound αCD3/CD28 for 5 days in the presence of 5 ng/mL TGF-β, 30 ng/mL IL-6, 10 μg/mL anti-IL-4, and 10 μg/mL anti-IFN-γ. IL-17A and IFN-γ production was assessed by flow cytometry following re-stimulation with PMA/ionomycin in the presence of brefeldin A, showing mean with SEM.
Unpaired two-tailed Student's t test, where ∗p < 0.05. See also Figure S7.
Another prediction of our model suggested that the increased expression of IL-7Rα on CD4+ T cells would enhance their differentiation into Th17 cells because IL-7Rα would antagonize the action of T cell-derived IL-2. Thus, to confirm a role for IL-7Rα in promoting Th17 differentiation, we assessed IL-17 production in WT and IL-7RαTg naive CD4+ T cells that were induced to differentiate into Th17 cells in vitro. Here, we found that increased expression of IL-7Rα significantly increased their ability to differentiate into IL-17-producing Th17 cells (Figure 6C). Collectively, these results demonstrate a negative regulatory role for IL-7Rα in IL-2R signaling.
Discussion
Foxp3+ Treg cells express high levels of IL-2Rβ but low levels of IL-7Rα (Seddiki et al., 2006; Liu et al., 2006; Cozzo et al., 2003). Such receptor distribution contrasts with the high levels of IL-7Rα and low levels of IL-2Rβ expression observed on conventional CD4+ T cells. IL-2Rβ upregulation is necessary to bestow Foxp3+ Treg cells with IL-2 responsiveness for their survival and suppressor function (Fontenot et al., 2005). However, it is not clear why Foxp3+ Treg cells downregulate IL-7Rα expression, and it remains unknown whether IL-7Rα downregulation is necessary for Foxp3+ Treg function or differentiation. Here, we show that IL-7Rα expression is suppressed as a direct consequence of Foxp3 protein expression, and that IL-7Rα downregulation was required for optimal IL-2 signaling. The failure to do so resulted in significantly blunted in vitro IL-2 responsiveness in Foxp3+ Treg cells. Collectively, the data from this study put forward a novel mechanism of cytokine receptor cross talk that augments IL-2 receptor signaling by suppressing IL-7Rα expression.
As a corollary, these results also expand the role of IL-7Rα beyond controlling IL-7 signaling in conventional T cells into affecting the effector functions of Foxp3+ Treg cells, of IL-17-producing Th17 cells, and potentially also of memory CD8 T cells. In the latter case, human CCR7-negative memory CD8 T cells were previously found to comprise two distinct subpopulations that expressed either high or low amounts of IL-7Rα (Kim et al., 2007). Notably, the IL-7Rαlow population displayed increased abundance of IL-2Rβ concomitant to increased expression of T-bet and Eomes, which was attributed to the increased responsiveness to IL-15 (Kim et al., 2007). These results demonstrated an inverse correlation of IL-7Rα and IL-2Rβ expression where the downregulation of IL-7Rα presumably facilitated IL-15 signaling and promoted the acquisition of an effector memory phenotype. Thus, in agreement with our model, the downregulation of IL-7Rα is associated and potentially also necessary to maximize IL-2Rβ expression and signaling.
The molecular explanation for IL-7Rα-mediated suppression of IL-2R signaling was unexpected, as this inhibition did not require signaling-competent IL-7Rα proteins to interfere. Instead, our data showed that the IL-7Rα extracellular domain was sufficient to suppress IL-2 signaling in vitro and that it did so by sequestering γc from associating with IL-2Rβ. IL-2 signaling requires heterodimerization of IL-2Rβ and γc, which induces the juxtaposition and transactivation of the receptor-associated kinases JAK1 and JAK3 to trigger downstream signaling (Miyazaki et al., 1994; Boussiotis et al., 1994). Heterodimerization of IL-2Rβ/γc, on the other hand, is proposed to be mediated by IL-2: first, by IL-2 binding to IL-2Rβ and then by the recruitment of γc to the IL-2/IL-2Rβ complex (Wang et al., 2009). Consequently, the failure to recruit γc results in impaired IL-2R signaling because the IL-2/IL-2Rβ complex itself is not sufficient to activate JAK and STAT5 (Nakamura et al., 1994). Molecular studies with human cytokine receptors also showed that γc itself does not bind IL-2 or any other cytokines (Rickert et al., 2004), so that the recruitment of γc to cytokine-proprietary receptors required pre-association of the ligand with its cognate receptor. Thus, it was not immediately apparent how ligand-free IL-7Rα proteins would sequester γc to prevent their association with IL-2 receptors and inhibit IL-2 signaling.
As a possible explanation, we considered that human IL-7Rα can associate with human γc independently of IL-7 (Rose et al., 2010). In agreement, we had previously found that γc binds to unoccupied surface IL-7Rα proteins even in the absence of ligand (Mcelroy et al., 2012; Hong et al., 2014). SPR binding studies also demonstrated that recombinant mouse γc ECD binds to immobilized IL-7Rα ECD proteins with a Kd of 50.8 μM, but binds to immobilized IL-2Rβ ECD with only a Kd of 695 μM, representing a 14-fold weaker binding affinity than that of IL-7Rα (Hong et al., 2014). Thus, the preferential association of γc with IL-7Rα rather than IL-2Rβ explains why a signaling-incompetent, tailless IL-7Rα protein suffices to interfere with IL-2 signaling. Notably, the truncated IL-7Rα311Tg still retains some membrane proximal intracellular residues that could be sufficient to bind JAK1. Therefore, we cannot definitively exclude the possibility that IL-7Rα overexpression also sequesters JAK1, which might disarm IL-2Rβ. JAK1 is a highly unstable protein with a half-life of only 1.5 h; therefore, continuous JAK1 synthesis is required to maintain sufficient JAK1 amounts for cytokine signaling (Katz et al., 2014). However, it is not known whether JAK1 is preferentially captured by IL-7Rα over IL-2Rβ, and it is also unclear whether JAK1 availability is indeed limited enough to fail furnishing IL-2Rβ proteins in IL-7RαTg Foxp3+ Treg cells. The generation of JAK1 transgenic mice would help resolve this issue, and we are currently in the process of producing such mice.
The uniquely low level of IL-7Rα expression in Treg cells was first reported by Caton and colleagues in a TCR-transgenic model (Cozzo et al., 2003) and then later described in further detail by the Fazekas de St. Groth (Seddiki et al., 2006) and Bluestone (Liu et al., 2006) groups, in both humans and mice. Importantly, IL-7Rα downregulation turned out to be a superior marker for Foxp3+ Treg cells than was conventional CD25 expression, permitting the identification of Foxp3+ regulatory cells in a more comprehensive fashion among CD4+ T cells (Banham, 2006). Mechanistic insights into the Foxp3+ Treg cell-specific suppression of IL-7Rα expression, however, have not been forthcoming. TCR stimulation downregulates IL-7Rα expression (Schluns et al., 2000; Alves et al., 2008), and Treg cells are generated by strong TCR signals such that their low IL-7Rα expression might be induced by strong, persistent TCR engagement (Jordan et al., 2001; Lee et al., 2012). Additionally, IL-2 signaling is known to suppress IL-7Rα transcription (Xue et al., 2002; Park et al., 2004). Thus, downregulation of IL-7Rα expression may be a direct consequence of IL-2 signaling during Foxp3+ Treg cell generation. Our analysis of Foxp3 transgenic T cells, however, indicated that Foxp3 protein expression alone sufficed to suppress IL-7Rα expression, even in resting naive CD4 T cells. Therefore, these results effectively dissociate the signals for Foxp3+ Treg cell lineage specification, i.e., strong TCR and/or IL-2 signaling, from the role of Foxp3 in IL-7Rα downregulation, and they indicate that Foxp3 is a direct effector molecule that suppresses IL-7Rα expression. In further support of this notion, exFoxp3 cells, which are CD4 effector T cells that are derived from Foxp3+ cells but have terminated Foxp3 expression, do express high levels of IL-7Rα, indicating that the loss of Foxp3 lifts the suppression of IL-7Rα expression (Zhou et al., 2009).
Foxp3 is a transcriptional repressor of the forkhead-box (Fox) gene family (Fontenot et al., 2003; Hori et al., 2003; Arvey et al., 2014). Fox-family proteins, such as Foxo1 and Foxp1, control IL-7Rα transcription, so that Foxo1 deficiency impairs, whereas Foxp1 deficiency upregulates IL-7Rα expression on T cells (Ouyang et al., 2009; Feng et al., 2011; Kerdiles et al., 2009). Foxo1 and Foxp1 regulate IL-7Rα expression through a 3.6-kb upstream putative enhancer element in the Il7r gene that contains a conserved forkhead box-binding site known as conserved non-coding sequence 1 (CNS1) (Lee et al., 2005). Because Foxp3 shares the same DNA-binding motif as Foxp1 (Feng et al., 2011), Foxp3-induced IL-7Rα downregulation could have been controlled through CNS1. Surprisingly, CNS1-deficient mice revealed that this was not the case (Abe et al., 2015). Although germline deletion of CNS1 resulted in significantly reduced IL-7Rα surface expression on mature T cells (Abe et al., 2015), it did not revert the repression by Foxp3, and it failed to restore IL-7Rα expression on Foxp3+ Treg cells. Instead, IL-7Rα on CNS1-deficient Foxp3+ Treg cells were still expressed, albeit in smaller amounts than on the naive CD4 T cells in the same mice (Abe et al., 2015). Altogether, these results indicate that Foxp3-induced IL-7Rα downregulation utilizes a mechanism that is distinct from that of other Fox family proteins. These data also suggest that CNS1 enhancer activity is required to establish steady-state IL-7Rα expression on T cells but is not sufficient for IL-7Rα suppression by Foxp3. Along this line, ChIP-qPCR assay results showed that anti-Foxp3 immunoprecipitates were highly enriched in DNA amplicons of the Il7r promoter region (Liu et al., 2006), suggesting that Foxp3 may directly suppress IL-7Rα promoter activity. However, we failed to identify a conserved forkhead box-binding motif in the mouse Il7r promoter; therefore, it remains unclear how Foxp3 interacts with the promoter region. Although Foxp3 might bind to the Il7r promoter by indirect association with transcription factors bound to the promoter element, the precise mechanism remains to be uncovered.
Reduced IL-7Rα expression is considered a characteristic feature of Foxp3+ Treg cells (Banham, 2006). However, it has not been made clear whether IL-7Rα downregulation has any physiological significance in Treg cell biology and, if it does, what its role might be. Because TCR stimulation downregulates IL-7Rα expression, IL-7Rα downregulation may simply reflect the fact that Foxp3+ Treg cells are constantly exposed to high-affinity TCR and CD28 costimulatory signals (Josefowicz et al., 2012; Tai et al., 2005). Alternatively, because we found that Foxp3 directly suppresses IL-7Rα expression, the downregulation of IL-7Rα may also represent a mere bystander event associated with Foxp3 protein expression in Treg cells. In the latter scenario, IL-7Rα downregulation would be a biomarker for Foxp3+ Treg cells without biological consequences. On the other hand, IL-7 receptor signaling can contribute to Foxp3+ Treg cell survival and function (Schmaler et al., 2015; Simonetta et al., 2014; Kim et al., 2012), such that diminished IL-7Rα expression would be detrimental for IL-7 signaling in Treg cells. Thus, it is counterintuitive to suggest that Foxp3+ Treg cells would suppress IL-7Rα expression when IL-7 could promote Treg cell survival and homeostasis.
Our findings resolve this conundrum by demonstrating that the downregulation of IL-7Rα expression has a biological purpose and that it represents a novel mechanism to maximize IL-2 receptor signaling in Foxp3+ Treg cells. In this regard, we would have expected that the in vivo survival or homeostasis of IL-7RαTg Foxp3+ Treg cells whose IL-7Rα expression is not suppressed would be markedly impaired because γc remains sequestered by IL-7Rα. However, we found the frequencies of Foxp3+ Treg cells unaffected in the LN and spleen of IL-7RαTg mice, suggesting that IL-7Rα-mediated suppression of IL-2R signaling is less effective under in vivo circumstances where the IL-7 availability is not limited, and when IL-7 signaling can compensate for the loss of IL-2 signaling. Moreover, the IL-7RαTg is expressed in all T lineage cells and not only in Foxp3+ Treg cells, so that understanding the role of IL-7Rα specifically in Treg cells becomes further complicated because of its compound effect on conventional T cells. We are currently in the process of generating IL-7Rα-transgenic mice where the transgene is driven by Foxp3 gene regulatory elements, and we aim to utilize these mice to further investigate the role of IL-7Rα in Treg cells.
Altogether, our current results reveal a previously unappreciated role of IL-7Rα as a negative regulator of IL-2/IL-2Rβ signaling and signify that modulating cytokine receptor expression has wide-ranging effects beyond tuning the signal strength of its cognate cytokine.
Limitations of the Study
Here, we showed that forced expression of IL-7Rα impairs the IL-2-mediated in vitro generation of Foxp3+ Treg cells. Mechanistically, we found that IL-7Rα interferes with IL-2 signaling in vitro, but the frequency and homeostasis of IL-7RαTg Foxp3+ Treg cells in vivo remain unaffected. Thus, the in vitro observation did not directly translate into an in vivo effect. Although we propose that increased IL-7 signaling would compensate for the loss of IL-2R signaling in vivo, further analyses are necessary to fully understand the role and consequences of IL-7Rα regulation in T cells.
Resource Availability
Lead Contact
Further information and requests for reagents should be directed to the Lead Contact, Jung-Hyun Park (parkhy@mail.nih.gov).
Materials Availability
Materials are available from the Lead Contact upon reasonable request but may require a Material Transfer Agreement.
Data and Code Availability
The data that support the findings of this study are available from the Lead Contact upon reasonable request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. Part of this study was also supported by contract UH2 CA-2055869 from the National Cancer Institute, NIH, to S.T.R.W.
Author Contributions
A.T.W., H.R.K., T.-H.K., and S.T.R.W. designed and performed the experiments, analyzed the data, and contributed to the writing of the manuscript. C.H. and M.A.L. performed experiments, analyzed the data, and commented on the manuscript. X.T. and C.M.-P. provided expertise and the reagents used, performed data analyses, and critically commented on the manuscript. J.-H.P. conceived the project, analyzed the data, and wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101421.
Supplemental Information
References
- Abe A., Tani-Ichi S., Shitara S., Cui G., Yamada H., Miyachi H., Kitano S., Hara T., Abe R., Yoshikai Y., Ikuta K. An enhancer of the IL-7 receptor alpha-chain locus controls IL-7 receptor expression and maintenance of peripheral T cells. J. Immunol. 2015;195:3129–3138. doi: 10.4049/jimmunol.1302447. [DOI] [PubMed] [Google Scholar]
- Akashi K., Kondo M., Von Freeden-Jeffry U., Murray R., Weissman I.L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Alves N.L., Van Leeuwen E.M., Derks I.A., Van Lier R.A. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J. Immunol. 2008;180:5201–5210. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- Arvey A., Van Der Veeken J., Samstein R.M., Feng Y., Stamatoyannopoulos J.A., Rudensky A.Y. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat. Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banham A.H. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–544. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Boussiotis V.A., Barber D.L., Nakarai T., Freeman G.J., Gribben J.G., Bernstein G.M., D'andrea A.D., Ritz J., Nadler L.M. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- Cho J.H., Kim H.O., Surh C.D., Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzo C., Larkin J., 3rd, Caton A.J. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J. Immunol. 2003;171:5678–5682. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- Egawa T., Tillman R.E., Naoe Y., Taniuchi I., Littman D.R. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J. Exp. Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Wang H., Takata H., Day T.J., Willen J., Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat. Immunol. 2011;12:544–550. doi: 10.1038/ni.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Gavin M.A., Rudensky A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Fry T.J., Mackall C.L. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- Gonnord P., Angermann B.R., Sadtler K., Gombos E., Chappert P., Meier-Schellersheim M., Varma R. A hierarchy of affinities between cytokine receptors and the common gamma chain leads to pathway cross-talk. Sci. Signal. 2018;11:eaal1253. doi: 10.1126/scisignal.aal1253. [DOI] [PubMed] [Google Scholar]
- Hong C., Luckey M.A., Ligons D.L., Waickman A.T., Park J.Y., Kim G.Y., Keller H.R., Etzensperger R., Tai X., Lazarevic V. Activated T cells secrete an alternatively spliced form of common gamma-chain that inhibits cytokine signaling and exacerbates inflammation. Immunity. 2014;40:910–923. doi: 10.1016/j.immuni.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C., Luckey M.A., Park J.H. Intrathymic IL-7: the where, when, and why of IL-7 signaling during T cell development. Semin. Immunol. 2012;24:151–158. doi: 10.1016/j.smim.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Johnson L.D., Jameson S.C. TGF-beta sensitivity restrains CD8+ T cell homeostatic proliferation by enforcing sensitivity to IL-7 and IL-15. PLoS One. 2012;7:e42268. doi: 10.1371/journal.pone.0042268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M.S., Boesteanu A., Reed A.J., Petrone A.L., Holenbeck A.E., Lerman M.A., Naji A., Caton A.J. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz G., Pobezinsky L.A., Jeurling S., Shinzawa M., Van Laethem F., Singer A. T cell receptor stimulation impairs IL-7 receptor signaling by inducing expression of the microRNA miR-17 to target Janus kinase 1. Sci. Signal. 2014;7:ra83. doi: 10.1126/scisignal.2005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdiles Y.M., Beisner D.R., Tinoco R., Dejean A.S., Castrillon D.H., Depinho R.A., Hedrick S.M. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.Y., Ligons D.L., Hong C., Luckey M.A., Keller H.R., Tai X., Lucas P.J., Gress R.E., Park J.H. An in vivo IL-7 requirement for peripheral Foxp3+ regulatory T cell homeostasis. J. Immunol. 2012;188:5859–5866. doi: 10.4049/jimmunol.1102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-R., Hwang K.-A., Kim K.-C., Kang I. Down-regulation of IL-7Rα expression in human T cells via DNA methylation. J. Immunol. 2007;178:5473–5479. doi: 10.4049/jimmunol.178.9.5473. [DOI] [PubMed] [Google Scholar]
- Kimura M.Y., Pobezinsky L.A., Guinter T.I., Thomas J., Adams A., Park J.H., Tai X., Singer A. IL-7 signaling must be intermittent, not continuous, during CD8(+) T cell homeostasis to promote cell survival instead of cell death. Nat. Immunol. 2013;14:143–151. doi: 10.1038/ni.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A., Tato C.M., Davidson T.S., Kanno Y., Chen Z., Yao Z., Blank R.B., Meylan F., Siegel R., Hennighausen L. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lee H.C., Shibata H., Ogawa S., Maki K., Ikuta K. Transcriptional regulation of the mouse IL-7 receptor alpha promoter by glucocorticoid receptor. J. Immunol. 2005;174:7800–7806. doi: 10.4049/jimmunol.174.12.7800. [DOI] [PubMed] [Google Scholar]
- Lee H.M., Bautista J.L., Scott-Browne J., Mohan J.F., Hsieh C.S. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligons D.L., Tuncer C., Linowes B.A., Akcay I.M., Kurtulus S., Deniz E., Atasever Arslan B., Cevik S.I., Keller H.R., Luckey M.A. CD8 lineage-specific regulation of interleukin-7 receptor expression by the transcriptional repressor Gfi1. J. Biol. Chem. 2012;287:34386–34399. doi: 10.1074/jbc.M112.378687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Putnam A.L., Xu-Yu Z., Szot G.L., Lee M.R., Zhu S., Gottlieb P.A., Kapranov P., Gingeras T.R., Fazekas De St Groth B. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccaughtry T.M., Etzensperger R., Alag A., Tai X., Kurtulus S., Park J.H., Grinberg A., Love P., Feigenbaum L., Erman B., Singer A. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J. Exp. Med. 2012;209:2263–2276. doi: 10.1084/jem.20121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelroy C.A., Holland P.J., Zhao P., Lim J.M., Wells L., Eisenstein E., Walsh S.T. Structural reorganization of the interleukin-7 signaling complex. Proc. Natl. Acad. Sci. U S A. 2012;109:2503–2508. doi: 10.1073/pnas.1116582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertsching E., Burdet C., Ceredig R. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int. Immunol. 1995;7:401–414. doi: 10.1093/intimm/7.3.401. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Kawahara A., Fujii H., Nakagawa Y., Minami Y., Liu Z.J., Oishi I., Silvennoinen O., Witthuhn B.A., Ihle J.N. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Russell S.M., Mess S.A., Friedmann M., Erdos M., Francois C., Jacques Y., Adelstein S., Leonard W.J. Heterodimerization of the IL-2 receptor beta- and gamma-chain cytoplasmic domains is required for signalling. Nature. 1994;369:330–333. doi: 10.1038/369330a0. [DOI] [PubMed] [Google Scholar]
- Ouyang W., Beckett O., Flavell R.A., Li M.O. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Oh S.A., Ma Q., Bivona M.R., Zhu J., Li M.O. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity. 2013;39:335–346. doi: 10.1016/j.immuni.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Yu Q., Erman B., Appelbaum J.S., Montoya-Durango D., Grimes H.L., Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Jo Y., Ko E., Luckey M.A., Park Y.K., Park S.H., Park J.H., Hong C. Soluble gammac cytokine receptor suppresses IL-15 signaling and impairs iNKT cell development in the thymus. Sci. Rep. 2016;6:36962. doi: 10.1038/srep36962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L.S., Martin U., Garka K., Gliniak B., Di Santo J.P., Muller W., Largaespada D.A., Copeland N.G., Jenkins N.A., Farr A.G. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peffault De Latour R., Dujardin H.C., Mishellany F., Burlen-Defranoux O., Zuber J., Marques R., Di Santo J., Cumano A., Vieira P., Bandeira A. Ontogeny, function, and peripheral homeostasis of regulatory T cells in the absence of interleukin-7. Blood. 2006;108:2300–2306. doi: 10.1182/blood-2006-04-017947. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Morrissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell J.C., Farkash E.A., Gao W., Thompson C.B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Rickert M., Boulanger M.J., Goriatcheva N., Garcia K.C. Compensatory energetic mechanisms mediating the assembly of signaling complexes between interleukin-2 and its alpha, beta, and gamma(c) receptors. J. Mol. Biol. 2004;339:1115–1128. doi: 10.1016/j.jmb.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Rochman Y., Spolski R., Leonard W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T., Pillet A.H., Lavergne V., Tamarit B., Lenormand P., Rousselle J.C., Namane A., Theze J. Interleukin-7 compartmentalizes its receptor signaling complex to initiate CD4 T lymphocyte response. J. Biol. Chem. 2010;285:14898–14908. doi: 10.1074/jbc.M110.104232. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schluns K.S., Kieper W.C., Jameson S.C., Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schmaler M., Broggi M.A., Lagarde N., Stocklin B.F., King C.G., Finke D., Rossi S.W. IL-7R signaling in regulatory T cells maintains peripheral and allograft tolerance in mice. Proc. Natl. Acad. Sci. U S A. 2015;112:13330–13335. doi: 10.1073/pnas.1510045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., Solomon M., Selby W., Alexander S.I., Nanan R. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta F., Gestermann N., Bloquet S., Bourgeois C. Interleukin-7 optimizes FOXP3+CD4+ regulatory T cells reactivity to interleukin-2 by modulating CD25 expression. PLoS One. 2014;9:e113314. doi: 10.1371/journal.pone.0113314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh C.D., Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tai X., Cowan M., Feigenbaum L., Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- Tai X., Erman B., Alag A., Mu J., Kimura M., Katz G., Guinter T., Mccaughtry T., Etzensperger R., Feigenbaum L. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38:1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Freeden-Jeffry U., Solvason N., Howard M., Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- Von Freeden-Jeffry U., Vieira P., Lucian L.A., Mcneil T., Burdach S.E., Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman A.T., Park J.Y., Park J.H. The common gamma-chain cytokine receptor: tricks-and-treats for T cells. Cell Mol. Life Sci. 2015;73:253–269. doi: 10.1007/s00018-015-2062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lupardus P., Laporte S.L., Garcia K.C. Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 2009;27:29–60. doi: 10.1146/annurev.immunol.24.021605.090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H.H., Bollenbacher J., Rovella V., Tripuraneni R., Du Y.B., Liu C.Y., Williams A., Mccoy J.P., Leonard W.J. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat. Immunol. 2004;5:1036–1044. doi: 10.1038/ni1117. [DOI] [PubMed] [Google Scholar]
- Xue H.H., Kovanen P.E., Pise-Masison C.A., Berg M., Radovich M.F., Brady J.N., Leonard W.J. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc. Natl. Acad. Sci. U S A. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Erman B., Bhandoola A., Sharrow S.O., Singer A. In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells. J. Exp. Med. 2003;197:475–487. doi: 10.1084/jem.20021765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Erman B., Park J.H., Feigenbaum L., Singer A. IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORgammat: impact on thymocyte development. J. Exp. Med. 2004;200:797–803. doi: 10.1084/jem.20032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Park J.H., Doan L.L., Erman B., Feigenbaum L., Singer A. Cytokine signal transduction is suppressed in preselection double-positive thymocytes and restored by positive selection. J. Exp. Med. 2006;203:165–175. doi: 10.1084/jem.20051836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Bailey-Bucktrout S.L., Jeker L.T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J.A. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Lead Contact upon reasonable request.