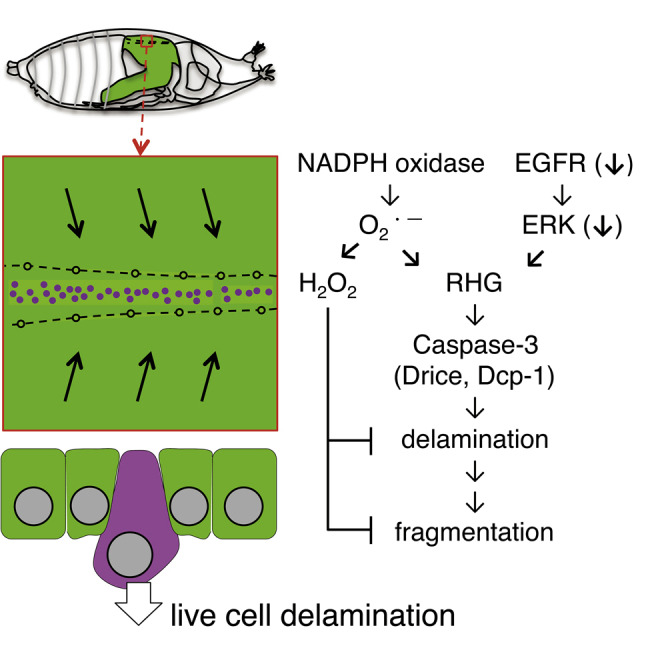
Summary
Thorax fusion occurs in the midline of the Drosophila pupal notum and involves epithelial cell delamination requiring apoptotic signaling. By genetic screening, we found that NADPH oxidases (Nox and Duox) associated with superoxide anion (O˙-2) are responsible for caspase-3 activation and delamination. We observed that Nox is upregulated in cells that undergo delamination and that delamination depends on caspase activation. However, the cell morphology and the almost complete lack of propidium iodide incorporation suggested little membrane disruption and signified apoptotic modulation. These results demonstrate that most delaminating cells undergo caspase activation, but this activation is not sufficient for apoptosis. We showed that the expression of Catalase, encoding an H2O2 scavenger in the cytosol, increases delamination and induces apoptotic nuclear fragmentation in caspase-3-activated cells. These findings suggest that the roles of O˙-2 and intracellular H2O2 for delamination differs before and after caspase-3 activation, which involves live cell delamination.
Subject Areas: Developmental Genetics, Molecular Genetics
Graphical Abstract
Highlights
-
•
NADPH oxidases are responsible for cell delamination in Drosophila pupal notum
-
•
Nox is upregulated in delaminating cells prior to caspase-3 activation
-
•
Nox promotes caspase-3 activation and cell delamination
-
•
H2O2 suppresses apoptotic nuclear fragmentation during delamination
Developmental Genetics; Molecular Genetics
Introduction
For organ morphogenesis, two distant tissues approach and combine to form one continuous structure. Various organs such as the palate, neural tube, heart, eyes, face, and body wall are developed by this process of “tissue fusion” (Ray and Niswander, 2012). A characteristic feature of tissue fusion is the appearance of many apoptotic cells near the fusion sites. However, the regulatory mechanisms and functions of apoptosis in this context remain to be elucidated (Cuervo and Covarrubias, 2004; Cuervo et al., 2002; Farbman, 1968; Hinrichsen, 1985; Martinez-Alvarez et al., 2000; Mori et al., 1994; Yamaguchi et al., 2011).
Drosophila melanogaster thorax fusion is a remarkable model for epithelial tissue fusion (Martin-Blanco and Knust, 2001; Martin-Blanco et al., 2000). We have previously shown that sub-lethal caspase activation regulates the speed of thorax closure (Fujisawa et al., 2019). Following thorax fusion in the midline, a monolayered epithelium called the pupal notum is formed. Epithelial cells along the fusion site of the midline frequently undergo basal extrusion (delamination, Koto et al., 2011; Figure 1A). The delamination rate escalates with increasing cell size via P110 overexpression and diminishes with decreasing cell size via Tsc1/2 overexpression (Marinari et al., 2012). This suggests that delamination is correlated with the local crowding status of the epithelium. Furthermore, upon laser wounding, the calculation of cell movement and direction via particle image velocimetry has demonstrated that delamination is caused by mechanical compaction of the midline cells (Levayer et al., 2016). This delamination is therefore designated “crowding-induced cell delamination.” Only approximately 30% of delamination is reported to be caspase dependent (Marinari et al., 2012). However, this caspase-dependent fraction has been found to be an underestimation (Levayer et al., 2016). Levayer et al. showed that major stress-sensitive pathways including p53, JNK, or Hippo Yap/Taz signaling are not involved in the delamination process (Levayer et al., 2016). The same group demonstrated that cell delamination is coupled with ERK downregulation and pro-apoptotic gene Hid upregulation (Moreno et al., 2019). Although ERK inactivation is limited to approximately 60% of delaminating cells with caspase-3 activation, other pathways involved in cell delamination have not yet been identified.
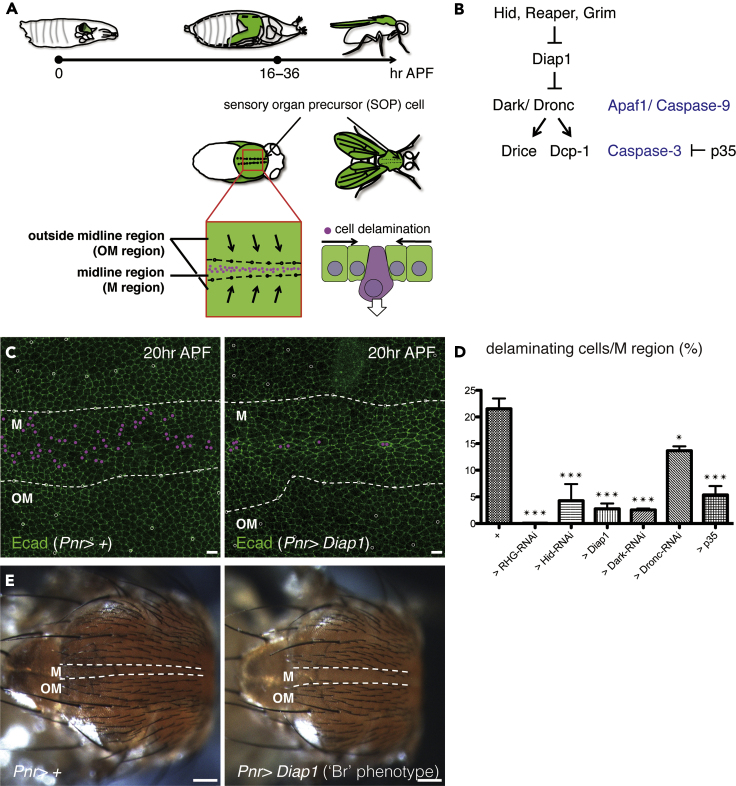
Figure 1.
Apoptotic Signaling Is Required for Cell Delamination
(A) A diagram representing the development of the Drosophila pupal notum and the timing of cell delamination upon crowding. The boundaries between the midline (M) and outside the midline (OM) regions are outlined with dotted lines. The anterior-to-posterior axes of all pupae and adults are oriented toward the left.
(B) A diagram representing the Drosophila apoptotic signaling pathway.
(C) Snapshots from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing Ecad:GFP (20 h after puparium formation; APF). Magenta dots indicate cells that delaminated in 10 h (from 20 to 30 h APF).
(D) The ratio of cell delamination (from 20 to 30 h APF; control: four nota, versus RHG-RNAi, Hid-RNAi, Diap1, Dark-RNAi, Dronc-RNAi, or p35: three nota under the control of Pnr-Gal4). The p value was calculated by one-way analysis of variance (ANOVA) with Dunnett's test. ∗, p < 0.05 and ∗∗∗, p < 0.005. Error bars indicate standard error of the mean.
(E) Images of the adult notum. Compared with the control, tissues with overexpressed Diap1 showed a “broaden (Br) phenotype” of the M region.
The boundaries between the M and OM regions are outlined with dotted white lines. The anterior-to-posterior axes of all pupae and adults are oriented toward the left. Scale bars: (C) 10 and (E) 100 μm.
See also Figure S1.
NADPH oxidases are involved in the generation of reactive oxygen species (ROS). These can attack a large number of biomolecules and are, therefore, associated with apoptotic cell death (Redza-Dutordoir and Averill-Bates, 2016; Wang et al., 2018). For example, spatiotemporal ROS production by NADPH oxidase is critical for tapetal programmed cell death in Arabidopsis (Xie et al., 2014) and is essential for follicle cell rupture during Drosophila ovulation (Li et al., 2018). Most studies exploring the involvement of NADPH oxidase in caspase activation and apoptosis are based on in vitro or ex vivo experimental models, and regulation of cellular functions by NADPH oxidases have not been specifically examined in vivo at the single cell level. The NADPH oxidase Nox is involved in superoxide anion (O˙-2) production.
In this study, we found, by genetic screening, that Nox regulates caspase-3 activation and delamination, independent of ERK downregulation. Furthermore, we showed that intracellular hydrogen peroxide (H2O2) enables cells to undergo delamination without apoptotic features downstream of caspase-3 activation. ROS generated by Nox (O˙-2) and intracellular H2O2 can thus differentially regulate cell delamination both upstream and downstream of caspase-3 activation.
Results
Genetic Screen for Cell Delamination
There are differing opinions regarding the requirement of caspase activation for crowding-induced cell delamination (Levayer et al., 2016; Marinari et al., 2012). We therefore tested whether apoptotic signaling is required for delamination (Figure 1B). Overexpressing Diap1 was sufficient to suppress the delamination rate in the midline region, which has been previously defined (M region; Figures 1C and 1D) (Levayer et al., 2016). In addition, the delamination rate was significantly reduced when Reaper, Hid, and Grim (RHG, three antagonists for an endogenous caspase inhibitor Diap1)-RNAi, Dark-RNAi, Dronc-RNAi, or p35 was expressed (Figure 1D). This confirmed that apoptotic signaling is necessary for delamination. Flies that overexpressed Diap1 showed a “broaden (Br) phenotype” on the adult notum (Figure 1E). This is consistent with a previous report, wherein the absence of caspase activation-induced delamination increased the final size of the M region (Levayer et al., 2016). Using this phenotype as an indicator for the lack of cell delamination, we sought to identify the molecules involved in cell delamination.
To this end, we conducted a genetic screen using Pnr-Gal4 as a control (Figure S1A). We crossed overexpression or inhibition lines based on genes selected from cellular functions such as cell death, mechano-sensing, cytoskeleton formation, and oxidative stress. As shown by “Br” in the first screening (Figures S1A and S1B), we found that some manipulations led to a Br phenotype. Among the first candidates, we found that the manipulation of apoptotic genes, ROS-related genes, Foxo-RNAi, and Atf3 led to a Br phenotype. None of the mechano-sensing genes were picked up in the first screening, suggesting differing mechanisms of cell delamination between Drosophila pupal notum and other models of live-cell delamination reported in vertebrates (Eisenhoffer et al., 2012). To test whether these phenotypes could be attributed to the suppression of delamination, we performed live imaging on the pupal notum. We identified that overexpression of Atf3, Sod1, or Sod2 or knockdown of Foxo, Nox, or Duox suppressed delamination. In contrast, Catalase or Keap1 overexpression and Hayan-RNAi did not suppress delamination. Although further analyses are required for each gene manipulation, given that Br phenotype is an integrated phenotype of both cell delamination and cell proliferation, these manipulations could enhance cell proliferation (which was not assessed in the screening). It is also possible that enhancement of cell death in the M region affects the crowding status and balance of cell proliferation. Cell elimination could therefore lead to a shift to the Br phenotype. Catalase overexpression is examined in detail below.
NADPH Oxidases Are Responsible for Cell Delamination and Caspase-3 Activation
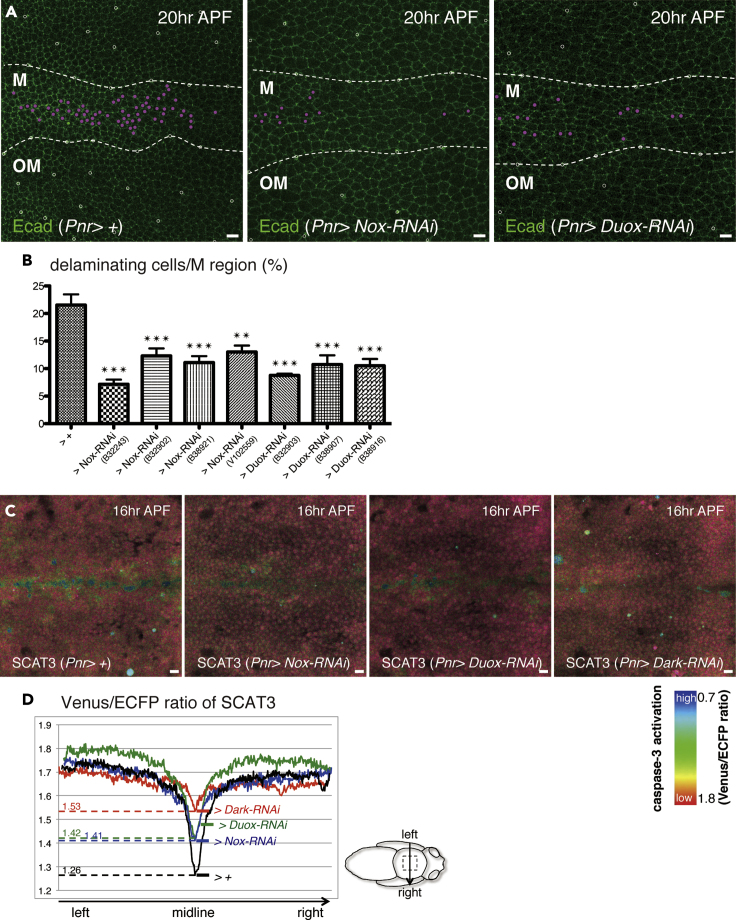
Among the selected genes, we focused on the suppressive effect of Nox or Duox knockdown. Both genes belong to the NADPH oxidase family and are the only two members present in Drosophila. NADPH oxidases have an NADPH-binding domain close to the C terminal, transfer an electron from NADPH to O2, and produce O˙-2. We confirmed the involvement of NADPH oxidases in delamination using four independent Nox-RNAi lines or three Duox-RNAi lines (Figures 2A, 2B, S1C). To investigate the contribution of NADPH oxidases to caspase-3 activation, we monitored the ECFP/Venus ratio of the FRET-based caspase activity indicator SCAT3 (Takemoto et al., 2003) at 16 h after puparium formation (APF), continuing until 36 h APF (Figure 1A). This is the point at which cells begin to undergo delamination in the M region. We found that Nox-RNAi or Duox-RNAi inhibited caspase-3 activation, although these inhibitory effects were smaller than those of Dark-RNAi (Figures 2C and 2D). These data suggest the involvement of NADPH oxidases in caspase-3 activation, which is required for delamination in the M region, in addition to the involvement of Hid modulation via ERK downregulation (Moreno et al., 2019).
Figure 2.
NADPH Oxidases Are Responsible for Cell Delamination and Caspase-3 Activation
(A) Snapshots from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing Ecad::GFP (20 h after puparium formation; APF). Magenta dots indicate cells that delaminated in 10 h (from 20 to 30 h APF).
(B) The ratio of cell delamination (from 20 to 30 h APF; control: four nota, versus Nox-RNAi or Duox-RNAi: three nota under the control of Pnr-Gal4). The p value was calculated by one-way analysis of variance (ANOVA) with Dunnett's test. ∗∗, p < 0.01 and ∗∗∗, p < 0.005. Error bars indicate standard error of the mean.
(C) Snapshots from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing SCAT3 under the control of Pnr-Gal4 (16 h APF).
(D) Averaged changes in the FRET ratio (the Venus signal to the ECFP signal) of SCAT3 (16 h APF; control: four nota, versus Nox-RNAi, Duox-RNAi, or Dark-RNAi: four nota under the control of Pnr-Gal4).
The boundaries between the midline (M) and outside the midline (OM) regions are outlined with dotted white lines. The anterior-to-posterior axes of all pupae are oriented toward the left. Scale bars: 10 μm.
See also Figure S2.
Nox Is Upregulated in Delaminating Cells
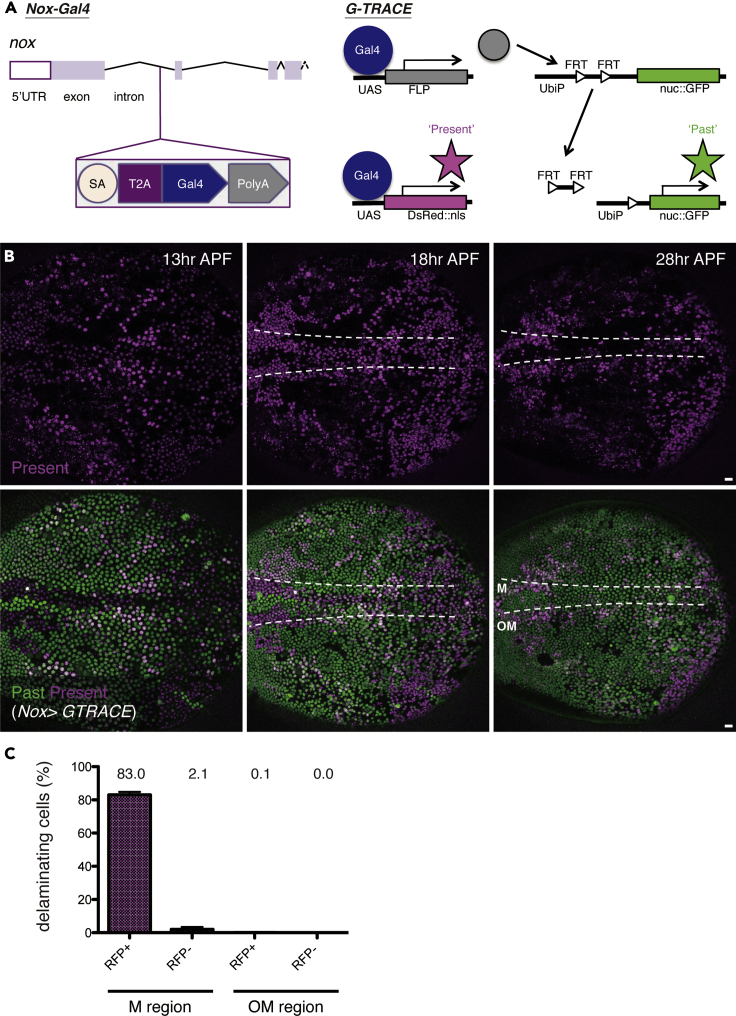
To validate the involvement of an NADPH oxidase in cell delamination, we first monitored Nox expression. This was done using a gene-trap Gal4 {CRIMIC}Nox-Gal4, in which the splicing acceptor, T2A, and the Gal4 sequence are inserted in-frame into the first intron of nox (Lee et al., 2018). As Gal4 is separated from the truncated (first exon) Nox protein after translation, this expression pattern is expected to reflect the endogenous transcription and translation patterns of the gene. Combining this construction with G-TRACE (Evans et al., 2009; Figure 3A), we monitored the spatiotemporal pattern of Nox expression from 13 h APF. This stage occurs approximately 3 h before the beginning of cell delamination (Figure 3B). Despite the time lag of approximately a few hours between Nox expression and Gal4/UAS-dependent labeling via fluorescence protein expression, cells marked with magenta (DsRed::nls) nuclei reflected “present” expression, whereas those with green (nuc::GFP) nuclei indicated cells that previously (potentially during larval stage) showed Nox expression (described as “past”; Figures 3A and 3B). Prior to the delamination stage (13–16 h APF; Figure 1A), GFP-expressing cells are rarely observed in the thorax fusion site of the M region. DsRed expression instead gradually became stronger in a subset of cells at specific locations, including the M region. Such DsRed+ cells with “present” Nox expression frequently underwent delamination in the M region (83.0%; Figure 3C, Video S1). These observations suggest that, in this region, Nox functions in a cell-autonomous manner during delamination. At the delamination stage (after 16 h APF), Nox expression in cells of the M region was confirmed in the pupal notum of flies carrying Nox::V5::TurboID, in which the C terminus of Nox is fused with a promiscuous biotin ligase (TurboID, Branon et al., 2018; Figure S2A) that biotinylates neighboring proteins and can sensitively label TurboID-expressing cells (Figures S2B–S2C′). To examine whether Nox-Gal4 “present” cells undergo delamination through caspase activation, we injected the pan-caspase inhibitor Z-VAD-fmk into the pupal notum and monitored the delamination process (Figures S3A and S3B). Consequently, Z-VAD-fmk treatment did not block the appearance of Nox-Gal4 “present” cells but prevented delamination and increased the number of these cells in the M region at 28 h APF (Figure S3B). This further supports the idea that Nox functions upstream of caspase-3 activation to promote delamination.
Figure 3.
Nox Is Upregulated in Delaminating Cells
(A) Nox-Gal4 locus and G-TRACE construction.
(B) Snapshots from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing Nox-Gal4 and G-TRACE (13, 18, and 28 h after puparium formation; APF).
(C) The ratio of cell delamination (from 18 to 28 h APF; three nota, midline (M): 699 DsRed+ cells and 638 DsRed− (GFP+) cells, versus outside the midline (OM): 3,816 DsRed + cells and 3,866 DsRed− (GFP+) cells). Error bars indicate standard error of the mean.
The boundaries between the M and OM regions are outlined with dotted white lines. The anterior-to-posterior axis of the pupa is oriented toward the left. Scale bars: 10 μm.
DsRed::nls was monitored from 18 to 35.5 h APF after puparium formation (APF) using an EMCCD camera (ImagEM X2, Hamamatsu) with an Olympus MVX10 macroscope.
Nox Is Not Involved in ERK Downregulation
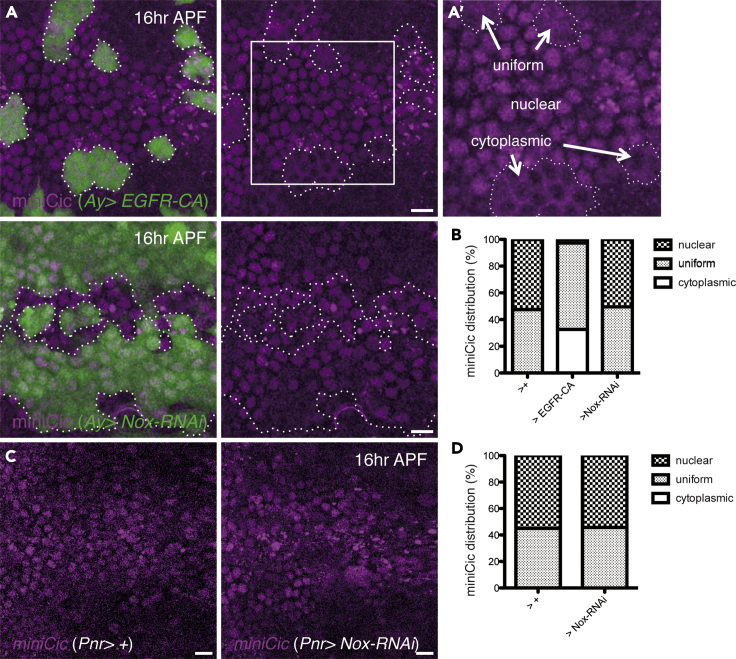
A previous study showed that ERK downregulation occurs in delaminating cells (Moreno et al., 2019). This is based on the fact that the mCherry signal of the nls::C1C2Cic::mCherry (miniCic) reporter, which is exported from the nucleus upon phosphorylation by ERK signaling, is observed in the nucleus during delamination. This reporter reveals that, among delaminating cells, ERK downregulation occurs in approximately 60% of the caspase-3-activated cells (Moreno et al., 2019). We therefore used this reporter to determine whether Nox regulates ERK downregulation. We combined heat shock-dependent flippase expression (Hs-flp) and Ay-Gal4 systems to conduct a mosaic analysis. We first expressed a constitutively active form of EGFR (EGFR-CA) with GFP. The mCherry signal was observed in a cytoplasmic or uniform manner in GFP+ cell populations with EGFR-CA expression but in a nuclear or uniform manner in those without gene manipulation (control; Figures 4A and 4B). This confirmed the translocation of the miniCic reporter upon ERK upregulation. We detected almost no modulation of mCherry localization in GFP+ cells with Nox knockdown compared with that in the control (Figures 4A and 4B). Consistent with this result, we found that Nox-RNAi via Pnr-Gal4 did not induce mCherry cytoplasmic signals in cells around the midline (Figures 4C and 4D). These findings indicate that Nox is not responsible for ERK downregulation and that these parallel pathways regulate cell delamination in the M region of the pupal notum.
Figure 4.
Nox Is Not Involved in ERK Downregulation
(A) Images of the z-projections of confocal stacks in the pupal notum of a live fly expressing miniCic, as well as green fluorescence protein (GFP) and EGFR-CA, under the control of Ay-Gal4 (16 h after puparium formation; APF). (A′) A magnified image of (A).
(B) Distribution of mCherry localization in the clones (16 h APF; control: three nota 222 cells, versus EGFR-CA: seven nota 181 cells or Nox-RNAi: three nota 269 cells).
(C) Images of the z-projections of confocal stacks in the pupal notum of a live fly expressing miniCic (16 h APF).
(D) Distribution of mCherry localization (16 h APF; control: three nota 244 cells, versus Nox-RNAi: three nota 188 cells). The anterior-to-posterior axes of all pupae are oriented toward the left. Scale bars: 10 μm.
ROS Generation Is Coupled with Cell Delamination
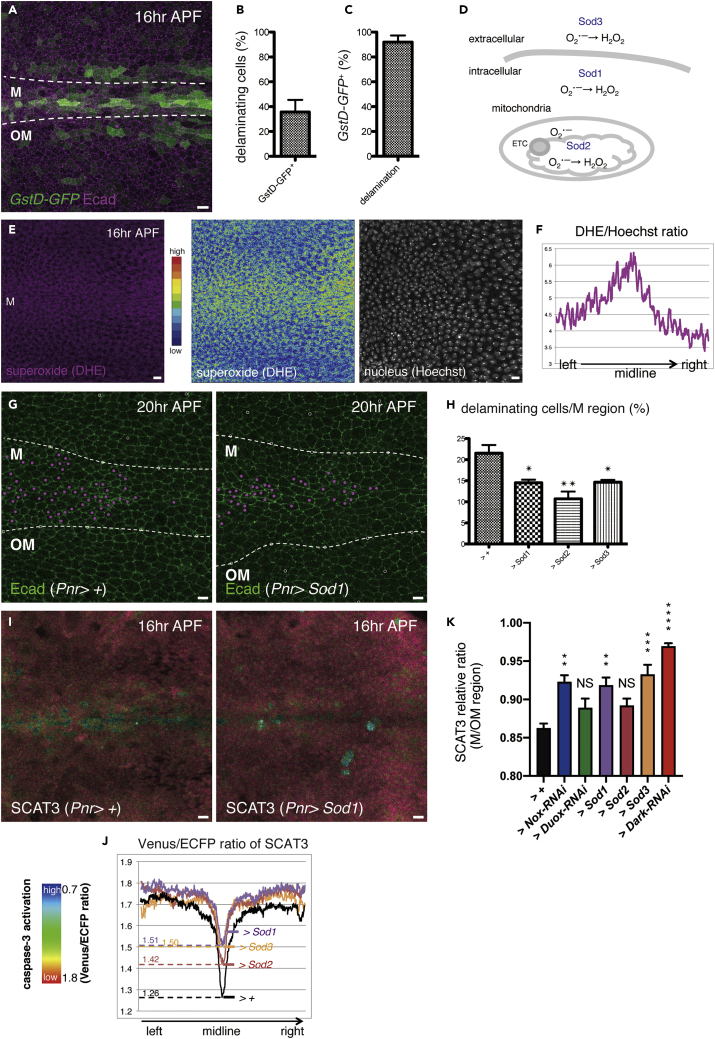
NADPH oxidases involve ROS production. To examine the ROS levels in this tissue, we used the in vivo sensor for oxidative stress, GstD-GFP (Sykiotis and Bohmann, 2008). The GFP signal pattern indicated a high level of ROS around the midline (Figure 5A). We thus tested the correlation between ROS generation and cell delamination. Although only approximately 40% of the cells positive for GstD-GFP underwent delamination (Figure 5B), almost all delaminating cells were labeled with GstD-GFP (Figure 5C). As in mammals, there are three superoxide dismutases (Sods) present in Drosophila. These include Sod1, Sod2, and Sod3, which are involved in the conversion of intracellular, mitochondrial, and extracellular O˙-2 to hydrogen peroxide (H2O2), respectively (Figure 5D). H2O2 oxidizes the sensor cysteine residues of Keap1, an E3-ligase of Nrf2. The oxidization of Keap1 inactivates E3-ligase activity and then Nrf2 is stabilized. Nrf2 translocates to nuclei, activates antioxidant response element, and induces the expression of genes such as GstD (Kansanen et al., 2013; Suzuki et al., 2019). Thus, H2O2 is likely to be accumulated in GstD-GFP-positive delaminating cells. To examine O˙-2 production, we injected dihydroethidium (DHE) with Hoechst 33342 (Figure 5E). When normalized to that of Hoechst 33342, the DHE signal demonstrated a higher level of O˙-2 generation in cells around the midline (Figure 5F). Besides, given that overexpressing each Sod solely suppressed the delamination rate (Figures 5G and 5H) and caspase-3 activation (Figures 5I and 5J), O˙-2 reduction and an increase of H2O2 could suppress delamination. To further confirm the requirement of O˙-2 production on caspase activation, we normalized the SCAT3 ratio of the M region by that of the OM region for comparison. Similar to in Dark-RNAi, we found that the relative ratios of SCAT3 in Nox-RNAi, Sod1, or Sod3 were significantly increased in M region cells compared with that of the control (Figure 5K). These data suggest an involvement of O˙-2 in caspase-3 activation around the midline.
Figure 5.
Reactive Oxygen Species (ROS) Generation Is Coupled with Cell Delamination
(A) An image of the z-projections of confocal stacks in the pupal notum of a live fly expressing GstD-GFP and Ecad:tdTomato (16 h after puparium formation; APF).
(B) The ratio of delamination in cells positive for GstD-GFP (from 16 to 30 h APF; three nota). Error bar indicates standard error of the mean.
(C) The ratio of GstD-GFP+ cells to delaminating cells (from 16 to 30 h APF; three nota). Error bar indicates standard error of the mean.
(D) A diagram representing the conversion of produced O˙-2 to H2O2 through superoxide dismutase (Sod).
(E) An image of the z-projections of confocal stacks in the pupal notum of a live fly after the injection of a mixture of DHE and Hoechst 33342.
(F) Averaged changes in the ratio of DHE signal to Hoechst 33342 signal (n = 4).
(G) Snapshots from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing Ecad::GFP (20 h APF). Magenta dots indicate cells that delaminated in 10 h (from 20 to 30 h APF).
(H) The ratio of cell delamination (from 20 to 30 h APF; control: four nota, versus Sod1, Sod2, or Sod3: three nota under the control of Pnr-Gal4). The p value was calculated by one-way analysis of variance (ANOVA) with Dunnett's test. ∗, p < 0.05 and ∗∗, p < 0.01. Error bars indicate standard error of the mean.
(I) Snapshots from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing SCAT3 under the control of Pnr-Gal4 (16 h APF).
(J) Averaged changes in the FRET ratio (the Venus signal to the ECFP signal) of SCAT3 (16 h APF; control: four nota, versus Sod1: six nota, Sod2: five nota, or Sod3: four nota, under the control of Pnr-Gal4).
(K) Averaged changes in the FRET ratio (the Venus signal to the ECFP signal) of SCAT3 (relative values of the M region to the OM region). The p value was calculated by one-way ANOVA with Dunnett's test. NS, not significant, ∗∗, p < 0.01, ∗∗∗, p < 0.001, and ∗∗∗∗, p < 0.0001. Error bars indicate standard error of the mean.
The boundaries between the midline (M) and outside the midline (OM) regions are outlined with dotted white lines. The anterior-to-posterior axes of all pupae are oriented toward the left. Scale bars: 10 μm. See also Figures S4 and S5.
H2O2 Blocks Cell Delamination after Caspase-3 Activation
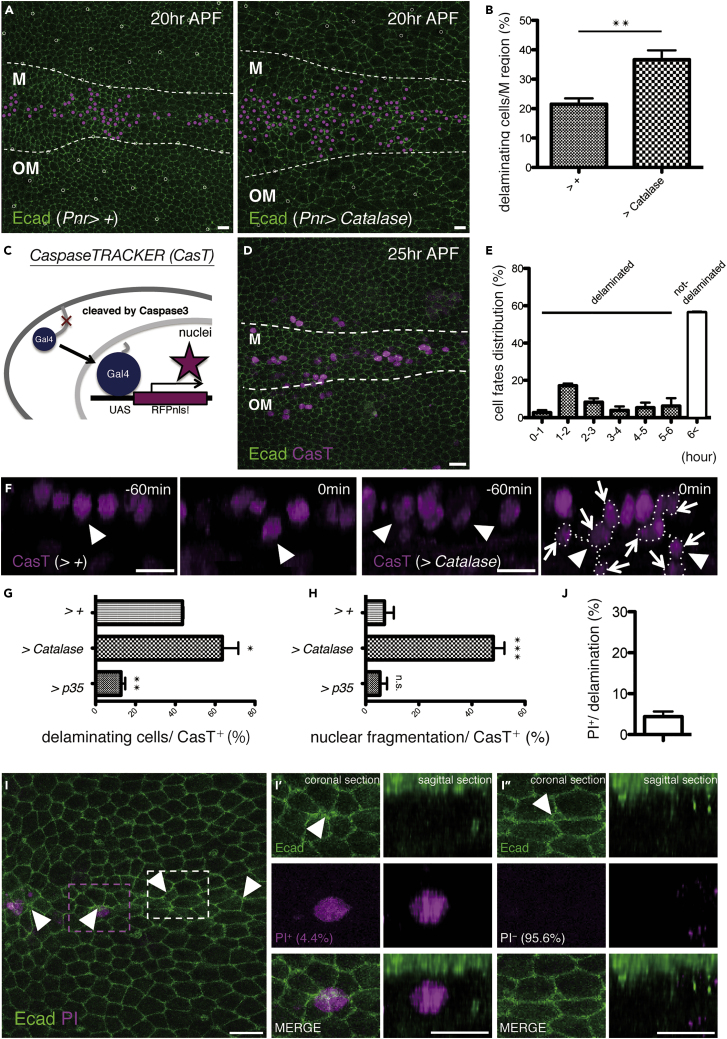
H2O2 is known to act in redox signaling without executing cell death (Sies, 2017). Given that the expression of the O˙-2 scavenger Sod promotes H2O2 generation, we can assume that delamination consists of the positive involvement of O˙-2 and negative involvement of H2O2. Indeed, we found that the H2O2 scavenger Catalase increased delamination (Figures 6A and 6B), suggesting the inhibitory impact of H2O2 on delamination. We therefore wished to determine the means by which H2O2 suppresses caspase-mediated cell delamination.
Figure 6.
H2O2 Prevents Delaminating Cells from Apoptosis
(A) Snapshots from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing Ecad::GFP (20 h after puparium formation; APF). Magenta dots indicate cells that delaminated in 10 h (from 20 to 30 h APF).
(B) The ratio of cell delamination (from 20 to 30 h APF; control: four nota, versus Catalase: three nota under the control of Pnr-Gal4). The p value was calculated by an unpaired two-tailed Student's t test. n.s., not significant and ∗∗, p < 0.01. Error bars indicate standard error of the mean.
(C) Diagram of CaspaseTRACKER.
(D and F) Snapshots from the movie, z-projections and y-projections of confocal stacks in the pupal notum of a live fly expressing and Ecad::GFP with CasT at 25 (D) and 31 h APF (F). Arrowheads and arrows indicate delaminating cells and cell fragmentation in delamination, respectively (F).
(E) Distribution of CasT+ cells that delaminated in the hours from 25 to 31 h APF (three nota, 71 cells). Error bars indicate standard error of the mean.
(G and H) The ratio of CasT+ cell delamination (G) and fragmentation in delamination (H) for 6 h (control: three nota, versus Catalase or p35: three nota under the control of Pnr-Gal4). The p value was calculated by one-way analysis of variance (ANOVA) with Dunnett's test. n.s., not significant, ∗, p < 0.05, ∗∗, p < 0.01, and ∗∗∗, p < 0.005. Error bars indicate standard error of the mean.
(I) A snapshot from the movie, z-projections of confocal stacks in the pupal notum of a live fly expressing Ecad::GFP after the injection of a mixture of propidium iodide (PI) and Hoechst 33342. A magnified image of delaminating cells stained (I′) or unstained (I″) with PI as shown in coronal and sagittal sections.
(J) The ratio of PI+ cells to delaminating cells (from 18 ± 2 to 40 ± 2 h APF; three nota, 230 cells). Error bar indicates standard error of the mean.
The boundaries between the midline (M) and outside the midline (OM) regions are outlined with dotted white lines. The anterior-to-posterior axes of all pupae are oriented toward the left. Scale bars: 10 μm.
See also Figures S4–S6 and Video S2 and Video S3.
SCAT3 monitoring revealed that Catalase expression did not affect caspase-3 activation (Figures S4A and S4B). Consistently, H2O2 and paraquat, which produce O˙-2 and H2O2, similarly triggered caspase-3 activation administered to cultured S2 Drosophila cells (Figures S5A and S5B). These data support the notion that both O˙-2 and H2O2 have the positive potential to drive caspase-3 activation. These results therefore suggest that O˙-2 reduction by Sods inhibits caspase-3 activation and H2O2 prevents delamination after caspase-3 activation. CaspaseTracker/CasExpress (thereafter, described as “CasT”) is a Gal4-based caspase-3 activation sensor (Ding et al., 2016; Tang et al., 2015). Here, 56.4% of the CasT+ cells detected at 25 h APF did not undergo delamination, even after 6 h (Figures 6C–6E). We then investigated whether CasT-induced genetic manipulations alter the cell delamination rate by expressing p35, thus inhibiting the caspase activation required for delamination. As expected, p35 expression under the control of CasT effectively prevented delamination (Figure 6G). Notably, CasT-driven Catalase expression significantly increased the delamination rate (Figures 6F and 6G). These findings demonstrate the inhibitory effect of H2O2 on cell delamination after caspase-3 activation.
H2O2 Prevents Delaminating Cells from Apoptotic Nuclear Fragmentation
Next, we found that less than 10% of the delaminating CasT+ cells showed nuclear fragmentation, a feature of typical apoptosis (Figures 6F and 6H, Video S2). In addition, the injection of a marker of late apoptosis, namely, propidium iodide (PI), into the pupa revealed that only 4.3% of the delaminating cells turned PI-positive (Figures 6I and 6J, Video S3). These results suggest that, although caspase-3 is activated in delaminating cells, this activation could be insufficient for executing apoptotic cell death. When Catalase was expressed in CasT+ cells, nuclear fragmentation was increased (Figures 6F and 6H), supporting the idea that H2O2 inhibits events occurring after initial caspase-3 activation (such as nuclear fragmentation). Therefore, cells undergo delamination without apparent apoptotic features. Taking this into consideration, the presence of both O˙-2 and intracellular H2O2 before and after caspase-3 activation leads to live cell delamination (Figure 7).
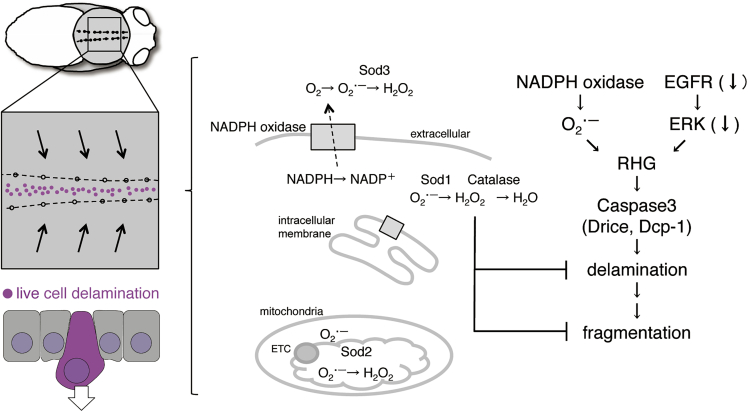
Figure 7.
A Model of the Mechanisms of Cell Delamination
Our summarized model for cell delamination in the midline region of Drosophila pupal notum. NADPH oxidase is shown as gray square. ETC, electron transfer chain; RHG, Reaper, Hid, and Grim.
GFP and mCherry signal were monitored after puparium formation (APF)
GFP and PI signals were monitored from 20 to 42 h after puparium formation (APF). Laser ablation was performed on the notum at 38 h APF. Upon the ablation, PI was immediately accumulated in cells. This indicates that PI was sufficiently supplied in the M region
Discussion
Caspase-activated cells have been identified in several tissue fusions (Ray and Niswander, 2012). However, the regulatory mechanisms and functions of caspases are poorly understood. After thorax fusion in Drosophila, epithelial crowding induces cell delamination. Here, we showed the regulation of NADPH oxidases for caspase-3 activation and delamination and suggested contrasting controls for caspase-dependent cell delamination via two types of ROS (O˙-2 and H2O2). Nox is localized on intracellular membranes (ER or redoxosome) as well as plasma membrane in mammalian cells (Bedard and Krause, 2007; Spencer and Engelhardt, 2014). Distribution of Nox neighboring proteins labeled by Nox::V5::TurboID suggests Nox localization not only on plasma membrane but also on intracellular membranes in M region (Figure S2C′). Thus, the suppressive effect of Sod1 and Sod3 on cell delamination indicates the involvement of intracellular and extracellular O˙-2 produced by Nox (Figure 7). Duox localizes on plasma membrane and produces O˙-2 in the extracellular space. The inhibitory effect of Duox-RNAi on cell delamination supports the idea that extracellular O˙-2 also promotes cell delamination. However, we could not deny the possibility that extracellular ROS promote cell delamination in a non-cell autonomous fashion.
H2O2 is known to be an activator of redox signaling and is involved in protective cellular functions. This includes an anti-apoptotic role in NF-κB activation (Sies, 2017). Given that the expression of the H2O2 scavenger Catalase significantly increased delamination and nuclear fragmentation (Figures 6F–6H), H2O2 likely blocks the events that occur after initial caspase-3 activation. Caspases cleave inhibitor of caspase-activated DNase (ICAD), and activated CAD promotes DNA fragmentation (Enari et al., 1998; Vaux and Korsmeyer, 1999). H2O2-driven oxidation of targets other than caspases, such as DNases like CAD, may affect nuclear fragmentation during delamination. In Caenorhabditis elegans, high oxidative stress delayed the appearance of apoptotic corpses (Lin et al., 2016). H2O2 prevents dimer formation of the Endonuclease G (EndoG) homolog CPS-6, which is involved in apoptotic DNA fragmentation, and the oxidized monomeric CPS-6 shows reduced nuclease activity (Lin et al., 2016). Notably, proline (P207) oxidation of EndoG/CPS-6 is suggested to be critical for the dissociation of the dimer into a monomeric form. This residue is conserved in Drosophila and humans.
MDCK cell extrusion initiated by apoptotic stimulation (caspase-8 activation or UV irradiation) is suppressed by inhibiting the calcium wave (Takeuchi et al., 2020) or via loss of desmosomal junctions (Thomas et al., 2020). Given that these extruding cells do not show apoptotic bodies, a common preventive mechanism could underlie cell fragmentation during M region cell delamination in the Drosophila pupal notum. Interestingly, two ROS may regulate distinct extrusion processes, with O˙-2 regulating calcium wave or desmosomal reorganization and H2O2 preserving the extruding cell shape.
Although most delamination is reported to be independent of caspase activation (Marinari et al., 2012), more detailed observations and genetic manipulations have revealed that apoptotic signaling is required for delamination (Levayer et al., 2016). These discrepant results are likely attributable to Gal4 drivers, specifically Pnr-Gal4 and the stronger driver Act-Gal4, and/or the UAS lines used for caspase inhibition. In the present study, although caspase dependency was confirmed, we still sought to determine whether caspase-dependent delamination is accompanied by apoptosis. Considering the CasT and PI experiments, the integrities of the nuclear and plasma membranes appeared to be intact during delamination (Figures 6D–6J).
In this study, we utilized several live imaging reporters for visualizing in vivo signaling events during cell delamination. We briefly summarized how respective reporters can be used for predicting cell delamination in Figure S6F. CasT can preferentially detect transient or weak caspase activation in cells (Figure 6C) but cannot detect apoptotic cells if CasT-cleaved cells are rapidly extruded and/or removed via engulfment before CasT-driven expression of reporter proteins. Although its sensitivity for detecting caspase activation appears to be lower than CasT, we used another indicator for caspase-3 activation (Schott et al., 2017). VC3Ai enables single cell visualization of caspase-3 activation to monitor real-time caspase activation and cell morphological dynamics during delamination (Figure S6A). Live imaging of delamination showed that caspase-3 is indeed activated during delamination (Figures S6B and S6C). Although the real-time reporter VC3Ai appears to be less sensitive to caspase-3 activation than CasT, it can detect the caspase-3 activity required to execute apoptosis (Zhang et al., 2013). Observations with this reporter determined that most VC3Ai+ cells in the M region were not fragmented, whereas those in the OM region showed typical apoptotic cell fragmentation (Figures S6D and S6E). The latter cells could be dead before or immediately after delamination, further highlighting the unique characteristics of caspase-3 activated cells in the M region. These findings also support the fact that the majority of delamination in the M region is live cell extrusion, despite caspase-3 activation (Figure 7).
Moreno et al. revealed that ERK downregulation in the pupal notum does not depend on the Spitz/EGF sequestration factor Argos (Moreno et al., 2019). This suggests that mechanical cues, but not diffusible factors, are involved in cell delamination. Here, we monitored Nox upregulation in delaminating cells. It has been reported that mechanical strain to embryoid bodies from embryonic stem cells induces expression of the NADPH oxidases Nox-1 and Nox-4, along with ROS generation. This leads to ERK1, ERK2, and JNK activation to promote cardiovascular differentiation (Schmelter et al., 2006). Although the involvement of mechanical stress in the EGFR/ERK and Nox pathways is largely unknown, such pathways activating cell survival and/or apoptotic signaling may be broadly used in multicellular organisms. The molecular mechanisms identified here in Drosophila may be evolutionally conserved and may therefore be beneficial for understanding how cell extrusion functions in epithelial tissue development and homeostasis.
Limitation of the Study
We conducted genetic manipulation for ROS generating and scavenging genes to test the involvement of ROS for cell delamination along the midline in the notum. Although our genetic analysis clearly showed the requirement of O2.--generating enzyme Nox and Duox for delamination, we have not detected the changes in the levels of O2.- during delamination. Given that O2.- has short lifetime, the detection of O2.- at the single cell level was technically very difficult. Thus, we cannot deny the possibility that Nox and/or Duox regulates delamination independent of O2.- generation. H2O2 is relatively stable, and the GstD-GFP reporter can be used for detection of accumulation of H2O2. Again, however, we could not exactly reveal the dynamics of H2O2 at the single cell level over time. Therefore, it is necessary to develop the monitoring technique for the dynamics of ROS with high spatiotemporal resolution to prove the differential associations of O2.- and H2O2 with cell delamination in vivo.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Masayuki Miura (miura@mol.f.u-tokyo.ac.jp).
Materials Availability
Materials and protocols used in this study are available from the authors upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Bohmann D, Hardwick JM, Chen CH, Hay BA, Richardson H, Freeman M, Uhlirova M, Levayer R, Moreno E, Kuranaga E, Nakamura Y, Nagata S, Schott S, Monier B, Suzanne M, Tabata T, the Bloomington Drosophila Stock Center (BDSC), the Vienna Drosophila Resource Center (VDRC), the Drosophila Genetic Resource Center (DGRC) Kyoto, and the FlyORF for providing fly strains. We also thank all members of the Miura laboratory for their valuable discussions, especially Kashio S, Katsuyama T, Koto A, Obata F, and Yamaguchi Y. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI Grant Number 16H06385 to M.M. and 15H04375 to T.C.). This study was also supported by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP17gm0610004 and JP20gm5010001 awarded to M.M. Y.F. is a research fellow of the Japan Society for the Promotion of Science.
Author Contributions
Y.F. and M.M. conceived this study. Y.F. and M.M. designed the experiments. Y.F. and N.S. performed the experiments and analyzed the data. M.M. and T.C. supervised the study. Y.F., N.S., T.C., and M.M. wrote the manuscript. All authors have edited and approved the final manuscript.
Declarations of Interests
The authors declare no competing or financial interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101413.
Supplemental Information
References
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Branon T.C., Bosch J.A., Sanchez A.D., Udeshi N.D., Svinkina T., Carr S.A., Feldman J.L., Perrimon N., Ting A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018;36:880–887. doi: 10.1038/nbt.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo R., Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- Cuervo R., Valencia C., Chandraratna R.A.S., Covarrubias L. Programmed cell death is required for palate shelf fusion and is regulated by retinoic acid. Dev. Biol. 2002;245:145–156. doi: 10.1006/dbio.2002.0620. [DOI] [PubMed] [Google Scholar]
- Ding A.X., Sun G., Argaw Y.G., Wong J.O., Easwaran S., Montell D.J. CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo. Elife. 2016;5:e10936. doi: 10.7554/eLife.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhoffer G.T., Loftus P.D., Yoshigi M., Otsuna H., Chien C.B., Morcos P.A., Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Evans C.J., Olson J.M., Ngo K.T., Kim E., Lee N.E., Kuoy E., Patananan A.N., Sitz D., Tran P., Do M.T. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat. Methods. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman A.I. Electron microscope study of palate fusion in mouse embryos. Dev. Biol. 1968;18:93–116. doi: 10.1016/0012-1606(68)90038-9. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y., Kosakamoto H., Chihara T., Miura M. Non-apoptotic function of Drosophila caspase activation in epithelial thorax closure and wound healing. Development. 2019;146:dev160937. doi: 10.1242/dev.169037. [DOI] [PubMed] [Google Scholar]
- Hinrichsen K. The early development of morphology and patterns of the face in the human-embryo. Adv. Anat. Embryol. Cell. 1985;98:1–76. doi: 10.1007/978-3-642-70754-4. [DOI] [PubMed] [Google Scholar]
- Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koto A., Kuranaga E., Miura M. Apoptosis ensures spacing pattern formation of Drosophila sensory organs. Curr. Biol. 2011;21:278–287. doi: 10.1016/j.cub.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Lee P.T., Zirin J., Kanca O., Lin W.W., Schulze K.L., Li-Kroeger D., Tao R., Devereaux C., Hu Y.H., Chung V. A gene-specific T2A-GAL4 library for Drosophila. Elife. 2018;7:e35574. doi: 10.7554/eLife.35574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levayer R., Dupont C., Moreno E. Tissue crowding induces caspase-dependent competition for space. Curr. Biol. 2016;26:670–677. doi: 10.1016/j.cub.2015.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Young J.F., Sun J.J. NADPH oxidase-generated reactive oxygen species in mature follicles are essential for Drosophila ovulation. Proc. Natl. Acad. Sci. U S A. 2018;115:7765–7770. doi: 10.1073/pnas.1800115115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.L.J., Nakagawa A., Skeen-Gaar R., Yang W.Z., Zhao P., Zhang Z., Ge X., Mitani S., Xue D., Yuan H.S. Oxidative stress impairs cell death by repressing the nuclease activity of mitochondrial endonuclease G. Cell Rep. 2016;16:279–287. doi: 10.1016/j.celrep.2016.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinari E., Mehonic A., Curran S., Gale J., Duke T., Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484:542–545. doi: 10.1038/nature10984. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E., Knust E. Epithelial morphogenesis: filopodia at work. Curr. Biol. 2001;11:R28–R31. doi: 10.1016/s0960-9822(00)00039-7. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E., Pastor-Pareja J.C., Garcia-Bellido A. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc. Natl. Acad. Sci. U S A. 2000;97:7888–7893. doi: 10.1073/pnas.97.14.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Alvarez C., Tudela C., Perez-Miguelsanz J., O'Kane S., Puerta J., Ferguson M.W.J. Medial edge epithelial cell fate during palatal fusion. Dev. Biol. 2000;220:343–357. doi: 10.1006/dbio.2000.9644. [DOI] [PubMed] [Google Scholar]
- Moreno E., Valon L., Levillayer F., Levayer R. Competition for space induces cell elimination through compaction-driven ERK downregulation. Curr. Biol. 2019;29:23–34 e8. doi: 10.1016/j.cub.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori C., Nakamura N., Okamoto Y., Osawa M., Shiota K. Cytochemical identification of programmed cell-death in the fusing fetal mouse palate by specific labeling of DNA fragmentation. Anat. Embryol. 1994;190:21–28. doi: 10.1007/BF00185843. [DOI] [PubMed] [Google Scholar]
- Ray H.J., Niswander L. Mechanisms of tissue fusion during development. Development. 2012;139:1701–1711. doi: 10.1242/dev.068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Schmelter M., Ateghang B., Helmig S., Wartenberg M., Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. Faseb J. 2006;20:1182. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- Schott S., Ambrosini A., Barbaste A., Benassayag C., Gracia M., Proag A., Rayer M., Monier B., Suzanne M. A fluorescent toolkit for spatiotemporal tracking of apoptotic cells in living Drosophila tissues. Development. 2017;144:3840–3846. doi: 10.1242/dev.149807. [DOI] [PubMed] [Google Scholar]
- Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N.Y., Engelhardt J.F. The basic biology of redoxosomes in cytokine-mediated signal transduction and implications for disease-specific therapies. Biochemistry. 2014;53:1551–1564. doi: 10.1021/bi401719r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Muramatsu A., Saito R., Iso T., Shibata T., Kuwata K., Kawaguchi S.I., Iwawaki T., Adachi S., Suda H. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. 2019;28:746–758 e744. doi: 10.1016/j.celrep.2019.06.047. [DOI] [PubMed] [Google Scholar]
- Sykiotis G.P., Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K., Nagai T., Miyawaki A., Miura M. Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J. Cell Biol. 2003;160:235–243. doi: 10.1083/jcb.200207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Narumi R., Akiyama R., Vitiello E., Shirai T., Tanimura N., Kuromiya K., Ishikawa S., Kajita M., Tada M. Calcium wave promotes cell extrusion. Curr. Biol. 2020;30:670–681.e6. doi: 10.1016/j.cub.2019.11.089. [DOI] [PubMed] [Google Scholar]
- Tang H.L., Tang H.M., Fung M.C., Hardwick J.M. In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity. Sci. Rep. 2015;5:9015. doi: 10.1038/srep09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Ladoux B., Toyama Y. Desmosomal junctions govern tissue integrity and actomyosin contractility in apoptotic cell extrusion. Curr. Biol. 2020;30:682–690.e5. doi: 10.1016/j.cub.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Vaux D.L., Korsmeyer S.J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wang Y., Branicky R., Noe A., Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H.T., Wan Z.Y., Li S., Zhang Y. Spatiotemporal production of reactive oxygen species by NADPH Oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell. 2014;26:2007–2023. doi: 10.1105/tpc.114.125427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Shinotsuka N., Nonomura K., Takemoto K., Kuida K., Yosida H., Miura M. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J. Cell Biol. 2011;195:1047–1060. doi: 10.1083/jcb.201104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang X., Cui W., Wang W., Zhang H., Liu L., Zhang Z., Li Z., Ying G., Zhang N. Visualization of caspase-3-like activity in cells using a genetically encoded fluorescent biosensor activated by protein cleavage. Nat. Commun. 2013;4:2157. doi: 10.1038/ncomms3157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DsRed::nls was monitored from 18 to 35.5 h APF after puparium formation (APF) using an EMCCD camera (ImagEM X2, Hamamatsu) with an Olympus MVX10 macroscope.
GFP and mCherry signal were monitored after puparium formation (APF)
GFP and PI signals were monitored from 20 to 42 h after puparium formation (APF). Laser ablation was performed on the notum at 38 h APF. Upon the ablation, PI was immediately accumulated in cells. This indicates that PI was sufficiently supplied in the M region