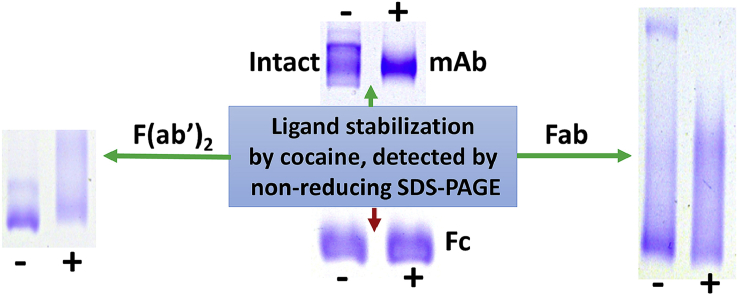
Abstract
Monoclonal antibodies are very useful tools in experimental biology, as well as being valuable and effective therapeutic drugs. They can be targeted against proteins with varied functions, or against small molecules of interest to both researchers and clinicians, such as drugs of abuse, including cocaine. Since there is no currently FDA approved pharmacological treatment for cocaine abuse, our laboratory has developed an anti-cocaine mAb for the treatment of cocaine use disorders. This humanized anti-cocaine antibody, named h2E2, has been thoroughly characterized both functionally and structurally, in preparation for the start of clinical development. We previously showed that this mAb could be characterized by sequential thermal unfolding of antibody domains using non-reducing SDS-PAGE. We also demonstrated that ligand-induced protein stabilization can be used to quantitatively measure cocaine and cocaine metabolite binding to the h2E2 mAb, utilizing differential scanning fluorimetry. Here, we demonstrate the utility of non-reducing SDS-PAGE for the qualitative assessment of binding of cocaine and some of its metabolites, both to the intact mAb, as well as to fragments containing the antigen binding site (Fab and F(ab’)2 fragments). These results clearly show a ligand concentration dependence of the stabilization of the cocaine binding domain in non-reducing SDS-PAGE, as well as visually differentiating the relative binding affinities of various cocaine metabolites. Thus, non-reducing SDS-PAGE is a simple and widely available technique that is useful as a measure of binding of cocaine and its metabolites to the h2E2 mAb, and it is likely that this technique will also be applicable to other small molecule-directed mAbs.
Keywords: Monoclonal antibody, Non-reducing SDS-PAGE, Cocaine binding, Cocaine metabolites, Electrophoretic migration, Antibody domain unfolding
Abbreviations: mAb, monoclonal antibody; h2E2, humanized anti-cocaine monoclonal antibody; NR SDS-PAGE, non-reducing SDS-PAGE; DSF, differential scanning fluorimetry; CE, cocaethylene; COC, cocaine; BE, benzoyl ecgonine; NC, norcocaine; EME, ecgonine methyl ester; EG, ecgonine
Graphical abstract
Highlights
-
•
Cocaine and metabolite mAb binding are visually assessed by non-reducing SDS-PAGE.
-
•
Ligand-induced changes are observed with the intact mAb, F(ab’)2, and Fab fragments.
-
•
No ligand-induced changes in gel bands are observed for the Fc mAb fragment.
-
•
The ligand-induced differential banding patterns are ligand concentration dependent.
-
•
High affinity cocaine metabolites cause the effect, low affinity metabolites do not.
1. Introduction
Monoclonal antibodies (mAbs) are important experimental and therapeutic agents, widely used in basic and translational research. Most mAbs are directed against proteins, where they are used to identify, localize, and/or modulate the activity of their targets. Unlike most small molecule drugs, mAbs typically have very good selectivity for their targets, and thus the potential for fewer clinical side effects and misleading research results due to decreased cross-reactivities with unintended targets.
In contrast to opioid addiction for which the antagonist naloxone and the partial agonist buprenorphine are widely used, there is a lack of suitable small molecule pharmacotherapies for the treatment of cocaine abuse [1]. This has stimulated the research and development of vaccines and mAbs directed against other drugs of abuse, including cocaine. Thus, our laboratory has developed and characterized a high affinity (nM) anti-cocaine mAb, which also binds the active metabolites of cocaine, cocaethylene (CE) and benzoyl ecgonine (BE) with high affinity, but has low affinity for other non-pharmacologically active metabolites of cocaine, such as norcocaine (NC), ecgonine methyl ester (EME), and, ecgonine (EG). Development of this h2E2 anti-cocaine mAb has successfully progressed to the point where it will soon be entering Phase 1 clinical trials.
In addition to functional studies in rats and mice [[2], [3], [4], [5]], our laboratory has also performed many structural and binding studies, using a variety of techniques [6,7]. We developed a method for measuring ligand binding by intrinsic mAb fluorescence quenching [8], as well as by ligand stabilization against thermal denaturation, using extrinsic fluorescent dyes and the differential scanning fluorimetry (DSF) method [9]. In addition, we demonstrated that non-reducing SDS-PAGE can be used to measure sequential, discrete denaturation events, as evidenced by bands of differing mobilities on gels, which can be associated with the differential thermal stabilities of the various protein domains of the h2E2 mAb (i.e., the CH3, CH2, and the Fab protein structural domains [10,11]).
In this current work, we utilized the technique of non-reducing SDS-PAGE to demonstrate that cocaine and some of its metabolites can stabilize the Fab h2E2 mAb domain against denaturation by SDS, and that the degree of this stabilization is related to both the concentration of the ligand, and to the affinity for the ligand recognition site of the compound used. The relative affinities of the cocaine-derived ligands qualitatively measured by non-reducing SDS-PAGE are consistent with results obtained using other established methods to quantitate ligand binding and affinity, including radioligand binding, ELISA, fluorescence quenching, and differential scanning fluorimetry assays. It is likely that the approach presented in this study will also be applicable to other mAbs, and some stable proteins, which are capable of binding small molecules and drugs with high affinity.
2. Materials and methods
2.1. Materials
The generation, production, and purification of the h2E2 anti-cocaine monoclonal antibody was previously described [2], and the recombinant h2E2 mAb was used as supplied by the manufacturer, Catalent PharmaSolutions, Inc., (Madison, WI). The purity, structure, and function of the recombinant mAb protein have been well characterized in our laboratory [6,8,12,13]. Generation and purification of the h2E2 Fab fragment by Endo-Lys-C proteolytic cleavage was described previously [8], as was the generation and purification of the h2E2 F(ab’)2 and Fc fragments by IdeS proteolysis using the FragIt kit purchased from Genovis [12]. Acrylamide, bisacrylamide, and all reagents used to pour, run, and stain SDS-PAGE gels were from BioRad. Pre-stained SDS-PAGE molecular weight standards (10–180 kDa) were purchased from SMOBIO Technology, Inc., catalogue # PM1600. Peptide N-glycosidase-F (PNGase-F) deglycosylation enzyme was purchased from New England Biolabs. For all h2E2 mAb ligands used in this study, 10 mM ligand stock solutions were made in distilled water from solids obtained from Research Triangle Institute (RTI): CE, cocaethylene (RTI batch 9885-1022-125B); COC, cocaine (RTI batch 14201-12A); BE, benzoyl ecgonine (RTI batch 7474-1022-85A); NC, norcocaine (RTI batch 7778-1022-36G), EME, ecgonine methyl ester (RTI batch 8542-1022-8A); and EG, ecgonine (RTI batch 7593-1022-183A).
2.2. Methods
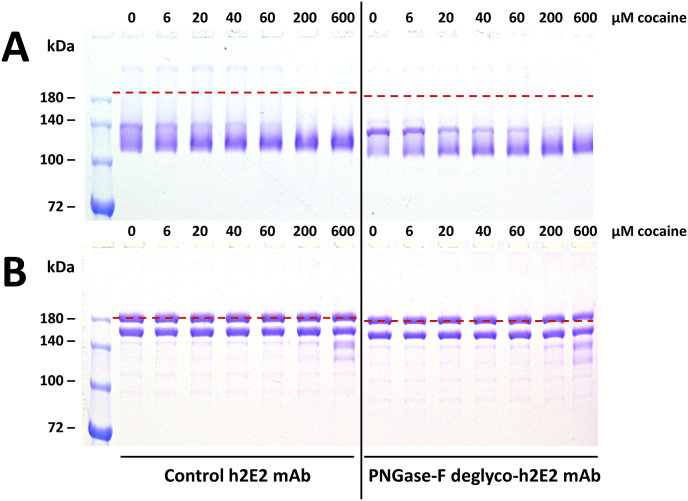
The 7% SDS-PAGE gels were poured, run, and stained with Coomassie Blue, basically as described by Laemmli [14], following our previously published protocols [10,11]. For some samples shown in Fig. 4, deglycosylation of the single glycan site on each heavy chain of the intact h2E2 mAb was performed as described previously, using peptide N-glycosidase-F [8]. The completeness of deglycosylation in this sample was demonstrated by electrophoretic mobility shift of the heavy chain on reducing SDS-PAGE after deglycosylation (data not shown).
Fig. 4.
The effect of 0, 6, 20, 40, 60, 200, and 600 μM cocaine on the non-reducing SDS-PAGE banding pattern for the control intact h2E2 mAb and the deglycosylated intact h2E2 mAb. 10 μL, containing 2 μg of each sample was loaded in each well of a 7% acrylamide non-reducing SDS-PAGE gel, and stained with Coomassie blue. The concentration of cocaine added to the samples are indicated above each well. The red dashed lines indicate the electrophoretic migration positions of the totally denatured proteins (proteins boiled for 5 min in the SDS sample buffer without a reductant). No bands were visible in the bottom sections of these two gels, which were cropped to make a more compact figure. The vertical black line was superimposed on the image of the two aligned gels shown in Panels A and B, and each Panel image was from a single gel, with the samples loaded in the order shown. Panel A (top) shows the banding pattern gel results when the samples were run less than 5 min after dilution into the non-reducing SDS-PAGE sample buffer (the same sample treatment used in Fig. 1, Fig. 2, Fig. 3), while Panel B (bottom) shows the banding pattern of aliquots of the same samples shown in Panel A, after incubation of those samples in the sample buffer for 24 h at 22 °C. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
At ambient temperature (22 °C), antibodies and antibody fragments (at 5–20 mg/ml) were incubated with concentrated ligands for 5 min in the buffers in which the mAb or fragments were purified, after which the samples were diluted with Laemmli non-reducing sample buffer (containing 5% SDS, 125 mM Tris-Cl, pH = 6.8, 12.5% glycerol, and 0.005% bromophenol blue tracking dye), to yield final protein concentrations of 0.2 mg/ml, and the final concentration of ligands indicated in each figure or figure legend (if not specified, a final ligand concentration of 600 μM ligand in non-reducing sample buffer was used). The samples were diluted in SDS sample buffer at ambient temperature, and loaded onto the room temperature SDS-PAGE gel as soon as possible (within 5 min) after dilution. 10 μL (2 μg) of each sample was loaded into individual wells of 15 well, 1.5 mm thick 7% acrylamide gels. As usual, no SDS or ligand was included in the gel polymerization solutions used to pour the gels. Most experiments shown were performed on a single gel, with the exception of the data shown in Fig. 1, which is the combination of 2 gels run on different days (Fig. 1 was designed to be directly comparable to Fig. 3, and there are not enough sample wells to run all the samples analyzed in Fig. 1 on the same gel). In all cases, electrophoresis was performed until just before the bromophenol blue tracking dye reached the bottom of the gel. Pre-stained molecular weight standards (10 μL) were also run on each gel. Gels were stained at room temperature for 30–45 min with Coomassie blue, and destained for 4–20 h prior to photography.
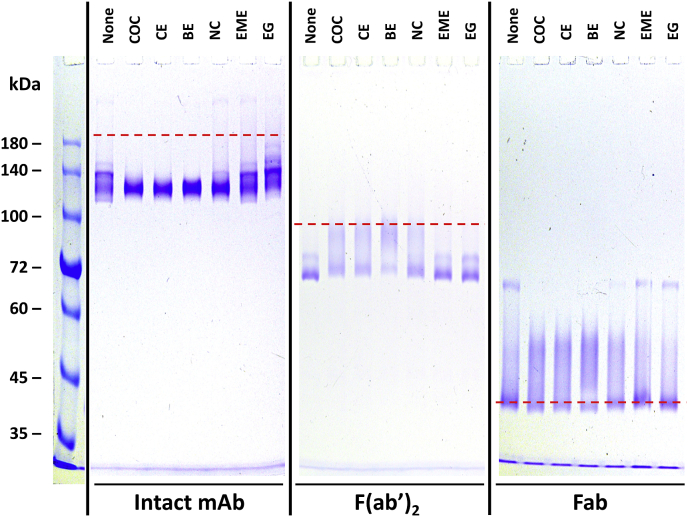
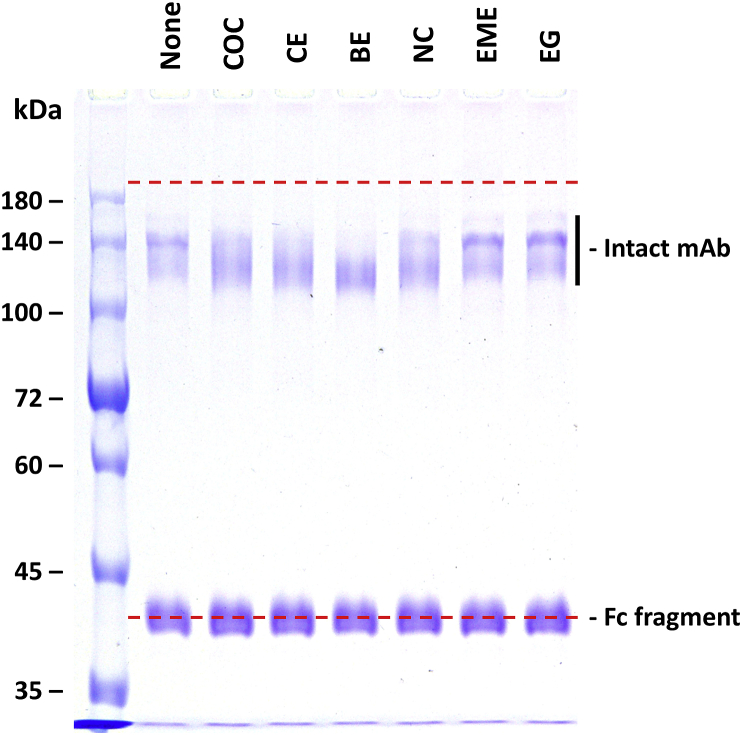
Fig. 1.
The effect of 600 μM ligand on the non-reducing SDS-PAGE banding pattern for the intact h2E2 mAb and its F(ab’)2 and Fab fragments. 10 μL, containing 2 μg of each sample was loaded in each well of a 7% acrylamide non-reducing SDS-PAGE gel, and stained with Coomassie blue. The red dashed lines indicate the electrophoretic migration positions of the totally denatured proteins (proteins boiled for 5 min in the SDS sample buffer without a reductant). The ligand included in each sample is indicated at the top of each sample well: None, no ligand; COC, cocaine; CE, cocaethylene; BE, benzoyl ecgonine; NC, norcocaine, EME, ecgonine methyl ester; EG, ecgonine. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
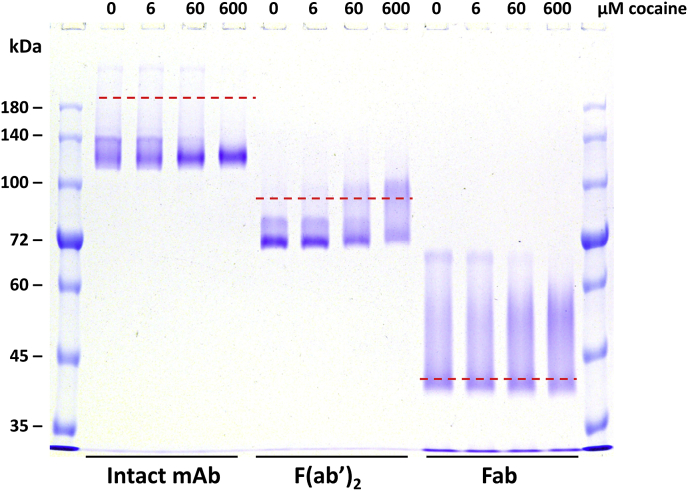
Fig. 3.
The effect of 0, 6, 60, and 600 μM cocaine on the non-reducing SDS-PAGE banding pattern for the intact h2E2 mAb and its F(ab’)2 and Fab fragments. 10 μL, containing 2 μg of each sample was loaded in each well of a 7% acrylamide non-reducing SDS-PAGE gel, and stained with Coomassie blue. The red dashed lines indicate the electrophoretic migration positions of the totally denatured proteins (proteins boiled for 5 min in the SDS sample buffer without a reductant). The ligand included in each sample is indicated at the top of each sample well: None, no ligand; COC, cocaine; CE, cocaethylene; BE, benzoyl ecgonine; NC, norcocaine, EME, ecgonine methyl ester; EG, ecgonine. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Results
Fig. 1 shows the no sample heating, non-reducing SDS-PAGE results obtained with prior incubation with 600 μM of cocaine or with one of the five cocaine metabolite ligands, using either purified intact h2E2 anti-cocaine mAb, the F(ab’)2 fragment, or the Fab fragment of this h2E2 mAb. The dashed red lines were superimposed on the gel images to indicate the electrophoretic migration positions of the non-reduced, fully denatured (boiled for 5 min) intact mAb, the F(ab’)2 fragment, and the Fab fragment of this h2E2 mAb, respectively from left to right (previously published data, see Ref. [10,11]). “None” means that no ligand was added to the protein sample before electrophoresis. The results without ligands present are consistent with our previously reported, non-reducing SDS-PAGE results in the absence of ligands without heating prior to electrophoresis [10,11]. However, it is obvious that some of the ligands change the banding pattern of each protein species analyzed. In addition, the high affinity ligands, cocaine (COC, KD ≈ 4 nM), cocaethylene (CE, KD ≈ 1 nM), and benzoyl ecgonine (BE, KD ≈ 20 nM) are all seen to yield similar banding patterns for the intact mAb, the F(ab’)2 fragment, and the Fab fragment, which are different from the no ligand controls. The intermediate affinity ligand, norcocaine (NC, KD ≈ 8 μM) gives rise to an intermediate banding pattern between the no ligand control and the high affinity ligand patterns, and the low affinity ligands, ecgonine methyl ester (EME, KD > 40 μM) and ecgonine (EG, KD > 200 μM) (ligand affinity estimates are derived from Ref. [8,9]), give rise to electrophoretic banding patterns that appear the same as the no ligand controls (“None” in the figure). For the intact mAb, this ligand stabilization effect is seen as a tighter, faster migrating band at about 120 kDa, while for the F(ab’)2 fragment and the Fab fragment, the ligand stabilized bands are observed as slower migrating, broad bands, at about 80–90 kDa and 45–55 kDa, respectively. In addition, in the case of the Fab fragment, a slow migrating tight band of apparent Mw of ≈65 kDa is observed with no ligand or the low affinity ligands (EME and EG), but disappears in the presence of the high affinity ligands, COC, CE, and BE, and is fainter in the presence of the intermediate affinity ligand, norcocaine (NC).
The data shown in Fig. 2 reiterate the differential ligand stabilization effects seen of the intact mAb, and importantly, in the exact same sample, show a complete lack of ligand stabilization for the non-ligand binding Fc fragment portion of the antibody. The sample analyzed on the gel shown in Fig. 2 was the result of an incomplete cleavage of the intact antibody using the FragIt IdeS protease kit, which normally generates the F(ab’)2 and the Fc fragments by completely cleaving the intact antibody, but in this case did not proteolyze all of the intact mAb present. This incomplete digest was purified using the Capture Select cartridge column contained in the FragIt kit, which specifically binds and purifies only Fc containing species (which is why the F(ab’)2 fragment which was also generated is not present in this sample).
Fig. 2.
The effect of 600 μM ligand on the non-reducing SDS-PAGE banding pattern for the intact h2E2 mAb and its Fc fragment. 10 μL, containing 2 μg of each sample was loaded in each well of a 7% acrylamide non-reducing SDS-PAGE gel, and stained with Coomassie blue. The red dashed lines indicate the electrophoretic migration positions of the totally denatured proteins (proteins boiled for 5 min in the SDS sample buffer without a reductant). The ligand included in each sample is indicated at the top of each sample well: None, no ligand; COC, cocaine; CE, cocaethylene; BE, benzoyl ecgonine; NC, norcocaine, EME, ecgonine methyl ester; EG, ecgonine. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To further characterize the ligand stabilization and show that it is dependent on the ligand (cocaine) concentration added prior to electrophoresis, 0, 6, 60, and 600 μM final concentrations of cocaine were added to the intact mAb, the F(ab’)2 fragment, and the Fab fragment, and the samples analyzed by NR SDS-PAGE (Fig. 3). The same effects on the electrophoretic mobilities of all three species which are seen in Fig. 1 are observed, but clearly in a concentration dependent fashion. The change in banding pattern is evident at 60 and 600 μM cocaine, but virtually non-existent at 6 μM cocaine (the concentrations of the three proteins are all 0.2 mg/ml, which correspond to about 1.4 μM, 2.1 μM, and 4.4 μM for intact mAb, F(ab)2 and Fab fragment, respectively).
Fig. 4 recapitulates the concentration dependence of cocaine stabilization of the intact mAb, using more concentrations of the cocaine ligand, as well as showing the ligand-induced stabilization effect on both natively glycosylated and PNGase-F deglycosylated h2E2 mAb (Fig. 4A). The concentration-dependent, cocaine-induced mobility shift to a faster migrating, more compact species is even more pronounced in the deglycosylated mAb sample, and the concentration dependence is apparent, and very similar, in both samples. To determine the time sensitivity of the cocaine-induced stabilization of the intact antibody in the SDS non-reducing sample buffer prior to electrophoresis, aliquots of the very same samples that were run on the gel after less than 5 min following dilution with the SDS sample buffer (Panel A) were run on an identical 7% NR SDS-PAGE gel after incubation of these samples for 24 h at room temperature (22 °C) in the SDS sample buffer, as shown in Panel B. The slight shift in electrophoretic mobility due to deglycosylation is more evident in Panel B, due to the tighter bands. Also, the bands and apparent molecular weights have shifted upwards compared to the 5-min samples, due to more complete denaturation of the domains in the mAb after 24 h of incubation in SDS. These bands observed in Panel B are consistent with previously published results, with the slowest migrating, highest apparent Mw band seen in Panel B migrating at the same position (apparent Mw) as the boiled, completely denatured h2E2 mAb (≥180 kDa, see Ref. [10,11]), indicated by the dashed red lines in the figure. Only at the highest cocaine concentrations (200 and 600 μM) are there traces of the 2 lower apparent Mw bands indicative of more incompletely denatured mAb.
4. Discussion
Our previous studies indicated that non-reducing SDS-PAGE can be used to monitor sequential denaturation of antibody domains in the presence of SDS, and demonstrated the presence of multiple, discrete staining bands on non-reducing SDS-PAGE that are attributable to antibody species with varying domains unfolded by SDS as a function of time and temperature [10,11]. Another study published by our laboratory demonstrated that ligand-induced protein stabilization to thermal denaturation can be used to measure ligand binding affinities using the rotor fluorescent dye DASPMI (4-(4-(Dimethylamino)styryl)-N-Methylpyridinium Iodide), and the differential scanning fluorimetry technique (DSF, see Ref. [9]). In that work, the quantitative relative binding affinities of cocaine and the same 5 cocaine metabolites used in the current study were determined by DSF. In the present study, we investigated whether the non-reducing SDS-PAGE technique could be used as a qualitative measure of comparative ligand binding to the same anti-cocaine h2E2 mAb, via ligand stabilization against SDS-induced unfolding of the ligand binding domain in the mAb at ambient temperature. We used the DSF-derived relative ligand binding affinities from the earlier study to correlate the relative quantitative ligand binding affinities with the present qualitative gel banding results. This involved visual inspection of the differential gel banding patterns for the intact antibody, as well as the F(ab’)2, and Fab antibody fragments, after incubation with SDS at ambient temperature in the presence of various ligands. We noted protection from SDS-induced denaturation conveyed by the high affinity (nM affinity ligands such as cocaine, cocaethylene and benzoyl ecgonine), but not by the low affinity (high μM KD ligands, ecgonine methyl ester and ecgonine). The intermediate affinity ligand, norcocaine, gave rise to intermediate stability changes and banding pattern effects. These effects were demonstrable for all antibody species capable of binding ligand (the intact mAb, the F(ab’)2 fragment, and the Fab fragment), but importantly, not for the non-ligand binding Fc fragment.
The ligand concentration dependent nature of the stabilization and consequent change in gel banding pattern was also clearly demonstrated using cocaine as the ligand (see Fig. 3, Fig. 4). It should be noted that the ligand concentrations needed to achieve these banding pattern effects are much higher than the affinities for the ligands measured under non-denaturing conditions (i.e., in the absence of SDS). This is due to at least three factors. First, the ligand affinities are undoubtedly lower in the presence of this high concentration of SDS, just as they were at the elevated temperatures used to measure them by differential scanning fluorimetry (measured at the midpoint denaturation temperature of about 70 °C, see Ref. [9]). Second, ligands were introduced only with the samples, with no ligands co-polymerized in the gels. This would lead to dissociation of ligand as the protein-ligand complex is electrophoresed down the gel, and also to lower free concentrations of ligands as the antibody/antibody fragment migrates down the gel. Third, the concentration of protein (and therefore the number of ligand binding sites) was relatively high in the original samples (≈3–5 μM binding sites), necessitating high concentrations of ligands (≥100 μM) to saturate the ligand binding sites in the samples applied to the gels, and causing some ligand depletion reduction of free ligand concentrations. The use of about 2 μg of protein sample (10 μL) loaded per well was deemed optimal for these experiments, since that amount resulted in sufficient staining intensity by Coomassie blue dye, while allowing very good resolution of the protein bands on the gels.
The current study and technique do not allow the quantitation of ligand binding constants or the assignment of stained gel bands to different partially denatured species of antibody and antibody fragments. However, the data shown in this work suggests that the simple, inexpensive, and ubiquitous method of non-reducing SDS-PAGE is useful to qualitatively assess the relative binding affinities of cocaine and its metabolites to the h2E2 anti-cocaine mAb and its fragments. Although obviously this is a qualitative technique, it is still useful to ascertain and evaluate large differences in affinities of ligands for their respective antibodies or binding proteins. In addition, one application not demonstrated here would be the detection of a relatively large loss of ligand affinity occurring after some chemical or sequence modification has been performed on the antibody or binding protein. The current results suggest that the KD of the ligands may need to be less than several hundred nM for this technique to demonstrate substantial banding pattern differences evident on non-reducing gels (and, if a binding protein other than an antibody is used, that the protein must be fairly stable to denaturation by SDS at room temperature). Regardless, such a simple, fast, inexpensive, and available analysis may prove generally useful for the qualitative ligand binding characterization of antibodies and other proteins having sub-micromolar affinities for their small molecule targets.
Declaration of competing interest
Dr. Norman is named as a co-inventor on patents for the matter and use of the h2E2 humanized anti-cocaine monoclonal antibody.
Acknowledgements
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse Grant U01DA039550. We are grateful to Catalent PharmaSolutions, Inc. (Madison, WI) for providing the recombinant humanized h2E2 anti-cocaine mAb protein expressed using their GPex® technology.
Contributor Information
Terence L. Kirley, Email: terry.kirley@uc.edu.
Andrew B. Norman, Email: rew.norman@uc.edu.
References
- 1.Vocci F., Ling W. Medications development: successes and challenges. Pharmacol. Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Norman A.B., Gooden F.C., Tabet M.R., Ball W.J. A recombinant humanized anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in rats. Drug Metabol. Dispos.: Biol. Fate. Chem. 2014;42:1125–1131. doi: 10.1124/dmd.114.057034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wetzel H.N., Tabet M.R., Ball W.J., Norman A.B. The effects of a humanized recombinant anti-cocaine monoclonal antibody on the disposition of cocaethylene in mice. Int. Immunopharm. 2014;23:387–390. doi: 10.1016/j.intimp.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wetzel H.N., Tsibulsky V.L., Norman A.B. The effects of a repeated dose of a recombinant humanized anti-cocaine monoclonal antibody on cocaine self-administration in rats. Drug Alcohol Depend. 2016;168:287–292. doi: 10.1016/j.drugalcdep.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wetzel H.N., Zhang T., Norman A.B. A mathematical model of a recombinant humanized anti-cocaine monoclonal antibody's effects on cocaine pharmacokinetics in mice. Life Sci. 2017;184:81–86. doi: 10.1016/j.lfs.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzel H.N., Webster R.P., Saeed F.O., Kirley T.L., Ball W.J., Norman A.B. Characterization of a recombinant humanized anti-cocaine monoclonal antibody produced from multiple clones for the selection of a master cell bank candidate. Biochem. Biophys. Res. Commun. 2017;487:690–694. doi: 10.1016/j.bbrc.2017.04.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan K., Zhou M., Ahrendt A.J., Duke N.E.C., Tabaja N., Ball W.J., Kirley T.L., Norman A.B., Joachimiak A., Schiffer M., Wilton R., Pokkuluri P.R. Structural analysis of free and liganded forms of the Fab fragment of a high-affinity anti-cocaine antibody, h2E2, Acta crystallographica. Sec F, Struct. Biol. Commun. 2019;75:697–706. doi: 10.1107/S2053230X19013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirley T.L., Norman A.B. Characterization of a recombinant humanized anti-cocaine monoclonal antibody and its Fab fragment. Hum. Vaccines Immunother. 2015;11:458–467. doi: 10.4161/21645515.2014.990856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirley T.L., Norman A.B., Wetzel H.N. A novel differential scanning fluorimetry analysis of a humanized anti-cocaine mAb and its ligand binding characteristics. J. Immunol. Methods. 2020;476:112676. doi: 10.1016/j.jim.2019.112676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirley T.L., Greis K.D., Norman A.B. Domain unfolding of monoclonal antibody fragments revealed by non-reducing SDS-PAGE. Biochem. Biophys. Rep. 2018;16:138–144. doi: 10.1016/j.bbrep.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirley T.L., Norman A.B. Unfolding of IgG domains detected by non-reducing SDS-PAGE. Biochem. Biophys. Res. Commun. 2018;503:944–949. doi: 10.1016/j.bbrc.2018.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirley T.L., Greis K.D., Norman A.B. Structural characterization of expressed monoclonal antibodies by single sample mass spectral analysis after IdeS proteolysis. Biochem. Biophys. Res. Commun. 2016;477:363–368. doi: 10.1016/j.bbrc.2016.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirley T.L., Greis K.D., Norman A.B. Selective disulfide reduction for labeling and enhancement of Fab antibody fragments. Biochem. Biophys. Res. Commun. 2016;480:752–757. doi: 10.1016/j.bbrc.2016.10.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]