Abstract
Background
Triple negative breast cancer encompasses several biological entities with different outcomes and is a priority to identify which patients require more treatment to reduce the risk of recurrence and which patients need less treatment.
Patients and methods
Among the 210 women with first primary invasive apocrine non metastatic breast cancer operated on between January 1998 and December 2016 at the European Institute Oncology, Milan, we identified 24 patients with a pT1-pT2, node-negative, triple negative subtype and Ki-67 ≤ 20% who did not receive adjuvant chemotherapy (CT). We compared the outcome of this cohort with a similar group of 24 patients with ductal tumors who received adjuvant chemotherapy, matched by pathological stage and biological features and also with a similar group of 12 patients with apocrine tumors who received adjuvant chemotherapy.
Results
The median age was 64 and 61 years in the apocrine (w/o CT) and ductal group, respectively. The median value of Ki-67 expression was 12% in the apocrine group (w/o CT) and 16% in the ductal group (p < 0.001). After a median follow-up of 7.5 years, no patients in the apocrine group (w/o CT) experienced a breast cancer related event compared with 4 events in the ductal carcinoma group (Gray test p-value = 0.11).
Conclusions
The outcome of selected apocrine triple negative breast cancer patients who did not received adjuvant chemotherapy is excellent and supports a treatment de-escalation. Multicenter projects focusing on the possibility of avoiding adjuvant chemotherapy in selected subtypes of triple negative breast cancers with favorable outcome are warranted.
Keywords: Apocrine carcinoma, Special type, Adjuvant chemotherapy
Highlights
-
•
Triple negative breast cancer is a eterogeneous disease.
-
•
The outcome of selected apocrine triple negative breast cancer patients is excellent despite the omission of chemotherapy.
-
•
A treatment de-escalation should be discussed.
-
•
Multicenter projects focusing on selected subtypes of triple negative breast cancers are warranted.
1. Introduction
Triple negative breast cancer (TNBC) is defined by the absence of immunostaining for estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2). Generally, TNBCs are more likely to have aggressive features and exhibit an invasive phenotype with rapid progression; for this subgroup of tumors chemotherapy remains critical in reducing the risk of recurrence [[1], [4]]. Three-year invasive disease-free survival (IDFS) rates of 81% have been reported for patients with TNBC who have received adjuvant anthracycline/taxanetherapy [4]. However, emerging data clearly indicate that TNBC is a heterogeneous disease with variable prognosis according to clinical, pathologic, and genetic factors. In particular, histologic subtypes might play a role in the outcome [5]. If compared with invasive ductal carcinoma with similar biological features and stages, it is possible to identify TNBCs with extremely good or extremely poor prognoses. In a previous study at the European Institute of Oncology (IEO) we investigated the outcome of 781 patients with TNBC. We reported a five-year disease free survival (DFS) of 56% in patients with metaplastic tumors. Conversely, the five-year DFS for patients with adenoid cystic and medullary subtypes was 100% [6]. The different outcome for different subtypes of TNBC indicates that TNBC encompasses several biological entities and for a minority of cases should be possible to avoid adjuvant chemotherapy. Focal apocrine differentiation is a common feature in invasive carcinoma. However, apocrine carcinoma is a rare form of breast malignancy, comprising ∼1% of all breast cancers [7]. Apocrine tumors are typically ER, PgR and HER2 negative and express androgen receptor [8]. One study suggested a better outcome of apocrine tumors with distinct clinic-pathological entity and a less aggressive behavior [8]. In our previous experience, the apocrine tumors had a similar outcome if compared with ductal carcinomas. Similarly Takeuchi et al. reported no difference in survival rates between apocrine carcinoma and non apocrine carcinoma at 10 years after surgery [9].
In our study we focused the attention on a selected group of apocrine TNBC to value the role of a de-escalation treatment. In particular, we decided to investigate the outcome of patients with apocrine TNBC with pT1-pT2, low Ki-67 expression and node negative who underwent to radical surgery. These patients were not candidate to adjuvant chemotherapy after interdisciplinary evaluation. The outcome of this group of patients was compared with those of a ductal carcinoma patients group with similar pathological stage and biological features, who received adjuvant chemotherapy, and also with those of an apocrine carcinoma patients group with similar pathological stage and biological features, who received adjuvant chemotherapy.
2. Materials and methods
2.1. Study patients
All consecutive women operated for breast cancer at IEO were referred for interdisciplinary evaluation and their data were included in the institutional database. We selected the ductal and apocrine breast cancer patients. We extracted information for 29,237 ductal and 210 apocrine early breast cancer patients operated between January 1998 and December 2016. We included in our analysis only women with available characterization of breast cancer (i.e., with evaluation of ER, PgR, HER2 and Ki-67), without a previous primary tumor and without a preoperative chemotherapy. The shared selection criteria were: triple negative subtype, pT1-pT2, node-negative tumor, and Ki-67 ≤ 20%. Both apocrine tumor who did not receive adjuvant chemotherapy and who received adjuvant chemotherapy and ductal carcinomas who received adjuvant chemotherapy were considered.
Twenty-four patients with apocrine tumors who did not received adjuvant chemotherapy and 26 patients with ductal tumors who received adjuvant chemotherapy fulfilled the inclusion criteria for the analysis. In order to have two balanced groups, a cohort of 24 women with ductal tumors was randomly extracted and matched (1:1 ratio) to the apocrine cohort who did not received adjuvant chemotherapy. A third group of 12 apocrine tumors who received adjuvant chemotherapy fulfilled the inclusion criteria and was included for supplementary analysis.
Flowchart for apocrine groups selection was reported in Supplementary Figure 1.
Pure apocrine differentiation was diagnosed according to WHO tumors classification morphological criteria and to immunoprofile status, ER and PgR negative and androgen receptor (AR) positive, as previously described [10].
2.2. Statistical methods
Categorical variables were reported with absolute and relative frequencies; continuous variables were reported with median and interquartile range (IQR). Fisher’s exact test and Wilcoxon rank-sum test were used to compare categorical and continuous variables, respectively.
The endpoints evaluated were invasive disease-free survival (IDFS), overall survival (OS) and cumulative incidence of invasive breast cancer related events (IBCRE). Events considered in the IDFS computations were invasive relapse, appearance of a second primary cancer, or death, whichever occurred first. OS was defined as the time from surgery until the date of death (from any cause). Events considered for IBCRE were loco-regional relapses, contralateral relapses and distant metastases. Surviving patients were censored at the last follow-up visit.
The IDFS and OS functions were estimated using the Kaplan–Meier method. The log-rank test was used to assess differences between groups.
The IBCRE curve function was estimated according to methods described by Kalbfleisch and Prentice, taking into account the competing causes of recurrence (not breast related events). The Gray’s test was used to assess cumulative incidence differences between groups.
All analyses were performed with SAS software v. 9.4 (SAS Institute, Cary, NC). All tests were two-sided and p-values <0.05 were considered statistically significant.
3. Results
Table 1 reports the clinical and biological features of apocrine group who did not received adjuvant chemotherapy and ductal carcinoma group who received adjuvant chemotherapy. The median age was 64 and 61 years in the apocrine and ductal group, respectively (p = 0.04). The median value of Ki-67 expression was 12% in the apocrine group and 16% in the ductal group (p < 0.001) 96% of apocrine breast patients were in menopausal compared with 67% of patients in the ductal group (p = 0.02). No significant differences were reported for the tumor size between apocrine and ductal carcinoma since in both groups most patients had breast tumor size less than 2 cm: 83% of apocrine and 71% of ductal (p = 0.49).
Table 1.
Biological and clinical features of apocrine (w/o CT) and ductal (w CT) tumors.
| Apocrine w/o CT N = 24 |
Matched ductal w CT N = 24 |
P-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Year of surgery | 0.01 | ||||
| Before 2003 | 0 | – | 8 | 33 | |
| 2003–2006 | 6 | 25 | 4 | 17 | |
| 2007–2010 | 7 | 29 | 5 | 21 | |
| After 2010 | 11 | 46 | 7 | 29 | |
| Menopausal status | 0.02 | ||||
| Premenopausal | 1 | 4 | 8 | 33 | |
| Postmenopausal | 23 | 96 | 16 | 67 | |
| pT | 0.49 | ||||
| pT1 | 20 | 83 | 17 | 71 | |
| pT2 | 4 | 17 | 7 | 29 | |
| Local treatment | 0.54 | ||||
| Quadrantectomy w/o RT | 1 | 4 | 0 | – | |
| Quadrantectomy w RT | 21 | 88 | 20 | 83 | |
| Mastectomy w/o RT | 2 | 8 | 2 | 8 | |
| Mastectomy w RT | 0 | – | 2 | 8 | |
| Peritumoral vascular invasion | 0.11 | ||||
| No | 24 | 100 | 20 | 83 | |
| Yes | 0 | – | 4 | 17 | |
| Age median (IQR) | 64.3 (58.2–66.9) | 61.2 (45.5–64.5) | 0.04 | ||
| Ki67 median (IQR) | 12.0 (10.0–15.0) | 16.0 (13.0–17.5) | <0.001 | ||
Median follow-up was 7.5 years.
All patients with ductal carcinoma received adjuvant chemotherapy. Specifically, 18 patients received CMF for six cycles (75%), 1 patient received metronomic cyclophosphamide and methotrexate (4.2%), 4 patients received anthracycline based chemotherapy (16.6%), 1 patient received taxane chemotherapy (4.2%).
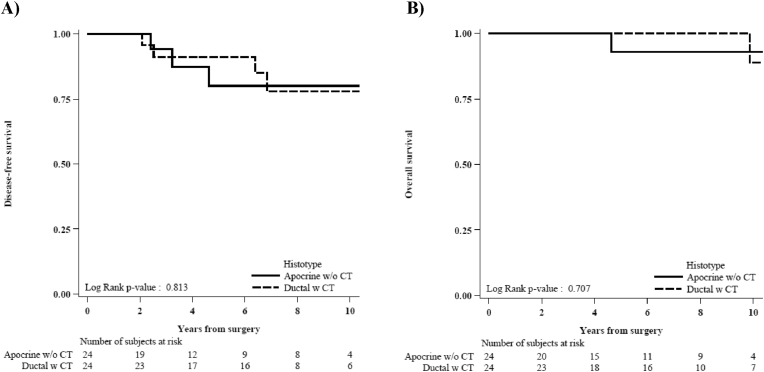
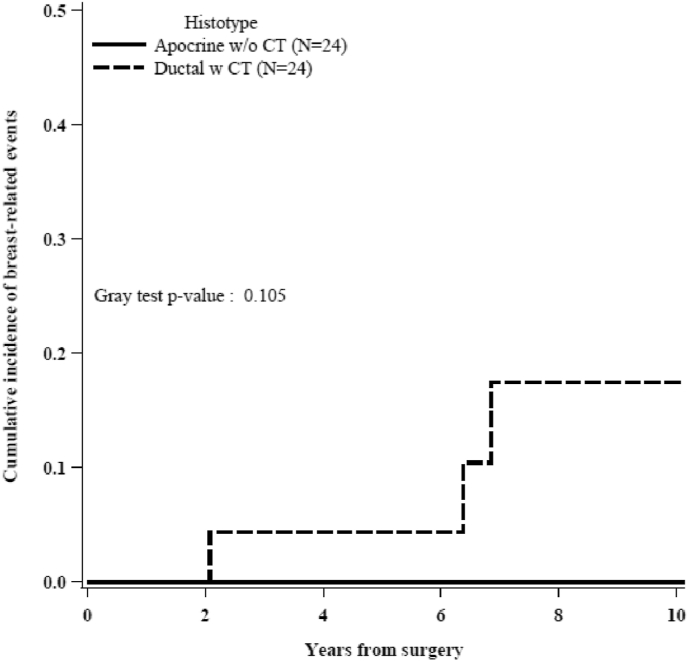
Fig. 1A shows the IDFS in both group. The 5-years IDFS was 80.1% for apocrine and 91.1% for ductal. In details, 3 events were reported in the apocrine group (1 death not cancer related as first event and 2 other primitive cancers) and 6 events in the ductal group (3 loco-regional recurrences, 2 other primitive cancers and 1 contralateral breast cancer). The 5-years OS was 92.9% and 100% in the apocrine and ductal carcinoma group, respectively, as shown in Fig. 1B. Fig. 2 shows the cumulative incidence of IBCRE in both groups. In detail: no patients in the apocrine group experienced an invasive breast cancer related event compared with 4 events (including 3 loco-regional recurrences and 1 contralateral tumors) in the ductal carcinoma group (Gray test p-value = 0.11). All these 4 patients with ductal carcinoma that experienced breast-related events received CMF for six cycles.
Fig. 1.
Invasive disease-free survival (A) and overall survival (B) in apocrine (w/o CT) and ductal (w CT) carcinoma.
Fig. 2.
Cumulative incidence of breast-related events in apocrine (w/o CT) and ductal (w CT) carcinoma.
Supplementary Table 1 reports the clinical and biological features of apocrine groups with and without adjuvant chemotherapy.
Supplementary Figure 2A shows the IDFS in apocrine groups with and without adjuvant chemotherapy. The 5-years IDFS for apocrine tumors that received adjuvant chemotherapy was 80.8%; 3 events were reported: 1 invasive contralateral breast recurrence, 1 invasive ipsilateral breast recurrence and 1 other primitive cancer. No differences in the IDFS for apocrine carcinoma with and without adjuvant chemotherapy was observed (Log-rank p-value = 0.814). Supplementary Figure 3 shows the cumulative incidence of IBCRE in apocrine groups with and without adjuvant chemotherapy. In the apocrine group that received adjuvant chemotherapy, 2 breast-related events were reported: 1 invasive contralateral breast recurrence, 1 invasive ipsilateral breast recurrence (Gray test p-value = 0.10).
The adjuvant chemotherapy in special types of apocrine tumors was prescribed more frequently during the initial years (i.e. before 2010) and in younger patients [Median age (IQR) 64.3 years (58.2–66.9) in the no adjuvant chemotherapy group and 60.8 years (44.0–65.6) in the chemotherapy group.]
4. Discussion
TNBC encompasses several biological entities with different outcomes. In this heterogeneous scenario, it is a priority to identify which patients require more treatment to reduce the risk of recurrence and which patients need less treatment. The reported prevalence of apocrine carcinoma markedly differs between several reports and ranges from 0.4% to 62% [[11], [12], [13], [14]].
This considerable variability seems to occur because of the absence of a uniform definition of apocrine carcinoma. Such a definition would be important to distinguish apocrine tumors from apocrine-like carcinomas, the latter predominantly belonging to the luminal phenotype [15,16].
The absence of standardized criteria and the suboptimal reproducibility of the histopathologic classification limits the use of available information on special types of breast cancer in tailoring adjuvant therapy [17].
In addition to these observations, our study is unique in that it evaluates the outcome of special types of breast cancer within the apocrine tumor subgroups and this includes the large (albeit limited) number of patients who are not treated with adjuvant chemotherapy. Moreover, this is a single institution study. All included patients had a histological evaluation carried out at the IEO by the same team of pathologists thereby ensuring consistent pathological reporting. The median follow-up was 7.5 years which should also be considered appropriate for the triple-negative group.
Of the apocrine group who did not receive chemotherapy, none of the patietns experienced an invasive breast cancer related event compared with 4 events (including 3 loco-regional recurrences and 1 contralateral tumors) in the ductal carcinoma group (Gray test p-value = 0.11). All these 4 patients with ductal carcinoma who experienced breast-related events received CMF for six cycles. This regime was administered primarily in earlier years in place of anthracycline and taxane chemotherapy which represents the current standard of care for TNBC: In our sample, 70% of patients in the ductal group underwent surgery before 2010. Morevoer no difference in the IDFS for apocrine carcinoma with or without adjuvant chemotherapy was observed (Log-rank p-value = 0.814) confirming the possibility to spare chemotherapy in selected TNBC patients.
On the other hand, data indicate that it is possible to identify by gene expression array a molecular apocrine breast cancer subtype characterized by the expression of androgen receptor and the absence of estrogen receptor [18]. One third of these cases are also HER2 negative.
Some of these tumors, but not all, are morphological hallmarks of apocrine. Recently the outcome of patients with molecular apocrine breast cancer included in the preoperative EORTC1099 study was analyzed. The Authors identified ninety-three cases with molecular apocrine subtype between the 846 eligible patients. Within the molecular apocrine subtype the 5-years recurrence free interval was 59% and the authors concluded that the outcome remains poor and suggested adjuvant trials be conducted to evaluate antiandrogens [19].
The results of our study indicate that an accurate and reliable histopathologic assessment is crucial in order to detect special types of cancer. The prognosis of selected apocrine triple negative breast cancer patients may be favorable even in the absence of adjuvant chemotherapy.
In our series, as previously reported, apocrine tumors were diagnosed more often in older women than was ductal carcinoma; the median age was 64 vs 61 years in the apocrine and ductal groups, respectively. The lack of need for chemotherapy should be particularly relevant as older women have a greater probability of having concomitant disease and treatments. However, the retrospective nature of our study and the very low number of patients does not allow us to draw definite conclusions regarding the prognosis of special types of TNBC. To support a treatment de-escalation in selected groups of special types of breast cancer, multicenter projects are warranted. Such tailored research will require international cooperation to solidify consensus on how to treat or not individual patients with special types of breast cancer.
5. Conclusion
The outcome of selected apocrine triple negative breast cancer patients is excellent and supports a treatment de-escalation. Multicenter projects focusing on the possibility of avoiding adjuvant chemotherapy in selected subtypes of triple negative breast cancers with favorable outcome are warranted.
We would like to thank William Russell-Edu for his assistance with the English text.
Funding source
No funding source for the study.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of competing interest
Emilia Montagna and Giuseppe Cancello received a honorarium for consultant/advisory role from Pierre Fabre.
Elisabetta Munzone received a honorarium for consultant/advisory role from Pierre Fabre and Genomic health.
Giuseppe Viale has received a honorarium for consultant/advisory role from Dako, Roche, Astellas Pharma, Novartis and he has received funding from Roche, Ventane, Dako.
Marco Colleoni has received a honorarium for consultant/advisory role from Novartis, Pierre Fabre, Pfizer, Obi-Pharma, Puba biotechnology, Celldex, Astrazeneca.
Eleonora Pagan, Vincenzo Bagnardi, Silvia Dellapasqua, Monica Iorfida, Manueliata Mazza, Giovanni Mazzarol, Paolo Veronesi, Viviana Galimberti, Giorgia Santomauro have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.07.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Trivers K.F., Lund M.J., Porter P.L., Liff J.M., Flagg E.W., Coates R.J., Eley J.W. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;Sep;20(7):1071–1082. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irshad S., Ellis P., Tutt A. Molecular heterogeneity of triple-negative breast cancer and its clinical implications. Curr Opin Oncol. 2011;23:566–577. doi: 10.1097/CCO.0b013e32834bf8ae. [DOI] [PubMed] [Google Scholar]
- 5.Montagna E., Maisonneuve P., Rotmensz N., Cancello G., Iorfida M., Balduzzi A. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Canc. 2013;Feb;13(1):31–39. doi: 10.1016/j.clbc.2012.09.002. 10.1016. [DOI] [PubMed] [Google Scholar]
- 6.Page D.L. Special types of invasive breast cancer, with clinical implications. Am J Surg Pathol. 2003;27:832–835. doi: 10.1097/00000478-200306000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Mills A.M.1, E Gottlieb C., Wendroth S M., Brenin C M., Atkins K.A. Pure apocrine carcinomas represent a clinicopathologically distinct androgen receptor-positive Subset of triple-negative breast cancers. Am J Surg Pathol Aug. 2016;40(8):1109–1116. doi: 10.1097/PAS.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi H., Tsuji K., Ueo H. Clinicopathological features and long-term prognosis of apocrine carcinoma of the breast in Japanese women. Breast Canc Res Treat. 2004;88:46–54. doi: 10.1007/s10549-004-9495-z. [DOI] [PubMed] [Google Scholar]
- 9.Dellapasqua S., Maisonneuve P., Viale G., Pruneri G. Immunohistochemically defined subtypes and outcome of apocrine breast cancer. Clin Breast Canc. 2013;Apr;13(2):95–102. doi: 10.1016/j.clbc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Gayatri G., Mondita B., Asha B., Vishal A. Study on apocrine carcinoma of breast: histomorphologic features and immunohistochemical behavior. International Journal of Basic and Applied Medical Sciences. 2012;2(3):190–193. [Google Scholar]
- 11.Yerushalmi R., Hayes M.M., Gelmon K.A. Breast carcinoma—rare types: review of the literature. Ann Oncol. 2009;20(11):1763–1770. doi: 10.1093/annonc/mdp245. [DOI] [PubMed] [Google Scholar]
- 12.Eusebi V., Millis R.R., Cattani M.G., Bussolati G., Azzopardi J.G. Apocrine carcinoma of the breast. A morphologic and immunocytochemical study. Am J Pathol. 1986;1986 Jun;123(3):532–541. Erratum in: Am J Pathol Sep;124(3):following 563. [PMC free article] [PubMed] [Google Scholar]
- 13.Japaze H., Emina J., Diaz C., Schwam R.J., Gercovich N., Demonty G., Morgenfeld E., Rivarola E., Gil Deza E., Gercovich F.G. Pure’ invasive apocrine carcinoma of the breast: a new clinicopathological entity? Breast. Feb. 2005;14(1):3–10. doi: 10.1016/j.breast.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Tsutsumi Y. Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol. 2012;42(5):375–386. doi: 10.1093/jjco/hys034. [DOI] [PubMed] [Google Scholar]
- 15.Durham J.R., Fechner R.E. The histologic spectrum of apocrine lesions of the breast. Am J Clin Pathol. 2000;113(supplement 1):S18. doi: 10.1309/7A2P-YMWJ-B1PD-UDN9. S3. [DOI] [PubMed] [Google Scholar]
- 16.Weigelt B., Reis-Filho J.S. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6:718–730. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 17.Farmer P., Bonnefoi H., Becette V., Tubiana-Hulin M. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 18.Guedj M., Marisa L., de Reynies A., Orsetti B., Schiappa R. A refined molecular taxonomy of breast cancer. Oncogene. 2012;mar 1;31(9):1196–1206. doi: 10.1038/onc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnefoi H., MacGrogan G., Poncet C., Iggo R. EORTC 10994/BIG 1-00 study investigators. Molecular apocrine tumours in EORTC 10994/BIG 1-00 phase III study: pathological response after neoadjuvant chemotherapy and clinical outcomes. Br J Cancer. Apr. 2019;120(9):913–921. doi: 10.1038/s41416-019-0420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.