Abstract
Fungal mycelia can eliminate almost all cocultured cyanobacterial cells within a short time. However, molecular mechanisms of algicidal fungi are poorly understood. In this study, a time‐course transcriptomic analysis of algicidal fungus Bjerkandera adusta T1 was applied to investigate gene expression and regulation. A total of 132, 300, 422, and 823 differentially expressed genes (DEGs) were identified at 6, 12, 24, and 48 hr, respectively. Most DEGs exhibited high endopeptidase activity, cellulose catabolic process, and transmembrane transporter activity by using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. Many decomposition genes encoding endopeptidases were induced a little later in B. adusta T1 when compared with previously investigated algicidal fungus Trametes versicolor F21a. Besides, the accumulated expression of Polysaccharide lyases8 (PL8) gene with peptidoglycan and alginate decomposition abilities was greatly delayed in B. adusta T1 relative to T. versicolor F21a. It was implied that endopeptidases and enzymes of PL8 might be responsible for the strong algicidal ability of B. adusta T1 as well as T. versicolor F21a.
Keywords: Algicidal fungi, Algicidal mechanism, Decomposition, Endopeptidase, Polysaccharide lyases8, Transcriptomic analysis
Algicidal process of fungus Bjerkandera adusta T1 was investigated by a time‐course transcriptomic analysis. The results showed that most differentially expressed genes exhibited high endopeptidase activity, cellulose catabolic process, and transmembrane transporter activity. Comparison of gene expression between B. adusta T1 and Trametes versicolor F21a during the algicidal process suggested that endopeptidases together with enzymes of the polysaccharide lyases family 8 might be responsible for the strong algicidal activity of B. adusta T1 as well as T. versicolor F21a.

1. INTRODUCTION
The occurrence of algal blooms or cyanobacterial blooms not only leads to the asphyxiation of aquatic fauna, but also releases highly toxic compounds, including microcystins, threatening the health of human beings and other organisms (Dai et al., 2018; Sun, Sun, Zhang, Esquivel‐Elizondo, & Wu, 2018). Biological methods are known to be simple and efficient to control algal blooms, with less pollution compared with the physical and chemical methods (Hou et al., 2019; Yu et al., 2019; Zhang et al., 2018). In addition to the inhibition of cyanobacterial growth, algicidal bacteria and viruses can affect the water clarity and aquatic ecosystem (Wang et al., 2010). Recently, a new method for the removal of cyanobacteria by fungi was reported (Jia et al., 2010). Further, it has been reported that the mycelia of fungus Trichaptumabietinum 1302BG could enclose and eliminate almost all cocultivated cyanobacterial cells within a short time (Jia et al., 2010), and the color of cyanobacterial medium turned transparent (Han et al., 2011). Other fungi, such as Trametes versicolor F21a, Bjerkandera adusta T1, Lophariaspadicea, Phanerochaete chrysosporium, Trichoderma citrinoviride, and Irpexlacteus T2b have been reported to exhibit algicidal ability (Han et al., 2011; Shu et al., 2016; Wang et al., 2010; Zeng, Wang, & Wang, 2015; Zeng et al., 2019). Among these, T. versicolor F21a and B. adusta T1 were considered as the two best algicidal fungi (Dai et al., 2018; Han et al., 2011; Zeng et al., 2015, 2019).
Previous studies have reported that both living and dead cyanobacterial cells first adhere to fungal mycelia before being eliminated by surrounding mycelia (Dai et al., 2018; Jia et al., 2010). It has been further demonstrated that the membranes of cyanobacterial cells and the pyrrole ring of chlorophyll a were extensively disrupted by mycelia of P. chrysosporium (Zeng et al., 2015). Transcriptomic and proteomic analyses of the algicidal mechanism of T. versicolor F21a showed that several biological processes, such as glucan 1,4‐α‐glucosidase activity, hydrolase activity, lipase activity, and endopeptidase activity, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, including glycolysis/gluconeogenesis, pyruvate metabolism, starch and sucrose metabolism, and amino acids biosynthesis, are involved in the elimination cyanobacterial cells (Dai et al., 2018; Gao et al., 2017). The expression of all Carbohydrate‐Active enZYmes (CAZyme) genes significantly increased during the algicidal process in T. versicolor F21a (Dai et al., 2018; Gao et al., 2017). Several members of CAZyme, such as AA5, GH18, GH5, GH79, GH128, and PL8, might play key roles in the decomposition of cyanobacterial cells at different eliminating stages (Dai et al., 2018). Although the underlying molecular mechanism of algicidal fungus T. versicolor F21a was elucidated, there are no reports on the mechanism of other efficient algicidal fungi.
B. adusta is a widely distributed “white rot” fungus, which has been often associated with the decomposition of hardwoods (Moody, Dudley, Hiscox, Boddy, & Eastwood, 2018). The components of wood cell walls, such as cellulose, hemicellulose, and recalcitrant lignin, can be degraded by this fungus (Moody et al., 2018). Besides, this fungus has been reported to decompose a wide range of environmental pollutants (Bouacem et al., 2018; Han et al., 2011; Sugawara, Igeta, Amano, Hyuga, & Sugano, 2019). In our previous study, B. adusta T1 was found to be one of the best algicidal fungi (Han et al., 2011). In this study, gene expression in the mycelia of B. adusta T1, cocultivated with and without cyanobacterial cells during the algicidal process, was compared by a time‐serial transcriptomic analysis. Differentially expressed genes (DEGs) were used to identify key decomposition gene(s) and pathway(s) in B. adusta T1, and the results were compared with that of T. versicolor F21a reported in a previous study (Dai et al., 2018).
2. MATERIALS AND METHODS
2.1. Fungal and algal strains
The previously isolated fungus B. adusta T1 from Zijinshan Mountain was used in this study (Han et al., 2011). Cyanobacterial strain (Microcystis aeruginosa PCC7806) was provided by the Institute of Hydrobiology of the Chinese Academy of Sciences (Wuhan, China).
2.2. Cocultivation of fungal mycelia and cyanobacterial cells
The cyanobacterial strain was cultivated at 25°C under 12‐hr light and 12‐hr dark cycles with ~90 μmol/m2 s‐1 of photons in BG‐11 medium (Jia et al., 2010). Round fungal mycelium (seven mm in diameter) was inoculated onto a nine‐cm plate, containing 15 ml of potato liquid medium, and incubated under static conditions for five days. Then, fungal mycelia were taken and transferred into 250‐mL Erlenmeyer flasks containing 100 ml of algal solution or medium. The cocultures were incubated at 25°C, 90 μmol photons/m2 s‐1, and 120 rpm to investigate differentially expressed fungal genes. Total chlorophyll a was measured according to the Standard Methods for the Examination of Water and Wastewater (Standard Methods for the Examination of Water & Wastewater, 1998).
2.3. RNA isolation and sequencing
Mycelia of B. adusta T1 were collected from cocultures after 6, 12, 24, and 48 hr of incubation. Two biological replicates of each treatment were used for RNA sequencing. Total RNA was extracted from each sample with TRIzol reagent following the manufacturer's instructions (Takara, Dalian, China). Then, crude RNA was digested via 10 U DNase I (TaKaRa, Japan) at 37°C for 30 min, and then, mRNA was isolated using Dynabeads® Oligo (dT) 25 (Life, America) following the manufacturer's instructions. One hundred ng mRNA of each sample was used to construct a sequencing library using NEBNext® UltraTM RNA Library Prep Kit (NEB, America). Paired‐end sequencing of cDNA fragments (~300 bp) was performed using Illumina HiSeq 4,000 platform at BGI‐Shenzhen, China.
2.4. Transcriptomic analysis
In this study, RNA‐Seq data of B. adusta T1 at 6, 12, 24, and 48 hr were analyzed. The quality of 150‐bp reads was assessed using the FASTQC program (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The paired‐end raw reads from RNA sequencing were trimmed using the pipeline Trimmomatic (v0.33) with parameters (LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 HEADCROP:12 MINLEN:36) (Bolger, Lohse, & Usadel, 2014). The clean reads were mapped to the B. adusta genome (v1.0) using STAR software (v2.5.3a) (Binder et al., 2013; Dobin et al., 2013). Expression value in FPKM (fragments per kilobase of exon model per million reads mapped) and DEGs were calculated via Cuffdiff (v2.2.1) using default parameters (p < .05, a fold change ≥ 2) (Si et al., 2019; Trapnell et al., 2012). Gene function was annotated using BLAST against reference protein‐encoding sequences from the Nr database of GenBank, Gene Ontology (GO), and KEGG (Ashburner et al., 2000; Kanehisa, Furumichi, Tanabe, Sato, & Morishima, 2017; Kanehisa & Goto, 2000; Kanehisa, Sato, Kawashima, Furumichi, & Tanabe, 2016). Fisher's exact test was used to obtain enriched functional terms at p < .05.
2.5. CAZyme and Secretome Annotation
All putative protein sequences of B. adusta were annotated with hmmscan against dbCAN database (Cantarel et al., 2009; Johnson, Eddy, & Portugaly, 2010; Yin et al., 2012) and further classified according to mycoCLAP database (Strasser et al., 2015). Signal information of the proteins was predicted by Target P 1.1 Server (Emanuelsson, Brunak, von Heijne, & Nielsen, 2007).
2.6. Quantitative PCR (qPCR) validation
qPCR was used to validate the gene expression calculated from RNA‐Seq data. A few randomly selected lignocellulose‐active enzyme genes were used in this study, and the β‐actin gene of B. adusta T1 was used as the endogenous control. The 20 μl reaction mixture consisted of 10 μl SYBR® Fast qPCR Mix (2x), 0.5 μl of each primer (10 μmolL−1), and 120–150 ng cDNA (Table A1). The qRT‐PCR program was set as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 20 s, and 72°C for 30 s. Relative expression levels were calculated using 2−ΔΔCT method (Livak & Schmittgen, 2001). Three biological replicates were used for qRT‐PCR.
3. RESULTS
3.1. Elimination rate during the algicidal process
The algicidal process of B. adusta T1 was monitored via spectrophotometer. As shown in Figure 1, the chlorophyll a content gradually decreased with the increase in incubation time. Approximately 86% of cyanobacterial cells were eliminated within 48 hr. The cyanobacterial cells were almost disappeared in the flask cocultivated with living fungal mycelia while the cyanobacterial cells were almost not affected by dead fungal mycelia compared with the blank control (Figure 1).
FIGURE 1.
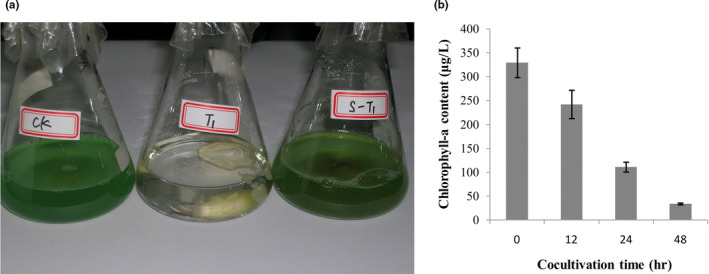
Changes in the algicidal process of B. adusta T1. Note: (a) Images of cocultivation after 48 hr; CK, the cyanobacterial cells as control;T1, the cocultivation of cyanobacterial cells and B. adusta T1 mycelia; S‐T1, the cocultivation of cyanobacterial cells and died fungal mycelia. (b) Changes in chlorophyll a content during the algicidal process
3.2. RNA‐Seq data generation and mapping
Mycelia of B. adusta T1 that was cocultivated with cyanobacterial cells at 6, 12, 24, and 48 hr were used for RNA sequencing. Fungal mycelia without cyanobacterial cells at the same time point were used as a control. Good quality RNA was isolated and used for RNA sequencing (Figure A1). A total of 63,437,015 pairs of raw reads (SRA accession: PRJNA543936) were generated (Table A2). Approximately 96% of reads were retained after the removal of adaptor and low‐quality bases (Table A2). More than 64% of reads were uniquely mapped to the reference genome by pipeline STAR (Table A2), suggesting that the results of mapping can be used for the identification of fungal DEGs.
3.3. Identification of fungal DEGs involved in the algicidal process
Boxplot of FPKM values across all samples showed the consistency of biological replicates of each treatment (Figure A2). Multi‐dimensional scaling (MDS) showed that the gene expression in mycelia cocultured with cyanobacterial cells was distinctly separated from that of mycelia without cyanobacterial cells (Figure 2). The difference became highly apparent with the increase in cocultivation time (Figure 2). A total of 132, 300, 422, and 823 fungal DEGs were identified at 6, 12, 24, and 48 hr in the mycelia cocultivated with cyanobacterial cells compared with the control, respectively (Figure 3). The expression of six randomly selected lignocellulose‐active enzyme genes, that is, a gene of esterase family, two genes of hydrolase family, a gene of hydrolase family 5, a radical oxidase encoding gene, a gene of hydrolase family 128, and a gene of hydrolase family 13, were further investigated via qRT‐PCR (Table A1). Similar expression patterns were observed between qRT‐PCR and transcriptomic analysis (Figure A3), indicating that DEGs identified by the transcriptomic analysis were suitable for further analyses.
FIGURE 2.
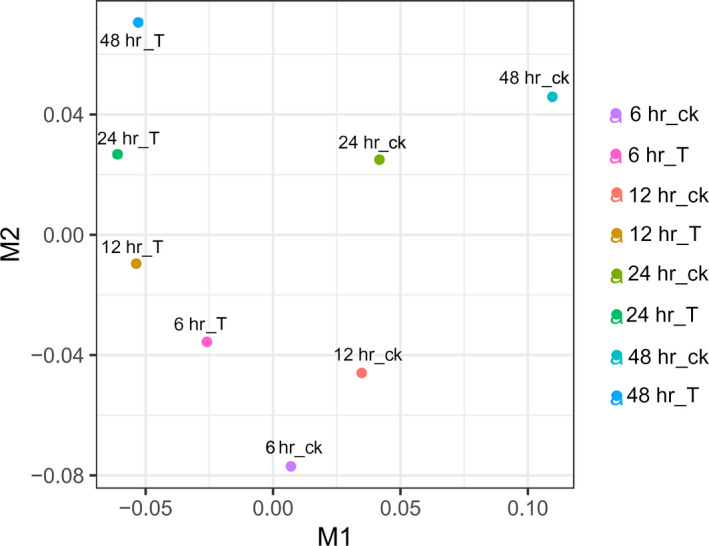
Multi‐dimensional scaling of gene expression data. Note: 6h_ck, control sample at 6h; 6h_T, treatment sample at 6 hr; 12h_ck, control sample at 12 hr; 12h_T, treatment sample at 12 hr; 24h_ck, control sample at 24 hr; 24h_T, treatment sample at 24 hr; 48h_ck, control sample at 48 hr; 48h_T, treatment sample at 48 hr
FIGURE 3.
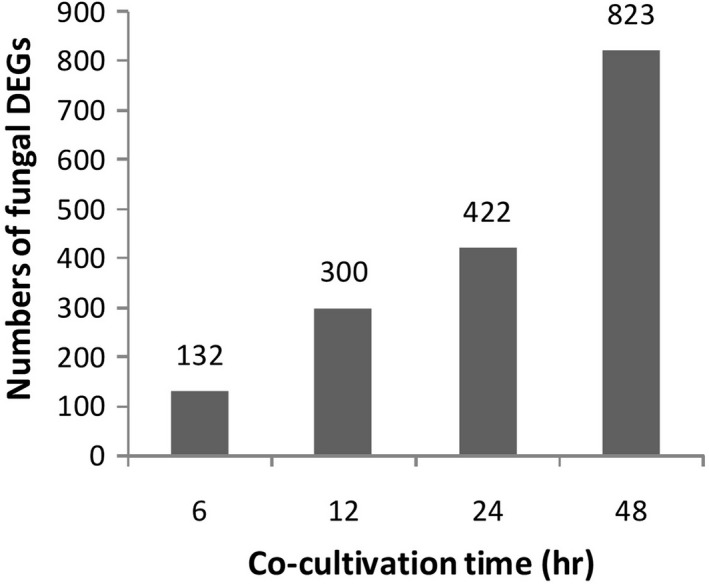
Number of fungal DEGs during the algicidal process of B. adusta T1
3.4. Annotation and enrichment analyses of fungal DEGs
After the comparison of candidate genes with Nr from NCBI, GO, and KEGG databases, DEGs were used to obtain enriched terms by Fisher's exact test (p < .05). The GO terms of DEGs were enriched in the extracellular region, cell wall, signal recognition particle, proteasome core complex, prefold in complex, ribosome, and other cellular components categories (Figure 4). Similarly, DEGs were found to be enriched on transport and catabolic processes in the biological process category, particularly cellulose catabolism and carbohydrate transport (Figure 5). Further, DEGs were enriched on decomposition and transporter activities in the molecular function category that included the activities of triglyceride lipase, serine‐type peptidase, manganese peroxidase, carboxypeptidase, cellulose 1,4‐β‐cellobiosidase, β‐glucosidase, aspartic‐type endopeptidase, α‐amylase, glycolipid transporter, amino acid transmembrane transporter, and other (Figure 6). The KEGG analysis showed that DEGs were enriched on glycerolipid metabolism, starch and sucrose metabolism, metabolism of xenobiotics by cytochrome P450, galactose metabolism, and ascorbate and aldarate metabolism in different stages of the algicidal process (Figure 7).
FIGURE 4.
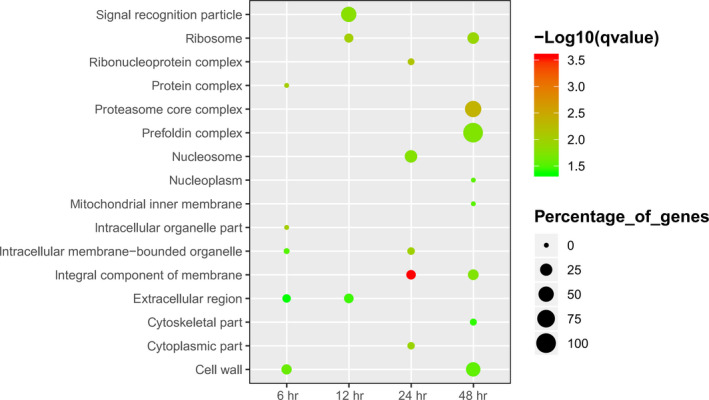
GO term enrichment of fungal DEGs in the cellular component category
FIGURE 5.
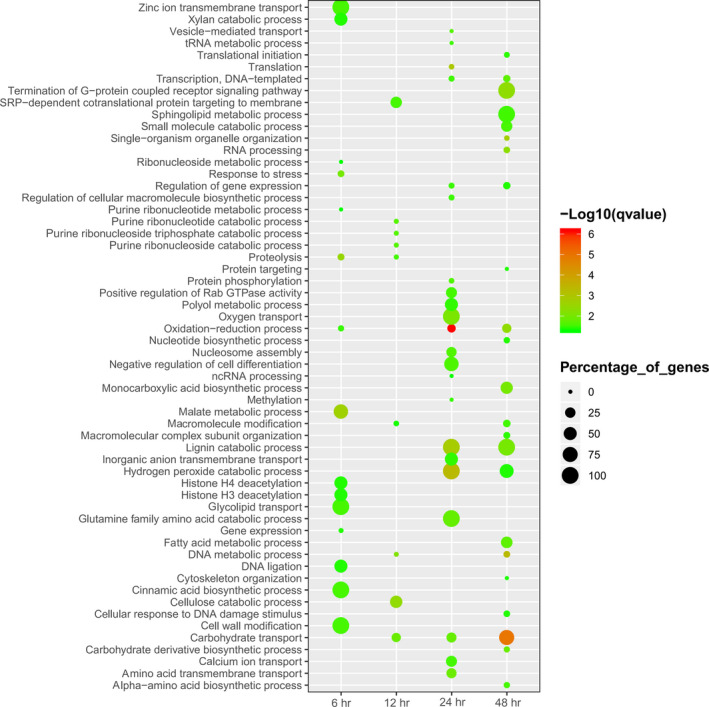
GO term enrichments of fungal DEGs in the biological process category
FIGURE 6.
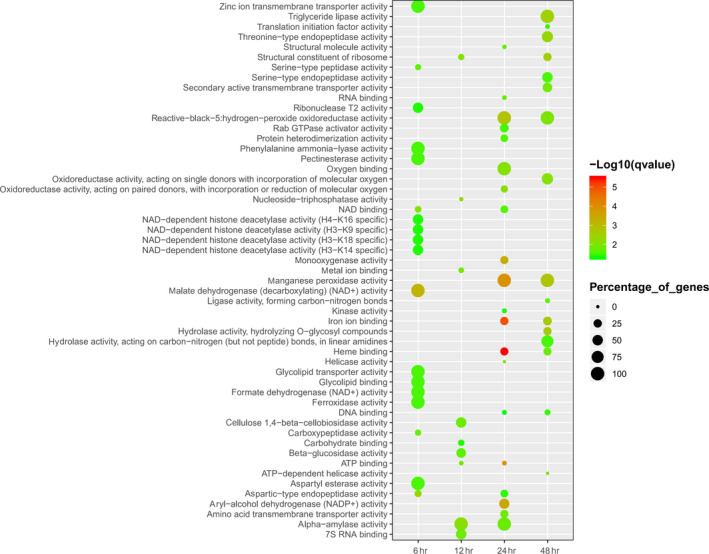
GO term enrichments of fungal DEGs in the molecular function category
FIGURE 7.
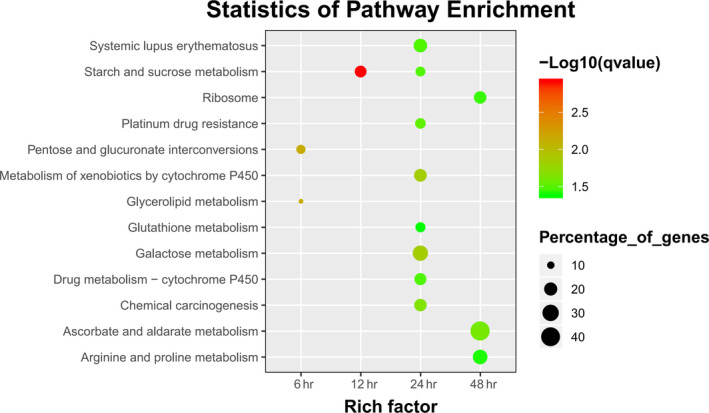
KEGG term enrichments of fungal DEGs during the algicidal process
3.5. Composition and expression of CAZyme genes of B. adusta T1 and its comparison with that of T. versicolor F21a
A total of401 CAZyme genes were identified in the genome of B. adusta by hmmscan against the dbCAN database (Table 1). The lignocellulose‐active genes can be divided into 77 CAZyme modules (Table 1). Most of the genes belonged to Glycoside Hydrolases (GH) family and Auxiliary Activities (AA) family. About 312 CAZyme genes were identified in the genome of T. versicolor F21a (Dai et al., 2018). The number of CAZyme genes in B. adusta T1 genome (401 CAZyme genes) was higher than that of T. versicolor F21a (312 CAZyme genes). Seventy CAZyme modules were detected in B. adusta T1, compared to 43 CAZyme modules in T. versicolor F21a in the previous study (Dai et al., 2018). However, the algicidal effects of T. versicolor F21a were slightly more efficient than that of B. adusta T1 (Han et al., 2011).
TABLE 1.
The number of decomposition enzymes detected by RNA‐Seq
| Enzyme classes | CAZyme module | No. of decomposition enzymes in the genome | No. of decomposition enzymes detected by RNA‐Seq | No. of decomposition enzymes in DEGs by RNA‐Seq |
|---|---|---|---|---|
| Auxiliary activities | AA1 | 1 | 1 | |
| AA2 | 21 | 19 | 10 | |
| AA3 | 38 | 30 | 12 | |
| AA4 | 1 | |||
| AA5 | 7 | 8 | 6 | |
| AA6 | 5 | 4 | 3 | |
| AA7 | 10 | 6 | 3 | |
| AA8 | 2 | 2 | ||
| AA9 | 27 | 20 | 7 | |
| Carbohydrate esterases | CE1 | 18 | 11 | 3 |
| CE10 | 42 | 31 | 6 | |
| CE12 | 3 | 2 | ||
| CE14 | 1 | 1 | ||
| CE15 | 2 | 2 | ||
| CE16 | 14 | 6 | 3 | |
| CE2 | 1 | 1 | ||
| CE3 | 1 | 1 | ||
| CE4 | 5 | 3 | 3 | |
| CE8 | 2 | 2 | 1 | |
| CE9 | 1 | |||
| GH1 | 2 | 2 | 1 | |
| Glycoside hydrolases | GH10 | 4 | 5 | 4 |
| GH105 | 3 | 3 | 1 | |
| GH109 | 8 | 8 | 5 | |
| GH115 | 2 | 2 | 1 | |
| GH12 | 2 | 1 | ||
| GH125 | 1 | 1 | ||
| GH127 | 1 | 1 | ||
| GH128 | 5 | 3 | 2 | |
| GH13 | 9 | 9 | 6 | |
| GH131 | 3 | |||
| GH15 | 2 | 2 | ||
| GH16 | 19 | 17 | 5 | |
| GH17 | 1 | 1 | ||
| GH18 | 13 | 10 | 3 | |
| GH2 | 3 | 2 | 2 | |
| GH20 | 4 | 2 | ||
| GH23 | 1 | |||
| GH24 | 1 | 1 | ||
| GH25 | 1 | 1 | ||
| GH27 | 3 | 3 | 1 | |
| GH28 | 6 | 4 | ||
| GH3 | 8 | 8 | 4 | |
| GH30 | 1 | 1 | 1 | |
| GH31 | 4 | 5 | 3 | |
| GH35 | 4 | 4 | ||
| GH37 | 2 | 1 | 1 | |
| GH38 | 1 | |||
| GH43 | 6 | 6 | 4 | |
| GH47 | 6 | 3 | ||
| GH5 | 20 | 16 | 8 | |
| GH51 | 2 | 2 | 1 | |
| GH53 | 1 | 1 | ||
| GH55 | 3 | 3 | 1 | |
| GH6 | 1 | 1 | 1 | |
| GH63 | 2 | 1 | ||
| GH7 | 5 | 4 | 1 | |
| GH71 | 3 | 3 | 1 | |
| GH72 | 1 | 1 | ||
| GH74 | 3 | 3 | ||
| GH76 | 2 | 1 | ||
| GH78 | 2 | 2 | ||
| GH79 | 7 | 9 | 6 | |
| GH85 | 1 | 1 | ||
| GH88 | 1 | 1 | ||
| GH89 | 1 | 1 | ||
| GH9 | 1 | 1 | ||
| GH92 | 3 | 3 | 1 | |
| GH95 | 1 | 1 | ||
| GH99 | 1 | |||
| Polysaccharide lyases | PL1 | 1 | 1 | |
| PL12 | 1 | 1 | ||
| PL14 | 5 | 6 | 5 | |
| PL3 | 2 | 2 | ||
| PL4 | 1 | |||
| PL5 | 1 | 2 | 2 | |
| PL8 | 1 | 1 | ||
| Total | 401 | 324 | 128 |
The identified 128 differentially expressed CAZyme genes in B. adusta T1 were found to belong to 37 modules (Table 1). The genes within the same module exhibited diverse expression profiles during the algicidal process of B. adusta T1 (Figure 8). It was observed that module GH128, AA7, AA6, and GH109 had the highest accumulated expression during the algicidal process. The sublocation analysis showed that ~ 61% (245/401) of lignocellulose‐active proteins contained secretory pathway signal peptides that can be secreted outside of fungal mycelia (Table A3). Genes within GH128 that encoded endo‐1,3‐β‐glucanase (EC3.2.1.39) could decompose xyloglucans and β‐1,3‐glucans into xylose and glucose, respectively. The enzymes of GH128, AA7, AA6, and GH109 were less efficient in cyanobacterial cell disruption. It is noteworthy that the accumulated expression of Polysaccharide lyases genes, particularly the PL8 module was highly up‐regulated during the later stage of the algicidal process of B. adusta T1, which was much delayed when compared to T. versicolor F21a (Dai et al., 2018).
FIGURE 8.
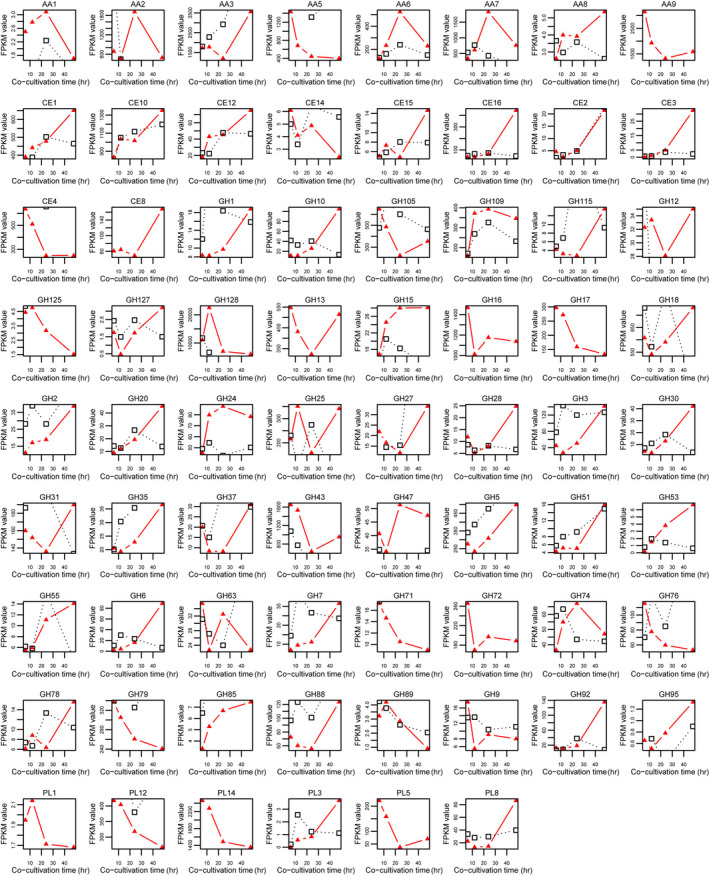
Total expression levels of each CAZyme module during the algicidal process
3.6. Expression of other decomposition genes in B. adusta T1 and their comparison with that of T. versicolor F21a
Only a few serine‐type peptidase, carboxypeptidase, and aspartic‐type endopeptidase, with strong ability in cyanobacterial cells disruption, were enriched in the DEGs list during the early stage of the algicidal process (6 hr) (Figure 6). However, no strong decomposition enzyme was enriched during the later stage of the algicidal process until 24 hr (Figure 6). During the later stage (24 hr), proteins with aspartic‐type endopeptidase activity and manganese peroxidase activity were the main decomposition enzymes (Figure 6). Various types of decomposition enzymes, such as threonine‐type endopeptidase and serine‐type endopeptidase, were induced after 48 hr of cocultivation. In this study, proteases with Protein ID jgi|Bjead1_1|36244|fgenesh1_kg.4_#_443_#_Locus8459v1_medCvg1568.9s and jgi|Bjead1_1|342083|CE153752_10262, and jgi|Bjead1_1|110676|e_gw1.8.836.1 were observed to be the main degradation genes that might be involved in cyanobacterial cells disruption (Figure 9). Thus, these proteases can play significant roles in the algicidal process. The decomposition genes showed delayed expression compared with that of T. versicolor F21a.
FIGURE 9.
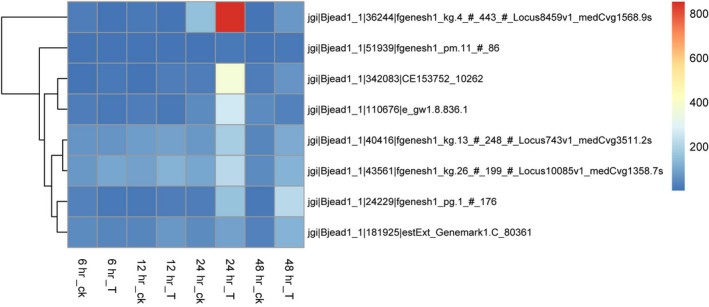
Time‐course change of protease genes expression level of T1 cocultivation with cyanobacteria. Note: 6h_ck, control sample at 6h; 6h_T, treatment sample at 6 hr; 12h_ck, control sample at 12 hr; 12h_T, treatment sample at 12 hr; 24h_ck, control sample at 24 hr; 24h_T, treatment sample at 24 hr; 48h_ck, control sample at 48 hr; 48h_T, treatment sample at 48 hr
4. DISCUSSION
Although several fungi showed a strong algicidal activity (Han et al., 2011), the underlying molecular mechanisms for algicidal capacities are largely less investigated. Interestingly, a few fungi from the Polyporales order of Basidiomycota exhibited a strong algicidal activity (Han et al., 2011). Comparative genome analyses found that the genomes of white rot fungi contain more genes encoding plant cell wall degrading enzymes than that of brown rot and mycorrhizal fungi (Kohler et al., 2015; Tisserant et al., 2013). White rot fungi including the order Polyporales can degrade lignin as well as cellulose (Kohler et al., 2015). In the present study, we observed that the number of CAZyme genes and expressed CAZyme genes of B. adusta T1 was great than that of T. versicolor F21a. However, the algicidal effects of B. adusta T1 were slightly less efficient than that of T. versicolor F21a (Han et al., 2011). More genome sequences of fungi with diverse algicidal abilities are available now, and we also compared the number of CAZyme genes in the genome of different algicidal fungi. No direct correlation was found between algicidal efficiency and several CAZyme genes (Data not shown). A similar result was observed in the study of Pilgaard et al., 2019. This suggested that the high efficiencies of algicidal fungi are not attributed to the number of genes encoding CAZyme in the fungal genome. High lignocellulose degradation ability of white rot fungi, in comparison with that of brown rot fungi and mycorrhizal fungi, can be attributed to the number of genes encoding plant cell wall degrading enzymes in fungal genomes as a result of long term natural selection (Kohler et al., 2015). The numbers of CAZyme genes were not directly correlated with algicidal abilities, which might be due to the fact that most algicidal fungi were isolated from terrestrial environments and lacked evolution selection pressure in the water system (Han et al., 2011).
Direct contact between fungal mycelia and cyanobacterial cells was required for eliminating cyanobacterial cells by fungi (Han et al., 2011; Jia et al., 2010). Previous studies showed that a few decomposition enzymes might play important roles in eliminating cyanobacterial cells by T. versicolor F21a. In particular, cellulase, β‐glucanase, and protease were supposed to efficiently disrupt cyanobacterial cells by T. versicolor F21a (Dai et al., 2018; Gao et al., 2017). In the present study, a large number of decomposition enzymes belonging to 37 modules were observed during the algicidal process of B. adusta T1. Among them, GH128, AA7, AA6, and GH109 were the highest accumulated expression module. However, the enzymes of GH128, AA7, AA6, and GH109 were not able to efficiently disrupt the macromolecules (Ekstrom, Taujale, McGinn, & Yin, 2014; Yin et al., 2012), such as cellulose in the cell wall of cyanobacterial cells. This suggested that lignocellulose‐active proteins of B. adusta T1 might not be the key enzymes for the breakdown of cyanobacterial cells.
Previous studies showed that chondroitin ABC lyase (EC4.2.2.1) of PL8 and alginate lyase (EC4.2.2.3) of PL14 were able to decompose peptidoglycan and alginate (Lombard, Golaconda Ramulu, Drula, Coutinho, & Henrissat, 2014), and the expression level was also significantly up‐regulated during the algicidal process of T. versicolor F21a (Dai et al., 2018; Gao et al., 2017). Chondroitin AC lyase (chondroitin sulfate) and alginate lyase were unique to a known saprophytic marine fungus Paradendryphiella salina in the breakdown of dried brown algae in the medium compared with its terrestrial counterparts (Pilgaard et al., 2019). Recombinant expression of Chondroitin AC lyase of the marine fungus P.salina reveals that alginate lyase can degrade several types of brown algae polysaccharides (Pilgaard et al., 2019). A putative PL8 of P.salina with a similar sequence should also decompose brown macroalgae (Pilgaard et al., 2019). Proteomic analysis of the secretome of P. salina grown on three species of brown algae and under carbon limitation implied that the basic CAZyme repertoire of saprobic fungi belongs to ascomycetes, with the addition of PL7 alginate lyases, provide P. salina with sufficient enzymatic capabilities to degrade several types of brown algae polysaccharides (Pilgaard et al., 2019). In the present study, the total expression level of PL14 was down‐regulated during the algicidal process of B. adusta T1, while no gene, belonging to PL7, was detected in the genome of B. adusta. The accumulated expression level of PL8 was highly up‐regulated in the later stage of the algicidal process of B. adusta T1, which was much delayed when compared with T. versicolor F21a (Dai et al., 2018). All the evidence indicated that enzymes of PL8 with strong peptidoglycan and alginate decomposition abilities might be a vital genetic factor for the determination of the algicidal ability of T. versicolor F21a as well as B. adusta T1.
Analysis of the enriched GO terms and KEGG pathways showed that several types of peptidases were enriched during the algicidal process of B. adusta T1. In particular, proteases (protein ID jgi|Bjead1_1|36244|fgenesh1_kg.4_#_443_#_Locus8459v1_medCvg1568.9s, jgi|Bjead1_1|342083|CE153752_10262, and jgi|Bjead1_1|110676|e_gw1.8.836.1) were highly up‐regulated during the later stages of cocultivation. Proteomic analysis of P. salina also implied that the PL7 and PL8 enzymes, abundantly secreted together with enzymes of P.salina, were necessary for degradation of laminarin, cellulose, lipids, and peptides of brown algae (Pilgaard et al., 2019). Different types of peptides were detected in P. salina grown on three species of brown algae (Pilgaard et al., 2019). Additionally, several fungal proteins belonging to peptidase were also up‐regulated during the algicidal process of T. versicolor F21a (Gao et al., 2017). Besides, four homologous decomposition enzymes of other species with endo‐glycosidase and endopeptidase activities were selected to investigate their effects on cyanobacterial cells, and one type of protease was found to effectively disrupt cyanobacterial cells (Dai et al., 2018). Comparison of the gene expression during the algicidal process of B. adusta T1 and T. versicolor F21a demonstrated that majority of decomposition genes with endopeptidase and endo‐glycosidase activities in B. adusta T1 were expressed in the later stage of cocultivation, while the similar genes in T. versicolor F21a were induced in the early stage (Dai et al., 2018). Thus, protease together with enzymes of PL8 might play a key role in the elimination of cyanobacterial cells both by B. adusta T1 and T. versicolor F21a. The expression of enzymes of PL8 and peptidases in B. adusta T1 was little delayed compared with that of T. versicolor F21a, which should be the reason why the algicidal efficiency of T. versicolor F21a is better than that of B. adusta T1.
The production of microcystins (MC) by cyanobacterial blooms often severely threatens human and ecosystems health (Li, Li, & Li, 2017). Biodegradation is an efficient and sustainable biological strategy for MC removal (Li et al., 2017). A large number of bacteria and several fungi were reported with MC removal or degrading capabilities (Dziga, Wasylewski, Wladyka, Nybom, & Meriluoto, 2013; Jia, Du, Song, Zhao, & Tian, 2012; Li et al., 2017; Mohamed, Hashem, & Alamri, 2014; Qin et al., 2019). Four mlr genes (i.e., mlrC, A, D, and B) located sequentially in a gene cluster in the genome of Sphingomonas sp. ACM‐3962 strain were identified for MC biodegradation (Bourne et al., 1996; Bourne, Riddles, Jones, Smith, & Blakeley, 2001). The enzymatic pathway involves at least three intracellular enzymes and two intermediate products (Li et al., 2017). Heterologous expression of the mlrA gene originated from Novosphingobium sp. THN1 showed that the recombinant MlrA hydrolyzed microcystin‐RR into a linear intermediate product by cleaving the peptide bond between Adda and arginine residue, which is also the first step involved in MC degradation pathway (Wang et al., 2017). Site‐directed mutants of MlrA suggested that MlrA is likely not a metalloprotease but a glutamate protease belonging to type II CAAX prenyl endopeptidases (Xu et al., 2019). A few fungi, for example, T. abietinum 1302BG, T.citrinoviride, and Mucor hiemalis were reported with MC removal or degrading capability (Esterhuizen‐Londt, Hertel, & Pflugmacher, 2017; Jia et al., 2012; Mohamed et al., 2014; Stephan, 2015); however, the enzymatic pathway was poorly understood compared with that of bacteria. In our study, many genes with endopeptidase activities were enriched during the algicidal process, and a gene encoding aflatoxin‐detoxifizyme with peptidase activity (Protein ID: jgi|Bjead1_1|37717|fgenesh1_kg.7_#_39_#_Locus4370v1_medCvg2101.1s) was up‐regulated during the algicidal process of B. adusta T1. Further mining the gene expression during the algicidal process of T. versicolor F21a identified a homolog gene (Protein ID: jgi|Trave1|56726|estExt_fgenesh1_pm.C_3_t10209) that was slightly up‐regulated in the later stage. In consideration bacterial MlrA encoding a protease, fungal aflatoxin‐detoxifizyme could be a possible candidate enzyme involving in MC degradation. In order to investigate the mechanism for MC degradation in fungi, there is more work need to be done.
5. CONCLUSIONS
In this study, the algicidal process of B. adusta T1 was investigated by a time‐serial transcriptomic analysis, and the results were compared with these from T. versicolor F21a, reported in our previous study. The identified DEGs were enriched in endopeptidase activity, cellulose catabolic process, and transmembrane transporter activity. Endopeptidases together with enzymes of PL8 might play a key role in the elimination of cyanobacterial cells by both algicidal fungi, B. adusta T1 and T. versicolor F21a.
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Guomin Han: Conceptualization (equal); Software (lead); Writing‐original draft (equal). Hui Ma: Investigation (equal). Shenrong Ren: Investigation (supporting). Xueyan Gao: Investigation (supporting). Xiaolong He: Investigation (supporting). Suwen Zhu: Resources (equal); Validation (supporting). Ruining Deng: Validation (supporting). Shihua Zhang: Conceptualization (equal); Writing‐review & editing (equal).
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
This study was financially supported by the National Natural Science Foundation of China (Project Nos. 31601289, 31470465, and 51309003).
APPENDIX 1.
TABLE A1.
Primers used in this study
| Protein ID | Annotation | Primer |
|---|---|---|
| jgi|Bjead1_1|459664|MIX10988_17319_14 | Radical oxidase | GTCGAAGCGGGTGGTCTTAA |
| CCTCTCCTCGTTGCCGTTT | ||
| jgi|Bjead1_1|34143|fgenesh1_kg.1_#_945_#_Locus732v1_medCvg1115.6s | Esterase family 1 protein | CCTCCCTGCAAACATCTCACA |
| GGAGACGTGTCGGGAAAGAG | ||
| jgi|Bjead1_1|172436|gm1.8875_g | Hydrolase family 5 protein | TACGAGGGCGACGATTGG |
| CTCACCGGACACGTAAACCA | ||
| jgi|Bjead1_1|35099|fgenesh1_kg.2_#_711_#_Locus118v3_medCvg9284.2s | Hydrolase family 5 protein | CTCGTTGACCCGCACAACTT |
| GGGAATATCGTGAGGCTCGTT | ||
| jgi|Bjead1_1|355947|CE167616_517 | Hydrolase family 128 protein | AGCGCGGTGTGTCATACAAC |
| TGTGTCCGGCATCGGTATT | ||
| jgi|Bjead1_1|38229|fgenesh1_kg.7_#_551_#_Locus8080v1_medCvg1578.8s | Hydrolase family 13 protein | CACGCCCGACTATTCGAAGT |
| GTCGGGTTTTCCGTGTCAAG |
TABLE A2.
Statistics of RNA‐Seq reads mapping results
| Sample | Raw reads | Number of input reads | Cleaned length | Uniquely mapped reads number | Uniquely mapped reads (%) |
|---|---|---|---|---|---|
| 6h_ck1 | 3,713,910 | 3,531,468 | 129.69 | 2,491,981 | 70.57 |
| 6h_ck2 | 3,618,291 | 3,416,852 | 129.055 | 2,205,290 | 64.54 |
| 6h_T1 | 3,644,390 | 3,577,152 | 128.745 | 2,802,466 | 78.34 |
| 6h_T2 | 4,379,858 | 4,280,639 | 128.835 | 3,282,569 | 76.68 |
| 12h_ck1 | 3,832,620 | 3,691,873 | 129.505 | 2,706,111 | 73.30 |
| 12h_ck2 | 3,806,801 | 3,651,869 | 129.09 | 2,603,461 | 71.29 |
| 12h_T1 | 3,493,777 | 3,325,461 | 125.865 | 2,458,201 | 73.92 |
| 12h_T2 | 4,020,571 | 3,899,676 | 128.9 | 2,967,516 | 76.10 |
| 24h_ck1 | 3,609,635 | 3,388,118 | 128.875 | 2,326,955 | 68.68 |
| 24h_ck2 | 3,684,973 | 3,497,767 | 129.655 | 2,466,212 | 70.51 |
| 24h_T1 | 4,831,627 | 4,684,474 | 128.62 | 3,554,342 | 75.87 |
| 24h_T2 | 4,567,295 | 4,436,833 | 128.9 | 3,395,508 | 76.53 |
| 48h_ck1 | 3,638,776 | 3,456,573 | 129.255 | 2,500,095 | 72.33 |
| 48h_ck2 | 3,594,592 | 3,405,731 | 128.51 | 2,473,909 | 72.64 |
| 48h_T1 | 4,499,718 | 4,347,471 | 128.275 | 3,264,957 | 75.10 |
| 48h_T2 | 4,500,181 | 4,353,762 | 128.58 | 3,277,084 | 75.27 |
The number of reads were expressed in pairs.
TABLE A3.
Sublocation of CAZyme proteins of B. adusta
| Protein ID | Len | mTP | SP | Other | Loc | RC |
|---|---|---|---|---|---|---|
| 39948 | 1,041 | 0.095 | 0.093 | 0.844 | _ | 2 |
| 170203 | 646 | 0.083 | 0.105 | 0.872 | _ | 2 |
| 229483 | 319 | 0.049 | 0.917 | 0.053 | S | 1 |
| 113359 | 295 | 0.045 | 0.95 | 0.032 | S | 1 |
| 40021 | 320 | 0.081 | 0.908 | 0.028 | S | 1 |
| 40040 | 465 | 0.053 | 0.942 | 0.029 | S | 1 |
| 230253 | 1,024 | 0.014 | 0.966 | 0.07 | S | 1 |
| 230354 | 1,005 | 0.342 | 0.705 | 0.024 | S | 4 |
| 183239 | 385 | 0.671 | 0.027 | 0.355 | M | 4 |
| 62585 | 305 | 0.147 | 0.104 | 0.761 | _ | 2 |
| 113961 | 604 | 0.534 | 0.055 | 0.439 | M | 5 |
| 452849 | 310 | 0.077 | 0.037 | 0.944 | _ | 1 |
| 170455 | 322 | 0.091 | 0.068 | 0.894 | _ | 1 |
| 237378 | 316 | 0.042 | 0.949 | 0.058 | S | 1 |
| 40461 | 244 | 0.054 | 0.954 | 0.043 | S | 1 |
| 183509 | 612 | 0.558 | 0.024 | 0.588 | _ | 5 |
| 240122 | 301 | 0.092 | 0.873 | 0.031 | S | 2 |
| 40615 | 587 | 0.037 | 0.159 | 0.919 | _ | 2 |
| 241975 | 605 | 0.068 | 0.073 | 0.901 | _ | 1 |
| 52811 | 537 | 0.063 | 0.913 | 0.03 | S | 1 |
| 40743 | 377 | 0.103 | 0.892 | 0.017 | S | 2 |
| 170929 | 704 | 0.088 | 0.048 | 0.937 | _ | 1 |
| 170934 | 551 | 0.063 | 0.897 | 0.086 | S | 1 |
| 244200 | 674 | 0.027 | 0.93 | 0.065 | S | 1 |
| 244246 | 669 | 0.018 | 0.971 | 0.054 | S | 1 |
| 62986 | 499 | 0.058 | 0.906 | 0.041 | S | 1 |
| 71431 | 617 | 0.491 | 0.658 | 0.014 | S | 5 |
| 245049 | 604 | 0.442 | 0.655 | 0.01 | S | 4 |
| 40812 | 611 | 0.087 | 0.044 | 0.906 | _ | 1 |
| 245297 | 598 | 0.061 | 0.81 | 0.11 | S | 2 |
| 171002 | 606 | 0.196 | 0.68 | 0.028 | S | 3 |
| 84503 | 373 | 0.101 | 0.05 | 0.922 | _ | 1 |
| 171059 | 593 | 0.14 | 0.872 | 0.019 | S | 2 |
| 156054 | 596 | 0.044 | 0.887 | 0.074 | S | 1 |
| 114954 | 574 | 0.084 | 0.115 | 0.897 | _ | 2 |
| 40886 | 614 | 0.079 | 0.052 | 0.904 | _ | 1 |
| 136631 | 614 | 0.123 | 0.045 | 0.86 | _ | 2 |
| 114902 | 593 | 0.423 | 0.556 | 0.029 | S | 5 |
| 52983 | 613 | 0.052 | 0.044 | 0.95 | _ | 1 |
| 52991 | 597 | 0.044 | 0.914 | 0.052 | S | 1 |
| 183896 | 599 | 0.014 | 0.93 | 0.089 | S | 1 |
| 53087 | 1,011 | 0.036 | 0.969 | 0.05 | S | 1 |
| 41108 | 696 | 0.159 | 0.081 | 0.841 | _ | 2 |
| 41113 | 573 | 0.093 | 0.207 | 0.634 | _ | 3 |
| 171368 | 478 | 0.069 | 0.079 | 0.9 | _ | 1 |
| 41241 | 396 | 0.274 | 0.84 | 0.018 | S | 3 |
| 454703 | 402 | 0.63 | 0.021 | 0.452 | M | 5 |
| 41251 | 337 | 0.075 | 0.736 | 0.21 | S | 3 |
| 41305 | 371 | 0.11 | 0.094 | 0.754 | _ | 2 |
| 41306 | 538 | 0.094 | 0.099 | 0.816 | _ | 2 |
| 256509 | 423 | 0.468 | 0.891 | 0.004 | S | 3 |
| 184224 | 600 | 0.103 | 0.101 | 0.838 | _ | 2 |
| 41490 | 303 | 0.052 | 0.147 | 0.93 | _ | 2 |
| 260893 | 199 | 0.086 | 0.089 | 0.914 | _ | 1 |
| 184394 | 582 | 0.803 | 0.053 | 0.115 | M | 2 |
| 157149 | 768 | 0.029 | 0.956 | 0.036 | S | 1 |
| 41596 | 266 | 0.069 | 0.929 | 0.046 | S | 1 |
| 171769 | 774 | 0.021 | 0.96 | 0.057 | S | 1 |
| 261859 | 808 | 0.085 | 0.06 | 0.922 | _ | 1 |
| 116111 | 281 | 0.069 | 0.143 | 0.873 | _ | 2 |
| 263236 | 400 | 0.054 | 0.958 | 0.066 | S | 1 |
| 29758 | 400 | 0.043 | 0.995 | 0.011 | S | 1 |
| 263252 | 398 | 0.053 | 0.98 | 0.022 | S | 1 |
| 41686 | 427 | 0.307 | 0.369 | 0.337 | S | 5 |
| 41708 | 649 | 0.18 | 0.862 | 0.014 | S | 2 |
| 41754 | 647 | 0.053 | 0.182 | 0.858 | _ | 2 |
| 41763 | 404 | 0.094 | 0.768 | 0.13 | S | 2 |
| 53682 | 693 | 0.255 | 0.759 | 0.029 | S | 3 |
| 41854 | 517 | 0.021 | 0.968 | 0.058 | S | 1 |
| 41863 | 491 | 0.11 | 0.913 | 0.016 | S | 1 |
| 41869 | 336 | 0.222 | 0.908 | 0.016 | S | 2 |
| 41896 | 447 | 0.713 | 0.025 | 0.412 | M | 4 |
| 138203 | 774 | 0.05 | 0.127 | 0.857 | _ | 2 |
| 184697 | 372 | 0.085 | 0.874 | 0.045 | S | 2 |
| 116816 | 362 | 0.078 | 0.863 | 0.061 | S | 2 |
| 172102 | 377 | 0.068 | 0.887 | 0.051 | S | 1 |
| 41961 | 329 | 0.044 | 0.92 | 0.064 | S | 1 |
| 456042 | 328 | 0.038 | 0.942 | 0.045 | S | 1 |
| 268970 | 386 | 0.025 | 0.953 | 0.056 | S | 1 |
| 29957 | 283 | 0.019 | 0.958 | 0.067 | S | 1 |
| 116945 | 203 | 0.143 | 0.062 | 0.889 | _ | 2 |
| 157771 | 401 | 0.037 | 0.944 | 0.045 | S | 1 |
| 157775 | 304 | 0.039 | 0.933 | 0.056 | S | 1 |
| 63838 | 343 | 0.015 | 0.974 | 0.051 | S | 1 |
| 41982 | 373 | 0.156 | 0.807 | 0.026 | S | 2 |
| 172152 | 362 | 0.046 | 0.927 | 0.051 | S | 1 |
| 269481 | 367 | 0.124 | 0.787 | 0.049 | S | 2 |
| 269524 | 373 | 0.13 | 0.823 | 0.028 | S | 2 |
| 41997 | 618 | 0.09 | 0.958 | 0.02 | S | 1 |
| 172246 | 372 | 0.117 | 0.854 | 0.028 | S | 2 |
| 157924 | 374 | 0.155 | 0.857 | 0.027 | S | 2 |
| 117149 | 396 | 0.013 | 0.496 | 0.863 | _ | 4 |
| 184935 | 309 | 0.034 | 0.944 | 0.061 | S | 1 |
| 138704 | 475 | 0.096 | 0.071 | 0.887 | _ | 2 |
| 172436 | 486 | 0.083 | 0.049 | 0.928 | _ | 1 |
| 42291 | 347 | 0.056 | 0.938 | 0.021 | S | 1 |
| 81341 | 141 | 0.059 | 0.274 | 0.852 | _ | 3 |
| 158334 | 414 | 0.237 | 0.054 | 0.674 | _ | 3 |
| 42434 | 421 | 0.05 | 0.914 | 0.051 | S | 1 |
| 54172 | 363 | 0.017 | 0.977 | 0.039 | S | 1 |
| 185179 | 452 | 0.09 | 0.805 | 0.059 | S | 2 |
| 117666 | 259 | 0.056 | 0.913 | 0.05 | S | 1 |
| 42534 | 327 | 0.084 | 0.883 | 0.031 | S | 2 |
| 42539 | 270 | 0.19 | 0.044 | 0.855 | _ | 2 |
| 296151 | 848 | 0.178 | 0.112 | 0.76 | _ | 3 |
| 185311 | 397 | 0.037 | 0.706 | 0.59 | S | 5 |
| 42617 | 504 | 0.024 | 0.239 | 0.872 | _ | 2 |
| 42631 | 975 | 0.141 | 0.86 | 0.023 | S | 2 |
| 117772 | 330 | 0.454 | 0.018 | 0.718 | _ | 4 |
| 54399 | 313 | 0.027 | 0.948 | 0.043 | S | 1 |
| 172925 | 348 | 0.068 | 0.979 | 0.031 | S | 1 |
| 172926 | 355 | 0.031 | 0.968 | 0.045 | S | 1 |
| 158817 | 338 | 0.037 | 0.936 | 0.087 | S | 1 |
| 158842 | 1,102 | 0.131 | 0.059 | 0.88 | _ | 2 |
| 185485 | 287 | 0.099 | 0.149 | 0.826 | _ | 2 |
| 118319 | 648 | 0.118 | 0.832 | 0.057 | S | 2 |
| 139564 | 387 | 0.947 | 0.041 | 0.047 | M | 1 |
| 42889 | 285 | 0.055 | 0.192 | 0.895 | _ | 2 |
| 302552 | 344 | 0.191 | 0.044 | 0.811 | _ | 2 |
| 305292 | 253 | 0.084 | 0.889 | 0.033 | S | 1 |
| 43095 | 366 | 0.129 | 0.816 | 0.031 | S | 2 |
| 306404 | 366 | 0.113 | 0.849 | 0.032 | S | 2 |
| 43114 | 348 | 0.099 | 0.818 | 0.057 | S | 2 |
| 306863 | 366 | 0.041 | 0.901 | 0.056 | S | 1 |
| 118718 | 363 | 0.052 | 0.884 | 0.049 | S | 1 |
| 119037 | 314 | 0.05 | 0.91 | 0.069 | S | 1 |
| 43329 | 364 | 0.143 | 0.843 | 0.03 | S | 2 |
| 173495 | 364 | 0.337 | 0.782 | 0.013 | S | 3 |
| 311850 | 437 | 0.082 | 0.904 | 0.031 | S | 1 |
| 54893 | 416 | 0.149 | 0.835 | 0.047 | S | 2 |
| 185921 | 568 | 0.19 | 0.847 | 0.046 | S | 2 |
| 459664 | 777 | 0.052 | 0.777 | 0.257 | S | 3 |
| 43446 | 386 | 0.099 | 0.882 | 0.061 | S | 2 |
| 313682 | 859 | 0.047 | 0.95 | 0.031 | S | 1 |
| 173673 | 260 | 0.015 | 0.968 | 0.041 | S | 1 |
| 119350 | 399 | 0.033 | 0.941 | 0.062 | S | 1 |
| 119522 | 575 | 0.905 | 0.042 | 0.13 | M | 2 |
| 119593 | 1,000 | 0.12 | 0.864 | 0.027 | S | 2 |
| 43812 | 1,034 | 0.027 | 0.834 | 0.427 | S | 3 |
| 43892 | 615 | 0.019 | 0.971 | 0.047 | S | 1 |
| 323280 | 369 | 0.239 | 0.763 | 0.019 | S | 3 |
| 43929 | 258 | 0.026 | 0.965 | 0.084 | S | 1 |
| 186344 | 320 | 0.122 | 0.849 | 0.039 | S | 2 |
| 120002 | 362 | 0.08 | 0.87 | 0.048 | S | 2 |
| 55334 | 249 | 0.034 | 0.907 | 0.075 | S | 1 |
| 43966 | 742 | 0.024 | 0.946 | 0.05 | S | 1 |
| 324420 | 819 | 0.032 | 0.934 | 0.049 | S | 1 |
| 186388 | 434 | 0.136 | 0.879 | 0.039 | S | 2 |
| 44047 | 495 | 0.025 | 0.966 | 0.045 | S | 1 |
| 44072 | 557 | 0.412 | 0.595 | 0.018 | S | 5 |
| 326659 | 470 | 0.38 | 0.617 | 0.062 | S | 4 |
| 141290 | 460 | 0.062 | 0.147 | 0.889 | _ | 2 |
| 120399 | 298 | 0.117 | 0.362 | 0.535 | _ | 5 |
| 344867 | 663 | 0.092 | 0.927 | 0.021 | S | 1 |
| 141539 | 663 | 0.101 | 0.904 | 0.022 | S | 1 |
| 345914 | 804 | 0.294 | 0.842 | 0.009 | S | 3 |
| 44370 | 571 | 0.236 | 0.777 | 0.041 | S | 3 |
| 44376 | 532 | 0.037 | 0.964 | 0.029 | S | 1 |
| 44391 | 385 | 0.022 | 0.964 | 0.048 | S | 1 |
| 141648 | 466 | 0.369 | 0.686 | 0.046 | S | 4 |
| 55696 | 466 | 0.59 | 0.689 | 0.03 | S | 5 |
| 462628 | 730 | 0.223 | 0.087 | 0.657 | _ | 3 |
| 120968 | 1,020 | 0.018 | 0.966 | 0.057 | S | 1 |
| 161363 | 452 | 0.052 | 0.912 | 0.047 | S | 1 |
| 174734 | 531 | 0.054 | 0.857 | 0.206 | S | 2 |
| 161500 | 326 | 0.05 | 0.927 | 0.032 | S | 1 |
| 353490 | 284 | 0.807 | 0.044 | 0.166 | M | 2 |
| 353489 | 254 | 0.422 | 0.051 | 0.632 | _ | 4 |
| 44803 | 1,134 | 0.105 | 0.028 | 0.928 | _ | 1 |
| 355947 | 264 | 0.021 | 0.946 | 0.08 | S | 1 |
| 73811 | 287 | 0.489 | 0.745 | 0.016 | S | 4 |
| 121664 | 369 | 0.079 | 0.874 | 0.033 | S | 2 |
| 31936 | 332 | 0.211 | 0.783 | 0.03 | S | 3 |
| 73869 | 357 | 0.123 | 0.12 | 0.842 | _ | 2 |
| 463744 | 931 | 0.029 | 0.972 | 0.025 | S | 1 |
| 45029 | 958 | 0.016 | 0.969 | 0.057 | S | 1 |
| 187270 | 615 | 0.044 | 0.957 | 0.029 | S | 1 |
| 56225 | 960 | 0.166 | 0.202 | 0.582 | _ | 4 |
| 32051 | 406 | 0.186 | 0.073 | 0.732 | _ | 3 |
| 361367 | 713 | 0.08 | 0.063 | 0.949 | _ | 1 |
| 73972 | 481 | 0.159 | 0.698 | 0.092 | S | 3 |
| 175283 | 521 | 0.208 | 0.945 | 0.004 | S | 2 |
| 45135 | 778 | 0.166 | 0.895 | 0.011 | S | 2 |
| 121936 | 537 | 0.043 | 0.918 | 0.053 | S | 1 |
| 45153 | 588 | 0.043 | 0.845 | 0.111 | S | 2 |
| 122105 | 500 | 0.097 | 0.128 | 0.838 | _ | 2 |
| 56307 | 208 | 0.323 | 0.115 | 0.412 | _ | 5 |
| 45281 | 340 | 0.341 | 0.59 | 0.036 | S | 4 |
| 45314 | 601 | 0.082 | 0.12 | 0.844 | _ | 2 |
| 465711 | 611 | 0.071 | 0.279 | 0.729 | _ | 3 |
| 175513 | 700 | 0.052 | 0.367 | 0.644 | _ | 4 |
| 143000 | 604 | 0.101 | 0.128 | 0.746 | _ | 2 |
| 56449 | 403 | 0.04 | 0.96 | 0.034 | S | 1 |
| 175536 | 379 | 0.127 | 0.872 | 0.031 | S | 2 |
| 74164 | 587 | 0.144 | 0.145 | 0.649 | _ | 3 |
| 162505 | 587 | 0.081 | 0.165 | 0.796 | _ | 2 |
| 56499 | 798 | 0.368 | 0.808 | 0.011 | S | 3 |
| 56525 | 330 | 0.449 | 0.653 | 0.023 | S | 4 |
| 45516 | 850 | 0.067 | 0.891 | 0.081 | S | 1 |
| 162602 | 215 | 0.09 | 0.086 | 0.866 | _ | 2 |
| 384658 | 698 | 0.142 | 0.879 | 0.033 | S | 2 |
| 45570 | 1,468 | 0.018 | 0.965 | 0.042 | S | 1 |
| 187728 | 605 | 0.116 | 0.157 | 0.718 | _ | 3 |
| 45647 | 404 | 0.1 | 0.692 | 0.179 | S | 3 |
| 66377 | 626 | 0.158 | 0.64 | 0.062 | S | 3 |
| 66400 | 890 | 0.144 | 0.023 | 0.921 | _ | 2 |
| 122937 | 361 | 0.08 | 0.919 | 0.03 | S | 1 |
| 66493 | 204 | 0.046 | 0.304 | 0.687 | _ | 4 |
| 143585 | 374 | 0.045 | 0.094 | 0.947 | _ | 1 |
| 123323 | 650 | 0.114 | 0.295 | 0.801 | _ | 3 |
| 45905 | 313 | 0.127 | 0.925 | 0.028 | S | 2 |
| 403554 | 339 | 0.262 | 0.075 | 0.74 | _ | 3 |
| 56859 | 320 | 0.081 | 0.863 | 0.059 | S | 2 |
| 176420 | 458 | 0.019 | 0.972 | 0.037 | S | 1 |
| 46260 | 847 | 0.035 | 0.189 | 0.908 | _ | 2 |
| 188241 | 862 | 0.038 | 0.161 | 0.94 | _ | 2 |
| 33215 | 801 | 0.067 | 0.777 | 0.182 | S | 3 |
| 101267 | 513 | 0.022 | 0.946 | 0.089 | S | 1 |
| 33263 | 449 | 0.021 | 0.9 | 0.099 | S | 1 |
| 102985 | 338 | 0.067 | 0.974 | 0.027 | S | 1 |
| 448899 | 540 | 0.413 | 0.042 | 0.616 | _ | 4 |
| 177450 | 748 | 0.043 | 0.939 | 0.031 | S | 1 |
| 196330 | 544 | 0.05 | 0.961 | 0.019 | S | 1 |
| 33636 | 717 | 0.168 | 0.175 | 0.701 | _ | 3 |
| 125362 | 239 | 0.358 | 0.042 | 0.545 | _ | 5 |
| 100935 | 396 | 0.091 | 0.352 | 0.583 | _ | 4 |
| 164180 | 836 | 0.303 | 0.845 | 0.012 | S | 3 |
| 102479 | 750 | 0.243 | 0.06 | 0.721 | _ | 3 |
| 199563 | 388 | 0.678 | 0.27 | 0.052 | M | 3 |
| 145317 | 557 | 0.044 | 0.799 | 0.201 | S | 3 |
| 95645 | 99 | 0.09 | 0.193 | 0.743 | _ | 3 |
| 33906 | 312 | 0.19 | 0.087 | 0.732 | _ | 3 |
| 201958 | 607 | 0.826 | 0.018 | 0.328 | M | 3 |
| 33959 | 594 | 0.708 | 0.032 | 0.35 | M | 4 |
| 33963 | 340 | 0.025 | 0.921 | 0.133 | S | 2 |
| 203296 | 422 | 0.12 | 0.923 | 0.024 | S | 1 |
| 34143 | 292 | 0.061 | 0.079 | 0.934 | _ | 1 |
| 47402 | 390 | 0.028 | 0.942 | 0.071 | S | 1 |
| 164550 | 682 | 0.506 | 0.434 | 0.072 | M | 5 |
| 126363 | 785 | 0.226 | 0.14 | 0.716 | _ | 3 |
| 34175 | 506 | 0.937 | 0.026 | 0.099 | M | 1 |
| 126440 | 419 | 0.058 | 0.137 | 0.91 | _ | 2 |
| 207338 | 523 | 0.042 | 0.926 | 0.053 | S | 1 |
| 34226 | 479 | 0.071 | 0.086 | 0.922 | _ | 1 |
| 207890 | 208 | 0.042 | 0.926 | 0.053 | S | 1 |
| 24753 | 992 | 0.257 | 0.036 | 0.723 | _ | 3 |
| 101242 | 366 | 0.165 | 0.049 | 0.851 | _ | 2 |
| 209426 | 255 | 0.073 | 0.226 | 0.761 | _ | 3 |
| 47558 | 367 | 0.035 | 0.916 | 0.062 | S | 1 |
| 164740 | 337 | 0.048 | 0.798 | 0.118 | S | 2 |
| 47647 | 744 | 0.034 | 0.946 | 0.075 | S | 1 |
| 103882 | 413 | 0.153 | 0.052 | 0.854 | _ | 2 |
| 24940 | 781 | 0.104 | 0.077 | 0.907 | _ | 1 |
| 34577 | 474 | 0.223 | 0.03 | 0.844 | _ | 2 |
| 24950 | 374 | 0.049 | 0.978 | 0.015 | S | 1 |
| 275330 | 650 | 0.465 | 0.629 | 0.023 | S | 5 |
| 34622 | 607 | 0.147 | 0.12 | 0.719 | _ | 3 |
| 34651 | 526 | 0.127 | 0.039 | 0.875 | _ | 2 |
| 34705 | 653 | 0.061 | 0.067 | 0.905 | _ | 1 |
| 165147 | 577 | 0.063 | 0.086 | 0.896 | _ | 1 |
| 34805 | 391 | 0.914 | 0.035 | 0.118 | M | 2 |
| 280856 | 545 | 0.204 | 0.073 | 0.755 | _ | 3 |
| 104675 | 505 | 0.097 | 0.869 | 0.039 | S | 2 |
| 282706 | 466 | 0.089 | 0.952 | 0.038 | S | 1 |
| 34945 | 466 | 0.261 | 0.722 | 0.022 | S | 3 |
| 35099 | 397 | 0.034 | 0.957 | 0.044 | S | 1 |
| 35123 | 603 | 0.73 | 0.055 | 0.209 | M | 3 |
| 35255 | 335 | 0.09 | 0.869 | 0.039 | S | 2 |
| 128174 | 279 | 0.124 | 0.95 | 0.01 | S | 1 |
| 331356 | 251 | 0.066 | 0.79 | 0.195 | S | 3 |
| 35327 | 233 | 0.037 | 0.887 | 0.112 | S | 2 |
| 35330 | 235 | 0.04 | 0.881 | 0.096 | S | 2 |
| 104983 | 435 | 0.365 | 0.084 | 0.512 | _ | 5 |
| 165879 | 280 | 0.063 | 0.095 | 0.909 | _ | 1 |
| 165993 | 546 | 0.072 | 0.707 | 0.106 | S | 2 |
| 338580 | 678 | 0.048 | 0.947 | 0.019 | S | 1 |
| 35711 | 528 | 0.089 | 0.177 | 0.79 | _ | 2 |
| 105145 | 362 | 0.361 | 0.72 | 0.031 | S | 4 |
| 48723 | 546 | 0.07 | 0.964 | 0.021 | S | 1 |
| 35742 | 516 | 0.053 | 0.457 | 0.796 | _ | 4 |
| 340063 | 377 | 0.193 | 0.176 | 0.709 | _ | 3 |
| 105469 | 346 | 0.204 | 0.051 | 0.772 | _ | 3 |
| 105723 | 203 | 0.124 | 0.097 | 0.841 | _ | 2 |
| 48765 | 461 | 0.582 | 0.076 | 0.307 | M | 4 |
| 105560 | 432 | 0.053 | 0.904 | 0.049 | S | 1 |
| 68408 | 564 | 0.03 | 0.956 | 0.04 | S | 1 |
| 25772 | 321 | 0.025 | 0.966 | 0.043 | S | 1 |
| 106998 | 404 | 0.052 | 0.172 | 0.887 | _ | 2 |
| 35876 | 325 | 0.046 | 0.921 | 0.085 | S | 1 |
| 35880 | 488 | 0.054 | 0.182 | 0.909 | _ | 2 |
| 106046 | 321 | 0.103 | 0.937 | 0.018 | S | 1 |
| 106351 | 275 | 0.118 | 0.059 | 0.875 | _ | 2 |
| 59360 | 863 | 0.079 | 0.83 | 0.127 | S | 2 |
| 25843 | 321 | 0.303 | 0.882 | 0.013 | S | 3 |
| 166233 | 323 | 0.181 | 0.857 | 0.033 | S | 2 |
| 35905 | 325 | 0.061 | 0.937 | 0.04 | S | 1 |
| 129150 | 325 | 0.167 | 0.909 | 0.032 | S | 2 |
| 364963 | 340 | 0.055 | 0.899 | 0.036 | S | 1 |
| 365447 | 826 | 0.077 | 0.893 | 0.039 | S | 1 |
| 365822 | 509 | 0.036 | 0.921 | 0.071 | S | 1 |
| 106230 | 542 | 0.345 | 0.068 | 0.679 | _ | 4 |
| 464718 | 327 | 0.1 | 0.856 | 0.041 | S | 2 |
| 49096 | 474 | 0.051 | 0.961 | 0.044 | S | 1 |
| 129655 | 389 | 0.274 | 0.187 | 0.411 | _ | 5 |
| 49205 | 779 | 0.028 | 0.954 | 0.046 | S | 1 |
| 166629 | 301 | 0.351 | 0.134 | 0.447 | _ | 5 |
| 180053 | 292 | 0.4 | 0.1 | 0.395 | M | 5 |
| 107081 | 1,018 | 0.021 | 0.962 | 0.059 | S | 1 |
| 106859 | 396 | 0.04 | 0.484 | 0.631 | _ | 5 |
| 387673 | 192 | 0.127 | 0.115 | 0.864 | _ | 2 |
| 107229 | 219 | 0.14 | 0.06 | 0.876 | _ | 2 |
| 107188 | 503 | 0.053 | 0.868 | 0.154 | S | 2 |
| 108447 | 882 | 0.021 | 0.947 | 0.069 | S | 1 |
| 36572 | 867 | 0.082 | 0.087 | 0.879 | _ | 2 |
| 389256 | 583 | 0.057 | 0.958 | 0.017 | S | 1 |
| 49473 | 238 | 0.019 | 0.964 | 0.071 | S | 1 |
| 180279 | 701 | 0.433 | 0.747 | 0.012 | S | 4 |
| 107702 | 742 | 0.189 | 0.042 | 0.841 | _ | 2 |
| 150151 | 257 | 0.413 | 0.059 | 0.588 | _ | 5 |
| 49748 | 386 | 0.015 | 0.906 | 0.209 | S | 2 |
| 150399 | 588 | 0.123 | 0.817 | 0.031 | S | 2 |
| 36985 | 453 | 0.051 | 0.294 | 0.694 | _ | 4 |
| 36994 | 589 | 0.045 | 0.917 | 0.036 | S | 1 |
| 130948 | 590 | 0.038 | 0.942 | 0.053 | S | 1 |
| 36996 | 588 | 0.066 | 0.925 | 0.036 | S | 1 |
| 37005 | 203 | 0.044 | 0.261 | 0.726 | _ | 3 |
| 37023 | 753 | 0.068 | 0.477 | 0.496 | _ | 5 |
| 396825 | 410 | 0.022 | 0.966 | 0.049 | S | 1 |
| 37051 | 681 | 0.056 | 0.101 | 0.905 | _ | 1 |
| 167339 | 723 | 0.081 | 0.047 | 0.918 | _ | 1 |
| 108031 | 840 | 0.039 | 0.298 | 0.854 | _ | 3 |
| 60306 | 510 | 0.96 | 0.018 | 0.089 | M | 1 |
| 94900 | 199 | 0.349 | 0.054 | 0.575 | _ | 4 |
| 150787 | 929 | 0.174 | 0.491 | 0.328 | S | 5 |
| 108631 | 254 | 0.7 | 0.029 | 0.417 | M | 4 |
| 151004 | 587 | 0.056 | 0.645 | 0.36 | S | 4 |
| 408988 | 1,119 | 0.13 | 0.887 | 0.023 | S | 2 |
| 37467 | 467 | 0.03 | 0.984 | 0.031 | S | 1 |
| 131760 | 370 | 0.03 | 0.705 | 0.316 | S | 4 |
| 412878 | 510 | 0.049 | 0.888 | 0.057 | S | 1 |
| 109222 | 337 | 0.086 | 0.929 | 0.02 | S | 1 |
| 420841 | 296 | 0.43 | 0.109 | 0.29 | M | 5 |
| 167984 | 273 | 0.256 | 0.065 | 0.614 | _ | 4 |
| 37832 | 314 | 0.294 | 0.736 | 0.057 | S | 3 |
| 181255 | 402 | 0.021 | 0.96 | 0.068 | S | 1 |
| 37882 | 458 | 0.034 | 0.945 | 0.064 | S | 1 |
| 168122 | 772 | 0.03 | 0.949 | 0.05 | S | 1 |
| 132435 | 597 | 0.138 | 0.364 | 0.28 | S | 5 |
| 424941 | 446 | 0.025 | 0.965 | 0.038 | S | 1 |
| 69748 | 2,350 | 0.114 | 0.907 | 0.026 | S | 2 |
| 38169 | 565 | 0.021 | 0.958 | 0.058 | S | 1 |
| 38189 | 796 | 0.115 | 0.147 | 0.695 | _ | 3 |
| 109757 | 566 | 0.908 | 0.033 | 0.094 | M | 1 |
| 38208 | 806 | 0.057 | 0.966 | 0.015 | S | 1 |
| 50823 | 892 | 0.053 | 0.066 | 0.95 | _ | 1 |
| 38229 | 528 | 0.123 | 0.923 | 0.014 | S | 2 |
| 69931 | 375 | 0.044 | 0.99 | 0.027 | S | 1 |
| 61232 | 400 | 0.265 | 0.256 | 0.365 | _ | 5 |
| 38397 | 558 | 0.019 | 0.947 | 0.08 | S | 1 |
| 133171 | 563 | 0.194 | 0.78 | 0.019 | S | 3 |
| 38406 | 253 | 0.17 | 0.863 | 0.026 | S | 2 |
| 38407 | 253 | 0.12 | 0.822 | 0.058 | S | 2 |
| 110758 | 322 | 0.172 | 0.383 | 0.274 | S | 5 |
| 152728 | 384 | 0.062 | 0.798 | 0.143 | S | 2 |
| 110978 | 740 | 0.279 | 0.141 | 0.526 | _ | 4 |
| 168656 | 547 | 0.041 | 0.97 | 0.032 | S | 1 |
| 111162 | 528 | 0.041 | 0.316 | 0.851 | _ | 3 |
| 434943 | 523 | 0.146 | 0.921 | 0.011 | S | 2 |
| 61366 | 344 | 0.068 | 0.951 | 0.018 | S | 1 |
| 38562 | 579 | 0.048 | 0.961 | 0.032 | S | 1 |
| 61437 | 560 | 0.053 | 0.951 | 0.021 | S | 1 |
| 111196 | 150 | 0.118 | 0.118 | 0.85 | _ | 2 |
| 38632 | 752 | 0.11 | 0.714 | 0.134 | S | 3 |
| 38673 | 589 | 0.045 | 0.9 | 0.09 | S | 1 |
| 38796 | 890 | 0.051 | 0.889 | 0.068 | S | 1 |
| 111761 | 408 | 0.065 | 0.959 | 0.023 | S | 1 |
| 441800 | 339 | 0.362 | 0.212 | 0.32 | M | 5 |
| 169026 | 714 | 0.062 | 0.987 | 0.015 | S | 1 |
| 153331 | 307 | 0.041 | 0.53 | 0.552 | _ | 5 |
| 153350 | 869 | 0.293 | 0.063 | 0.66 | _ | 4 |
| 111954 | 463 | 0.02 | 0.981 | 0.054 | S | 1 |
| 61758 | 254 | 0.03 | 0.337 | 0.811 | _ | 3 |
| 51514 | 257 | 0.043 | 0.288 | 0.734 | _ | 3 |
| 111348 | 534 | 0.03 | 0.978 | 0.038 | S | 1 |
| 169283 | 531 | 0.071 | 0.944 | 0.017 | S | 1 |
| 39120 | 460 | 0.039 | 0.139 | 0.91 | _ | 2 |
| 153798 | 467 | 0.061 | 0.158 | 0.896 | _ | 2 |
| 182393 | 468 | 0.038 | 0.133 | 0.935 | _ | 1 |
| 112304 | 545 | 0.154 | 0.113 | 0.64 | _ | 3 |
| 39290 | 605 | 0.079 | 0.129 | 0.844 | _ | 2 |
| 214618 | 503 | 0.081 | 0.056 | 0.926 | _ | 1 |
| 39296 | 269 | 0.061 | 0.915 | 0.031 | S | 1 |
| 39375 | 890 | 0.055 | 0.865 | 0.069 | S | 2 |
| 51842 | 588 | 0.112 | 0.122 | 0.793 | _ | 2 |
| 51888 | 475 | 0.314 | 0.663 | 0.039 | S | 4 |
| 182705 | 511 | 0.053 | 0.883 | 0.083 | S | 1 |
| 219817 | 369 | 0.317 | 0.328 | 0.142 | S | 5 |
| 219843 | 336 | 0.45 | 0.866 | 0.004 | S | 3 |
| 182872 | 668 | 0.034 | 0.956 | 0.057 | S | 1 |
| 39816 | 471 | 0.021 | 0.96 | 0.064 | S | 1 |
| 227734 | 617 | 0.118 | 0.049 | 0.865 | _ | 2 |
Abbreviation: cTP, chloroplast transit peptide; Len, Sequence length; Loc, prediction of localization; M, Mitochondrion; RC, Reliability class; S, secretory pathway; SP, signal peptide.
FIGURE A1.
Total RNAs extracted from mycelia co‐cultivated with cyanobacterial cells (Treatment) and without cyanobacterial cells (Control) of 6, 12, 24, and 48 hr samples
FIGURE A2.
Boxplots showing the distribution of the FPKM values of each sample. Note: 6h_ck, control sample at 6 hr; 6h_T, treatment sample at 6 hr; 12h_ck, control sample at 12 hr; 12h_T, treatment sample at 12 hr; 24h_ck, control sample at 24 hr; 24h_T, treatment sample at 24 hr; 48h_ck, control sample at 48 hr; 48h_T, treatment sample at 48 hr. “_0” and “_1” represent repeat samples
FIGURE A3.
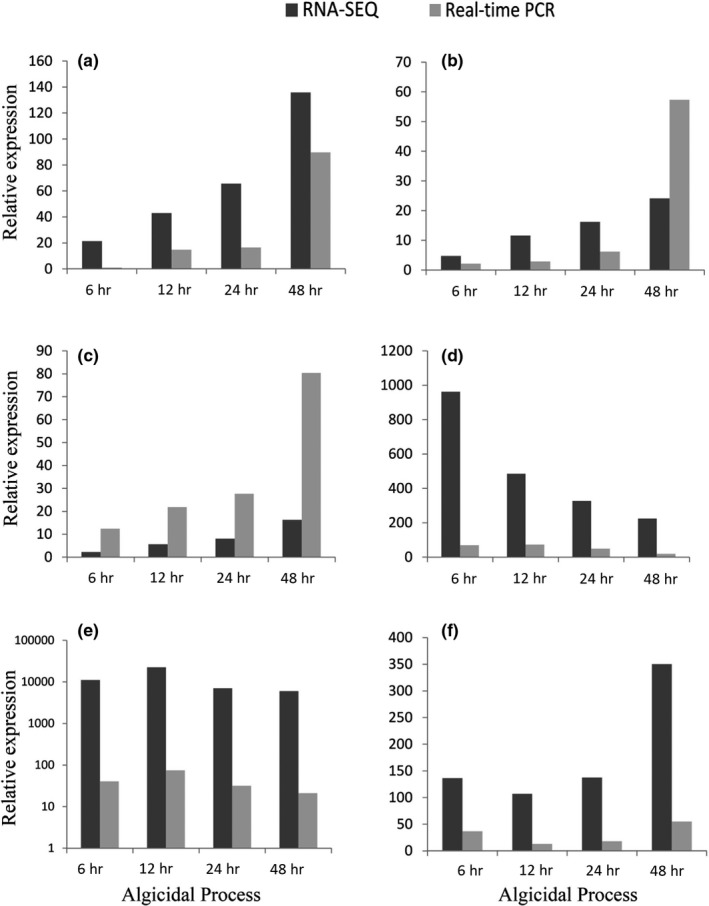
Comparison of expression changes between Real‐time PCR and RNA‐Sequencing. Note: A, jgi|Bjead1_1|34143|fgenesh1_kg.1_#_945_#_Locus732v1_medCvg1115.6s (a protein of esterase family 1); B, jgi|Bjead1_1|35099|fgenesh1_kg.2_#_711_#_Locus118v3_medCvg9284.2s (a protein of hydrolase family 5); C, jgi|Bjead1_1|172436|gm1.8875_g (a protein of hydrolase family 5); D, jgi|Bjead1_1|459664|MIX10988_17319_14 (a radical oxidase); E, jgi|Bjead1_1|355947|CE167616_517 (a protein of hydrolase family 128); F, jgi|Bjead1_1|38229|fgenesh1_kg.7_#_551_#_Locus8080v1_medCvg1578.8s (a protein of hydrolase family 13)
Han G, Ma H, Ren S, et al. Insights into the mechanism of cyanobacteria removal by the algicidal fungi Bjerkandera adusta and Trametes versicolor . MicrobiologyOpen. 2020;9:e1042 10.1002/mbo3.1042
Guomin Han and Hui Ma authors contributed equally to this work
Contributor Information
Guomin Han, Email: guominhan@ahau.edu.cn, Email: zhangshihua@ahau.edu.cn.
Shihua Zhang, Email: zhangshihua@ahau.edu.cn.
DATA AVAILABILITY STATEMENT
The raw paired‐end sequences from the Bjerkandera adusta isolate T1 are available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA543936. The genome annotations of Bjerkandera adusta and Trametes versicolor can be found at the JGI MycoCosm: https://mycocosm.jgi.doe.gov/Bjead1_1/Bjead1_1.home.html and https://mycocosm.jgi.doe.gov/Trave1/Trave1.home.html, respectively.
REFERENCES
- Ashburner, M. , Ball, C. A. , Blake, J. A. , Botstein, D. , Butler, H. , Cherry, J. M. , … Sherlock, G. (2000). Gene ontology: Tool for the unification of biology. The gene ontology Consortium. Nature Genetics, 25(1), 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, M. , Justo, A. , Riley, R. , Salamov, A. , Lopez‐Giraldez, F. , Sjökvist, E. , … Hibbett, D. S. (2013). Phylogenetic and phylogenomic overview of the Polyporales. Mycologia, 105(6), 1350–1373. 10.3852/13-003 [DOI] [PubMed] [Google Scholar]
- Bolger, A. M. , Lohse, M. , & Usadel, B. (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30(15), 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouacem, K. , Rekik, H. , Jaouadi, N. Z. , Zenati, B. , Kourdali, S. , El Hattab, M. , … Jaouadi, B. (2018). Purification and characterization of two novel peroxidases from the dye‐decolorizing fungus Bjerkandera adusta strain CX‐9. International Journal of Biological Macromolecules, 106, 636–646. 10.1016/j.ijbiomac.2017.08.061 [DOI] [PubMed] [Google Scholar]
- Bourne, D. G. , Jones, G. J. , Blakeley, R. L. , Jones, A. , Negri, A. P. , & Riddles, P. (1996). Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin microcystin LR. Applied and Environment Microbiology, 62(11), 4086–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne, D. G. , Riddles, P. , Jones, G. J. , Smith, W. , & Blakeley, R. L. (2001). Characterisation of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin LR. Environmental Toxicology, 16(6), 523–534. 10.1002/tox.10013 [DOI] [PubMed] [Google Scholar]
- Cantarel, B. L. , Coutinho, P. M. , Rancurel, C. , Bernard, T. , Lombard, V. , & Henrissat, B. (2009). The Carbohydrate‐Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Research, 37(Database), D233–D238. 10.1093/nar/gkn663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, W. , Chen, X. , Wang, X. , Xu, Z. , Gao, X. , Jiang, C. , … Han, G. (2018). The Algicidal fungus trametes versicolor F21a eliminating blue algae via genes encoding degradation enzymes and metabolic pathways revealed by transcriptomic analysis. Frontiers in Microbiology, 9, 826 10.3389/fmicb.2018.00826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin, A. , Davis, C. A. , Schlesinger, F. , Drenkow, J. , Zaleski, C. , Jha, S. , … Gingeras, T. R. (2013). STAR: Ultrafast universal RNA‐seq aligner. Bioinformatics, 29(1), 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziga, D. , Wasylewski, M. , Wladyka, B. , Nybom, S. , & Meriluoto, J. (2013). Microbial degradation of microcystins. Chemical Research in Toxicology, 26(6), 841–852. 10.1021/tx4000045 [DOI] [PubMed] [Google Scholar]
- Ekstrom, A. , Taujale, R. , McGinn, N. , & Yin, Y. (2014). PlantCAZyme: A database for plant carbohydrate‐active enzymes. Database (Oxford), 2014, 10.1093/database/bau079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson, O. , Brunak, S. , von Heijne, G. , & Nielsen, H. (2007). Locating proteins in the cell using TargetP. SignalP and Related Tools. Nature Protocols, 2(4), 953–971. 10.1038/nprot.2007.131 [DOI] [PubMed] [Google Scholar]
- Esterhuizen‐Londt, M. , Hertel, S. , & Pflugmacher, S. (2017). Uptake and biotransformation of pure commercial microcystin‐LR versus microcystin‐LR from a natural cyanobacterial bloom extract in the aquatic fungus Mucor hiemalis. Biotechnology Letters, 39(10), 1537–1545. 10.1007/s10529-017-2378-2 [DOI] [PubMed] [Google Scholar]
- Gao, X. , Wang, C. , Dai, W. , Ren, S. , Tao, F. , He, X. , … Wang, W. (2017). Proteomic analysis reveals large amounts of decomposition enzymes and major metabolic pathways involved in algicidal process of Trametes versicolor F21a. Scientific Reports, 7(1), 3907 10.1038/s41598-017-04251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, G. , Feng, X. , Jia, Y. , Wang, C. , He, X. , Zhou, Q. , & Tian, X. (2011). Isolation and evaluation of terrestrial fungi with algicidal ability from Zijin Mountain, Nanjing, China. The Journal of Microbiology, 49(4), 562–567. 10.1007/s12275-011-0496-4 [DOI] [PubMed] [Google Scholar]
- Hou, X. , Huang, J. , Tang, J. , Wang, N. A. , Zhang, L. U. , Gu, L. , … Huang, Y. (2019). Allelopathic inhibition of juglone (5‐hydroxy‐1,4‐naphthoquinone) on the growth and physiological performance in Microcystis aeruginosa. Journal of Environmental Management, 232, 382–386. 10.1016/j.jenvman.2018.11.105 [DOI] [PubMed] [Google Scholar]
- Jia, Y. , Du, J. , Song, F. , Zhao, G. , & Tian, X. (2012). A fungus capable of degrading microcystin‐lr in the algal culture of Microcystis aeruginosa PCC7806. Applied Biochemistry and Biotechnology, 166(4), 987–996. 10.1007/s12010-011-9486-6 [DOI] [PubMed] [Google Scholar]
- Jia, Y. , Han, G. , Wang, C. , Guo, P. , Jiang, W. , Li, X. , & Tian, X. (2010). The efficacy and mechanisms of fungal suppression of freshwater harmful algal bloom species. Journal of Hazardous Materials, 183(1–3), 176–181. 10.1016/j.jhazmat.2010.07.009 [DOI] [PubMed] [Google Scholar]
- Johnson, L. S. , Eddy, S. R. , & Portugaly, E. (2010). Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinformatics, 11, 431 10.1186/1471-2105-11-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa, M. , Furumichi, M. , Tanabe, M. , Sato, Y. , & Morishima, K. (2017). KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research, 45(D1), D353–D361. 10.1093/nar/gkw1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa, M. , & Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research, 28(1), 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa, M. , Sato, Y. , Kawashima, M. , Furumichi, M. , & Tanabe, M. (2016). KEGG as a reference resource for gene and protein annotation. Nucleic Acids Research, 44(D1), D457–462. 10.1093/nar/gkv1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, A. , Kuo, A. , Nagy, L. G. , Morin, E. , Barry, K. W. , Buscot, F. , … Martin, F. (2015). Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics, 47(4), 410–415. 10.1038/ng.3223 [DOI] [PubMed] [Google Scholar]
- Li, J. , Li, R. , & Li, J. (2017). Current research scenario for microcystins biodegradation ‐ A review on fundamental knowledge, application prospects and challenges. Science of the Total Environment, 595, 615–632. 10.1016/j.scitotenv.2017.03.285 [DOI] [PubMed] [Google Scholar]
- Livak, K. J. , & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lombard, V. , Golaconda Ramulu, H. , Drula, E. , Coutinho, P. M. , & Henrissat, B. (2014). The carbohydrate‐active enzymes database (CAZy) in 2013. Nucleic Acids Research, 42(D1), D490–D495. 10.1093/nar/gkt1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, Z. A. , Hashem, M. , & Alamri, S. A. (2014). Growth inhibition of the cyanobacterium Microcystis aeruginosa and degradation of its microcystin toxins by the fungus Trichoderma citrinoviride. Toxicon, 86, 51–58. 10.1016/j.toxicon.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Moody, S. C. , Dudley, E. , Hiscox, J. , Boddy, L. , & Eastwood, D. C. (2018). Interdependence of primary metabolism and xenobiotic mitigation characterizes the proteome of Bjerkandera adusta during Wood Decomposition. Applied and Environment Microbiology, 84(2), 10.1128/AEM.01401-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgaard, B. , Wilkens, C. , Herbst, F. A. , Vuillemin, M. , Rhein‐Knudsen, N. , Meyer, A. S. , & Lange, L. (2019). Proteomic enzyme analysis of the marine fungus Paradendryphiella salina reveals alginate lyase as a minimal adaptation strategy for brown algae degradation. Scientific Reports, 9, ARTN12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, L. , Zhang, X. , Chen, X. , Wang, K. , Shen, Y. , & Li, D. (2019). Isolation of a novel microcystin‐degrading bacterium and the evolutionary origin of mlr Gene Cluster. Toxins (Basel), 11(5), 10.3390/toxins11050269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, W. , Zhao, L. , Hou, S. , Yu, Q. J. , Tan, S. , & Yin, P. (2016). Toxic effect on the membrane system and cell proliferation of Prorocentrum donghaiense caused by the novel algicidal fungus Talaromyces purpurogenus YL13. Journal of Applied Phycology, 29(1), 275–284. 10.1007/s10811-016-0878-4 [DOI] [Google Scholar]
- Si, W. , Hang, T. , Guo, M. , Chen, Z. , Liang, Q. , Gu, L. , & Ding, T. (2019). Whole‐Genome and Transposed Duplication Contributes to the Expansion and Diversification of TLC Genes in Maize. International Journal of Molecular Sciences, 20(21), 5484 10.3390/ijms20215484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater (1998). (20th, edn ed). Wanshington, DC, USA: American Public Health Association/ American Water Works Association/ Water Environment Federation.
- Stephan, P. (2015). Toxin Resistance in Aquatic Fungi Poses Environmentally Friendly Remediation Possibilities: A Study on the Growth Responses and Biosorption Potential of Mucor hiemalis EH5 against Cyanobacterial Toxins. International Journal of Water and Wastewater Treatment (ISSN 2381‐5299), 1(1), 2381–5299. 10.16966/2381-5299.101 [DOI] [Google Scholar]
- Strasser, K. , McDonnell, E. , Nyaga, C. , Wu, M. , Wu, S. , Almeida, H. , … Tsang, A. (2015). mycoCLAP, the database for characterized lignocellulose‐active proteins of fungal origin: Resource and text mining curation support. Database (Oxford), 2015, 10.1093/database/bav008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara, K. , Igeta, E. , Amano, Y. , Hyuga, M. , & Sugano, Y. (2019). Degradation of antifungal anthraquinone compounds is a probable physiological role of DyP secreted by Bjerkandera adusta. AMB Express, 9(1), 56 10.1186/s13568-019-0779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, R. , Sun, P. , Zhang, J. , Esquivel‐Elizondo, S. , & Wu, Y. (2018). Microorganisms‐based methods for harmful algal blooms control: A review. Bioresource Technology, 248(Pt B), 12–20. 10.1016/j.biortech.2017.07.175 [DOI] [PubMed] [Google Scholar]
- Tisserant, E. , Malbreil, M. , Kuo, A. , Kohler, A. , Symeonidi, A. , Balestrini, R. , … Martin, F. (2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proceedings of the National Academy of Sciences of the United States of America, 110(50), 20117–20122. 10.1073/pnas.1313452110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, C. , Roberts, A. , Goff, L. , Pertea, G. , Kim, D. , Kelley, D. R. , … Pachter, L. (2012). Differential gene and transcript expression analysis of RNA‐seq experiments with TopHat and Cufflinks. Nature Protocols, 7(3), 562–578. 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Su, M. , Zhu, W. , Li, X. , Jia, Y. , Guo, P. , … Tian, X. (2010). Growth inhibition of Microcystis aeruginosa by white‐rot fungus Lopharia spadicea. Water Science and Technology, 62(2), 317–323. 10.2166/wst.2010.214 [DOI] [PubMed] [Google Scholar]
- Wang, R. , Li, J. , Jiang, Y. , Lu, Z. , Li, R. , & Li, J. (2017). Heterologous expression of mlrA gene originated from Novosphingobium sp. THN1 to degrade microcystin‐RR and identify the first step involved in degradation pathway. Chemosphere, 184, 159–167. 10.1016/j.chemosphere.2017.05.086 [DOI] [PubMed] [Google Scholar]
- Xu, Q. , Fan, J. , Yan, H. , Ahmad, S. , Zhao, Z. , Yin, C. , … Zhang, H. (2019). Structural basis of microcystinase activity for biodegrading microcystin‐LR. Chemosphere, 236, 124281 10.1016/j.chemosphere.2019.07.012 [DOI] [PubMed] [Google Scholar]
- Yin, Y. , Mao, X. , Yang, J. , Chen, X. , Mao, F. , & Xu, Y. (2012). dbCAN: A web resource for automated carbohydrate‐active enzyme annotation. Nucleic Acids Research, 40(W1), W445–W451. 10.1093/nar/gks479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Zeng, Y. , Li, J. , Yang, C. , Zhang, X. , Luo, F. , & Dai, X. (2019). An algicidal Streptomyces amritsarensis strain against Microcystis aeruginosa strongly inhibits microcystin synthesis simultaneously. Science of the Total Environment, 650(Pt 1), 34–43. 10.1016/j.scitotenv.2018.08.433 [DOI] [PubMed] [Google Scholar]
- Zeng, G. , Wang, P. , & Wang, Y. (2015). Algicidal efficiency and mechanism of Phanerochaete chrysosporium against harmful algal bloom species. Algal Research, 12, 182–190. 10.1016/j.algal.2015.08.019 [DOI] [Google Scholar]
- Zeng, G. , Zhang, M. , Wang, P. , Li, X. , Wu, P. , & Sun, D. (2019). Genotoxicity effects of Phanerochaete chrysosporium against harmful algal bloom species by micronucleus test and comet assay. Chemosphere, 218, 1031–1042. 10.1016/j.chemosphere.2018.11.148 [DOI] [PubMed] [Google Scholar]
- Zhang, F. X. , Ye, Q. , Chen, Q. L. , Yang, K. , Zhang, D. Y. , Chen, Z. R. , … Xu, H. (2018). Algicidal activity of novel marine Bacterium Paracoccus sp Strain Y42 against a harmful algal‐bloom‐causing dinoflagellate, Prorocentrum donghaiense. Applied and Environmental Microbiology, 84(19), e01015‐18 UNSP [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw paired‐end sequences from the Bjerkandera adusta isolate T1 are available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA543936. The genome annotations of Bjerkandera adusta and Trametes versicolor can be found at the JGI MycoCosm: https://mycocosm.jgi.doe.gov/Bjead1_1/Bjead1_1.home.html and https://mycocosm.jgi.doe.gov/Trave1/Trave1.home.html, respectively.


