Abstract
Antibiotic‐resistant strains of bacteria such as methicillin‐resistant Staphylococcus aureus are a threat to human health, and effective treatment options against them are needed. This study aimed to determine whether the insecticide permethrin was capable of inhibiting the growth of S. aureus or if some other component of a permethrin cream was responsible for a decrease in scabies associated bacterial infection previously observed. Ten S. aureus strains were grown in the presence of permethrin and formaldehyde both alone and in combination with percent inhibition determined by viable counts. Also, a time‐kill assay was conducted on S. aureus exposed to the same conditions. Finally, the morphology of S. aureus grown in the presence of permethrin was examined by scanning electron microscopy. Bacterial inhibition by permethrin ranged from 0% to 41% whereas inhibition by formaldehyde was 100%. The time‐kill curves of permethrin exposed cells were very similar to the positive growth control while the formaldehyde and combination exposure showed complete inhibition even at the 0‐hr time point. The scanning electron micrographs of permethrin grown S. aureus showed healthy cocci cells with no sign of cell damage. Our results show that permethrin is not capable of inhibiting the growth of bacteria enough for it to be termed bactericidal. Formaldehyde is a known antiseptic and therefore was responsible for the antibacterial effect observed after the use of permethrin cream.
Keywords: antibacterial activity, formaldehyde, methicillin‐resistant Staphylococcus aureus, permethrin
Antibiotic resistance is an increasing threat to human health. Alternative treatment options are needed. This study investigated the effect of permethrin and formaldehyde on the growth of Staphylococcus aureus, in particular MRSA strains. While permethrin alone was not bactericidal, formaldehyde both in the presence and absence of permethrin was. This was at concentrations of formaldehyde and permethrin found in the anti‐scabies permethrin cream and suggests a potential use of such creams for treating bacterial skin infections, particularly refractory impetigo.

1. INTRODUCTION
Antibiotics have been a mainstay for treatment of bacterial infections since their discovery. However, the continued misuse and overuse of antibiotics has led to the rapid development and spread of antibiotic‐resistant strains of bacteria. As a result, antibiotic resistance is rising more rapidly than new antibiotics are being developed. This threatens to end the golden age of antibiotics we are currently living in and begin the postantibiotic era (Perry, Waglechner, & Wright, 2016). One such bacterial pathogen that has become a problem in the clinical setting due to the wide variety of antibiotics it has developed resistances to is Staphylococcus aureus.
Staphylococcus aureus is a Gram‐positive cocci bacterium that can be found as a commensal on human skin and nares. Approximately 30% of the population live asymptomatically colonized with S. aureus. However, it can also act as a pathogenic bacterium in humans causing diseases that range from minor skin infections to more serious infections such as impetigo, osteomyelitis, and endocarditis (Rojo, Barrios, Palacios, Gomez, & Chaves, 2010). Infections with S. aureus are typically treated with antibiotics but the spread of increasingly resistant strains of S. aureus has made treatment with antibiotics difficult. One of the most widespread antibiotic‐resistant strains of S. aureus is methicillin‐resistant S. aureus (MRSA) that is resistant to β‐lactam antibiotics including penicillin and methicillin. As many as 60% of clinically isolated strains of S. aureus are resistant to methicillin (Romaniuk & Cegelski, 2015).
Even antibiotics like vancomycin and daptomycin, which were considered last‐resort antibiotics for MRSA infections, have become ineffective against certain strains of S. aureus (Bayer, Schneider, & Sahl, 2013; McGuinness, Malachowa, & DeLeo, 2017; Walters et al., 2015). As resistances continue to develop, alternative treatment options are required. This includes the development of new antibiotics as well as novel treatment methods such as anti‐virulence medication and phage therapy (Khodaverdian et al., 2013; Zhang et al., 2018).
Infections with S. aureus are often associated with scabies infections. Scabies is an infectious skin disease caused by the Sarcoptes scabiei mite and is spread primarily by skin‐to‐skin contact. The mites infect a host and burrow into the skin causing itching and irritation which can disrupt the skin barrier. Skin barrier disruption allows for the development of secondary bacterial infections, typically of Group A Streptococci or S. aureus and can lead to diseases such as impetigo and endocarditis (May et al., 2016). Permethrin is a synthetic pyrethroid and the preferred treatment for scabies infections. Permethrin's mode of action against scabies mites involves binding to the mite's voltage‐gated sodium channels in a hydrophobic pocket formed by the helices of two separate protein domains (Field, Davies, O'Reilly, Williamson, & Wallace, 2017). This binding prevents the transition from an activated state to an inactivated state allowing an influx of sodium ions, causing prolonged depolarization, paralysis, and death of the mite (Andriantsoanirina et al., 2014).
Impetigo is a skin infection commonly found in children that, as mentioned above, can accompany scabies infections. It is usually caused by S. aureus but can also be caused by Streptococcus pyogenes and presents on the skin as blisters or ulcers (Ghazvini, Treadwell, Woodberry, Nerette, & Powery, 2017). Impetigo contributes to a high burden of disease (especially in resource‐poor communities) with an estimated global burden of 162 million children in low to low‐middle income countries being affected by impetigo. In Australia alone, it is estimated that over 15,000 indigenous children suffer from impetigo at any one time (Bowen et al., 2015). Impetigo is usually treated with topical antibiotic creams but in more serious infections an oral antibiotic may be administered instead. The first‐choice creams for the treatment of impetigo are fusidic acid and mupirocin but resistances to even these antibiotics have led to the use of retapamulin as an alternative treatment option (Alsterholm, Flytström, Bergbrant, & Faergemann, 2010; Pereira, 2014; Poovelikunnel, Gethin, & Humphreys, 2015).
A prior study by Whitehall, Kuzulugil, Sheldrick, and Wood (2013) assessed the health burden associated with scabies and pyoderma in children at Mt Isa Hospital. This study also identified the bacteria present in the infected patients (Group A streptococcus, S. aureus and Group C streptococcus). The treatment methods included soap baths, administration of the antibiotic flucloxacillin, application of a 5% permethrin cream, and an adequate diet with iron supplementation. This treatment regimen was sufficient in curing both scabies and associated bacterial infections. However, subsequent analysis of the bacterial strains found that most of the staphylococci strains were resistant to flucloxacillin. This was despite all the patients recovering and implicates either the soap and water baths or the 5% permethrin cream as having a major benefit for treating antibiotic‐resistant bacterial infections.
This study aimed to identify the cause of the observed antibacterial effect in Whitehall et al. (2013). In doing so, a new antibacterial treatment method could be identified for use in combating antibiotic‐resistant strains of bacteria. The effect of permethrin on insects is well characterized but there are no reports of it possessing antibacterial activity. Therefore, this study focused on the 5% permethrin cream used in Whitehall et al. (2013) looking for an antibacterial effect in its components. Initially, permethrin itself was the sole focus but after failing to identify substantial antibacterial effect with permethrin alone, formaldehyde (used as a preservative in the 5% permethrin cream) was investigated. As a result, it was identified that formaldehyde both alone and in the presence of permethrin can inhibit the growth of S. aureus and was, therefore, the most likely cause of the antibacterial activity in Whitehall et al. (2013). This article highlights the potential for the already used permethrin cream with a formaldehyde preservative as a treatment for antibiotic‐resistant bacterial skin infections, particularly refractory impetigo.
2. MATERIALS AND METHODS
2.1. Bacterial isolates and growth conditions
A total of ten strains of S. aureus including five methicillin‐susceptible (MSSA 1, MSSA 2, MSSA 4, MSSA 6 and MSSA 8) and five methicillin‐resistant (MRSA 1, MRSA 2, MRSA 8, MRSA 9 and MRSA 13; Table 1; Turnidge, Coombs, Daley, & Nimmo, 2016) were obtained from Prof Iain Gosbell (Liverpool Hospital) and used throughout the experiments. Before use, each strain was freshly streaked on lysogeny broth (LB) agar plates (Difco Laboratories) and incubated overnight at 35 ± 2°C in a Binder Drying and Heating Chamber Model ED 115. After overnight incubation, 2–3 colonies were suspended in Mueller‐Hinton Broth (MHB) (Difco Laboratories) to OD595 0.09 (≈1 × 108 CFU/ml matching a 0.5 McFarland turbidity standard) using a spectrophotometer (SPECTROstar Nano; BMG Labtech). These cells were then diluted to 1 × 107 CFU/ml, and appropriate volumes were used to have 0.5 × 105 CFU/ml in the experiments.
TABLE 1.
Characteristics of methicillin‐resistant strains of Staphylococcus aureus used in the experiments (Turnidge et al., 2016)
| Strain | Name | Type (HA/CA) a | Origin | Resistance pattern | PVL b |
|---|---|---|---|---|---|
| MRSA 1 | QLD1 | CA‐MRSA | Australia | Typically no additional resistances (susceptible to non‐β‐lactam classes of antimicrobial) | + |
| MRSA 2 | Aus‐2 | HA‐MRSA | Australia |
Macrolides/Lincosamides Tetracyclines Trimethoprim‐sulfamethoxazole Gentamicin Fluoroquinolones |
− |
| MRSA 8 | USA300 | CA‐MRSA | USA |
Macrolides/Lincosamides Fluoroquinolones (variable) |
+ |
| MRSA 9 | SWP | CA‐MRSA | New Zealand | Typically no additional resistances | + |
| MRSA 13 | WA‐1 | CA‐MRSA | Australia |
Typically, no additional resistances Macrolides/lincosamides variable Fusidic acid variable |
_ |
Healthcare Associated/Community Associated.
Panton Valentine Leucocidin production.
2.2. In vitro antimicrobial testing
To identify the effect of permethrin and formaldehyde on the growth of S. aureus both alone and in combination, each strain was exposed to four concentrations of permethrin, four concentrations of formaldehyde and a combination of permethrin and formaldehyde. The four concentrations of permethrin and formaldehyde used were twofold concentrations and included the concentration present in the commercial permethrin cream. For the combination treatment, the concentration of permethrin and formaldehyde as present in the commercial permethrin cream was used.
Permethrin was obtained from Sigma, and its stock was made in MHB by dissolving in an ultrasonic cleaner (Scientifix) at 37°C for 10 min or until the solution was homogenous. Organic solvents like DMSO and methanol were not used for dissolving permethrin because they interfered with the growth of the test organisms. The bacterial inoculum, as prepared above, was added to each of 1.25%, 2.5%, 5%, and 10% permethrin set in a 96‐well microplate. Also, positive growth control of cells in MHB without permethrin was included. The cells were incubated on a benchtop orbital shaker (Thermo Scientific) for 20 hr at 35°C and 100 rpm. Each experiment was repeated three times independently and viable counts determined by a drop plate method as described in Section 2.4.
Formaldehyde solution was obtained from Electron Microscopy Sciences and diluted in MHB to make a stock. The bacterial inoculum was added to each of 0.07%, 0.15%, 0.3%, and 0.6% formaldehyde, and the experiment was conducted as was done for permethrin described above.
To identify whether permethrin and formaldehyde affected each other's activity when present together, combination exposure experiments were conducted in which each strain was tested in the presence of a combination of permethrin and formaldehyde at the concentrations found in the permethrin cream. The bacterial inoculum was added to each of 5% permethrin, 0.3% formaldehyde and both 5% permethrin and 0.3% formaldehyde together, and the experiment was conducted as was done for permethrin described above.
2.3. Time‐kill assay
To determine the time at which formaldehyde exerts its antibacterial effect on S. aureus, whether permethrin delays bacterial growth and whether permethrin interferes with the effect that formaldehyde exerts against S. aureus, a time‐kill assay was conducted. The bacterial inoculum was set up as was done for the combined exposure experiment described above and samples incubated on a benchtop orbital shaker at 35°C and 100 rpm. At 0, 4, 8, and 24 hr postincubation, samples were taken and viable counts determined as described in Section 2.4. Each experiment was repeated three times independently.
2.4. Viable counts
Viable counts were determined using a drop plate method (modified from Miles, Misra, and Irwin (1938)). Each cell suspension was serially diluted tenfold and the appropriate dilutions dispensed onto LB agar plates in triplicate. The plates were incubated overnight (19–20 hr) in an incubator at 35°C. After incubation, the number of colonies was counted to determine the average CFU/ml and percent inhibition. Bactericidal activity was defined as a decrease in growth of ≥99.9% (≥3log10 CFU/ml reduction).
2.5. Scanning electron microscopy
To visualize any effect that permethrin had on the growth and morphology of S. aureus cells, each strain was grown in the presence of 5% permethrin, fixed, and dehydrated for imaging by scanning electron microscopy (SEM). Cells were first grown either in the presence of 5% permethrin or in MHB alone as a positive growth control and incubated on a benchtop orbital shaker for 20 hr at 35°C and 100 rpm. The cell suspension was then washed three times in 0.1 M sodium phosphate buffer (pH 7.4) (made by adding 0.1 M NaH2PO4 [VWR Chemicals] to 0.1 M Na2HPO4 [Merck]). Washed cells were then resuspended in 2.5% glutaraldehyde (ProSciTech) in 0.1 M sodium phosphate buffer and incubated overnight at 4°C to fix the cells. Following overnight incubation at 4°C, the cells were again washed three times in 0.1 M sodium phosphate buffer before being dehydrated in graded ethanol (Chem‐Supply) series (30%, 50%, 70%, 80%, 90% 10 min each and 100% for one hour). The cell suspension was then transferred to a silica wafer for imaging by SEM.
Cells were examined by SEM using a Zeiss Merlin FE‐SEM under low vacuum with a beam strength of 1 kV, an aperture of 30 µm, and with a working distance of 5 mm. A high‐efficiency secondary electron (HE‐SE2) detector was used to image cells.
3. RESULTS
The effect of permethrin on the growth of S. aureus cells was tested at four concentrations to determine if the concentration of permethrin found in a commonly used permethrin cream was capable of inhibiting bacterial growth. The focus was not on the determination of minimum inhibitory concentration (MIC) of permethrin but rather on the concentration found in the permethrin cream. The results of these experiments are summarized in Figure 1 as an average percent inhibition of three independent runs. 1.25% permethrin showed inhibition ranging from 0% to 34%, 2.5% permethrin showed inhibition ranging from 0% to 23%, 5% permethrin showed inhibition ranging from 0% to 32%, and 10% permethrin showed inhibition ranging from 0% to 32%. In several cases, no inhibition was observed as was an increase in cells in the permethrin‐treated samples when compared to the positive growth control. The observed inhibition was also not concentration‐dependent. Poor aqueous solubility and unavoidable use of multiple batches of permethrin across experiments could have been responsible for large variations seen in percent inhibition in repeats of experiments but none of them was high enough to warrant bactericidal effects of permethrin.
FIGURE 1.
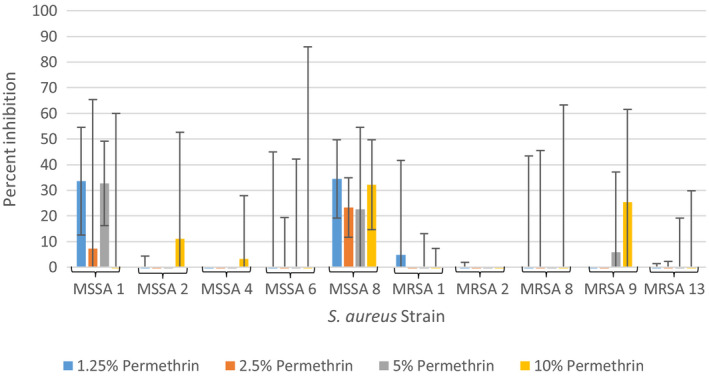
Average percent inhibition of methicillin‐sensitive and methicillin‐resistant Staphylococcus aureus grown in 1.25%, 2.5%, 5%, and 10% permethrin from three independent runs with standard deviation of the mean error bars
The effect of formaldehyde on the growth of S. aureus cells was also tested at four concentrations to determine if the concentration of formaldehyde in a commonly used permethrin cream was capable of inhibiting bacterial growth. The results of these experiments are summarized in Figure 2 as an average percent inhibition of three independent runs. All four concentrations of formaldehyde (0.07%, 0.15%, 0.3%, and 0.6%) were capable of inhibiting 100% of growth in all ten tested S. aureus strains.
FIGURE 2.
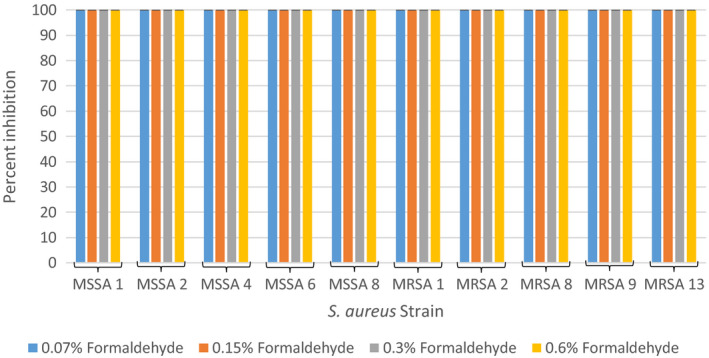
Average percent inhibition of methicillin‐sensitive and methicillin‐resistant Staphylococcus aureus grown in 0.07%, 0.15%, 0.3%, and 0.6% formaldehyde from three independent runs with standard deviation of the mean error bars
The effect of 0.3% formaldehyde, 5% permethrin and a combination of 0.3% formaldehyde and 5% permethrin on the growth of S. aureus was investigated to determine the effect of permethrin in combination with formaldehyde (at the concentrations found in a commonly used permethrin cream) to inhibit bacterial growth. The results of these experiments are summarized in Figure 3 as average percent inhibition of three independent runs. Both 0.3% formaldehyde alone and in combination with 5% permethrin were capable of inhibiting 100% of growth in all ten tested S. aureus strains. 5% permethrin alone exhibited similar inhibition as seen in the permethrin experiments with inhibition ranging from 0% to 41%.
FIGURE 3.
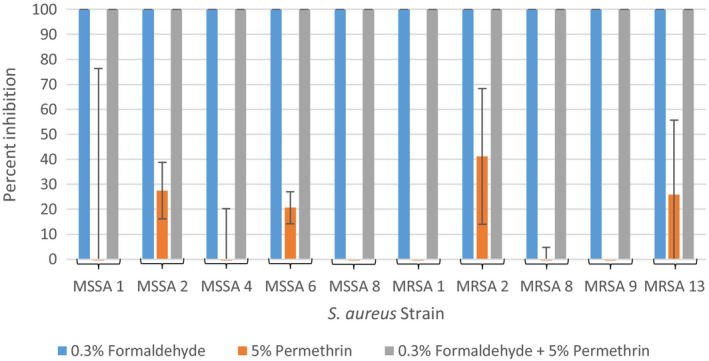
Average percent inhibition of methicillin‐sensitive and methicillin‐resistant Staphylococcus aureus grown in 0.3% formaldehyde and 5% permethrin alone, and combined from three independent runs with standard deviation of the mean error bars
To identify the time at which formaldehyde and permethrin alone and in combination exert their activity against S. aureus, a time‐kill assay was conducted on MSSA 1 and MRSA 1 in the presence of 0.3% formaldehyde, 5% permethrin and a combination of 0.3% formaldehyde and 5% permethrin. In both experiments, 5% permethrin exposure resulted in growth rates similar to the positive growth control (Figure 4). However, 0.3% formaldehyde and the combination of 0.3% formaldehyde and 5% permethrin exhibited zero growth at all‐time points including the zero‐hour time point. This meant that on contact with formaldehyde, all bacterial cells were rendered nonviable with no latency in this activity.
FIGURE 4.
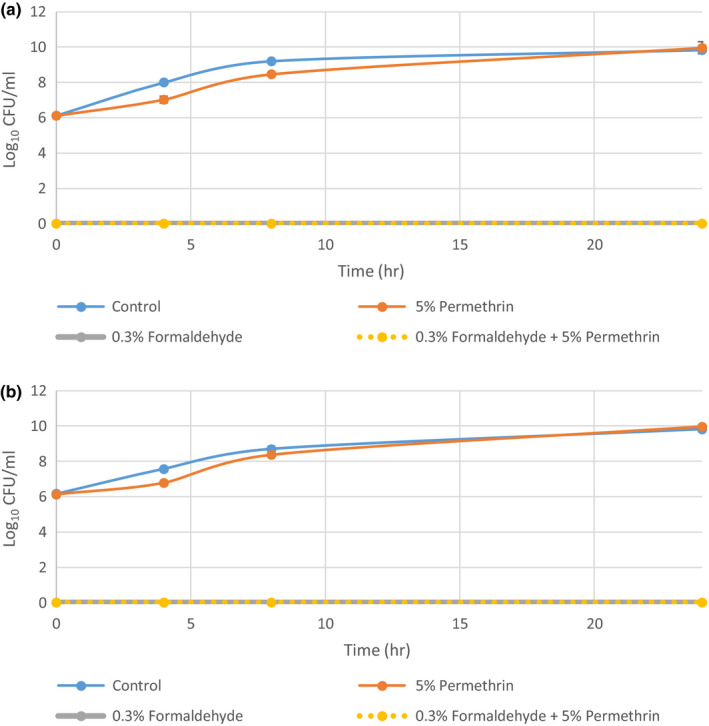
Time‐kill curves of methicillin‐sensitive and methicillin‐resistant Staphylococcus aureus grown in 0.3% formaldehyde and 5% permethrin alone, and combined from three independent runs with standard deviation of the mean error bars. (a) MSSA 1 (b) MRSA 1
After confirming that 5% permethrin exerts no significant effect on the growth of S. aureus in the above time‐kill curve experiment, the remaining eight strains were only exposed to the combination of 0.3% formaldehyde and 5% permethrin. The results for the four methicillin‐sensitive strains are summarized in Figure A1, and the results for the four methicillin‐resistant strains are summarized in Figure A2. All eight strains exhibited zero growth in the combination treatment at all‐time points.
All ten strains were grown in the presence of MHB alone and permethrin to compare and identify any changes to their morphology as a result of permethrin exposure. The methicillin‐sensitive strains can be seen in Figure 5, and the methicillin‐resistant strains can be seen in Figure 6. As can be seen in these representative SEM images, there was no difference in the morphology between the untreated and permethrin‐treated cells. There were also no obvious signs of forced cell death, cell shrinkage, lysis, or other forms of cell damage in the permethrin‐treated cells. Furthermore, there appear to be cells that are embedded within the permethrin itself and capable of growth.
FIGURE 5.
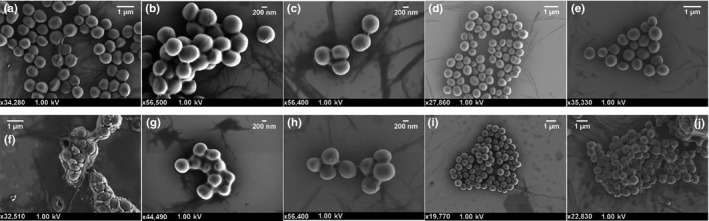
Scanning electron micrographs of methicillin‐sensitive Staphylococcus aureus grown in Mueller‐Hinton broth or permethrin. The top row shows control cells, and the bottom row shows cells treated with 5% permethrin. (a & f) MSSA 1; (b & g) MSSA 2; (c & h) MSSA 4; (d & i) MSSA 6; and (e & j) MSSA 8
FIGURE 6.
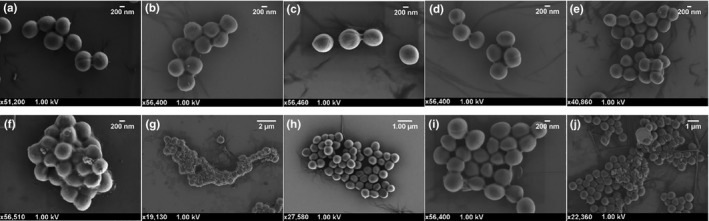
Scanning electron micrographs of methicillin‐resistant Staphylococcus aureus grown in Mueller Hinton broth or permethrin. The top row shows control cells, and the bottom row shows cells treated with 5% permethrin. (a & f) MRSA 1; (b & g) MRSA 2; (c & h) MRSA 8; (d & i) MRSA 9; and (e & j) MRSA 13
4. DISCUSSION
In an earlier study, the use of a 5% permethrin cream in patients with scabies and impetigo was associated with a decrease in infection with antibiotic‐resistant S. aureus (Whitehall et al., 2013). Permethrin is an insecticide used to treat scabies infections (Currie & McCarthy, 2010) and while its insecticidal effects are well characterized, the literature does not contain any mention of permethrin possessing direct antibacterial properties. There is however some evidence that it can affect the composition of bacterial populations (Dada et al., 2019; Jin, Wu, Zeng, & Fu, 2017). This study, therefore, attempted to identify what component of the permethrin cream was responsible for the observed antibacterial effect and found that while permethrin itself does not possess antibacterial activity; the formaldehyde preservative was more than sufficient for the killing of S. aureus.
In this study, it was observed that none of the tested concentrations of permethrin was able to significantly inhibit the growth of S. aureus for it to be termed as antibacterial. None of the tested concentrations exhibited more than 41% inhibition. As this is well below the ≥99.9% inhibition required for it to be termed bactericidal, permethrin itself was not responsible for the observed antibacterial effect in Whitehall et al. (2013). However, the permethrin cream used in Whitehall et al. (2013) contained 0.3% formaldehyde as a preservative. Formaldehyde is known to possess strong antibacterial properties (with a MIC of 156 mg/L [0.02%] against S. aureus) and is hence used as a preservative (Mazzola, Jozala, Novaes, Moriel, & Penna, 2009). Formaldehyde may, therefore, have been responsible for the observed antibacterial activity. This is supported by all four tested concentrations of formaldehyde completely inhibiting the growth of all ten S. aureus strains as well as 0.3% formaldehyde being able to completely inhibit bacterial growth even in the presence of permethrin. This indicates that permethrin does not interfere with the antibacterial action of formaldehyde. The conducted time‐kill assay was able to confirm that the rate of growth of S. aureus was unaffected by permethrin as well as that formaldehyde both alone and in combination with permethrin exhibits complete inhibition of S. aureus very rapidly with no growth seen even at the 0‐hr time point.
While the above results indicate that permethrin has no bactericidal effect, it may have the ability to alter the growth of S. aureus cells in some other way. However, as can be seen in the representative electron micrographs, both the control and permethrin‐treated cells exhibit similar morphology with no indication of cell damage. Furthermore, healthy S. aureus cells embedded within the permethrin imply that S. aureus is unaffected by exposure to permethrin.
Through combination exposure and time‐kill assays, this study has shown that 5% permethrin does not possess strong bactericidal properties, nor does it act antagonistically against the action of formaldehyde. Therefore, the observed antibacterial activity in the earlier study (Whitehall et al., 2013) was most likely due to the presence of 0.3% formaldehyde in the 5% permethrin cream. While the results presented here show that permethrin cannot be used as a novel antimicrobial, they do bring to attention the potential for antibacterial treatment using formaldehyde at low concentrations in medicinal creams.
Formaldehyde is widely used in cosmetics and medicines but its risks need to be investigated further than what is currently available. While the airborne formaldehyde gas is known to cause nasopharyngeal cancer (Nielsen, Larsen, & Wolkoff, 2017) and allergic contact dermatitis (De Groot, Flyvholm, Lensen, Menné, & Coenraads, 2009), and its effects in allergic individuals are well characterized (Hauksson et al., 2016), the effects of dermally applied formaldehyde in nonallergic individuals are not as well understood (most studies published in the 1980s). Similarly, the rate of formaldehyde absorption through human skin is unknown with no in vivo human studies available. Only animal studies and studies on excised human skin, published in the 1980s, indicate that dermal application of low concentrations of formaldehyde results in minimal absorption through unbroken skin (Bartnik, Gloxhuber, & Zimmermann, 1985; Iversen, 1986; Lodén, 1986). A more recent study showed that 2% formaldehyde caused swelling in mice ears but the minimal concentration required for this effect was not identified (Saito et al., 2011). Widespread use of formaldehyde in cosmetics and medicinal creams may highlight its relative safety in the dermal application at low concentrations.
It is, therefore, possible that a formaldehyde cream could serve as a last‐resort treatment in infections with antibiotic‐resistant bacteria that are not responsive to any other form of therapy. A benefit of using formaldehyde in this manner is that it is difficult for bacteria to develop resistance to it. No Gram‐positive bacteria have been reported to be resistant to formaldehyde and while bacteria, such as Amycolatopsis methanolica and Mycobacterium gastri, are tolerant to formaldehyde, both are still susceptible at concentrations above 0.8 mM (Bystrykh et al., 1993).
The results presented here, combined with the potent antibacterial activity of formaldehyde, the difficulty bacteria have in developing adequate resistance to formaldehyde and the implication that low concentrations of formaldehyde may be safe for use in humans, highlight that formaldehyde‐containing cream such as a permethrin cream can be used for treating refractory infections (Nikolic, Mudgil, & Whitehall, 2019). By treating infections with multi‐resistant strains of S. aureus, formaldehyde could help to combat antibiotic resistance by both curing what may be considered incurable infections and reducing the use of antibiotics. For example, in scabies infections, a permethrin cream with a formaldehyde preservative would be sufficient in treating both scabies and the associated bacterial infections, rather than using permethrin cream for scabies and a separate antibiotic for the bacterial infections.
While permethrin creams containing formaldehyde are already medically approved for dermal application, it is recommended that more research is conducted to confirm what was published in the 80s and to fill the current gaps present in the literature before using it for treating antibiotic‐resistant bacterial skin infections. This would include research into how formaldehyde affects humans after dermal application, the rate of absorption through human skin and whether or not dermally applied formaldehyde can release dangerous levels of airborne formaldehyde. However, the currently available information does indicate that the dermal application of low concentrations of formaldehyde is unlikely to cause significant harm.
5. CONCLUSION
The results presented here conclude that permethrin does not possess strong antibacterial properties and that formaldehyde was responsible for the observed antibacterial effect after administration of a 5% permethrin cream. Formaldehyde is a known antiseptic, and it could serve as a last resort in bacterial infections with no other treatment option. However, the risks associated with its use would need to be considered and, in the case of dermal contact in nonallergic individuals, investigated further. The continued use of formaldehyde in dermal creams suggests lower risk than currently thought.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Philip Nikolic: Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (supporting); Validation (lead); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (equal). Poonam Mudgil: Conceptualization (lead); Methodology (lead); Project administration (lead); Resources (lead); Supervision (lead); Validation (supporting); Visualization (supporting); Writing‐review & editing (equal). John Whitehall: Conceptualization (supporting); Writing‐review & editing (supporting).
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Richard Wuhrer, WSU, Advanced Materials Characterisation Facility (AMCF), for training on scanning electron microscopy and the use of their facilities. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
APPENDIX 1.
FIGURE A1.
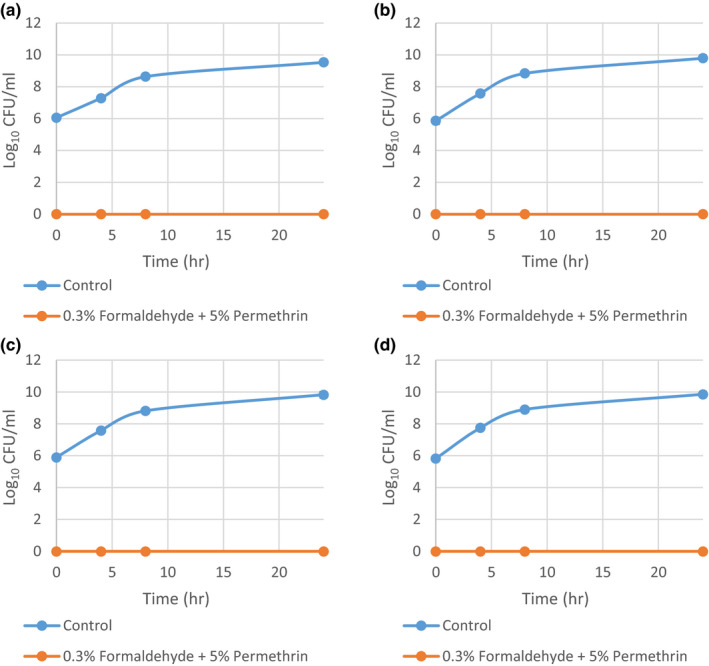
Time‐kill curves of methicillin‐sensitive Staphylococcus aureus grown in 0.3% formaldehyde and 5% permethrin combined from three independent runs with standard deviation of the mean error bars. (a) MSSA 2; (b) MSSA 4; (c) MSSA 6; and (d) MSSA 8
FIGURE A2.
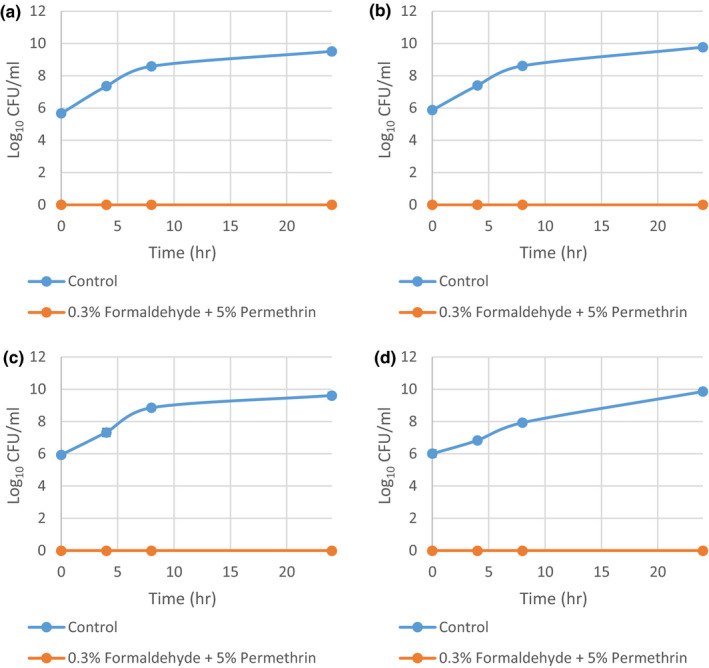
Time‐kill curves of methicillin‐resistant Staphylococcus aureus grown in 0.3% formaldehyde and 5% permethrin combined from three independent runs with standard deviation of the mean error bars. (a) MRSA 2; (b) MRSA 8; (c) MRSA 9; and (d) MRSA 13
Nikolic P, Mudgil P, Whitehall J. The in vitro antibacterial effect of permethrin and formaldehyde on Staphylococcus aureus . MicrobiologyOpen. 2020;9:e1054 10.1002/mbo3.1054
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- Alsterholm, M. , Flytström, I. , Bergbrant, I. , & Faergemann, J. (2010). Fusidic acid‐resistant Staphylococcus aureus in impetigo contagiosa and secondarily infected atopic dermatitis. Acta Dermato‐Venereologica, 90(1), 52–57. 10.2340/00015555-0771 [DOI] [PubMed] [Google Scholar]
- Andriantsoanirina, V. , Izri, A. , Botterel, F. , Foulet, F. , Chosidow, O. , & Durand, R. (2014). Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clinical Microbiology and Infection, 20(2), 139–141. 10.1111/1469-0691.12334 [DOI] [PubMed] [Google Scholar]
- Bartnik, F. G. , Gloxhuber, C. , & Zimmermann, V. (1985). Percutaneous absorption of formaldehyde in rats. Toxicology Letters, 25(2), 167–172. 10.1016/0378-4274(85)90078-5 [DOI] [PubMed] [Google Scholar]
- Bayer, A. S. , Schneider, T. , & Sahl, H. (2013). Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Annals of the New York Academy of Sciences, 1277(1), 139–158. 10.1111/j.1749-6632.2012.06819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen, A. C. , Mahé, A. , Hay, R. J. , Andrews, R. M. , Steer, A. C. , Tong, S. Y. C. , & Carapetis, J. R. (2015). The global epidemiology of impetigo: A systematic review of the population prevalence of impetigo and pyoderma. PLoS ONE, 10(8), e0136789 10.1371/journal.pone.0136789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrykh, L. V. , Govorukhina, N. I. , van Ophem, P. W. , Hektor, H. J. , Dijkhuizen, L. , & Duine, J. A. (1993). Formaldehyde dismutase activities in Gram‐positive bacteria oxidizing methanol. Microbiology, 139(9), 1979–1985. 10.1099/00221287-139-9-1979 [DOI] [Google Scholar]
- Currie, B. J. , & McCarthy, J. S. (2010). Permethrin and ivermectin for scabies. The New England Journal of Medicine, 362(8), 717–725. 10.1056/NEJMct0910329 [DOI] [PubMed] [Google Scholar]
- Dada, N. , Lol, J. C. , Benedict, A. C. , López, F. , Sheth, M. , Dzuris, N. , … Lenhart, A. (2019). Pyrethroid exposure alters internal and cuticle surface bacterial communities in Anopheles albimanus . The ISME Journal, 13(1), 2447–2464. 10.1038/s41396-019-0445-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot, A. C. , Flyvholm, M. , Lensen, G. , Menné, T. , & Coenraads, P. (2009). Formaldehyde‐releasers: Relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde‐releasers. Contact Dermatitis, 61(2), 63–85. 10.1111/j.1600-0536.2009.01582.x [DOI] [PubMed] [Google Scholar]
- Field, L. M. , Davies, T. E. , O'Reilly, A. O. , Williamson, M. S. , & Wallace, B. A. (2017). Voltage‐gated sodium channels as targets for pyrethroid insecticides. European Biophysics Journal, 46(7), 675–679. 10.1007/s00249-016-1195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini, P. , Treadwell, P. , Woodberry, K. , Nerette, E. Jr , & Powery, H. II (2017). Impetigo in the pediatric population. Journal of Dermatology and Clinical Research, 5(1), 1092. [Google Scholar]
- Hauksson, I. , Pontén, A. , Isaksson, M. , Hamada, H. , Engfeldt, M. , & Bruze, M. (2016). Formaldehyde in cosmetics in patch tested dermatitis patients with and without contact allergy to formaldehyde. Contact Dermatitis, 74(3), 145–151. 10.1111/cod.12493 [DOI] [PubMed] [Google Scholar]
- Iversen, O. H. (1986). Formaldehyde and skin carcinogenesis. Environment International, 12(5), 541–544. 10.1016/0160-4120(86)90148-0 [DOI] [Google Scholar]
- Jin, Y. , Wu, S. , Zeng, Z. , & Fu, Z. (2017). Effects of environmental pollutants on gut microbiota. Environmental Pollution, 222(1), 1–9. 10.1016/j.envpol.2016.11.045 [DOI] [PubMed] [Google Scholar]
- Khodaverdian, V. , Pesho, M. , Truitt, B. , Bollinger, L. , Patel, P. , Nithianantham, S. , … Shoham, M. (2013). Discovery of antivirulence agents against methicillin‐resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 57(8), 3645–3652. 10.1128/AAC.00269-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodén, M. (1986). The in vitro permeability of human skin to benzene, ethylene glycol, formaldehyde, and n‐hexane. Acta Pharmacologica et Toxicologica, 58(5), 382–389. 10.1111/j.1600-0773.1986.tb00126.x [DOI] [PubMed] [Google Scholar]
- May, P. , Bowen, A. , Tong, S. , Steer, A. , Prince, S. , Andrews, R. , … Carapetis, J. (2016). Protocol for the systematic review of the prevention, treatment and public health management of impetigo, scabies and fungal skin infections in resource‐limited settings. Systematic Reviews, 5, 162 10.1186/s13643-016-0335-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola, P. G. , Jozala, A. F. , Novaes, L. C. , Moriel, P. , & Penna, T. C. V. (2009). Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Brazilian Journal of Pharmaceutical Sciences, 45(2), 241–248. 10.1590/S1984-82502009000200008 [DOI] [Google Scholar]
- McGuinness, W. A. , Malachowa, N. , & DeLeo, F. R. (2017). Vancomycin resistance in Staphylococcus aureus . The Yale Journal of Biology and Medicine, 90(2), 269–281. [PMC free article] [PubMed] [Google Scholar]
- Miles, A. A. , Misra, S. , & Irwin, J. (1938). The estimation of the bactericidal power of the blood. Epidemiology & Infection, 38(6), 732–749. 10.1017/S002217240001158X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, G. D. , Larsen, S. T. , & Wolkoff, P. (2017). Re‐evaluation of the WHO (2010) formaldehyde indoor air quality guideline for cancer risk assessment. Archives of Toxicology, 91(1), 35–61. 10.1007/s00204-016-1733-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic, P. , Mudgil, P. , & Whitehall, J. (2019). Formaldehyde as an alternative to antibiotics for treatment of refractory impetigo and other infectious skin diseases. Expert Review of Anti‐infective Therapy, 17(9), 681–687. 10.1080/14787210.2019.1654376 [DOI] [PubMed] [Google Scholar]
- Pereira, L. B. (2014). Impetigo ‐ Review. Anais Brasileiros de Dermatologia, 89(2), 293–299. 10.1590/abd1806-4841.20142283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, J. , Waglechner, N. , & Wright, G. (2016). The prehistory of antibiotic resistance. Cold Spring Harbour Perspectives in Medicine, 6(6), a025197 10.1101/cshperspect.a025197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovelikunnel, T. , Gethin, G. , & Humphreys, H. (2015). Mupirocin resistance: Clinical implications and potential alternatives for the eradication of MRSA. Journal of Antimicrobial Chemotherapy, 70(10), 2681–2692. 10.1093/jac/dkv169 [DOI] [PubMed] [Google Scholar]
- Rojo, P. , Barrios, M. , Palacios, A. , Gomez, C. , & Chaves, F. (2010). Community‐associated Staphylococcus aureus infections in children. Expert Review of Anti‐infective Therapy, 8(5), 541–554. 10.1586/eri.10.34 [DOI] [PubMed] [Google Scholar]
- Romaniuk, J. A. H. , & Cegelski, L. (2015). Bacterial cell wall composition and the influence of antibiotics by cell‐wall and whole‐cell NMR. Philosophical Transactions of the Royal Society B Biological Sciences, 370(1679), 20150024 10.1098/rstb.2015.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, A. , Tanaka, H. , Usuda, H. , Shibata, T. , Higashi, S. , Yamashita, H. , … Nagai, H. (2011). Characterization of skin inflammation induced by repeated exposure of toluene, xylene, and formaldehyde in mice. Environmental Toxicology, 26(3), 224–232. 10.1002/tox.20547 [DOI] [PubMed] [Google Scholar]
- Turnidge, J. , Coombs, G. , Daley, D. , & Nimmo, G. (2016). Australian group on antimicrobial resistance (AGAR) participants, 2000–14. MRSA: A tale of three types 15 years of survey data from AGAR. Sydney, NSW: Australian Commission on Safety and Quality in Health Care. [Google Scholar]
- Walters, M. S. , Eggers, P. , Albrecht, V. , Travis, T. , Lonsway, D. , Hovan, G. , … Kallen, A. (2015). Vancomycin‐resistant Staphylococcus aureus ‐ Delaware, 2015. MMWR. Morbidity and Mortality Weekly Report, 64(37), 1056 10.15585/mmwr.mm6437a6 [DOI] [PubMed] [Google Scholar]
- Whitehall, J. , Kuzulugil, D. , Sheldrick, K. , & Wood, A. (2013). Burden of paediatric pyoderma and scabies in North West Queensland. Journal of Paediatrics and Child Health, 49(2), 141–143. 10.1111/jpc.12095 [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Zhao, Y. , Paramasivan, S. , Richter, K. , Morales, S. , Wormald, P. J. , & Vreugde, S. (2018). Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients. International Forum of Allergy & Rhinology, 8(3), 406–414. 10.1002/alr.22046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


