Abstract
Objective
Investigate associations of early-life residence and school segregation with cognitive change in the Minority Aging Research Study.
Methods
Four hundred ninety-eight blacks (age ~ 73.5; 75% = women) without dementia at baseline self-reported State of birth, residence at age 12, and school segregation status. Census Bureau definitions of South and Northeast/Midwest were used to categorize early-life residence. We evaluated global cognition and five cognitive domains at baseline and annually for ~7.5 years. Linear mixed effects models examined the associations of region of birth and residence at age 12 with baseline level and longitudinal change in cognition. Additional models examined school segregation experience.
Results
~65% of Southern-born participants still lived in the South at age 12. Southern birth was associated with lower baseline global cognition and all cognitive domains (p-values ≤ .02) compared to Northern birth, but not cognitive change. A similar profile was seen for Southern residence at age 12. Segregation experience significantly modified associations of residence at age 12 on levels of cognition. Participants residing in the South attending a legally desegregated school demonstrated lower baseline levels of cognition (global, semantic, and working memory) than their Northeast/Midwest counterparts attending a legally desegregated or segregated school as well as their Southern counterparts attending a legally segregated school. This profile for participants attending a desegregated school in the South held for processing speed and visuospatial ability in comparisons to Northeast/Midwest counterparts, particularly those attending a legally desegregated school.
Conclusion
Baseline cognition was poorer in individuals born and residing in the South, particularly those attending desegregated schools at age 12.
Keywords: Cognition, Epidemiology, Life events and context, Longitudinal change, Segregation
Older non-Latino blacks perform more poorly on cognitive testing than older non-Latino whites, independent of dementia (Barnes & Bennett, 2014). Factors including increased prevalence of cardiovascular disease risk factors (CVD-RFs) and lower socioeconomic status (SES) (Schwartz et al., 2004) among older blacks compared to older whites have been reported to contribute to disparities in cognition. Historic events of the 20th century that directly affected the black experience in the United States may also, and uniquely, influence cognition and brain aging for older blacks. Most prior research is based solely on birth region. Therefore, investigating how factors associated with the unique experiences of older blacks growing up in the United States relate to level and change in cognitive functioning may provide important information about additional contributors to brain aging in this population.
During the 20th century, several historic events affected early-life residential and educational experiences of many blacks growing up in the United States. Beginning in the early 1900s and peaking in the 1940s, over 6 million blacks migrated from the Southern to the Northern United States settling primarily in large industrialized cities such as New York and Chicago. This “Great Migration” was in response to economic opportunities in the North as well as segregation and violence in the Jim Crow South. Also during this time (1900–1970s), the U.S. Supreme Court declared an end to segregation in schools with their 1954 ruling in Brown v. Board of Education (Brown v. Bd. of Educ. of. Topeka, 347 US 483, 1954). Although this ruling officially ended the former constitutionally sanctioned laws that required separate but equal educational experiences for blacks and whites, southern schools remained racially segregated for at least another decade, and race-based distinctions in the educational experiences of individuals attending schools across the South persisted (Frisvold & Golberstein, 2011). For example, older blacks born and educated in Alabama during the time of Brown v. Board of Education experienced higher student–teacher ratios, lower school funding per student, and shorter school years than whites (Crowe et al., 2013), not to mention the psychological stressors associated with desegregation of Southern schools and the community as a whole (Allen, Brown, Jackson, & Lewis, 1977; Travis & Anthony, 1978; Walker & Archung, 2003). It has been suggested that the educational experience of segregation in schools, greater in the South of the United States compared to the North, may contribute to late-life cognitive outcomes, particularly for older black adults (Allaire & Whitfield, 2004; Liu, Glymour, et al., 2015).
In addition to place of birth, a growing number of studies have investigated early-life contextual factors that may help to explain differences in cognition between blacks and whites. Educational quality has emerged as a consistent and considerable contributor (Crowe et al., 2013; Fyffe et al., 2011; Manly, Jacobs, Touradji, Small, & Stern, 2002) even after controlling for CVD-RFs (Carvalho et al., 2015) and/or SES (Dotson, Kitner-Triolo, Evans, & Zonderman, 2009). Likewise, early-life residence (Liu, Glymour, et al., 2015; Sellers, Burns, & Guyrke, 2002; Sisco et al., 2015) and early-life adversity (Barnes, Wilson, et al., 2012) have been documented to affect late-life cognitive differences between older blacks and older whites (Everson-Rose et al., 2003; Fritsch et al., 2007; Liu, Glymour, et al., 2015; Wilson, Scherr, Bienias, et al., 2005; Wilson, Scherr, Hoganson, et al., 2005). The majority of these studies, however, have compared older blacks to older whites. While comparison studies are important for documenting racial differences, given the profoundly different social environment that blacks experienced at that time, within race investigations are needed to further knowledge about how specific contextual factors may uniquely influence late-life cognition among blacks.
In the current study, we investigated the role of early-life residence and educational experiences, that is, school segregation, on level and change in cognitive function over time in older black adults without known dementia participating in the Minority Aging Research Study (MARS). Work to date investigating early-life residential and educational experiences related to late-life cognition within older black adults has been limited and primarily cross-sectional (Allaire & Whitfield, 2004). While most prior research is based solely on birth region, we are aware of only one longitudinal study (Aiken-Morgan, Gamaldo, Sims, Allaire, & Whitfield, 2015) which reported that persons who attended segregated schools performed more poorly on baseline measures of language and perceptual speed, but the experience of segregation did not influence the degree of cognitive change from baseline when compared to an evaluation conducted approximately 2.75 years later. MARS provides an average of 7.5 years (range 2–10 years) of annual data collection allowing for the longest follow-up to date on this topic. Additionally, we examined the combined effect of early-life residence and the educational experience of segregation on level and change in cognitive functioning. We hypothesized that older black adults born in the South would show lower levels of late-life cognitive functioning and greater cognitive decline, particularly for semantic memory (akin to the language domain used in previous studies; Aiken-Morgan et al., 2015) and perceptual speed, compared to older black adults born in the North after adjusting for relevant covariates. We further hypothesized that geographical differences in cognition would remain when considering residence at age 12. Lastly, we hypothesized that the experience of school segregation would alter cognition and cognitive decline in affected individuals regardless of residence at age 12.
Method
Participants
Participants were enrolled in the Minority Aging Research Study (MARS) (Barnes, Shah, Aggarwal, Bennett, & Schneider, 2012). First begun in 2004, MARS is an ongoing longitudinal community-based, clinical-pathological cohort study of aging in older blacks. As described elsewhere (Barnes, Shah, et al., 2012), participants are recruited following a presentation on healthy aging from a variety of community-based settings including churches, subsidized senior housing facilities, retirement communities, African American clubs, organizations, fraternities and sororities, as well as social service centers that cater to seniors in the metropolitan Chicago area and outlying suburbs. Participants enroll without known dementia and agree to detailed annual clinical evaluations and cognitive testing. The Institutional Review Board of Rush University Medical Center approved this study and participants gave written informed consent for all aspects of the study in accordance with the Declaration of Helsinki.
At the time of these analyses, 745 participants had enrolled in MARS and 737 had completed baseline evaluation including a cognitive evaluation. Of these 737 participants, 670 had two or more follow-up evaluations whereas 67 had only a baseline evaluation at the time of these analyses. Of this group, data on early-life residence were available for 513 participants with baseline cognitive testing. Based on a uniform structured clinical evaluation (Barnes, Shah, et al., 2012), we excluded 15 participants who had a diagnosis of dementia (Bennett et al., 2006) following the NINDS/ADRDA criteria (McKhann et al., 1984), or mild cognitive impairment, that is, cognitive impairment that did not meet criteria for dementia (Bennett et al., 2002), at the time of their baseline evaluation. This left 498 individuals in our analyses.
Birth and Early-Life Residence
At the time of the baseline evaluation, MARS participants were asked their full street address including city, state, county, and country at birth and where they resided at age 12. We focused on age 12 because it is an age when nearly all children are in school (Wilson, Barnes, et al., 2005). For this analysis, we used state level information only. We designated Southern born or the “South” to correspond with the U.S. Census Bureau definition of the South that includes Alabama, Arkansas, Delaware, the District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia. Following the definitions of the U.S. Census Bureau, the Northeast region includes Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont and the Midwest includes Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin. Given the location of this study, we designated Northern born or the “North” to include both Northeast and Midwest regions and hereafter referred to as Northeast/Midwest. These same definitions were used to designate place of residence at age 12. We excluded one individual who was born and resided outside of our regions of interest. Of the remaining 497 participants in this study, 61 did not have information on residence at age 12 and one had moved to a state outside our geographic areas of interest at age 12.
Early-Life Educational Experience
Information was collected on the type of academic setting participants experienced during their primary education, that is, public, public magnet school, private Catholic, other private, and other, and their level of enrollment, that is, full-time versus part-time. Participants were asked if they attended a “legally segregated school” during their primary education with responses coded as yes, no, and do not know (only one individual did not know). If participants responded in the affirmative, information was collected on the number of years they attended a legally segregated school.
Neuropsychological Assessment
All participants underwent an annual cognitive evaluation administered in an identical fashion at annual evaluations (Barnes, Shah, et al., 2012). The Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) was used only to describe the cohort whereas the remaining 19 tests were selected to assess five specific cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. Episodic memory consisted of scores from immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, and Word List Recognition. The semantic memory domain consisted of performance on a 15-item version of the Boston Naming Test, Verbal Fluency, and a 15-item reading test. As previously stated, for the purposes of this study the semantic memory domain is seen as akin to the language domain used in previous research (Aiken-Morgan et al., 2015). The working memory domain score was comprised of scores for Digit Span Forward, Digit Span Backward, and Digit Ordering whereas perceptual speed consisted of performance on Symbol Digit Modalities Test, Number Comparison, and two indices from a modified version of the Stroop Neuropsychological Screening Test. Visuospatial ability was represented by a 15-item version of Judgment of Line Orientation and a 16-item version of Standard Progressive Matrices.
Raw scores on each of the individual tests were converted to standard z scores using the baseline mean (SD) of the entire cohort, and the z scores of all tests for each domain were then averaged for the five cognitive domains outlined earlier. A global cognitive function score was also derived averaging a person’s standard scores across all 19 test scores outlined earlier. Psychometric information on these summary scores has been deemed adequate and is contained in previous publications (e.g., Barnes, Wilson, et al., 2012; Wilson et al., 2002).
Covariates
In addition to age, sex, and years of education, we adjusted for body mass index (BMI), and CVD-RF burden at study baseline. BMI was calculated based on height and weight determined by direct measurement to the nearest inch and pound, respectively. We created a composite measure of CVD-RF burden (Boyle, Buchman, Wilson, Leurgans, & Bennett, 2009) based on any self-reported current or past history of hypertension, diabetes, and/or smoking (max score = 3; higher score = higher burden).
We chose to adjust for these additional covariates for several reasons. First, the current study was interested in the predictive utility of region of residence rather than the causal effect of region of residence. Additionally, given that the majority of the states self-reported by participants who were included in the Southern region (approximately 90%)—and one in the Northeast/Midwest region, that is, Indiana—may be localized within the Stroke Belt of the United States (Feinleib et al., 1993; Howard, Labarthe, Hu, Yoon, & Howard, 2007; National Heart, 2015; Sergeev, 2011). Furthermore, there is consensus that the impact of being overweight or obese on stroke risk is most significant for blacks in America (Howard et al., 2006; Miller et al., 2008). Not only is there an interplay of CVD-RF burden on stroke risk, but there is also a negative impact of exposure to segregation and discrimination on later cardiac health (Chae, Nuru-Jeter, & Adler, 2012; Hoggard, Hill, Gray, & Sellers, 2015).
Statistical Analysis
Descriptive summaries of all variables of interest including covariates were conducted for the overall study sample, then recalculated after stratification by South versus Northeast/Midwest region of birth and residence at age 12 (separately). We tested for differences in key participant characteristics using independent sample t-tests or chi-square testing (as appropriate) for region of birth and residence at age 12 (separately). We also tested whether region of birth and residence at age 12 could be included in the same model. However, tetrachoric correlational analyses for binary variables revealed a high degree of collinearity between these variables (rtet = 0.97) that precluded including both terms in the same model.
Linear mixed effects regression models were conducted to examine associations of early-life residence (region of birth or residence at age 12, separately) with baseline level and longitudinal change in global cognition and cognitive domains (separately). Early-life residence was a dichotomous predictor (0 = Northeast/Midwest, 1 = South), and each cognitive measure was a continuous longitudinal outcome. All models included the terms for time in years since baseline, age, sex, education, BMI, CVD-RF burden, and interaction terms of each of these demographic variables by time (Model 1). In analyses for residence at age 12 only, we augmented Model 1 by adding a binary variable representing whether a participant attended a segregated school (0 = no, 1 = yes) and an interaction term of this variable by time (Model 2). Lastly, because a significantly greater proportion of participants attended a legally segregated school in the South compared to the North/Midwest, we investigated whether the association of residence at age 12 with cognition differed by school segregation status. Thus, we added the interaction term for residence at age 12*school segregation as well as the 3-way interaction term with residence at age 12, segregation status, and time to Model 2 outlined earlier. Analyses were conducted using SAS/STAT software, Version 9.4 of the SAS System for Linux (SAS Institute, Cary, NC) and significance was set at p < .05 with the exception that we corrected for multiple comparisons when considering the five cognitive domains (0.05/5, p ≤ .01).
Results
Participants (N = 497) were on average 73 years of age, primarily female (75%), and reported an average 15 years of education. Approximately 90% of our study sample went to public schools with 40% reporting having attended a segregated school for an average of 9 years. Of the 213 Southern born participants, 178 had available data for residence at age 12, of whom 136 (76.4%) were still living in the South at age 12. Only 1 participant reported residence at age 12 outside of our regions of interest. Based on this data, our sample showed relative residential stability for the time points in question. Additional information may be found in Table 1.
Table 1.
Participant Characteristics
| N = 497 | |
|---|---|
| Key demographics | |
| Age (years) | 73.5 (6.2) |
| Sex (male:female ratio) | 124:374 |
| Education (years) | 14.9 (3.5) |
| Mini-Mental State Examination | 27.8 (2.3) |
| Body mass index | 30.0 (6.4) |
| CVD-RF Burden | 1.5 (0.86) |
| Early-life educational experience | |
| Attended Public School (%) | 91.5 |
| Full-time (%) | 94.8 |
| Legally segregated (Y:N:DK) | 189:308:1 |
| Segregation exposure (years) | 8.9 (3.8) |
| Cognitive composite scores | |
| Global cognition | 0.03 (0.54) |
| Episodic memory | 0.03 (0.65) |
| Semantic memory | 0.04 (0.72) |
| Working memory | 0.03 (0.75) |
| Perceptual speed | 0.02 (0.77) |
| Visuospatial ability | 0.03 (0.78) |
Notes. CVD-RF = cardiovascular disease risk factor; Y = yes; N = no; DK = do not know. All values are mean (SD), unless otherwise noted.
Regional Differences in Participant Characteristics
Compared to Southern-born participants, Northeast/Midwest born participants were younger, had higher levels of education, and performed better on the MMSE (all p-values ≤ .01; Table 2). This profile remained the same when participants were compared based on their state of residence at age 12 (Table 2) with the exception of age. Additionally, participants reporting a Northeast/Midwest residence at age 12 (n = 299) were less likely to have attended public school including public magnet schools (10.3% reported attending a private Catholic school) or a legally segregated school when compared to the 136 participants reporting a Southern residence at age 12 (both p-values ≤ .01; Table 2). Specific information about these as well as other variables are outlined by South versus Northeast/Midwest region of birth and residence at age 12 (separately) in Table 2.
Table 2.
Participant Characteristics Stratified by Region of Birth and Residence at Age 12 (Separately)
| Region of birth | Residence at age 12 | |||
|---|---|---|---|---|
| South (n = 213) | North/Midwest (n = 284) | South (n = 136) | North/Midwest (n = 299) | |
| Key demographics | ||||
| Age (years) | 74.4 (6.6) | 72.8 (5.7)** | 73.4 (6.1) | 73.1 (5.8) |
| Sex (male:female ratio) | 58:155 | 66:218 | 39:97 | 71:228 |
| Education (years) | 14.0 (3.2) | 15.6 (3.6)*** | 14.1 (3.1) | 15.4 (3.6)** |
| Mini-Mental State Exam | 27.2 (2.8) | 28.2 (1.8)*** | 27.3 (3.2) | 28.0 (1.8)** |
| Body mass index | 30.0 (6.4) | 30.1 (6.4) | 29.5 (5.7) | 30.0 (6.4) |
| CVD-RF burden | 1.5 (0.8) | 1.4 (0.8) | 1.5 (0.8) | 1.4 (0.8) |
| Educational experience | ||||
| Attended public school (%) | n/a | n/a | 96.3 | 88.6** |
| Full-time (%) | n/a | n/a | 92.0 | 96.3 |
| Legally segregated (%) | n/a | n/a | 73.5 | 23.5*** |
| Segregation exposure (years) | n/a | n/a | 9.7 (3.3) | 7.7 (4.2)** |
| Cognitive composite scores | ||||
| Global cognition | −0.18 (0.52) | 0.20 (0.50)*** | −0.13 (0.53) | 0.16 (0.50)*** |
| Episodic memory | −0.11 (0.66) | 0.15 (0.62)*** | −0.03 (0.65) | 0.11 (0.65)* |
| Semantic memory | −0.24 (0.76) | 0.24 (0.61)*** | −0.18 (0.78) | 0.20 (0.63)*** |
| Working memory | −0.16 (0.70) | 0.18 (0.76)*** | −0.12 (0.68) | 0.17 (0.73)*** |
| Perceptual speed | −0.30 (0.71) | 0.25 (0.73)*** | −0.26 (0.73) | 0.20 (0.73)*** |
| Visuospatial ability | −0.17 (0.76) | 0.19 (0.77)*** | −0.15 (0.73) | 0.17 (0.77)*** |
Notes. CVD-RF = cardiovascular disease risk factor. Of the original 497 participants in our sample analyses, 61 did not have information on residence at age 12 and 1 had moved to a state outside our geographic areas of interest. All values are mean (SD) unless otherwise noted. Only key demographics and educational experience data were compared by region of birth and residence at age 12 (separately) with significance noted as *p < .05, **p < .01, and ***p ≤ .0001.
Early-Life Residence and Cognition
Region of birth
In linear mixed effects models including terms for time, age, sex, education, BMI, CVD-RF burden, and interaction terms of each of these demographic variables by time, Southern birth was a significant and negative predictor of level of global cognitive functioning (estimate = −0.22, SE = 0.04, p < .0001). Southern region of birth was also associated with lower levels of performance in episodic memory, semantic memory, working memory, perceptual speed and visuospatial ability (Table 3). Southern birth was not, however, associated with change in global cognitive functioning or any individual cognitive domain over time (Table 3).
Table 3.
Associations of Region of Birth With Global Cognition and the Five Cognitive Domains
| Global cognition | Episodic memory | Semantic memory | Working memory | Perceptual speed | Visuospatial ability | |
|---|---|---|---|---|---|---|
| Age | −0.02 | −0.03 | −0.02 | −0.01 | −0.04 | −0.02 |
| (0.003, p < .0001) | (0.004, p < .0001) | (0.004, p < .0001) | (0.005, p = .04) | (0.004, p < .0001) | (0.005, p < .0001) | |
| Sex | −0.07 | −0.17 | −0.03 | −0.03 | −0.15 | 0.28 |
| (0.04, p = .10) | (0.06, p = .004) | (0.06, p = .63) | (0.07, p = .62) | (0.06, p = .01) | (0.07, p = .002) | |
| Education | 0.05 | 0.03 | 0.06 | 0.05 | 0.06 | 0.05 |
| (0.006, p < .0001) | (0.007, p < .0001) | (0.008, p < .0001) | (0.009, p < .0001) | (0.007, p < .0001) | (0.008, p < .0001) | |
| BMI | −0.003 | 0.001 | −0.007 | −0.005 | −0.0001 | −0.006 |
| (0.003, p = .45) | (0.004, p = .80) | (0.004, p = .12) | (0.005, p = .31) | (0.004, p = .84) | (0.004, p = .23) | |
| CVD-RF burden | 0.01 | 0.03 | −0.03 | 0.04 | 0.004 | −0.04 |
| (0.02, p = .65) | (0.03, p = .34) | (0.03, p = .36) | (0.03, p = .26) | (0.03, p = .90) | (0.03, p = .25) | |
| South born | −0.22 | −0.13 | −0.33 | −0.20 | −0.35 | −0.25 |
| (0.04, p < .0001) | (0.05, p = .01) | (0.05, p < .0001) | (0.06, p = .001) | (0.05, p < .0001) | (0.06, p < .0001) | |
| Time*age | −0.004 | −0.004 | −0.005 | −0.003 | −0.003 | −0.002 |
| (0.001, p < .0001) | (0.001, p = .002) | (0.001, p < .0001) | (0.001, p = .0004) | (0.001, p < .0001) | (0.001, p = .001) | |
| Time*sex | −0.02 | −0.03 | −0.01 | −0.02 | −0.001 | −0.006 |
| (0.01, p = .07) | (0.01, p = .02) | (0.01, p = .23) | (0.01, p = .09) | (0.009, p = .92) | (0.009, p = .51) | |
| Time*education | −0.002 | −0.003 | −0.003 | −0.0001 | −0.001 | 0.001 |
| (0.001, p = .14) | (0.002, p = .12) | (0.002, p = .11) | (0.001, p = .80) | (0.001, p = .41) | (0.001, p = .61) | |
| Time*BMI | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 |
| (0.001, p = .16) | (0.001, p = .50) | (0.001, p = .22) | (0.001, p = .07) | (0.001, p = .31) | (0.001, p = .46) | |
| Time*CVD−RF Burden | 0.009 | 0.01 | 0.02 | 0.003 | −0.0001 | 0.008 |
| (0.006, p = .11) | (0.007, p = .14) | (0.007, p = .003) | (0.006, p = .60) | (0.004, p = .94) | (0.004, p = .09) | |
| Time*South born | −0.007 | −0.004 | 0.003 | −0.01 | −0.005 | −0.006 |
| (0.01, p = .50) | (0.01, p = .79) | (0.01, p = .82) | (0.01, p = .15) | (0.008, p = .57) | (0.008, p = .43) |
Note: BMI = Body mass index; CVD-RF = cardiovascular disease risk factor. Values are unstandardized coefficient (SE, p-value) from linear regression models with significance corrected for multiple comparisons at p < .01. Bolded terms and values denote variables of interest for this study, not significance levels.
Residence at age 12
In linear mixed effects models including terms for time, age, sex, education, BMI, CVD-RF burden, and interaction terms of each of these demographic variables with time (Model 1), Southern residence at age 12 was significantly associated with lower level of global cognitive functioning (estimate = −0.20, SE = 0.04, p < .0001), and all of the cognitive domains except episodic memory (Table 4). Like region of birth results, Southern residence at age 12 was not associated with change in global cognitive functioning over time, p-value = .74, or with any individual cognitive domain scores over time (Table 4).
Table 4.
Associations of Residence at age 12 With Global Cognition and the Five Cognitive Domains
| Global cognition | Episodic memory | Semantic memory | Working memory | Perceptual speed | Visuospatial ability | |
|---|---|---|---|---|---|---|
| Age | −0.02 | −0.03 | −0.01 | −0.006 | −0.04 | −0.02 |
| (0.003, p < .0001) | (0.005, p < .0001) | (0.005, p = .0006) | (0.005, p = .26) | (0.005, p < .0001) | (0.005, p < .0001) | |
| Sex | −0.12 | −0.21 | −0.10 | −0.07 | −0.19 | 0.24 |
| (0.05, p = .01) | (0.06, p = .001) | (0.07, p = .20) | (0.07, p = .30) | (0.06, p = .004) | (0.07, p = .0008) | |
| Education | 0.05 | 0.04 | 0.07 | 0.04 | 0.07 | 0.04 |
| (0.006, p < .0001) | (0.008, p < .0001) | (0.008, p < .0001) | (0.01, p < .0001) | (0.008, p < .0001) | (0.009, p < .0001) | |
| BMI | −0.003 | 0.001 | −0.007 | −0.005 | −0.001 | −0.007 |
| (0.003, p = .41) | (0.004, p = .92) | (0.005, p = .13) | (0.005, p = .31) | (0.005, p = .90) | (0.005, p = .18) | |
| CVD-RF burden | 0.03 | 0.04 | −0.02 | 0.06 | 0.03 | −0.02 |
| (0.02, p = .25) | (0.03, p = .20) | (0.03, p = .60) | (0.03, p = .08) | (0.03, p = .37) | (0.03, p = .50) | |
| South at age 12 | −0.19 | −0.07 | −0.27 | −0.21 | −0.32 | −0.25 |
| (0.04, p < .0001) | (0.06, p = .24) | (0.06, p < .0001) | (0.06, p = .001) | (0.06, p < .0001) | (0.06, p = .0001) | |
| Time*age | −0.004 | −0.004 | −0.005 | −0.003 | −0.003 | −0.003 |
| (0.001, p < .0001) | (0.001, p = .0006) | (0.001, p < .0001) | (0.001, p = .0007) | (0.001, p < .0001) | (0.001, p = .0005) | |
| Time*sex | −0.02 | −0.04 | −0.02 | −0.02 | −0.006 | −0.003 |
| (0.01, p = .04) | (0.01, p = .008) | (0.01, p = .15) | (0.01, p = .13) | (0.009, p = .50) | (0.01, p = .77) | |
| Time*education | −0.002 | −0.003 | −0.002 | 0.0001 | −0.0001 | 0.001 |
| (0.001, p = .25) | (0.002, p = .12) | (0.002, p = .33) | (0.002, p = .70) | (0.001, p = .76) | (0.001, p = .45) | |
| Time*BMI | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| (0.001, p = .17) | (0.001, p = .51) | (0.001, p = .19) | (0.001, p = .12) | (0.001, p = .34) | (0.009, p = .38) | |
| Time*CVD-RF Burden | 0.01 | 0.01 | 0.02 | 0.003 | 0.002 | 0.008 |
| (0.006, p = .08) | (0.008, p = .12) | (0.007, p = .001) | (0.006, p = .63) | (0.004, p = .67) | (0.005, p = .10) | |
| Time*South at age 12 | 0.004 | 0.004 | 0.02 | −0.006 | 0.006 | −0.003 |
| (0.01, p = .74) | (0.01, p = .77) | (0.01, p = .14) | (0.01, p = .62) | (0.009, p = .51) | (0.009, p = .73) |
Note: BMI = Body mass index; CVD-RF = cardiovascular disease risk factor. Values are unstandardized coefficient (standard error, p-value) from linear regression models with significance corrected for multiple comparisons at p < .01. Bolded terms and values denote variables of interest for this study, not significance levels.
School Segregation and Cognition
Next, we examined the main effect of school segregation status in models with residence at age 12 along with terms for time, age, sex, education, BMI, CVD-RF burden, residence at age 12, and interaction terms of each of these variables with time. Segregation status was not associated with either baseline levels or rates of change in any of the cognitive outcomes (p-values ≥ .08; data not shown).
Because a significantly greater proportion of participants attended a legally segregated school in the South compared to the North/Midwest, we next investigated whether the associations between where the participant lived at age 12 (i.e., residence at age 12) and cognition differed by school segregation status by conducting a subsequent model that included the interaction term of residence at age 12*school segregation status as well as the three-way interaction term with residence at age 12, segregation status, and time (Supplementary Table 1). This model also included terms for time, age, sex, education, BMI, CVD-RF burden, residence at age 12, school segregation status, and their interaction terms with time.
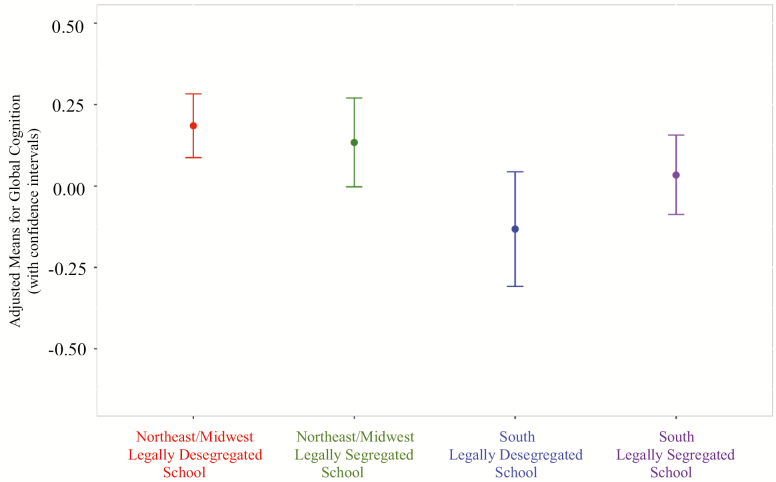
There was a significant interaction of residence at age 12*school segregation status on level (estimate = −0.24, SE = 0.10, p = .02) but not change (p = .82) in global cognitive performance. While individual follow-up analyses revealed that participants residing in the South at age 12 who reported attending a legally segregated school had lower baseline levels of global cognitive performance when compared to Northeast/Midwest at age 12 and reportedly attending a legally desegregated school (fully adjusted estimate = −0.15, SE = 0.05, p = .005), participants residing in the South at age 12 who reported attending a legally desegregated school had the lowest baseline levels of global cognitive performance compared to all other groups (Figure 1). More specifically, these individuals had lower baseline levels of global cognition compared to participants residing in the Northeast/Midwest at age 12 and reportedly attending a legally desegregated (fully adjusted model estimate = −0.35, SE = 0.08, p < .0001) or legally segregated (fully adjusted estimate = −0.31, SE = 0.09, p < .001) school, as well as when compared to their Southern counterparts attending a legally segregated school (fully adjusted model estimate = −0.20, SE = 0.08, p = .02).
Figure 1.
Level of global cognitive functioning for early-life residence at age 12 by educational experience of school segregation.
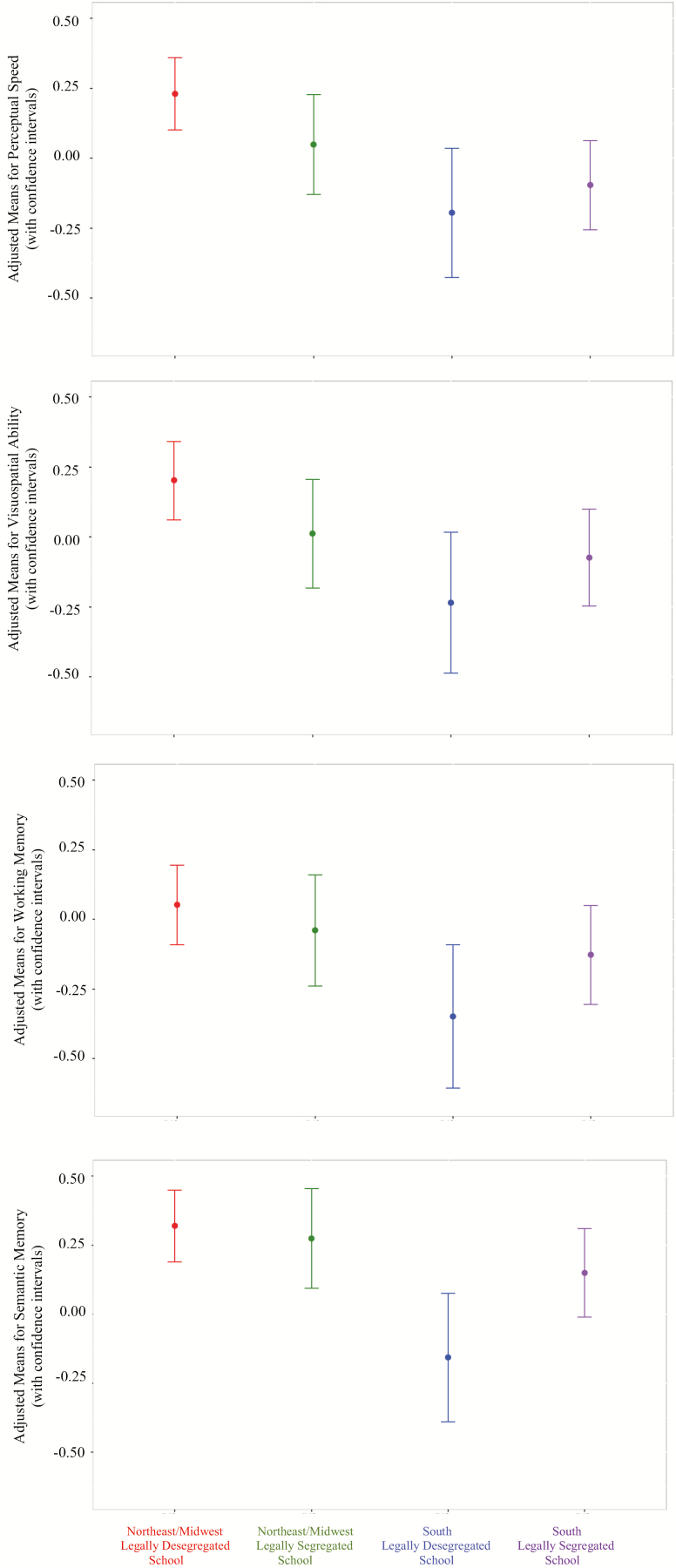
Likewise, analyses by cognitive domains revealed a significant residence at age 12*school segregation status interaction for level of semantic memory (fully adjusted Model estimate = −0.32, SE = 0.14, p = .03), working memory (fully adjusted Model estimate = −0.33, SE = 0.15, p = .03), perceptual speed (fully adjusted Model estimate = −0.30, SE = 0.14, p = .02), and visuospatial ability (fully adjusted Model estimate = −0.31, SE = 0.15, p = .04). Follow-up analyses in fully adjusted Models revealed that participants residing in the South at age 12 and reportedly attending a legally desegregated school had the lowest baseline levels of semantic memory and working memory compared to all other groups, i.e., when compared to their Northern counterparts also reportedly attending a legally desegregated (semantic memory: fully adjusted estimate = −0.50, SE = 0.11, p < .0001; fully adjusted working memory: estimate = −0.41, SE = 0.12, p = .0006) or a legally segregated (semantic memory: fully adjusted estimate = −0.50, SE = 0.12, p = .0001; working memory: fully adjusted estimate = −0.31, SE = 0.13, p = .02) school as well as other participants residing in the South at age 12 but attending a legally segregated school (semantic memory: estimate = −0.31, SE = 0.12, p = .009; working memory: estimate = −0.23, SE = 0.12, p = .06 although this last estimate did not reach significance) (Figure 2).
Figure 2.
Level of domains of cognitive functioning for early-life residence at age 12 by educational experience of school segregation.
As may be seen in Figure 2, follow-up analyses in fully adjusted Models for perceptual speed and visuospatial ability revealed that while participants residing in the South at age 12 who reported attending a legally segregated school had lower baseline levels on these two cognitive domains compared to participants residing in the Northeast/Midwest at age 12 and reportedly attending a legally desegregated school (perceptual speed: fully adjusted estimate = −0.33, SE = 0.07, p < .0001; visuospatial ability: fully adjusted estimate = −0.26, SE = 0.07, p = .0008), participants residing in the South at age 12 who reported attending a legally desegregated school had the lowest baseline levels of cognitive performance compared to participants residing in the Northeast/Midwest at age 12 and reportedly attending a legally desegregated (perceptual speed: estimate = −0.47, SE = 0.10, p < .0001; visuospatial ability: estimate = −0.40, SE = 0.11, p = .0005), or legally segregated school (perceptual speed: estimate = −0.31, SE = 0.12, p = .009; visuospatial ability: estimate = −0.24, SE = 0.13, p = .06 although this last estimate did not reach significance).
Discussion
In this cohort study of approximately 500 older black adults without dementia at baseline, we investigated the association of early-life residence (i.e., at birth and at age 12, separately) and educational experiences (i.e., school segregation), on levels of cognition and change in cognition over time. As hypothesized, being born and residing in the Southern United States (at least until age 12) was negatively associated with baseline levels of global cognition as well as semantic memory and perceptual speed after adjusting for age, sex, years of education, BMI and CVD-RF burden. Although not hypothesized, this finding extended to other domains of cognition to include lower baseline levels of working memory and visuospatial abilities after similar adjustments. Neither metric of early-life residence contributed to declines in cognition. While attending a segregated school at age 12 did not predict level or change in global cognition or any cognitive domain, there were significant interactive effects on cognition of segregated school status and depending upon where the participant resided at age 12. Thus, older blacks reporting residence in the South at age 12 and attendance at a legally desegregated school demonstrated the lowest baseline levels of global cognition, semantic and working memory, compared to all other groups. Furthermore, this profile for participants attending a desegregated school in the South held for processing speed and visuospatial ability in comparison to older blacks residing in the Northeast/Midwest, particularly those attending a legally desegregated school.
Results of this study contribute to the literature in several important ways. First, early-life residence, being born and/or raised in the South more specifically, has been linked to other organ damage (e.g., Liu, Manly, Capistrant, & Glymour, 2015) as well as adverse brain outcomes regardless of race including late-life cognitive alterations (Liu, Glymour, et al., 2015), dementia (Gilsanz, Mayeda, Glymour, Quesenberry, & Whitmer, 2017), stroke morbidity, and stroke mortality (Feinleib et al., 1993; Howard et al., 2007; National Heart, 2015). Older black adults, however, bear a disproportionately greater burden of disease compared to older white adults (Howard et al., 2007; Liu, Manly, et al., 2015). We extend this work as it relates to an early-life residence in the United States to include within race behavioral distinctions in global cognition and distinct cognitive domains. This is in keeping with a study of older black adults self-reporting childhood birth and residence until age 19 (Hall, Gao, Unverzagt, & Hendrie, 2000) that found an increased risk of Alzheimer’s disease on their outcome of interest only when coupled with birth and early-life residence in rural (versus urban) areas primarily of the United States South. While results by cognitive domain suggest an independent effect of Southern birth across all cognitive domains, residence at age 12 was not associated with episodic memory. This may suggest a less potent effect of this region over time on this particular cognitive domain. On the whole, however, our work and the work of others investigating within race distinctions suggests that there is something unique about the experiences of blacks born and growing up in the Southern region of the United States.
Race-based comparison studies have shown that early-life educational experiences for blacks including state governmental influences (Sisco et al., 2015) such as school funding (Crowe et al., 2013), as well as classroom characteristics (Crowe et al., 2013; Liu, Glymour, et al., 2015; Sisco et al., 2015) not only differ from whites, but may help to explain differences in early-life educational quality as it relates to racial disparities in late-life cognition. Thus, our second contribution to the literature on this topic is to extend these educational considerations to include within race distinctions in the experience of school segregation as an additional factor associated with cognition for older black adults. Previous work has reported on the negative effect of segregation on spatial abilities, language and perceptual speed in a sample of black adults from the Northeast (Aiken-Morgan et al., 2015), one of the first regions of the United States to enact desegregation policies post Brown v. Board of Education. Our profiles of cognition point toward similar results for worse visuospatial abilities and perceptual speed not only in older blacks reportedly residing in the South at age 12 attending segregated schools compared to Northeast/Midwest participants attending desegregated schools, but, surprisingly expanded the finding to older blacks reportedly residing in the South at age 12 attending legally desegregated schools compared to Northeast/Midwest participants regardless of school segregation status. Furthermore, our results also revealed that older blacks reportedly residing in the South at age 12 and attending a legally desegregated school had the lowest baseline levels of performance across other cognitive domains including semantic and working memory. While our study and others’ (Aiken-Morgan et al., 2015; Whitfield, Allaire, & Wiggins, 2004) report the negative impact of early-life residential and educational experiences on levels of cognition in later life but not cognitive decline, future studies should nonetheless combine early-life residence with early-life educational experiences of school segregation when considering associates with late-life cognition in older blacks.
We believe our results do not suggest that desegregation is bad for cognition, rather, given the time of desegregation for our participants residing in the South in and around 1954, context is key. Blacks age 65 and older born and residing in the South during the majority of their schooling may have had early-life and educational experiences including desegregation and the discrimination and chaos that accompanied it de facto if not de jure (Fairclough, 2006) that negatively affected their level of cognitive functioning in later life. While beyond the scope of this study, underlying mechanisms to explain these associations may be found in the literature. We (Barnes, Lewis, et al., 2012) and others (Zahodne, Manly, Smith, Seeman, & Lachman, 2017) have investigated the relationship between perceived discrimination and late-life cognitive functioning. Additionally, perceived discrimination has been shown to negatively affect vascular health (Lewis et al., 2009) and associated inflammatory processes independent of vascular health (Lewis, Aiello, Leurgans, Kelly, & Barnes, 2010). Thus, older blacks who attended legally desegregated schools in the South may have experienced a radical early-life transformation and with it, significant discrimination (Fairclough, 2006). Thus, these individuals, may have simultaneously experienced increases in inflammatory processes detrimental to brain health and associated cognitive functioning (Nilsson, Gustafson, & Hultberg, 2011). Given that racial discrimination has been shown to have a prolonged effect on cardiac health (Hoggard et al., 2015) and longevity (Barnes et al., 2008), the possibility that early-life educational experiences of desegregation in the South, may have a lasting impact on the underlying inflammatory mechanisms associated with brain health and cognitive functioning is possible.
Other possible mechanisms for our results that may also affect cognition for individuals reporting early-life residence in the South more generally include lower socioeconomic conditions in this region of the United States, and exposure to environmental toxins. Lower SES may result in undernutrition, an early-life influencer of late-life cognition (de Rooij, Wouters, Yonker, Painter, & Roseboom, 2010). Additionally, exposure to environmental toxins may have occurred through chemicals used in the primarily agrarian society of the South in the early 1900s (Hall et al., 2000), or poor water quality that may have contained levels of trace minerals known to negatively affect Alzheimer’s dementia (Sun, 2017). Additional work investigating these potential mechanisms is needed.
This study has several strengths and also some limitations that should be highlighted. Participants in our study showed relative stability in self-reported residency throughout early-life commensurate with previous reports in similarly aged minority population studies (Liu, Manly, et al., 2015) allowing us to have confidence that our study is addressing the role of at least a 12-year duration of early-life residence exposure on late-life cognition. The fact that all participants are now living in the Chicago area suggests that their migration may have occurred later in life and affected cognition at a later date. The comprehensive nature of our cognitive assessment allowed us to discuss domain-specific effects of early-life residence and educational experiences on cognition. Our inclusion of a brief reading test in the semantic memory composite does suggest that educational quality may be embedded in this composite score somewhat biasing those results; however, other domains of cognition devoid of this variable were also associated with early-life and educational experiences. While we adjusted for several variables thought to confound our effect of interest, additional variables including perceived discrimination, psychological stress, and childhood SES were not accounted for and may help to explain our results.
Lastly, while early-life residence and educational experiences for participants representing the Southern region were widespread across several southern states, not surprisingly, the majority of participants representing the Northern/Midwestern region were from the state of Illinois suggesting less regional representation for Northern/Midwestern participants than their Southern counterparts (Supplementary Table 2). Thus, future work may wish to explore whether the associations between early-life residence, educational experience, and cognition vary by states within the South, Northwest, or Midwest given that historical events, physical and social characteristics of context, and legislative policies may differ and have unique influences based on particular locations.
In conclusion, being born or residing in the South at least until age 12 are two early-life residence metrics associated with lower levels of baseline cognitive functioning, regardless of domain, in older black adults. Furthermore, residence at age 12 and the educational experience of segregation interacted, resulting in the worst outcomes for participants living in the South reportedly attending a legally desegregated school. Interestingly, these early-life markers were only associated with level of cognition and not change in cognition over time, suggesting that early life experiences have a negative affect on test-taking skills that affect where a person starts, but may not necessarily be a marker of disease leading to cognitive decline. Even if not a marker of disease, however, they nonetheless influence the point at which individuals begin their course of decline toward pathological aging, perhaps lowering the threshold for meeting criteria for dementia. Thus, providers working with older blacks in the United States need to quantify not only years of education and/or reading levels of their patients, but also probe early-life residence and educational experiences in order to fully understand the role of their patient’s historical context on the foundation of his or her cognitive health.
Funding
This work was supported by National Institute on Aging (grant number RF1 AG022018).
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
We thank all the participants in the Minority Aging Research Study and the staff of the Rush Alzheimer’s Disease Center.
References
- Aiken-Morgan A. T., Gamaldo A. A., Sims R. C., Allaire J. C., & Whitfield K. E (2015). Education desegregation and cognitive change in African American older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 70, 348–356. doi:10.1093/geronb/gbu153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire J. C., & Whitfield K. E (2004). Relationships among education, age, and cognitive functioning in older African Americans: The impact of desegregation. Aging, Neuropsychology, and Cognition, 11, 443–449. doi:10.2174/156720512801322627 [Google Scholar]
- Allen I. M., Brown J. L., Jackson J., & Lewis R (1977). Psychological stress of young black children as a result of school desegregation. Journal of the American Academy of Child Psychiatry, 16, 739–747. [DOI] [PubMed] [Google Scholar]
- Barnes L. L., & Bennett D. A (2014). Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Affairs (Project Hope), 33, 580–586. doi:10.1377/hlthaff.2013.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. L., de Leon C. F., Lewis T. T., Bienias J. L., Wilson R. S., & Evans D. A (2008). Perceived discrimination and mortality in a population-based study of older adults. American Journal of Public Health, 98, 1241–1247. doi:10.2105/AJPH.2007.114397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. L., Lewis T. T., Begeny C. T., Yu L., Bennett D. A., & Wilson R. S (2012). Perceived discrimination and cognition in older African Americans. Journal of the International Neuropsychological Society: JINS, 18, 856–865. doi:10.1017/S1355617712000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. L., Shah R. C., Aggarwal N. T., Bennett D. A., & Schneider J. A (2012). The minority aging research study: Ongoing efforts to obtain brain donation in African Americans without dementia. Current Alzheimer Research, 9, 734–745. doi:10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. L., Wilson R. S., Everson-Rose S. A., Hayward M. D., Evans D. A., & Mendes de Leon C. F (2012). Effects of early-life adversity on cognitive decline in older African Americans and whites. Neurology, 79, 2321–2327. doi:10.1212/WNL.0b013e318278b607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. A., Schneider J. A., Aggarwal N. T., Arvanitakis Z., Shah R. C., Kelly J. F.,…Wilson R. S (2006). Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology, 27, 169–176. doi:10.1159/000096129 [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Wilson R. S., Schneider J. A., Evans D. A., Beckett L. A., Aggarwal N. T.,…Bach J (2002). Natural history of mild cognitive impairment in older persons. Neurology, 59, 198–205. doi:https://doi.org/10.1212/WNL.59.2.198 [DOI] [PubMed] [Google Scholar]
- Boyle P. A., Buchman A. S., Wilson R. S., Leurgans S. E., & Bennett D. A (2009). Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Archives of Neurology, 66, 1339–1344. doi:10.1001/archneurol.2009.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho J. O., Tommet D., Crane P. K., Thomas M. L., Claxton A., Habeck C.,…Romero H. R (2015). Deconstructing racial differences: The effects of quality of education and cerebrovascular risk factors. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 70, 545–556. doi:10.1093/geronb/gbu086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae D. H., Nuru-Jeter A. M., & Adler N. E (2012). Implicit racial bias as a moderator of the association between racial discrimination and hypertension: A study of Midlife African American men. Psychosomatic Medicine, 74, 961–964. doi:10.1097/PSY.0b013e3182733665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe M., Clay O. J., Martin R. C., Howard V. J., Wadley V. G., Sawyer P., & Allman R. M (2013). Indicators of childhood quality of education in relation to cognitive function in older adulthood. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68, 198–204. doi:10.1093/gerona/gls122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson V. M., Kitner-Triolo M. H., Evans M. K., & Zonderman A. B (2009). Effects of race and socioeconomic status on the relative influence of education and literacy on cognitive functioning. Journal of the International Neuropsychological Society: JINS, 15, 580–589. doi:10.1017/S1355617709090821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose S. A., Mendes de Leon C. F., Bienias J. L., Wilson R. S., & Evans D. A (2003). Early life conditions and cognitive functioning in later life. American Journal of Epidemiology, 158, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Fairclough A. (2006). The costs of Brown: Black teachers and school integration. The Journal of American History, 91(1), 1–12. doi:10.2307/3659612 [Google Scholar]
- Feinleib M., Ingster L., Rosenberg H., Maurer J., Singh G., & Kochanek K (1993). Time trends, cohort effects, and geographic patterns in stroke mortality–United States. Annals of Epidemiology, 3, 458–465. doi:https://doi.org/10.1016/1047-2797(93)90096-M [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., & McHugh P. R (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:https://doi.org/10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Frisvold D., & Golberstein E (2011). School quality and the education-health relationship: Evidence from blacks in segregated schools. Journal of Health Economics, 30, 1232–1245. doi:10.1016/j.jhealeco.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch T., McClendon M. J., Smyth K. A., Lerner A. J., Friedland R. P., & Larsen J. D (2007). Cognitive functioning in healthy aging: The role of reserve and lifestyle factors early in life. The Gerontologist, 47, 307–322. doi: https://doi.org/10.1093/geront/47.3.307 [DOI] [PubMed] [Google Scholar]
- Fyffe D. C., Mukherjee S., Barnes L. L., Manly J. J., Bennett D. A., & Crane P. K (2011). Explaining differences in episodic memory performance among older African Americans and Whites: The roles of factors related to cognitive reserve and test bias. Journal of the International Neuropsychological Society: JINS, 17, 625–638. doi:10.1017/S1355617711000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz P., Mayeda E. R., Glymour M. M., Quesenberry C. P., & Whitmer R. A (2017). Association between birth in a high stroke mortality state, race, and risk of dementia. JAMA Neurology, 74, 1056–1062. doi:10.1001/jamaneurol.2017.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. S., Gao S., Unverzagt F. W., & Hendrie H. C (2000). Low education and childhood rural residence: Risk for Alzheimer’s disease in African Americans. Neurology, 54, 95–99. doi:http://dx.doi.org/10.1212/WNL.54.1.95 [DOI] [PubMed] [Google Scholar]
- Hoggard L. S., Hill L. K., Gray D. L., & Sellers R. M (2015). Capturing the cardiac effects of racial discrimination: Do the effects “keep going”? International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 97, 163–170. doi:10.1016/j.ijpsycho.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G., Labarthe D. R., Hu J., Yoon S., & Howard V. J (2007). Regional differences in African Americans’ high risk for stroke: The remarkable burden of stroke for Southern African Americans. Annals of Epidemiology, 17, 689–696. doi:10.1016/j.annepidem.2007.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G., Prineas R., Moy C., Cushman M., Kellum M., Temple E.,…Howard V (2006). Racial and geographic differences in awareness, treatment, and control of hypertension: The REasons for Geographic And Racial Differences in Stroke study. Stroke, 37, 1171–1178. doi:10.1161/01.STR.0000217222.09978.ce [DOI] [PubMed] [Google Scholar]
- Lewis T. T., Aiello A. E., Leurgans S., Kelly J., & Barnes L. L (2010). Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain, Behavior, and Immunity, 24, 438–443. doi:10.1016/j.bbi.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T. T., Barnes L. L., Bienias J. L., Lackland D. T., Evans D. A., & Mendes de Leon C. F (2009). Perceived discrimination and blood pressure in older African American and white adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 64, 1002–1008. doi:10.1093/gerona/glp062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y., Glymour M. M., Zahodne L. B., Weiss C., & Manly J. J (2015). Role of place in explaining racial heterogeneity in cognitive outcomes among older adults. Journal of the International Neuropsychological Society: JINS, 21, 677–687. doi:10.1017/S1355617715000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y., Manly J. J., Capistrant B. D., & Glymour M. M (2015). Historical differences in school term length and measured blood pressure: Contributions to persistent racial disparities among US-born adults. PLoS One, 10, e0129673. doi:10.1371/journal.pone.0129673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly J. J., Jacobs D. M., Touradji P., Small S. A., & Stern Y (2002). Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society: JINS, 8, 341–348. doi:https://doi.org/10.1017/S1355617702813157 [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., & Stadlan E. M (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939–944. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Schulz M. R., Bibeau D. L., Galka A. M., Spann L. I., Martin L. B.,…Chase C. M (2008). Factors associated with misperception of weight in the stroke belt. Journal of General Internal Medicine, 23, 323–328. doi:10.1007/s11606-007-0499-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute (2015). Stroke Belt Initiative: Project Accomplishments and Lessons Learned Author: Bethesda, MD: Retrieved from: https://www.nhlbi.nih.gov/files/docs/resources/heart/sb_spec.pdf [Google Scholar]
- Nilsson K., Gustafson L., & Hultberg B (2011). C-reactive protein level is decreased in patients with Alzheimer’s disease and related to cognitive function and survival time. Clinical Biochemistry, 44, 1205–1208. doi:10.1016/j.clinbiochem.2011.07.011 [DOI] [PubMed] [Google Scholar]
- de Rooij S. R., Wouters H., Yonker J. E., Painter R. C., & Roseboom T. J (2010). Prenatal undernutrition and cognitive function in late adulthood. Proceedings of the National Academy of Sciences of the United States of America, 107, 16881–16886. doi:10.1073/pnas.1009459107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. S., Glass T. A., Bolla K. I., Stewart W. F., Glass G., Rasmussen M.,…Bandeen-Roche K (2004). Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environmental Health Perspectives, 112, 314–320. doi:10.1289/ehp.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A. H., Burns W. J., & Guyrke J (2002). Differences in young children’s IQs on the Wechsler preschool and primary scale of intelligence-revised as a function of stratification variables. Applied Neuropsychology, 9, 65–73. doi:10.1207/S15324826AN0902_1 [DOI] [PubMed] [Google Scholar]
- Sergeev A. V. (2011). Racial and rural-urban disparities in stroke mortality outside the Stroke Belt. Ethnicity & Disease, 21, 307–313. [PubMed] [Google Scholar]
- Sisco S., Gross A. L., Shih R. A., Sachs B. C., Glymour M. M., Bangen K. J.,…Manly J. J (2015). The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 70, 557–567. doi:10.1093/geronb/gbt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. (2017). Associations of spatial disparities of Alzheimer’s disease mortality rates with soil selenium and sulfur concentrations and four common risk factors in the United States. Journal of Alzheimer’s Disease: JAD, 58, 897–907. doi:10.3233/JAD-170059 [DOI] [PubMed] [Google Scholar]
- Travis C. B., & Anthony S. E (1978). Some psychological consequences of integration. The Journal of Negro Education, 47(2), 151–158. [Google Scholar]
- Walker V. S., & Archung K. N (2003). The segregated schooling of Blacks in the southern United States and South Africa. Comparative Education Review, 47(1), 21–40. doi:10.1086/373961 [Google Scholar]
- Whitfield K. E., Allaire J. C., & Wiggins S. A (2004). Relationships among health factors and everyday problem solving in African Americans. Health psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 23, 641–644. doi:10.1037/0278-6133.23.6.641 [DOI] [PubMed] [Google Scholar]
- Wilson R. S., Barnes L. L., Krueger K. R., Hoganson G., Bienias J. L., & Bennett D. A (2005). Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society: JINS, 11, 400–407. doi:https://doi.org/10.1017/S1355617705050459 [PubMed] [Google Scholar]
- Wilson R. S., Barnes L. L., Mendes de Leon C. F., Aggarwal N. T., Schneider J. S., Bach J.,…Bennett D. A (2002). Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology, 59, 364–370. doi:https://doi.org/10.1212/WNL.59.3.364 [DOI] [PubMed] [Google Scholar]
- Wilson R. S., Scherr P. A., Bienias J. L., Mendes de Leon C. F., Everson-Rose S. A., Bennett D. A., & Evans D. A (2005). Socioeconomic characteristics of the community in childhood and cognition in old age. Experimental Aging Research, 31, 393–407. doi:10.1080/03610730500206683 [DOI] [PubMed] [Google Scholar]
- Wilson R. S., Scherr P. A., Hoganson G., Bienias J. L., Evans D. A., & Bennett D. A (2005). Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology, 25(1), 8–14. doi:10.1159/000085307 [DOI] [PubMed] [Google Scholar]
- Zahodne L. B., Manly J. J., Smith J., Seeman T., & Lachman M. E (2017). Socioeconomic, health, and psychosocial mediators of racial disparities in cognition in early, middle, and late adulthood. Psychology and Aging, 32, 118–130. doi:10.1037/pag0000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.